Abstract
Rationale
Evidence is increasing of a link between interferon (IFN) and pulmonary arterial hypertension (PAH). Conditions with chronically elevated endogenous IFNs such as systemic sclerosis are strongly associated with PAH. Furthermore, therapeutic use of type I IFN is associated with PAH. This was recognized at the 2013 World Symposium on Pulmonary Hypertension where the urgent need for research into this was highlighted.
Objective
To explore the role of type I IFN in PAH.
Methods and Results
Cells were cultured using standard approaches. Cytokines were measured by ELISA. Gene and protein expression were measured using reverse transcriptase polymerase chain reaction, Western blotting, and immunohistochemistry. The role of type I IFN in PAH in vivo was determined using type I IFN receptor knockout (IFNAR1−/−) mice. Human lung cells responded to types I and II but not III IFN correlating with relevant receptor expression. Type I, II, and III IFN levels were elevated in serum of patients with systemic sclerosis associated PAH. Serum interferon γ inducible protein 10 (IP10; CXCL10) and endothelin 1 were raised and strongly correlated together. IP10 correlated positively with pulmonary hemodynamics and serum brain natriuretic peptide and negatively with 6-minute walk test and cardiac index. Endothelial cells grown out of the blood of PAH patients were more sensitive to the effects of type I IFN than cells from healthy donors. PAH lung demonstrated increased IFNAR1 protein levels. IFNAR1−/− mice were protected from the effects of hypoxia on the right heart, vascular remodeling, and raised serum endothelin 1 levels.
Conclusions
These data indicate that type I IFN, via an action of IFNAR1, mediates PAH.
Keywords: chemokine CXCL10; endothelin-1; IFNAR1 subunit, interferon alpha-beta receptor; inflammation; interferon type I; pulmonary arterial hypertension; scleroderma, systemic
Pulmonary arterial hypertension (PAH) is a rare but devastating disease, which is defined as a mean pulmonary artery pressure of ≥25 mm Hg with a normal pulmonary capillary wedge pressure. It is characterized by remodeling of the muscular, precapillary vessels, leading to an increase in pulmonary vascular resistance. The associated strain exerted on the right heart ultimately results in right heart failure and premature death.1,2
Autoimmunity has long been implicated in PAH3 and, most recently, evidence has emerged implicating interferon (IFN).4 IFN is central to the innate immune response to viral infection, and 3 types have been identified; type I IFN (IFNα and IFNβ) that signals through a heterodimeric receptor consisting of IFNAR1 and IFNAR2, type II IFN (IFNγ) that signals through IFNGR1 and IFNGR2, and type III IFN (IFNλ) the receptor for which comprises interleukin (IL)10RB and IL28RA.
There is growing evidence that clinically proven PAH can be precipitated with type I IFN therapy.5–8 Furthermore, in as many as 48% of patients receiving IFNα treatment, lung function (diffusion capacity of lung for carbon monoxide [DLCO]) is reduced by ≥15%,9 which may be due to undiagnosed pulmonary vascular pathology.10 This is particularly relevant when considering that endothelin 1 (ET-1), a key mediator in the pathogenesis of PAH, is elevated in a subpopulation of patients receiving IFNα therapy for hepatitis C viral infection,11 and that our group has pioneered the idea that ET-1 is an IFN inducible gene in vascular smooth muscle cells.12,13 Concern surrounding IFN and PAH has reached such a level that, at the recent World Symposium on Pulmonary Hypertension (Nice, February 2013), type I IFN was added to the list of drugs that may be associated with PAH.
It is well appreciated that patients with HIV, where IFNs are chronically elevated, have an increased incidence of PAH.14 However, arguably the most compelling clinical case for endogenously produced IFN and PAH may be that associated with systemic sclerosis (SSc). SSc is a chronic autoimmune disease that affects up to 286 people per 1 million population15 and is associated with pulmonary vascular pathology in as many as half of patients16 manifesting as diagnosed PAH in 12% to 15%.17 The mechanisms underpinning SSc-associated PAH are still unclear. Patients with SSc have high levels of IFN18,19 and ET-120 but any link between IFN in SSc and PAH has not been fully addressed. Importantly, IFN induces a specific signature of genes, one of the most responsive being interferon γ–inducible protein 10 (IP10; CXCL10).21 IP10 is increasingly recognized as a potential mediator of inflammation, including that associated with the lung.22,23
The mounting evidence base for a link between IFN and PAH has led us to hypothesize that activation of IFN pathways is central to the pathobiology of PAH and, as such, could represent an important contributing factor to SSc-associated disease. We have used in vitro and in vivo experimental techniques as well as clinical samples from patients with SSc-PAH to address this hypothesis.
Methods
In Vitro Cell Culture
All cells were serum deprived for 24 hours and subsequently treated with IFNs in the presence and absence of tumor necrosis factor (TNF) α for 24 hours before supernatant was removed for analysis. IFNα and IFNγ were used at 10 or 30 ng/mL; whereas IFNλ was used at 1000 ng/mL to reflect its 100-fold lower specific activity. TNFα was used at 10 ng/mL. Cell culture supernatant levels of ET-1 and IP10 were measured by ELISA.
Isolation of Blood Outgrowth Endothelial Cells
Blood was collected from patients with PAH, and healthy controls and blood outgrowth endothelial cells (BOECs) were isolated as per previously published protocols.24,25 BOECs were treated with IFNα for 24 hours, and supernatant levels of IP10 and ET-1 were measured by ELISA. The number of colony forming units that emerged from peripheral blood mononuclear cell cultures within 3 days was counted, and proliferation of BOECs was measured during 24 hours using the alamarBlue method.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
Quantitative reverse transcriptase polymerase chain reaction for the type I IFN receptor (IFNAR1 and IFNAR2), the type II IFN receptor (IFNGR1 and IFNGR2), and the type III IFN receptor (IL10RB and IL28RA) was performed using TaqMan gene expression assays.
Lung Tissue Immunohistochemistry
IFNAR1, IFNGR1, and IL28RA expression was studied using immunohistochemistry performed on lung tissue from patients with SSc-PAH, idiopathic (I)PAH, and healthy controls.
Western Blotting
IFNAR1 expression in the lungs of patients with SSc-PAH, IPAH, and healthy controls was studied by Western blotting, and ImageJ software was used to quantify the level of protein expression.
Chronic Hypoxia–Induced Mouse Model of Pulmonary Hypertension
All studies were performed in accordance with UK Home Office Animals (Scientific Procedures) Act 1986 and institutional guidelines. Male C57Bl/6J and IFNAR1−/− mice (all 8 to 10 weeks old; ≈20 g) were housed in normal air or placed in a normobaric hypoxic chamber (FiO2 10%) for 14 days (n=8–15/group). Development of pulmonary hypertension was confirmed as previously described.26
Mouse Model of Acute Lipopolysaccharide–Induced ET-1 Release
Male C57Bl/6J and IFNAR1−/− mice (8 to 10 weeks old, weighing ≈20 g) were intraperitoneally injected with 8 μl/g of either vehicle control (0.9% sterile saline) or lipopolysaccharide (LPS) from Escherichia Coli 055:B5 (1.25 mg/mL). At 4 hours, mice were humanely euthanized. Blood was taken; serum obtained; and IP10, ET-1, IFNs, and keratinocyte-derived chemokine (KC) levels were measured by ELISA.
Human and Mouse Tissue Organ Culture
Segments of whole human pulmonary artery were freshly harvested from patients undergoing pulmonary resection. Pulmonary arteries were treated with IFNs for 24 hours before supernatants were removed, and IP10 was measured by ELISA.
Mouse aorta and lung tissue were obtained from C57Bl/6J mice after being humanely euthanized. Segments of vessel or lung were treated with recombinant human IFNα2b, mouse IFNα A, or human pegylated IFNα2b for 24 hours. Supernatant levels of IP10 were measured by ELISA.
Clinical Samples
Sixty-three patients with SSc (28 patients with SSc-PAH and 35 SSc patients without PAH) were recruited from 2 specialist centers: Royal Free Hospital, London, and Papworth Hospital Research Tissue Bank. Healthy controls were recruited from Royal Free Hospital and Royal Brompton Hospital, London. Serum levels of ET-1, IP10, IFNs, and related cytokines were measured by ELISA. Clinical data from each patient were collected. Patients with lung disease or left heart disease were excluded.
