Abstract
In Pseudomonas aeruginosa, the small RNA-binding, regulatory protein RsmA is a negative control element in the formation of several extracellular products (e.g., pyocyanin, hydrogen cyanide, PA-IL lectin) as well as in the production of N-acylhomoserine lactone quorum-sensing signal molecules. RsmA was found to control positively the ability to swarm and to produce extracellular rhamnolipids and lipase, i.e., functions contributing to niche colonization by P. aeruginosa. An rsmA null mutant was entirely devoid of swarming but produced detectable amounts of rhamnolipids, suggesting that factors in addition to rhamnolipids influence the swarming ability of P. aeruginosa. A small regulatory RNA, rsmZ, which antagonized the effects of RsmA, was identified in P. aeruginosa. Expression of the rsmZ gene was dependent on both the global regulator GacA and RsmA, increased with cell density, and was subject to negative autoregulation. Overexpression of rsmZ and a null mutation in rsmA resulted in quantitatively similar, negative or positive effects on target genes, in agreement with a model that postulates titration of RsmA protein by RsmZ RNA.
Pseudomonas aeruginosa is a ubiquitous saprophyte and an opportunistic human pathogen which synthesizes numerous extracellular products including elastase, LasA protease, alkaline protease, phospholipase C, lipase, exotoxin A, rhamnolipids, hydrogen cyanide (HCN), and pyocyanin (25, 63). The production of these exoproducts, most of which can act as virulence factors, is positively controlled by two quorum-sensing signal molecules, N-(3-oxododecanoyl)-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-homoserine lactone (C4-HSL), which activate the transcription factors LasR and RhlR, respectively (20, 26, 59). The las and rhl systems are organized in a hierarchical manner such that the las system exerts transcriptional control over both rhlR and rhlI (26). A third signal molecule, 2-heptyl-3-hydroxy-4(1H)-quinolone, the synthesis and activity of which is linked to the las and rhl circuitry, is also required for virulence factor production and, in particular, for rhl-dependent exoproducts including pyocyanin and PA-IL lectin (41).
Motility helps P. aeruginosa to colonize niches (10); three types of motility are observed, i.e., swimming, twitching, and swarming (24, 44). Whereas swimming in liquid media depends on flagella, twitching on solid media requires type IV pili. Swarming on semisolid media results from a combination of both types of motility and also requires rhamnolipid production (24, 46). Rhamnolipids are biosurfactants which not only enhance bacterial surface translocation by virtue of their wetting properties but also stimulate solubilization and degradation of hydrocarbons (35) and act as heat-stable hemolysins (21).
The production of N-acyl-homoserine lactones (AHLs) and the expression of many virulence determinants in P. aeruginosa is negatively controlled at a posttranscriptional level by the small RNA-binding protein RsmA (42, 43). This regulator is a homolog of CsrA in Escherichia coli and Salmonella enterica (27, 47) and of RsmA in Erwinia spp. and Pseudomonas fluorescens CHA0 (3, 7). When RsmA and CsrA exert a negative effect on gene expression, they are assumed to prevent translation initiation by binding at or near the ribosome binding site, and this can favor mRNA decay. A well-documented example for this regulation is provided by CsrA-mediated posttranscriptional repression of glycogen biosynthesis in E. coli, where CsrA binding to the 5′ leader transcript of the glgG gene inhibits translation (2, 28). In a number of cases, CsrA can also exert positive effects on gene expression in E. coli, directly or indirectly (39). For instance, flagellar motility of E. coli is positively regulated by CsrA at the level of the flhDC master operon. The binding of CsrA to a 5′ segment of flhDC mRNA stimulates its translation and enhances its half-life (57). The repressive action of CsrA and RsmA can be relieved by small regulatory RNAs such as CsrB and CsrC in E. coli, RsmB in Erwinia carotovora, or RsmY and RsmZ (PrrB) in P. fluorescens (1, 18, 29, 30, 55, 58). The present model postulates that the regulatory RNAs antagonize the effect of CsrA/RsmA by sequestering multiple copies of these proteins, thereby titrating their activity (17, 29, 47). This kind of posttranscriptional control may facilitate a rapid but potentially reversible regulation of diverse cellular functions.
RsmA/RsmY/RsmZ of P. fluorescens, RsmA/RsmB of E. carotovora, and CsrA/CsrB/CsrC of E. coli and S. enterica are all part of the GacS/GacA signal transduction pathway which operates an important metabolic switch from primary to secondary metabolism in many gram-negative bacteria and can also pleiotropically affect central carbon metabolism and enzyme secretion (3, 17, 19, 27, 39, 52, 58). In P. aeruginosa, the global response regulator GacA positively regulates the quorum-sensing machinery and the expression of several virulence factors via a mechanism involving the participation of RsmA as a negative control element (42, 43, 45). In this study, we demonstrate that RsmA of P. aeruginosa can also be a positive control element, as it is required for swarming motility, rhamnolipid synthesis, and lipase production. Both positive and negative effects of RsmA were found to be antagonized by the small regulatory RNA RsmZ, a homolog of P. fluorescens RsmZ.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Both E. coli and P. aeruginosa strains were routinely grown in nutrient yeast broth (NYB) or on nutrient agar plates at 37°C (45). Where required, antibiotics were added to media at the following concentrations: tetracycline, 25 μg ml−1 (E. coli) or 125 μg ml−1 (P. aeruginosa); gentamicin, 10 μg ml−1; chloramphenicol, 250 μg ml−1; spectinomycin, 1,000 μg ml−1 (P. aeruginosa). To counterselect E. coli S17-1 donor cells in matings with P. aeruginosa, chloramphenicol was used at a concentration of 10 μg ml−1; enrichment for tetracycline-sensitive strains was performed with carbenicillin (2,000 μg ml−1) and tetracycline (20 μg ml−1) (43, 45). Flagellar swimming was examined as described by Rashid and Kornberg (44) on NYB solidified with 0.3% (wt/vol) agar. Swarming was evaluated on plates containing 0.5% (wt/vol) agar, 8 g of nutrient broth (Oxoid) liter−1, and 5 g of glucose liter−1 (44). Twitching motility was assayed on 1% (wt/vol) agar supplemented with Luria broth (24).
