Abstract
The Deinococcus-Thermus group of species is currently recognized as a distinct phylum solely on the basis of their branching in 16S rRNA trees. No unique biochemical or molecular characteristics that can distinguish this group from all other bacteria are known at present. In this work, we describe eight conserved indels (viz., inserts or deletions) in seven widely distributed proteins that are distinctive characteristics of the Deinococcus-Thermus phylum but are not found in any other group of bacteria. The identified signatures include a 7-amino-acid (aa) insert in threonyl-tRNA synthetase, 1- and 3-aa inserts in the RNA polymerase β′ subunit, a 5-aa deletion in signal recognition particle (Ffh/SR54), a 2-aa insert in major sigma factor 70 (σ70), a 2-aa insert in seryl-tRNA synthetase (SerRS), a 1-aa insert in ribosomal protein L1, and a 2-aa insert in UvrA homologs. By using PCR primers for conserved regions, fragments of these genes were amplified from a number of Deinococcus-Thermus species, and all such fragments (except SerRS in Deinococcus proteolyticus) were found to contain the indicated signatures. The presence of these signatures in various species from all three known genera within this phylum, viz., Deinococcus, Thermus, and Meiothermus, provide evidence that they are likely distinctive characteristics of the entire phylum which were introduced in a common ancestor of this group. The signature in SerRS, which is absent in D. proteolyticus, was likely introduced after the branching of this species. Phylogenetic studies as well as the nature of the inserts in some of these proteins (viz., σ70 and SerRS) also support a sister group relationship between the Thermus and the Meiothermus genera. The identified signatures provide strong evidence for the monophyletic nature of the Deinococcus-Thermus phylum. These molecular markers should prove very useful in the identification of new species related to this group.
The Deinococcaceae are well known for their extreme resistance to UV, desiccation, and ionizing radiation (1, 28) and have been found in canned meat, soil, animal feces, dust, and irradiated medical instruments (27, 31, 35). These nonsporulating, polyploidic cocci are also resistant to hydrogen peroxide and other agents that damage DNA due to a highly efficient DNA repair system (1, 28). Although these species stain gram positive due to a thick layer of peptidoglycan in their cell wall (2, 30, 31), they are structurally similar to the gram-negative bacteria in that they contain an outer membrane (2, 31, 41). This outer layer, however, is unique in that it does not contain lipid A or heptoses typical of other gram-negative bacteria (2, 7, 31). Thermus-Meiothermus species are gram-negative thermophilic rods which have been isolated from thermally polluted streams, industrial and domestic water taps, and hydrothermal vents with neutral to alkaline pH (9, 35). Both the deinococci and the Thermus-Meiothermus groups have an atypical cell wall containing ornithine in place of diaminopimelic acid in their peptidoglycan, although the species of the Thermus-Meiothermus group have few other characteristics in common with the deinococci (2, 30, 31). Because of their unusual radiation resistance characteristics, the deinococci have been of great interest with regard to the bioremediation of sites contaminated with radiation and toxic chemicals (1, 3, 28). There is also much interest in this group due to the production of a number of thermostable enzymes of much biotechnological importance, e.g., Taq polymerase. These practical applications have increased the desire to understand the evolutionary relationships of the Deinococcus-Thermus group to other bacteria (10, 22, 26, 39, 42, 43).
The Deinococcus, Thermus, and Meiothermus genera have been grouped together as a distinct phylum within Bacteria based on their close clustering in 16S rRNA trees, despite morphological and physiological dissimilarity (2, 25, 31, 42, 44). With the rapid increase in the sequence database entries, it is becoming increasingly imprecise to assign species to different taxonomic groups based on branch patterns alone (25). Unfortunately, there are presently no other criteria or molecular means by which species belonging to this phylum can be unambiguously distinguished from other bacterial phyla (2, 9, 31, 33). We have described a new approach based on conserved indels (i.e., inserts or deletions) found in different proteins that is helpful in distinguishing the major bacterial phyla and to understand the interrelationships among them (13-15, 17). Recently, a large number of conserved indels (or signature sequences) which provide distinctive molecular markers for the identification of proteobacteria, chlamydiae, and cyanobacteria have been described (12, 14, 19).
The present communication describes for the first time a number of conserved indels in widely distributed proteins that are distinctive characteristics of the Deinococcus-Thermus phylum. The identified signatures include a 7-amino-acid (aa) insert in Thr-tRNA synthetase (ThrRS), a 5-aa deletion in the signal recognition particle protein Ffh, a 1- and a 3-aa insert in the β′ subunit of RNA polymerase RpoC, a 1-aa insert in the ribosomal protein L1, and 2-aa inserts in major sigma factor 70 (σ70), seryl-tRNA synthetase (SerRS), and UvrA homologs. The sequence information for these proteins was previously available from only a limited number of Deinococcus and Thermus species. As the Meiothermus genus has only recently been established, there is little sequence information currently available for this group (33). We have tested the specificity of the identified signatures by PCR amplifying and sequencing fragments of these genes from additional Deinococcus and Meiothermus species for which no sequence information was available. The presence of these signatures in all of the species examined (with a single exception) provide evidence that they are likely distinctive characteristics of the entire phylum and might be used as molecular markers for this group of species.
MATERIALS AND METHODS
Identification of signature sequences.
Deinococcus-Thermus-specific signatures were identified in global multiple sequence alignments by means of visual inspection. Alignments for different proteins were constructed by using the ALIGN PLUS 4 program (Scientific & Educational Software, Durham, N.C.) as described in earlier work (12, 14, 19). To qualify as a useful group-specific signature, any identified indel was required to be uniquely (or mainly) present in the Deinococcus-Thermus-Meiothermus group of species and to be flanked on both sides by conserved regions to ensure that the observed insertion or deletion was not a result of sequencing errors or alignment artifacts.
PCR amplification and sequencing.
Cultures of Meiothermus ruber (ATCC 35948), Meiothermus silvanus (DMSZ 9946), and Deinococcus grandis (DSMZ 3963) were generously supplied by Peter Gogarten and Lorraine Olenzenski (36). Deinococcus proteolyticus (ATCC 35074) high-molecular-weight DNA was prepared as previously described (6, 16). Oligonucleotide primers, in opposite orientations, were designed for conserved regions in the protein sequences that flanked these signatures based on sequence information from available Deinococcus-Thermus and other species. Degeneracy was incorporated into the primers to account for differences in codon usage among different species. The primers were synthesized at the Molecular Biology Central Facility (MOBIX) of McMaster University, Hamilton, Ontario, Canada.
