Summary
Recent studies have revealed remarkable species specificity of the Toll-Like Receptors (TLR) 11 and TLR12, and the Immunity Related GTPase (IRG) proteins that are essential elements for detection and immune control of Toxoplasma gondii in mice, but not in humans. The biological and evolutionary implications of these findings for the T. gondii host-pathogen relationship and for human disease are discussed.
Introduction
Host resistance and parasite virulence travel together through evolutionary time in a dynamic and often unstable equilibrium, each imposing unpredictable and intense selective pressures on the other. As a result, host-parasite relationships become remarkably specialized. General mechanisms of host immunity are now well understood, but for the parasite it is the details that matter, generating refined mechanisms of co-adaptation specific for each host-parasite complex.
Toxoplasma gondii is a ubiquitous protozoan that belongs to the phylum Apicomplexa, and provides an interesting example of parasite-host co-adaptation and successful transmission in nature (Elmore et al., 2010). The T. gondii sexual stages show restricted host specificity for feline species that act as definitive hosts. As a coccidian, what is unique in the T. gondii life cycle is the existence of an intermediate host. Felines are indiscriminate in their diets and the asexual stage of Toxoplasma is notable for its lack of host specificity, infecting hundreds of avian and mammalian species, and thus, favoring parasite spread in nature and transmission to the definitive host. The evolutionary importance of any intermediate host for Toxoplasma is a function of the frequency with which it contributes to the transmission of the parasite, or in other words, is prey for cats. A reasonable thesis is that this role falls particularly (though certainly not exclusively) on the rodents that are thought to be natural intermediate hosts for this parasite. As a result the rodent immune system adapted to better cope with T. gondii infection. By contrast, many vertebrates that are not regularly part of the felines’ food chain are considered accidental intermediate hosts. Humans, at least outside Kruger National Park, are certainly accidental intermediate hosts for Toxoplasma and play little or no part in its natural history and evolution. Thus the human immune system is not under selective pressure from Toxoplasma.
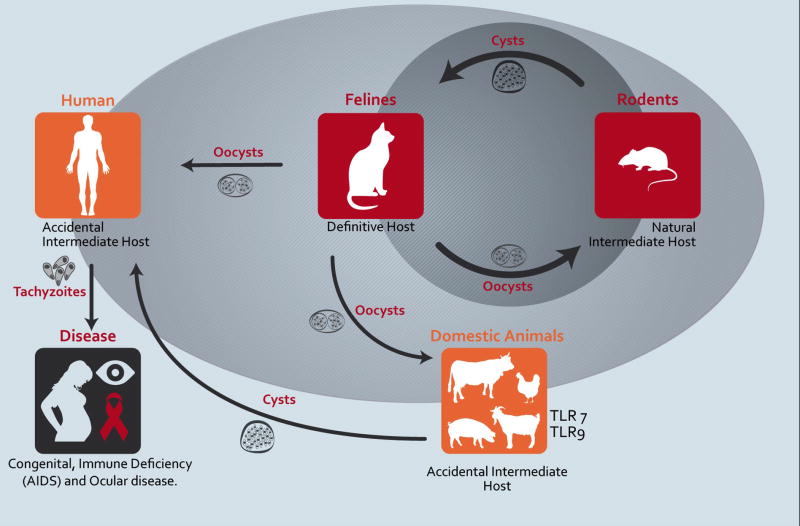
Toxoplasma sexual reproduction within feline gut epithelial cells generates oocysts that become highly infective when shed into the environment. After ingestion by the intermediate host, the parasite transforms into tachyzoites that rapidly multiply by endodyogeny within parasitophorous vacuoles (PV) of a large number of different cell types. If not controlled by the immune system, the tachyzoites cause a systemic and lethal disease. The immunological control of tachyzoites is accompanied by the development of slowly-replicating bradyzoites that persist isolated from the immune system in intracellular cysts typically residing in the central nervous system (CNS) and muscle. The life cycle is completed when an intermediate host infected with tissue cysts is eaten by a feline (Figure 1).
Figure 1. Toxoplasma gondii life cycle and the host specificity of TLR11/TLR12/IRG proteins.
