Abstract
The Drosophila melanogaster gustatory system consists of several neuronal pathways representing diverse taste modalities. The two predominant modalities are a sweet sensing pathway that mediates attraction, and a bitter sensing pathway that mediates avoidance. A central question is how flies integrate stimuli from these pathways and generate the appropriate behavioral response. We have developed a novel assay for induction of taste memories. We demonstrate that the gustatory response to fructose is suppressed when followed by the presence of bitter quinine. We employ optogenetic neural activation using infrared laser in combination with heat sensitive channel - TRPA1 to precisely activate gustatory neurons. This optogenetic system allows for spatially and temporally controlled activation of distinct neural classes in the gustatory circuit. We directly activated bitter-sensing neurons together with presentation of fructose for remote induction of aversive taste memories. Here we report that activation of bitter-sensing neurons in the proboscis suffices as a conditioning stimulus. Spatially restricted stimulation indicates that the conditioning stimulus is indeed a signal from the bitter neurons in the proboscis and it is independent of postingestive feedback. The coincidence of temporally specific activation of bitter-sensing neurons with fructose presentation is crucial for memory formation, establishing aversive taste learning in Drosophila as associative learning. Taken together, this optogenetic system provides a powerful new tool for interrogation of the central brain circuits that mediate memory formation.
1. Introduction
Distinct subsets of Drosophila gustatory receptor neurons are capable of detecting sweet or bitter stimuli (Dethier and Goldrich-Rachman, 1976, Meunier et al., 2000, Hiroi et al., 2002, Meunier et al, 2003, Hiroi et al., 2004). Sugar-sensing gustatory neurons express gustatory receptors (GRs) that detect nutritious substances and mediate attraction (Thorne et al., 2004, Wang et al., 2004, Wisotsky et al., 2011). Stimulation of sugar-sensing neurons induces the appetitive response of proboscis extension reflex (PER) (Marella et al., 2006). Bitter-sensing neurons express a larger group of GRs that sense noxious chemicals and mediate avoidance (Kim et al., 2010, Weiss et al., 2011). Stimulation of these neurons does not elicit PER and inhibits sugar stimulation-induced PER, suggesting bitter sensing GRs are hard-wired to inhibit feeding (Meunier et al, 2003, Marella et al., 2006). Flies express gustatory receptors in both the tarsi (feet) and the proboscis (mouth), and these two organs serve distinct functions in taste and foraging behavior (Dethier and Goldrich-Rachman, 1976, Stocker and Schorderet, 1981, Rajashekhar and Singh, 1994, Pollack and Balakrishnan, 1997, Singh, 1997, Mitchell et al, 1999). Neurons of the two different systems from both organs project to non-overlapping regions in subesophageal ganglion (SOG) in the brain (Miyazaki and Ito, 2010). Yet, flies can integrate the opposing gustatory signals to generate the appropriate behavior (Marella et al., 2006). Drosophila conditioning studies predominantly employ inter modality, e.g. odor and electric shock (Pitman et al., 2009). In taste conditioning, flies are capable of learning to pair gustatory stimuli with aversive heat reinforcement (Masek and Scott, 2010), or use the gustatory stimulus as the reinforcement (Tempel et al, 1983) but their ability to learn to pair two gustatory cues of the same modality remains minimally studied (Bouhouche A, 1995). We have developed a novel assay to determine how the Drosophila gustatory system is capable of intra-modality behavioral plasticity.
Extensive genetics and amenability to light and temperature-induced modulation of neural activity makes Drosophila a powerful system for analysis of the neural circuitry regulating behavior (Claridge-Chang et al., 2009, Krashes et al., 2009, Pulver et al., 2009). Activation of specific modulatory neurons through ectopic expression of the thermosensitive cation channel Transient Receptor Potential A1 (TRPA1) is sufficient to activate motor and modulatory taste neurons (Gordon and Scott, 2009, Marella et al., 2012). Furthermore, inducible neural activation by temperature-shift or by the light activated channel channelrhodopsin can substitute for reinforcing stimuli during olfactory conditioning (Schroll et al., 2006, Claridge-Chang et al., 2009, Krashes et al., 2009, Aso et al., 2010, Liu et al., 2012). In this study we employ a focused infrared (IR) laser beam to activate dTRPA1-expressing neurons in a spatially and temporally specific manner. We demonstrate that our IR-laser activation paradigm is capable of activating distinct subpopulations of neurons and provides a novel method for interrogating the neural circuitry underlying Drosophila memory.
2. Experimental procedures
Experimental Animals
Drosophila stocks were maintained on standard cornmeal/agar/molasses medium at 25ºC in a LD incubator with 12:12 light/dark cycle. The following lines were used: Gr5a-GAL4 (Chyb et al., 2003), Gr64f-GAL4 (Dahanukar et al., 2007), Gr66a-GAL4 (Dunipace et al, 2001), w; UAS-dTRPA1 (Hamada et al., 2008), w; ; TH-GAL4 (Friggi-Grelin et al, 2003), w; ; DDC-GAL4 (Li et al., 2000), w; TDC2-GAL4 (Cole et al., 2005). W1118 and Canton-S were used as wild type control.
