Abstract
Lipoprotein(a) [Lp(a)] is an independent risk factor for cardiovascular diseases, but the mechanism is unclear. The pathogenic risk of Lp(a) is associated with elevated plasma concentration, small isoforms of apolipoprotein [apo(a)], the unique apolipoprotein of Lp(a), and a mimic of plasminogen. Inflammation is associated with both the initiation and recovery of cardiovascular diseases, and plasminogen plays an important role in leukocyte recruitment. Because Lp(a)/apo(a) is expressed only in primates, transgenic mice were generated, apo(a)tg and Lp(a)tg mice, to determine whether Lp(a)/apo(a) modifies plasminogen-dependent leukocyte recruitment or whether apo(a) has an independent role in vivo. Plasminogen activation was markedly reduced in apo(a)tg and Lp(a)tg mice in both peritonitis and vascular injury inflammatory models, and was sufficient to reduce matrix metalloproteinase-9 activation and macrophage recruitment. Furthermore, neutrophil recruitment and the neutrophil cytokines, CXCL1/CXCL2, were suppressed in apo(a)tg mice in the abdominal aortic aneurysm model. Reconstitution of CXCL1 or CXCL2 restored neutrophil recruitment in apo(a)tg mice. Apo(a) in the plasminogen-deficient background and Lp(a)tg mice were resistant to inhibition of macrophage recruitment that was associated with an increased accumulation of apo(a) in the intimal layer of the vessel wall. These data indicate that, in inflammation, Lp(a)/apo(a) suppresses neutrophil recruitment by plasminogen-independent cytokine inhibition, and Lp(a)/apo(a) inhibits plasminogen activation and regulates matrix metalloproteinase-9 activation and macrophage recruitment.
Cardiovascular diseases (CVDs) are the leading cause of death in the United States, and numerous studies document the close association of inflammation in the initiation and progression of CVD.1,2 Strong evidence establishes elevated lipoprotein(a) [Lp(a)] is an independent risk factor for myocardial infarction, ischemic stroke, and peripheral vascular disease.3,4 Lp(a) is composed of the low-density lipoprotein (LDL) lipid core, with apolipoprotein (apoB) tethered to the unique apolipoprotein, apo(a). The pathogenic mechanism of Lp(a) in CVD is unclear, and unlike LDL and other lipoproteins, there is no effective therapy to lower plasma Lp(a).5
Although plasmin is the primary enzyme for the degradation of fibrin, studies in the plasminogen-deficient (Plg−/−) mice have consistently shown abnormalities in leukocyte migration in a variety of models [eg, transplant atherosclerosis,6 thioglycollate (TG)-induced peritonitis,7,8 myocardial infarction,9,10 and vascular injury11]. Plasminogen binds to multiple proteins,12–15 and many of these proteins are also plasmin substrates. In addition to direct proteolytic action on extracellular matrix components, plasmin activates matrix metalloproteinase- 9 (MMP-9), which is a key enzyme for collagen degradation. Macrophage infiltration in the peritonitis and abdominal aortic aneurysm (AAA) inflammatory models requires plasminogen activation of MMP-9.8,9 The goal of this study was to determine whether Lp(a)/apo(a) interferes with the plasminogen-dependent leukocyte recruitment in vivo in inflammation.
As a component of Lp(a), apo(a) contains kringle domains that are homologous to plasminogen. Apo(a) and plasminogen have lysine-binding sites (LBS) in the kringle domains that contribute to the competition of Lp(a) and plasminogen binding to fibrin, cells, and extracellular matrix.15–17 The LBS in the kringle IV type 10 domain, the primary LBS of apo(a), is similar, but not identical, to the LBS of plasminogen.18 Lp(a) binds to cells with both apo(a) and LDL inhibitable interactions.16 These dual components of Lp(a) make it difficult to distinguish their roles in vivo. Lp(a) is found only in humans and nonhuman primates, and in humans, Lp(a) with small isoforms in high concentration has the highest pathogenic risk.19,20 To isolate Lp(a) function from LDL, transgenic mice with a small isoform of apo(a) in high concentration were generated to investigate the interference of apo(a) with plasminogen in vivo.
The pathogenic mechanism of Lp(a)/apo(a) is not known. Herein, we investigate the hypothesis that, as a component of Lp(a), apo(a) interferes with plasminogen-dependent leukocyte recruitment in inflammation. The results indicate that Lp(a)/apo(a) interferes with the plasminogen/MMP-9 activation pathway and macrophage recruitment. Apo(a) also has plasminogen-independent suppression of neutrophil infiltration by inhibition of the release of cytokines, required for neutrophil emigration. Furthermore, Lp(a) inhibited plasminogen and MMP-9 activation and macrophage recruitment, but transgenic apo(a) [apo(a)tg] mice in the plasminogen-deficient background and transgenic Lp(a) [Lp(a)tg] mice were not resistant to macrophage recruitment when apo(a) was deposited in the intimal area of the vessel wall.
Materials and Methods
Mice
Plg−/− mice were developed, as previously described,7 and backcrossed over 10 generations into the C57BL/6J background. Apo(a) transgenic mice were generated with an apo(a) 6 KIV construct (KIV 5 to 10, plus KV and a protease-like domain)21 with a liver-specific promoter22 in a C57BL/6J background and maintained in Plg−/− or Plg+/+ background. Plg+/+ and Plg−/− or apo(a)tg:Plg+/+ and apo(a)tg:Plg−/− mice were maintained by heterozygous breeding pairs. Mice were genotyped for plasminogen by PCR.23 Human apoB transgenic mice in the C57BL/6 background were obtained from L. S. Huang (Columbia University, New York, NY), and genotyped as described.24,25 Lp(a) double-transgenic mice were generated by crossing our apo(a)tg mice and human apoB transgenic mice.25 We confirmed with a nonreducing gel that Lp(a) was formed. Apo(a) concentration in plasma was measured by an enzyme-linked immunosorbent assay (ELISA) that we verified by using Western blot analysis. The ELISA used a polyclonal antibody, V406, and apo(a) standard isolated from humans.23 Values of apo(a) in the mice ranged from 1 to 6 μmol/L (20 to 100 mg/dL), considered to be pathogenic in humans.19,20,23 The apo(a) from these transgenic mice was functionally active, measured in a quantitative immunoassay. These mice had lysine binding site activity.26 Plasminogen mutant (Plgmut) mice were obtained from Victoria A. Ploplis and Francis J. Castellino (University of Notre Dame, Notre Dame, IN), and genotyped as described.27 All mice were maintained on a C57BL/6J background and used at 8 to 10 weeks of age. Mice were bred, housed in sterilized isolator cages, maintained on a 14-hour light/10-hour dark cycle, and provided with sterilized food and water at the Biological Resource Unit of the Cleveland Clinic Lerner Research Institute (Cleveland, OH). All animal experiments were performed in accordance with protocols approved by the Cleveland Clinic Institutional Animal Research Committee.
Apo(a) Purification
Plasma from apo(a)tg:Plg−/− mice was pooled, and apo(a) was isolated on a mini lysine-Sepharose column (lysine Sepharose 4B; GE Healthcare, Pittsburgh, PA). The plasma from apo(a)tg:Plg−/− mice has the advantage that Plg will not interfere with the apo(a) binding to the lysine-Sepharose. After incubation with plasma overnight at 4°C, the column was washed with PBS four times and the last wash was with 0.3 mol/L NaCl in PBS and absorbance at A280 was <0.1. The column was eluted with 0.2 mol/L ε-aminocaproic acid (Sigma, St. Louis, MO) to release apo(a). The apo(a) was recovered by dialysis to remove the ε-aminocaproic acid and concentrated by Amicon Ultra Centrifugal Filters-50K (Millipore, Billerica, MA).
Assays
Plasminogen activity was measured in 10 μL of plasma and 10 μL of peritoneal lavage in 96-well plates in a total volume of 250 μL with 0.3 mmol/L S-2251 (S820332; ChromogenixDiapharma, West Chester, OH), without or with 5 IU urinary-type plasminogen activator (672081; Millipore) in 0.05 mol/L Tris buffer (pH 7.4) containing 0.1 mol/L NaCl. Plasmin standards ranged from 0 to 10 nmol/L. Plasmin generation was continually monitored at 405 nm every 10 minutes through hydrolysis of S2251 at 37°C for 1 hour and showed a linear increase. The rate of plasmin generation in lavage and plasma samples was calculated as OD change/minute and converted to concentration/minute, according to the standards. The slope of OD change was linear over 60 minutes. Plasma and aorta were collected at 1 or 3 days after CaCl2-induced AAA. Aortas were homogenized in liquid nitrogen and extracted by radioimmunoprecipitation assay lysis buffer (RIPA buffer) containing protease inhibitor (04693159001; Roche, Indianapolis, IN). Aortic protein concentration was measured by BCA protein assay (23225; Thermo Scientific, Rockford, IL). Following the manufacturer’s instructions (MCXCL100 and MM200; R&D, Minneapolis, MN), CXCL1 and CXCL2 concentrations were detected by ELISA. MMP-9 activity in the aortic tissues and peritoneal lavage were examined with a 10% gelatin zymogram gel (EC6175BOX; Life Technologies, Grand Island, NY). The gels were treated with Renaturing and Developing Buffer (LC2670 and LC2671; Life Technologies), according to the manufacturer’s instructions, and then stained with Gel-Code Blue Stain Reagent (24590; Thermo Scientific). The intensities of bands were quantified by ImageJ software version 1.1.6 (NIH, Bethesda, MD). THP-1, human monocytic cell line, and macrophages were used for the in vitro cell assays.
Histological, Immunohistochemistry, and Immunofluorescent Staining
The dissected abdominal aorta was fixed in 4% paraformaldehyde and embedded in Tissue-Tek CRYO-O.C.T. Compound (Torrance, CA). Cryostat sections (5 μm thick) were stained with H&E for histochemical examination, with Masson’s trichrome for collagen content, and with Elastica van Gieson for elastic lamina detection. Aorta sections (12 μm thick) were immunostained with mouse neutrophil antibody (CL8993AP; Cedarlane Laboratories Ltd, Burlington, NC) and macrophage antibody Mac-3 (550292; BD Biosciences, San Jose, CA) with a 1:200 dilution. The area of positive signal and the total area of each section were quantified using Image-Pro Plus Software version 7.0 (Media Cybernetics Inc, Silver Spring, MD). Three to four sections from each mouse were analyzed, and the values were averaged for seven to nine mice. Paraffin sections were immunostained with primary anti-apo(a) V406 (1:100), anti-apoB MB47 (1:100; obtained from L. K. Curtiss, The Scripps Research Institute, La Jolla, CA), and anti-smooth muscle actin (1:200; Sigma), and appropriate secondary antibodies, anti-rabbit conjugated with Alexa 488 (Life Technologies) or anti-mouse conjugated with Alexa 568, were added.