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides
| Strain, plasmid, or primer | Genotype, phenotype, or sequence (5′-3′) and description | Reference or origin |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO1 | Wild type | ATCC 15692 |
| PAO6281 | gacA::Ω-Sp/Sm | 45 |
| PAO6327 | gacS::Ω-Sp/Sm | C. Reimmann, unpublished data |
| PAO6343 | ΔrsmA, gacA′::Ω-Sp/Sm | This study |
| PAO6354 | ΔrsmZ | This study |
| PAO6385 | ΔrsmZ, gacA′::Ω-Sp/Sm | This study |
| PAZH13 | ΔrsmA | 43 |
| PT712 | rhlA::Ω-Gm | 24 |
| E. coli | ||
| DH5α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 deoR λ(φ80dlacZΔM15) | 48 |
| S17-1 | pro thi hsdR recA Tpr Smr; chromosome::RP4-2 Tc::Mu-Km::Tn7 | 49 |
| Plasmids | ||
| pBLS-II KS | Cloning vector; ColE1 replicon, Apr | Stratagene |
| pDB18R | pTZ18R, rpoS, Apr | 54 |
| pECP60 | rhlA′-′lacZ translational fusion on pSW205, Apr | 40 |
| pME3087 | Suicide vector, polylinker of pMMB67, ColE1-replicon, Tcr | 56 |
| pME3280b | Mini-Tn7 gene delivery vector based on pUX-BF5 with a HindIII-PstI-MluI-SpeI MCS, ColE1-replicon Gmr Apr | 64 |
| pME3328 | pBLS-II KS, with a 1.43-kb BamHI-XhoI fragment containing ′rpoS rsmZ and ′fdxA, Apr | This study |
| pME3331 | pME6016 derivative, containing the rsmZ promoter fused at +1 to lacZ, Tcr | This study |
| pME3332 | pME3087 carrying a 1.15-kb KpnI-BamHI ′rpoS-′fdxA insert with a 250-bp deletion in rsmZ, Tcr | This study |
| pME3337.1 | pME6000, with a 1.1-kb EcoRI-BamHI fragment of pME3328 containing rsmZ, Tcr | This study |
| pME3337.3 | pME6001, with a 1.1-kb EcoRI-BamHI fragment of pME3328 containing rsmZ, Gmr | This study |
| pME3838 | pME6016 derivative, containing the rhlA promoter fused 2 bp after the +1 site to lacZ, Tcr | This study |
| pME3839 | pME6032 carrying the rhlAB genes under Ptac control, Tcr | This study |
| pME3849 | pME6001 with rsmA, Gmr | 43 |
| pME6000 | Cloning vector derived from pBRR1MCS, Tcr | 31 |
| pME6001 | Cloning vector derived from pME6000, Gmr | 16 |
| pME6016 | Cloning vector for transcriptional lacZ fusions, pVS1-p15A replicon, Tcr | 18 |
| pME6032 | Cloning vector for overexpression under the IPTG-inducible tac promoter, Tcr | 16 |
| pME6111 | pME3088 suicide vector carrying a 4.8-kb EcoRI-KpnI fragment with gacA::ΩSm/Sp, Tcr, Cmr | 45 |
| pME6313 | Mini-Tn7 gene delivery vector based on pUX-BF5 with a HindIII-PstI-SmaI-SpeI MCS, Gmr Apr | H. Winteler and D. Haas, unpublished data |
| pSB536 | AHL biosensor, ahyR"-luxCDABE in pAHP13, Apr | 53 |
| pSB1075 | AHL reporter plasmid, P. aeruginosa lasRI fused with luxCDABE from Photorhabdus luminescens, Apr | 60 |
| Primers | ||
| DS5-EcoRI | AAAAGAATTCCAATACCACCAACC, with an underlined EcoRI restriction site, located in rhlA promoter | |
| DS6-PstI | AAAACTGCAGATGAACACTTTTTAGCC, with an underlined PstI restriction site, annealing to the +1 site (bold) region of rhlA | |
| RhlAB-KH3 | AAAAGAATTCATGCGGCGCGAAAGTC, with an underlined EcoRI restriction site, located in the start codon (bold) region of rhlA | |
| RhlAB-KH4 | CCCTGATCGATAAAATGC, with an underlined ClaI restriction site, located 108 bp after the stop codon of rhlB | |
| PRSMPAO1 | CCCTGTACGCTGCAGTGATATTAGCGATTCCC, with an underlined PstI restriction site, located in rsmZ promoter (Fig. 3) | |
| PRSMPAO2 | AAACGCTCGGTGAATTCAAGTAACTTATTG, located in rsmZ promoter region (Fig. 3) | |
| PRSMPAO4 | GATGAATTCTCGAGGATCAATC, with an underlined XhoI restriction site, located upstream of fdxA | |
| PRSMPAO7 | GGCCTCTCGAGTGACGCGCTGTTCC, with an underlined XhoI restriction site, located at the 3′ end of rpoS (Fig. 3) | |
| PRSMPAO8 | AATAAGCTTAATGCTTACAAGAGCAGACAC, with an underlined HindIII restriction site, located in the upstream activation sequence of rsmZ (Fig. 3) | |
| PRSMPAO9 | AATAAGCTTAGGGACTGAAGAGTGGGCGG, with an underlined HindIII restriction site, located 75 bp after rsmZ start (Fig. 3) | |
| PRSMZ1 | CGTACAGGGAACACGCAACC, corresponding to the +1 and the first 20 bases of rsmZ gene | |
| PRSMZ2 | AAAAAAAGGGGCGGGGTATT, located in the terminator of rsmZ gene |
DNA manipulation and cloning procedures.
Small-scale preparations of plasmid DNA were carried out by the cetyltrimethylammonium bromide method (8), and large-scale preparations were performed by using JetStar-Tips (Genomed, Basel, Switzerland). Chromosomal DNA was extracted from P. aeruginosa and purified as described elsewhere (14). Restriction enzyme digestions, ligations, and agarose gel electrophoresis were performed by standard methods (48). Restriction fragments were purified from agarose gels with the Gene Clean II kit (Bio 101). Transformation of E. coli and P. aeruginosa strains was carried out by electroporation (12). Cloned PCR products were sequenced with the Big Dye terminator cycle sequencing kit and an ABI-PRISM 373 automatic sequencer (Applied Biosystems). Alignment of nucleotide and deduced amino acid sequences was performed by using the Genetics Computer Group program GAP. Oligonucleotides used in this study are listed in Table 1.
Plasmid constructions.
For the construction of plasmid pME3838, a 369-bp fragment containing the promoter of rhlA was amplified by PCR from chromosomal DNA of P. aeruginosa with primer DS5-EcoRI, carrying an artificial restriction site for EcoRI, and primer DS6-PstI, annealing to the +1 region of the rhlA gene (32) and carrying an artificial restriction site for PstI. The PCR product digested with EcoRI and PstI was inserted into pME6016 carrying the lacZ gene with its own ribosome binding site. The resulting rhlA-lacZ transcriptional fusion contains 353 bp of the rhlA promoter region and lacks the rhlA translational control region, which is present in the translational rhlA′-′lacZ fusion on plasmid pECP60. To overexpress the rhamnolipids, we constructed plasmid pME3839 as follows: a 2.6-kb fragment containing the rhlA and rhlB genes was amplified by PCR from P. aeruginosa chromosomal DNA with primer RhlAB-KH3, annealing to the 5′ region of the rhlA gene and carrying an artificial restriction site for EcoRI, and primer RhlAB-KH4, carrying the natural restriction site for ClaI located 108 bp after the stop codon of rhlB. The PCR product was digested with EcoRI and ClaI and inserted into pME6032 cut with the same enzymes. The resulting plasmid carries the rhlAB genes under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible (1 mM) tac promoter, such that the ATG start codon of rhlA was located 41 bp downstream of the transcription start. This region contains the ribosome binding site of lacZ, which is not controlled by RsmA (3). Thus, pME3839 allows RsmA-independent overexpression of rhlAB.
Cloning, overexpression, and mutation of the P. aeruginosa rsmZ gene.
