Abstract
The objective of this study was to assess the efficacy of perianal infiltration of ropivacaine and dexmedetomidine added to ropivacainein in the relief of pain after hemorrhoidectomy. Patients in group C(placebo control group, n = 21) received perianal injections of normal saline and those in group RO(ropivacaine injection group, n = 21) received ropivacaine, those in group RD(ropivacaine with dexmedetomidine injection group, n = 19) were administered ropivacaine with dexmedetomidine, prior to the initiation of the operation. Reductions of the VAS score, the frequency with which the PCA button was pushed, and fentanyl consumption were assessed in groups RO and RD as compared to that of group C, and in group RD as compared to that of group RO(p < 0.05). We concluded that the use of perianal ropivacaine injection prior to surgical incision reduced both postoperative pain and fentanyl consumption following hemorrhoidectomy, and the addition of dexmedetomidine to ropivacaine may have an additive effect in postoperative analgesic care.
Keywords: Dexmedetomidine, Hemorrhoidectomy, Pain, Ropivacaine
Introduction
Treatment of hemorrhoidal disease depends on the stage of the disorder and the symptom [1]. Surgical hemorrhoidectomy is indicated for third and fourth-degree symptomatic hemorrhoids [2].
However, the postoperative course of hemorrhoidal surgery is commonly extremely painful.
Conventionally, nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates have often been used to control pain, but their use is confined to a short period of time and is associated with frequent side effects, including gastrointestinal problems, kidney dysfunction, nausea and vomiting, increased urinary retention, and reduced bowel mobility [3].
Consequently, continuous efforts have been made to reduce the use of NSAIDs and opiates.
Various attempts, including pudendal nerve block, perianal infiltration of local anesthetics, and application of topical preparations such as metronidazole, glyceryl nitrate, and calcium channel blockers, have been suggested to relieve the pain [4].
Among those methods, the perianal injection of local anesthetic (LA) has been shown to improve postoperative pain control after hemorrhoidectomy under general or spinal anesthesia [5–7].
Dexmedetomidine (Precedex®, Hospira, Inc., Lake Forest, IL) is a newly developed selective α2-adrenoceptor agonist used for continuous intravenous sedation in the intensive care setting and procedural sedation in nonintubated patients.
A previous study demonstrated that dexmedetomidine added to ropivacaine prolonged the duration of sensory and motor blockade in a sciatic nerve block [8] and intrathecal, intravenous dexmedetomidine enhanced the analgesic efficacy of regional blockade after operation [9, 10].
However, there are no clinical data regarding the effects of local infiltration of dexmedetomidine on postoperative pain.
We hypothesized that dexmedetomidine added to ropivacaine might potentiate the analgesic property of ropivacaine when employed in perianal injection.
Therefore, we designed a prospective randomized double-blind clinical study to evaluate the effects of perianal injection of ropivacaine and ropivacaine with dexmedetomidine on pain after hemorrhoidectomy.
Materials and Methods
Patients
The study protocol was approved by the local ethics committee and conducted in accordance with the principles set out in the Declaration of Helsinki of 2000. Written informed consent was obtained from all participants prior to their inclusion in the trial.
A total of 72 consecutive patients (age range 18–71 years) who were classified as American Society of Anesthesiologists (ASA) physical status I–III and who underwent closed hemorrhoidectomy for third or fourth-degree hemorrhoids at X Hospital, between March 2010 and December 2010, were considered eligible for the study. The exclusion criteria were a body weight of lower than 45 kg or greater than 100 kg, a history of pregnancy, use of analgesic medication in the week prior to surgery, contraindication to spinal anesthesia (e.g., coagulation defects, infection at puncture site, and preexisting neurological deficit in the lower extremities), previous anal operation, and known allergy to any of the test drugs. The decisions to enroll and exclude patients were made by the investigator, who did not otherwise participate in conducting the study or data collection.
