Abstract
Interferon (IFN)-γ displays a critical role in tuberculosis (TB), modulating the innate and adaptive immune responses. Previously, we reported that secretory leukocyte protease inhibitor (SLPI) is a pattern recognition receptor with anti-mycobacterial activity against Mycobacterium tuberculosis (Mtb). Herein, we determined whether IFN-γ modulated the levels of SLPI in TB patients. Plasma levels of SLPI and IFN-γ were studied in healthy donors (HDs) and TB patients. Peripheral blood mononuclear cells from HDs and patients with TB or defective IFN-γ receptor 1* were stimulated with Mtb antigen and SLPI, and IFN-γR expression levels were measured. Both SLPI and IFN-γ were significantly enhanced in plasma from those with TB compared with HDs. A direct association between SLPI levels and the severity of TB was detected. In addition, Mtb antigen stimulation decreased the SLPI produced by peripheral blood mononuclear cells from HDs, but not from TB or IFN-γR patients. Neutralization of IFN-γ reversed the inhibition of SLPI induced by Mtb antigen in HDs, but not in TB patients. Furthermore, recombinant IFN-γ was unable to modify the expression of SLPI in TB patients. Finally, IFN-γR expression was lower in TB compared with HD peripheral blood mononuclear cells. These results show that Mtb-induced IFN-γ down-modulated SLPI levels by signaling through the IFN-γR in HDs. This inhibitory mechanism was not observed in TB, probably because of the low expression of IFN-γR detected in these individuals.
Tuberculosis (TB) is among the most common causes of morbidity and mortality in patients with HIV infection. Although protective immunological mechanisms against Mycobacterium tuberculosis (Mtb) are not fully understood, resistance to mycobacterial infections is primarily mediated by the interaction of antigen-specific T cells and macrophages.1,2 This interaction depends on the cross talk of cytokines produced by these cells, and interferon (IFN)-γ is essential for protection.2,3 Thus, during the immune response of the host against Mtb, IFN-γ produced by type 1 helper T cells is recognized by its receptor on macrophages. The IFN-γ receptor (IFN-γR) is composed of two ligand-binding IFNGR1 chains associated with two signal-transducing IFNGR2 chains, and an associated signaling machinery.2–5 IFN-γ binds to its receptor and activates macrophages to efficient killing of intracellular mycobacteria. In humans, the loss-of-function mutations in IFNGR1 or IFNGR2 genes are closely associated with severe susceptibility to poorly virulent mycobacteria highlighted in childhood.4,6,7
Secretory leukocyte protease inhibitor (SLPI) is a serine protease inhibitor secreted by inflammatory and epithelial cells, mainly in the respiratory tract mucosa, and it is primarily active against neutrophilic elastase, cathepsin G, trypsin, and chymotrypsin.8 The expression and secretion of SLPI are down-modulated during chronic obstructive pulmonary disease.9–11 In addition, cathepsins B, L, and S and cigarette smoke exposure result in the cleavage and inactivation of SLPI.12,13 Moreover, it has been demonstrated that IFN-γ is a prominent stimulator of cathepsins and matrix metalloproteinase-12 and an inhibitor of SLPI.14 Remarkably, SLPI may also function as an endogenous immunomodulatory, anti-inflammatory, and/or antimicrobial substance.15–18 The antimicrobial effects of SLPI against several bacteria have been demonstrated.15 In particular, Nishimura et al17 described that recombinant mouse SLPI inhibited the growth of bacillus Calmette-Guérin (BCG) and Mtb through the disruption of the mycobacterial cell wall structure. Furthermore, we reported that human SLPI is a secreted pattern recognition receptor for mycobacteria that increases both the phagocytosis and killing of the pathogen.18 Remarkably, exposure of murine peritoneal macrophages to Mtb led to an increase in SLPI secretion.19 Thus, given the anti-inflammatory and anti-mycobacterial roles of SLPI in humans and taking into account the fact that SLPI is inhibited by IFN-γ,20 a crucial cytokine in the protective immunity against Mtb, herein we studied the effect of IFN-γ on the expression of SLPI during human active disease.