A 0.70-kb fragment containing rsmZ was amplified by PCR from P. aeruginosa chromosomal DNA with primers PRSMPAO2 and PRSMPAO4 (Table 1), digested with HindIII and XhoI, and inserted into pBluescript KS cut with the same enzymes. A 0.73-kb BamHI-HindIII fragment from pDB18R, carrying the upstream rpoS gene and part of the rsmZ promoter, was inserted into this plasmid, resulting in pME3328 (see Fig. 3). To overexpress the rsmZ gene, a 1.1-kb EcoRI-BamHI fragment from pME3328 carrying rsmZ with its promoter was subcloned into two multicopy plasmids, pME6000 and pME6001, cut with the same restriction enzymes, resulting in pME3337.1 and pME3337.3, respectively. To obtain an rsmZ deletion, an inverse PCR with the primers PRSMPAO8 and PRSMPAO9 (Table 1) was carried out with pME3328 as the template. The amplified fragment was digested with HindIII and recircularized. This produced a 250-bp deletion of rsmZ. The resulting 1.15-kb KpnI-BamHI ′rpoS ΔrsmZ ′fdxA fragment was inserted into the suicide plasmid pME3087, producing pME3332. Biparental conjugation with PAO1 cultivated overnight at 43°C as the recipient and E. coli S17-1/pME3332 as the donor allowed selection of chromosomal integration of the plasmid on nutrient agar containing chloramphenicol and tetracycline. Plasmid excision by a second crossover event was obtained after enrichment with carbenicillin. The rsmZ deletion in the recombinant strain was verified by PCR with the primers PRSMPAO4 and PRSMPAO7 (Table 1). For the construction of a transcriptional rsmZ-lacZ fusion, a 289-bp fragment containing the rsmZ promoter fragment was amplified from pME3328 by PCR with the primers PRSMPAO7 and PRSMPAO1 (Table 1), digested with XhoI and PstI, and ligated to a 1-kb PstI-ClaI fragment containing the 5′ end of lacZ from pME6522. The resulting 1.3-kb BamHI-ClaI fragment carrying rsmZ-lacZ′ was inserted into pME6016 cut with the same enzymes, resulting in pME3331 (Table 1). A chromosomal gacA::ΩSm/Sp mutation was introduced into PAZH13 and PAO6354 as previously described (45), with the ColE1-based suicide vector pME6111, resulting in PAO6343 and PAO6385, respectively.
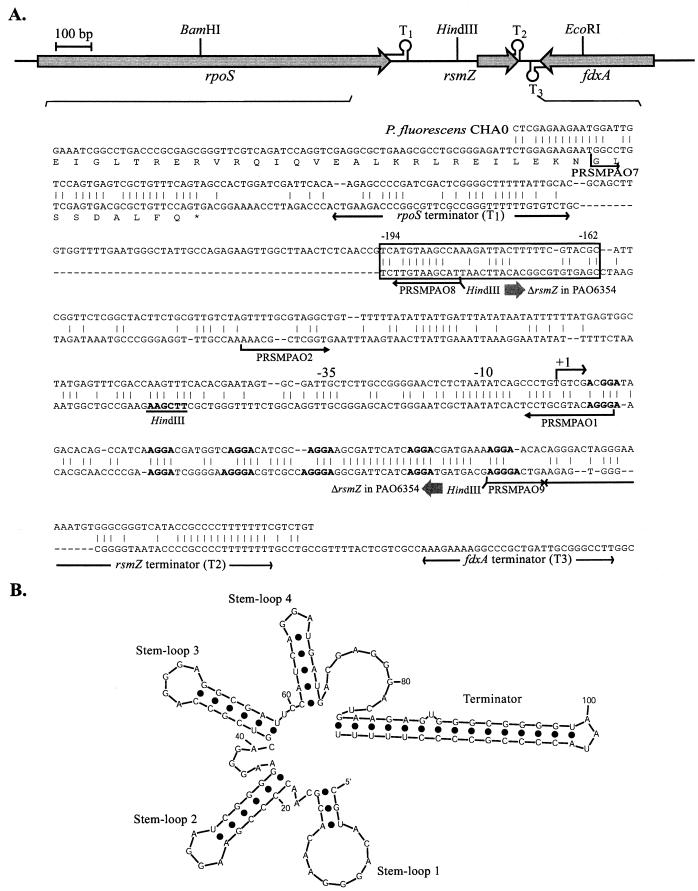
FIG. 3.
(A) The 1.8-kb region of P. aeruginosa PAO1 with rpoS, rsmZ, and fdxA (50). Nucleotide sequence identities with the rsmZ region of P. fluorescens CHA0 (18) are represented. The transcription start site (+1) and the −35/−10 promoter sequences are deduced from the similar prrB (rsmZ) gene of P. fluorescens F113 (1). T1 and T2 are rho-independent terminators. A well-conserved upstream activation sequence pointed out by Heeb et al. (18) located around position −180 is boxed. The rsmZ deletion of strain PAO6354 extends from −177 to +70 and was created between the two artificial HindIII restriction sites in primers PRSMPAO8 and PRSMPAO9 indicated below the sequence. (B) Predicted secondary structure of RsmZ at 37°C obtained by using the M-fold program (65).
Semiquantitative determination of rhamnolipid concentrations by the orcinol method.
Strains were grown at 37°C in 50-ml Erlenmeyer flasks containing 10 ml of M9 medium supplemented with glycerol (2% vol/vol), glutamate (0.05%), and Triton X-100 (0.05%) with shaking for 18 h. Rhamnolipids were extracted with three volumes of diethyl ether from culture supernatants filtered through a 0.22-μm-pore-size membrane. Rhamnolipid contents were quantified by the orcinol method as described by Pearson et al. (38), with an extract from the rhamnolipid-negative rhlA mutant PT712 as a blank.
Northern blot analysis.
RNA was prepared with the High Pure RNA isolation kit (Roche) according to the manufacturer's recommendations. RNA (4 μg) was electrophoretically separated on a denaturing urea-polyacrylamide gel in 1× Tris-borate-EDTA buffer and electrophoretically transferred to a Hybond-N membrane. Prehybridization, hybridization, and detection were performed as previously described (18). A 120-bp DNA probe for rsmZ RNA was produced by PCR (95°C for 5 min; 30 cycles of 95°C for 1 min, 48°C for 40 s, 72°C for 30 s; and 72°C for 5 min) where standard deoxynucleoside triphosphates were replaced with digoxigenin-labeled deoxynucleoside triphosphates (Roche), with primers PRSMZ1 and PRSMZ2 (Table 1) which amplify rsmZ from the +1 transcription start to the terminator. The rsmZ probe was purified by using a QIAquick purification kit (Qiagen).
β-Galactosidase assay.
P. aeruginosa reporter strains were routinely cultivated, in triplicate, in 50-ml Erlenmeyer flasks containing 20 ml of NYB supplemented with 0.05% (vol/vol) Triton X-100, with shaking at 37°C. β-Galactosidase specific activities were determined by the Miller method (33).
Lipase assay.
Lipase was quantified in P. aeruginosa cultures grown in triplicate in 50-ml Erlenmeyer flasks containing 20 ml of YEA medium (61) for 18 h. Culture samples were centrifuged, and 10 to 100 μl of the supernatant was assayed for soluble lipase activity with p-nitrophenol palmitate as the substrate (51). Cell-associated lipase was extracted and assayed as previously described (61). Lipase activities are the totals of soluble and cell-associated enzyme. Specific lipase activities are expressed as nanograms of enzyme per 109 cells. The following conversions were used: 1.0 A410 unit (absorption of p-nitrophenol) equals 0.212 ng of pure lipase, and 1.0 OD600 unit (optical density of cell cultures at 600 nm) corresponds to 109 cells ml−1.
Assays for lytic activities.
Total proteolytic activity was assayed as casein hydrolyzing activity (4). Elastolytic activity (LasB) of bacterial supernatants was determined with the elastin Congo red (Sigma) assay (37) as previously described (9). Staphylolytic protease activity (LasA) was assayed as previously described by Kessler et al. (23) by determining the ability of P. aeruginosa supernatants from NYB cultures, grown at 37°C with shaking to an OD600 of 2.5, to lyse boiled Staphylococcus aureus cells.