PCRs.
PCR was performed in a Techne Techgene thermocycler. The PCRs had a final volume of 10 μl, and all primer sets were optimized for Mg2+ concentration (in the range of 1.5 to 4 mM) for each DNA strain tested. PCR amplification was carried out over 30 cycles (15 s at 94°C, 15 s at 55 or 45°C, 1 min at 72°C) with an initial 1-min hot start at 94°C and a final extension step (15 s at 94°C, 15 s at 55°C, 7 min at 72°C) (12). The reaction mix also contained 2% dimethyl sulfoxide, which improves PCR performance by lowering the melting temperature of DNA. DNA fragments of the expected size were purified from 0.8% (wt/vol) agarose gels (using a GENECLEAN kit) and subcloned into the plasmid pDRIVE by using a TU cloning kit (Invitrogen). Escherichia coli JM109 cells were transformed with the ligated vector and insert, and the inserts from a number of positive clones were sequenced at MOBIX. Sequences of all cloned fragments were run through a BLAST search to ensure that the amplified gene was from a novel source. The primer sequences used for the amplification of different genes are as follows.
(i) σ70.
The following primers were successful in amplifying 504-bp inserts from D. grandis, D. proteolyticus, M. silvanus, and M. ruber: forward, 5′-ACNTAYGCNACNTGGTGGAT-3′; reverse, 5′-GRNGCYTTRTTYTCDATYTG-3′, where N represents A, G, C, or T; Y is C or T; R is A or G; and D is A, G, or T.
(ii) Threonyl tRNA synthetase.
Fragments 432 bp in length were generated from D. grandis, M. silvanus, and M. ruber genomic DNA with the following primers: forward, 5′-TTCCGSCACWCSCTGGSCCACGTCMTG-3′; reverse, 5′-CCNCKCCARTANGCNCC-3′, where S represents C or G, W is A or T, K is G or T, and M is A or C.
(iii) Signal recognition particle Ffh.
The following primers were used to amplify a 264-bp fragment from D. grandis: forward, 5′-ATHYTNGGNATGGGNGA-3′; reverse, 5′-CKYTCYTTNACNGTCAT-3′, where H represents A, C, or T.
(iv) SerRS.
Fragments from M. silvanus and D. proteolyticus of 234 bp in length were successfully amplified by using forward primer 5′-CACSARTTYCGYAARGTNGARCAG-3′ and reverse primer 5′-CGARCAGGARTGGGTYTCGCGRTC-3′.
(v) RNA polymerase β′ subunit RpoC.
RpoC gene fragments (645 bp) were amplified by PCR from M. silvanus, M. ruber, D. proteolyticus, and D. grandis by using the following primers: forward, 5′-GAYGGNGGNMGNTTYGC-3′; reverse, 5′-CATYTGRTCNCCRTCRAARTC-3′.
(vi) Ribosomal protein L1.
A 510-bp fragment was generated from M. silvanus and D. grandis by using the following primers: forward, 5′-ATGCCTAAGCACGGCAAGCGTTACC-3′; reverse, 5′-CCGGTCTTGTCGTTGCGGAACTC-3′.
(vii) Exinuclease ABC subunit A UvrA.
A 639-bp fragment was amplified from M. silvanus by using forward primer 5′-TGGCYTTYGACACCATCTACGCCGAGG-3′ and reverse primer 5′-AGGCGAACTTCTCSGAGWACAGCTCCTC-3′.
Phylogenetic analysis.
Phylogenetic analysis on protein sequences was carried out by procedures described in earlier work (6, 20). Multiple alignment of protein homologs from different groups of bacteria was created by using the ALIGN program. The data for the newly sequenced fragments were added to the alignment, and the fragments were all trimmed to the same length as the amplified fragments. Phylogenetic analyses were performed in both the presence and the absence of the signature region to determine its influence on the branching pattern. The aligned sequences were used to generate 100 bootstrapped data sets with the SEQBOOT program, and genetic distances were calculated by PROTDIST by using Kimura's method (23). Neighbor-joining trees from these distances were constructed by the NEIGHBOR program (40). A consensus tree for various bootstrapped sequences was obtained by using the CONSENSE program. All of these phylogenetic programs are part of the PHYLIP software package (version 3.5; J. Felsenstein, University of Washington, Seattle, Wash.).
Nucleotide sequence accession numbers.
The sequence data for all of the gene fragments cloned and sequenced in this work have been deposited in the GenBank database under accession numbers AY450950, AY452779, AY453862, AY489057, and AY453858 for D. grandis; AY450951 and AY453857 for D. proteolyticus; AY450952, AY452780, AY455864, AY489058, AY489059, and AY452782 for M. sylvanus; and AY452778, AY452781, and AY453861 for M. ruber.
RESULTS
Description of conserved indels that are distinctive of the Deinococcus-Thermus group.
Conserved indels that are shared by all members of one particular group (group-specific signatures), or are commonly present in species belonging to more than one taxa (main-line signatures), provide powerful means to identify individual taxa in molecular terms and to understand the interrelationships among them (13, 14, 17). Evolutionarily significant indels are generally of defined size, are present at a specific location, and are flanked by conserved regions to ensure their reliability. We describe below a number of conserved indels in widely distributed proteins that are distinctive characteristics of the Deinococcus-Thermus group (Deinococcus, Thermus, and Meiothermus) of species.
