Abstract
So far, no pediatric doses for indinavir combined with ritonavir have been defined. This study evaluated the pharmacokinetics of 400 mg of indinavir/m2 combined with 125 mg of ritonavir/m2 every 12 h (q12h) in 14 human immunodeficiency virus type 1-infected children. The area under the concentration-time curve from 0 to 24 h and the minimum concentration of drug in serum for indinavir were similar to those for 800 mg of indinavir-100 mg of ritonavir q12h in adults, while the maximum concentration of drug in serum was slightly decreased, with geometric mean ratios (90% confidence intervals in parentheses) of 1.1 (0.87 to 1.3), 0.96 (0.60 to 1.5), and 0.80 (0.68 to 0.94), respectively.
The human immunodeficiency virus (HIV) protease inhibitor indinavir has been licensed since 1996 for the treatment of HIV type 1 (HIV-1) infection in adults and children aged 4 years and older (7-10). Indinavir, if used as a sole protease inhibitor, should be administered every 8 h (q8h), preferably when the patient's stomach is empty or with a low-caloric meal. The addition of the HIV protease inhibitor ritonavir to therapy involving indinavir is known to increase levels of indinavir in plasma, especially at the 12-h trough level (minimum concentration of drug in serum [Cmin]) (2, 11, 12, 19). The increased Cmin of indinavir when combined with ritonavir can potentially improve the antiretroviral efficacy of the regimen (1).
Data on the use of indinavir as a component of highly active antiretroviral therapy for HIV-infected children are relatively limited and often based on small sample sizes (4, 6, 14-18, 22-24). Children generally show lower 8-h trough levels (Cmin) of indinavir than adults and may therefore be at higher risk of virologic failure (4, 6). For HIV-infected children, the combination of indinavir and ritonavir is promising in view of its higher Cmin and the possibility of q12h administration. However, this combination in children has very rarely been explored.
The present study was a prospective, one-armed, one-period, open-label study of HIV-1-infected children between 2 and 18 years of age. After enrollment, patients started study medication consisting of indinavir at 400 mg/m2 q12h with ritonavir at 125 mg/m2 q12h on study day 1. On the pharmacokinetic sampling day, 2 to 4 weeks after study day 1, a 12-h pharmacokinetic curve was recorded to obtain plasma levels of indinavir and ritonavir (13). Pharmacokinetic parameters of indinavir and ritonavir were calculated by noncompartmental methods. Safety was assessed at the baseline (before the study medication was started) and on the pharmacokinetic sampling day. All statistical tests were performed with SPSS (Chicago, Ill.), version 10.0.
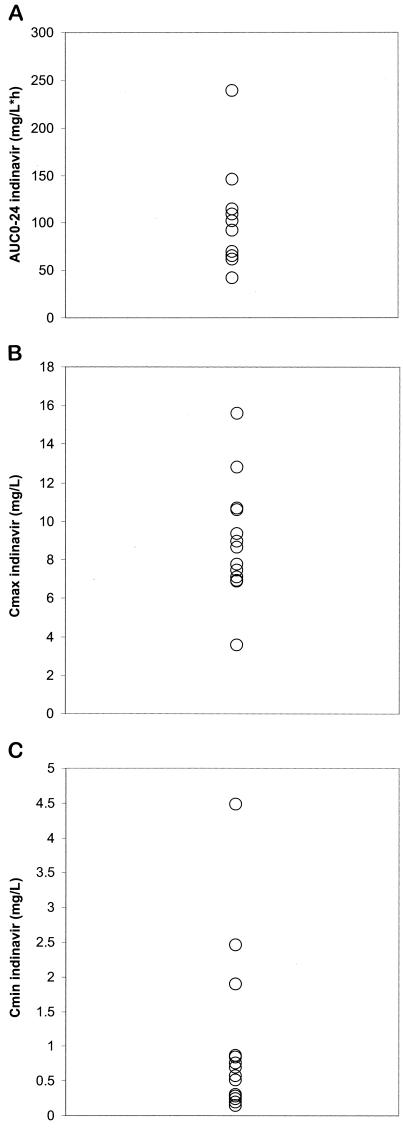
Baseline characteristics of the 14 HIV-infected children that were enrolled are given in Table 1. Pharmacokinetic parameters for indinavir and ritonavir for the 14 patients are summarized in Table 2. Overall, the regimen of indinavir at 400 mg/m2 combined with ritonavir at 125 mg/m2 q12h resulted in a significantly larger area under the concentration-time curve (AUC0-24) for indinavir than was reflected in reference data for indinavir q8h in both adults and children. This increase in AUC0-24 was reflected in a significantly higher geometric mean Cmin, while the maximum concentration of drug in serum (Cmax) was only marginally and nonsignificantly increased. For none of the patients was the Cmin of indinavir below 0.1 mg/liter, a value which has been associated with an increased virologic failure rate (Fig. 1) (3).