Materials and Methods
Subjects
Adult patients with active TB were evaluated at the Hospital F. J. Muñiz (Buenos Aires, Argentina). The diagnosis was established on the basis of clinical and radiological data, together with the identification of acid-fast bacilli in sputum. Physical examination, complete blood cell count, electrolyte determination, chest X-ray, and HIV test were performed for each patient. According to the extent and type of X-ray findings, the severity of lung involvement was classified into three groups: mild (a single lobe involved, and without visible cavities), moderate (unilateral involvement of two or more lobes and cavities, if present, reaching a total diameter ≤4 cm), and severe (massive involvement of both lungs and multiple cavities).21 Exclusion criteria included a positive HIV test result or the presence of concurrent infectious diseases. Healthy adult donors (HDs), individuals with no history of TB who had received BCG vaccination at birth, were also included in the study. The demographic characteristics of HD individuals and TB patients are shown in Supplemental Table S1. All participants provided a written informed consent for the collection of samples and subsequent analysis. All of the protocols were approved by the Ethical Committee of the Hospital F. J. Muñiz and the International Review Board Fundación Huésped. Two patients with inactivating mutations of the IFNGR1 were also included. Patient 1 (P1), a first child born from nonconsanguineous Mestizo Mexican parents, received BCG vaccine at birth without adverse reactions. At 2 years old, P1 was diagnosed with cutaneous TB and treated with anti-mycobacterial drugs during a year, with total cure of the skin lesions. At age 13 years, P1 required treatment for uveitis in the left eye during 1 year, with remission of symptoms. At the time of writing this article, the patient was 20 years old and reported doing well, with no infections. Patient 2 (P2), the second child from the same family, received BCG vaccine at birth, which caused him axillary lymphadenitis. A biopsy specimen of the lymph nodes was positive for acid-fast bacilli, and he received treatment with anti-TB drugs during 1 year, with complete remission. He did not have recurrence of any mycobacterial infection. At the time of writing this article, the patient was 15 years old, and he reported doing well, with no infections. Both P1 and P2 were studied to seek for immunological defects, and pieces of evidence were found about partial response to IFN-γ. Molecular studies demonstrated a heterozygous mutation in the IFNGR1 gene in both patients, affecting intracellular signaling. Epstein-Barr virus–transformed B-cell lines from both patients had been experimentally tested and showed no phosphorylation of Stat-1 when cells were treated with IFN-γ. Phosphorylation of Stat-1 in response to IFN-α demonstrated the specificity in the IFNGR defect.
Antigen
In vitro stimulation of cells throughout the study was performed with a cell lysate from the virulent Mtb H37Rv strain prepared by probe sonication [Mtb-antigen (Ag)] and obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases (Bethesda, MD) (NIH: Mtb, strain H37Rv, whole cell lysate, NR-14822).
Blood Samples, Cell Isolation, and Culture
Blood samples were obtained between 8 and 10 am in heparinized tubes. Plasma was obtained and preserved at −20°C. Peripheral blood mononuclear cells (PBMCs) were isolated over density gradient centrifugation on Ficoll-Paque (Amersham Biosciences, Piscataway, NJ). Then, 1 × 106 PBMCs/mL were cultured with RPMI 1640 medium (Gibco, Gaithersburg, MD) supplemented with l-glutamine, gentamicin, and 10% fetal bovine serum (Gibco). PBMCs were incubated in the presence or absence of 10 μg/mL Mtb-Ag for 48 hours. Afterward, media were removed and cell-free supernatants were assayed for SLPI by a homemade enzyme-linked immunosorbent assay (ELISA). In some experiments, 30 minutes before the stimulation with the antigen, PBMCs were incubated with 15 μg/mL blocking antibody against IFN-γ [functional grade mouse (IgG1κ) anti-human IFN-γ, clone MD-1; eBioscience, San Diego, CA] or 15 μg/mL purified mouse IgG1κ isotype control (clone P3.6.2.8.1; eBioscience). In other experiments, PBMCs were incubated in the presence or absence of 7.5 ng/mL recombinant IFN-γ (rIFN-γ; eBioscience).