Felines and rodents are the definitive and natural intermediate hosts for T. gondii, respectively. After sexual reproduction in intestinal epithelial cells, the oocysts are formed and shed to the environment in cat feces. Once in the environment the oocysts sporulate and become highly infective to a wide variety of vertebrates, including rodents and accidental intermediate hosts such as humans. The parasite life cycle is completed, when the cat eats its prey, most often small rodents, and become infected by ingestion of the dormant Toxoplasma cysts. While most infections are asymptomatic, toxoplasma infection is a threat when congenitally transmitted to the fetus in immunologically naïve pregnant women or in immunocompromised individuals.
The more recent co-evolution of T. gondii with rodents suggests that the murine immune response is well adapted to handle this parasite. Indeed, experimental infection of mice with T. gondii has become a widely exploited model for elucidating mechanisms of innate and acquired immunity to intracellular pathogens. However, since murine rodents, including the house mouse, are almost certainly evolutionarily significant hosts for T. gondii (at least in Eurasia where they are native), they should be under selective pressure from the parasite, resulting in significant modification of the immune system. Indeed recent findings show that humans and mice employ distinct innate immune pathways to control T. gondii infection. As reviewed here, elements required both for the initial detection and immune control of T. gondii in mice: the Toll-Like Receptors (TLR) 11 and TLR121, and the Immunity Related GTPase (IRG) proteins respectively are notably absent in humans. The alternative mechanisms that may replace these functions in humans, and the potential consequences for human disease are discussed along with the implications of these findings for the evolution of host defense pathways.
Mechanisms of innate immunity to T. gondii infection in mice
Mice deficient in essential common downstream elements of the TLR signaling pathway such as IRAK4 or MyD88 show impaired resistance to T. gondii due to a deficient cytokine response responsible for the control of the parasite (Bela et al., 2012; Scanga et al., 2002). TLR7 and TLR9, detect parasite RNA and DNA, whereas TLR11 and TLR12 sense the tachyzoite derived profilin-like protein (PRF) (Andrade et al., 2013; Koblansky et al., 2013; Yarovinsky et al., 2005). TLR activated dendritic cells (DCs) (Andrade et al., 2013; Goldszmid et al., 2012), and inflammatory monocytes (MØs) (Dunay et al., 2008) produce IL-12 that triggers the production of IFN-γ by NK cells and later by T lymphocytes. IFN-γ also primes innate immune cells to express pro-inflammatory mediators. This inflammatory milieu favors the development of Th1 lymphocytes that are the basis of the long-term host control of T. gondii (Gazzinelli and Denkers, 2006).
Two main IFN-γ-inducible mechanisms by which mouse cells limit tachyzoite growth have been studied. One of these is mediated by the inducible nitric oxide synthase (NOS2) and the release of nitric oxide (NO) by MØs (Hunter and Sibley, 2012). The second mechanism, which is essential for mice survival upon infection with T. gondii, involves the IRG proteins that are inducible by IFNγ both in non-myeloid and myeloid cells. The recruitment of members of this family of large GTPases results in rupture of the PV membrane and tachyzoite degradation in the host cell cytoplasm (Howard et al., 2011).
Based on recent studies, we hypothesize that TLR11 and TLR12 emerged in rodents as T. gondii sensors that more efficiently initiate IL-12 dependent IFNγ production, leading to a rapid activation of IRG proteins (Figure 2). Thus, while structurally distinct these two systems are connected to each other and serve to a common purpose, which is the control of the rapidly multiplying tachyzoites at the very first days of infection. While TLR11 and TLR12 sense a conserved Toxoplasma protein, it is the IRG proteins that deal with parasite diversity. As a consequence, the TLR11 and TLR12 genes are conserved, whereas IRG proteins interact antagonistically with polymorphic virulence factors of Toxoplasma and are themselves highly polymorphic.