Study Design and Randomization
This is a randomized, double-blinded, placebo-controlled study. Randomization into one of the three groups was based on Excel random-number generation. The details of the series, which were generated by a statistician who did not otherwise participate in this study, were unknown to both the investigators and the patients, and the numbers were hidden in a set of sealed envelopes. After admitting the patient into the operating room and just prior to the induction of anesthesia, the numbered envelope was opened and the card inside determined into which group the patient would be placed. In order to keep the surgeon and the anesthesiologist “blind” to the patient groups, the patients were administered ropivacaine, ropivacaine with dexmedetomidine, or normal saline as placebo, without a label, by an investigator who read the card. The patients were divided into three groups according to the perianal solutions administered. The patients assigned to group RO (ropivacaine perianal injection group) received perianal injections of 20 ml of 0.2 % ropivacaine. Those in group RD (ropivacaine with dexmedetomidine injection group) were administered 20 ml of 0.2 % ropivacaine and 5 μg/kg dexmedetomidine, and those in group C (the placebo control group) received identical amounts of normal saline.
One investigator who was blinded to the details of the study collected the postoperative data.
Anesthesia and Injection Technique
All patients were transferred to the operating room without premedication.
On arrival at the operation room, the patients were placed in the right decubitus position. Lumbar puncture was conducted at the L3-4 interspace via a midline approach, using a 25-gauge Quincke needle. Heavy marcaine (0.5 %, 5 mg) was injected. After intrathecal injection, the patients sat down for 5 min.
The noninvasive arterial blood pressure, electrocardiography, and pulse oximetry were continuously monitored. During the surgery, the patients received an intravenous infusion of lactated Ringer’s solution at a rate of 3–6 ml/kg/h. No additional intravenous opioids were injected.
Prior to skin incision, the surgeon infiltrated 20 ml of either solution to the surgical area with a 26-gauge needle. The solution was injected along the anal verges in the four quadrants (5 ml) and then more deeply in the posterior commissure (5 ml) and the two ischiorectal fossae (10 ml). During the procedure, careful aspirations were repeated to avoid intravascular injection of the solution.
Surgical Technique
One surgeon performed all operations under saddle block with a standard anesthesiologic procedure. All patients underwent closed hemorrhoidectomy in the jack-knife position, performed by one surgeon. The anal canal could be gently dilated to about two fingers’ width to permit adequate exposure. A Hill-Ferguson retractor was inserted into the anal canal. The hemorrhoidal masses were picked up with a straight hemostat. A triangular skin incision was cut around the hemorrhoidal mass with scissors (starting from the anal verge and extending to the dentate line). The mucosa was freed from the internal sphincter cephalad (close to the dentate line). The mucosa suspensory ligament was divided using the electrocautery. The proximal part of the internal sphincter was cut free and the hemorrhoid complex was removed. The wound was then closed with chromic 3-0 sutures.
Postoperative Pain Control
To control postoperative pain, intravenous fentanyl with a computerized intravenous patient-controlled analgesia (PCA) system (Automed 3300™, ACE Medical Corp. Ltd., Seoul, South Korea) was used. The mode of PCA was a bolus of 0.1 μg/kg, a lockout interval of 15 min, and a continuous infusion of 0.1 μg/kg/h (total regimen 10 μg/kg/100 ml). The patients were taught to push the button of the PCA system to receive a bolus of drug each time pain occurred. In the case of persistent pain greater than a visual analogue scale (VAS) pain score of 30 mm, an additional 50 μg of fentanyl was injected intravenously by the investigator until the pain was relieved to a level below a VAS pain score of 30 mm. No other analgesics, such as NSAIDs or acetaminophens, were included.
Studied Variables
For each patient, the age, gender, height, weight, the ASA physical status, and the grade of hemorrhoid were recorded.
The primary outcome measure of the study was the VAS (0–100 mm) pain score, which was measured by an investigator, who was blind to the study, at 4, 8, 12, 24, and 48 h after surgery. Additional analyses were conducted with regard to pain at first defecation, frequency with which the button of PCA was pushed (FPB), fentanyl consumption, the incidence of urinary retention, and postoperative nausea and vomiting (PONV).
The need for additional intravenous fentanyl was evaluated, and the integrated fentanyl consumption (PCA delivered + additional fentanyl) was assessed at the same intervals for each patient. The total amount of injected fentanyl for the postoperative period was compared between the groups.
Statistical Analysis
To estimate the group size, a pilot study was conducted for measuring the VAS pain score at 2 h after surgery in 10 patients who received perianal injection of normal saline. The standard deviation of the VAS pain score in this group was 19 mm. For our power calculation, we assumed an equal standard deviation in groups RO and RD. We wanted the capability to show a difference of 20 mm in the VAS pain score at 4 h after surgery among the groups. With α = 0.05, two-tailed and a power of 80 %, we needed 19 patients per group. Considering a compliance rate of 80 %, we asked 72 patients to participate in this study.