Optogenetic setup
We used infrared (IR, 808nm) high power laser diode module (type RLDB808-150-3, Roithner Lasertechnik GmbH, http://www.roithner-laser.com) with maximal output power: 150mW, operating voltage: 3V DC, operating current: ≤ 250 mA, divergence: 0.3mrad, with focusable 4 element glass lens (both sides AR coated) and output aperture: Ø 7mm. The module was positioned ~8cm from the fly. A camera with IR-sensitive CMOS sensor from Microsoft® LifeCam HD-3000 Web Camera (Microsoft Corporation) was fitted with inverted 50mm f:1.8 MD Minolta lens (Konica Minolta Holdings, Inc.) on an 8cm extension. The camera was placed 16cm from the fly with working distance of 8 cm. The image of the fly and the laser beam were streamed to computer (15fps) and displayed on screen using AMCap 9.2 software (http://noeld.com). This image was used to focus the laser beam on a spot placed in the center of the image and in the focus of the camera lens. The laser beam size was adjusted by the module lens down to 1mm2. The entire setup was attached to a 3-way micromanipulator to simultaneously positioning the laser beam and the focus of the laser on the fly. The laser module was connected to a custom made voltage controller (MPI Martinsried, Germany) with adjustable voltage range of 0–3VDC. A low energy setting (< 1V) was used for precise focusing and positioning the laser beam on the fly prior to the experiment. The laser was then switched off and power was set to the level used during the experiments. The laser was activated by foot switch for 0.5–5s depending on the experiment as described in the Results.
Behavioral experiments
One week-old mated females were collected and placed on fresh food for 24hrs, then starved for 24hrs in food-vials on wet Kimwipe paper. Flies were later anaesthetized on CO2 pads, glued with nail polish to dorsal side of thorax on a microscopy slide and allowed 3–6 hrs recovery in a box with wet Kimwipe paper prior to experiments. For experiments, the slide was mounted vertically under the dissecting microscope (LEICA) and PER was observed.
PER induction was performed as described previously (Wang et al., 2004). Briefly, flies were satiated with water before and during the experiment. Flies that did not initially water satiate within 5mins were excluded from conditioning studies. A 1ml syringe (Tuberculine, Becton Dickinson & Comp) was used for tastant presentation. We used purified water, 10mM fructose (Sigma), 100mMtsucrose (Merck) or 10mM quinine (Sigma) solutions. Each fly was given 10mM fructose on their tarsi three times with 10s inter-trial interval and the number of full proboscis extensions was recorded (Wang et al, 2004, Chabaud et al., 2006). For taste suppression experiments, a droplet of 10mM quinine was placed on the extended proboscis and flies were allowed to drink it for up to 2s or until they retracted their proboscis. After each session, tarsi and proboscis were washed with water and flies were allowed to drink to satiation. At the end of each experiment, flies were given 100mM sucrose to check retained ability for PER and non responders were excluded.
Inducible activation
Flies were prepared as described above for behavioral experiments. Each Gal4 line was crossed to UAS-dTRPA1 or control flies (w1118). Flies were water-satiated and the laser beam was focused and placed on antennae, head, neck, thorax, abdomen or the wing base on the mesothorax depending on experiments as described in the text. The laser was adjusted from 1.7-2.2V depending on the experiment. The PER response to laser stimulation was observed through the dissection microscope and full proboscis extensions were recorded. For learning experiments, flies were stimulated with 10mM fructose on tarsi and then stimulated with a laser focused on the head and proboscis area. The laser was activated every time fructose was presented during training, irrespective of proboscis extension to provide each fly with the same amount of reinforcement. The total PER number was recorded.
Statistics
Statistical analyses were performed using InStat software (GraphPad Software Inc.). 20–25 flies were tested for each experiment. Each fly was sampled three times with the same stimulus during pretest, training (three in total) or test. The response was binary (PER yes/no), and these three responses were pooled for values ranging from 0 to 3. Most tested groups violated the assumption of the normal distribution. Therefore, all the data were analyzed with non-parametric statistics. Kruskal-Wallis test (nonparametric ANOVA) was performed on the raw data from single flies and Dunn’s Multiple Comparisons test was used to compare different genotypes. Wilcoxon signed rank test (non-parametric) with two-tailed P value was used to test learning significance on single groups. In figures, graphs bars are mean values and error bars are standard error of the mean. P-values in figures are shown as * p < 0.05, ** p < 0005, ***p < 0.001.
3. Results
IR-laser system for TRPA1 activation
We have designed a novel IR-laser-induced neural activating system that is capable of targeting distinct populations of TRPA1 expressing neurons with acute temporal and spatial specificity (Fig. 1). Briefly, a 150 mW IR-laser is connected to a voltage controller for laser power adjustment. The laser module is equipped with a lens used for focusing the IR-beam in order to change the area of activation and the intensity of the stimulation. The laser is attached to a micromanipulator and is coupled to an IR-sensitive camera for targeting the laser beam to specific body regions. The laser is aligned to the target at a low energy setting (< 1V) sufficient for visualization with the IR-sensitive camera, but insufficient to activate TRPA1. The laser power is then increased during the experimental protocol. The IR-laser is triggered by a control-switch and coupled with LEDs for signaling the laser activation.