Results
AAA Formation in Apo(a)tg and Plg−/− Mice
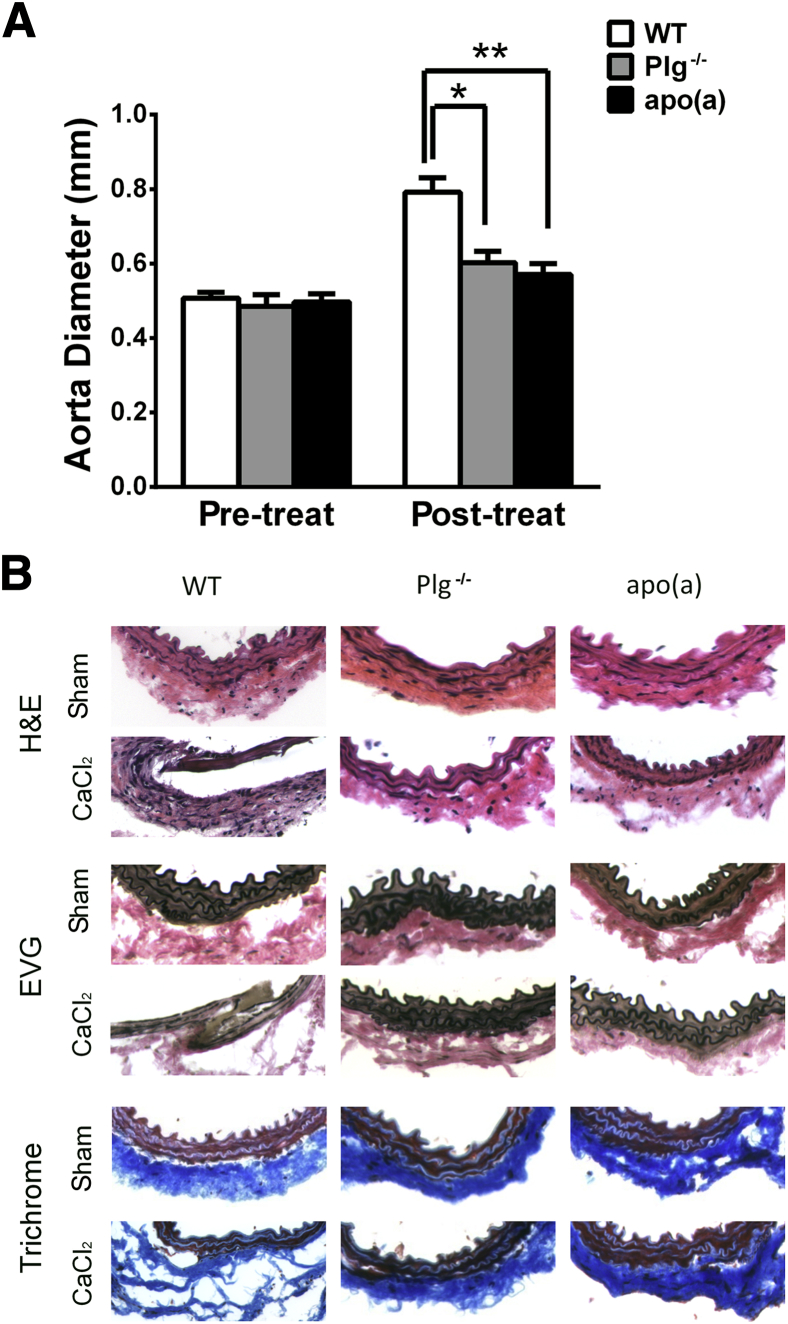
To determine whether apo(a), as a component of Lp(a), interferes with the suppression of plasminogen-dependent macrophage recruitment, wild-type (WT), plasminogen-deficient (Plg−/−), and apo(a)-transgenic [apo(a)tg] mice were evaluated after CaCl2-induced vascular injury. The abdominal aorta was dilated by 62% in WT mice, but only 24% in the Plg−/− mice and 15% in apo(a)tg mice 3 weeks after CaCl2 treatment (Figure 1A). No aortic dilation was observed in saline-treated sham controls of WT, Plg−/−, or apo(a)tg mice (Supplemental Figure S1A). Dilation of the abdominal aorta was accompanied by elevated leukocyte infiltration, thinning of the media layer, and disruption of elastic lamellae in WT mice (Figure 1B). For the saline-treated sham group, the vessel structure of Plg−/− and apo(a)tg mice was not different from that for WT mice. Compared with the sham, increased inflammatory cells in the adventitia of the WT mice vessel wall were observed at 3 weeks after CaCl2 treatment. In contrast, cellular infiltration was not elevated in peri-aortic tissue from Plg−/− mice or apo(a)tg mice. Trichrome staining of sections from WT mice revealed a loss of arterial architecture and decreased collagen deposition, whereas Plg−/− mice and apo(a)tg mice remained unchanged. Elastic lamella became fragmented and flattened in WT mice, but in apo(a)tg and Plg−/− mice, the elastic lamella was similar to that of the saline-treated mice (Figure 1B). The inhibition of dilation and lack of change in the morphological characteristics in the apo(a)tg mice (with Plg) were similar to those of the Plg−/− mice, suggesting that apo(a) interferes with Plg in the formation of AAA in the apo(a)tg:Plg+/+ mice.
Figure 1.
Inhibition of AAA development in apo(a)tg and Plg−/− mice. A: Abdominal aorta diameters measured before CaCl2 treatment and 3 weeks later. Bars are means ± SEM (n = 6 to 14). Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. B: Abdominal aortas collected 3 weeks after CaCl2 treatment. Aorta sections stained with H&E stain, Masson’s trichrome stain for collagen, and Elastica van Gieson (EVG) stain for elastic lamina from saline-treated sham and CaCl2-treated mice, representative sections from six mice per genotype. ∗P < 0.05, ∗∗P < 0.01 versus WT. Original magnification, ×10 (B).
Apo(a) Suppresses Macrophage Recruitment and MMP-9 Activation in AAA
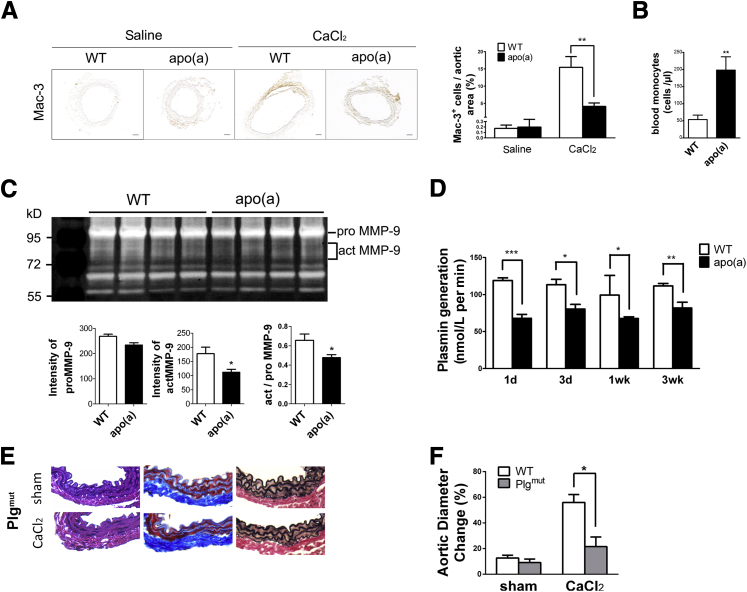
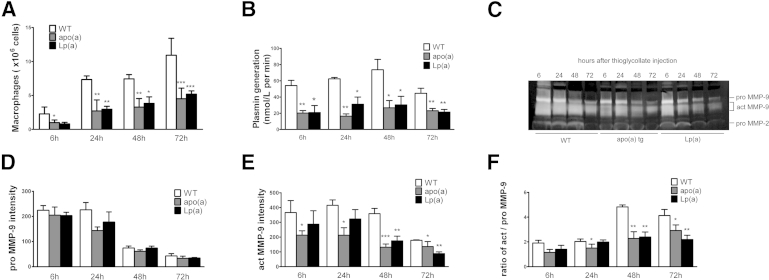
Previously, we found that abdominal aortic injury in plasminogen deficiency was accompanied by reduced macrophage infiltration and activation of MMP-9.8 To understand how apo(a) interfered with plasminogen-mediated inflammation, we assessed macrophage migration and MMP-9 activation in apo(a)tg mice. Few macrophages were observed in the saline-treated mice, because no inflammation was induced. With CaCl2 treatment, macrophage recruitment to the injured vessel wall, when compared with the saline control, was increased to 15% in WT mice, but was only 4% in apo(a)tg mice (Figure 2A). The percentage of macrophage was measured by positive Mac-3 area to the total aortic area. In contrast, blood monocytes were fourfold higher in apo(a)tg mice than in WT mice (Figure 2B) and remained higher at 3 weeks (3.7-fold higher). The accumulation of monocytes in the blood and reduced macrophages in the injured tissue of apo(a)tg mice suggested that migration from blood through the vessel wall was impaired. MMP-9 activity was determined in the abdominal aorta of WT and apo(a)tg mice. The proenzyme of MMP-9 (ProMMP-9) was not different in WT and apo(a)tg mice, and Western blot analysis of the plasma also indicated there was no difference in total MMP-9 (Supplemental Figure S1B). Active MMP-9 and proMMP-9 were determined by zymography (proMMP-9, 105 kDa; and actMMP-9, 95 and 88 kDa) in the aorta 1 day after treatment. ActMMP-9 was 37% lower in apo(a)tg mice than WT mice, and the ratio of actMMP-9/proMMP-9 was significantly decreased in apo(a)tg mice compared with WT mice (Figure 2C). Reduced MMP-9 activation also supported the observation in apo(a)tg mice that collagen was protected from degradation (Figure 1B). The reduced macrophage recruitment and activation of MMP-9 in apo(a)tg mice was similar to that found for Plg−/− mice in the AAA model.8 Taken together, these results indicate that apo(a) inhibits MMP-9 activation and macrophage recruitment and, consequently, AAA development.
Figure 2.
Suppressed macrophage recruitment, MMP-9 activation, and plasminogen activation in apo(a)tg mice. A: Abdominal aortas collected 3 weeks after CaCl2 treatment. Representative aorta sections stained with macrophage antibody Mac-3. Quantification of number of macrophages per area from three to four sections per mouse (n = 6 to 8). B: Blood monocytes 3 weeks after CaCl2 treatment. C: Aorta active and proMMP-9 in WT and apo(a)tg mice detected on day 1 by gelatin zymography and quantified, representative sections. Bars are means plusmn; SEM (n = 6 to 8), by t-test. D: Plasma was collected at 1 day, 3 days, 1 week, and 3 weeks after CaCl2 treatment. Plasminogen activation monitored as plasmin generation (n = 4 to 6). Bars are means ± SEM. Statistical analysis, one-way analysis of variance, and Newman-Kuels posttest were performed. E: Representative aorta sections with H&E, Trichrome, EVG staining from sham and CaCl2-treated Plgmut mice. F: Abdominal aorta diameters of Plgmut mice measured before CaCl2 treatment and 3 weeks later. Bars are means ± SEM (n = 6 to 7). Statistical analysis and t-test were performed. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 100 μm (A).
Plasmin Activity Is Impaired in Apo(a)tg Mice in an AAA Inflammatory Model
To determine whether the inhibition of macrophage recruitment and MMP-9 activation in apo(a)tg mice was dependent on plasminogen activation, the plasmin generation rate was determined. In untreated WT and apo(a)tg mice, plasminogen activation was not different in the plasma (Supplemental Figure S1C). However, with CaCl2 treatment, plasma plasminogen activation in apo(a)tg mice was significantly reduced compared with WT mice during AAA development (Figure 2D), suggesting that apo(a) interfered with the activation of plasminogen.