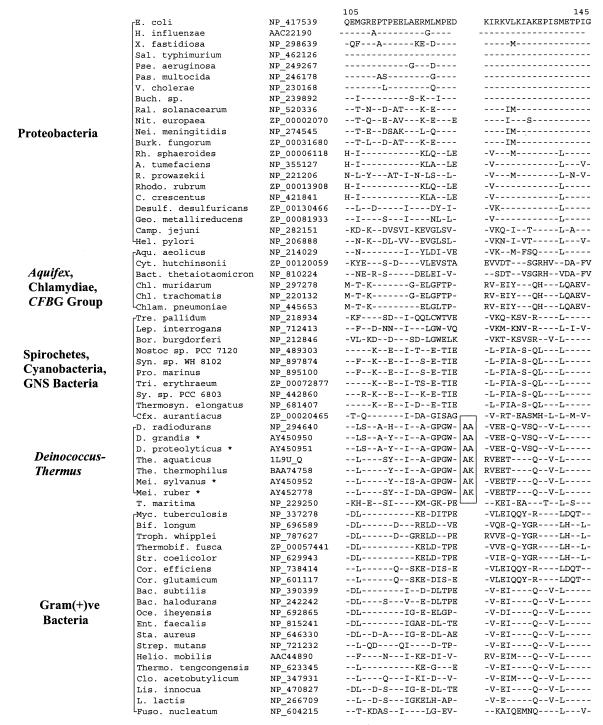
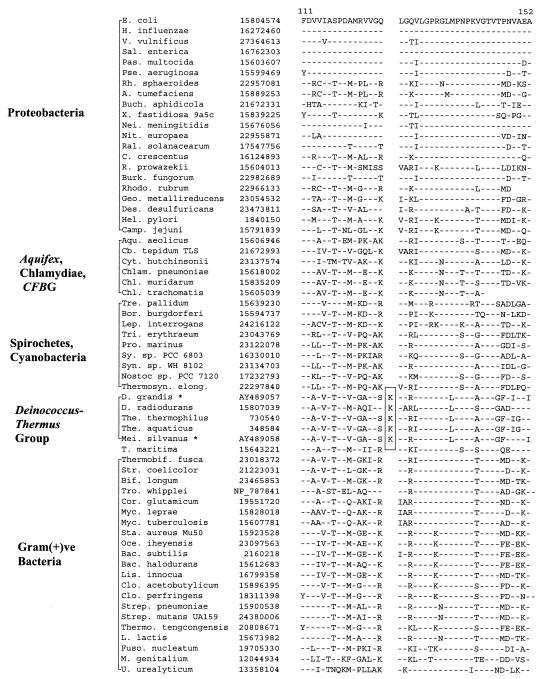
In σ70, which plays a central role in the transcription process by conferring promoter specificity to RNA polymerase (5), a 2-aa insert is present in a conserved region in various available Deinococcus-Thermus homologs (viz., Deinococcus radiodurans, Thermus aquaticus, and Thermus thermophilus) but not in any other bacteria. However, variable inserts are present in this region in Mycoplasma species (data not shown), which are likely of independent origin. The specificity of this insert for the Deinococcus-Thermus phylum was tested by PCR amplifying and sequencing fragments of the σ70 gene from four other members belonging to this group for which no sequence information was available. Results of these studies, which are included in Fig. 1, show that all four species tested, which included two Deinococcus (D. grandis and D. proteolyticus) and two Meiothermus (M. ruber and M. silvanus) species contained the identified signature. The sequence region which flanked the identified insert (Fig. 1, boxed region) was also found to be distinctive for Deinococcus-Thermus-Meiothermus species. Since sequence information for this signature is now available for representatives from all three genera within the Deinococcus-Thermus phylum, the shared presence of this insert in all of them strongly indicates that it is very likely a distinctive characteristic of the entire phylum.
FIG. 1.
Partial sequence alignment for σ70 proteins showing a 2-aa insert (boxed area) in a conserved region that is uniquely present in Deinococcus, Thermus, and Meiothermus homologs. Dashes in this and all other alignments indicate identity to the amino acid on the top line (E. coli protein). The position of this sequence region in the E. coli protein is indicated at the top. The accession numbers of different proteins are provided in the second column. Sequence information for only representative species from different bacterial groups is presented. The sequences marked with an asterisk were cloned and sequenced in the present work. Abbreviations for the species names are as follows: A., Agrobacterium; Aqu., Aquifex; Bac., Bacillus; Bact., Bacteroides; Bif., Bifidobacterium; Bor., Borrelia; Buch., Buchnera; C., Caulobacter; Camp., Campylobacter; Cfx., Chloroflexus; Chl., Chlamydia; Chlam., Chlamydophila; Clo., Clostridium; Cor., Corynebacterium; Cyt., Cytophaga; D., Deinococcus; Des., Desulfovibrio; E., Escherichia; Ent., Enterococcus; Fuso., Fusobacterium; Geo., Geobacter; H., Haemophilus; Hel., Helicobacter; Helio., Heliobacillus; L., Lactococcus; Lep., Leptospira; Lis., Listeria; M., Mycoplasma; Mei., Meiothermus; Myc., Mycobacterium; Nei., Neisseria; Nit., Nitrosomonas; Oce., Oceanobacillus; Pas., Pasteurella; Pse., Pseudomonas; Ral., Ralstonia; Rh., Rhodobacter; Rho., Rhodospirillum; R., Rickettsia; Sal., Salmonella; Sta., Staphylococcus; Str., Streptomyces; Strep., Streptococcus; Sy., Synechocystis; Syn., Synechococcus; T., Thermotoga; Thermo., Thermoanaerobacter; The., Thermus; Thermosyn., Thermosynechococcus; Tre., Treponema; Tri., Trichodesmium; Troph., Tropheryma; V., Vibrio; X., Xylella. GNS, green nonsulfur bacteria; Gram(+)ve, gram-positive.