Figure 1. Diagram of paradigm used for IR-laser activation.
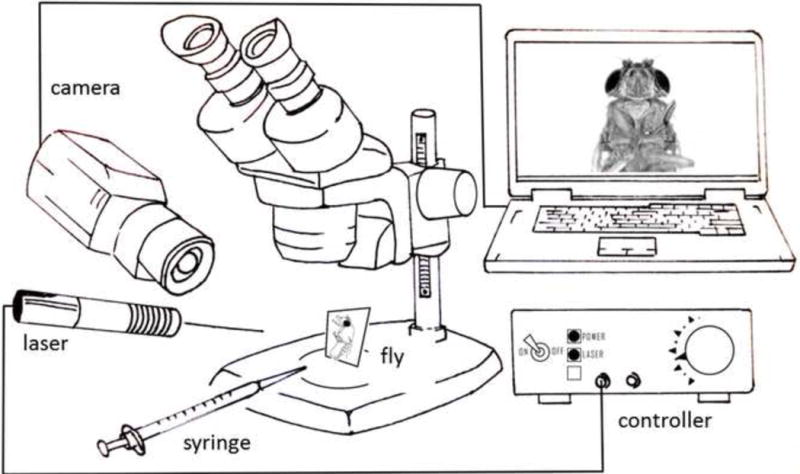
A fly is immobilized on a microscopy slide with its tarsi and proboscis accessible. A 1ml syringe containing water, fructose or quinine solutions is used to apply tastant to the tarsi or the proboscis. A 150mW infrared (IR) laser module attached to a custom-made voltage controller is targeted to a specific body region. An IR-sensitive camera is used for laser targeting displaying fly and laser dot on the computer screen. Tastant delivery to the fly and proboscis extension are observed through the dissection microscope.
To determine whether IR activation of gustatory neurons alone is sufficient to trigger PER, we expressed TRPA1 in sugar-sensing neurons using two independent Gal4 lines, Gr5a-GAL4 or Gr64f-GAL4, that reflect the expression of sweet-sensing gustatory receptors (Dahanukar et al., 2001, Dunipace et al., 2001). As a control, we expressed TRPA1 in bitter-sensing neurons under the control of Gr66a-GAL4 that reflect the expression of caffeine receptor (Jiao et al., 2007). Flies were mounted on the dorsal side of thorax and the laser was targeted to the ventral side of the head, the thorax, or the abdomen. The laser beam was focused on the body surface with an area of 1mm2 and activation lasted for 1s (Fig. 2A). Activation of both Gr5a and Gr64f neurons in the head or thorax resulted in significant PER, whereas targeting the laser on the abdomen did not elicit any PER (Fig. 2B). We also expressed another heat sensitive channel (TRPV1) in Gr5a neurons and obtained comparable results (data not shown). Activating bitter-sensing neurons did not lead to PER irrespective of the targeted body region (Fig. 2B). Taken together, these results indicate that the IR-laser is capable of activating subpopulations of gustatory neurons.
Figure 2. Activation of sensory, modulatory, and motor neurons with IR-laser.
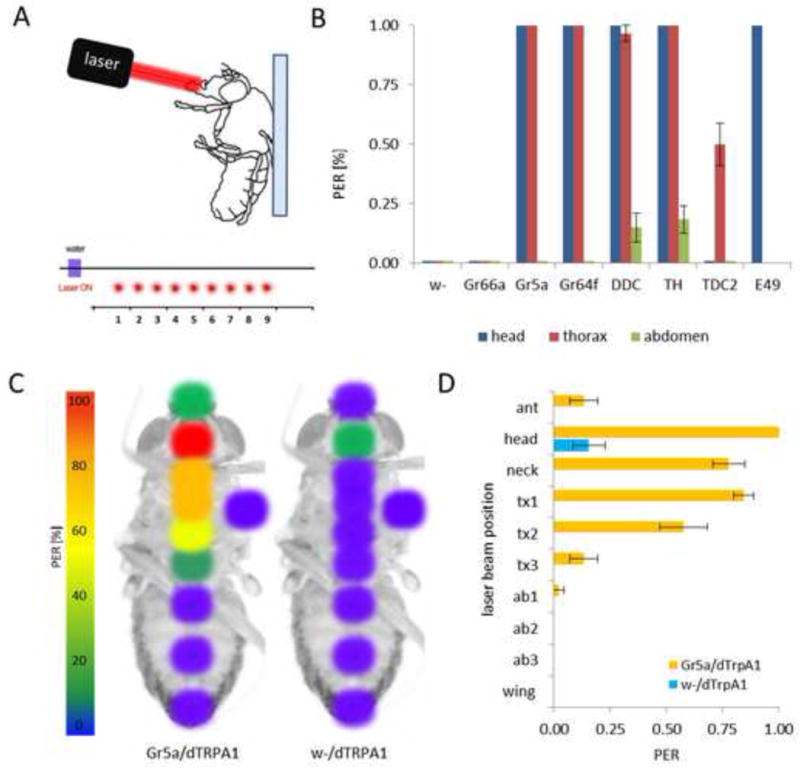
A) For IR-laser-induced induction of proboscis extension reflex (PER), a fly is glued on the dorsal side of the thorax to a slide and the laser beam is targeted on the ventral side. The laser is focused to a specific body region and activated three times with 5s intervals between activations. B) Flies harboring sensory (Gr66a, Gr5a and Gr64f), dopaminergic (TH and DDC), octopaminergic (TDC2) or motor (E49) neurons Gal4 driving dTRPA1 were stimulated with the laser targeted to the proboscis (blue), ventral thorax (red), or ventral abdomen (green). A laser activation area of 1mm2 was stimulated for 1s. The response is displayed as percentage of PER response following the stimulation. C) Fine resolution of laser activation of sugar-sensing neurons. Positions of the laser beam on ventral side of the fly are shown. The color-coding shows the probability of laser-elicited PER after stimulation at the respective site (warmer colors means higher response). Left is fly expressing dTRPA1 in sugar neurons (Gr5a/dTRPA1) and on the right is control fly (w-/dTRPA1). D) Laser stimulation on head, neck, first and second regions on thorax induced significant PER response in flies expressing dTRPA1 in sugar-sensing neurons (p < 0.001). Stimulation of the other regions did not result in significant responses. Control flies with dTRPA1 alone did not significantly respond to any laser stimulation (p > 0.05).