Lectin detection.
PA-IL lectin was detected by Western blotting with polyclonal antibodies raised against the purified P. aeruginosa lectin (62) in cells grown in NYB at 37°C with shaking, as previously described (43).
Assays for pyocyanin and HCN.
Pyocyanin was extracted with chloroform from culture supernatants of strains grown in 50-ml Erlenmeyer flasks containing 20 ml of glycerol-alanine medium (13) with shaking at 37°C for 20 h. Pyocyanin was quantified spectrophotometrically at 520 nm (11). HCN was quantified (15) in culture supernatants of strains grown in tightly closed 125-ml bottles containing 60 ml glycine minimal medium (5); samples were taken after 10 h of incubation (at ∼109 cells ml−1).
AHL detection and analysis.
Samples (900 μl) were taken from culture supernatants of P. aeruginosa strains grown in NYB at 37°C with shaking to OD600s of 0.6 and 2.5, filter sterilized, and treated for AHL quantification as described by Diggle et al. (9). Detection and quantification of 3-oxo-C12-HSL or C4-HSL were done after separation by normal- or reverse-phase thin-layer chromatography (silica gel 60 F254, or RP-18 F245; Merck), respectively. E. coli AHL biosensor strains with reporter plasmid pSB1075 (for 3-oxo-C12-HSL detection) or pSB536 (for C4-HSL detection) were used, and bioluminescence was quantified with a Luminograph LB 980 photon video camera (EG & G Berthold) (9). AHL concentrations were estimated by comparison with standards, i.e., 0.13, 0.25, 0.50, or 1.00 μM for 3-oxo-C12-HSL and 1.56, 3.13, 6.25, or 12.50 μM for C4-HSL.
RESULTS
RsmA can act as a positive control element.
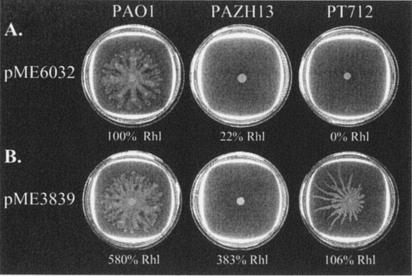
When compared with the wild type P. aeruginosa PAO1, the rsmA mutant PAZH13 was unable to swarm (Fig. 1A; Table 2). Swarming of P. aeruginosa requires flagella, pili, and rhamnolipids (24). The absence of rhamnolipids in strain PT712 (rhlA) sufficed to abolish swarming (Fig. 1A); the rhlA gene is proximal in the rhlAB rhamnolipid biosynthetic operon (36). The fact that the rsmA mutation in strain PAZH13 had no marked effect on swimming and twitching motility (data not shown), but resulted in a fivefold reduction of rhamnolipid synthesis compared to the wild type (Fig. 1A), is consistent with the concept that rhamnolipids sustain swarming motility. However, when pME3839, a rhlAB+ plasmid allowing RsmA-independent rhamnolipid production (see Materials and Methods), was introduced into strains PT712 and PAZH13, swarming ability was restored to the rhlA mutant but not to the rsmA mutant (Fig. 1B). This finding suggests that lack of swarming of the rsmA mutant is not solely a consequence of reduced rhamnolipid synthesis but may also be caused by cellular clumping.
FIG. 1.
Influence of an rsmA mutation on swarming ability in the absence or presence of rhamnolipids. Inocula of 2 μl from overnight cultures of PAO1 (wild type), PAZH13 (ΔrsmA), or PT712 (rhlA′::Ω-Gm) with the control vector pME6032 (A) or plasmid pME3839 carrying the rhlAB genes under the control of the inducible tac promoter (B) were spotted onto 0.5% agar supplemented with 1 mM IPTG and incubated at 37°C overnight. The amounts of rhamnolipids (Rhl) produced by each strain were assayed in a separate experiment as described in Materials and Methods and are indicated below each swarming plate. The value obtained for PAO1/pME6032 (1.21 ± 0.04 μg/ml) was set at 100%.
TABLE 2.
RsmA- and RsmZ-dependent production of lipase, pyocyanin, and HCN and swarming ability in P. aeruginosa strains
| Strain (genotype) | Swarminga | Lipaseb (ng/109 cells) | Pyocyaninc (μg/109 cells) | HCNd (μM) |
|---|---|---|---|---|
| PAO1 (wild type) | + | 1.48 ± 0.52 | 1.24 ± 0.21 | 6.25 ± 0.42 |
| PAZH13 (rsmA) | − | 0.77 ± 0.19 | 4.89 ± 0.13 | 32.50 ± 1.88 |
| PAO6354 (rsmZ) | + | 1.69 ± 0.39 | 0.92 ± 0.26 | 5.57 ± 0.92 |
| PAO1/pME6000 (control for pME3337.1) | + | 1.18 ± 0.39 | 0.76 ± 0.08 | 5.28 ± 1.32 |
| PAO1/pME3337.1 (rsmZ++) | − | 0.21 ± 0.02 | 5.92 ± 0.98 | 16.53 ± 0.98 |
Swarming ability was tested on semisolid medium incubated at 37°C, as described in Materials and Methods. +, ability to swarm and completely invade the plate within 24 h; −, no swarming.
Concentrations of lipase were measured for strains grown in YEA medium to about 4 × 109 cells ml−1 (see Materials and Methods).
Pyocyanin was extracted and quantitated for P. aeruginosa strains grown in glycerol-alanine medium (see Materials and Methods).
Concentrations of HCN were determined for strains grown under semianaerobic conditions in MMC medium to about 1.1 × 109 cells ml−1, as described in Materials and Methods.
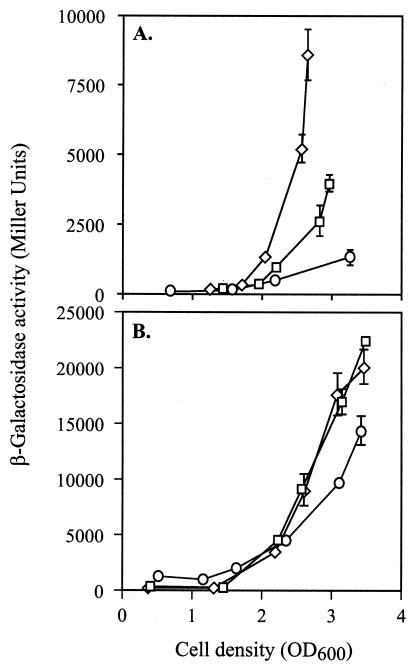
The expression of a translational rhlA′-′lacZ fusion on pECP60 was reduced two- to threefold in the rsmA mutant PAZH13 compared to the wild type, PAO1 (Fig. 2A), confirming the observed reduction of rhamnolipid production in the mutant. By contrast, a transcriptional rhlA-lacZ fusion on pME3838 was expressed almost equally well in the wild type and in the rsmA mutant (Fig. 2B). This result indicates that the positive effect of RsmA on rhlA expression occurs essentially at a posttranscriptional level.
FIG. 2.
Influence of rsmA and rsmZ mutations on rhlA expression. β-Galactosidase expression from a translational rhlA′-′lacZ fusion on pECP60 (A) and a transcriptional fusion on pME3838 (B) was determined in PAO1 (□), PAO6354 (rsmZ) (⋄), and PAZH13 (rsmA) (○). Each result is the mean ± standard deviation of the results from three measurements. Bacterial growth in NYB medium reached a plateau at an OD600 of about 3.