For intergroup comparisons, the distribution of the data was initially evaluated for normality via the Shapiro–Wilk test. The normally distributed data are expressed herein as the mean ± standard deviation, and groups were compared using analysis of variance and a post-hoc Tukey test. The nonnormally distributed data are expressed as medians (interquartile range) and were analyzed using the Kruskal–Wallis test with Bonferroni’s correction. Descriptive variables were subjected to χ2 analysis or Fischer’s exact test, as appropriate, and P values of <0.05 were regarded as statistically significant. The data in the figures were reported as the mean ± standard error. Statistical analysis was conducted using SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
No differences were noted among the groups in terms of age, height, weight, the ASA class, grade of hemorrhoid, and the operation time (Table 1).
Table 1.
Demographic data
| Group C (n = 21) | Group IP (n = 21) | Group IV (n = 19) | P value | |
|---|---|---|---|---|
| ASA 1/2/3 (n) | 14/5/2 | 15/3/3 | 14/2/3 | 0.815 |
| Age (yr) | 35.0 (25.5–46.5) | 32.0 (22.0–46.5) | 43.0 (22.0–52.0) | 0.528* |
| Gender M/F (n) | 15/6 | 12/9 | 12/7 | 0.626 |
| Height (cm) | 164.1 ± 8.7 | 169.2 ± 6.4 | 167.2 ± 7.4 | 0.097 |
| Weight (kg) | 66.0 ± 8.1 | 68.1 ± 9.2 | 63.5 ± 7.2 | 0.233 |
| Grade (3/4) | 16/5 | 15/6 | 14/5 | 0.940 |
ASA American society of anaesthesiologist physical status; Values are expressed as mean ± SD, except for ASA grade, gender and grade of hemorrhoid which are number of patients. * Kruskal–Wallis test is used and expressed as median (interquartile range) because of abnormal distribution. No significant differences between groups
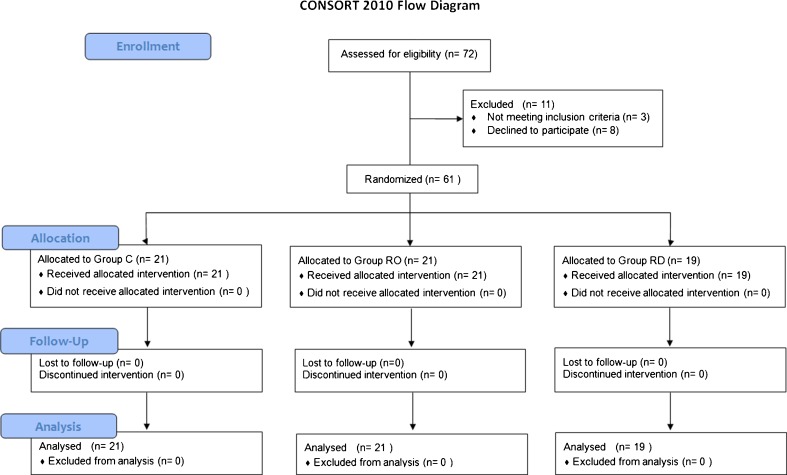
Among the 72 patients who were eligible for the study from March 2010 to December 2010, 8 patients refused to participate and 3 patients were excluded due to history of pregnancy, previous anal operation, and use of analgesic medication in the week prior to surgery (Fig. 1).
Fig. 1.
CONSORT diagram of the study
Of the 61 patients, 21 were randomized to group C, 21 to group RO, and 22 to group RD.