Statistical Analysis
Data are presented as mean±SEM. For all data, the Kolmogorov–Smirnov test of normality was applied. All normally distributed data were analyzed by 1-way ANOVA followed by Bonferroni post-test adjustment for multiple comparisons and for correlations with clinical parameters, the Pearson correlation test was applied. Nonparametric data were analyzed by the Kruskal–Wallis test followed by Dunn multiple comparison test and, for correlations, the Spearman rank test was used. Graphpad Prism was used for all statistical analysis.
An expanded Methods is available in the Online Data Supplement.
Results
Effect of IFNs on IP10 and ET-1 Release by Human Lung Cells in Culture
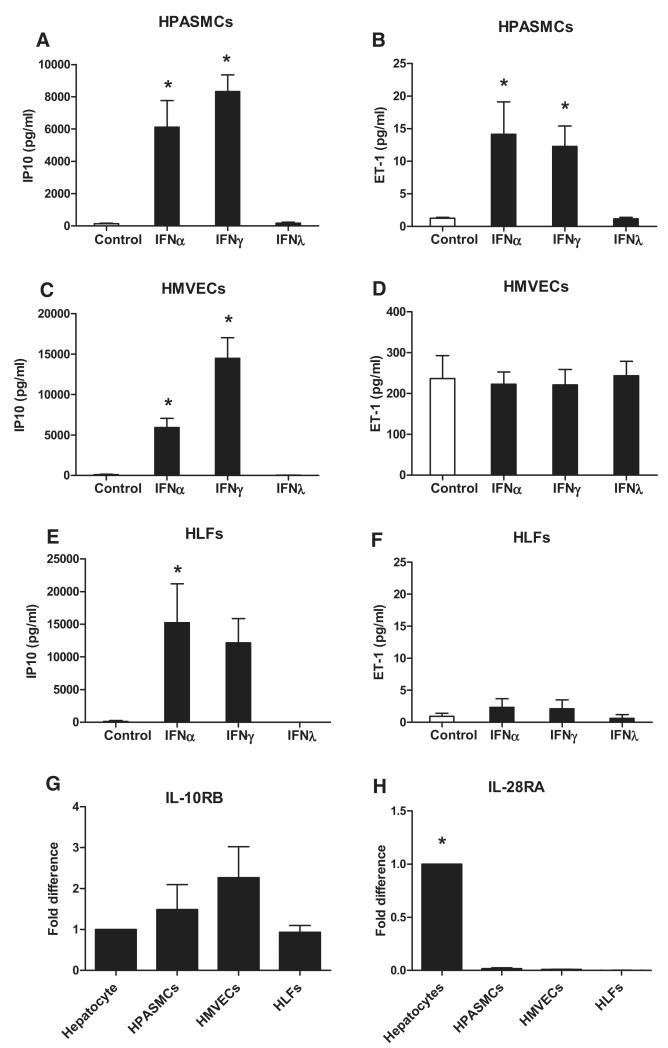
As we have shown previously,27 type I IFNα and type II IFNγ induced IP10 and ET-1 release from TNFα-primed human pulmonary artery smooth muscle cells (HPASMCs) (Figure 1A and 1B). Again, as we have seen before, type III IFNλ did not induce appreciable levels of IP10 or ET-1 release from HPASMCs. IP10 release in response to type I and type II IFNs was also seen in human lung microvascular endothelial cells and in human lung fibroblasts (Figure 1C and 1E). ET-1 release by human lung microvascular endothelial cells was predictably high28 and not increased by treatment with any of the IFNs studied (Figure 1D). ET-1 release by human lung fibroblasts was relatively low and similar to that seen from HPASMCs; however, unlike vascular smooth muscle, it was not increased by IFN treatment (Figure 1F). Based on full concentration response experiments (Online Figures I–III), for type I and type II IFNs, threshold concentrations were in the low ng/mL range, whereas type III IFNλ remained inactive at concentrations up to 1000 ng/mL. Although TNFα coadministration accentuated IFN-induced responses, it is not an absolute prerequisite for IFN sensing in some cells,27 particularly with regard to the current study for IFNα or IFNγ-induced ET-1 release by HPASMCs (Online Figures IB and ID). As predicted by the strong cellular response to type I and II IFNs, HPASMCs, human lung microvascular endothelial cells, and human lung fibroblasts expressed both receptor subtypes for IFNAR and IFNGR (Online Figure IV). By contrast, IFNλ was inactive in all 3 lung cell types tested. Using human hepatocytes, which respond well to IFNλ (Online Figure V) as a reference, we found that although human lung cells expressed comparable levels of IL10RB, they expressed low levels of IL28RA (Figure 1G and 1H).
Figure 1.
Response of pulmonary vascular cells to types I, II, and III interferons (IFNs) and type III IFN receptor expression in pulmonary vascular cells as compared with hepatocytes. Human pulmonary artery smooth muscle cells (HPASMCs; A and B), human lung microvascular endothelial cells (HMVECs; C and D), and human lung fibroblasts (HLFs; E and F) were treated with IFNα (10 ng/mL), IFNγ (10 ng/mL), and IFNλ (1000 ng/mL) in the presence of tumor necrosis factor (TNFα; 10 ng/mL) and assayed for IFN γ inducible protein 10 (IP10; A, C, and E) and endothelin 1 (ET-1; B, D, and F). Data are presented as mean±SEM from n=3 to 6 experiments performed in singlicate. Statistical significance (*P<0.05) compared with control was determined by 1-way ANOVA with Dunnett multiple comparison post-test adjustment. IL10RB (G) and IL28RA (H) gene expressed as mean±SEM fold difference compared with hepatocytes from n=3 experiments in the absence of TNFα. Statistical significance (*P<0.05) compared with hepatocytes was determined by 1-way ANOVA with Dunnett multiple comparison post-test adjustment.
