Abstract
Immune challenge to the insect Podisus maculiventris induces synthesis of a 21-residue peptide with sequence homology to frog skin antimicrobial peptides of the brevinin family. The insect and frog peptides have in common a C-terminally located disulfide bridge delineating a cationic loop. The peptide is bactericidal and fungicidal, exhibiting the largest antimicrobial spectrum observed so far for an insect defense peptide. An all-D-enantiomer is nearly inactive against Gram-negative bacteria and some Gram-positive strains but is fully active against fungi and other Gram-positive bacteria, suggesting that more than one mechanism accounts for the antimicrobial activity of this peptide. Studies with truncated synthetic isoforms underline the role of the C-terminal loop and flanking residues for the activity of this molecule for which we propose the name thanatin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessalle R., Kapitkovsky A., Gorea A., Shalit I., Fridkin M. All-D-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990 Nov 12;274(1-2):151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- Bulet P., Cociancich S., Dimarcq J. L., Lambert J., Reichhart J. M., Hoffmann D., Hetru C., Hoffmann J. A. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem. 1991 Dec 25;266(36):24520–24525. [PubMed] [Google Scholar]
- Bulet P., Dimarcq J. L., Hetru C., Lagueux M., Charlet M., Hegy G., Van Dorsselaer A., Hoffmann J. A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem. 1993 Jul 15;268(20):14893–14897. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Tempst P. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J Biol Chem. 1993 Apr 5;268(10):7044–7054. [PubMed] [Google Scholar]
- Casteels P., Tempst P. Apidaecin-type peptide antibiotics function through a non-poreforming mechanism involving stereospecificity. Biochem Biophys Res Commun. 1994 Feb 28;199(1):339–345. doi: 10.1006/bbrc.1994.1234. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. P., Durell S., Maloy W. L., Zasloff M. Ranalexin. A novel antimicrobial peptide from bullfrog (Rana catesbeiana) skin, structurally related to the bacterial antibiotic, polymyxin. J Biol Chem. 1994 Apr 8;269(14):10849–10855. [PubMed] [Google Scholar]
- Cociancich S., Dupont A., Hegy G., Lanot R., Holder F., Hetru C., Hoffmann J. A., Bulet P. Novel inducible antibacterial peptides from a hemipteran insect, the sap-sucking bug Pyrrhocoris apterus. Biochem J. 1994 Jun 1;300(Pt 2):567–575. doi: 10.1042/bj3000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cociancich S., Ghazi A., Hetru C., Hoffmann J. A., Letellier L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993 Sep 15;268(26):19239–19245. [PubMed] [Google Scholar]
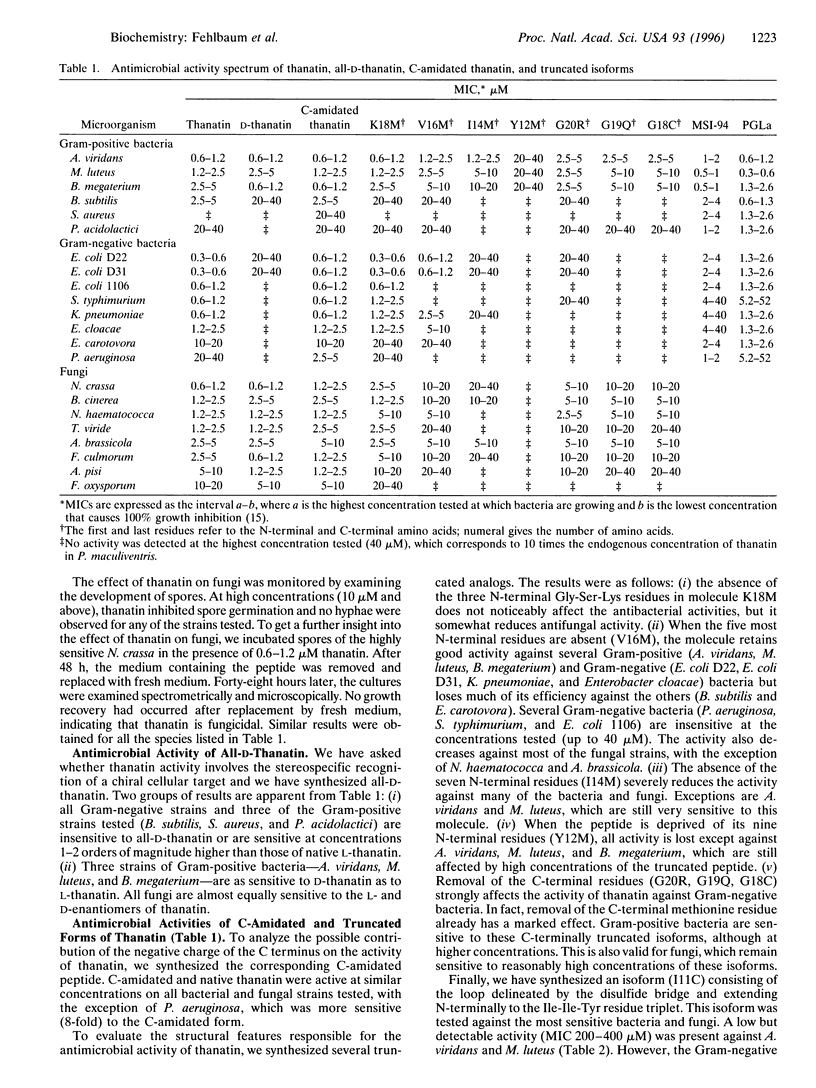
- Fehlbaum P., Bulet P., Michaut L., Lagueux M., Broekaert W. F., Hetru C., Hoffmann J. A. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994 Dec 30;269(52):33159–33163. [PubMed] [Google Scholar]
- Hoffmann J. A. Innate immunity of insects. Curr Opin Immunol. 1995 Feb;7(1):4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- King D. S., Fields C. G., Fields G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990 Sep;36(3):255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Kari U. P. Structure-activity studies on magainins and other host defense peptides. Biopolymers. 1995;37(2):105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Hagiwara K., Nakajima T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun. 1992 Nov 30;189(1):184–190. doi: 10.1016/0006-291x(92)91542-x. [DOI] [PubMed] [Google Scholar]
- Neimark J., Briand J. P. Development of a fully automated multichannel peptide synthesizer with integrated TFA cleavage capability. Pept Res. 1993 Jul-Aug;6(4):219–228. [PubMed] [Google Scholar]
- Park J. M., Jung J. E., Lee B. J. Antimicrobial peptides from the skin of a Korean frog, Rana rugosa. Biochem Biophys Res Commun. 1994 Nov 30;205(1):948–954. doi: 10.1006/bbrc.1994.2757. [DOI] [PubMed] [Google Scholar]
- Simmaco M., Mignogna G., Barra D., Bossa F. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J Biol Chem. 1994 Apr 22;269(16):11956–11961. [PubMed] [Google Scholar]
- Simmaco M., Mignogna G., Barra D., Bossa F. Novel antimicrobial peptides from skin secretion of the European frog Rana esculenta. FEBS Lett. 1993 Jun 14;324(2):159–161. doi: 10.1016/0014-5793(93)81384-c. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]