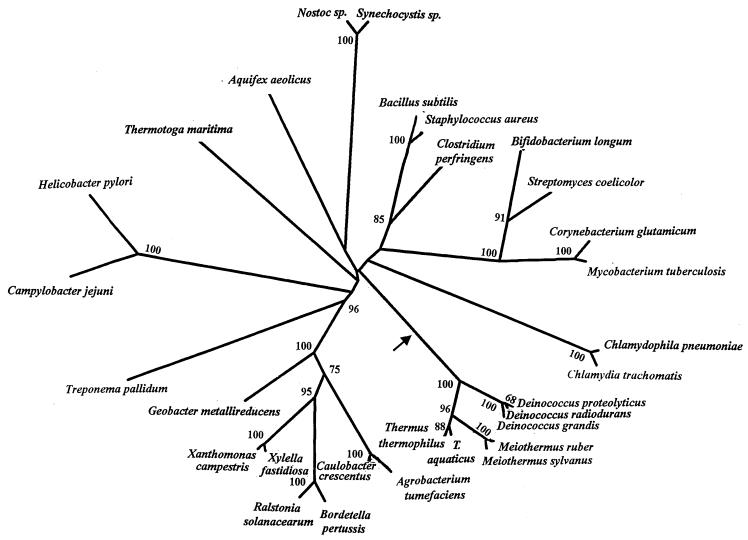
We also performed phylogenetic analysis based on σ70 sequences from different bacteria. For these purposes, 169 aa positions for which σ70 sequence information was available from different species were utilized. The sequence alignment data were used to generate 100 bootstrapped data sets, and a consensus neighbor-joining tree was obtained from these data. At the same time, a neighbor-joining distance tree showing branch lengths, shown in Fig. 2, was also constructed. The bootstrap scores for different nodes which were >50 are marked on this tree. As shown in Fig. 2, most bacterial groups are clearly distinguished from each other in the tree (as shown by their high bootstrap score), but their branching orders or interrelationships are not resolved, which is a common problem with phylogenetic trees (13, 25). Importantly, in the present context, all of the Deinococcus-Thermus-Meiothermus species formed a well-defined group, branching together 100% of the time. Within this group, different Deinococcus-Thermus genera (viz., Deinococcus, Thermus, and Meiothermus) formed distinct clusters. Of these genera, Deinococcus was found to be the earliest branching lineage, whereas a closer relationship was seen between the Thermus and Meiothermus genera. A similar relationship among these groups is seen in the 16S rRNA trees (2, 39). It is noteworthy that the insert sequence in Deinococcus species consists of two alanine residues, whereas in Thermus and Meiothermus species, the insert sequence is comprised of one alanine and one lysine residue (i.e., AK), again indicating a closer relationship between these two genera. Thus, the inference from signature sequences is in accordance with results from phylogenetic analysis (2, 6, 39, 42). We have also performed phylogenetic analysis on these sequences after omitting the insert region. The tree obtained in this case was very similar to that in Fig. 2 (results not shown), indicating that the observed relationship is not dependant upon or affected by the presence of the insert.
FIG. 2.
A neighbor-joining distance tree with branch lengths based on σ70 sequences. The tree is based on 169 aa positions for which sequence information was available for various species. The bootstrap scores (out of 100) of various nodes which were >50 are indicated. The arrow marks the suggested position where the identified insert in this gene was introduced.
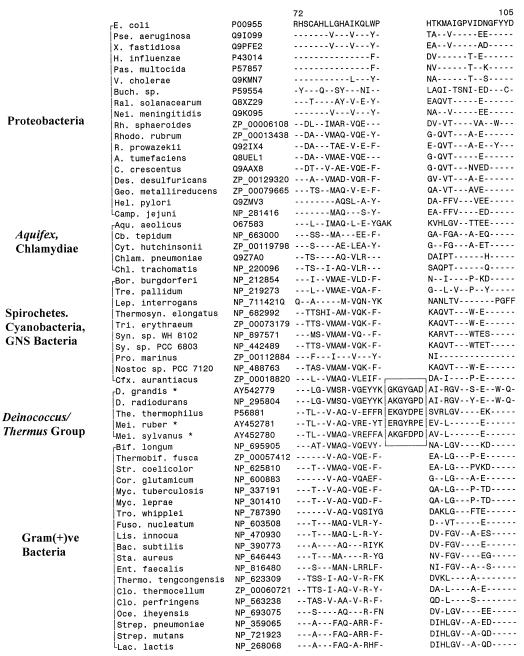
Aminoacyl-tRNA synthetases play an essential role in protein synthesis by catalyzing the attachment of correct amino acids to the 3′-terminal ends of their cognate tRNA to form the aminoacyl-tRNA, which provide the basic substrate for protein synthesis (21). We have identified a 7-aa insert in a conserved region of ThrRS which is uniquely present in all three available sequences from Deinococcus-Thermus species (D. radiodurans, T. thermophilus, and T. aquaticus) but is not found in any other bacterial homologs (Fig. 3). The specificity of this signature was tested by PCR amplifying fragments of the ThrRS gene from several additional species, viz., D. grandis, M. silvanus, and M. ruber. The sequences for these species are included in Fig. 3, and all of the sequences were found to contain the identified signature. These results strongly indicate that the identified indel in ThrRS is likely a group-specific signature for the Deinococcus-Thermus group. In a phylogenetic tree based on ThrRS sequences (data not shown), all of the Deinococcus-Thermus species were found to group together with high affinity (95% bootstrap score), supporting the inference that they form a monophyletic group.
FIG. 3.
Excerpt from a sequence alignment of threonyl-tRNA synthetase showing a 7-aa insert (boxed areas) that is distinctive of the Deinococcus-Thermus-Meiothermus species. Cb., Chlorobium; Pro., Prochlorococcus. See the legend to Fig. 1 for an explanation of additional abbreviations used.
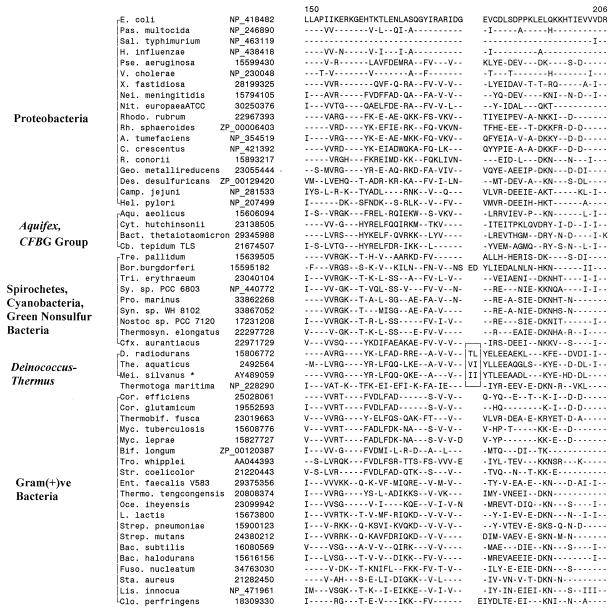
The core subunits of the RNA polymerase (i.e., α, β, and β′) are evolutionarily conserved in sequence, structure, and function in all species ranging from bacteria to humans (24, 37). In the β′ subunit of RNA polymerase, which is encoded by the rpoC gene, we have identified a 1- and a 3-aa insert in conserved regions that are only present in D. radiodurans, T. aquaticus, and T. thermophilus but are not found in any other bacteria or species (Fig. 4). Further studies on this indel were carried out by cloning and sequencing fragments of the rpoC gene from three other Deinococcus-Thermus species (D. grandis, M. ruber, and M. silvanus). Results of these studies, which are included in Fig. 4, show that both of these inserts were present in all of these species, indicating that they are distinctive characteristics of the Deinococcus-Thermus phylum. Furthermore, as seen in the case of σ70 homologs, the sequence of the 3-aa insert in various Meiothermus and Thermus species (i.e., KDE) was identical and differed from that seen in the Deinococcus species, pointing to a closer relationship between the Meiothermus and Thermus species.