Levels of IFNs, IP10, and ET-1 in Serum of SSc Patients With or Without PAH
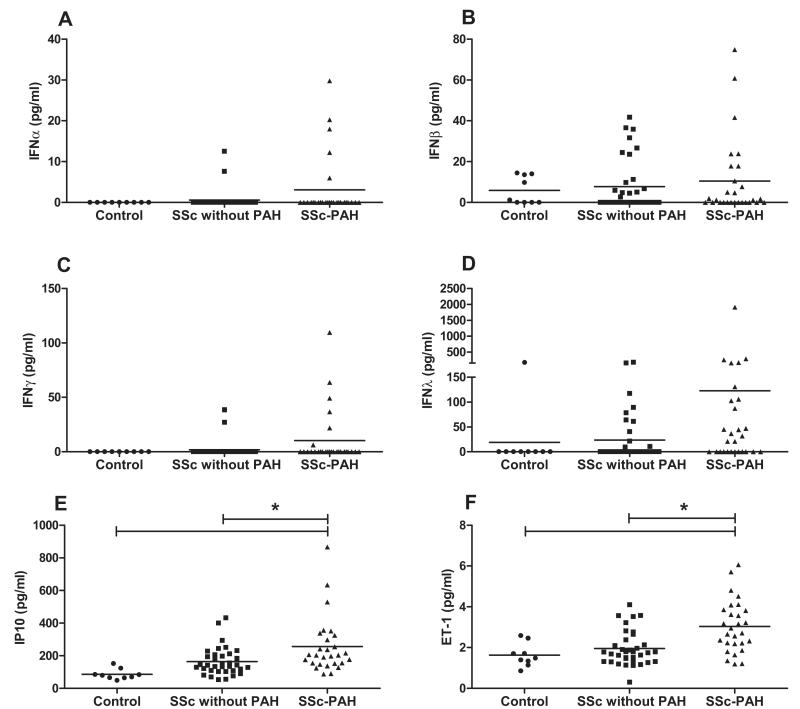
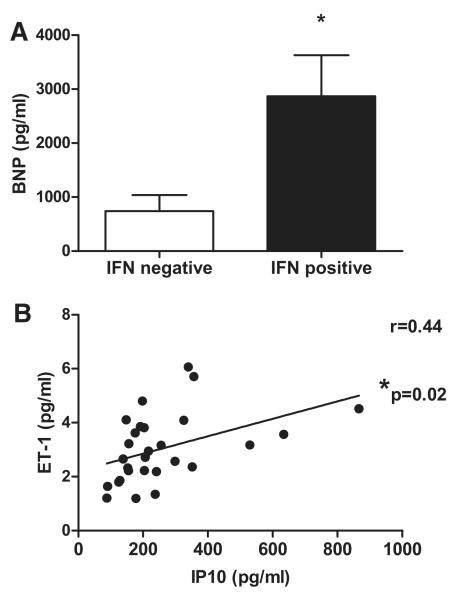
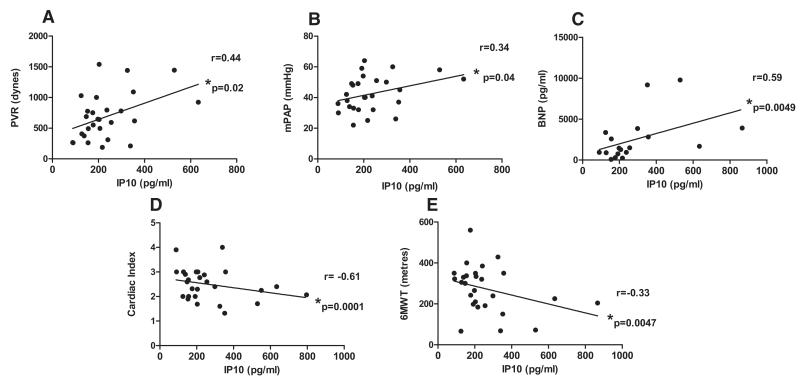
Five (18%) patients in the SSc-PAH group had detectable levels of IFNα, whereas only 2 (5.7%) patients had detectable levels in the SSc without PAH group. IFNα was below the level of detection in serum samples of all control subjects. Similarly 6 (21%) patients with SSc-PAH had detectable levels of IFNγ, whereas this was the case in only 2 (5.7%) patients with SSc and no PAH. IFNγ was not detectable in any controls. While IFNβ levels were similar in all 3 groups, there was a clear but non significant trend to increased levels of IFNλ in serum of patients with SSc-PAH compared with those without PAH or controls (Figure 2A−2D). In line with these data, we found that levels of our 2 key IFN-stimulated gene products, IP10 and ET-1, were significantly increased in the serum of patients with SSc-PAH as compared to controls and to SSc patients without PAH (Figure 2E and 2F). Patients in whom serum levels of ≥1 of the IFNs measured were detectable, so-called IFN-positive patients, had significantly raised levels of serum brain natriuretic peptide as compared with IFN-negative patients (Figure 3A). We hypothesized that levels of IP10 and ET-1 were linked because there was a strong correlation between them in the serum of patients with SSc-PAH (r=0.44; P=0.02; Figure 3B). In this patient group, there were positive correlations between IP10 and pulmonary vascular resistance (r=0.44; P=0.02; Figure 4A), between IP10 and mean pulmonary artery pressure (r=0.34; P=0.04; Figure 4B), and between IP10 and serum levels of brain natriuretic peptide (r=0.59; P=0.0049; Figure 4C), and strong negative correlations between IP10 and cardiac index (r=−0.61; P=0.0001; Figure 4D) and IP10 and 6-minute walk test (6MWT; r=−0.33; P=0.047; Figure 4E). Importantly, there was no relationship between serum levels of IP10 and ET-1 in SSc patients without PAH, suggesting that this axis has specific relevance to SSc-PAH (Online Figure VI). As has previously been demonstrated in patients with IPAH29 and SSc-PAH,30 we found increased levels of proinflammatory cytokines, such as IL-1α and IL-8, and statistically significant increases in levels of IL-6, IL-12p70, and TNFα (P<0.05) in the serum of patients with SSc-PAH (Online Figure VII).
Figure 2.
Serum interferon (IFN) γ inducible protein 10 (IP10), endothelin 1 (ET-1), and IFN levels in patients with systemic sclerosis (SSc)-pulmonary arterial hypertension (PAH), SSc without PAH, and healthy controls. Serum levels of IFNα (A), IFNβ (B), IFNγ (C), IFNλ (D), IP10 (E), and ET-1 (F) were analyzed from controls, n=9, patients with SSc without PAH, n=35, and patients with SSc-PAH, n=28. Individual data points refer to each patient, and means of all patients in the cohort are represented by horizontal lines. Statistical significance (*P<0.05) for all 3 groups compared with each other was determined by Kruskal–Wallis test followed by Dunn multiple comparison post-test for nonparametric data (A–D) and by 1-way ANOVA followed by Bonferroni multiple comparison post-test for normally distributed data (E and F).
Figure 3.
Serum brain natriuretic peptide (BNP), interferon (IFN) γ inducible protein 10 (IP10), and endothelin 1 (ET-1) levels measured in a cohort of patients with systemic sclerosis (SSc)-pulmonary arterial hypertension (PAH). Serum BNP levels in IFN-positive and IFN-negative patients (A). Data expressed as mean±SEM. Statistical significance (*P<0.05) determined using a t test. Serum IP10 and ET-1 levels measured in patients with SSc-PAH, n=28 (B). Data points represent individual patient readouts. Correlation was determined using Pearson correlation coefficient, and r and P values are shown.
Figure 4.
Correlation of interferon γ inducible protein 10 (IP10) with clinical and hemodynamic parameters in patients with systemic sclerosis (SSc)-pulmonary arterial hypertension (PAH). Pearson correlation coefficient was determined between IP10 and pulmonary vascular resistance (PVR; dynes), n=27 (A); and mean pulmonary artery pressure (mPAP; mm Hg), n=27 (B); Spearman rank correlation coefficient was determined between IP10 and serum brain natriuretic peptide (BNP) levels (pg/mL), n=18 (C); cardiac index, n=27 (D); and 6-minute walk test (6MWT; meters), n=27 (E). Data points represent individual patient readouts.
Immunohistochemistry, Western Blotting, and Response of Blood Outgrowth Endothelial Cells to IFNα
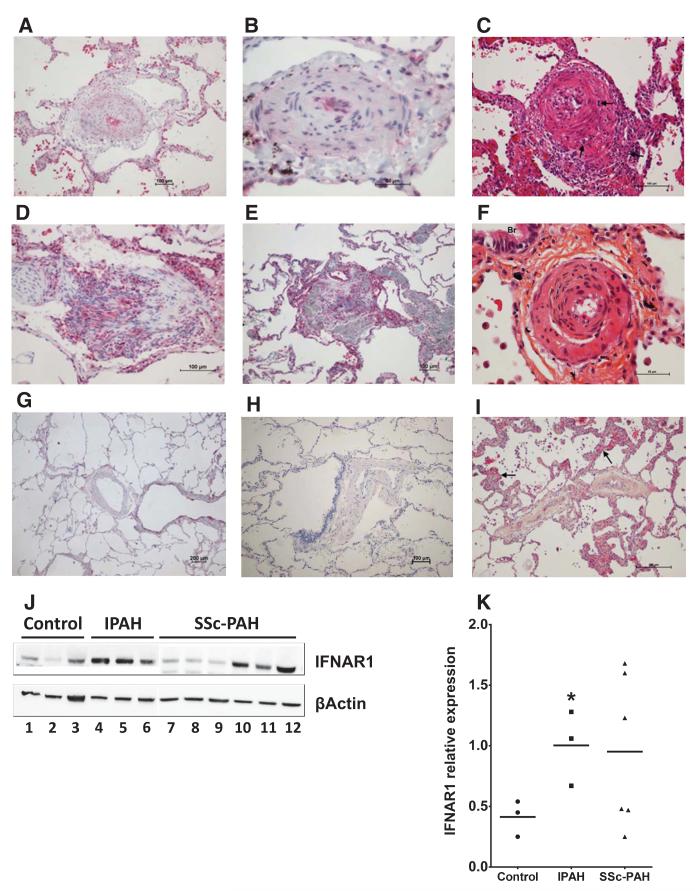
The biological consequences of increased IFN are not only governed by tissue and plasma concentrations, but also by the level of receptor expression. To explore further the relevance that IFNα may have in SSc-PAH, we looked at type I IFN receptor expression in the pulmonary vasculature of patients with SSc-PAH and compared it with expression in lung sections from control patients without PAH or SSc. We found a greatly increased level of IFNAR1 staining in the lung sections of patients with SSc-PAH (Figure 5A and 5B) as compared with controls (Figure 5G and 5H). There was increased IFNAR1 expression within the remodeled pulmonary arteries of lungs from patients with SSc-PAH. This increased staining was most abundant within the smooth muscle layer, endothelium, and vascular interstitium as well as within inflammatory intravascular cells. Interestingly, this was also the case in patients with IPAH (Figure 5D and 5E), which led us to consider that type I IFN may play a pathogenic role in other forms of PAH and not exclusively those associated with SSc.