Plasmin has both signaling and proteolytic activity, and apo(a) may inhibit the signaling and/or proteolytic activity of plasminogen to inhibit AAA formation. Plgmut mice have a single amino acid mutation (Ser 743 to Ala) in the protease domain and lack proteolytic activity, but retain signaling activity.27 The aorta diameter dilated 20% in Plgmut mice after CaCl2 treatment and was significantly lower compared with WT mice (Figure 2F). The morphological characteristics of the Plgmut mice were similar to those of Plg−/− mice (Figures 1B and 2E) and suggest that plasmin activity is required for the AAA formation. The inhibition of dilation and lack of changes in morphological features in apo(a)tg mice were also similar to Plgmut mice and suggest apo(a) may inhibit plasmin proteolytic activity rather than plasminogen signaling. Li et al28 reported plasmin activation of Akt, extracellular signal–regulated kinase, and c-Jun kinase pathways in THP-1 macrophages, but these pathways were not different in apo(a)tg mice compared with WT mice (Supplemental Figure S2A). The prevention of AAA in Plgmut mice suggested plasminogen proteolytic activity is required for AAA development. Apo(a)tg mice were also protected from AAA. In addition, inhibition of the plasmin proteolytic activity by apo(a) may account for the reduced MMP-9 activation.
To determine whether monocyte/macrophage chemokines were reduced in the apo(a)tg mice and contributed to the reduced recruitment into the aorta, CCL2, the primary monocyte/macrophage chemoattractant, was measured in the plasma of the sham- and CaCl2-treated mice at 1 day and 3 weeks (Supplemental Figure S2, B and C). CCL2 was not different between WT and apo(a)tg sham or CaCl2-treated mice 1 day after treatment. However, at 3 weeks after treatment, plasma CCL2 was nearly threefold higher in apo(a)tg mice than WT mice. Expression of Ccl2 mRNA, determined by quantitative RT-PCR in the aorta of WT and apo(a)tg mice 1 day after sham and CaCl2 treatment, was twofold higher in the apo(a)tg mice than WT mice. Expression of Ccl3 mRNA, also a monocyte/macrophage chemoattractant, was also increased in apo(a)tg mice compared with WT mice (Supplemental Figure S2, D and E). Thus, a decrease in the monocyte/macrophage chemoattractants could not account for reduced macrophage recruitment in the apo(a)tg mice.
Apo(a) Inhibits MMP-9 Activation and Macrophage Migration in Vitro
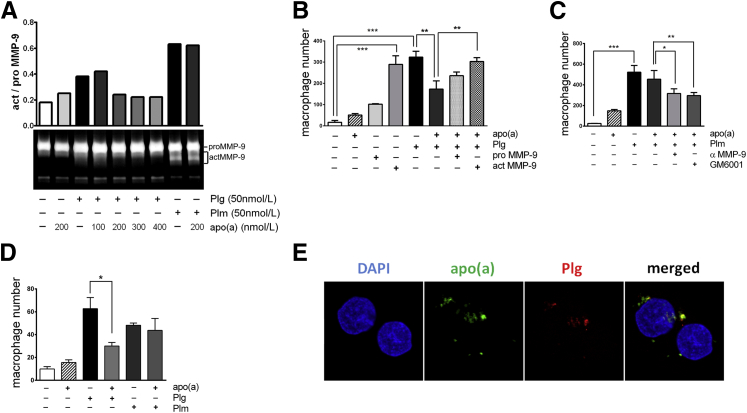
To understand if apo(a), as a component of Lp(a), inhibits MMP-9 activation directly or competes with plasminogen to inhibit MMP-9 activation, THP-1 cells were incubated with increasing concentrations of apo(a), from 100 to 400 nmol/L, in the presence of 50 nmol/L plasminogen. Plasminogen and plasmin enhanced MMP-9 activation compared with the control, and apo(a) alone did not affect MMP-9 activation (Figure 3A). Plasminogen-induced MMP-9 activation was inhibited by apo(a) in a dose-dependent manner. MMP-9 activation was remarkably diminished by adding ≥200 nmol/L concentrations of apo(a) to 50 nmol/L plasminogen. However, 50 nmol/L plasmin-induced MMP-9 activation was not affected with addition of 200 nmol/L apo(a), suggesting that apo(a) suppressed conversion of plasminogen to plasmin.
Figure 3.
Apo(a) inhibited plasminogen-induced MMP-9 activation and macrophage migration in vitro. A: THP-1 cells incubated with 50 nmol/L of plasminogen or plasminogen with 100 to 400 nmol/L of apo(a) for 48 hours are shown. Zymography and intensity of secreted MMP-9 in conditioned media, representative of three independent experiments. B: Macrophages were isolated from mouse peritoneal lavage seeded on transmembrane-coated collagen IV inserts, and macrophage number was calculated. Cells incubated with 50 nmol/L plasminogen and 200 nmol/L apo(a) with proMMP-9 or actMMP-9 in the top chamber overnight. C: Boyden chamber assay macrophage number for migrated cells stimulated with 50 nmol/L plasmin and 200 nmol/L apo(a) with MMP inhibitor, GM6001, or MMP-9–neutralizing antibody. D: Macrophage invasion through the transmembrane-coated Matrigel inserts. Three independent experiments performed in triplicate. Bars are means ± SEM of each averaged experiment. Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. E: Representative confocal image of colocalization (yellow) of 50 nmol/L apo(a) (green) and 50 nmol/L plasminogen (red) binding on THP-1 cells. THP-1 cells were incubated with plasminogen and apo(a) in the media for 16 hours, and cells were washed before fluorescent staining. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 10 μm (E).
To determine whether apo(a) suppresses plasminogen-mediated cell migration or invasion in vitro, primary macrophages were isolated from mouse peritoneal lavage. The Boyden chamber was coated with collagen IV for migration assay (Figure 3, B and C) or with Matrigel for invasion assay (Figure 3D). Apo(a), plasminogen, and plasmin were added in the top chamber, with the chemoattractant MCP-1 in the bottom chamber. Both plasminogen and actMMP-9 markedly promoted macrophage migration, compared with the control (media only). Plasminogen-stimulated migration was decreased by 50% with apo(a) addition. This inhibition was restored by actMMP-9, but not proMMP-9 (Figure 3B). Interestingly, plasmin promoted cell migration as well, but was not inhibited by apo(a) addition. The MMP inhibitor, GM6001, or MMP-9–neutralizing antibody significantly reduced migration in the presence of plasminogen and apo(a), indicating MMP-9 activation was not directly modulated by apo(a) but through plasminogen activation (Figure 3C). In the invasion assay, plasminogen promoted THP-1 cell invasion that was inhibited by apo(a) addition, but plasmin-stimulated invasion was not suppressed by apo(a) (Figure 3D), consistent with the observations in the migration assay.
Taken together, these results indicate that apo(a) inhibits plasminogen activation and MMP-9 activation, and macrophage migration or invasion was consequently impaired. We demonstrated plasminogen-regulated macrophage migration was suppressed by apo(a) both in vivo and in vitro. Syrovets et al29 reported Lp(a) and apo(a) were chemoattractants for monocytes and promoted cell migration in vitro. In their experiments, they added apo(a)/Lp(a) to the bottom chamber to attract THP-1 cells. We added apo(a) to the top chamber, and apo(a) alone did not affect migration. However, apo(a) blocked plasminogen (also added to the top chamber) activity and, consequently, inhibited macrophage migration induced by plasminogen. Our results showed that macrophage recruitment was inhibited in apo(a)tg mice by CaCl2-induced AAA inflammation, and this function acts through apo(a)-regulated plasminogen and MMP-9 activity ablation.
Numerous studies demonstrate that plasminogen activation occurs most efficiently bound to the cell membrane, rather than in solution. Apo(a) competes with plasminogen binding to cells in vitro, but whether apo(a) and plasminogen bind to the same site is not clear. By incubating THP-1 cells with 50 nmol/L plasminogen and 50, 100, or 200 nmol/L apo(a), we observed apo(a) and plasminogen colocalized on the cells, implying apo(a) may interact with plasminogen receptors to suppress plasminogen activation on the cell surface (Figure 3E).
Apo(a) with Plasminogen Deficiency Promotes Macrophage Recruitment
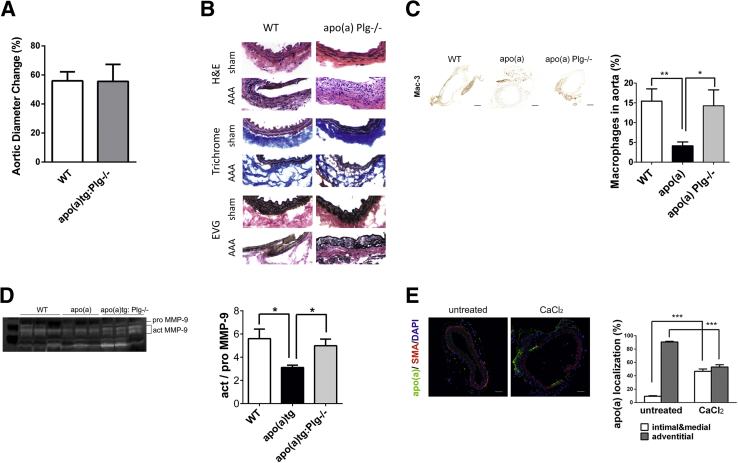
To determine the role of apo(a) in the plasminogen-deficient background, macrophage recruitment and susceptibility to aneurysm formation were assessed in apo(a)tg:Plg−/− mice. The apo(a)tg:Plg−/− mice were susceptible to AAA formation, similar to the WT mice (Figure 4A). In the apo(a)tg:Plg−/− mice, the dilation of the aorta and the vessel architecture were similar to WT mice (Figure 4B), and there was no difference between the WT and apo(a)tg:Plg−/− mice in the saline-treated control aorta (Supplemental Figure S1A). Macrophage recruitment and MMP-9 activation were not different in apo(a)tg:Plg−/− mice compared with WT mice (Figure 4, C and D), suggesting apo(a) in the absence of plasminogen promoted MMP-9 activation and macrophage migration by a non–plasmin-dependent mechanism to promote AAA formation by a plasminogen-independent pathway.
Figure 4.
Aorta dilation was similar in WT and apo(a)tg:Plg−/− mice. A: Change in abdominal aorta diameter measured before CaCl2 treatment and 3 weeks later (n = 9). B: Cell infiltration, collagen degradation, and elastic lamina disruption 3 weeks after CaCl2 treatment (representative sections shown). C: Representative sections of macrophages immunostained (Mac-3) in injured aorta 3 weeks after CaCl2 treatment (n = 3 to 9). Macrophages are given as percentage total area. D: MMP-9 activity assessed in the peritoneal lavage 72 hours after TG injection (n = 3). E: Deposition of vessel wall apo(a) before and 3 weeks after CaCl2 treatment (n = 4). A–E: Bars are means ± SEM. Statistical analysis, one-way analysis of variance, and Newman-Kuels posttest were performed. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 200 μm (C). EVG, Elastica van Gieson; SMA, α-smooth muscle actin (a marker for smooth muscle cells).
To test where the apo(a) accumulated in the injured aorta of the apo(a)tg:Plg−/− mice, sections were immunostained (Figure 4E) and apo(a) was found in the intimal layer. In apo(a)tg:Plg−/− mice without CaCl2 treatment, apo(a) was found primarily in the adventitial layer. The results from these mice suggest that the accumulation of apo(a) in the intimal layer of injured aorta may accelerate with CaCl2-induced AAA formation.