TABLE 1.
Baseline characteristics of 14 enrolled patients
| Characteristic | Value for characteristic |
|---|---|
| Gendera | |
| Male | 6 (43) |
| Female | 8 (57) |
| Age (years)b | 8.4 (4.3) |
| Racea | |
| White | 2 (14) |
| Black | 9 (64) |
| Asian | 1 (7) |
| Other | 2 (14) |
| Dose of indinavir (mg/m2 q12h)b | 392 (34) |
| Dose of ritonavir (mg/m2 q12h)b | 124 (8) |
| No. (%) of patients having undergone one or more prior antiretroviral therapies (%) | 10 (71) |
| Indinavir q8hc | 8 (80) |
| Indinavir-ritonavir q12hc | 1 (10) |
| Nelfinavirc | 1 (10) |
| Zidovudine + lamivudinec | 10 (100) |
| CD4 cell countb | |
| Cells/mm3 | 824 (613) |
| % of age-specific median cell counts | 75 (44) |
| Plasma HIV RNA viral load (log10 copies/ml)b | 3.60 (1.05) |
| No. of subjects undergoing concomitant treatment | |
| Zidovudine-lamivudine | 12 |
| Didanosine-stavudine | 2 |
| Fluconazole | 1 |
| Terbinafine | 1 |
| Co-trimoxazole | 2 |
Number (%) of subjects (total no. of subjects, 14).
Mean (standard deviation) for 14 subjects.
Number (%) of subjects (total no. of subjects, 10).
TABLE 2.
Pharmacokinetic parameters for indinavir and low-dose ritonavir for 14 children in this study and comparisons with those for historical controlsa
| Value | Value for parameter:
|
|||
|---|---|---|---|---|
| AUC0-24 | Cmax | Cmin | Tmax | |
| Indinavir | ||||
| Geometric mean for 14 patients in this study | 92.9 (75.4-114.5) | 8.6 (7.3-10.1) | 0.63 (0.40-1.0) | 2.8 (1.8-3.1) |
| GMR of parameter for this study to that for two previous protocolsb | 1.8 (1.5-2.3)* | 1.1 (0.97-1.4) | 8.4 (5.3-13.4)* | NA |
| GMR of parameter for this study to that for Merck protocol 021c | 1.8 (1.4-2.2)* | 1.2 (0.99-1.4) | 4.9 (3.1-7.9)* | NA |
| GMR of parameter for this study to that for a previous studyd | 1.1 (0.87-1.3) | 0.80 (0.68-0.94)* | 0.96 (0.60-1.5) | ND |
| Ritonavir | ||||
| Geometric mean for 14 patients in this study | 76.8 (61.0-96.7) | 6.0 (4.7-7.8) | 1.1 (0.84-1.6) | 3.6 (1.5-4.2) |
| GMR of parameter for this study to that for a previous studyd | 3.8 (3.0-4.8)* | 2.9 (2.2-3.7)* | 3.2 (2.4-4.3)* | ND |
For AUC0-24, Cmax, and Cmin, ranges in parentheses are 90% confidence intervals; for Tmax (time to minimum concentration of drug in serum), ranges in parentheses are interquartile ranges. GMR, geometric mean ratio; NA, not available; ND, not determined; *, statistically significant.
Combined protocols Merck 068 and PACTG395, involving 500 mg of indinavir/m2 q8h in children.
Merck protocol 021 involved 800 mg of indinavir q8h in adults.
The study by Arnaiz et al. (2), involving 800 mg of indinavir plus 100 mg of ritonavir q12h in adults.
FIG. 1.
AUC0-24 (A), Cmax (B), and Cmin(C) values for indinavir in 14 patients.
Compared to the pharmacokinetic data for 800 mg indinavir with 100 mg ritonavir q12h in adults, the geometric means of AUC0-24 and Cmin for indinavir were not significantly different, while the geometric mean of Cmax was modestly but significantly decreased.