Figure 5.
Immunohistochemistry and Western blotting demonstrating type I interferon receptor (IFNAR1) in pulmonary arterial hypertension (PAH) lung. IFNAR1 staining in lung sections from a patient with systemic sclerosis (SSc)-PAH (A and B), Idiopathic (I) PAH (D and E), and non-PAH controls (G and H). Figures shown are representative images from n=3 patients. Lung samples from patients with SSc-PAH with high (C and F) and low (I) IFNAR1 expression; hematoxylin and eosin and hematoxylineosin-saffron staining. C, A small pulmonary artery displaying arteritis with transmural inflammatory infiltrate (arrows) and important intimal thickening. F, A pulmonary artery adjacent to a small bronchiole (Br) showing quasiocclusive fibrosis of the intima. I, Small pulmonary vein displaying important fibrotic occlusive remodeling, a feature frequently encountered in SSc-PAH; note the surrounding alveolar septa with capillary hemangiomatosis-like appearance (arrows). IFNAR1 expression (J); 1 to 3, n=3 controls; 4 to 6, n=3 patients with IPAH; (separate blot) 7 to 9, n=3 SSc-PAH with predominantly venous disease and 10 to 12, n=3 SSc-PAH with predominantly arterial disease. Blot (J) quantified as expression relative to β actin (K). Statistical significance (*P<0.05) was determined by 1-way ANOVA with Bonferroni multiple comparison post-test.
To allow direct quantification of protein expression, we performed Western blot experiments from total lung homogenate of patients with IPAH, SSc-PAH, and controls. We found that IFNAR1 expression was significantly raised in all patients with IPAH tested and in some, but not all, patients with SSc-PAH (Figure 5J and 5K). It is known that all patients with SSc-PAH display histological features of pulmonary arterial inflammation with varying degrees of pulmonary venous occlusive disease, and that those patients with a greater propensity to venous disease are less responsive to specific PAH therapies.31,32 Our interesting observation that there seemed to be a heterogeneity of IFNAR1 expression within patients with SSc-PAH led us to hypothesize that those patients with high IFNAR1 gross lung levels might be those that morphologically resemble an IPAH pattern of arterial involvement as opposed to pulmonary venous disease. To some extent, all 6 of the patients with SSc-PAH examined displayed important venous involvement. However, of these, the 3 patients with high gross lung IFNAR1 expression displayed profound arterial inflammation and concentric laminar fibrosis of the intima, both features consistent with true PAH and histomorphologically closest to IPAH (Figure 5C and 5F). Patients with low IFNAR1 expression in total lung homogenates had a predominance of venous involvement with fibrotic occlusive remodeling and surrounding alveolar septa with capillary hemangiomatosis-like appearance, features frequently encountered in SSc-PAH (Figure 5I).
Because levels of IFNγ and IFNλ were also raised in the serum of patients with SSc-PAH, we performed further immunohistochemistry experiments for IFNGR1 and IL28RA staining. There was an increase in IFNGR1 staining in the pulmonary vasculature of patients with SSc-PAH compared with controls (Online Figure VIIIA–C) and, although far less profound, it followed similar morphological patterns to that of IFNAR1. Pulmonary vascular IL28RA staining was also increased in patients with SSc-PAH; however, the pattern of receptor expression differed with staining predominantly seen within the epithelial cell layer (Online Figure VIIID–F).
The finding that the type I IFN receptor is upregulated in the lungs of patients with PAH is consistent with our hypothesis that endogenous type I IFNs may have a role in driving PAH and is highly relevant to our understanding of how therapeutic IFN preparations may be associated with pulmonary toxicity. In line with increased staining of IFNAR1 in the vasculature, we found that endothelial cells grown from blood of a heterogeneous group of patients with PAH (1 IPAH, 1 connective tissue disease-PAH, 1 congenital heart disease-PAH, 1 sarcoid-PAH) were more sensitive to stimulation with IFNα even in the absence of TNFα (Figure 6). As with other cell types used in this study, TNFα coadministration enhanced the sensitivity of BOECs to IFNα in all cells, and this response was exaggerated in cells from patients with PAH (Online Table I). Interestingly, there was a trend for BOECs from patients with PAH to release higher levels of ET-1 than those from healthy individuals under basal conditions and in response to IFNα (Online Figure IXA and IXB). We went on to assess the angiogenic activity of these cells and found that although there was a trend to an increased number of colony forming units of BOECs grown from the blood of patients with PAH as compared with controls, this did not reach statistical significance and, over a range of seeding densities, no difference in proliferation of these cells was noted (Online Figure XA and XB).
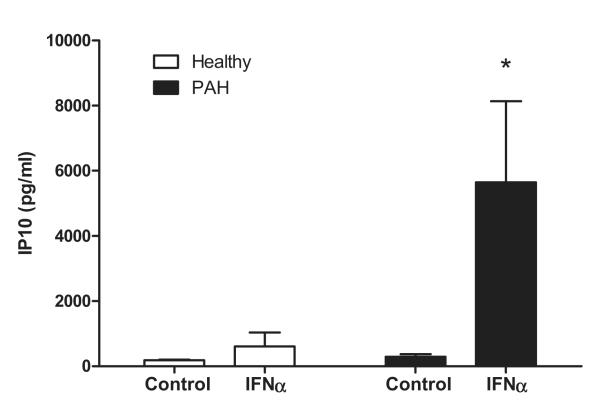
Figure 6.
Interferon γ inducible protein 10 (IP10) release from interferon α (IFNα)-stimulated endothelial cells grown from the blood of patients with pulmonary arterial hypertension (PAH) and healthy controls. Endothelial cells grown from the blood of patients (blood outgrowth endothelial cells from patients with PAH, n=4 and healthy controls, n=4 were treated with IFNα (30 ng/mL) and assayed for IP10. Data are presented as mean±SEM, and statistical significance (*P<0.05) was determined by 2-way ANOVA with Bonferroni multiple comparison post-test.