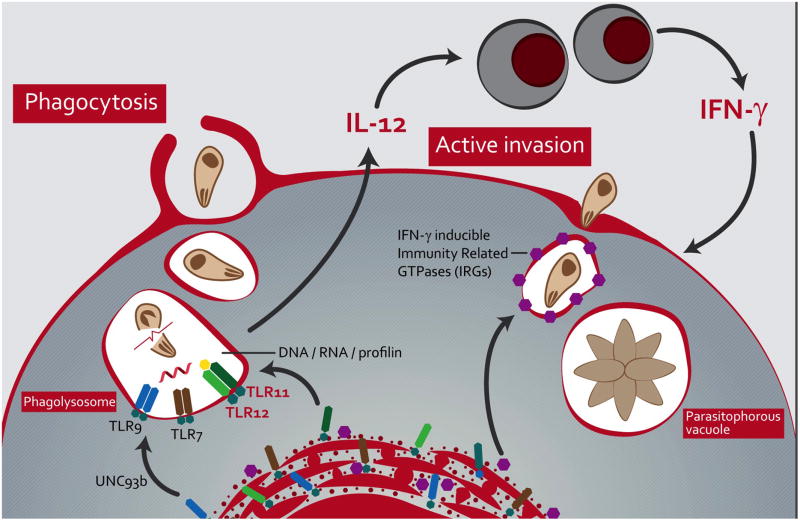
Figure 2. TLR11, TLR12 and IRG protein loop is essential for mouse resistance to Toxoplasma infection.
Uptake of parasite debris or alternatively, destruction of engulfed tachyzoites leads to activation of TLR7, TLR9 as well as TLR11/TLR12 heterodimers by Toxoplasma RNA, DNA and profilin (PRF) released in phagolysosomes. The activation of these innate immune receptors, and in particular TLR11 and TLR12, induces the production of IL-12 by dendritic cells and consequent induction of IFNγ by NK cells and T lymphocytes. IFNγ then leads to expression of IRG proteins that are recruited to the parasitophorous vacuole and destroy T. gondii tachyzoites that have actively invaded the host cells.
TLR11/TLR12 specificity and function
TLR11 was shown to mediate mouse resistance to gram-negative bacteria that are relevant to human disease (Mathur et al., 2012). Nevertheless, considering the central role of rodents in the T. gondii life cycle, and that TLR11 responds to minute amounts (in the range of 1–10 ng/ml) of PRF (Yarovinsky et al., 2005), we favor the hypothesis that this TLR emerged as a consequence of the selective pressure of T. gondii or perhaps closely related coccidian parasites. Experiments performed with genetically manipulated parasites lacking PRF indicate that this protein is involved in tachyzoite motility and host cell invasion (Plattner et al., 2008). Hence, TLR11 senses a protein that is essential for parasite survival and perpetuation in the host, and thus, highly conserved among different strains of Toxoplasma.
Two recent studies also indicate that TLR12, the TLR most closely related to TLR11, is also required for innate recognition of Toxoplasma tachyzoites (Andrade et al., 2013; Koblansky et al., 2013). Both TLR11 and TLR12 are expressed by DCs, and the lack of either dramatically reduces IL-12 production in response to PRF in vitro, and results in impaired IL-12 production in T. gondii infected mice. Moreover, upon activation with PRF, TLR11 and TLR12 form heterodimers that may represent the dominant form of the two proteins involved in signaling in myeloid DCs (Andrade et al., 2013). Intriguingly, both TLR11 and TLR12 are necessary for the activation of macrophages and CD11c+ DCs by PRF, whereas TLR12 alone is sufficient for activation and Type I IFN production by Flt3+ plasmacytoid DCs exposed to PRF (Koblansky et al., 2013).
Another important observation is that the response to PRF is completely ablated in DCs from mice functionally mutated in UNC93B1 which encodes for a chaperone required to bring nucleic acid sensing TLRs 3, 7 and 9 from the endoplasmic reticulum (ER) to the endocytic system. These animals are also highly susceptible to Toxoplasma infection (Melo et al., 2010). Nevertheless, it was demonstrated that TLR11 and TLR12 also co-localize with UNC93B1, both in the ER and endosomal compartment, suggesting that the extreme phenotype of the UNC93B1 mutant mice is due to a combined defect in the nucleic acid sensing- and PRF sensing-TLRs. Consistent with this hypothesis, quadruple KO mice deficient in TLRs 3, 7, 9 and 11 closely resemble UNC93B1 mutant mice in their complete loss of resistance to T. gondii (Andrade et al., 2013).