FIG. 4.
Partial sequence alignment of RNA polymerase β′ subunit (RpoC) showing 1- and 3-aa conserved inserts (boxed area) that are specific for the Deinococcus-Thermus-Meiothermus species. This sequence region is highly divergent in Thermotoga maritima (data not shown); hence, it is difficult to infer the presence or absence of the inserts in this species. See the legends to Fig. 1 and 3 for abbreviations used.
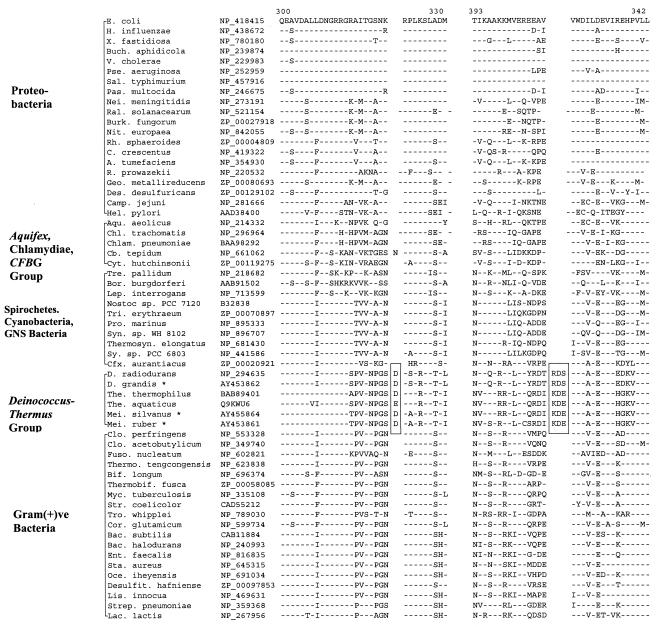
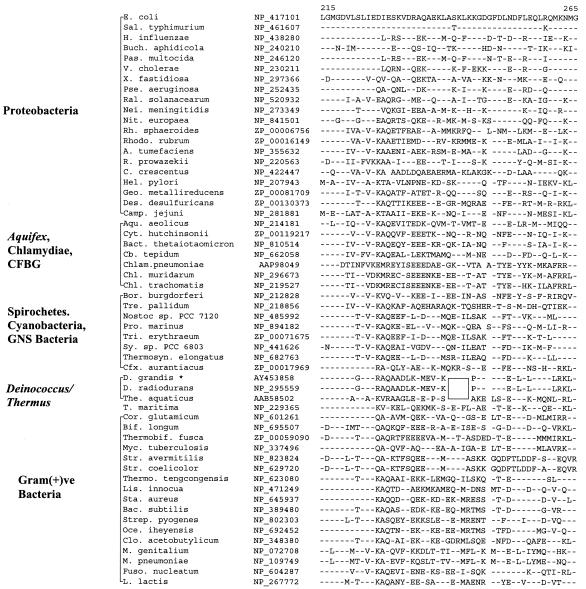
The L1 protein of the 50S ribosomal subunit has been implicated in the release and removal of deacylated tRNA from the E site (32). Our studies have revealed a conserved 1-aa insert in L1 protein which is present in the available Deinococcus-Thermus species (Fig. 5). We have amplified 510-bp fragments of the L1 protein gene from two other members of this group (M. silvanus and D. grandis), and both were found to contain the indel. The insert in all cases is a lysine residue, indicating that it was introduced only once in a common ancestor of these species.
FIG. 5.
Sequence alignment of ribosomal L1 protein showing a conserved 1-aa insert (boxed area) that is distinctive of the Deinococcus-Thermus-Meiothermus species. Burk., Burkholderia. See the legends to Fig. 1 and 3 for additional abbreviations used.
A 2-aa insert is also found in the exinuclease ABC subunit A homologs (i.e., UvrA protein) of the Deinococcus-Thermus group (Fig. 6). UvrA is one of the two subunits of the damage recognition complex required for nucleotide excision during repair of UV light-induced DNA damage (8). Previously, sequences were available only from two species belonging to this phylum (D. radiodurans and T. aquaticus), and no information existed for the Meiothermus group of species. To bridge this gap, we have amplified a 639-bp fragment of the uvrA gene from M. silvanus. The amplified fragment contained the 2-aa insert, providing evidence that this signature is also a distinctive characteristic of the Deinococcus-Thermus group. In addition to the Deinococcus-Thermus species, a 2-aa insert is also present in this position in Borrelia burgdorferi, which may have originated either independently or through lateral gene transfer (LGT).
FIG. 6.
Partial sequence alignment of UvrA protein showing a 2-aa insertion in different Deinococcus-Thermus-Meiothermus homologs (boxed area). The insert seen in B. burgdorferi could either have occurred independently or have been derived by means of LGT. See the legends to Fig. 1 and 3 for abbreviations.