SLPI and IFN-γ Determination
The levels of IFN-γ were determined by sandwich ELISA (lower limit of detection, 36 pg/mL) following the manufacturer’s instructions (eBioscience). The concentrations of SLPI in plasma and cell culture supernatants were measured by sandwich ELISA, with a lower limit of detection of 0.31 ng/mL, as previously described.22
Real-Time PCR
PBMCs were stimulated in the presence or absence of Mtb-Ag for 0, 16, 24, and 48 hours. Total RNA was then isolated with TRIzol (Invitrogen Life Technologies, Buenos Aires, Argentina), followed by reverse transcription, according to the manufacturer’s instructions (Promega, Madison, WI). Real-time PCR was performed with Mezcla Real Master Mix (Biodynamics SRL, Buenos Aires, Argentina) and specific primers for human IFN-γR (Integrated DNA Technologies, Biodynamics, Buenos Aires, Argentina). Relative RNA expression was normalized to the expression of GAPDH (encoding glyceraldehyde-3-phosphate dehydrogenase), calculated by the change-in-threshold (−ΔΔCT) method. The primers used for IFN-γR and GAPDH were as follows: 5′-TGTGTATGTGAGAATGAACGGAAG-3′ (forward) and 5′-AGGATACTGGAATCGCTAACTGG-3′ (reverse); and 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ (forward) and 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ (reverse), respectively.
Statistical Analysis
Analysis of variance and post hoc Dunnett’s multiple comparisons test were used as indicated in the figure legend. Wilcoxon rank sum test was used to analyze differences between unpaired samples. Correlation analyses were performed using the Pearson correlation test. P < 0.05 was considered significant.
Results
Expression of SLPI in HDs and TB Patients
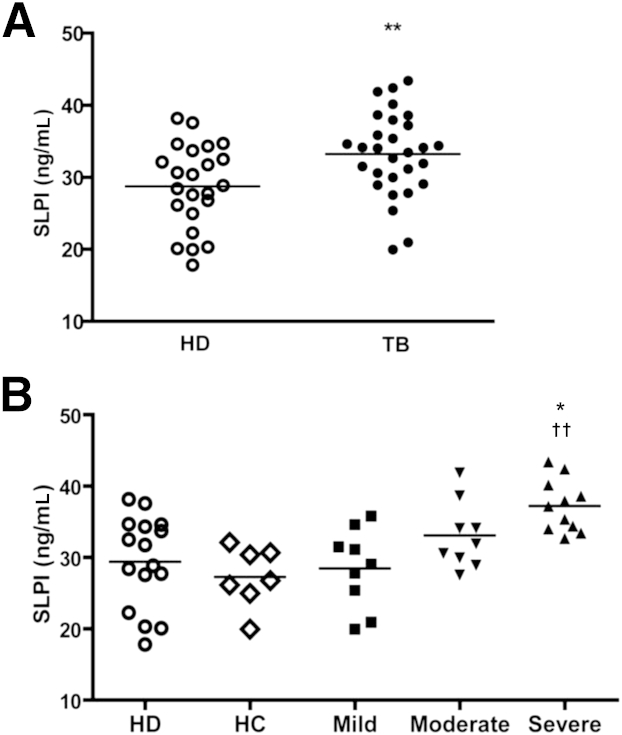
Exposure of murine peritoneal macrophages to Mtb led to an increase in SLPI secretion.19 To assess whether Mtb also induces the production of SLPI in humans, we analyzed the levels of SLPI in plasma of TB patients and HD subjects. Figure 1A shows that SLPI was significantly higher in patients’ plasma compared with HDs. To determine a potential relationship between SLPI concentration and the severity of TB, we investigated the levels of SLPI in patients classified according to their radiological lesions (mild, moderate, and severe), as previously described.21 Interestingly, we found that patients with severe TB displayed higher levels of SLPI compared with patients with mild TB and with subjects in contact with TB patients (HCs) and HDs (Figure 1B). Patients with moderate TB showed slightly higher levels of SLPI than HDs, but significant differences were not detected (Figure 1B).
Figure 1.
Plasma levels of SLPI in HDs and TB patients. A: Plasmatic SLPI levels. SLPI levels were analyzed by sandwich ELISA in plasma from HDs (n = 23) and TB patients (n = 29). B: Association between plasma SLPI levels and disease severity. HD data from A were plot as subjects who had been in contact with TB patients (HCs) and those who had not been in contact with patients (HDs). Patients with TB were also classified as mild, moderate, and severe.21 Student’s t-test (A) or analysis of variance post hoc Dunnett’s multiple comparisons test (B) was used. ∗P < 0.05, between severe and mild TB patients; ∗∗P < 0.01 (A); ††P < 0.01 between severe TB patients and HDs or HCs (B).