The neurotransmitters dopamine and octopamine regulate appetitive and aversive memories as well as feeding behavior (Schwaerzel et al., 2003, Krashes et al, 2009, Aso et al, 2010, Marella et al., 2012). To determine whether the IR laser is capable of activating central brain circuits, we measured the PER of animals expressing TRPA1 in dopaminergic (TH-GAL4 and DDC-Gal4) or octopaminergic neurons (TDC2-GAL4) (Li et al., 2000, Friggi-Grelin et al., 2003, Cole et al., 2005, Yarali and Gerber, 2010). Targeting the head and thorax region of flies expressing TRPA1 in dopaminergic neurons induced significant PER, whereas flies expressing TRPA1 in octopaminergic neurons induced PER only when the thorax was targeted (Fig. 2B).
The E-49 GAL4 line expresses in proboscis motor neurons that mediate PER (Gordon and Scott, 2009). The direct activation of these neurons alone is sufficient to elicit PER (Gordon and Scott, 2009). IR laser stimulation of these neurons induced robust PER (Fig. 2B), confirming that laser induce activation of TRPA1 neurons functions in gustatory motor neurons. Taken together, these findings indicate that IR-laser activation of sensory, modulatory, and/or motor neurons induces a rapid PER. Therefore, optogenetic modulation of neural activity is capable of spatially restricted activation of central and peripheral cells that confer feeding.
Spatially restricted activation of neurons in the taste circuit
To determine the spatial resolution of IR-laser activation, we selectively activated Gr5a neurons in specific body regions and observed resulting PER. The laser beam was focused to a 1mm2 point in nine distinct locations over the longitudinal axis on the ventral side of the fly’s body. Activation was directed to the antennae, proboscis, ‘neck’, three spots on thorax, and three spots on the abdomen. We also targeted the base of left wing because gustatory receptors are expressed in the wing margins (Thorne et al., 2004) (Fig. 2C). All stimulations lasted for 0.5s. Only stimulation of the proboscis yielded 100% PER (Fig. 2D). Focusing on the thorax also resulted in significant PER responses (> 50%). Lengthening the stimulation time to 1s resulted in 100% PER, indicating that activation of thoracic Gr5a-neurons is sufficient for induction of PER (data not shown). Targeting the antennae did not induce significant PER. Next, we asked if the stimulation of thoracic regions was eliciting PER by activating the neurons in the thorax, or indirectly through activation of SOG We stimulated the left wing-base that is equal distance to SOG as the stimulated sites on thorax but we did not observe any PER. This implies that activation of TRPA1 neurons requires direct targeting with the IR laser. This experiment also revealed that the stimulation of Gr5a neurons in the wing did not induce a feeding response, suggesting that these sugar-sensing neurons are not connected to the PER circuit. Gr5a is expressed in the abdomen together with other sugar-sensing GRs where they have chemosensory roles in the intestine to regulate physiological functions such as food uptake, nutrient absorption, or sugar homeostasis (Park and Kwon, 2011). We did not observe any PER response following stimulation of the abdomen, even with extended exposure times of up to 5s (data not shown). Targeting flies harboring the TRPA1 transgene in the absence of a GAL4 driver did not induce significant PER, confirming that the observed PER was specifically due to activation of Gr5a neurons. Taken together, these findings reveal that laser-induced activation of TRPA1-expressing gustatory neurons allows for localized activation of neuronal populations.