IRG proteins: specificity and function
IRG proteins are a family of IFNγ inducible mouse GTPases with an approximate molecular weight of 47 kDa that are essential for the control of tachyzoite replication and host survival in mice infected with T. gondii (Howard et al., 2011). While the IRG gene family is widely distributed in vertebrates, the IRG gene diversity found in rodents is remarkable. Mouse IRG proteins fall into five sub-families, IRGA, IRGB, IRGC, IRGD and IRGM, encoded by about 23 genes in the C57BL/6 genome. All except IRGC are massively inducible in all nucleated cells by IFNγ. Disruption of individual IRG genes results in a profound (Irgm1, Irgm3) or partial (Irgd, Irga6) enhancement of susceptibility to T. gondii. The three members of the IRGM (GMS) subfamily, Irgm1, Irgm2 and Irgm3, are negative regulators of the IRGA, IRGB and IRGD effector (GKS) subfamilies (Hunn et al 2008) that damage the parasitophorous vacuole membrane. The regulatory GMS proteins prevent off-target activation on organellar membranes (Haldar et al., 2013). Members of the effector GKS subfamilies assemble on the PV membrane. This becomes corrugated, limiting the internal volume available for the parasite and pulling it tight against the elastic cytoskeleton of the parasite cytoskeleton. The PV membrane ultimately ruptures releasing the tachyzoite into the cytosol (Zhao et al., 2009), where it dies within 20 minutes. The infected cell itself undergoes necrotic death about an hour later. For unknown reasons some PVs are not coated with IRG proteins, and become permissive to parasite replication. The survivors are probably destined to differentiate into bradyzoites and encyst in brain and muscle tissues.
The IRG system can also defend itself against attack by T. gondii virulence factors. Certain strains of T. gondii secrete a pseudokinase (ROP5) and a kinase (ROP18) forming a complex that phosphorylates and inactivates IRG proteins, resulting in high virulence (Hunter and Sibley, 2012). Some mouse strains are resistant to the ROP5/ROP18 kinase complex of these virulent T. gondii strains, which they achieve via an IFNγ-inducible “tandem” IRG protein of the IRGB subfamily built of two GTPase units, that server as a decoy for the ROP18/ROP5 kinase complex sparing the effector IRG proteins (Lilue et al., 2013). Indeed, the IRG system of the mouse is at least as polymorphic as the MHC, indicating the presence of selection pressures acting on this system. The IRG allele-specific differential resistance of mouse strains to certain T. gondii strains supports the argument that T. gondii may be responsible for at least some of this pressure on the IRG system.
While IRG proteins of the mouse appear to play no role in resistance to a many bacterial and protozoal pathogens, T. gondii is not the only organism that can impact on the IRG system and potentially exert selection pressure. Resistance of mice to certain strains of Chlamydia also requires an intact IRG system (Howard et al., 2011; Miyairi et al., 2007). It is therefore to be expected that the IRG system will provide immunity against other groups of organisms.
Lack of functional TLR11, TLR12 and IRG genes in the human genome
While we have learned a lot about how mice control T. gondii infections, how do humans respond to this parasite? Although normally asymptomatic in healthy individuals, T. gondii infection is a serious and often fatal disease in immunodeficient individuals. In non-immune pregnant women congenital transmission may cause serious damage to the child before and after birth. In addition, in South America eye disease and other symptoms of systemic inflammation are not uncommon outcomes of primary infection even in immunocompetent individuals. From the dramatically enhanced susceptibility of AIDS patients and patients on immunodepletion therapy it is clear that the adaptive immune system plays an essential role in immunity to human toxoplasmosis.
Less well understood is the nature or relevance of innate immunity against T. gondii infection in humans, a situation compounded by the absence in humans of the critical mediators of innate immunity discovered in the mouse. The segment of mouse chromosome 4 that contains the TLR11 gene is found in human chromosome 1 but the human TLR11 gene contains three stop codons and does not encode a functional protein. Similarly, the TLR12 gene, present in mouse chromosome 14, is not present in the human genome. Thus recognition of PRF, which is a strong activator in the mouse, apparently plays no role in human resistance to infection. Nevertheless, it is possible that the nucleic acid sensing TLRs play an important role in Toxoplasma recognition by human cells. Consistent with this hypothesis, human monocytes and DCs are activated by parasite ssRNA and DNA, probably via TLR7, TLR8 and TLR9, to produce large amounts of pro-inflammatory cytokines (Andrade et al., 2013).
More puzzling, however, is the absence from humans of the IRG system, the essential effector mechanism against T. gondii in mice. The IFNγ-inducible IRG proteins are encoded in two gene clusters on mouse chromosomes 11 and 18. These clusters are in genomic segments syntenic with a region of human chromosome 5. The only vestige of the entire IRG family in this region is a fragment carrying most of a G-domain with typical sequence features of the IRGM regulatory subfamily of the mouse. Humans and mice share one orthologous and highly conserved full-length IRG gene, IRGC, expressed exclusively in the testis in both species, which is not regulated by cytokines and unlikely to play a role in host resistance to T. gondii (Bekpen et al., 2005).