IFN-γ Levels in Plasma from HD and TB Patients
Because the severity of TB disease is associated with a reduced T-cell IFN-γ production,23 we next analyzed the plasma levels of this cytokine in TB patients and HD subjects. Figure 2A shows that IFN-γ levels were significantly higher in patients compared with HDs. Therefore, although Mtb-Ag–stimulated PBMCs from TB patients had diminished IFN-γ production, compared with healthy tuberculin reactors,23 plasma levels of this cytokine are augmented during active disease, as previously reported.24,25 Furthermore, we found a positive correlation between IFN-γ and SLPI levels in plasma of TB patients (Pearson coefficient: r = 0.532, P < 0.05), but not in HD subjects (Pearson coefficient: r = 0.024, P > 0.05) (Figure 2B).
Figure 2.
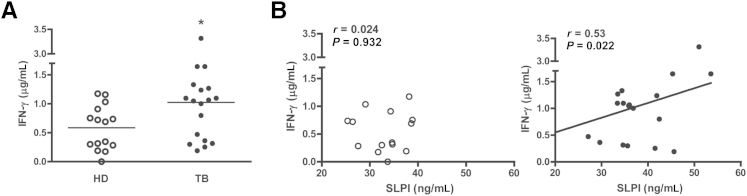
Correlation between the levels of IFN-γ and SLPI in HDs and TB patients. IFN-γ plasma levels in HD and TB patients were determined. A: ELISA results for plasmatic IFN-γ levels from HDs (n = 15) and TB patients (n = 19). B: Correlation between the levels of SLPI and IFN-γ in the plasma of HDs (white circles; n = 15) and TB patients (black circles; n = 19). Student’s t-test (A) and Pearson correlation coefficient (B). ∗P < 0.05.
Effect of IFN-γ on SLPI Levels
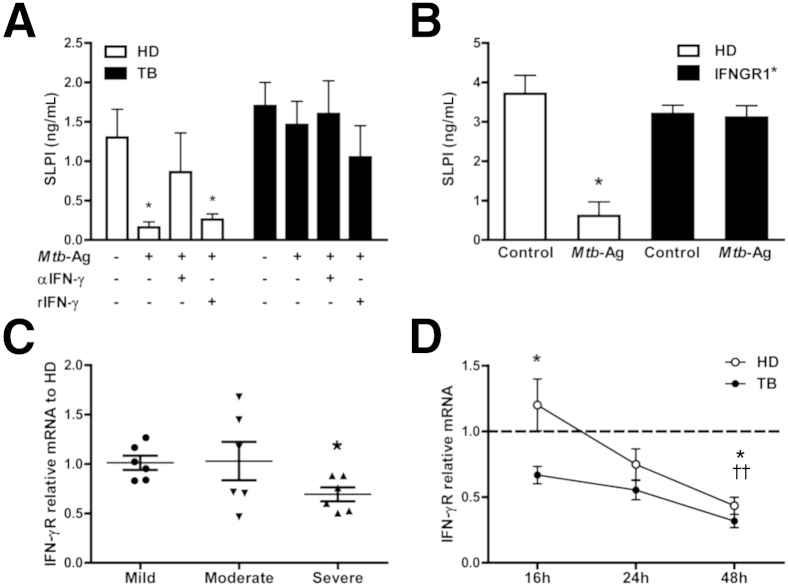
It is known that IFN-γ inhibits the production of SLPI in HD subjects20 and that Mtb-Ag–stimulated PBMCs increase IFN-γ production.26 Therefore, we speculated that the increased secretion of IFN-γ induced by Mtb-Ag in vitro might decrease the production of SLPI in HD and TB patients. To investigate this, PBMCs were cultured with Mtb-Ag and SLPI levels were measured in cell culture supernatants. Mtb-Ag stimulation of PBMCs from HDs decreased SLPI levels in culture supernatants (Figure 3A). Moreover, when Mtb-Ag–stimulated cells from HDs were incubated with a blocking antibody against IFN-γ, the levels of SLPI were restored (Figure 3A). However, when PBMCs from TB patients were stimulated with Mtb-Ag or blocking anti–IFN-γ antibody, no modification of the levels of SLPI was detected (Figure 3A). Even more, the addition of rIFN-γ to cultures with Mtb-Ag did not modify the levels of SLPI production by TB patients.