For ritonavir, AUC0-12, Cmax, and Cmin values were significantly higher than those reflected in historical references for 100 mg ritonavir q12h combined with indinavir in adults (Table 2).
Of the 14 children enrolled, 8 (57%) underwent adverse clinical or laboratory experiences between study days 1 and 2. In total, 22 adverse experiences were recorded (Table 3).
TABLE 3.
Description of adverse experiences
| Adverse experience | Frequencya |
|---|---|
| Gastrointestinal disorder | 3 (14) |
| Ear, nose, and throat infection | 1 (5) |
| Skin disorder | 2 (9) |
| Urinary tract disorders (nephrolithiasis and leucocyturia) | 2 (9) |
| Jaundice/hepatomegaly | 2 (9) |
| Abnormal liver function test | 4 (18) |
| Abnormal blood cell count | 1 (5) |
| Increased cholesterol/triglyceride levels | 5 (23) |
| Increased amylase levels | 1 (5) |
| Decreased platelet count | 1 (5) |
| Total | 22 (100) |
Number of experiences, with percentage of total number of experiences in parentheses.
Published data on HIV-infected children indicate that a pediatric dose of indinavir q8h should be on the order of 500 to 600 mg/m2 (4, 17). The pharmacokinetics of indinavir as a sole protease inhibitor in children is characterized by a higher Cmax than that for adults, followed by relatively high drug clearance, often resulting in a lower Cmin than that for adults (4-6, 15). In substantial numbers of children, the Cmin of indinavir is below 0.1 mg/liter, a concentration which has been associated with virologic efficacy and approximates the in vitro 95% inhibitory concentration of indinavir for wild-type virus (1, 3). While doses of indinavir above 500 to 600 mg/m2 q8h result in higher Cmin values and possibly better clinical responses, the toxicity of indinavir seems to be dose limiting in children (4, 20). In the 14 HIV-1 infected children described here, the regimen of 400 mg of indinavir/m2 q12h with 125 mg of ritonavir/m2 q12h resulted in higher AUC0-24 and Cmin values than those reflected in pharmacokinetic data on indinavir q8h in both adults and children. The explored dose combination resulted in AUC0-24 and Cmin values for indinavir that were approximately similar to those reflected in the pharmacokinetic data for indinavir with low-dose ritonavir in adults (2).
In our study, AUC0-24 and Cmax values for indinavir were dose-proportionally lower than those reflected in published data from a case series involving 4 children between 0.8 and 10 years old treated with 500 mg of indinavir/m2 and 100 mg of ritonavir/m2 q12h (21). In contrast, the Cmin of indinavir was approximately 40% higher, and not lower, as might have been expected, than that found in the case series. In our study, the AUC0-24 and Cmin values for indinavir were 59 and 50% higher, respectively, than those reflected in preliminary pharmacokinetic data from a pediatric study of 11 patients between 3 and 11 years old who used 350 mg of indinavir/m2 with 125 mg of ritonavir/m2 q12h, while Cmax was 16% higher in our study (E. G. Chadwick, J. H. Rodman, P. Samson, T. Fenton, E. J. Abrams, B. Nowak, S. I. Pelton, S. Lavoie, K. Knapp, M. Bambji, and R. Yogev, 10th Conf. Retrovir. Opportunistic Infect., abstr. 875, 2003). The differences in AUC0-24 and Cmin values were especially remarkable, since the two regimens were distinguished from each other only by a slight difference (14%) in indinavir doses (400 versus 350 mg/m2) (25). When considering our data with respect to the other data on pharmacokinetics of indinavir combined with ritonavir in children, it should be taken into account that differences in outcome may be partly due to high interindividual variability and the limited sample sizes used in these studies. In conclusion, the investigated doses of indinavir combined with ritonavir resulted in a higher-than-expected AUC0-24, combined with a favorably increased Cmin values for indinavir (>0.1 mg/liter in all children). While the short-term tolerability of the study medication was generally good, data on the long-term safety and efficacy of this regimen in children are needed for a better evaluation.
Acknowledgments
The study nurses and medical students of the Erasmus MC/Sophia Children's Hospital, Division of Pediatric Infectious Diseases and Immunology, Rotterdam, are kindly acknowledged for their collection of the samples. The technicians of the Department of Clinical Pharmacy, University Medical Center Nijmegen, are kindly acknowledged for their analysis of the plasma samples.