Quinine-mediated conditioned gustatory suppression
We employed a modified taste-conditioning paradigm that trained flies to inhibit feeding in response to sweet fructose application to the tarsi (Masek and Scott, 2010) (Fig. 3A). Briefly, mounted flies were tested with repeated stimulation of 10mM fructose leading to a robust PER. Fructose presentation to the tarsi was then paired with the application of bitter quinine to an extended proboscis. Tarsi were touched three times with fructose, and for every proboscis extension following tarsal stimulation, flies were given quinine to drink. After the session, tarsi and proboscis were washed and the fly was kept satiated with water. This protocol was repeated three times. During testing, fructose was presented to tarsi in the absence of quinine and PER was measured. This training protocol for quinine-mediated conditioned gustatory suppression inhibited the PER response to fructose (Fig. 3A). A single pairing of the aversive quinine resulted in a significant PER reduction to attractive fructose (Fig. 3B). We tested flies’ response to sucrose at the end of each experiment to assure that the general PER response was retained and the PER suppression was specific to fructose. This training protocol significantly suppressed PER for 30 min after the initial test, consistent with induction of short-term memories (Heisenberg, 2003, Keene and Waddell, 2007, Davis, 2011) (Fig. 3C). These results show that flies are capable of learning to pair two stimuli within the same sensory modality.
Figure 3. Quinine-mediated conditioned gustatory suppression.
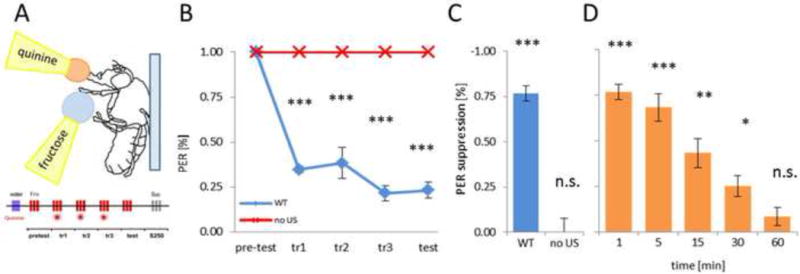
A) Drop of 10mM fructose is applied on tarsi three times during the pretest. During training, fructose is applied on tarsi and after PER response, 10mM quinine is applied on the extended proboscis, and flies are allowed to drink up to 2s. During testing, only fructose is applied on tarsi and resulting PER is measured. B) Fructose-induced PER was significantly reduced in quinine-conditioned flies after the initial training session (p < 0.001). C) PER suppression during test phase after pairing fructose with quinine or without quinine revealed a significant PER suppression only when quinine was presented (p < 0.001). D) Quantification of PER suppression was measured for intervals from 1 to 60min following training. Flies showed significant PER suppression lasting at least 30min, indicating establishment of short-term memory (p < 0.05). P-values are displayed as * p < 0.05, ** p < 0005, *** p < 0.001.
Direct activation of bitter-sensing neurons is sufficient for associative learning
The induction of conditioned memories requires simultaneous presentation of two paired stimuli. Unfortunately, during gustatory conditioning, the residues of tastants remain on the tarsi, at the proboscis or ingested even after the intended stimulation has ended. To overcome this limitation, we employed direct laser activation of bitter-sensing neurons to induce gustatory memories. In quinine-mediated paradigm, flies reduced their response to fructose after the sweet stimulus was paired with the intake of quinine. To determine whether activation of bitter-sensing sensory neurons in the absence of ingestion is sufficient for the induction of aversive taste memory, the perception of bitter tastant was initiated by laser-induced activation of Gr66a-neurons. Gr66a is expressed in the majority of bitter-sensing neurons (Moon et al., 2006, Weiss et al., 2011) (Moon et al., 2006, Weiss et al, 2011) (Moon et al., 2006, Weiss et al., 2011), and these neurons are involved in perception of all tested bitter substances (Marella et al., 2006, Weiss et al, 2011). This technique substitutes inducible neural activation of bitter-tasting neurons expressing TRPA1 for the presence of quinine solution on the proboscis.
The IR-laser beam was focused on the proboscis to selectively activate Gr66a-expressing neurons in the head. Tarsi were stimulated with 10mM fructose while the bitter-sensing neurons were simultaneously activated for 0.5s with the laser (Fig. 4A). Laser stimulation significantly reduced PER response to fructose stimulation in flies expressing TRPA1 under the control of Gr66a-Gal4 (Fig. 4B,C). A single training session was sufficient to reduce response to fructose, leading to a significant PER suppression (Fig. 4B). Maximal suppression was achieved after second training session (Fig. 4B). Control flies harboring either the TRPA1 or Gr66a-Gal4 transgenes alone did not reduce their PER response, confirming that the PER suppression observed after the laser stimulation is not caused by heating the fly but by direct activation of the heat sensitive channels. Also targeting the laser on the abdomen instead on the proboscis did not lead to PER suppression (Fig. 4C) indicating that heat from the laser itself is not sufficient to induce memory. To test whether coincidence of fructose and bitter-sensing neurons activation is necessary for establishing the aversive taste memory, we presented the two stimuli unpaired. Activating the laser prior to tarsal stimulation with fructose did not cause any PER suppression, confirming the requirement for simultaneous pairing of conditioning stimuli during the memory formation (Fig. 4B,C).