Figure 3.
Effect of IFN-γ on SLPI levels. A: PBMCs from HDs and TB patients were cultured for 48 hours in the presence or absence of 10 μg/mL Mtb-Ag, ±7.5 ng/mL rIFN-γ, and ±15 μg/mL blocking monoclonal antibody against IFN-γ (30 minutes) or 15 μg/mL isotype control. B: PBMCs from IFNGR1∗ patients and age-matched HDs were cultured for 5 days in the presence or absence of Mtb-Ag. A and B: After culture, medium was removed and cell-free supernatants were collected and assayed for SLPI. Data are expressed as the means ± SEM in HDs (n = 6) and TB patients (n = 6) (A) and in HDs (n = 4) and IFNGR1∗ patients (n = 2) (B). Analysis of variance post hoc Dunnett’s multiple comparisons test. C and D: Real-time PCR for IFN-γR expression on PBMCs. C: PBMCs were obtained from patients with TB classified as mild (n = 6), moderate (n = 6), and severe (n = 6)21 and HD subjects (n = 6). IFN-γR expression was determined by quantitative real-time PCR. Values are represented as fold of increase using the comparative method for relative quantification. Expression of IFN-γR was calculated as follows (a comparative method for relative quantification after normalization to GAPDH expression): where and Mann-Whitney test. C: Significant differences between mild and severe TB patients. D: PBMCs from patients with TB (n = 9) and HDs (n = 11) were cultured by 16, 24, or 48 hours in the presence or absence of Mtb-Ag. Cells were harvested, and IFN-γR expression was determined as in C. Expression of IFN-γR was calculated as follows (a comparative method for relative quantification after normalization to GAPDH expression): where and ΔΔCt = [ΔCtstimulated−ΔCtunstimulated]. Unpaired t-test, for significant differences between HDs and TB patients at 16 hours and for significant differences between 16 and 48 hours in TB patients. ∗P < 0.05 (A–D); ††P < 0.01, for significant differences between 16 and 48 hours in HDs (D).
IFN-γ signals are mediated through its heterodimeric receptor, a molecule down-modulated in patients with active TB but restored on anti-TB therapy.27 Then, we wondered whether the absence of signaling through IFN-γR could be responsible for the ineffectiveness of IFN-γ on modifying the production of SLPI by TB patients. To this end, we investigated the levels of SLPI produced by two patients with IFNGR1 mutation, in response to Mtb-Ag stimulation. Mtb did not modify the amount of SLPI produced by PBMCs from IFNGR1∗ patients, suggesting that signaling through IFN-γR modulates SLPI expression and secretion (Figure 3B). Given that SLPI levels were associated with TB severity, we further measured the expression of IFN-γR by real-time PCR in patients with mild, moderate, and severe TB. Among TB patients, individuals with severe disease displayed the lowest amounts of IFN-γR (Figure 3C).
Finally, to verify in our physiological model that the absence of SLPI inhibition in TB patients was mediated by the down-modulation of the expression of IFN-γR, we next evaluated by real-time PCR the levels of IFN-γR in PBMCs from TB patients and HDs, treated with Mtb-Ag. PBMCs from TB patients cultured overnight with Mtb-Ag expressed significantly lower levels of IFN-γR compared with HDs (Figure 3D). Interestingly, these differences were not observed after 24 or 48 hours of antigen stimulation of the cells (Figure 3D), probably because in vitro, the levels of IFN-γR were down-modulated during the time of cell culture. Taken together, our findings indicate that TB patients are unable to modulate the expression of SLPI, further suggesting that the anti-mycobacterial activity of SLPI would not be compromised.
Discussion
SLPI is a secreted pattern recognition receptor for Mtb detected in sputum from patients with active disease.18 In the present study, we found that both SLPI and IFN-γ were significantly enhanced in plasma from TB patients compared with HDs. Furthermore, our findings demonstrated a direct association between SLPI levels and the severity of TB disease. Stimulation of PBMCs with Mtb antigen significantly diminished the levels of SLPI produced by HDs but did not modify the production of SLPI by patients with active disease or patients with mutation in IFNGR1. Moreover, neutralization of endogenous IFN-γ reversed the inhibition of SLPI induced by Mtb in HDs, whereas rIFN-γ was unable to modify the expression of SLPI in TB patients. Furthermore, our data showed that IFN-γR was expressed at lower levels in TB patients.