Financial support for this study was received from Merck & Co., Inc., West Point, Pa.
REFERENCES
- 1.Acosta, E. P., T. N. Kakuda, R. C. Brundage, P. L. Anderson, and C. V. Fletcher. 2000. Pharmacodynamics of human immunodeficiency virus type 1 protease inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S151-S159. [DOI] [PubMed] [Google Scholar]
- 2.Arnaiz, J. A., J. Mallolas, D. Podzamczer, J. Gerstoft, J. D. Lundgren, P. Cahn, G. Fatkenheuer, A. D'Arminio-Monforte, A. Casiro, P. Reiss, D. M. Burger, M. Stek, and J. M. Gatell. 2003. Continued indinavir versus switching to indinavir/ritonavir in HIV-infected patients with suppressed viral load. AIDS 17:831-840. [DOI] [PubMed] [Google Scholar]
- 3.Burger, D. M., R. M. W. Hoetelmans, P. W. H. Hugen, J. W. Mulder, P. L. Meenhorst, P. P. Koopmans, K. Brinkman, M. Keuter, W. Dolmans, and Y. A. Hekster. 1998. Low plasma concentrations of indinavir are related to virological treatment failure in HIV-1 infected patients on indinavir-containing triple therapy. Antivir. Ther. 3:215-220. [PubMed] [Google Scholar]
- 4.Burger, D. M., A. M. van Rossum, P. W. Hugen, M. H. Suur, N. G. Hartwig, S. P. Geelen, H. J. Scherpbier, R. M. Hoetelmans, A. G. Vulto, and R. de Groot. 2001. Pharmacokinetics of the protease inhibitor indinavir in human immunodeficiency virus type 1-infected children. Antimicrob. Agents Chemother. 45:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher, C. V., R. C. Brundage, R. P. Remmel, L. M. Page, D. Weller, N. R. Calles, C. Simon, and M. W. Kline. 2000. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob. Agents Chemother. 44:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatti, G., A. Vigano, N. Sala, S. Vella, M. Bassetti, D. Bassetti, and N. Principi. 2000. Indinavir pharmacokinetics and pharmacodynamics in children with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 44:752-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, L. Jonas, A. Meibohm, D. Holder, W. A. Schleif, J. H. Condra, E. A. Emini, R. Isaacs, J. A. Chodakewitz, and D. D. Richman. 1998. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA 280:35-41. [DOI] [PubMed] [Google Scholar]
- 8.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 9.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, A. Meibohm, J. H. Condra, F. T. Valentine, D. McMahon, C. Gonzalez, L. Jonas, E. A. Emini, J. A. Chodakewitz, R. Isaacs, and D. D. Richman. 2000. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 133:35-39. [DOI] [PubMed] [Google Scholar]
- 10.Hammer, S. M., K. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, J. E. Feinberg, H. H. Balfour, L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 11.Hsu, A., G. R. Granneman, G. Cao, L. Carothers, A. Japour, T. El-Shourbagy, S. Dennis, J. Berg, K. Erdman, J. M. Leonard, and E. Sun. 1998. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob. Agents Chemother. 42:2784-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugen, P. W., D. M. Burger, H. J. ter Hofstede, P. P. Koopmans, M. Stek, Y. A. Hekster, P. Reiss, and J. M. Lange. 2000. Dose-finding study of a once-daily indinavir/ritonavir regimen. J. Acquir. Immune Defic. Syndr. 25:236-245. [DOI] [PubMed] [Google Scholar]
- 13.Hugen, P. W. H., C. P. W. G. M. Verwey-van Wissen, D. M. Burger, E. W. Wuis, P. P. Koopmans, and Y. A. Hekster. 1999. Simultaneous determination of the HIV-protease inhibitors indinavir, nelfinavir, saquinavir and ritonavir in human plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. B 727:139-149. [DOI] [PubMed] [Google Scholar]