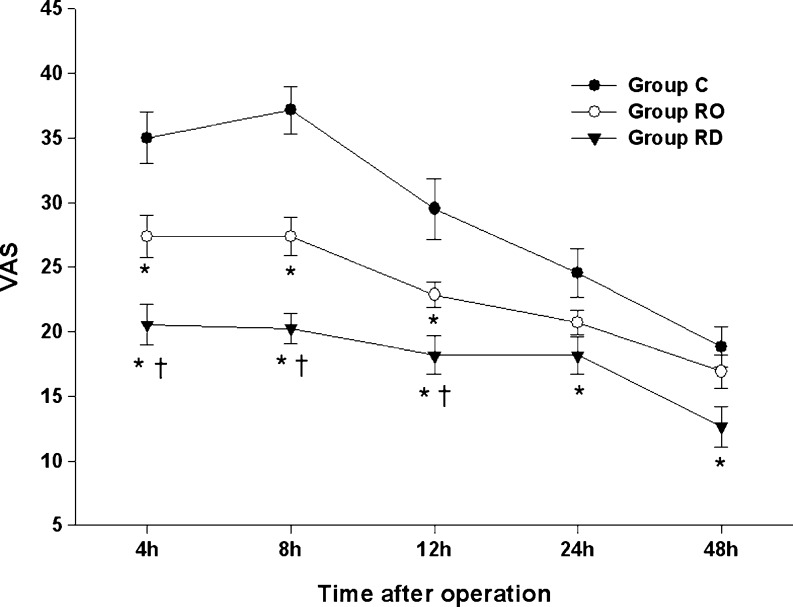
The results of the VAS pain scores are shown in Fig. 2. Despite the administration of rescue analgesic, the VAS pain score in group C was above 30 mm until 8 h. Pain scores were significantly reduced in group RD compared with those of group C at all time points (P < 0.05). The VAS was lower in group RO than in group C until 12 h (P < 0.05). Significant reductions in VAS were seen in group RD as compared to group RO until 12 h. In all groups, the pain was relieved gradually, except at 8 h in group C, when it was slightly increased over the previous time interval. VAS at first defecation was significantly lower in group RO and group RD than in group C, and was lower in group RD than in group RO (Table 2).
Fig. 2.
VAS pain score. Values are expressed as mean ± SE. * P < 0.05 compared with group C. † P < 0.05 comparison between group RO and group RD
Table 2.
Postoperative parameters
| Group C (n = 21) | Group RO (n = 21) | Group RD (n = 19) | P value | |
|---|---|---|---|---|
| VAS (first defecation) | 41.00 | 29.00 | 21.00 | <0.001* |
| (30.50–45.00) | (22.00–36.00) † | (19.00–24.00) †‡ | ||
| Fentanyl (ug) | 765.95 ± 106.34 | 635.19 ± 82.73† | 537.74 ± 65.88†‡ | <0.001 |
| Frequency to push the button of PCA (n) | 30.00 | 12.00 | 10.00 | <0.001* |
| (25.00–35.00) | (9.00–23.00) † | (6.00–12.00) †‡ | ||
| PONV (n) | 4 (19.0) | 3(14.3) | 2(10.5) | 0.748 |
| Urinary retention (n) | 2 (9.5) | 2 (9.5) | 1(5.3) | 0.854 |
VAS Visual Analogue Score of pain; PCA Patient-controlled analgesia machine; n number of patients; PONV postoperative nausea and vomiting. Values are expressed as mean ± SD, except for PONV, gender and urinary retention which are number of patients (proportion). * Kruskal–Wallis test is used and expressed as median (interquartile range) because of abnormal distribution. † P < 0.05 compared with Group C, ‡ P < 0.05 comparison between Group RO and Group RD
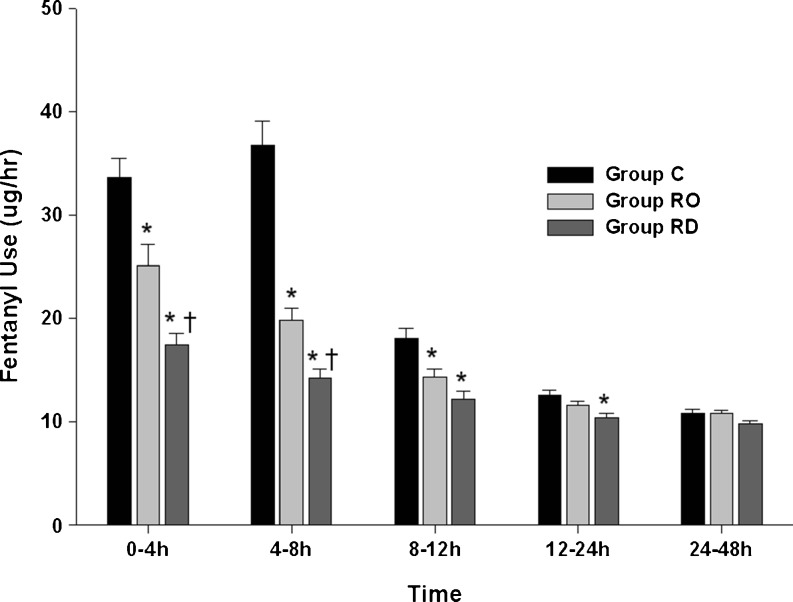
Fentanyl use for analgesia was the highest in group C and the lowest in group RD (Fig. 3) for all time intervals. The requirements decreased gradually, except at 4–8 h in group C, in all groups up to 24–48 h. Fentanyl use was significantly lower in group RD than in group C until 12–24 h (P < 0.05). Group RO received less fentanyl than group C until 8–12 h (P < 0.05), and group RD received less fentanyl than group RO until 4–8 h (P < 0.05).