Human cells appear to dispose of a range of IFNγ-inducible toxoplasmastatic mechanisms but it remains unclear which, if any, are essential for the usually benign course of T. gondii infection. Both NO-dependent control and non-NO-dependent control mechanisms (Nagineni et al., 1996) have been reported in different systems. There is also considerable evidence for a link between the interferon-inducible tryptophan catalyzing enzyme, indoleamine dioxygenase (IDO), tryptophan depletion and a toxoplasmastatic activity in a variety of human cellular systems (Pfefferkorn, 1984). However, these activities are not universally observed in human cells, suggesting that an unidentified NO- and IDO-independent mechanism induced by IFNγ may also play an important role in human resistance to T. gondii infection.
Although absent in humans, one can imagine that TLR11, TLR12 and IRG proteins in other animals may indirectly influence human disease by controlling the zoonotic dissemination of the parasite. These elements operating together in small rodents should select and strongly impact the abundance of different parasites strains in the environment, and thereby the ones that most often infect humans. However, a major gap in our present information and parasite transmission to the intermediate hosts is the nature of resistance against T. gondii in the domestic cat and other felines, which expresses neither TLRs 11 and 12 nor IRG proteins.
Evolution and host specificity of TLR11, TLR12, and IRG proteins
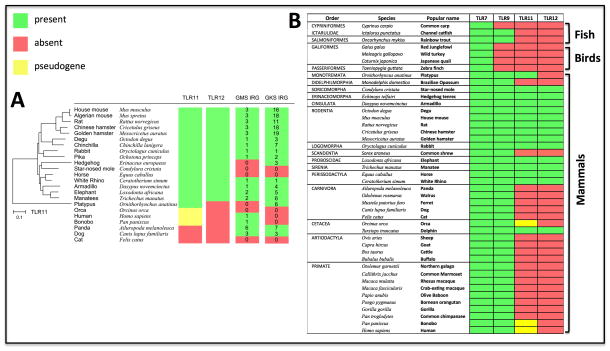
Considering the ubiquity of Toxoplasma in nature, it is intriguing that genes encoding TLR11, TLR12 as well as IRG proteins are not found in many mammalian species. Interestingly, if the representation of IRG gene numbers is superimposed on the species that encode TLR11 and TLR12 genes some consistency is apparent, particularly with the rodents (Figure 3A). When first infected with T. gondii, felines shed around hundred million oocysts that may persist in the environment for years. In addition, parasite genetic crosses in intestinal epithelial cells from cats often generate virulent clones (Saeij et al., 2007). Due to intimate contact with felines, small rodents, the natural intermediate hosts, are often exposed to higher dose and more virulent parasites than other groups of mammals. It may therefore be that TLR11, TLR12 and the polymorphism of IRG proteins have been positively selected for in rodents and other small mammals, because of their critical importance in host resistance against high infective doses or more virulent clones of T. gondii.
Figure 3. Distribution of TLR11, TLR12 and IRG protein genes in mammalian species.
(A) Phylogenetic tree of TLR11 and overlapping expression of TLR12 as well as IRG GMS and IRG GKS gene families. (B) Ubiquitous versus confined distribution of TLR7/TLR9 and TLR11/TLR12 genes in vertebrate genomes, respectively. TLRs 7, 9, 11 and 12 coding sequences were downloaded from Genbank from species that have complete genome sequences. Retrieved sequences from mammals, fishes and birds were analyzed by ORFfinder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) to identify pseudogenes by looking for frameshifts or premature stop codons. Multiple and global sequence alignments for each TLR were performed by Muscle. Neighbor-joining trees were designed by MEGA 5.2.2 from Muscle alignments.
The TLRs compose an ancient multigene family that fits better with a “swiss army knife” model of evolution. Each TLR subfamily evolved to detect components of a distinct nature that are essential for viability of different categories of microorganisms, and therefore, cannot easily mutate. Because of their critical importance for host resistance to microbial pathogens, TLRs are kept under selective pressure to maintain their specificity, and thus selection is dominant over mutation (Roach et al., 2005). While these genes are evolving slowly, the evolutionary changes seen in the TLR repertoire may reflect changes in the spectrum of species-specific pathogens and structural adaptations to relevant microbial components, as is the case for PRF recognition by TLR11 and TLR12 in rodents.