The production of extracellular lipase was also shown to be positively controlled by RsmA in P. aeruginosa, as lipase specific activity in a culture supernatant was reduced about twofold in the rsmA mutant PAZH13 compared to the wild type (Table 2). This parallel positive regulation of rhamnolipids and lipase is striking and will be considered in Discussion.
Cloning and mutational inactivation of the rsmZ gene of P. aeruginosa PAO1.
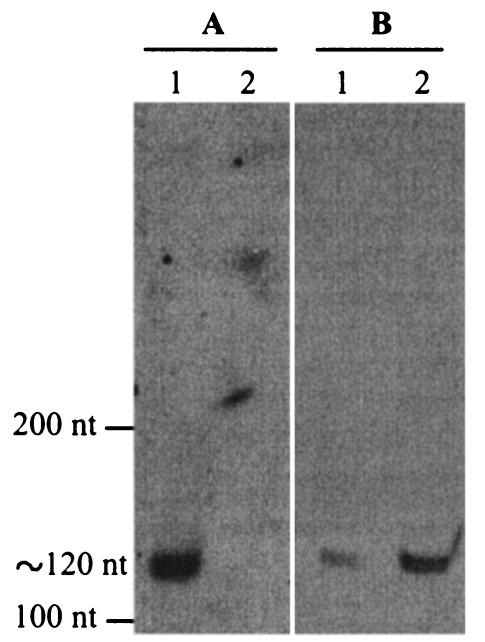
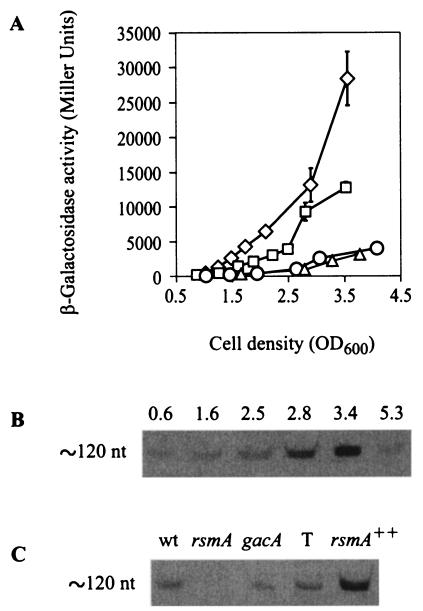
Since in other gram-negative bacteria the effects of RsmA are antagonized by small noncoding RNAs such as RsmY and RsmZ of P. fluorescens CHA0 (18, 55), we carried out a nucleotide sequence alignment of the conserved rpoS-rsmZ-fdxA region of different Pseudomonas species, i.e., P. putida Corvallis and WCS358, P. syringae pv. syringae B728a, P. fluorescens F113 and CHA0, and P. aeruginosa PAO (data not shown). Although within the rsmZ gene of these organisms, nucleotide sequence conservation was only about 45%, a repeated GGA motif, which is also characteristic of CsrB, CsrC, RsmB, and RsmY, clearly stands out, as illustrated in RsmZ of P. fluorescens CHA0 and P. aeruginosa PAO1 (Fig. 3A). Moreover, despite primary sequence differences, the predicted secondary structures of RsmZ RNAs from both bacterial strains are highly conserved. In particular, both RsmZ RNAs show four stem-loop structures with GGA motifs in the loops and an additional hairpin structure formed by the 3′ region (Fig. 3B) (18). Using sequence information from the complete genome of P. aeruginosa PAO, we PCR amplified and cloned the rsmZ region including its promoter and its terminator, and a chromosomal 250-bp deletion in rsmZ was constructed in strain PAO6354 (as described in Materials and Methods). RsmZ RNA was revealed as a single band corresponding to ca. 120 nucleotides in the wild type, PAO1, and the absence of this RNA from strain PAO6354 was confirmed in the same Northern blot experiment (Fig. 4A).
FIG. 4.
Northern blot revealing RsmZ RNA. Total RNA (4 μg) from P. aeruginosa PAO1 and PAO6354 was electrophoresed in a urea-8% acrylamide-0.4% bisacrylamide gel and hybridized with an rsmZ digoxigenin-labeled probe (see Materials and Methods). (A) Lane 1, PAO1; lane 2, PAO6354 (ΔrsmZ). (B) Lane 1, PAO1/pME6000 vector control; lane 2, PAO1/pME3337.1 (rsmZ++). nt, nucleotides.
RsmZ as an antagonist of RsmA.
We studied the phenotypic effects of deletion and overexpression of rsmZ and compared them with those observed for deletion of rsmA, to verify the prediction that RsmZ RNA antagonizes RsmA activity in P. aeruginosa. The rsmZ deletion mutant PAO6354 grew as well as the wild type, PAO1, and did not exhibit any morphological changes in colony phenotype after growth on nutrient agar. Production of RsmA protein, as determined by Western blotting, was similar in the rsmZ mutant and in the wild type (data not shown). For overexpression, rsmZ was cloned with its promoter into the multicopy vector pME6000, giving pME3337.1. Overproduction of RsmZ RNA was confirmed by Northern blotting (Fig. 4B). We examined the role of RsmZ in the regulation of rhamnolipid and lipase production, which in this study, have been shown to be positively controlled by RsmA. We also determined the importance of RsmZ for extracellular enzymes and AHLs whose production is known to be negatively regulated by RsmA in P. aeruginosa (43). These experiments gave the following results.
Overexpression of rsmZ in PAO1/pME3337.1 resulted in loss of swarming ability, comparable with that of an rsmA mutant, whereas an rsmZ deletion had no visible effect on swarming (Table 2). The expression of a translational rhlA′-′lacZ fusion in strain PAO1 was enhanced about twofold in the rsmZ mutant PAO6354, at an OD600 of ≥2.5, compared to the wild type, PAO1 (Fig. 2A).
Whereas the rsmZ mutant PAO6354 was not different from the wild type for lipase production (Table 2), the rsmZ-overexpressing strain PAO1/pME3337.1 produced about six-times-less lipase than did the control PAO1/pME6000 (Table 2). In this case, overproduction of RsmZ RNA resulted in an effect that was even greater than that of an rsmA mutation (Table 2); the reasons for this difference are not yet evident.
The rsmZ-overexpressing strain PAO1/pME3337.1, in common with the rsmA mutant PAZH13, produced more pyocyanin than did the wild type, PAO1 (Table 2). In contrast, the rsmZ mutation in strain PAO6354 did not exert any significant effect on pyocyanin levels (Table 2).
Cyanogenesis was compared in the same strains. Both the rsmA mutant and the rsmZ-overexpressing strain produced more HCN than did the PAO1 control or the rsmZ mutant (Table 2).
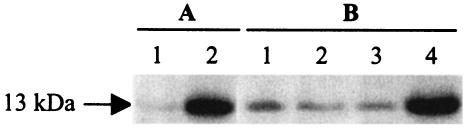
The cytotoxic internal lectin PA-IL (LecA) followed the same expression pattern as that observed for pyocyanin and HCN. Mutation of rsmA or overexpression of rsmZ resulted in strongly enhanced production compared to the wild type and the rsmZ mutant (Fig. 5).
FIG. 5.
Influence of an rsmZ mutation on lectin production. Cells were grown in NYB to an OD600 of 2.5, and lectin was visualized by Western blotting as previously described (43). (A) Lane 1, PAO1 (wild type); lane 2, PAZH13 (ΔrsmA). (B) Lane 1, PAO1 (wild type); lane 2, PAO6354 (ΔrsmZ); lane 3, PAO1/pME6000 (control for pME3337.1); lane 4, PAO1/pME3337.1 (rsmZ++).