Fig. 3.
Fentanyl consumption. Values are expressed as mean ± SE. *P < 0.05 compared with group C. †P < 0.05 comparison between group RO and group RD
The total amount of fentanyl injected over 48 h was compared among groups (Table 2). Groups RO and RD required significantly less analgesia than group C during this observation period (P < 0.01). The total fentanyl use was lower in group RD than in group RO (P < 0.05).
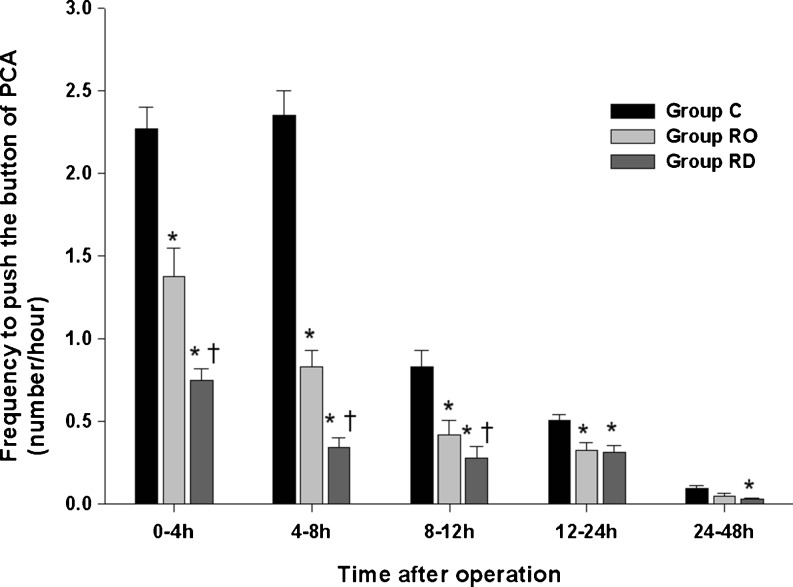
As with fentanyl use, the FPB was highest in group C and lowest in group RD at every time point (Fig. 4). The FPB values were significantly lower in group RO than in group C except over 24–48 h; group RD showed a lower FPB than group C in all measured intervals (P < 0.05). The FPB was significantly lower in group RD than in group RO until 8–12 h (P < 0.05). The overall rates of FPB were recorded (Table 2). Compared with group C, groups RO and RD showed lower FPB values (P < 0.05). Group RD also evidenced a significantly lower FPB than group RO (P < 0.05).
Fig. 4.
Frequency of pushing the button of PCA (FPB). Values are expressed as mean ± SE. *P < 0.05 compared with group C. †P < 0.05 comparison between group RO and group RD
Nausea was less frequent in groups RO and RD than in group C, but not statistically significantly so (Table 2). No significant differences were noted among groups with regard to urinary retention.
Discussion
In this prospective study of patients who underwent hemorrhoidectomy at our hospital, we found that preemptive perianal ropivacaine injection was associated with reduced VAS pain score, reduced need for postoperative fentanyl consumption, and decreased FPB, and the addition of dexmedetomidine to ropivacaine potentiated the analgesic properties of perianal ropivacaine injection.
During the study, none of the patients were excluded from the study due to undesirable surgical outcomes or patient intolerance. To the best of our knowledge, this is the first study to compare the postoperative pain outcomes of perianal injection of ropivacaine and ropivacaine with dexmedetomidine in patients undergoing hemorrhoidectomy.
Hemorrhoidectomy is usually associated with considerable pain during the postoperative period, which may delay discharge, recovery, and return to work. Accordingly, a variety of methods for reducing pain after hemorrhoidectomy have been investigated, including pudendal nerve block, perianal infiltration of local anesthetics or botulinum toxin, and application of topical preparation such as metronidazole, glyceryl nitrate, and calcium channel blocker [4].