Most likely TLR11 diverged from the TLRs 21–23 genes found in fish and frogs. Interestingly, TLR11 and TLR12 are the most divergent of all TLRs and found only in mammals, suggesting that they are relatively new and fast evolving genes (Roach et al., 2005). The Platypus genome contains an open reading frame with 40% homology to mouse TLR11, which is likely the mammalian ancestral gene for TLR11. A limited number of mammal species encode functional TLR11 and TLR12 genes, which include rodents (mice, rats, degu and hamsters) and other orders of small mammals (Lagomorpha, Erinaceomorpha, and Soricomorpha) that are potential prey for cats and other felines. The high similarity and remarkable overlapping distribution in different species, favor the hypothesis that TLR12 is a duplication of TLR11 and the two genes co-evolved to exert a similar function. Consistently, the three species that have TLR11 pseudogenes lack the TLR12 gene (Figure 3). Published studies indicate that TLR11 and TLR12 work primarily as a heterodimer (Andrade et al. 2013). Therefore, it is possible that in the absence of TLR12, the TLR11 gene was lost because it could not function as a homodimer.
The IRG gene family is more widely distributed in vertebrates, present in fish and mammals but apparently absent in birds. In mammals IRG genes appear in all orders, but erratically, for example they are represented in the dog genome and absent from the cat. The IRGs are, however, consistently well represented and in large gene numbers in rodents. Since control of the mouse IRG system requires all 3 members of the IRGM regulator subfamily, and since the system is weakened by loss of individual IRG effector proteins, we cannot interpret a genome such as that of American pika or the rabbit which appear to contain only one IRGM gene and only one or two effector IRG genes and cannot say whether these reduced sets can function in immunity against T. gondii. Where the IRG system is absent or non-functional, as in humans, we assume that other mechanisms effectively control the parasite.
Because Toxoplasma PRF is a conserved molecule, TLR11 / TLR12 heterodimers are likely to sense most genetic variants of T. gondii that continuously attack rodents. In contrast, IRG diversity seems necessary to protect mice against the activity of the highly polymorphic members of parasite ROP proteins. Consistently, certain IRG haplotypes confer resistance to T. gondii strains described as highly virulent in laboratory mice (Lilue et al., 2013) generating a host phenotype that allows encystment and transmission of otherwise lethal clones. These data suggest that the generation of diversity in the T. gondii ROP proteins is a continuous selective force for IRG protein evolution in the wild, even if the selective balance in this complex system is unclear. The interacting surfaces of the IRG and ROP5 proteins are the most polymorphic regions of both proteins (Fleckenstein et al., 2012; Lilue et al., 2013), also consistent with the argument that these proteins exert reciprocal selection pressures on each other. Because diverse rodent species have many IRG genes (Figure 3A), we speculate that the arms race with ROP proteins started in an ancestral species and it is still a dynamic process in contemporary rodents. One could predict that the arms race would be dampened in geographic areas where few clonal populations of Toxoplasma became dominant. However, the presence of TLR11 and 12 and a sizeable collection of IRG genes in the elephant and other larger species unlikely to be evolutionarily significant for T. gondii seem to argue against such an oversimplified view and, one cannot discard a more complex scenario that a constellation of infections together imposes a special demand on expansion of IRG families in small rodents.
Although one can imagine the evolutionary pressures that gave rise to TLR11, TLR12 and the diversification of the IRG system, a major question remains unanswered (Lauw et al., 2005). Why are these genes absent or downgraded to non-coding status in the genome of humans and other vertebrates? A likely explanation is that only recently, after cat domestication, did T. gondii become a relevant pathogen for humans and other domestic animals. As a consequence these hosts did not co-evolve with Toxoplasma, and thus, have not been under selective pressure to develop these highly specific mechanisms of host defense against the parasite.
An alternative explanation for the absence of TLR11, TLR12 and IRG host defense mechanisms in a range of vertebrates would be the deliberate deletion of the relevant genes. Certainly IRG genes, which are already present in fish, were removed from the primate lineage sometime before the evolution of monkeys. While found only in mammals, it is clear that TLR11 gene has downgraded to a pseudogene in several species. In addition, both TLR11 and TLR12 genes are absent in the genomes of more recently evolved mammals (Figure 3).