Thus, loss of RsmA and overexpression of RsmZ had similar effects on the formation of these exoproducts, and this RsmA-RsmZ antagonism was observed for both positive and negative control exerted by RsmA. In only one case (rhlA expression) did the rsmZ mutation result in a measurable effect; in the other examples studied, mutational loss of rsmZ did not have significant consequences, suggesting a possible redundancy of genes encoding small regulatory RNAs able to interact with RsmA.
With respect to AHL production and to expression of the quorum-sensing genes lasR, lasI, rhlR, and rhlI, the consequences of rsmZ overexpression were slight (induction factors of ≤2) (data not shown). Furthermore, rsmZ overexpression had only minor effects on total extracellular protease, elastase (LasB), and staphylolytic enzyme (LasA). All of these phenotypes revealed no apparent change in an rsmZ null mutant (data not shown).
Regulation of rsmZ expression.
We constructed a transcriptional fusion of the lacZ reporter gene to the rsmZ promoter on pME3331, in which the +1 nucleotide of lacZ corresponds to the transcription start site of the rsmZ promoter (Fig. 3A), as described in Materials and Methods. Expression of this rsmZ-lacZ fusion in the wild type, PAO1, was cell density dependent, with optimal transcription of the rsmZ gene at the end of exponential growth phase (Fig. 6A). This result was corroborated by a Northern blot showing increasing RsmZ RNA concentrations in parallel with increasing cell densities. Interestingly, in the stationary phase (after 24 h of growth), most of the RsmZ RNA was degraded (Fig. 6B). When either gacA or rsmA was inactivated in the mutants PAO6281 and PAZH13, respectively, expression of the rsmZ-lacZ fusion on pME3331 was abolished, indicating that both GacA and RsmA have positive effects on the rsmZ promoter, directly or indirectly (Fig. 6A). Again, a Northern blot confirmed the positive regulatory roles of GacA and RsmA. Overexpression of RsmA resulted in overexpression of RsmZ (Fig. 6C). This rsmZ expression pattern prompted us to examine the possibility that rsmZ might be controlled via AHL-dependent quorum sensing; however, addition of 5 μM 3-oxo-C12-HSL or 10 μM C4-HSL had no effect on the expression of rsmZ-lacZ in strain PAO1 (data not shown).
FIG. 6.
Influence of different mutations on rsmZ expression. (A) β-Galactosidase expression from the transcriptional fusion rsmZ-lacZ on pME3331 was determined in the wild type, PAO1 (□), the rsmZ mutant PAO6354 (⋄), the rsmA mutant PAZH13(○), and the gacA mutant PAO6281 (▵). Each result is the mean ± standard deviation of the results from three measurements. Bacterial growth reached a plateau at an OD600 of 2 to 3. (B) Northern blot showing RsmZ RNA. Total RNA (4 μg) from P. aeruginosa PAO1 was prepared from cultures grown to different densities, as indicated by OD600 values. (C) Northern blot showing RsmZ RNA in different genetic contexts. T, pME6001 vector control; nt, nucleotides; wt, wild type.
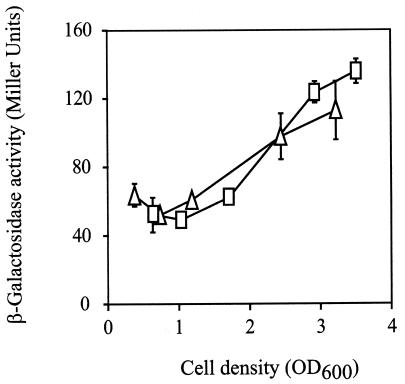
Deletion of rsmZ in the mutant PAO6354 resulted in a threefold-enhanced expression of rsmZ-lacZ (Fig. 6A), suggesting that rsmZ can negatively regulate its own expression. The double mutants PAO6343 (gacA rsmA) and PAO6385 (gacA rsmZ) both showed the same low levels of rsmZ expression, as did the single gacA mutant PAO6281 (data not shown). We considered the possibility that RsmA might exert its positive effect on rsmZ expression via GacA, similar to the situation described for E. coli where the RsmA homolog CsrA appears to regulate the GacA homolog UvrY (58). However, a chromosomal translational gacA′-′lacZ fusion showed no significant difference in its expression when inserted into either the wild type, PAO1, or the rsmA mutant PAZH13 (Fig. 7). Moreover, the gacA′-′lacZ fusion was expressed normally in the gacA mutant PAO6281 and the gacS mutant PAO6327 (data not shown). Together, these data indicate that RsmZ and RsmA have opposite effects on the expression of rsmZ and that these effects are not mediated by the GacS/GacA two-component system.
FIG. 7.
Influence of an rsmA mutation on gacA expression. β-Galactosidase activities from a translational gacA′-′lacZ fusion inserted at the Tn7 attachment site of the chromosome were determined in the wild type, PAO1 (□), and the rsmA mutant PAZH13 (▵). Each result is the mean ± standard deviation of the results from three measurements. Bacterial growth reached a plateau at an OD600 of about 3.
DISCUSSION
In P. aeruginosa, the small RNA-binding protein RsmA has previously been shown to control negatively the expression of several genes involved in the production of extracellular virulence factors, e.g., HCN, pyocyanin, lectin (LecA), elastase (LasB), and staphylolytic enzyme (LasA) (43). Moreover, RsmA modulates negatively the synthesis of 3-oxo-C12-HSL and C4-HSL, by exerting some kind of transient repression on the lasI and rhlI genes (43). Here we report positive effects of RsmA on swarming and on lipase and rhamnolipid production in P. aeruginosa (Fig. 2; Table 2). The positive effect on swarming may involve the production of rhamnolipids (Fig. 2), i.e., surfactants which lower the surface tension and facilitate the spreading of bacteria on semisolid surfaces. However, the inability of rhamnolipid production in strain PAZH13 (rsmA) carrying pME3839 (rhlA+B+) to restore swarming suggests that an rsmA mutation may cause cell-cell aggregation that rhamnolipids cannot dissolve. Whether RsmA regulates the formation of flagella and pili, which are also required for swarming (24), is uncertain. However, judging from the swimming and twitching abilities of an rsmA mutant, we believe that a major involvement of RsmA in the control of these processes is unlikely. By contrast, the RsmA homolog CsrA positively regulates the synthesis of flagella and swimming in E. coli (57), whereas in Erwinia spp., RsmA negatively affects motility (34).
Negative control exerted by RsmA on pyocyanin, HCN, and lectin formation in P. aeruginosa (43) was confirmed in the present study (Table 2; Fig. 5). The positive and negative effects of RsmA were revealed to be antagonized by overexpression of a small noncoding RNA termed RsmZ. This regulatory RNA is similar to RsmZ (alternatively designated PrrB) of P. fluorescens strains (1, 18). Although nucleotide sequence identities between RsmZ of P. aeruginosa PAO1 and RsmZ of P. fluorescens CHA0 are only 58%, the conserved neighborhood of the rsmZ genes and the conserved secondary structures of the RNAs encoded in both organisms (Fig. 3) clearly indicate homology and suggest an analogous mode of action, i.e., sequestration of the RNA-binding protein RsmA. This general model is supported by data for CsrA/CsrB/CsrC in E. coli, RsmA/RsmB in Erwinia spp., and RsmA/RsmY/RsmZ in P. fluorescens (3, 6, 18, 47, 55, 58). Moreover, akin to the situation in E. coli, E. carotovora, and P. fluorescens (18, 19, 52), the GacS/GacA two-component system is strictly required for expression of rsmZ in P. aeruginosa (Fig. 6A and C).