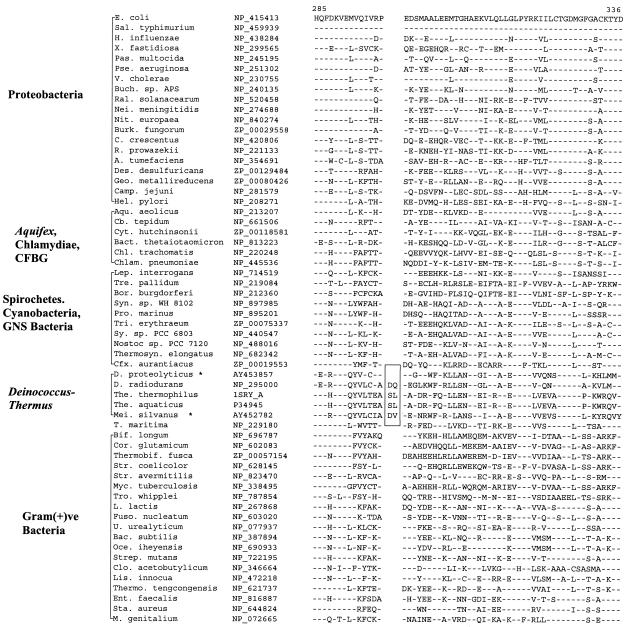
All sequenced organisms contain an Ffh/SRP54 family member, which forms part of the signal recognition particle and coordinates the cotranslational targeting of secretory and membrane proteins to either the membrane of the endoplasmic reticulum or the plasma membrane in bacteria (29). In E. coli, the signal recognition particle is composed of Ffh protein and the 4.5S RNA. A 5-aa deletion is present in a conserved region of the Ffh protein that is only seen in Deinococcus-Thermus homologs but is not found in any other bacteria (Fig. 7). Since sequence information for Deinococcus-Thermus was available only from D. radiodurans and T. aquaticus, we have amplified and sequenced a fragment of the Ffh gene from D. grandis. The fragment from this species was also found to contain the deletion (Fig. 7), indicating that this signature may also be specific for the entire Deinococcus-Thermus phylum. Due to DNA limitation, sequence information for this signature for Meiothermus species was not obtained.
FIG. 7.
Partial sequence alignments of Ffh protein showing a 5-aa deletion (boxed area) that is a unique characteristic of the Deinococcus-Thermus-Meiothermus homologs. See the legends to Fig. 1 and 3 for abbreviations.
Another signature for the Deinococcus-Thermus group is present in the protein SerRS (21). The signature in this case consists of a 2-aa insert in a conserved region that is commonly present in the SerRS homologs from various available Deinococcus-Thermus species (D. radiodurans, T. aquaticus, and T. thermophilus) (Fig. 8). By means of PCR amplification, we have obtained sequence information for the SerRS gene from two additional species, viz., M. silvanus and D. proteolyticus. Interestingly, while the M. silvanus homolog contained the signature, this insert was not found in the fragment derived from D. proteolyticus (Fig. 8). The most parsimonious explanation for these results is that the insert was introduced in a common ancestor of D. radiodurans, T. aquaticus, T. thermophilus, and M. silvanus after the divergence of D. proteolyticus. However, the possibility that the insert has been lost from D. proteolyticus cannot be excluded.
FIG. 8.
Excerpt from SerRS sequence alignment showing a 2-aa insert (boxed area) that is present in various Deinococcus-Thermus-Meiothermus species, except D. proteolyticus. This insert was likely introduced in a common ancestor of this group after the branching of D. proteolyticus. U., Ureaplasma. See the legends to Fig. 1, 3, and 5 for additional abbreviations used.
DISCUSSION
In 16S rRNA and various protein trees, the Deinococcus-Thermus phylum represents one of the earliest branching groups within the Bacteria (10, 16, 19, 25, 38, 39, 42, 44). In the past, this phylum consisted of only two genera (Deinococcus and Thermus); however, a third genus (Meiothermus) has recently been established (34). According to branch patterns, species belonging to the genus Meiothermus form a sister lineage with Thermus species, forming the order Thermales (family Thermaceae), which clusters together with the distantly related Deinococcales in a single lineage (2, 34, 39). Although Deinococcales shows 77.5 to 81% 16S rRNA sequence similarity with the Thermus-Meiothermus group, and species of the Deinococcus-Thermus group share an A3β murein-type peptidoglycan (l-ornithine as the diamino acid and glycylglycine as the interpeptide bridge) and menaquinone-8 as their major respiratory quinone, these characteristics are not unique to these groups, and they share few other characteristics in common (2, 4, 30, 31). Although a few unique base pairs that appear limited to the genus Deinococcus have been identified in the16S rRNA sequences (2), currently there is no molecular or structural marker known that is distinctive to the entire Deinococcus-Thermus phylum which might be used to distinguish or define this group of bacteria from all others.
In the present work, we have identified eight conserved indels in seven widely distributed proteins that are distinctive characteristics of the Deinococcus-Thermus phylum. Based on the work reported here and information available in the databases, information for six of these proteins containing seven signatures (viz., SerRs, ThrRS, σ70, RpoC, UvrA, and ribosomal L1 protein) is available from all three genera within the Deinococcus-Thermus phylum. The sequence information for Ffh/SR54 is currently available from only Deinococcus and Thermus genera, but based on the observation that Meiothermus forms a sister lineage with Thermus species (9, 33, 39), it is expected that this signature will also be found in Meiothermus organisms. Except for the absence of the SerRS insert in D. proteolyticus, the identified signatures are present in all Deinococcus-Thermus species examined but not in other bacteria. These signatures thus provide molecular markers for distinguishing the Deinococcus-Thermus phylum from all other bacteria and for identifying new species related to them based simply on the presence or absence of these signatures. The presence of these distinctive signatures also provides strong evidence for the monophyletic nature of the Deinococcus-Thermus phylum as indicated by 16S rRNA trees (38, 42, 44). The most likely explanation for these signatures is that they were introduced in a common ancestor of this lineage and then were passed on to all descendants. This inference is also supported by phylogenetic analysis based on a number of these proteins. The presence of the insert in SerRS in various Deinococcus-Thermus species, but not D. proteolyticus, might be accounted for by two different possibilities. First, it is possible that this insert was introduced in a common ancestor of the other Deinococcus-Thermus species after the branching of D. proteolyticus. Alternatively, this insert may have been introduced in a common ancestor of the entire phylum but then subsequently lost from D. proteolyticus. We favor the first of these possibilities, based on the observation that in phylogenetic trees derived from 16S rRNA sequences, a branch comprised of D. proteolyticus and D. radiophilus forms the deepest group within the Deinococcus-Thermus phylum (2, 39).