The anti-mycobacterial activity of SLPI either in vitro or in vivo has been described.17,18 SLPI was also shown to decrease cell-mediated immunity.28,29 More important, SLPI inhibited IFNγ-induced NF-κB activation in macrophages of patients with chronic obstructive pulmonary disease.30 Considering that cell-mediated immunity is critical in the immune response of the host to Mtb, in particular IFN-γ production,31 the mentioned reported results preclude the possible use of SLPI as a new therapeutic drug in human TB. However, our present findings show, for the first time to our knowledge, that, in contrast to HD subjects, TB patients are unable to modify SLPI production in response to IFN-γ. Consistent with our results, differences in the behavior of other immune cells from HD subjects and TB patients have been previously shown,32,33 although the mechanisms underlying those defects were unclear. Herein, we observed an association between the levels of plasma SLPI and the severity of TB disease (Figure 1B). Actually, the levels of IFN-γ in the serum of TB patients correlated with SLPI concentration (Figure 2B). In particular, this later correlation reinforces our in vitro results showing that IFN-γ fails to inhibit SLPI production in TB patients, in contrast to data described for murine cells.34 Our results also demonstrated an absence of modulation of SLPI levels by IFN-γ in Mtb-stimulated cells from TB patients (Figure 3A).
Functional IFN-γR included two ligand-binding IFNGR1 chains associated with two signal-transducing IFNGR2 chains and belongs to the class II cytokine receptor family, a class of receptors that bind ligand in the small angle of a V formed by the two Ig-like folds that constitute the extracellular domain.4 One of the major mechanisms used by the body to regulate the strength and duration of IFN-γ responses is through the modulation of the levels of its receptor. Accordingly, the down-modulation of IFN-γR surface protein and mRNA expression has been demonstrated in PBMCs from TB patients exposed to live Mtb.27 Consistent with those reports, we also observed a decrease in IFN-γR mRNA levels associated with the severity of TB disease (Figure 3C) and a time-dependent down-modulation of IFNGR transcripts in TB patients (Figure 3D). Probably, those changes in the expression of IFN-γR might be related to the levels of IFN-γ present in the medium.
Loss of functional IFNGR1 appears to be associated with an increased susceptibility to some viruses and intracellular bacterial infection. Patients with inactivating mutations of IFNGR1 or IFNGR2 chains often present severe susceptibility to poorly virulent mycobacteria, such as early-onset bacille Calmette-Guerin infection in childhood.4,35 Experiments conducted with PBMCs from IFNGR1∗ patients suggested that signaling through this receptor would trigger the mechanism required to perform SLPI inhibition, because the absence of signaling through IFN-γR impaired the decrease in the levels of SLPI (Figure 3B). Similar to other receptor-cytokine systems, the ability of a cell to respond to IFN-γ depends on the level of expression of its receptor and on its functionality.36 Thus, the low expression of IFN-γR in PBMCs from TB patients would alter the IFN-γ–specific responsiveness of the target cell. Finally, we postulate that the high levels of SLPI in patients with active disease might help to eliminate Mtb through its microbicidal activity.17,18 However, further work would be necessary to unravel the effect of SLPI on IFN-γ expression.
Acknowledgment
We thank Dr. Oscar Bottasso for tuberculosis patients' plasma samples.
Footnotes
Supported by Agencia Nacional de Promoción Científica y Tecnológica (the National Agency for the Promotion of Science and Technology) Public Health Service grants PAE-PID-2007-00127 (H.E.C. and V.E.G.), PICT 2331 (H.E.C.), and PICT 01384 (V.E.G.); University of Buenos Aires grants UBACYT2011-2014 M940 (H.E.C.) and UBACYT 20020100100221 (V.E.G.), CONACYT (Mexico) grant 182817 (S.P.-S.), CONICET grant PIP0901 (H.E.C.), and National Institutes of Allergy and Infectious Diseases NIH grant R01 AI079007 (V.E.G.).
V.E.G. and H.E.C. contributed equally as senior authors of this work.
Disclosures: None declared.