The results were diverse, yet many authors support the efficacy of perianal LA in relieving post-hemorrhoidectomy pain [5–7]. These observations corroborate our results.
Interestingly, although the blockage of central sensitization at the spinal cord level has already been accomplished by spinal anesthesia, additional LA injection can enhance the postoperative course of pain.
This may be explained by the following facts:
Despite the suppression of central sensitization by spinal anesthesia, perianal LA injection may prevent peripheral sensitization, thus additive effect of the blockage of central and peripheral sensitization may improve the postoperative pain course.
As LA has anti-inflammatory properties [11], the injection of LA may inhibit the action of inflammatory mediators released into wound tissue, which is believed to be the one of the factors in post-hemorrhoidectomy pain [12].
Although the result of pain control by perianal LA injection is positive in the majority of cases, shortcomings still exist.
Vinson-Bonnet et al. previously reported that perianal infiltration improved immediate postoperative pain control after hemorrhoidectomy, but only for up to 6 h [6]. Hussein et al. reported that perianal injection made patients more comfortable after surgery, but could not alleviate analgesic requirements [13].
Our preemptive application of LA (Hussein et al. injected LA after surgery) and different anesthetic techniques (Binson-Bonnet et al. injected perianal LA under general anesthesia) may have caused differences in the results.
Ropivacaine is a member of the amino amide class of LA agents and is supplied as the pure S-(–)-enantiomer. In this study, ropivacaine was used as the LA because it produces a long-lasting block comparable to that provided by bupivacaine and less cardiotoxicity than bupivacaine [14, 15]. Moreover, infiltration with ropivacaine may induce local vasoconstriction, which may impair vascular absorption [16]. Thus, it may prolong the duration of local anesthetic and guarantee a good margin of safety between effective and toxic doses, as a high vascular absorption is supposed to occur after perineal injection with LA in hemorrhoidectomy.
Dexmedetomidine provides analgesia and sedation without respiratory depression when intravenously administered [17], and synergistic interactions between dexmedetomidine and LA have been observed in previous studies.
Memis et al. reported that the addition of 0.5 μg/kg dexmedetomidine to lidocaine for intravenous regional anesthesia shortened sensory and motor block onset times and prolonged sensory and motor block recovery times without causing side effects [18].
Coskuner et al. previously demonstrated that intravenous administration of dexmedetomidine might prolong the recovery time of the sensory blockade of bupivacaine-induced sensorial blockade during epidural anesthesia [19].
Kaya et al. previously demonstrated that the intravenous administration of dexmedetomidine prolonged spinal bupivacaine sensory blockade and provided additional analgesia [20].
However, there are currently no clinical data regarding the effects of additional local infiltration of dexmedetomidine.
Although this study showed that additional local infiltrations of dexmedetomidine could improve postoperative pain, thus reducing the need for analgesic, the underlying mechanism of this effect remains unclear.
Supraspinal analgesic secondary to absorption to systemic circulation, potentiating the blockade of C-fibers, or augmenting the effects of local anesthetics, vasoconstriction action, and preventing norepinephrine release of dexmedetomidine are suggested to be involved in this mechanism [21, 22]
Our study has some limitations. First, we did not estimate the blood levels of ropivacaine and dexmedetomidine, but our dose did not exceed the dose used in the previous study, which reported no toxic effects, and no systemic toxic effects were recorded in our patients; this may support the safety of the agents we used. Second, we did not find significantly reduced PONV or urinary retention in group RO and group RD. One of the reasons that the data did not decrease significantly may be that this was not the primary criterion. As we determined the optimal group size by calculating the power based on the VAS pain score, the number of patients included may have been too few to reach a level of significance with regard to other categories. Third, none of our patients had severe underlying disease. Therefore, the results of our study should not be generalized to other patients with severe underlying disease.
On the other hand, some advantages of the current study are worth highlighting. We included only elective, closed hemorrhoidectomy in our study to avoid the type, nature, and duration of pain associated with different types of surgery. Moreover, all the surgeries were performed by the same surgeon to minimize the differences in tissue handling. Furthermore, all the observations were carried out by a single observer to eliminate any interobserver variability. Thus, we can assume that the difference in pain relief reflects only the efficacy of the antinociceptive measures.