LGT is indicated to have played an important role in the evolution of the Deinococcus-Themus group. These organisms are thought to have received genes from a number of other phyla such as the Archaea, Eucarya, and cyanobacteria (11, 26, 36, 43). However, for the various genes studied in the present work, which contain identified signatures, there is no evidence of lateral gene exchange between the Deinococcus-Thermus group and other bacterial phyla, except possibly the UvrA gene in B. burgdorferi. If these genes were subjects of LGTs, one would expect a more random distribution of these signature sequences in which these indels would have been present in other groups of bacteria and at the same time several Deinococcus-Thermus species would be lacking them, which is clearly not the case here. However, in contrast to these genes, a number of genes studied in earlier work contained signature sequences that were commonly shared by cyanobacteria and the Deinococcus-Thermus species, which may be the results of LGTs (13, 18).
We have also previously described many main-line signatures (i.e., indels commonly shared by a number of different bacterial phyla), which provide useful information concerning the phylogenetic placement of the Deinococcus-Thermus group within the bacterial domain (13, 15, 17). The distribution patterns of these signatures in bacterial sequences indicate that the Deinococcus-Thermus phylum has evolved after the divergence of various gram-positive phyla (viz., Firmicutes, Actinobacteria, Clostridia, and relatives) but before the emergence of Aquifex, Chloroflexi, cyanobacteria, spirochetes, the Chlamydia-Cytophaga-Flavobacteria-Bacteroides-green sulfur bacteria group, and proteobacteria (15, 17). The branching of the Deinococcus-Thermus phylum in between the gram-positive bacteria and gram-negative bacteria also accounts for a hitherto puzzling characteristic of Deinococcus. Although all Deinococcus-Thermus species are surrounded by an outer membrane, which is a distinguishing property of the gram-negative bacteria, most species belonging to the genus Deinococcus (all except D. grandis) exhibit positive Gram staining and contain a thick sacculus characteristic of gram-positive bacteria (2, 30, 31, 41). These seemingly contradictory properties are readily explained by the suggested placement of the Deinococcus-Thermus phylum between the gram-positive bacteria (monoderm bacteria surrounded by a single membrane) and gram-negative bacteria (diderm bacteria bound by both inner and outer membranes), and they indicate that this group of species may represent evolutionary intermediates in the transition between these two structurally distinct groups of bacteria (13).
Acknowledgments
This work was supported by a research grant from the National Science and Engineering Research Council of Canada.
We are thankful to Peter Gogarten and Lorraine Olenzenski for providing us bacterial strains and Melanine Havers for assistance in the creation of some of the signature sequence files.
REFERENCES
- 1.Battista, J. R. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203-224. [DOI] [PubMed] [Google Scholar]
- 2.Battista, J. R., and F. A. Rainey. 2001. Family I. Deinococcaceae Brooks and Murray 1981, 356VP, emend. Rainey, Nobre, Schumann, Stackebrandt and da Costa 1997, 513, p. 395-403. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, N.Y.
- 3.Brim, H., S. C. McFarlan, J. K. Fredrickson, K. W. Minton, M. Zhai, L. P. Wackett, and M. J. Daly. 2000. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 18:85-90. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, B. W., R. G. E. Murray, J. L. Johnson, E. Stackebrandt, C. R. Woese, and G. E. Fox. 1980. Red-pigmented micrococci: a basis for taxonomy. Int. J. Syst. Bacteriol. 30:627-646. [Google Scholar]
- 5.Burgess, R. R., and L. Anthony. 2001. How sigma docks to RNA polymerase and what sigma does. Curr. Opin. Microbiol. 4:126-131. [DOI] [PubMed] [Google Scholar]
- 6.Bustard, K., and R. S. Gupta. 1997. The sequences of heat shock protein 40 (DnaJ) homologs provide evidence for a close evolutionary relationship between the Deinococcus-Thermus group and cyanobacteria. J. Mol. Evol. 45:193-205. [DOI] [PubMed] [Google Scholar]
- 7.Counsell, T., and R. G. E. Murray. 1986. Polar lipid profiles of the genus Deinococcus. Int. J. Syst. Bacteriol. 36:202-206. [Google Scholar]
- 8.Courcelle, J., J. R. Donaldson, K.-H. Chow, and C. T. Courcelle. 2003. DNA damage-induced replication fork regression and processing in Escherichia coli. Science 299:1064-1067. [DOI] [PubMed] [Google Scholar]
- 9.da Costa, M. S., M. F. Nobre, and F. A. Rainey. 2001. Genus I. Thermus brock and freeze, 1969, 295AL, emend. Nobre, Truper and da Costa 1996b, 605, p. 404-414. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, N.Y.