Neutrophil Recruitment and Cytokines Are Suppressed in Apo(a)tg Mice
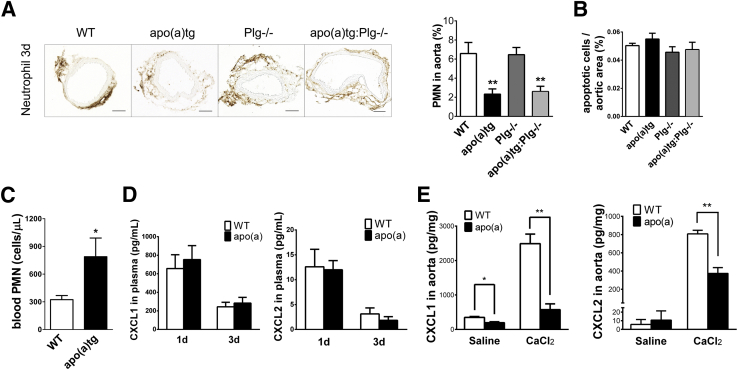
Neutrophils are among the first cells recruited during inflammation, and depletion in animal models inhibits AAA formation.30 Previously, we reported that apo(a) inhibited neutrophil recruitment in TG-induced peritonitis and was not plasminogen dependent. To investigate the modulation of apo(a) in neutrophil recruitment in the AAA vascular injury model, neutrophils were determined in the injured aorta. Initially, neutrophils were measured in WT mice aorta to determine the time course of recruitment. Neutrophils were highest at 1 day after CaCl2 treatment and declined to undetectable by day 6 (Supplemental Figure S3A). Thus, neutrophils were examined at 1 and 3 days after CaCl2 treatment in both aorta and circulation in WT, apo(a)tg, Plg−/−, and apo(a)tg:Plg−/− mice. At day 1, neutrophil recruitment in the aorta of apo(a)tg mice was not different from that in WT mice (Supplemental Figure S3, B and C). At day 3, neutrophils in the aorta adventitia of apo(a) mice were 2.3% (of aortic area), significantly lower than 6.6% in WT mice (P < 0.01). Neutrophils were also less in the aorta of apo(a)Plg−/− mice (2.6%). However, neutrophil recruitment in Plg−/− mice was equivalent to that in WT mice (Figure 5A). Apo(a) inhibited neutrophil recruitment in either a WT or Plg−/− background. Consistent with the findings in the peritonitis inflammatory model, plasminogen does not affect neutrophil number at the early stages of inflammation, and apo(a) suppresses neutrophil recruitment independent of plasminogen. However, circulating neutrophils in apo(a)tg mice at day 3 were twofold higher than in WT mice, but did not remain significant at 3 weeks (WT versus apo(a)tg mice, 0.36 ± 0.12 versus 0.69 ± 0.15). The circulating monocytes in apo(a)tg mice were not only significantly higher at 3 weeks, but were also higher at day 3. The elevated circulating leukocytes suggest that, despite the reduced recruitment to the injured aorta, systemic inflammation may be prolonged in the apo(a)tg mice.
Figure 5.
Suppressed neutrophil recruitment and CXCL1 and CXCL2 expressed in CaCl2-treated aorta of apo(a)tg mice. A: Neutrophils in the vessel wall 3 days after CaCl2 treatment, with three sections for each mouse (n = 5 to 8 mice). B: Apoptotic cells in injured aorta at day 3. WT, apo(a)tg, Plg−/−, and apo(a)tg:Plg−/− mice are shown (P > 0.05, ns). C: Neutrophil number in blood at day 3. D: Plasma CXCL1 and CXCL2 at 1 and 3 days after CaCl2 treatment. E: CXCL1 and CXCL2 in aortas collected at day 1 after saline (sham control) or CaCl2 treatment and homogenized. Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. ∗P < 0.05, ∗∗P < 0.01. Scale bar = 200 μm (A). PMN, polymorphonuclear leukocyte.
The apo(a) suppression of neutrophils may be due to reduced entry into the injured vessel wall or increased exit from the tissue. Apoptotic cells were not different in the abdominal aorta of WT and apo(a)tg, Plg−/−, or apo(a)tg:Plg−/− mice at day 3 (Figure 5B). More neutrophils in the circulation were observed in apo(a)tg mice than in WT mice, suggesting that the entry into the vessel wall was impaired (Figure 5C).
CXCL1 and CXCL2, neutrophil chemoattractants, are required for neutrophil recruitment.31,32 We measured CXCL1 and CXCL2 in the circulation and injured aorta. Circulating CXCL1 and CXCL2 were not different in WT and apo(a)tg mice at 1 or 3 days after CaCl2 treatment (Figure 5D). Compared with the saline-treated mice, CXCL1 and CXCL2 in the abdominal aorta were enhanced by more than fivefold in WT mice with CaCl2 treatment at day 1. Although neutrophil number was equivalent in WT and apo(a)tg mice, WT cytokines were significantly higher than in apo(a)tg mice, with CXCL1 fourfold higher and CXCL2 twofold higher in aortic tissue at day 1 after CaCl2 treatment (Figure 5E). Impaired neutrophil recruitment was observed in apo(a)tg mice at day 3 as a consequence of decreased cytokines detected at day 1. Cytokines in the apo(a)tg:Plg−/− mice were similar to those in the apo(a)tg mice in both the plasma and aorta at days 1 and 3 (data not shown), confirming that apo(a) regulation of CXCL1/CXCL2 was independent of plasminogen. The cytokine expression in the injured tissue, but not in plasma, was different between WT and apo(a)tg mice, implying suppressed CXCL1 and CXCL2 in the aortic tissue were responsible for impaired neutrophil recruitment in the apo(a)tg mice.
AAA Formation in WT Mice Is Prevented with CXCL2 Neutralization
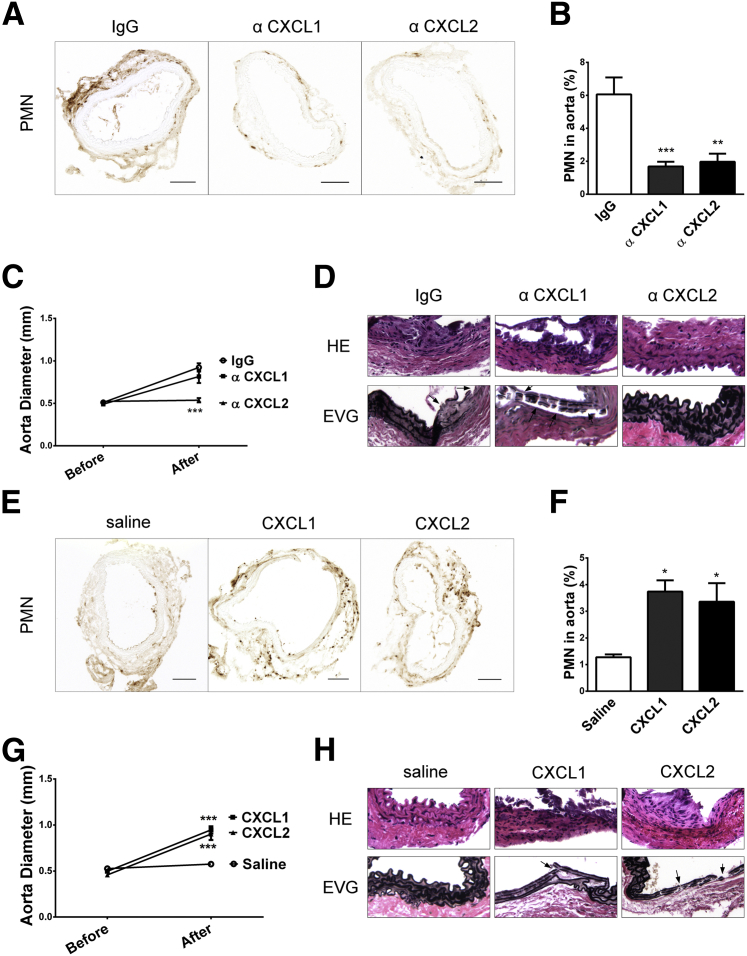
To investigate the role of CXCL1 and CXCL2 in AAA formation, neutralizing antibodies to CXCL1 or CXCL2 were injected into WT mice daily for 3 days after CaCl2 treatment. The neutrophil recruitment was reduced by threefold in the WT mice injected with CXCL1 or CXCL2 neutralizing antibody, compared with IgG injection control (Figure 6, A and B). AAA formation was blocked with CXCL2 antibody, but not CXCL1 antibody, at 3 weeks in WT mice. The aorta dilation of mice injected with CXCL1 antibody was similar to that of the IgG-injected mice. In contrast, the aorta dilation was completely abolished in the mice with CXCL2 antibody injection (Figure 6C and Supplemental Figure S3D). Although diminishing CXCL1 or CXCL2 significantly suppressed neutrophil recruitment, only the injection of CXCL2 antibody inhibited AAA formation, protecting mice from elastic lamina disruption and AAA formation (Figure 6D).
Figure 6.
Inhibition in WT mice and restoration in apo(a)tg mice of cytokines and AAA. A–D: WT mice treated with IgG, anti-CXCL1, or anti-CXCL2 antibodies at 0 to 2 days after CaCl2 treatment. Neutrophils in aortas collected at day 3. A: Representative images. B: Neutrophil number in aorta, mean of four images per mouse (n = 3 to 4 mice per group). C: Diameter measured both before CaCl2 treatment and 3 weeks later. D: H&E- and EVG-stained abdominal aorta sections; arrows indicate fragmented elastic lamina. E–H: Apo(a)tg mice injected with saline, cytokine CXCL1, or CXCL2 at 0 to 2 days after CaCl2 treatment. E: Neutrophils in aorta collected at day 3. F: Neutrophil number quantified. G: Aorta diameter measured both before CaCl2 treatment and 3 weeks later. H: Increased cell infiltration and elastic lamina fragmentation. Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnifications: ×20 (D and H); ×10 (E). EVG, Elastica van Gieson; PMN, polymorphonuclear leukocyte.
Reconstitution of Cytokines CXCL1 and CXCL2 Restores AAA Formation in Apo(a) Mice
CXCL1 and CXCL2 expression in the aorta of apo(a)tg mice was lower than in WT mice in the AAA model (Figure 5A). To determine whether restoration of CXCL1 and CXCL2 in apo(a)tg mice was sufficient to restore AAA formation, CXCL1 or CXCL2 was injected. Either CXCL1 or CXCL2 injection enhanced neutrophil recruitment to the injured aorta of apo(a)tg mice (Figure 6, E and F). CXCL1 or CXCL2 significantly restored neutrophil recruitment compared with the saline control (P < 0.05) (Figure 6F). Within 3 weeks after CaCl2 treatment, AAA was severe in the apo(a)tg mice with CXCL1 or CXCL2 injection compared with saline injection. The aorta dilation in the apo(a)tg mice rescued with CXCL1 or CXCL2 was similar to that in WT mice (Figure 6G). CXCL1 and CXCL2 reconstitution also promoted inflammatory cell infiltration, evident with H&E staining of the aorta, and the elastic lamina fragmentation in apo(a)tg mice (Figure 6H). In summary, both CXCL1 and CXCL2 efficiently restored AAA formation in apo(a)tg mice. In conclusion, apo(a) suppressed neutrophil recruitment by inhibiting the expression of CXCL1/CXCL2 in the injured aorta.