Figure 4. IR-laser mediated conditioned gustatory suppression.
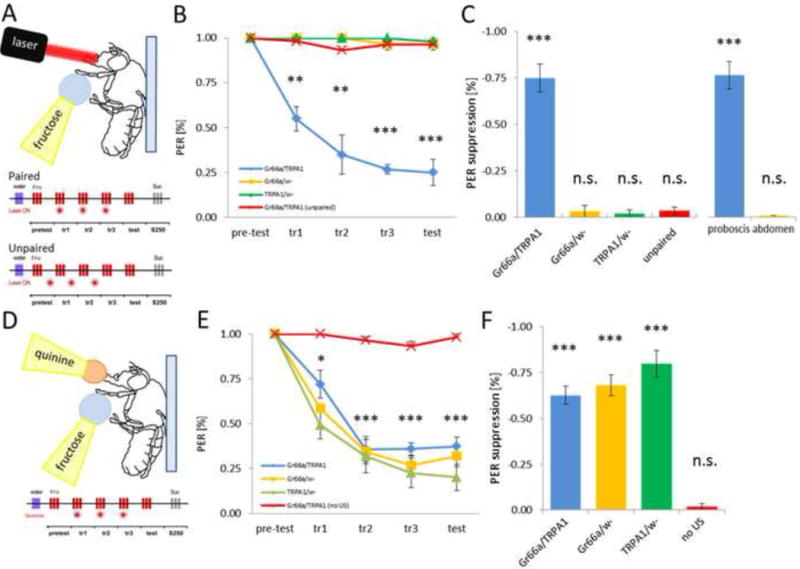
A) For optogenetic conditioning, a fly is mounted on a glass slide and stimulated with 10mM fructose applied to tarsi. The IR-laser is focused on the proboscis area and is active for 0.5s. For paired conditioning, laser stimulation is delivered together with fructose stimulation on tarsi. For unpaired conditioning, laser stimulation precedes the onset of fructose stimulation. B) PER of Gr66a/dTRPA1 flies and control flies in response to 10mM fructose was scored before, during and after stimulation with the IR-laser (laser power set to 1.72V). Both groups showed 100% PER in pre-test, but only flies expressing dTRPA1 significantly reduced PER response already after the first training session (p < 0.005) and showed even higher reduction in the test (p < 0.001). Fl ea s with matching genotypes but with laser stimulation preceding fructose presentation (unpaired group) did not change PER response compared to pretest levels. Flies carrying GAL4 or UAS alone and having fructose presentation paired with laser stimulation also did not reduce PER following training. C) Quantification of PER suppression during testing reveals that only flies expressing dTRPPA1 in Gr66a-neurons suppressed PER response when in the paired group (p < 0.001). Unpairing of the IR-laser with fructose did not lead to PER suppression in the same flies. Also targeting the laser beam on abdomen (as opposed to proboscis) did not reduce PER response to fructose. D) Gr66a/dTRPA1 flies and controls were conditioned for quinine-mediated suppression of PER. E) Time course of fructose-elicited tarsal PER response during and after simultaneous application of quinine on proboscis. PER response to fructose was significantly reduced after the initial training session for all genotypes tested (p < 0.05). Flies presented with fructose alone did not show PER suppression during training or test. F) Quantification of PER response during test phase. All genotypes achieved significant PER suppression when paired with quinine (p < 0.05). P-values are displayed as * p < 0.05, ** p < 0005, *** p < 0.001.
To confirm that optogenetic activation of Gr66a-expressing neurons phenocopies quinine-induced stimulation, we tested flies of matching genotypes in quinine-mediated gustatory paradigm (Fig. 4D). Conditioned PER suppression in these flies was indistinguishable from control and wild-type flies (Fig. 4E,F). Only flies offered fructose without the quinine reinforcement initiated PER at the level of untreated flies, ruling out habituation or adaptation to repeated stimulation of fructose as a reason for reduction in PER response (Fig. 4E,F). Therefore, quinine stimulation and optogenetic activation of bitter-tasting neurons appear to have comparable efficacy in inducing PER suppression and acting as the conditioning stimulus in aversive taste memory.
4. Discussion
We have developed a novel memory assay where mounted flies are trained to suppress sugar-induced PER by the simultaneous presentation of bitter quinine to the proboscis. We find that these memories can remain stable for approximately 30 minutes, consistent with the notion that these are protein synthesis independent short-term memories (Margulies et al, 2005, Keene and Waddell, 2007). These memories were induced with three training trials in succession, and it is possible that varying the number or spacing of training trials may result in the induction of longer lasting memories.
Using an IR-laser to activate bitter-sensing neurons appears to mimic natural stimulation of the same neurons with quinine and leads to a comparable taste suppression response. These findings reveal that memories can be induced with a bitter tastant or through optogenetic activation of the bitter sensing neurons. Using this IR-laser paradigm and restricting the stimulation of bitter-sensing neurons to the proboscis revealed that activity of Gr66a neurons in the proboscis alone is sufficient for conditioned gustatory suppression. These findings suggest that Gr66a neurons also convey the aversive signal in the quinine-mediated gustatory conditioning paradigm.