In Vivo Experiments and Animal Model of PAH
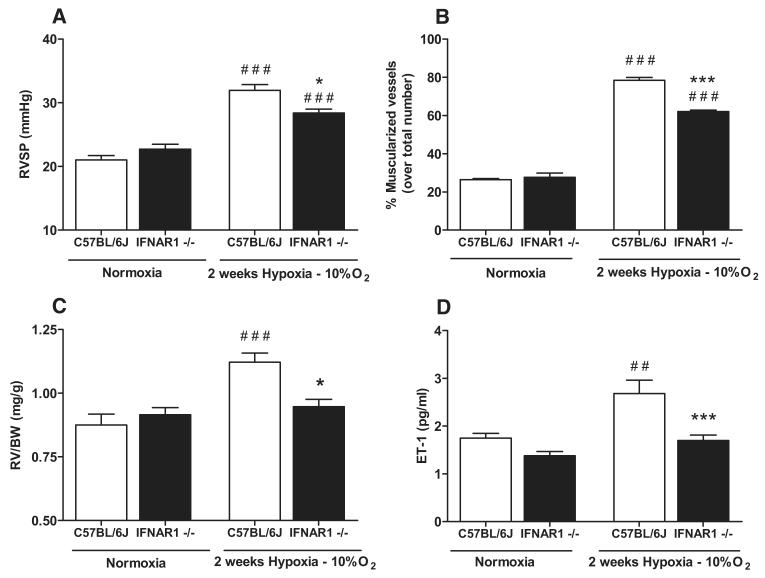
Although consistent with our hypothesis, the above data are observational and do not provide definitive proof for a direct association between IFN and PAH. As previously discussed, the type I IFN receptor consists of a heterodimeric IFNAR1–IFNAR2 complex. It has recently been demonstrated that these 2 subunits have different functions, and that IFNAR1 (and not IFNAR2) via an IFNAR1–IFN complex can independently transduce proinflammatory signals under the control of type I IFN.33 Thus, to explore the effect of type I IFN signaling directly on the development of PAH, we investigated the response to chronic hypoxia of mice lacking functional IFNAR1 (IFNAR1−/−). Under hypoxic conditions, wild-type (C57Bl/6J) mice developed elevations in right ventricular (RV) systolic pressure (Figure 7A) and an increase in the percentage of muscularized pulmonary vessels (Figure 7B). Along with pulmonary vascular changes, C57Bl/6J mice exposed to hypoxia developed RV hypertrophy (Figure 7C) and had higher circulating levels of ET-1 in their serum (Figure 7D). In line with our hypothesis, IFNAR1−/− mice were protected from the effects of hypoxia with a significant reduction in RV systolic pressure, percentage of muscularized vessels, and ratio of RV to body weight (Figure 7A–7C). Furthermore, consistent with the development of PAH, hypoxia induced raised serum levels of ET-1 in C57Bl/6J mice but not in IFNAR1−/− mice (Figure 7D). Serum IP10, IFNα, and IFNγ levels were undetectable in all mice. Serum IFNλ levels could be measured but were not influenced by the development of PAH (Online Figure XI). There was no significant difference in body weight between the C57Bl/6J (27.6±2.2 g) and IFNAR1−/− mice (25.6±2.7 g), and IFNAR1−/− mice were also protected from developing RV hypertrophy when calculated using the Fulton Index (RV/[left ventricle+septum]); (Online Figure XIIA). Representative images of pulmonary arteries from a C57Bl/6J mouse and an IFNAR1−/− mouse housed in hypoxia are shown (Online Figures XIIB and XIIC, respectively). Importantly, under control conditions, IFNAR1−/− mice have previously been well characterized and are known to have similar cardiovascular physiology, systemic blood pressure, and ventricular function to C57Bl/6J mice.34
Figure 7.
Influence of type I interferon (IFN) signaling on the development of pulmonary arterial hypertension (PAH) explored using the chronic hypoxic mouse model and mice lacking a functional type I IFN receptor. Mice lacking a functional type I IFN receptor (IFNAR1−/−) exposed to hypoxia (10% O2) or normoxia (room air) compared with wild-type (C57Bl/6J) mice exposed to the same conditions. Data presented as mean±SEM from n=4 to 15 mice. Right ventricular systolic pressure (RVSP; mm Hg; A), percentage of muscularized pulmonary vessels over total number of vessels (B), ratio of right ventricular (RV) mass to body weight (BW) (RV/BW; mg/g; C), and serum endothelin (ET)-1 levels (pg/mL; D) were measured. Statistical significance was determined by 1-way ANOVA followed by Bonferroni multiple comparison post-test (###P<0.0001 and ##P<0.005 for normoxic vs hypoxic conditions) and (***P<0.0001, **P<0.005, and *P<0.05 for IFNAR1−/− vs C57Bl/6J mice).
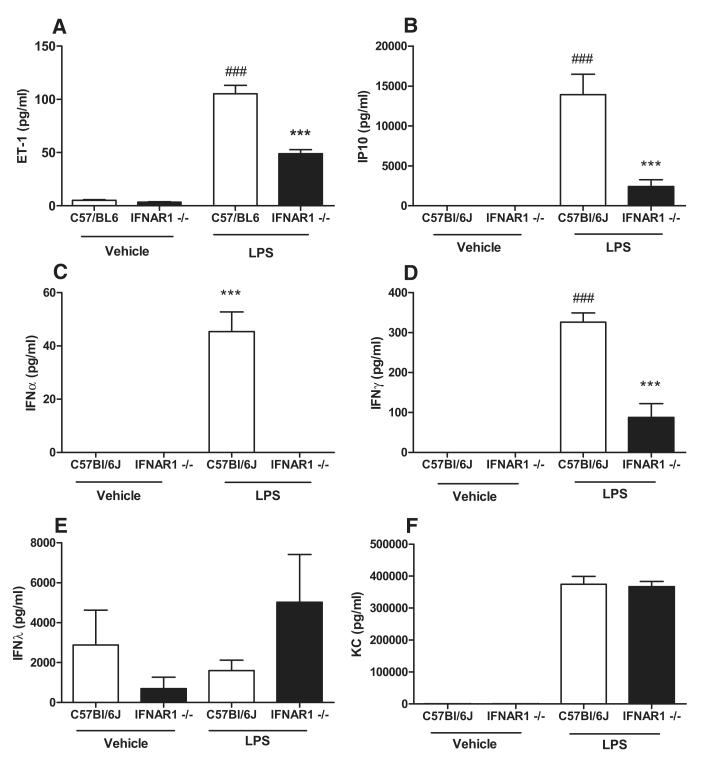
These data confirm our earlier findings implicating IFNs in the development of PAH and more specifically highlight a potential pathological role for type I IFN signaling in PAH, which may be mediated by ET-1. To further explore the underlying mechanisms, we attempted to create a model of PAH secondary to exogenous IFN administration. Given the short half-life of IFN (≈8 hours), for a 14-day chronic hypoxia study, it would be necessary to use pegylated IFN. Because pegylated forms of mouse IFN are not commercially available, in the first instance, we undertook experiments using pegylated human IFNα and compared responses with authentic (unpegylated) human IFNα and with mouse IFNα. We found that human IFNα (both pegylated and native forms) are inactive in mouse tissue as compared with mouse IFN (Online Figures XIIIA and XIIIB). Consequently, we elected to use a different approach in which to provide further mechanistic evidence for the relevance of IFN in the generation of ET-1 and hence PAH. Systemic levels of ET-1 are increased experimentally in 2 animal models: (1) hypoxia-driven PAH (demonstrated above) and (2) LPS-induced endotoxemia.35 LPS, via Toll like receptor 4 (TLR4), activates 2 adapter proteins, which give rise to separate groups of genes. Toll like receptor 4 activates TIR domain-containing adaptor inducing IFN-beta (TRIF), which is associated with expression of IFNs and IFN-related genes, such as IP10. Toll like receptor 4 also activates myeloid differentiation primary response gene 88 (MyD88), which activates nuclear factor κB–regulated genes including KC (the mouse homolog of IL-8/CXCL8). In C57Bl/6J mice, LPS induced systemic release of IFNα, IFNγ, ET-1, IP10, and KC but not IFNλ (Figure 8A–8F). Importantly, in IFNAR1−/− mice, LPS-induced release of IFNα, IFNγ, ET-1, and IP10 was significantly abrogated. By contrast, levels of KC induced by LPS were similar in both C57BL/6J and IFNAR1−/− mice (Figure 8A–8F). This provides further mechanistic evidence that ET-1 production is mediated by type I IFN via the type I IFN receptor IFNAR1 but is independent of IFNλ.
Figure 8.
Influence of type I interferon (IFN) signaling on lipopolysaccharide (LPS)-induced endothelin 1 (ET-1) generation explored using mice lacking a functional type I IFN receptor. Mice lacking a functional type I IFN receptor (IFNAR1−/−) injected with either LPS or vehicle control for 4 hours and compared with wild-type (C57Bl/6J) mice. Data presented as mean±SEM from n=6 mice. Serum levels of ET-1 (A), interferon γ inducible protein 10 (IP10) (B), IFNα (C), IFNγ (D), IFNλ (E), and keratinocyte-derived chemokine (KC) (F) were measured. Statistical significance determined by 1-way ANOVA followed by Bonferroni multiple comparison post-test (###P<0.0001 for LPS vs Vehicle) and (***P<0.0001 for IFNAR1−/− vs C57Bl/6J mice).