Lp(a) and Apo(a) Inhibit Macrophage Recruitment and MMP-9 Activation in Peritonitis
Apo(a) circulates in the plasma as free apo(a) or bound to LDL as Lp(a). To determine whether our findings in the apo(a)tg mice were also present in Lp(a)tg mice, Lp(a)tg mice were assessed in the TG-induced peritonitis and the CaCl2-induced AAA formation. The lavage was collected from WT, apo(a)tg, and Lp(a)tg mice at time points of 6, 24, 48, and 72 hours after TG injection. In WT mice, macrophage recruitment to the peritoneal cavity gradually increased and peaked at 72 hours. In contrast, macrophages in the lavage were reduced by 50% in apo(a)tg mice and by 40% in Lp(a)tg mice (Figure 7A); plasmin generation in apo(a)tg and Lp(a)tg mice was 50% to 70% lower than in WT mice at 6 to 72 hours after TG injection, suggesting plasminogen activation was suppressed by apo(a) (Figure 7B). MMP-9 activation was detected in the lavage by zymography of proMMP-9 (105 kDa) and actMMP-9 (95 and 88 kDa). ProMMP-9 was not significantly different in WT and apo(a)tg mice (Figure 7D). Compared with WT mice, actMMP-9 in apo(a)tg and Lp(a)tg mice was significantly lower at each time point from 6 to 72 hours and was reduced by 50% at 48 hours (Figure 7E). In WT mice, the ratio of actMMP-9/proMMP-9 increased by 2.5-fold at 48 hours compared with 6 hours, but in apo(a)tg and Lp(a)tg mice, this peak did not occur (Figure 7F). The ratio of actMMP-9/proMMP-9 was significantly lower in apo(a)tg mice, indicating that the activation but not the expression was decreased (Figure 7F). The response of the Lp(a)tg mice to the inflammatory stimulus was analogous to the response in the apo(a)tg mice, suggesting that the Lp(a) response was mediated by the apo(a) component.
Figure 7.
Macrophage recruitment and plasminogen/MMP-9 activation impaired in Lp(a)tg mice in the TG peritonitis model. A: Peritoneal lavage collected at 6, 24, 48, and 72 hours after TG injection, with macrophages measured by non-specific esterase assay (n = 5 to 9 for each genotype at each time point). B: Peritoneal lavage plasmin activity. C: Representative zymogram gel of lavage. D and E: Band intensity quantified from three zymogram gels (lavage samples from three mice per genotype at each time point were run on three gels separately). F: Ratio of actMMP-9/proMMP-9. Bars are means ± SEM. Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 when compared to WT at same time point.
AAA Formation in Lp(a)tg Mice
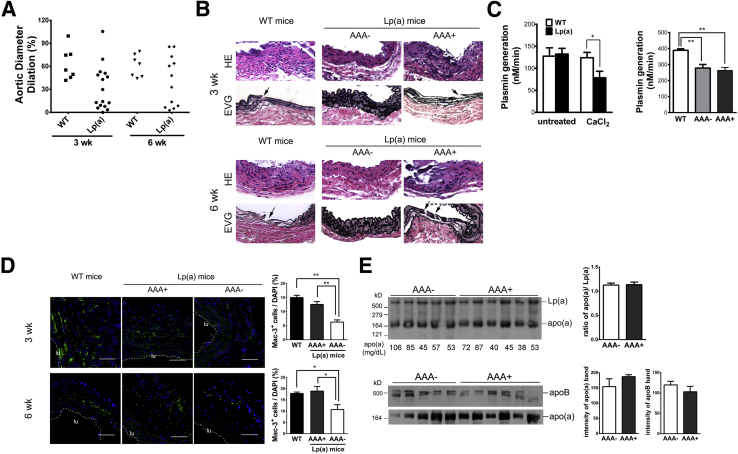
Because our study suggests apo(a) inhibits AAA formation, we also performed AAA on Lp(a)tg mice. Interestingly, 8 of 16 Lp(a)tg mice had a dilated aorta, similar to the WT mice, and the other 8 mice showed little dilation, similar to apo(a)tg mice. The phenotypes of Lp(a)tg mice were separated into two groups, resistant to AAA (AAA−) and prone to AAA (AAA+). To determine whether some mice needed a longer time to develop the aneurysm, the post-surgery time was extended to 6 weeks. In another group of Lp(a)tg mice, 58% (7 of 12) of the mice formed the aneurysm. Aortic dilation of seven Lp(a)tg mice was more like WT mice, and minimal dilation was observed in the other five Lp(a)tg mice (Figure 8A).
Figure 8.
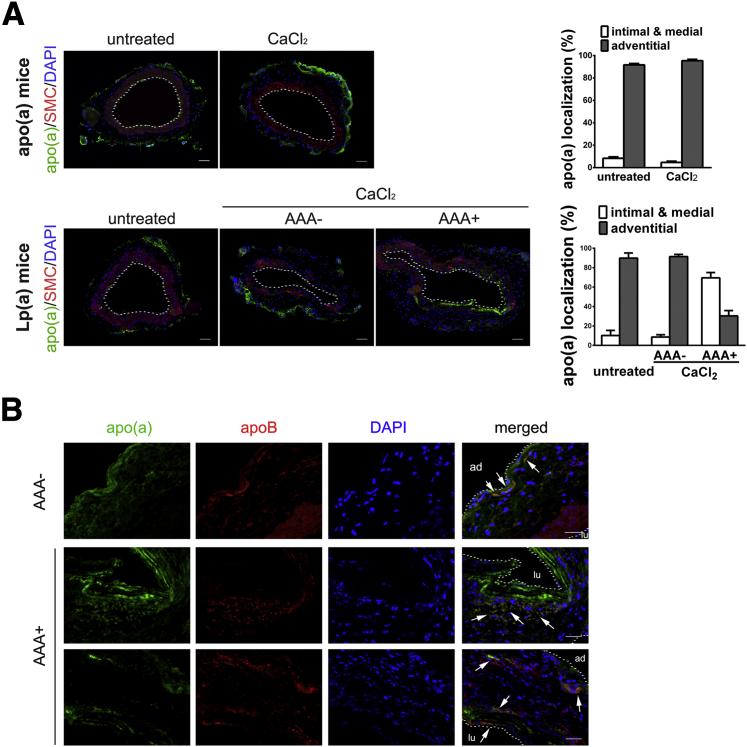
AAA development in Lp(a)tg mice. A and B: AAA 3 and 6 weeks after CaCl2 treatment. A: Aorta dilation. B: Histochemical staining and representative aorta sections from WT, Lp(a)tg AAA-resistant (AAA−), or susceptible (AAA+) mice. The fragmented laminae (arrow) in the intimal aorta was observed in WT or Lp(a) AAA+ mice but not in Lp(a) AAA− mice. C: Plasminogen activation in plasma of untreated or CaCl2-treated (at 1 week) WT and Lp(a)tg mice (left panel). Statistical analysis, by t-test (ns, P > 0.05). Plasminogen activation in plasma in WT, AAA−, or AAA+ Lp(a)tg mice at 6 weeks (n = 7 to 9) (right panel). D: Macrophages stained with Mac-3 in aorta collected at 3 or 6 weeks after CaCl2 treatment. A dashed line marked the inner boundary of the vessel. Percentage Mac-3+ cells per area quantified from three sections per mouse (n = 3 to 5 mice per group). Bars are means ± SEM. Statistical analysis, one-way analysis of variance, and Newman-Kuels posttest were performed. E: Plasma of Lp(a)tg mice collected at 6 weeks after CaCl2 treatment. Top panel, analyzed with a nonreducing gel. Apo(a) immunoblotting of free apo(a) and Lp(a) complex. Circulating apo(a) measured by ELISA and concentration (mg/dL) indicated at the bottom of gel. Bottom panel, Western blot analysis of plasma with reducing gel and immunostained for apo(a) or apoB. Bars are means ± SEM. Statistical analysis, by t-test (ns P > 0.05). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×10 (B). Scale bar = 100 μm (D). lu, Lumen area.
Examination of the aorta tissue sections of Lp(a)tg mice collected 3 or 6 weeks after CaCl2 treatment revealed that only the mice that formed an aneurysm [AAA+ Lp(a)tg mice] had increased cell infiltration and disrupted elastic lamina, similar to WT mice. In the Lp(a)tg mice that did not form an aneurysm [AAA− Lp(a)tg mice], the reduced cell infiltration and the vessel architecture were similar to apo(a)tg mice (Figure 8B). Plasminogen activation was significantly reduced in Lp(a)tg mice after CaCl2 treatment, compared with WT mice. AAA− and AAA+ Lp(a)tg mice did not show any difference in plasminogen activation 6 weeks after CaCl2 treatment (Figure 8C). Macrophage recruitment was also assessed in WT and Lp(a)tg mice. At 3 weeks, macrophages were not different in the arterial wall of WT and AAA+ Lp(a)tg mice, but were reduced by twofold in AAA− Lp(a)tg mice, suggesting macrophage recruitment contributed to AAA formation in Lp(a)tg mice. At 6 weeks, macrophages were also significantly lower in AAA− Lp(a)tg mice than WT and AAA+ Lp(a)tg mice (Figure 8D). Thus, AAA+ Lp(a)tg mice had the potential to develop AAA, such as the WT mice, and AAA− Lp(a)tg mice were more like apo(a)tg mice that were protected from AAA formation.
To determine whether the distribution of the apo(a) in the mice determined susceptibility to AAA, plasma from CaCl2-treated Lp(a)tg mice was subjected to Western blot analysis with nonreduced conditions, and there was no difference between free and bound apo(a) in the AAA− and AAA+ Lp(a)tg mice. Reduced apo(a) or apoB was also not different between two groups (Figure 8E). Fluorescent immunostaining of the aorta indicated that >90% of apo(a) was located in the adventitial layer of apo(a)tg mice before or after CaCl2 treatment. α-Smooth muscle actin was assessed to delineate the medial layer. In untreated Lp(a)tg mice, apo(a) was also localized predominately in the adventitia, as found in the apo(a)tg mice. With CaCl2 treatment, in AAA− Lp(a)tg mice, apo(a) was localized in the adventitia, whereas AAA+ Lp(a)tg mice had more apo(a) accumulated in the intimal and medial layers compared with the adventitial layer (Figure 9A). In AAA+ Lp(a)tg mice, apo(a) was deposited in the intimal layer, where the elastic lamina was fragmented. The difference in susceptibility for AAA between AAA− and AAA+ Lp(a) mice could be explained by apo(a) localization in the vessel wall. In apo(a)tg mice and AAA− Lp(a)tg mice, the vessel wall was intact and may be the reason they were protected from apo(a) deposition in the intimal/medial layer. Apo(a) accumulation was accelerated in the intimal layer of injured tissue in AAA+ Lp(a)tg mice and may lead to the degradation of the elastic lamina and dilation of the vessel. The AAA+ Lp(a)tg mice are similar to the apo(a)tg:Plg−/− mice that also formed AAA with degradation of the elastic lamina, similar to WT mice. In the apo(a)tg:Plg−/− mice, apo(a) also localized to the intimal/medial area. The formation of the AAA in the AAA+ Lp(a)tg mice (reduced plasminogen activation) and apo(a)tg:Plg−/− mice (no plasminogen) suggests apo(a) acts independent of plasminogen.
Figure 9.