Expression of the rsmZ gene strongly depends on RsmA and is derepressed in an rsmZ mutant of P. aeruginosa (Fig. 6A). As these effects were observed with a construct consisting of only the rsmZ promoter fused to the lacZ reporter, a possible effect of RsmA on RsmZ RNA stability cannot explain the observed regulation. Given the strict GacA dependence of the promoter (Fig. 6A and C), we considered the possibility that RsmA might have a positive feedback regulation effect on the GacS/GacA system. However, using a gacA′-′lacZ fusion, we could not detect any significant stimulation of gacA expression by RsmA (Fig. 7). It could be hypothesized that the rsmZ promoter might additionally be under negative control mediated by one or several regulatory proteins, which in turn would be repressed by RsmA. The model (Fig. 8) which we derive from this study and previous work (42, 43, 45) is a variant of similar models that have been proposed for the BarA/UvrY (= GacS/GacA)-CsrA/CsrB and the GacS/GacA-RsmA/RsmB regulatory circuitries of E. coli and Erwinia spp., respectively (6, 52).
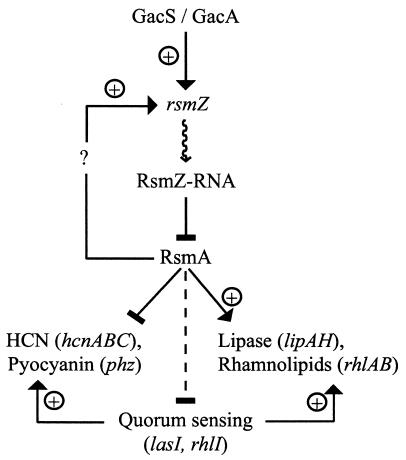
FIG. 8.
Model of the GacA/RsmA signal transduction pathway in P. aeruginosa PAO1. Expression of the untranslated regulatory RNA RsmZ depends on the presence of GacA. The function of RsmZ is to antagonize the action of the small RNA-binding protein RsmA. RsmA positively controls rsmZ expression, thus forming a negative autoregulatory circuit whose mechanism is not understood at present. RsmA also negatively controls AHL-dependent quorum sensing as well as a number of quorum-sensing-dependent genes, some of which code for secondary metabolites and virulence determinants; these are regulated indirectly at the transcriptional level via quorum sensing but probably also directly at the translational level, as is the case for hcnA (42). Lipase and rhamnolipid production are controlled positively by RsmA, independently of the quorum-sensing control. Dotted line, modulating negative effect; solid bar, negative effect; arrow, positive effect.
The role of the quorum-sensing machinery in the GacS/GacA-RsmZ/RsmA regulatory network of P. aeruginosa deserves a closer look. Production of C4-HSL and, to a lesser extent, of 3-oxo-C12-HSL is controlled positively by GacA (45) and negatively by RsmA (43). Extracellular products whose synthesis is positively regulated by AHLs may therefore be expected to be under positive control by GacA and under negative control by RsmA. Such is indeed observed in a number of examples, e.g., in the case of the hcnABC genes encoding HCN synthase (42). However, the GacA-RsmA 3-oxo-C12-HSL/C4-HSL regulatory pathway contributes <30% of the observed cell density-dependent regulation of HCN synthesis (42). A second regulatory pathway involving direct RsmA-mediated posttranscriptional repression of the hcnA 5′ leader mRNA has a more pronounced effect and accounts for >70% of the positive effect of GacA (42). This dual GacA control may also apply to the regulation of rhamnolipid and lipase formation, where an RsmA-stimulated direct pathway appears to have a stronger overall impact than the RsmA-repressed quorum-sensing branch (Fig. 8).
The mechanism by which RsmA brings about a positive effect on rhamnolipid and lipase synthesis remains to be elucidated. By analogy with positive CsrA control of flagellar mobility in E. coli, which results from a stabilization of the flhDC mRNA by CsrA binding (57), we propose that RsmA might stabilize the rhlA and lipA mRNAs and/or facilitate their translation initiation. However, an alternative possibility is that RsmA might act indirectly. Previously, the product of the dksA gene has been found to exert positive posttranscriptional control on the expression of rhlI, rhlAB, and lasB in P. aeruginosa (22).
The parallel positive impact of RsmA on rhamnolipid and lipase production may be beneficial to P. aeruginosa, as rhamnolipids improve the availability of hydrophobic compounds such as lipids to the bacteria, resulting in accelerated degradation of such compounds (35).
The rsmZ mutant PAO6354 was phenotypically similar to the wild type, PAO1, in most assays conducted (Table 2) and also with respect to AHL production. Overexpression of rsmZ from the multicopy plasmid pME6000, by contrast, had significant effects on the formation of several exoproducts, similar to those caused by an rsmA mutation (Table 2). These results suggest that RsmZ might not be the only regulatory RNA in the GacS/GacA cascade of P. aeruginosa. The fact that rsmZ expression is subject to negative autoregulation in wild-type P. aeruginosa should be a reason for cautious interpretation of the rsmZ overexpression data. Whereas these data support the model of RsmA sequestration by RsmZ, they do not reflect the natural, finely balanced situation in vivo.
The small regulatory RNAs CsrB and CsrC of E. coli, CsrB of Erwinia spp., RsmY and RsmZ of P. fluorescens (1, 18, 29, 55, 58), and RsmZ of P. aeruginosa (Fig. 3B) all have elaborate secondary structures, and they share limited nucleotide sequence identities. As a common denominator, single-stranded GGA motifs stand out and might be important for binding of RsmA/CsrA (58).
Acknowledgments
We thank Cornelia Reimmann for providing PAO6327 Thilo Köhler for providing PT712, and Klaus Winzer, Matt Holden, Steve Diggle, and Claudia Matz for help in discussion of this work and in organizing F.W.'s data.
This study was supported by the Swiss National Foundation for Scientific Research (project 31-56608.99), the European project Nanofoldex (QLK3-CT-2002-0286), and the Biotechnology and Biological Sciences Research Council, United Kingdom.
Footnotes
This work is dedicated to the memory of Faye Williams who was an ever-smiling collaborator in this project. She died tragically on 14 November 2001.