- 14.Jankelevich, S., B. U. Mueller, C. L. Mackall, S. Smith, S. Zwerski, L. V. Wood, S. L. Zeichner, L. Serchuck, S. M. Steinberg, R. P. Nelson, J. W. Sleasman, B. Y. Nguyen, P. A. Pizzo, and R. Yarchoan. 2001. Long-term virologic and immunologic responses in human immunodeficiency virus type 1-infected children treated with indinavir, zidovudine, and lamivudine. J. Infect. Dis. 183:1116-1120. [DOI] [PubMed] [Google Scholar]
- 15.Kline, M. W., C. V. Fletcher, A. T. Harris, K. D. Evans, R. C. Brundage, R. P. Remmel, N. R. Calles, S. B. Kirkpatrick, and C. Simon. 1998. A pilot study of combination therapy with indinavir, stavudine (d4T), and didanosine (ddI) in children infected with human immunodeficiency virus. J. Pediatr. 132:543-546. [DOI] [PubMed] [Google Scholar]
- 16.Monpoux, F., N. Sirvent, J. Cottalorda, R. Mariani, and J. C. Lefbvre. 1997. Stavudine, lamivudine and indinavir in children with advanced HIV-1 infection: preliminary experience. AIDS 11:1523-1525. [PubMed] [Google Scholar]
- 17.Mueller, B. U., J. Sleasman, R. P. Nelson, S. Smith, P. Deutsch, W. Ju, S. M. Steinberg, F. M. Balis, P. F. Jarosinski, P. Brouwers, G. Mistry, G. Winchell, S. Zwerski, S. Sei, L. V. Wood, S. Zeichner, and P. A. Pizzo. 1998. A phase I/II study of the protease inhibitor indinavir in children with HIV infection. Pediatrics 102:101-109. [DOI] [PubMed] [Google Scholar]
- 18.Rutstein, R., A. Feingold, D. Meislich, B. Word, and B. Rudy. 1997. Protease inhibitor therapy in children with perinatally acquired HIV infection. AIDS 11:F107-F111. [DOI] [PubMed] [Google Scholar]
- 19.Saah, A. J., G. A. Winchell, M. L. Nessly, M. A. Seniuk, R. R. Rhodes, and P. J. Deutsch. 2001. Pharmacokinetic profile and tolerability of indinavir-ritonavir combinations in healthy volunteers. Antimicrob. Agents Chemother. 45:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rossum, A. M., J. P. Dieleman, P. L. Fraaij, K. Cransberg, N. G. Hartwig, D. M. Burger, I. C. Gyssens, and R. de Groot. 2002. Persistent sterile leukocyturia is associated with impaired renal function in human immunodeficiency virus type 1-infected children treated with indinavir. Pediatrics 110:e19. [DOI] [PubMed] [Google Scholar]
- 21.van Rossum, A. M. C., R. de Groot, N. G. Hartwig, C. M. Weemaes, S. Head, and D. M. Burger. 2000. Pharmacokinetics of indinavir and low-dose ritonavir in children with HIV-1 infection. AIDS 14:2209-2219. [DOI] [PubMed] [Google Scholar]
- 22.van Rossum, A. M. C., H. G. M. Niesters, S. P. M. Geelen, H. J. Scherpbier, N. G. Hartwig, C. M. Weemaes, A. J. P. Veerman, M. H. Suur, E. R. De Graeff-Meeder, W. A. T. Slieker, W. C. J. Hop, A. D. M. E. Osterhaus, D. M. Burger, and R. de Groot. 2000. Clinical and virologic response to combination treatment with indinavir, zidovudine, and lamivudine in children with human immunodeficiency virus-1 infection: a multicentre study in the Netherlands. J. Pediatr. 136:780-788. [DOI] [PubMed] [Google Scholar]
- 23.Vigano, A., L. Dally, D. Bricalli, N. Sala, M. Pirillo, M. Saresella, D. Trabattoni, S. Vella, M. Clerici, and N. Principi. 1999. Clinical and immuno-virologic characterization of the efficacy of stavudine, lamivudine, and indinavir in human immunodeficiency virus infection. J. Pediatr. 135:675-682. [DOI] [PubMed] [Google Scholar]
- 24.Wintergerst, U., F. Hoffmann, B. Solder, G. Notheis, T. Petropoulou, J. Eberle, L. Gurtzler, and B. H. Belohradsky. 1998. Comparison of two antiretroviral triple combinations including the protease inhibitor indinavir in children infected with human immunodeficiency virus. Pediatr. Infect. Dis. J. 17:495-499. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe, S. J., and J. V. Aranda (ed.). 1992. Pediatric pharmacology: therapeutic principles in practice, 2nd ed., W. B. Saunders Co., Philadelphia, Pa.