Apo(a) and apoB localization in aorta of Lp(a)tg mice with CaCl2 treatment. A: Apo(a) localization in aorta of untreated or CaCl2-treated (3 weeks) apo(a)tg and Lp(a)tg (AAA− and AAA+) mice. Aorta immunostained for apo(a) (green), α-smooth muscle actin (SMA; red), and merged with DAPI. Dashed line delineates the inner boundary of the vessel. Apo(a) expression in intimal/medial or adventitial area quantified as percentage of total apo(a) in section. Bars are means ± SEM (n = 6). Apo(a) and apoB colocalization in vessel wall of AAA− Lp(a)tg mice (a) and AAA+ Lp(a) mice (b and c) 3 weeks after CaCl2 treatment. B: Apo(a) and apoB colocalization indicated as arrows in the adventitial aorta of AAA− Lp(a) mice (a) 3 weeks after CaCl2 treatment (top panel). Representative images of AAA+ Lp(a) mice with colocalization of apo(a) and apoB indicated by arrows in the media layer (middle panel) or both intimal and adventitial layers (bottom panel). Scale bars: 100 μm (A); 50 μm (B). ad, Adventitial boundary; lu, lumen.
ApoB was also detected in the arterial wall, and localized in each layer of aorta from intimal to adventitial. Because apo(a) dominated in the adventitial layer of AAA− Lp(a)tg mice, the colocalization of apo(a) and apoB [Lp(a) complex] was also observed predominantly in the adventitia. AAA+ Lp(a)tg mice had apo(a) and apoB colocalized in adventitia and the intimal and medial layers (Figure 9B), indicating that Lp(a) localization in the intimal/media layer, but not the adventitial layer, was related to AAA incidence. However, the increased apo(a) accumulation in the injured intimal and medial layers may accelerate fragmentation of the elastic lamina and dilation of the aorta that specifically occurred in AAA+ Lp(a)tg mice. Thus, Lp(a)/apo(a) exhibit both plasminogen-dependent and plasminogen-independent effects on AAA formation.
Discussion
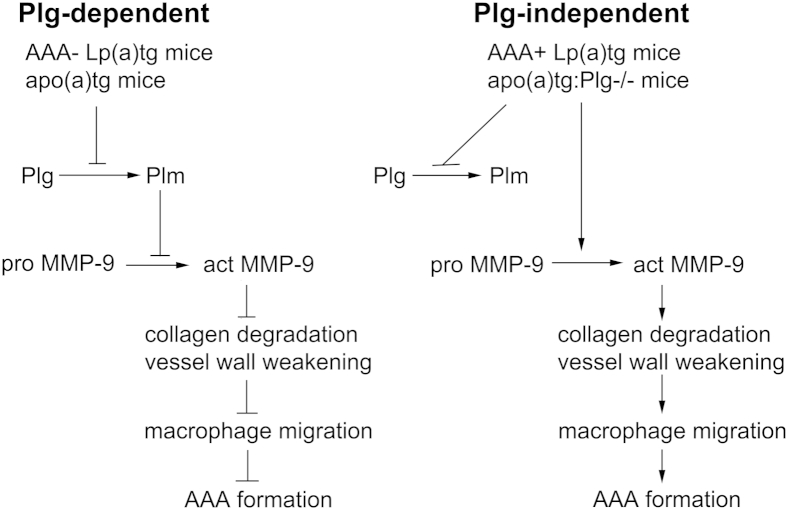
Elevated Lp(a) is an independent risk factor for cardiovascular and inflammatory diseases.3,4 However, the pathophysiological role of Lp(a) in these diseases is unclear. The goal of this study was to determine whether apo(a)/Lp(a) modified plasminogen-dependent leukocyte recruitment in vivo in inflammation. Our findings of macrophage recruitment are summarized in Figure 10. By using the mouse models of apo(a)tg mice and Lp(a) double-transgenic mice, this study provides evidence that Lp(a) and apo(a) interfere with plasminogen-dependent macrophage recruitment in inflammation by inhibition of plasminogen and MMP-9 activation. Reduced macrophage recruitment was required for protection from aneurysm formation in the apo(a)tg, Plg−/−, Plgmut, and Lp(a)tg-AAA resistant mice (plasminogen-dependent pathway), and was due to decreased plasma plasminogen activation, aorta MMP-9 activation, collagen accumulation, and macrophage recruitment in the aorta. However, dilation of the aorta developed in mice with vascular macrophage recruitment in apo(a)tg:Plg−/− and Lp(a)+AAA-susceptible mice (plasminogen-independent pathway). Although plasma plasminogen activation was reduced in these mice, MMP-9 activation and macrophage recruitment were not different from findings for WT mice, suggesting a plasminogen-independent pathway of MMP-9 activation, macrophage recruitment, and AAA formation. Furthermore, we verify an independent role of apo(a) in neutrophil recruitment to the injured aorta. Although plasminogen deficiency does not inhibit neutrophil recruitment in TG-induced peritonitis or CaCl2-induced AAA, apo(a) inhibits neutrophil recruitment in both inflammatory models by inhibiting cytokine release and reducing entry of neutrophils into the vessel wall. Both macrophages and neutrophils play important roles in AAA initiation and in the repair of damaged tissue through phagocytosis and stimulation of paracrine factors for wound healing and tissue remodeling after injury.33,34 Our novel findings of Lp(a)/apo(a) of MMP-9–dependent regulation of macrophage recruitment and cytokine-dependent neutrophil recruitment in vivo and the leukocytosis in the peripheral blood may account for many of the pathological consequences of elevated Lp(a).
Figure 10.
Apo(a) regulation of macrophage recruitment in inflammation. Apo(a) acts with plasminogen-dependent and plasminogen-independent effects on macrophage recruitment and AAA formation. Plasminogen-dependent pathway: Apo(a) inhibits plasminogen activation, MMP-9 activation, macrophage migration, and AAA formation. Plasminogen-independent pathway: Apo(a) inhibits plasminogen activation, but not MMP-9 activation, macrophage migration, or AAA formation.
Because mice do not carry the gene for apo(a), transgenic mice were generated to express human apo(a), and mice with genotypes of WT, apo(a)tg:Plg−/−, apo(a)tg:Plg−/− [apo(a)tg in a Plg−/− background], and Lp(a)tg:[apo(a)/apoB double transgenic] were used in this study. There are several reasons to think that our mouse model is biologically relevant and offers several advantages: a small apo(a) construct (smaller isoforms are more pathogenic in patients); expression of apo(a) at high concentrations; in mice, apo(a) does not covalently bind to mouse LDL so that effects can be assigned directly to the apo(a) moiety; and the apo(a) form expressed has high LBS activity, which is associated with pathogenic effects of apo(a) and its ability to inhibit plasminogen functions. We also believe our apo(a) mouse model, when crossed into a plasminogen-deficient and human apoB background [intact apo(a) assembles Lp(a)], is a useful tool for dissecting the plasminogen-dependent mechanisms of Lp(a). The TG-induced peritonitis model is a widely used inflammatory model, and the CaCl2-induced AAA model is reproducible and induces an inflammatory response and dilation of the aorta in mice with the C57BL/6J background.
Several studies demonstrate that plasminogen-dependent macrophage recruitment in inflammation requires the binding to receptors (α-enolase, histone H2B, S100A10, and plasminogen-R KT) on the cell surface and activation of plasminogen to plasmin.35 Plasminogen deficiency or deficiency of specific receptors abolishes or attenuates plasmin generation in vivo in inflammation and reduces leukocyte recruitment.35 Although baseline values of plasmin activation before treatment were not different in apo(a)tg mice compared with WT mice, plasmin generation was markedly inhibited by Lp(a)tg or apo(a)tg mice after treatment in the TG-induced peritonitis and CaCl2-induced AAA inflammatory models. Lp(a)/apo(a) inhibits binding of plasminogen to cells and has a marked inhibition of activation after stimulation in inflammation.
MMP-9 activation by plasmin is crucial for macrophage migration and stem cell mobilization,8,9 and in this study, plasmin activation of MMP-9 was suppressed by either apo(a) or Lp(a) in the peritonitis inflammatory model. Although an inverse association of actMMP-9 and Lp(a) serum level was found in AAA patients,36 there was no direct evidence of whether Lp(a) or apo(a) regulated MMP-9 activation. In this study, the modulation of MMP-9 activation by apo(a) was tested not only in vivo, but also in vitro, and suggests that apo(a) does not inhibit MMP-9 directly, but via inhibition of plasminogen activation. MMP-9 activation has been reported to be important for recovery after hind limb ischemia, myocardial infarction, and stroke.37,38 The inhibition of MMP-9 activation by elevated Lp(a)/apo(a) may impair recovery from CVD. Although the major activator of MMP-9 is plasmin, in the apo(a)tg:Plg−/− mice and Lp(a)tg AAA+ mice, plasmin was not required for the activation of MMP-9, suggesting that there may be an alternative activator for MMP-9. Activation of MMP-9 by other MMPs has been suggested, but these studies were not evaluated in vivo in inflammation.39,40
Neutrophils are the first leukocytes to arrive at the site of injury, and CXCL1 and CXCL2 are the major cytokine chemoattractants for neutrophils. Mice treated with anti-neutrophil antibodies failed to form an aneurysm after elastase stimulation.41 In Plg−/− mice, neutrophils are not reduced in either the peritonitis or AAA models, suggesting plasminogen is not required for early-stage neutrophil recruitment. The inhibition of neutrophil recruitment by apo(a) occurred on either a plasminogen-replete or a plasminogen-deplete background, indicating that the suppressed neutrophil recruitment by apo(a) was independent of plasminogen. Suppression of neutrophil recruitment by apo(a) contributed to the inhibition of aneurysm formation. CXCL1 and CXCL2 expression may be cell or stimulus dependent.31,32 The major sources of CXCL1 and CXCL2 are macrophages and neutrophils. Neither macrophage nor neutrophil number was different in apo(a)tg and WT mice at the time of cytokine expression on day 1 after treatment, suggesting that the major sources of the cytokines were not limiting in the apo(a)tg mice, and that the release of the cytokines may be impaired in the apo(a)tg mice. Another potential source of the cytokines is the resident cells, such as endothelial cells and smooth muscle cells in the vessel wall.30 Although circulating CXCL1 and CXCL2 were not different, aortic CXCL1 and CXCL2 were reduced in apo(a)tg mice compared with WT mice, suggesting that cytokine secretion by cells in the aorta was regulated by apo(a).
Apo(a)tg mice in the Plg+/+ background were protected from AAA formation, but Lp(a)tg mice had a 50% incidence of AAA, such as the WT mice, and 50% had no AAA-like apo(a)tg mice. The difference between the AAA+ and AAA− Lp(a)tg mice was not due to a difference in age, plasmin generation, plasma apo(a), or plasma apoB concentration, or the free/bound apo(a) ratio in the plasma, but may be related to apo(a) location in the vessel wall. Apo(a) was predominantly localized in the adventitia of AAA− Lp(a)tg mice, but in the AAA+ Lp(a)tg mice, more apo(a) was deposited in the intimal/medial layer, where elastic lamina was disrupted. Because apo(a) localized exclusively in the adventitial layer of all untreated Lp(a)tg mice, we postulate that its distribution into the fragmented intimal layer of AAA+ Lp(a) mice promotes the dilation of the aorta and AAA progression. The apo(a)tg:Plg−/− mice were also susceptible to AAA formation, and the distribution of apo(a) in the vessel wall was similar to that of Lp(a)AAA+ mice, with apo(a) differentially expressed in the intimal/medial layer, rather than the adventitia, such as the untreated mice.