Further Study of In Vitro Roles of Types I, II, and III IFN
We have demonstrated that pulmonary vascular cells respond strongly to types I and II IFN to release IP10 and ET-1, and that serum levels of these IFNs, as well as IP10, and ET-1 are raised in patients with SSc-PAH. It was interesting therefore to observe that IFNλ was inactive in cells because of restricted receptor expression but raised in the serum of this patient group and we consequently investigated this phenomenon further. It is well established that type I IFN strongly induces IFNλ.36 Although this raises the possibility that IFNλ may represent a marker of type I IFN signaling, we wished to explore further its potential to play a pathological role in its own right. Having found IFNλ to retain activity only in hepatocyte cells in culture, we wished to exclude the possibility that primary cells in culture might display an altered phenotype and therefore assessed the activity of IFNs −α, −γ, and −λ on segments of freshly harvested human pulmonary artery. In direct corroboration of our data with cultured pulmonary vascular cells, we found that only IFNs −α and −γ induced IP10 release from whole vessel and that IFNλ was inactive (Online Figure XIV). Using immunohistochemistry, we then looked for the presence of the specific IFNλ receptor, IL28RA, within the pulmonary vasculature of patients with SSc-PAH and found that there was increased receptor expression predominantly within the lung epithelium (Online Figure VIIID and VIIIE), which reflects what is known of the IFNλ receptor in that it is expressed solely on epithelial surfaces and hepatocytes.4 Based on these findings, we assessed for the presence of the 2 IFNλ receptor subunits in the human epithelial lung carcinoma cell line (A549) cell line and found that although the IL10RB subunit seemed to be widely expressed, the IL28RA subunit was only expressed on hepatocytes and A549 cells (Online Figure XVA and XVB). Interestingly, we found that although these cells express relatively high levels of both IFNλ receptor subunits, they did not respond to IFNλ but responded strongly to IFNα and IFNγ releasing IP10 (Online Figure XVI) and ET-1 (Online Figure XVII) in the presence of TNFα. This was in contrast to hepatocytes that did respond to IFNλ to release IP10 (Online Figure VA). We repeated these experiments in 2 further epithelial cell types and as with A549 cells, we found that neither the immortalized human type I alveolar epithelial (TT1) cell line (Online Figure XVIII), nor the bronchial epithelial (Beas2B) cell line (Online Figure XIX) responded to IFNλ in the presence or absence of TNFα.
Discussion
In the current study, we have systematically addressed the role of IFN in PAH. To demonstrate the sensing of IFNs by cells in vitro, the pathological relevance of the IFN system in patients with PAH and the underlying IFN pathways at play in an in vivo model of PAH, we have extensively explored the roles of IP10 and ET-1. Previous work from our group has shown that ET-1 is an IFN gene,4 and it is well established that ET-1 is a critical mediator and therapeutic target in PAH. Furthermore, IP10 is one of the best characterized of all the IFN-stimulated genes. It is the cognate ligand of CXCR3 and is an antiviral and chemoatttractant that promotes the formation of lymphoid infiltrates commonly seen in viral infection and autoimmune disease37 by attracting T lymphocytes, monocytes, and natural killer cells.22,38 IP10, which is secreted by endothelial cells (among other cell types), also potentiates the adhesion of T lymphocytes to the endothelium,22 promotes the migration of CXCR3+ cells to the lung,23 and has been shown to play a pathogenic role in the development of interstitial lung disease.39 Indeed CXCR3 expression at both the gene and protein level has previously been demonstrated to be upregulated in patients with PAH, and IP10 has been shown to mediate endothelial dysfunction by disrupting calcium homeostasis.40
Our data suggest that human lung cells sense type I and type II IFNs readily but are insensitive to type III IFN, and that this is explained by relative IFN receptor expression across these cell types. Taken together with our in vivo data, we suggest that although IFNλ is raised in the serum of patients with SSc-PAH, it does not play a pathological role. Elevated systemic type I IFN induces the expression of many IFN-stimulated genes including IFNλ. Some, such as ET-1 and potentially IP10, play an important immunopathological role in PAH. However, many other IFN-stimulated genes, such as IFNλ, are not involved in the disease process, and we suggest that their upregulation may be a reflection of type I IFN activity driven by an underlying dysregulated innate immune system. Although it is not the subject of this study, our finding that by contrast to human hepatocytes human lung cells are insensitive to IFNλ is interesting and potentially clinically relevant because pegylated IFNλ is currently in Phase III clinical trials for the treatment of chronic Hepatitis C virus. It is tempting to speculate that these drugs may spare the lungs, thereby displaying an improved side effect profile as compared with existing IFNα preparations.
The data we present in this article are consistent with the large body of work that now recognizes the importance of inflammation in PAH. As others have previously demonstrated,29,41,42 the concentrations of IFN required to activate cells in vitro are much higher than levels detected in serum of patients with PAH. It is well established that serum IFN levels may not accurately represent cytokine activity at the tissue level.43 The half-life of IFNα is between 10 and 20 minutes, and low levels are found in the circulation as a result of dilution in bodily fluids, diffusion from plasma to extracellular fluid compartments, binding to cell surface receptors and rapid catabolism by the kidneys, liver, muscle, and lungs.44 This means that only a small amount of IFNα is in steady state in the circulation. Detectable circulating levels reflect increased tissue activity and, therefore, serum levels can only provide a representative and relative readout of the dynamic ongoing homeostatic balance between production and excretion.
The ability for extrapulmonary vascular cells to sense IFNs is as yet not fully characterized. We know that endothelial cells45 and vascular smooth muscle cells46 from systemic vessels also sense IFNs and, although these observations illustrate that IFNs are not selective for pulmonary vascular cells in healthy individuals, it is known that vessels that are primed for proliferation and remodeling (such as is the case in PAH) will respond far more profoundly to inflammatory stimuli.47 Furthermore, the pulmonary vasculature is a low-pressure system and, therefore, small changes to vascular resistance carry far greater clinical significance than they would do systemically.
In this study, we found clear evidence that IFN, IFNAR1, and downstream mediators are increased in PAH and correlate strongly with disease severity and established biomarkers of disease. In line with these observations, we also found that endothelial cells grown from the blood (BOECs) of patients with PAH have an increased sensitivity to exogenous IFNα than cells from control donors. It was interesting to note that patients with IPAH and patients with SSc-PAH with a histological predominance of arterial disease express far higher amounts of lung IFNAR1 than SSc-PAH patients with a greater burden of venous disease. By discovering this heterogeneity of IFNAR1 protein expression, we may have identified specific groups of patients with PAH where targeting type I IFN in the form of novel therapies might be most successful. Whether or not raised pulmonary vascular IFNAR1 expression is mirrored by serum evidence of IFN activation now requires further investigation. If that proves to be the case, serum IP10, for example, could represent a circulating marker of arterial pathology in SSc-PAH and therefore be a predictor of increased likelihood of response to specific PAH therapies.
To demonstrate a causal relationship for type I IFN in PAH, we employed the use of genetically modified mice lacking a functional type I IFN receptor. We found that type I IFN signaling mediates the deleterious effects of hypoxia on the pulmonary vasculature, on the RV and on increased serum ET-1 levels. As mentioned above, our group has pioneered the concept that ET-1 is an IFN-driven gene, and these data are fully supportive of this. However, to better understand whether the reduction in circulating ET-1 levels was principally because of the amelioration in pulmonary vascular pathology observed or could be ascribed to the specific effects of IFN signaling on ET-1 release, we performed additional experiments where mice were treated with LPS. Here, as predicted, IP10 but not KC release induced by LPS was mediated by IFNAR1. KC is the mouse homolog of human IL-8 and is released by LPS independently of IFN. Importantly LPS-induced ET-1 release, like IP10, was dependent on IFNAR1. In addition to supporting and validating our hypothesis linking type I IFN with PAH, this is the first in vivo demonstration of ET-1 as an IFN dependent gene.