Supplemental Data
References
- 1.Flynn J.L. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russell D.G., Orme I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spellberg B., Edwards J.E., Jr. Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 4.Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 5.Bazan J.F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doffinger R., Jouanguy E., Dupuis S., Fondaneche M.C., Stephan J.L., Emile J.F., Lamhamedi-Cherradi S., Altare F., Pallier A., Barcenas-Morales G., Meinl E., Krause C., Pestka S., Schreiber R.D., Novelli F., Casanova J.L. Partial interferon-gamma receptor signaling chain deficiency in a patient with bacille Calmette-Guerin and Mycobacterium abscessus infection. J Infect Dis. 2000;181:379–384. doi: 10.1086/315197. [DOI] [PubMed] [Google Scholar]
- 7.Jouanguy E., Lamhamedi-Cherradi S., Lammas D., Dorman S.E., Fondaneche M.C., Dupuis S., Doffinger R., Altare F., Girdlestone J., Emile J.F., Ducoulombier H., Edgar D., Clarke J., Oxelius V.A., Brai M., Novelli V., Heyne K., Fischer A., Holland S.M., Kumararatne D.S., Schreiber R.D., Casanova J.L. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21:370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 8.Hiemstra P.S. Novel roles of protease inhibitors in infection and inflammation. Biochem Soc Trans. 2002;30:116–120. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 9.Luo B.L., Niu R.C., Feng J.T., Hu C.P., Xie X.Y., Ma L.J. Downregulation of secretory leukocyte proteinase inhibitor in chronic obstructive lung disease: the role of TGF-beta/Smads signaling pathways. Arch Med Res. 2008;39:388–396. doi: 10.1016/j.arcmed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Higashimoto Y., Yamagata Y., Iwata T., Ishiguchi T., Okada M., Masuda M., Satoh H., Itoh H. Adenoviral E1A suppresses secretory leukoprotease inhibitor and elafin secretion in human alveolar epithelial cells and bronchial epithelial cells. Respiration. 2005;72:629–635. doi: 10.1159/000089579. [DOI] [PubMed] [Google Scholar]
- 11.Jaumann F., Elssner A., Mazur G., Dobmann S., Vogelmeier C., Munich Lung Transplant Group Transforming growth factor-beta1 is a potent inhibitor of secretory leukoprotease inhibitor expression in a bronchial epithelial cell line. Eur Respir J. 2000;15:1052–1057. doi: 10.1034/j.1399-3003.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 12.Taggart C.C., Lowe G.J., Greene C.M., Mulgrew A.T., O’Neill S.J., Levine R.L., McElvaney N.G. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 13.Taggart C.C., Cryan S.A., Weldon S., Gibbons A., Greene C.M., Kelly E., Low T.B., O’Neill S.J., McElvaney N.G. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005;202:1659–1668. doi: 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Zheng T., Zhu Z., Homer R.J., Riese R.J., Chapman H.A., Jr., Shapiro S.D., Elias J.A. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams S.E., Brown T.I., Roghanian A., Sallenave J.M. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 16.Sallenave J.M. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol. 2010;42:635–643. doi: 10.1165/rcmb.2010-0095RT. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura J., Saiga H., Sato S., Okuyama M., Kayama H., Kuwata H., Matsumoto S., Nishida T., Sawa Y., Akira S., Yoshikai Y., Yamamoto M., Takeda K. Potent antimycobacterial activity of mouse secretory leukocyte protease inhibitor. J Immunol. 2008;180:4032–4039. doi: 10.4049/jimmunol.180.6.4032. [DOI] [PubMed] [Google Scholar]
- 18.Gomez S.A., Arguelles C.L., Guerrieri D., Tateosian N.L., Amiano N.O., Slimovich R., Maffia P.C., Abbate E., Musella R.M., Garcia V.E., Chuluyan H.E. Secretory leukocyte protease inhibitor: a secreted pattern recognition receptor for mycobacteria. Am J Respir Crit Care Med. 2009;179:247–253. doi: 10.1164/rccm.200804-615OC. [DOI] [PubMed] [Google Scholar]