The roles of apo(a) and Lp(a) in AAA were not identical. Thompson et al42 investigated the variation of AAA in inbred mice and found that, although all of the C57BL/6J mice had aortic dilation, there was a twofold variation in the amount of dilation. In our study, Lp(a)tg mice were both susceptible and resistant to AAA formation, and this is consistent with variation that occurs in human populations. The observation that not all of apoB colocalized with apo(a) suggested deposition of free apo(a) in the vessel wall. This is consistent with the studies in humans of the differential localization of LDL, Lp(a), and free apo(a) in the atherosclerotic lesions.43–46 Numerous studies reported elevated serum LDL was associated with AAA in patients.47 LDL is elevated in mice with the human apoB transgene24 and may contribute to the distinct roles of apo(a) and Lp(a) in AAA in our mouse model. Our findings of the mechanism of apo(a)/Lp(a) in inflammation and AAA formation have not previously been reported.
Clinical Significance
Whether Lp(a) is a risk factor in AAA patients has been controversial.48–51 Because our study was performed in mice, regulation of plasminogen, MMP-9 activation, and cytokine release will need to be verified in humans with elevated Lp(a). Although AAA animal models recapitulate many features of the human AAA, the animal models are primary inflammatory models that address formation of the AAA. The results of these models that induce the formation of AAA are short-term and may not predict the long-term effects that occur in humans. In patients, AAA is chronic and, more important, it is a process of atherothrombosis.52,53 In the long-term, Lp(a)/apo(a) may contribute to anti-fibrinolysis that promotes atherothrombosis development, leading to deleterious AAA outcome. The protective role of Lp(a)/apo(a) inhibition of inflammatory cell recruitment, protease activity, and neutrophil chemoattractants in early inflammation might be counteracted with its impact on intraluminal thrombus, which may occur with the progression of dilation. In addition, the leukocytosis due to the reduced recruitment in the AAA model may be detrimental. Studies have provided strong evidence that leukocyte number54 and markers of leukocyte activation55,56 are independent risk factors for myocardial infarction and other CVDs. As new therapeutic drugs are developed to reduce elevated Lp(a), these outcomes can be evaluated.
In conclusion, this study demonstrates that elevated Lp(a)/apo(a) interferes with plasminogen cell binding and markedly reduces plasminogen activation and inhibits MMP-9 activation in inflammation. Although previous studies recognized the consequences of Lp(a) in reduced fibrinolysis, our study suggests that Lp(a)/apo(a) sufficiently inhibits plasminogen activation and that its downstream pathway, such as MMP-9 activation and macrophage recruitment, is suppressed. Identification of the initial formation and potentially new risk factors for AAA has been feasible with new techniques to visualize the intact aorta.57,58 In addition, our study suggests Lp(a)/apo(a) may also have plasminogen-independent effects on macrophage recruitment in Lp(a)tg and apo(a):Plg−/− mice. Our study suggests targets for AAA, such as variation in plasminogen activation, MMP-9 activation, leukocyte infiltration, and leukocytosis, that may affect the response to elevated Lp(a). Suppression of MMP-9 activation may serve as a therapeutic tool. Apo(a) localization in the vessel wall may be considered a critical indicator for Lp(a)-induced vascular injury.
Footnotes
Supported by NIH grants, National Heart, Lung, and Blood Institute grants R01HL078701 and R01HL17964 (J.H.P.) and American Heart Association grants 09BGIA2050157 and 12SDG9050018 (Y.G.).
Disclosures: None declared.
Current address of M.H., Department of Radiation Oncology, University of Pennsylvania School of Medicine, Philadelphia, PA; of Y.G., Division of Translational Medicine and Human Genetics, University of Pennsylvania School of Medicine, Philadelphia, PA.
Supplemental Data
Pretreatment aorta diameter, baseline MMP-9, and pretreatment plasmin generation. A: Diameter change in sham (saline)–treated mice. WT, Plgmut, Plg−/−, apo(a)tg, apo(a)tg;Plg−/−, and Lp(a)tg mice were treated with saline applied to the abdominal aorta, rather than CaCl2 in the AAA model, and did not show a difference in aorta dilation after 3 weeks. Bars are means ± SEM (n = 4 to 16). Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. B: MMP-9 expression in the aorta of WT and apo(a)tg mice, detected by using Western blot analysis, 1 day after CaCl2 treatment, and normalized by β-actin expression. Bars are means ± SEM (n = 4). A statistical analysis and t-test were performed. C: Plasmin generation is not different in the plasma of WT and apo(a)tg mice before CaCl2 treatment. Bars are means ± SEM (n = 3 to 5). A statistical analysis and t-test were performed.
Signaling pathways and cytokine expression. A: Akt, extracellular signal–regulated kinase (ERK), and C-Jun kinase (JNK) were not different in the aorta of WT and apo(a)tg mice 1 day after CaCl2 treatment. Bars are means ± SEM (n = 3). A statistical analysis and t-test were performed (ns = P > 0.05). B: CCL2, a macrophage chemokine, was detected by ELISA in the plasma of WT and apo(a)tg mice collected 1 day after saline (sham) or CaCl2 treatment. C: CCL2 in the plasma 3 weeks after CaCl2 treatment. D: Quantitative RT-PCR (RT-qPCR) of Ccl2 mRNA level in the homogenized aorta collected 1 day after CaCl2 treatment. E: RT-qPCR of Ccl3 mRNA 1 day after CaCl2 treatment. Bars are means ± SEM (n = 6). Statistical analysis between WT and apo(a)tg mice, by t-test, was performed. ∗P < 0.05.
Neutrophil recruitment and dilation of aorta after cytokine antibody or antigen treatment. A: Neutrophils (PMNs) in treated abdominal aorta. Time points of neutrophil recruitment in CaCl2-treated abdominal aortas in WT mice (n = 4 to 13 per time point). B and C: Neutrophils immunostained in the aorta of WT and apo(a)tg mice 1 day after CaCl2 treatment. B: Representative sections. C: Neutrophil number (n = 6). D: AAA formation of WT and apo(a)tg mice rescued with antibodies or cytokines. WT mice were treated with IgG, and anti-CXCL1 and anti-CXCL2, neutralizing antibodies, for the first 3 days, and the aorta was collected 3 weeks after CaCl2 treatment. Dilation was observed in WT mice with IgG or anti-CXCL1, but not in the mice with anti-CXCL2 antibodies (n = 6). E: Dilation of aorta of apo(a)tg mice with saline or cytokines collected 3 weeks after CaCl2 treatment. CXCL1 or CXCL2 induced AAA formation in apo(a)tg mice compared with saline injection (n = 6).
References
- 1.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu K., Mitchell R.N., Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 3.Jones G.T., van Rij A.M., Cole J., Williams M.J., Bateman E.H., Marcovina S.M., Deng M., McCormick S.P. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem. 2007;53:679–685. doi: 10.1373/clinchem.2006.079947. [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup P.R., Benn M., Tybjaerg-Hansen A., Nordestgaard B.G. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen city heart study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 5.Hoover-Plow J., Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moons L., Shi C., Ploplis V., Plow E., Haber E., Collen D., Carmeliet P. Reduced transplant arteriosclerosis in plasminogen-deficient mice. J Clin Invest. 1998;102:1788–1797. doi: 10.1172/JCI3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploplis V.A., French E.L., Carmeliet P., Collen D., Plow E.F. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 8.Gong Y., Hart E., Shchurin A., Hoover-Plow J. Inflammatory macrophage migration requires mmp-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y., Fan Y., Hoover-Plow J. Plasminogen regulates stromal cell-derived factor-1/cxcr4-mediated hematopoietic stem cell mobilization by activation of matrix metalloproteinase-9. Arterioscler Thromb Vasc Biol. 2011;31:2035–2043. doi: 10.1161/ATVBAHA.111.229583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creemers E., Cleutjens J., Smits J., Heymans S., Moons L., Collen D., Daemen M., Carmeliet P. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol. 2000;156:1865–1873. doi: 10.1016/S0002-9440(10)65060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P., Moons L., Ploplis V., Plow E., Collen D. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J Clin Invest. 1997;99:200–208. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salonen E.M., Saksela O., Vartio T., Vaheri A., Nielsen L.S., Zeuthen J. Plasminogen and tissue-type plasminogen activator bind to immobilized fibronectin. J Biol Chem. 1985;260:12302–12307. [PubMed] [Google Scholar]
- 13.Salonen E.M., Zitting A., Vaheri A. Laminin interacts with plasminogen and its tissue-type activator. FEBS Lett. 1984;172:29–32. doi: 10.1016/0014-5793(84)80866-2. [DOI] [PubMed] [Google Scholar]
- 14.Stack M.S., Moser T.L., Pizzo S.V. Binding of human plasminogen to basement-membrane (type iv) collagen. Biochem J. 1992;284:103–108. doi: 10.1042/bj2840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles L.A., Sebald M.T., Fless G.M., Scanu A.M., Curtiss L.K., Plow E.F., Hoover-Plow J.L. Interaction of lipoprotein (a) with the extracellular matrix. Fibrinolysis Proteolysis. 1998;12:79–87. [Google Scholar]
- 16.Miles L.A., Fless G.M., Scanu A.M., Baynham P., Sebald M.T., Skocir P., Curtiss L.K., Levin E.G., Hoover-Plow J.L., Plow E.F. Interaction of lp(a) with plasminogen binding sites on cells. Thromb Haemost. 1995;73:458–465. [PubMed] [Google Scholar]
- 17.Angles-Cano E., Hervio L., Rouy D., Fournier C., Chapman J.M., Laplaud M., Koschinsky M.L. Effects of lipoprotein(a) on the binding of plasminogen to fibrin and its activation by fibrin-bound tissue-type plasminogen activator. Chem Phys Lipids. 1994;67-68:369–380. doi: 10.1016/0009-3084(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 18.Hoover-Plow J.L., Miles L.A., Fless G.M., Scanu A.M., Plow E.F. Comparison of the lysine binding functions of lipoprotein(a) and plasminogen. Biochemistry. 1993;32:13681–13687. doi: 10.1021/bi00212a037. [DOI] [PubMed] [Google Scholar]
- 19.Erqou S., Thompson A., Di Angelantonio E., Saleheen D., Kaptoge S., Marcovina S., Danesh J. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 20.Simo J.M., Joven J., Vilella E., Ribas M., Pujana M.A., Sundaram I.M., Hammel J.P., Hoover-Plow J.L. Impact of apolipoprotein(a) isoform size heterogeneity on the lysine binding function of lipoprotein(a) in early onset coronary artery disease. Thromb Haemost. 2001;85:412–417. [PubMed] [Google Scholar]
- 21.Bonen D.K., Hausman A.M., Hadjiagapiou C., Skarosi S.F., Davidson N.O. Expression of a recombinant apolipoprotein(a) in hepg2 cells: evidence for intracellular assembly of lipoprotein(a) J Biol Chem. 1997;272:5659–5667. doi: 10.1074/jbc.272.9.5659. [DOI] [PubMed] [Google Scholar]