One hallmark of conditioning is the requirement of two stimuli (classical conditioning) or behavior and stimulus (operant conditioning) to be presented simultaneously (Rescorla, 1972, Heisenberg, 2003, Terry, 2006). A confounding effect of applying tastants to tarsi or proboscis is that the stimulation continues even after the tastant stimulus is removed, probably due to traces of the tastant solution being left on the taste sensillae. Furthermore, bitter substances may also be detected post-ingestively by internal sensing organs, such as in crop or gut. These residues on the sensory sensillae and post-ingestive behaviors prevent the full dissociation between conditioning stimuli. The use of an IR-laser allowed for quick activation of the taste neurons and did not indicate any aftereffect after switching off. The unpairing of fructose presentation and the laser-activation of Gr66a neurons failed to cause PER suppression, arguing that the laser-mediated conditioning produces associative learning (Fig. 4B,C).
Pairing the aversive bitter reinforcing stimulus with presentation of fructose can lead either to classical conditioning where flies associate the sensory perception of fructose or to operant conditioning where flies associate the proboscis extension (behavior) with the presence of the reinforcement (bitter). In our experiments, flies suppressed PER elicited by fructose stimulation. The PER following sucrose stimulation remained unchanged, indicating a form of classical conditioning. The separation of gustatory sensory information and proboscis extension could also be achieved through our inducible system by directly activating modulatory or motor neurons in the PER pathway together with activation of bitter sensing neurons. Operant gustatory conditioning might also play a role and a further work is necessary addressing the role of classical versus operant conditioning in our paradigm.
Current methods to activate TRPA1-expressing neurons require heating the entire animal (Aso et al., 2010). Laser stimulation provides a number of advantages including near immediate on/off kinetics, adjustability of the laser power, and reproducibility of the stimulation strength. Spatially-restricted activation of neurons can be achieved genetically (Simpson, 2009), and the precise laser focusing which allows for stimulation of selected body regions provides improved spatial resolution for targeted neural activation. The area stimulated by the laser beam can be readily adjusted to cover the whole fly or just an area of about 1mm2. With improved lenses, the laser can be focused to an even smaller area, and selective activation of a single sensilla or specific central brain region may be possible.
A number of other optogenetic methods allowing for direct neural activation have been described including ChR2, P2X2 and TRPV1 (Miesenböck, 2011). ChR2-based neural activation is capable of eliciting a PER response (Gordon and Scott, 2009), innate avoidance (Suh et al., 2007), or stimulating a learning circuit (Schroll et al., 2006). P2X2 and TRPV1 have been utilized as artificial photoreceptors, operated optically by releasing extracellular ATP or capsaicin from caged precursors (Zemelman et al, 2003, Lima and Miesenbock, 2005, Zhao et al, 2006, Gilbert et al., 2007). A strength of our method is that it does not require injection or feeding of a substrate required for light activation. Using IR light for neural activation may be advantageous in behavioral studies because it is invisible to flies and in addition, its longer wavelength should provide better tissue penetration than blue light used for activation of ChR2 and P2X2. Finally, this assay is relatively simple to build with minimal cost, making it accessible to study neural circuits and behavior, thus providing an additional tool in the growing optogenetic arsenal of Drosophila.
Future studies using this technique will allow for the precise mapping of the central brain circuits that confer feeding and gustatory memories. For example, probing TDC2-expressing neurons for PER reveals thorax stimulation of octopaminergic neurons induces feeding behavior. Combining our technique with genetic tools available for spatially restricted gene expression, such as TSH-GAL80, which blocks transgene expression selectively in the thorax, will allow for the characterization of complex neural circuitry. IR-laser activation of TRPA1 can also be adapted for activation of other sensory and behavioral circuitry, including olfactory, auditory or visual systems and cleaning, aggression or courtship behavior.
We have developed a system suitable for acute spatial and temporal neural activation using targeted IR-laser beam stimulation and restricted ectopic expression of heat-sensing receptors, either TRPA1 or TRPV1. We confirmed these findings by showing that different sensory and central brain neurons can be activated by this system. We showed that activation of spatially defined body regions result in differential behavioral responses. Additionally, our findings employ precise temporal control of neural activation to demonstrate a gustatory learning paradigm substituting natural bitter stimulus with a virtual bitter percept. This system will allow functional dissection of the neural tissues that regulate associative conditioning behavioral studies precisely manipulating the presence of an unconditioned stimulus.
Highlights.