- 10.Ferreira, A. C., M. F. Nobre, F. A. Rainey, M. T. Silva, R. Wait, J. Burghardt, A. P. Chung, and M. S. da Costa. 1997. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 47:939-947. [DOI] [PubMed] [Google Scholar]
- 11.Goldman, B. S., and R. G. Kranz. 1998. Evolution and horizontal transfer of an entire biosynthetic pathway for cytochrome c biogenesis: Helicobacter, Deinococcus, Archaea and more. Mol. Microbiol. 27:871-873. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths, E., and R. S. Gupta. 2002. Protein signatures distinctive of chlamydial species: horizontal transfers of cell wall biosynthesis genes glmU from archaea to chlamydiae and murA between chlamydiae and Streptomyces. Microbiology 148:2541-2549. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R. S. 1998. Protein phylogenies and signature sequences: a reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol. Mol. Biol. Rev. 62:1435-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, R. S. 2000. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol. Rev. 24:367-402. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, R. S. 2003. Evolutionary relationships among photosynthetic bacteria. Photosynth. Res. 76:173-183. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, R. S., K. Bustard, M. Falah, and D. Singh. 1997. Sequencing of heat shock protein 70 (DnaK) homologs from Deinococcus proteolyticus and Thermomicrobium roseum and their integration in a protein-based phylogeny of prokaryotes. J. Bacteriol. 179:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, R. S., and E. Griffiths. 2002. Critical issues in bacterial phylogeny. Theor. Popul. Biol. 61:423-434. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, R. S., and V. Johari. 1998. Signature sequences in diverse proteins provide evidence of a close evolutionary relationship between the Deinococcus-Thermus group and cyanobacteria. J. Mol. Evol. 46:716-720. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, R. S., M. Pereira, C. Chandrasekera, and V. Johari. 2003. Molecular signatures in protein sequences that are characteristic of cyanobacteria and plastid homologues. Int. J. Syst. Evol. Microbiol. 53:1833-1842. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, R. S., and B. Singh. 1994. Phylogenetic analysis of 70 kD heat shock protein sequences suggests a chimeric origin for the eukaryotic cell nucleus. Curr. Biol. 4:1104-1114. [DOI] [PubMed] [Google Scholar]
- 21.Ibba, M., A. W. Curnow, and D. Soll. 1997. Aminoacyl-tRNA synthesis: divergent routes to a common goal. Trends Biochem. Sci. 22:39-42. [DOI] [PubMed] [Google Scholar]
- 22.Karlin, S., and J. Mrazek. 2001. Predicted highly expressed and putative alien genes of Deinococcus radiodurans and implications for resistance to ionizing radiation damage. Proc. Natl. Acad. Sci. USA 98:5240-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 24.Klenk, H. P., and W. Zillig. 1994. DNA-dependent RNA polymerase subunit B as a tool for phylogenetic reconstructions: branching topology of the archaeal domain. J. Mol. Evol. 38:420-432. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig, W., and H.-P. Klenk. 2001. Overview: a phylogenetic backbone and taxonomic framework for prokaryotic systematics, p. 49-65. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology. Springer-Verlag, New York, N.Y.
- 26.Makarova, K. S., L. Aravind, Y. I. Wolf, R. L. Tatusov, K. W. Minton, E. V. Koonin, and M. J. Daly. 2001. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 65:44-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masters, C. I., R. G. Murray, B. E. Moseley, and K. W. Minton. 1991. DNA polymorphisms in new isolates of ‘Deinococcus radiopugnans’. J. Gen. Microbiol. 137:1459-1469. [DOI] [PubMed] [Google Scholar]
- 28.Minton, K. W. 1994. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol. Microbiol. 13:9-15. [DOI] [PubMed] [Google Scholar]
- 29.Muller, M., H. G. Koch, K. Beck, and U. Schafer. 2001. Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog. Nucleic Acid Res. Mol. Biol. 66:107-157. [DOI] [PubMed] [Google Scholar]
- 30.Murray, R. G. E. 1986. Family II. Deinococcaceae Brooks and Murray 1981, 356VP, p. 1035-1043. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. Lippincott Williams & Wilkins, Baltimore, Md.
- 31.Murray, R. G. E. 1992. The family Deinococcaceae, p. 3732-3744. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 32.Nikulin, A., I. Eliseikina, S. Tishchenko, N. Nevskaya, N. Davydova, O. Platonova, W. Piendl, M. Selmer, A. Liljas, D. Drygin, R. Zimmermann, M. Garber, and S. Nikonov. 2003. Structure of the L1 protuberance in the ribosome. Nat. Struct. Biol. 10:104-108. [DOI] [PubMed] [Google Scholar]
- 33.Nobre, M. F., and M. S. da Costa. 2001. Genus II. Meiothermus Nobre, Truper and da Costa 1996b, 605VP, p. 414-420. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, N.Y.
- 34.Nobre, M. F., H. G. Truper, and M. S. da Costa. 1996. Transfer of Thermus ruber (Loginova et al. 1994), Thermus silvanus (Tenreiro et al. 1995) and Thermus chliarophilus (Tenreiro et al. 1995) to Meiothermus gen. nov. as Meiothermus silvanus comb. nov., and Meiothermus chliarophilus comb. nov., respectively, and emendation of the genus Thermus. Int. J. Syst. Bacteriol. 46:604-606. [Google Scholar]
- 35.Nold, S. C., and D. M. Ward. 1995. Diverse Thermus species inhabit a single hot spring microbial mat. Syst. Appl. Microbiol. 18:274-278. [DOI] [PubMed] [Google Scholar]
- 36.Olendzenski, L., L. Liu, O. Zhaxybayeva, R. Murphey, D. G. Shin, and J. P. Gogarten. 2000. Horizontal transfer of archaeal genes into the Deinococcaceae: detection by molecular and computer-based approaches. J. Mol. E vol. 51:587-599. [DOI] [PubMed] [Google Scholar]
- 37.Olsen, G. J., and C. R. Woese. 1997. Archaeal genomics: an overview. Cell 89:991-994. [DOI] [PubMed] [Google Scholar]
- 38.Olsen, G. J., C. R. Woese, and R. Overbeek. 1994. The winds of (evolutionary) change: breathing new life into microbiology. J. Bacteriol. 176:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rainey, F. A., M. F. Nobre, P. Schumann, E. Stackebrandt, and M. S. da Costa. 1997. Phylogenetic diversity of the deinococci as determined by 16S ribosomal DNA sequence comparison. Int. J. Syst. Bacteriol. 47:510-514. [DOI] [PubMed] [Google Scholar]
- 40.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, B. G., and R. G. Murray. 1981. Isolation and characterization of the plasma membrane and the outer membrane of Deinococcus radiodurans strain Sark. Can. J. Microbiol. 27:729-734. [DOI] [PubMed] [Google Scholar]
- 42.Weisburg, W. G., S. J. Giovannoni, and C. R. Woese. 1989. The Deinococcus-Thermus phylum and the effect of rRNA composition on phylogenetic tree construction. Syst. Appl. Microbiol. 11:128-134. [DOI] [PubMed] [Google Scholar]
- 43.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, D. L. Richardson, K. S. Moffat, H. Y. Qin, L. X. Jiang, W. Pamphile, M. Crosby, M. Shen, J. J. Vamathevan, P. Lam, L. McDonald, T. Utterback, C. Zalewski, K. S. Makarova, L. Aravind, and M. J. Daly. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]