Perhaps a deleterious inflammatory response that follows activation of TLR11 and 12 carries a net cost in the absence of intense infection, while activation of the IRG system can induce host cell death by necrosis and could also be detrimental in certain species. Figure 3B illustrates the wide distribution in vertebrates of TLR7 and TLR9 that recognize both Toxoplasma RNA and DNA. Hence, it is possible that the nucleic acid-sensing TLRs are sufficient to control parasite replication in the initial stages of infection in many mammal species, including perhaps felines that are critical to the Toxoplasma life cycle. As we have seen, the IRG system is not the only effector system available that mediates control of T. gondii infection. Hence, negative selection of TLR11, TLR12 and IRG genes/functions may contribute to host tolerance to infection and represent a gain of net fitness cost, unless intense exposure to T. gondii and other relevant infections impose unusual pressure demanding the maintenance of these unique immunological functions for host survival.
Regardless, the studies reviewed are consistent with a model in which the co-evolution of T. gondii and rodents led to the emergence TLR11, TLR12 and a family of diversified IRG genes as highly specialized innate immune mechanisms of host resistance to this parasite. Further studies on rodent toxoplasmosis where powerful parasite genetic and host immunologic tools can be readily deployed should help provide new insights into this fascinating example of parasite-host co-adaptation.
Acknowledgments
We are grateful to the members of our laboratories as well as Sankar Ghosh, Greg Taylor and Felix Yarovinsky for important discussions and their key contributions in defining the role of TLR11, TLR12 and IRG proteins in mouse resistance to T. gondii. We also thank Daniel Caffrey for critically reading this manuscript. RTG is recipient of the Visiting Professor Scholarship from CAPES and the David Rockefeller Center for Latin America Studies at the Harvard School of Public Health and supported by the National Institute of Science and Technology on Vaccines and the National Institutes of Health (NIAID R01 AI071319-01). JH is recipient of the SPP1399 and SFB680 grants from the German Research Council while AS is supported by the Intramural Program of the National Institutes of Allergy and Infectious Disease.
Footnotes
There is a discrepancy in the designation of TLR11 and TLR12 gene/proteins. Here we follow the nomenclature defined in the functional studies of these TLRs (Yarovinsky et al. J.F., 2005; Andrade et al., 2013; Koblansky et al., 2013). According to this definition, TLR11 is a pseudogene and TLR12 is absent in human genome. Both TLRs are functional in mice. In the NCBI genebank definition, what we designate as TLR11 is defined as TLR12 and vice-versa.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Andrade WA, do Souza MC, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13:42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bela SR, Dutra MS, Mui E, Montpetit A, Oliveira FS, Oliveira SC, Arantes RM, Antonelli LR, McLeod R, Gazzinelli RT. Impaired innate immunity in mice deficient in interleukin-1 receptor-associated kinase 4 leads to defective type 1 T cell responses, B cell expansion, and enhanced susceptibility to infection with Toxoplasma gondii. Infect Immun. 2012;80:4298–4308. doi: 10.1128/IAI.00328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Fleckenstein MC, Reese ML, Konen-Waisman S, Boothroyd JC, Howard JC, Steinfeldt T. A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol. 2012;10:e1001358. doi: 10.1371/journal.pbio.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013;9:e1003414. doi: 10.1371/journal.ppat.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol. 2011;14:414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 2005;26:509–511. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Lilue J, Muller UB, Steinfeldt T, Howard JC. Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife. 2013;2:e01298. doi: 10.7554/eLife.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R, Oh H, Zhang D, Park SG, Seo J, Koblansky A, Hayden MS, Ghosh S. A mouse model of Salmonella typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo MB, Kasperkovitz P, Cerny A, Konen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 2010;6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyairi I, Tatireddigari VR, Mahdi OS, Rose LA, Belland RJ, Lu L, Williams RW, Byrne GI. The p47 GTPases Iigp2 and Irgb10 regulate innate immunity and inflammation to murine Chlamydia psittaci infection. J Immunol. 2007;179:1814–1824. doi: 10.4049/jimmunol.179.3.1814. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Pardhasaradhi K, Martins MC, Detrick B, Hooks JJ. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]