Thus, using several in vitro and in vivo experimental techniques as well as studies in patients, we have demonstrated for the first time that type I IFN is associated with human PAH and mechanistically linked to the development of PAH in mice. We have shown that this link is associated with IP10 and ET-1 and is regulated by the type I IFN receptor, IFNAR1. These findings contribute significantly to our understanding of PAH mechanisms and help to explain why IFN therapies can cause pulmonary vascular pathology, which in extreme cases may lead to PAH. Furthermore, we conclude that modulation of IFN pathways may represent a novel therapeutic target in the treatment of PAH.
Supplementary Material
Online Table I: IP10 release from Blood Outgrowth Endothelial Cells grown from PAH patients
Online Figure I: Response of pulmonary artery smooth muscle cells to types I, II and III IFN with and without TNFα
Online Figure II: Response of human lung microvascular endothelial cells to types I, II and III IFN with and without TNFα
Online Figure III: Response of human lung fibroblasts to types I, II and III IFN with and without TNFα
Online Figure IV: Type I and II IFN receptor gene expression assessed in pulmonary vascular cells and compared to a standard reference point - human hepatocytes
Online Figure V: Response of hepatocytes to types I, II and III IFNs
Online Figure VI: Correlation between IP10 and ET-1 in patients with SSc but no PAH
Online Figure VII: Serum cytokine levels in patients with SSc-PAH, patients with SSc without PAH and healthy controls
Online Figure VIII: Immunohistochemistry for type II and type III IFN receptor expression in SSc-PAH lung
Online Figure IX: ET-1 release from BOECs grown from PAH patients and healthy controls
Online Figure X: Angiogenic functions of BOECs from healthy individuals and patients with PAH
Online Figure XI: Serum IFNλ levels in hypoxic mouse model
Online Figure XII: Further characterisation of hypoxic mouse model of PAH
Online Figure XIII: Response of mouse tissue to human and mouse IFNα
Online Figure XIV: Human pulmonary artery organ culture
Online Figure XV: IFNλ receptor gene expression assessed in pulmonary vascular and epithelial cells and compared to a standard reference point - human hepatocytes
Online Figure XVI: Response of A549 epithelial cells to IFNs – IP10 release
Online Figure XVII: Response of A549 epithelial cells to IFNs – ET-1 release
Online Figure XVIII: Effect of IFNs on Alveolar type I epithelial cells
Online Figure XIX: Effect of IFNs on Beas2B epithelial cells
Novelty and Significance.
What Is Known?
Type I interferon (IFN) is an effective treatment; however, it causes adverse effects in the lung including, in rare cases, severe and sometimes irreversible pulmonary vascular inflammation.
IFN induces several genes including interferon γ inducible protein 10 (IP10) (which is implicated in lung inflammation) and endothelin 1 (which is central to the pathogenesis of pulmonary arterial hypertension [PAH]).
Autoimmune conditions where type I IFN is chronically overproduced such as systemic sclerosis (SSc) show a robust association with PAH but mechanisms are poorly understood.
What New Information Does This Article Contribute?
IFNs activate human lung cells and along with associated downstream targets correlate with known biomarkers and clinical end points of PAH, particularly that associated with SSc.
The type I IFN receptor (IFNAR1) mediates the pathological hallmarks of pulmonary hypertension induced by hypoxia in mice and the induction of endothelin 1 by lipopolysaccharide in vivo is mediated by type I IFN signaling.
Type I IFN receptor expression is increased in defined cases of PAH and the link between type I IFN and PAH extends beyond that associated with SSc having relevance to other forms of the disease including idiopathic (I)PAH.
Type I IFN treatment for hepatitis C causes detectable changes in the lung in ≈48% of patients. In some individuals, this can manifest as severe and irreversible pulmonary hypertension. Conditions such as SSc, which are strongly associated with PAH, are typified by chronically increased endogenous type I IFN production. However, the mechanisms linking IFN to PAH are poorly understood. Here, we demonstrate the involvement of type I IFN in the pathogenesis of PAH. This may be mediated by specific IFN-stimulated genes, including interferon γ inducible protein 10 (IP10) and endothelin 1, via the type I IFN receptor IFNAR1. We found that the relationship between IFN and PAH may extend beyond SSc-associated disease and may have broader relevance to other etiologies including IPAH. Our findings significantly advance the scientific understanding of the relationship between dysregulated innate immunity and pulmonary vascular pathology and suggest that modulation of type I IFN pathways could be a novel strategy for the treatment of PAH.
Acknowledgments
We acknowledge Neil Galloway-Phillipps who assisted with cell culture work and Nicolas Raymond who assisted with immunohistochemistry. Dr Stephanie MacNeill provided statistical advice.
Sources of Funding P.M. George is supported by a Medical Research Council clinical research training fellowship. M. Southwood is funded by a National Institute for Health Research Healthcare Scientist Fellowship. The French pulmonary hypertension pharmacovigilance network VIGIAPATH is chaired by M. Humbert and supported by the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM). The study was supported by the National Institute for Health Research (NIHR) Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College.
Nonstandard Abbreviations and Acronyms
- BOEC
blood outgrowth endothelial cell
- ET-1
endothelin 1
- HPASMC
human pulmonary artery smooth muscle cell
- IP10
interferon γ inducible protein 10
- PAH
pulmonary arterial hypertension
- RV
right ventricular
- SSc
systemic sclerosis
- TNF
tumor necrosis factor
Footnotes
Disclosures P.M. George reports personal fees from GlaxoSmithKline (GSK) and educational funding from Actelion Pharmaceuticals. P. Dorfmuller reports personal fees from Actelion Pharmaceuticals. B.E. Schreiber reports personal fees from GSK, Actelion, Pfizer, and Eli Lily. S.J. Wort reports personal fees from Actelion Pharmaceuticals, grants from Bayer Pharmaceuticals and personal fees from GSK. N.W. Morrell reports grants from Novartis plc. M. Humbert reports personal fees from Actelion, Bayer, GSK, Novartis, Pfizer, and United Therapeutics. J.A. Mitchell reports grants and personal fees from Actelion, personal fees from United Therapeutics, grants and personal fees from Roche. The other authors report no conflicts.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.114.302221/-/DC1.