We have established a single fly conditioned taste aversion paradigm
We have developed an optogenetic method using a focused infrared laser
Laser activation provides high spatial and temporal specificity
We demonstrate activation of different classes of neurons by laser stimulation
Laser stimulation of bitter-sensing neurons expressing TRPA1 induces memory
Acknowledgments
We would like to thank the Tanimoto laboratory for sharing their lab space and providing support and valuable discussion while conducting the research. We would like to thank the workshops of Max-Planck-Istitute, Martinsried, Germany for the help with building the experimental setup and Jens Rister and Robert Renden for helpful discussion and comments on the manuscript. We also thank John Carlson and Ron Tanimoto for generously providing the flies. This work was made possible by NIH grants from the NCRR (5P20RR016464) and the NIGMS (8 P20 GM103440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhouche A EA, Vaysse G, Choulli MK. The role of quinine chlorhydrate in the conditioned inhibition of the tarsal reflex in. Drosophila melanogaster Can J Exp Psychol. 1995;49:9. doi: 10.1037/1196-1961.49.4.520. [DOI] [PubMed] [Google Scholar]
- Chabaud MA, Devaud JM, Pham-Delegue MH, Preat T, Kaiser L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:1335–1348. doi: 10.1007/s00359-006-0160-3. [DOI] [PubMed] [Google Scholar]
- Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei Y-t, Kwon JY, Carlson JR. Two Gr Genes Underlie Sugar Reception in Drosophila. Neuron. 2007:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Review Traces of Drosophila Memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG, Goldrich-Rachman N. Anesthetic stimulation of insect water receptors. Proc Natl Acad Sci USA. 1976;73:3315–3319. doi: 10.1073/pnas.73.9.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Funk K, Dekowski B, Lechler R, Keller S, Mohrlen F, Frings S, Hagen V. Caged capsaicins: New tools for the examination of TRPV1 channels in somatosensory neurons. Chembiochem. 2007;8:89–97. doi: 10.1002/cbic.200600437. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. MUSHROOM BODY MEMOIR: Group 4. 2003:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-poll F, Tanimura T. Differentiated Response to Sugars among Labellar Chemosensilla in Drosophila. Scanning Electron Microscopy. 2002;1018:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Meunier N, Tanimura T. Responding to Sweet-Salty and Bitter Taste in Drosophila ABSTRACT. Journal of Neurobiology. 2004:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nature reviews Neuroscience. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Liu C, Plaçais PY, Yamagata N, Pfeiffer BD, Aso Y, Siwanowicz I, Friedrich AB, Rubin GM, Préat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. doi: 10.1038/nature11304. in press. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Current biology. 2005;CB 15:R700–713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Scott K. Limited taste discrimination in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N, Ferveur JF, Marion-Poll F. Sex-specific non-pheromonal taste receptors in Drosophila. Current biology. 2000;CB 10:1583–1586. doi: 10.1016/s0960-9822(00)00860-5. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars J-P, Tanimura T. Peripheral coding of bitter taste in Drosophila. Journal of neurobiology. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Miesenböck G. Optogenetic control of cells and circuits. Annual review of cell and developmental biology. 2011;27:731–758. doi: 10.1146/annurev-cellbio-100109-104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BK, Itagaki H, Rivet MP. Peripheral and central structures involved in insect gustation. Microscopy research and technique. 1999;47:401–415. doi: 10.1002/(SICI)1097-0029(19991215)47:6<401::AID-JEMT4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Ito K. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. The Journal of comparative neurology. 2010;518:4147–4181. doi: 10.1002/cne.22433. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Current biology. 2006;CB 16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Park J-H, Kwon JY. Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PloS one. 2011;6:e29022. doi: 10.1371/journal.pone.0029022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman JL, DasGupta S, Krashes MJ, Leung B, Perrat PN, Waddell S. There are many ways to train a fly. Fly (Austin) 2009;3:3–9. doi: 10.4161/fly.3.1.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack GS, Balakrishnan R. Taste sensilla of flies: function, central neuronal projections, and development. Microsc Res Tech. 1997;39:532–546. doi: 10.1002/(SICI)1097-0029(19971215)39:6<532::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J Comp Neurol. 1994;349:633–645. doi: 10.1002/cne.903490410. [DOI] [PubMed] [Google Scholar]
- Rescorla RAW AR. A theory of Pavlovian conditioning. Variations in effectiveness of reinforcement and non-reinforcement. In: Black A, Prokasky WF, editors. Classical Conditioning. II. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Current biology. 2006;CB 16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH. Mapping and manipulating neural circuits in the fly brain. Adv Genet. 2009;65:79–143. doi: 10.1016/S0065-2660(09)65003-3. [DOI] [PubMed] [Google Scholar]
- Singh RN. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc Res Tech. 1997;39:547–563. doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–523. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry WS. Learning and Memory: Basic principles, processes, and procedures. Boston: Pearson Education, Inc; 2006. [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H, Building C, Carolina N. Taste Perception and Coding in Drosophila. Current. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss La, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisotsky Z, Medina A, Freeman E, Dahanukar A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nature neuroscience. 2011;14:1534–1541. doi: 10.1038/nn.2944. [DOI] [PubMed] [Google Scholar]
- Yarali A, Gerber B. A Neurogenetic Dissociation between Punishment-, Reward-, and Relief-Learning in Drosophila. Front Behav Neurosci. 2010;4:189. doi: 10.3389/fnbeh.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc Natl Acad Sci USA. 2003;100:1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Gover TD, Muralidharan S, Auston DA, Weinreich D, Kao JP. Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation. Biochemistry. 2006;45:4915–4926. doi: 10.1021/bi052082f. [DOI] [PMC free article] [PubMed] [Google Scholar]