REFERENCES
- 1.Aarons, S., A. Abbas, C. Adams, A. Fenton, and F. O'Gara. 2000. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolites genes in Pseudomonas fluorescens F113. J. Bacteriol. 182:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, C. S., I. Morozov, K. Suzuki, T. Romeo, and P. Babitzke. 2002. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 44:1599-1610. [DOI] [PubMed] [Google Scholar]
- 3.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brint, J. M., and D. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castric, P. A. 1975. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 21:613-618. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. Regulation of Erwinia carotovora hrpLEcc (sigma-LEcc), which encodes an extracytoplasmic function subfamily of sigma factor required for expression of the HRP regulon. Mol. Plant-Microbe Interact. 15:971-980. [DOI] [PubMed] [Google Scholar]
- 7.Cui, Y., A. Chatterjee, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora ssp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 177:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Sal, G., G. Manfioletti, and C. Schneider. 1988. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 16:9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diggle, S. P., K. Winzer, A. Lazdunski, P. Williams, and M. Cámara. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 184:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake, D., and T. C. Montie. 1988. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J. Gen. Microbiol. 134:43-52. [DOI] [PubMed] [Google Scholar]
- 11.Essar, D. W., L. Eberly, A. Hadero, and I. Crawford. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farinha, M. A., and A. M. Kropinski. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 58:221-225. [DOI] [PubMed] [Google Scholar]
- 13.Frank, L. H., and R. D. De Moss. 1959. On the biosynthesis of pyocyanine. J. Bacteriol. 77:776-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamper, M., B. Ganter, M. R. Polito, and D. Haas. 1992. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J. Mol. Biol. 226:943-957. [DOI] [PubMed] [Google Scholar]
- 15.Gewitz, H. S., E. K. Pistorius, H. H. Voss, and B. Vennesland. 1976. Cyanide formation in preparations from Chlorella vulgaris Beijerinck: effect of sonication and amygdalin addition. Planta 131:145-148. [DOI] [PubMed] [Google Scholar]
- 16.Heeb, S., Y. Itoh, T. Nishijiyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria CHA0. Mol. Plant-Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 17.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 18.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyytiäinen, H., M. Montesano, and E. T. Palva. 2001. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 14:931-938. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, Y., M. Cámara, S. R. Chhabra, K. R. Hardie, B. W. Bycroft, A. Lazdunski, G. P. Salmond, G. S. Stewart, and P. Williams. 1998. In vitro biosynthesis of the Pseudomonas aeruginosa quorum-sensing signal molecule N-butanoyl-L-homoserine lactone. Mol. Microbiol. 28:193-203. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, M. K., and D. Boese-Marrazzo. 1980. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 29:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jude, F., T. Köhler, P. Branny, K. Perron, M. P. Mayer, R. Comte, and C. van Delden. 2003. Posttranscriptional control of quorum-sensing-dependent virulence genes by DksA in Pseudomonas aeruginosa. J. Bacteriol. 185:3558-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler, E., M. Safrin, J. C. Olson, and D. E. Ohman. 1993. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 268:7503-7508. [PubMed] [Google Scholar]
- 24.Köhler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechère. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.König, B., K. E. Jaeger, A. E. Sage, M. L. Vasil, and W. König. 1996. Role of Pseudomonas aeruginosa lipase in inflammatory mediator release from human inflammatory effector cells (platelets, granulocytes, and monocytes). Infect. Immun. 64:3252-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 27.Lawhon, S. D., R. Maurer, M. Suyemoto, and C. Altier. 2002. Intestinal short-chain fatty acids Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46:1451-1464. [DOI] [PubMed] [Google Scholar]
- 28.Liu, M. Y., and T. Romeo. 1997. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J. Bacteriol. 179:4639-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M. Y., Y. Cui, B. Wei, J. F. Preston III, L. Oakford, U. Yuksel, D. P. Giedroc, and T. Romeo. 1997. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 28:17502-17510. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. Carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219-234. [DOI] [PubMed] [Google Scholar]
- 31.Maurhofer, M., C. Reimmann, P. Schmidli-Sacherer, S. Heeb, D. Haas, and G. Défago. 1998. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology 88:678-684. [DOI] [PubMed] [Google Scholar]
- 32.Medina, G., K. Juárez, B. Valderrama, and G. Soberón-Chávez. 2003. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol. 185:5976-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Mukherjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1996. Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherichia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology 142:427-434. [DOI] [PubMed] [Google Scholar]
- 35.Noordman, W. H., and D. B. Janssen. 2002. Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 68:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochsner, U. A., A. Fiechter, and J. Reiser. 1994. Isolation, characterization, and expression in Eschericia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J. Biol. Chem. 269:19787-19795. [PubMed] [Google Scholar]
- 37.Ohman, D. E., S. J. Cryz, and B. H. Iglewsi. 1980. Isolation and characterizarion of a Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pernestig, A.-K., D. Georgellis, T. Romeo, K. Suzuki, H. Tomenius, S. Normark, and Ö. Melefors. 2003. The Escherichia coli BarA-UvrY two-component system is needed for efficient switching between glycolytic and gluconeogenic carbon sources. J. Bacteriol. 185:843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S., Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pessi, G., and D. Haas. 2001. Dual control of hydrogen cyanide biosynthesis by the global activator GacA in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 200:73-78. [DOI] [PubMed] [Google Scholar]
- 43.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Cámara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:4885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 46.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Défago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 47.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-790. [Google Scholar]
- 50.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 51.Stuer, W., K.-E. Jaeger, and U. K. Winkler. 1986. Purification of extracellular lipase from Pseudomonas aeruginosa. J. Bacteriol. 168:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki, K., X. Wang, T. Weilbacher, A.-K. Pernestig, Ö. Melefors, D. Georgellis, P. Babitzke, and T. Romeo. 2002. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J. Bacteriol. 184:5130-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swift, S., A. V. Karlyshev, E. L. Durant, M. K. Winson, P. Williams, S. Macintyre, and G. S. A. B. Stewart. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicidia: identification of the LuxRI homologues AhyRI and AsaRI and their cognate signal molecules. J. Bacteriol. 179:5271-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka, K., and H. Takahashi. 1994. Cloning, analysis and expression of an rpoS homologue gene from Pseudomonas aeruginosa PAO1. Gene 150:81-85. [DOI] [PubMed] [Google Scholar]
- 55.Valverde, C., S. Heeb, C. Keel, and D. Haas. 2003. RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol. Microbiol. 50:1361-1379. [DOI] [PubMed] [Google Scholar]
- 56.Voisard, C., C. T. Bull, C. Keel, J. Laville, M. Maurhofer, U. Schnider, G. Défago, and D. Haas. 1994. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches, p. 67-89. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms. VCH, Weinheim, Germany.
- 57.Wei, B. L., A.-M. Brun-Zinkernagel, J. W. Simecka, B. M. Prüss, B. P. Babitzke, and T. Romeo. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245-256. [DOI] [PubMed] [Google Scholar]
- 58.Weilbacher, T., K. Suzuki, A. K. Dubey, X. Wang, S. Gudapaty, I. Morozov, C. S. Baker, D. Georgellis, P. Babitzke, and T. Romeo. 2003. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol. Microbiol. 48:657-670. [DOI] [PubMed] [Google Scholar]
- 59.Winson, M. K., M. Cámara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, B. W. Bycroft, G. P. C. Salmond, A. Lazdunski, G. S. A. B. Stewart, and P. Williams. 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. A. B. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum-sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]
- 61.Winteler, H. V., B. Schneidinger, K.-E. Jaeger, and D. Haas. 1996. Anaerobically controlled expression system derived from the arcDABC operon of Pseudomonas aeruginosa: application to lipase production. Appl. Environ. Microbiol. 62:3391-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winzer, K., C. Falconer, N. C. Garber, S. P. Diggle, M. Cámara, and P. Williams. 2000. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 182:6401-6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winzer, K., and P. Williams. 2001. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291:131-143. [DOI] [PubMed] [Google Scholar]
- 64.Zuber, S., F. Carruthers, C. Keel, A. Mattart, C. Blumer, G. Pessi, C. Gigot-Bonnefoy, U. Schnider-Keel, S. Heeb, C. Reimmann, and D. Haas. 2003. GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant-Microbe Interact. 16:634-644. [DOI] [PubMed] [Google Scholar]
- 65.Zuker, M. 1989. On finding all suboptimal foldings of an RNA molecule. Science 244:48-52. [DOI] [PubMed] [Google Scholar]