- 22.Fan J., Ji Z.S., Huang Y., de Silva H., Sanan D., Mahley R.W., Innerarity T.L., Taylor J.M. Increased expression of apolipoprotein e in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J Clin Invest. 1998;101:2151–2164. doi: 10.1172/JCI1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha J., McCullough B., Hart E., Nassir F., Davidson N.O., Hoover-Plow J. Apo(a) promotes thrombosis in a vascular injury model by a mechanism independent of plasminogen. J Thromb Haemost. 2005;3:2281–2289. doi: 10.1111/j.1538-7836.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 24.Ko C., O’Rourke S.M., Huang L.S. A fish oil diet produces different degrees of suppression of apob and triglyceride secretion in human apob transgenic mouse strains. J Lipid Res. 2003;44:1946–1955. doi: 10.1194/jlr.M300172-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Callow M.J., Stoltzfus L.J., Lawn R.M., Rubin E.M. Expression of human apolipoprotein b and assembly of lipoprotein(a) in transgenic mice. Proc Natl Acad Sci U S A. 1994;91:2130–2134. doi: 10.1073/pnas.91.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoover-Plow J.L., Boonmark N., Skocir P., Lawn R., Plow E.F. A quantitative immunoassay for the lysine-binding function of lipoprotein(a): application to recombinant apo(a) and lipoprotein(a) in plasma. Arterioscler Thromb Vasc Biol. 1996;16:656–664. doi: 10.1161/01.atv.16.5.656. [DOI] [PubMed] [Google Scholar]
- 27.Iwaki T., Malinverno C., Smith D., Xu Z., Liang Z., Ploplis V.A., Castellino F.J. The generation and characterization of mice expressing a plasmin-inactivating active site mutation. J Thromb Haemost. 2010;8:2341–2344. doi: 10.1111/j.1538-7836.2010.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Laumonnier Y., Syrovets T., Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- 29.Syrovets T., Thillet J., Chapman M.J., Simmet T. Lipoprotein(a) is a potent chemoattractant for human peripheral monocytes. Blood. 1997;90:2027–2036. [PubMed] [Google Scholar]
- 30.Garcia-Ramallo E., Marques T., Prats N., Beleta J., Kunkel S.L., Godessart N. Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J Immunol. 2002;169:6467–6473. doi: 10.4049/jimmunol.169.11.6467. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong D.A., Major J.A., Chudyk A., Hamilton T.A. Neutrophil chemoattractant genes Cxcl1 and Cxcl2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75:641–648. doi: 10.1189/jlb.0803370. [DOI] [PubMed] [Google Scholar]
- 32.Call D.R., Nemzek J.A., Ebong S.J., Bolgos G.R., Newcomb D.E., Wollenberg G.K., Remick D.G. Differential local and systemic regulation of the murine chemokines Cxcl1 and mip2. Shock. 2001;15:278–284. doi: 10.1097/00024382-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 33.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ardi V.C., Kupriyanova T.A., Deryugina E.I., Quigley J.P. Human neutrophils uniquely release timp-free mmp-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles L.A., Parmer R.J. Plasminogen receptors: the first quarter century. Semin Thromb Hemost. 2013;39:329–337. doi: 10.1055/s-0033-1334483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen E., Wagberg F., Angquist K.A. Does lipoprotein(a) inhibit elastolysis in abdominal aortic aneurysms? Eur J Vasc Endovasc Surg. 2003;26:423–428. doi: 10.1016/s1078-5884(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 37.Liu P., Sun M., Sader S. Matrix metalloproteinases in cardiovascular disease. Can J Cardiol. 2006;22(Suppl B)):25B–30B. doi: 10.1016/s0828-282x(06)70983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos-Fernandez M., Bellolio M.F., Stead L.G. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Fridman R., Toth M., Pena D., Mobashery S. Activation of progelatinase b (mmp-9) by gelatinase a (mmp-2) Cancer Res. 1995;55:2548–2555. [PubMed] [Google Scholar]
- 40.Ogata Y., Enghild J.J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 41.Eliason J.L., Hannawa K.K., Ailawadi G., Sinha I., Ford J.W., Deogracias M.P., Roelofs K.J., Woodrum D.T., Ennis T.L., Henke P.K., Stanley J.C., Thompson R.W., Upchurch G.R., Jr. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 42.Thompson R.W., Curci J.A., Ennis T.L., Mao D., Pagano M.B., Pham C.T. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 43.Cushing G.L., Gaubatz J.W., Nava M.L., Burdick B.J., Bocan T.M., Guyton J.R., Weilbaecher D., DeBakey M.E., Lawrie G.M., Morrisett J.D. Quantitation and localization of apolipoproteins [a] and b in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis. 1989;9:593–603. doi: 10.1161/01.atv.9.5.593. [DOI] [PubMed] [Google Scholar]
- 44.Pepin J.M., O’Neil J.A., Hoff H.F. Quantification of apo[a] and apob in human atherosclerotic lesions. J Lipid Res. 1991;32:317–327. [PubMed] [Google Scholar]
- 45.Fortunato J.E., Bassiouny H.S., Song R.H., Kocharian H., Glagov S., Edelstein C., Scanu A.M. Apolipoprotein (a) fragments in relation to human carotid plaque instability. J Vasc Surg. 2000;32:555–563. doi: 10.1067/mva.2000.107757. [DOI] [PubMed] [Google Scholar]
- 46.Papagrigorakis E., Iliopoulos D., Asimacopoulos P.J., Safi H.J., Weilbaecher D.J., Ghazzaly K.G., Nava M.L., Gaubatz J.W., Morrisett J.D. Lipoprotein(a) in plasma, arterial wall, and thrombus from patients with aortic aneurysm. Clin Genet. 1997;52:262–271. doi: 10.1111/j.1399-0004.1997.tb04343.x. [DOI] [PubMed] [Google Scholar]
- 47.Takagi H., Manabe H., Kawai N., Goto S.N., Umemoto T. Serum high-density and low-density lipoprotein cholesterol is associated with abdominal aortic aneurysm presence: a systematic review and meta-analysis. Int Angiol. 2010;29:371–375. [PubMed] [Google Scholar]
- 48.Lindholt J.S., Heegaard N.H., Vammen S., Fasting H., Henneberg E.W., Heickendorff L. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised ldl, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;21:51–56. doi: 10.1053/ejvs.2000.1262. [DOI] [PubMed] [Google Scholar]
- 49.Norrgard O., Angquist K.A., Dahlen G. High concentrations of lp(a) lipoprotein in serum are common among patients with abdominal aortic aneurysms. Int Angiol. 1988;7:46–49. [PubMed] [Google Scholar]
- 50.Golledge J., Tsao P.S., Dalman R.L., Norman P.E. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118:2382–2392. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takagi H., Manabe H., Kawai N., Goto S.N., Umemoto T. Circulating lipoprotein(a) concentrations and abdominal aortic aneurysm presence. Interact Cardiovasc Thorac Surg. 2009;9:467–470. doi: 10.1510/icvts.2009.208843. [DOI] [PubMed] [Google Scholar]
- 52.Michel J.B., Martin-Ventura J.L., Egido J., Sakalihasan N., Treska V., Lindholt J., Allaire E., Thorsteinsdottir U., Cockerill G., Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiernicki I., Stachowska E., Safranow K., Cnotliwy M., Rybicka M., Kaczmarczyk M., Gutowski P. Enhanced matrix-degrading proteolytic activity within the thin thrombus-covered wall of human abdominal aortic aneurysms. Atherosclerosis. 2010;212:161–165. doi: 10.1016/j.atherosclerosis.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Bhatt D.L., Chew D.P., Lincoff A.M., Simoons M.L., Harrington R.A., Ommen S.R., Jia G., Topol E.J. Effect of revascularization on mortality associated with an elevated white blood cell count in acute coronary syndromes. Am J Cardiol. 2003;92:136–140. doi: 10.1016/s0002-9149(03)00527-7. [DOI] [PubMed] [Google Scholar]
- 55.Brennan M.L., Hazen S.L. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–359. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Zhang R., Brennan M.L., Shen Z., MacPherson J.C., Schmitt D., Molenda C.E., Hazen S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 57.Kontopodis N., Georgakarakos E., Metaxa E., Pagonidis K., Papaharilaou Y., Ioannou C.V. Estimation of wall properties and wall strength of aortic aneurysms using modern imaging techniques: one more step towards a patient-specific assessment of aneurysm rupture risk. Med Hypotheses. 2013;81:212–215. doi: 10.1016/j.mehy.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 58.Ramaswamy A.K., Hamilton M., 2nd, Joshi R.V., Kline B.P., Li R., Wang P., Goergen C.J. Molecular imaging of experimental abdominal aortic aneurysms. ScientificWorldJournal. 2013;2013:973150. doi: 10.1155/2013/973150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pretreatment aorta diameter, baseline MMP-9, and pretreatment plasmin generation. A: Diameter change in sham (saline)–treated mice. WT, Plgmut, Plg−/−, apo(a)tg, apo(a)tg;Plg−/−, and Lp(a)tg mice were treated with saline applied to the abdominal aorta, rather than CaCl2 in the AAA model, and did not show a difference in aorta dilation after 3 weeks. Bars are means ± SEM (n = 4 to 16). Statistical analysis, one-way analysis of variance, and Dunnett’s posttest were performed. B: MMP-9 expression in the aorta of WT and apo(a)tg mice, detected by using Western blot analysis, 1 day after CaCl2 treatment, and normalized by β-actin expression. Bars are means ± SEM (n = 4). A statistical analysis and t-test were performed. C: Plasmin generation is not different in the plasma of WT and apo(a)tg mice before CaCl2 treatment. Bars are means ± SEM (n = 3 to 5). A statistical analysis and t-test were performed.
Signaling pathways and cytokine expression. A: Akt, extracellular signal–regulated kinase (ERK), and C-Jun kinase (JNK) were not different in the aorta of WT and apo(a)tg mice 1 day after CaCl2 treatment. Bars are means ± SEM (n = 3). A statistical analysis and t-test were performed (ns = P > 0.05). B: CCL2, a macrophage chemokine, was detected by ELISA in the plasma of WT and apo(a)tg mice collected 1 day after saline (sham) or CaCl2 treatment. C: CCL2 in the plasma 3 weeks after CaCl2 treatment. D: Quantitative RT-PCR (RT-qPCR) of Ccl2 mRNA level in the homogenized aorta collected 1 day after CaCl2 treatment. E: RT-qPCR of Ccl3 mRNA 1 day after CaCl2 treatment. Bars are means ± SEM (n = 6). Statistical analysis between WT and apo(a)tg mice, by t-test, was performed. ∗P < 0.05.
Neutrophil recruitment and dilation of aorta after cytokine antibody or antigen treatment. A: Neutrophils (PMNs) in treated abdominal aorta. Time points of neutrophil recruitment in CaCl2-treated abdominal aortas in WT mice (n = 4 to 13 per time point). B and C: Neutrophils immunostained in the aorta of WT and apo(a)tg mice 1 day after CaCl2 treatment. B: Representative sections. C: Neutrophil number (n = 6). D: AAA formation of WT and apo(a)tg mice rescued with antibodies or cytokines. WT mice were treated with IgG, and anti-CXCL1 and anti-CXCL2, neutralizing antibodies, for the first 3 days, and the aorta was collected 3 weeks after CaCl2 treatment. Dilation was observed in WT mice with IgG or anti-CXCL1, but not in the mice with anti-CXCL2 antibodies (n = 6). E: Dilation of aorta of apo(a)tg mice with saline or cytokines collected 3 weeks after CaCl2 treatment. CXCL1 or CXCL2 induced AAA formation in apo(a)tg mice compared with saline injection (n = 6).