We conclude by recommending that perianal ropivacaine with dexmedetomidine is not only effective but is also a safe procedure, and can be a better alternative strategy for reducing the pain of patients who are undergoing hemorrhoidectomy.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1003700).
References
- 1.Holzheimer RG. Hemorrhoidectomy: indications and risks. Eur J Med Res. 2004;9:18–36. [PubMed] [Google Scholar]
- 2.Nisar PJ, Scholefield JH. Managing haemorrhoids. BMJ. 2003;327:847–851. doi: 10.1136/bmj.327.7419.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehlet H, Rung GW, Callesen T. Postoperative opioid analgesia: time for a reconsideration? J Clin Anesth. 1998;8:441–445. doi: 10.1016/0952-8180(96)00131-6. [DOI] [PubMed] [Google Scholar]
- 4.Cheetham MJ, Phillips RK. Evidence-based practice in haemorrhoidectomy. Color Dis. 2001;3:126–134. doi: 10.1046/j.1463-1318.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 5.Morisaki H, Masuda J, Fukushima K, Iwao Y, Suzuki K, Matsushima M. Wound infiltration with lidocaine prolongs postoperative analgesia after haemorrhoidectomy with spinal anaesthesia. Can J Anaesth. 1996;43:914–918. doi: 10.1007/BF03011804. [DOI] [PubMed] [Google Scholar]
- 6.Vinson-Bonnet B, Coltat JC, Fingerhut A, Bonnet F. Local infiltration with ropivacaine improves immediate postoperative pain control after hemorrhoidal surgery. Dis Colon Rectum. 2002;45:104–108. doi: 10.1007/s10350-004-6121-4. [DOI] [PubMed] [Google Scholar]
- 7.Brunat G, Pouzeratte Y, Mann C, Didelot JM, Rochon JC, Eledjam J. Posterior perineal block with ropivacaine 0.75 % for pain control during and after hemorrhoidectomy. Reg Anesth Pain Med. 2003;28:228–232. doi: 10.1097/00115550-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–1119. doi: 10.1097/ALN.0b013e3181bbcc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinbroum AA, Ben-Abraham R. Dextromethorphan and dexmedetomidine: new agents for the control of perioperative pain. Eur J Surg. 2001;167:563–569. doi: 10.1080/110241501753171146. [DOI] [PubMed] [Google Scholar]
- 10.Elcicek K, Tekin M, Kati I. The effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaine anesthesia. J Anesth. 2010;24:544–548. doi: 10.1007/s00540-010-0939-9. [DOI] [PubMed] [Google Scholar]
- 11.Hollmann MW, Gross A, Jelacin N. Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology. 2001;95:113–122. doi: 10.1097/00000542-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 13.Hussein MK, Taha AM, Haddad FF. Bupivacaine local injection in anorectal surgery. Int Surg. 1998;83:56–57. [PubMed] [Google Scholar]
- 14.Scott DB, Lee A, Fagan D. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–569. [PubMed] [Google Scholar]
- 15.Santos AC, Arthur GR, Wlody D, De Armas P, Morishima HO, Finster M. Comparative systemic toxicity of ropivacaine and bupivacaine in nonpregnant and pregnant ewes. Anesthesiology. 1995;82:734–740. doi: 10.1097/00000542-199503000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Cederholm I, Evers H, Lofstrom JB. Skin blood flow after intradermal injection of ropivacaine in various concentrations with and without epinephrine evaluated by laser Doppler flowmetry. Reg Anesth. 1992;17:322–328. [PubMed] [Google Scholar]
- 17.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–790. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Memis D, Turan A, Karamanlioglu B, Pamukcu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98:835–840. doi: 10.1213/01.ane.0000100680.77978.66. [DOI] [PubMed] [Google Scholar]
- 19.Coskuner I, Tekin M, Kati I, Yagmur C, Elcicek K. Effects of dexmedetomidine on the duration of anaesthesia and wakefulness in bupivacaine epidural block. Eur J Anaesthesiol. 2007;24:535–540. doi: 10.1017/S0265021506002237. [DOI] [PubMed] [Google Scholar]
- 20.Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, Ozcan B. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth. 2010;57:39–45. doi: 10.1007/s12630-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 21.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Choyce A, Peng P. A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth. 2002;49:32–45. doi: 10.1007/BF03020416. [DOI] [PubMed] [Google Scholar]