References
- 1.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–1565. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 4.George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012;135:44–53. doi: 10.1016/j.pharmthera.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Al-Zahrani H, Gupta V, Minden MD, Messner HA, Lipton JH. Vascular events associated with alpha interferon therapy. Leuk Lymphoma. 2003;44:471–475. doi: 10.1080/1042819021000055066. [DOI] [PubMed] [Google Scholar]
- 6.Jochmann N, Kiecker F, Borges AC, Hofmann MA, Eddicks S, Sterry W, Baumann G, Trefzer U. Long-term therapy of interferon-alpha induced pulmonary arterial hypertension with different PDE-5 inhibitors: a case report. Cardiovasc Ultrasound. 2005;3:26. doi: 10.1186/1476-7120-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledinek AH, Jazbec SS, Drinovec I, Rot U. Pulmonary arterial hypertension associated with interferon beta treatment for multiple sclerosis: a case report. Mult Scler. 2009;15:885–886. doi: 10.1177/1352458509104593. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon S, Kaker A, Dosanjh A, Japra D, Vanthiel DH. Irreversible pulmonary hypertension associated with the use of interferon alpha for chronic hepatitis C. Dig Dis Sci. 2010;55:1785–1790. doi: 10.1007/s10620-010-1220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster GR, Zeuzem S, Pianko S, Sarin SK, Piratvisuth T, Shah S, Andreone P, Sood A, Chuang WL, Lee CM, George J, Gould M, Flisiak R, Jacobson IM, Komolmit P, Thongsawat S, Tanwandee T, Rasenack J, Sola R, Messina I, Yin Y, Cammarata S, Feutren G, Brown KK. Decline in pulmonary function during chronic hepatitis c virus therapy with modified interferon alfa and ribavirin. J Viral Hepat. 2013;20:e115–e123. doi: 10.1111/jvh.12020. [DOI] [PubMed] [Google Scholar]
- 10.George PM, Mitchell JA. Decline in pulmonary function during chronic hepatitis c virus therapy with modified interferon alfa and ribavirin therapy. J Viral Hepat. 2013;20:592. doi: 10.1111/jvh.12020. [DOI] [PubMed] [Google Scholar]
- 11.George PM, Cunningham ME, Galloway-Phillipps N, Badiger R, Alazawi W, Foster GR, Mitchell JA. Endothelin-1 as a mediator and potential biomarker for interferon induced pulmonary toxicity. Pulm Circ. 2012;2:501–504. doi: 10.4103/2045-8932.105039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods M, Mitchell JA, Wood EG, Barker S, Walcot NR, Rees GM, Warner TD. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902–909. [PubMed] [Google Scholar]
- 13.Wort SJ, Ito M, Chou PC, Mc Master SK, Badiger R, Jazrawi E, de Souza P, Evans TW, Mitchell JA, Pinhu L, Ito K, Adcock IM. Synergistic induction of endothelin-1 by tumor necrosis factor alpha and interferon gamma is due to enhanced NF-kappaB binding and histone acetylation at specific kappaB sites. J Biol Chem. 2009;284:24297–24305. doi: 10.1074/jbc.M109.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 15.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 16.Young RH, Mark GJ. Pulmonary vascular changes in scleroderma. Am J Med. 1978;64:998–1004. doi: 10.1016/0002-9343(78)90455-2. [DOI] [PubMed] [Google Scholar]
- 17.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eloranta ML, Franck-Larsson K, Lövgren T, Kalamajski S, Rönnblom A, Rubin K, Alm GV, Rönnblom L. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69:1396–1402. doi: 10.1136/ard.2009.121400. [DOI] [PubMed] [Google Scholar]
- 19.Christmann RB, Hayes E, Pendergrass S, Padilla C, Farina G, Affandi AJ, Whitfield ML, Farber HW, Lafyatis R. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum. 2011;63:1718–1728. doi: 10.1002/art.30318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachulla E, Coghlan JG. A new era in the management of pulmonary arterial hypertension related to scleroderma: endothelin receptor antagonism. Ann Rheum Dis. 2004;63:1009–1014. doi: 10.1136/ard.2003.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, Moore BB, Kuziel WA, Liu C, Cooke KR. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173:2050–2059. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 25.Starke RD, Paschalaki KE, Dyer CE, Harrison-Lavoie KJ, Cutler JA, McKinnon TA, Millar CM, Cutler DF, Laffan MA, Randi AM. Cellular and molecular basis of von Willebrand disease: studies on blood outgrowth endothelial cells. Blood. 2013;121:2773–2784. doi: 10.1182/blood-2012-06-435727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 27.Badiger R, Mitchell JA, Gashaw H, Galloway-Phillipps NA, Foser S, Tatsch F, Singer T, Hansel TT, Manigold T. Effect of different interferonα2 preparations on IP10 and ET-1 release from human lung cells. PLoS One. 2012;7:e46779. doi: 10.1371/journal.pone.0046779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wort SJ, Mitchell JA, Woods M, Evans TW, Warner TD. The prostacyclin-mimetic cicaprost inhibits endogenous endothelin-1 release from human pulmonary artery smooth muscle cells. J Cardiovasc Pharmacol. 2000;36:S410–S413. doi: 10.1097/00005344-200036051-00120. [DOI] [PubMed] [Google Scholar]
- 29.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 30.Pendergrass SA, Hayes E, Farina G, Lemaire R, Farber HW, Whitfield ML, Lafyatis R. Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLoS One. 2010;5:e12106. doi: 10.1371/journal.pone.0012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorfmüller P, Humbert M, Perros F, Sanchez O, Simonneau G, Müller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Overbeek MJ, Vonk MC, Boonstra A, Voskuyl AE, Vonk-Noordegraaf A, Smit EF, Dijkmans BA, Postmus PE, Mooi WJ, Heijdra Y, Grünberg K. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34:371–379. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 33.de Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, Zaker-Tabrizi L, Fung KY, Forster SC, Beddoe T, Reid HH, Rossjohn J, Hertzog PJ. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14:901–907. doi: 10.1038/ni.2667. [DOI] [PubMed] [Google Scholar]
- 34.Tsushima K, Osawa T, Yanai H, Nakajima A, Takaoka A, Manabe I, Ohba Y, Imai Y, Taniguchi T, Nagai R. IRF3 regulates cardiac fibrosis but not hypertrophy in mice during angiotensin II-induced hypertension. FASEB J. 2011;25:1531–1543. doi: 10.1096/fj.10-174615. [DOI] [PubMed] [Google Scholar]
- 35.Shindo T, Kurihara H, Kurihara Y, Morita H, Yazaki Y. Upregulation of endothelin-1 and adrenomedullin gene expression in the mouse endotoxin shock model. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S541–S544. doi: 10.1097/00005344-199800001-00156. [DOI] [PubMed] [Google Scholar]
- 36.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 38.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 39.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabini D, Nagaraj C, Stacher E, Lang IM, Nierlich P, Klepetko W, Heinemann A, Olschewski H, Bálint Z, Olschewski A. Angiostatic factors in the pulmonary endarterectomy material from chronic thromboembolic pulmonary hypertension patients cause endothelial dysfunction. PLoS One. 2012;7:e43793. doi: 10.1371/journal.pone.0043793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 42.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 43.Prinz M, Schmidt H, Mildner A, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Bocci V. Pharmacokinetic studies of interferons. Pharmacol Ther. 1981;13:421–440. doi: 10.1016/0163-7258(81)90023-1. [DOI] [PubMed] [Google Scholar]
- 45.Indraccolo S, Pfeffer U, Minuzzo S, Esposito G, Roni V, Mandruzzato S, Ferrari N, Anfosso L, Dell’Eva R, Noonan DM, Chieco-Bianchi L, Albini A, Amadori A. Identification of genes selectively regulated by IFNs in endothelial cells. J Immunol. 2007;178:1122–1135. doi: 10.4049/jimmunol.178.2.1122. [DOI] [PubMed] [Google Scholar]
- 46.Woods M, Bishop-Bailey D, Pepper JR, Evans TW, Mitchell JA, Warner TD. Cytokine and lipopolysaccharide stimulation of endothelin-1 release from human internal mammary artery and saphenous vein smooth-muscle cells. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S348–S350. doi: 10.1097/00005344-199800001-00097. [DOI] [PubMed] [Google Scholar]
- 47.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table I: IP10 release from Blood Outgrowth Endothelial Cells grown from PAH patients
Online Figure I: Response of pulmonary artery smooth muscle cells to types I, II and III IFN with and without TNFα
Online Figure II: Response of human lung microvascular endothelial cells to types I, II and III IFN with and without TNFα
Online Figure III: Response of human lung fibroblasts to types I, II and III IFN with and without TNFα
Online Figure IV: Type I and II IFN receptor gene expression assessed in pulmonary vascular cells and compared to a standard reference point - human hepatocytes
Online Figure V: Response of hepatocytes to types I, II and III IFNs
Online Figure VI: Correlation between IP10 and ET-1 in patients with SSc but no PAH
Online Figure VII: Serum cytokine levels in patients with SSc-PAH, patients with SSc without PAH and healthy controls
Online Figure VIII: Immunohistochemistry for type II and type III IFN receptor expression in SSc-PAH lung
Online Figure IX: ET-1 release from BOECs grown from PAH patients and healthy controls
Online Figure X: Angiogenic functions of BOECs from healthy individuals and patients with PAH
Online Figure XI: Serum IFNλ levels in hypoxic mouse model
Online Figure XII: Further characterisation of hypoxic mouse model of PAH
Online Figure XIII: Response of mouse tissue to human and mouse IFNα
Online Figure XIV: Human pulmonary artery organ culture
Online Figure XV: IFNλ receptor gene expression assessed in pulmonary vascular and epithelial cells and compared to a standard reference point - human hepatocytes
Online Figure XVI: Response of A549 epithelial cells to IFNs – IP10 release
Online Figure XVII: Response of A549 epithelial cells to IFNs – ET-1 release
Online Figure XVIII: Effect of IFNs on Alveolar type I epithelial cells
Online Figure XIX: Effect of IFNs on Beas2B epithelial cells