- 19.Ding A., Yu H., Yang J., Shi S., Ehrt S. Induction of macrophage-derived SLPI by Mycobacterium tuberculosis depends on TLR2 but not MyD88. Immunology. 2005;116:381–389. doi: 10.1111/j.1365-2567.2005.02238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin F.Y., Nathan C., Radzioch D., Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 21.Mahuad C., Bay M.L., Farroni M.A., Bozza V., Del Rey A., Besedovsky H., Bottasso O.A. Cortisol and dehydroepiandrosterone affect the response of peripheral blood mononuclear cells to mycobacterial antigens during tuberculosis. Scand J Immunol. 2004;60:639–646. doi: 10.1111/j.0300-9475.2004.01514.x. [DOI] [PubMed] [Google Scholar]
- 22.Tateosian N.L., Costa M.J., Guerrieri D., Barro A., Mazzei J.A., Eduardo Chuluyan H. Inflammatory mediators in exhaled breath condensate of healthy donors and exacerbated COPD patients. Cytokine. 2012;58:361–367. doi: 10.1016/j.cyto.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Lin Y., Iyer D.V., Gong J., Abrams J.S., Barnes P.F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rey A.D., Mahuad C.V., Bozza V.V., Bogue C., Farroni M.A., Bay M.L., Bottasso O.A., Besedovsky H.O. Endocrine and cytokine responses in humans with pulmonary tuberculosis. Brain Behav Immun. 2007;21:171–179. doi: 10.1016/j.bbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Verbon A., Juffermans N., Van Deventer S.J., Speelman P., Van Deutekom H., Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquinelli V., Quiroga M.F., Martinez G.J., Zorrilla L.C., Musella R.M., Bracco M.M., Belmonte L., Malbran A., Fainboim L., Sieling P.A., Garcia V.E. Expression of signaling lymphocytic activation molecule-associated protein interrupts IFN-gamma production in human tuberculosis. J Immunol. 2004;172:1177–1185. doi: 10.4049/jimmunol.172.2.1177. [DOI] [PubMed] [Google Scholar]
- 27.Singhal A., Jaiswal A., Arora V.K., Prasad H.K. Modulation of gamma interferon receptor 1 by Mycobacterium tuberculosis: a potential immune response evasive mechanism. Infect Immun. 2007;75:2500–2510. doi: 10.1128/IAI.01743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X., Zeng L., Jin W., Thompson J., Mizel D.E., Lei K., Billinghurst R.C., Poole A.R., Wahl S.M. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J Exp Med. 1999;190:535–542. doi: 10.1084/jem.190.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guazzone V.A., Guerrieri D., Jacobo P., Glisoni R.J., Chiappetta D., Lustig L., Chuluyan H.E. Micro-encapsulated secretory leukocyte protease inhibitor decreases cell-mediated immune response in autoimmune orchitis. Life Sci. 2011;89:100–106. doi: 10.1016/j.lfs.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Geraghty P., Greene C.M., O’Mahony M., O’Neill S.J., Taggart C.C., McElvaney N.G. Secretory leucocyte protease inhibitor inhibits interferon-gamma-induced cathepsin S expression. J Biol Chem. 2007;282:33389–33395. doi: 10.1074/jbc.M706884200. [DOI] [PubMed] [Google Scholar]
- 31.Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterling T.R., Dorman S.E., Chaisson R.E., Ding L., Hackman J., Moore K., Holland S.M. Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clin Infect Dis. 2001;33:976–982. doi: 10.1086/322670. [DOI] [PubMed] [Google Scholar]
- 33.Caccamo N., Guggino G., Meraviglia S., Gelsomino G., Di Carlo P., Titone L., Bocchino M., Galati D., Matarese A., Nouta J., Klein M.R., Salerno A., Sanduzzi A., Dieli F., Ottenhoff T.H. Analysis of Mycobacterium tuberculosis-specific CD8 T-cells in patients with active tuberculosis and in individuals with latent infection. PLoS One. 2009;4:e5528. doi: 10.1371/journal.pone.0005528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Odaka C., Mizuochi T., Yang J., Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol. 2003;171:1507–1514. doi: 10.4049/jimmunol.171.3.1507. [DOI] [PubMed] [Google Scholar]
- 35.Newport M.J., Huxley C.M., Huston S., Hawrylowicz C.M., Oostra B.A., Williamson R., Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 36.de Weerd N.A., Nguyen T. The interferons and their receptors: distribution and regulation. Immunol Cell Biol. 2012;90:483–491. doi: 10.1038/icb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.