Abstract
Drug transporters mediate the movement of endobiotics and xenobiotics across biological membranes in multiple organs and in most tissues. As such, they are involved in physiology, development of disease, drug pharmacokinetics, and ultimately the clinical response to myriad medications. Genetic variants in transporters cause population-specific differences in drug transport and are responsible for considerable inter-individual variation in physiology and pharmacotherapy. The purpose of this review is to provide a broad overview of how inherited variants in transporters are associated with disease etiology, disease state, and the pharmacological treatment of diseases. Given that there are thousands of published papers related to the interplay between transporter genetics and medicine, this review will provide examples that exemplify the broader focus of the literature.
Keywords: ABC, SLC, transporter, clinical pharmacology
INTRODUCTION
The ultimate goal of pharmacogenetics research on transporters is to better understand human diseases and to optimize therapeutics with drug transporters in mind. Pharmacogenetic approaches tailor therapies to patients based on their individual genetic background, and transporters are quintessential to pharmacogenetics research. The present review will: (i) provide a basic background of selected transporters, their polymorphisms, and their inheritance based on racial background; (ii) discuss how transporters mediate clinical pharmacology within various organs based on genetic variation; and (iii) provide a general discussion of how inter-individual variation in transporters influences normal physiology, diseases, and clinical pharmacology. Rather than providing a comprehensive overview, examples will be provided to demonstrate how transporter polymorphisms alter drug disposition and outcome.
Background and genetic variation of selected transporters
In general, transporters move substrates in an intracellular to extracellular direction (“efflux” transporters) or vice versa (“influx transporters”). There are a variety of efflux transporters (e.g. the ATP-binding cassette transporter family (ABCs), and multidrug toxin extrusion proteins (MATEs)), and influx transporters (e.g., the organic anion transporters (OATs and OATPs), organic cation transporters (OCTs), oligopeptide transporters (PEPTs), etc.), some of which are bidirectional. Moreover, these transporters are often arranged in polarized cells such that they facilitate the movement of substrates at a single membrane, either basolateral or apical, thereby regulating how substrates distribute across epithelial barriers. There is significant genetic variation in nearly every drug transporter, which has various consequences on their expression, mRNA stability, protein folding, intracellular localization, degradation, substrate binding, and/or transport kinetics. Herein we discuss the most commonly characterized polymorphisms and their consequences. For more comprehensive overviews of transporter SNPs see Boivin et al. (2010), Cascorbi (2008), Choi and Song (2008), and Niemi et al. (2011).
ABCB1/MDR1
ABCB1 is the most thoroughly studied efflux transporter. It is expressed in most human and rodent tissues with the greatest expression at the apical surface of enterocytes, the canalicualar plasma membrane of hepatocytes, and the proximal renal tubule (Fojo et al., 1987; Schellens et al., 2000; Schinkel et al., 1997; Sharom, 2011; Thiebaut et al., 1987). In these tissues ABCB1 serves to modulate the absorption, distribution, metabolism and excretion (ADME) of a multitude of exogenous substrates (Leslie et al., 2005) (Table 1). ABCB1 is also expressed in hematopoietic stem cells, the blood-brain barrier (BBB), the heart, nerves, testes, and placenta. ABCB1 effluxes substrates away from these tissues, and therefore limits the penetration of toxins (Chaudhary et al., 1992; Eichelbaum et al., 2004; Fromm, 2004; Meissner et al., 2002; Rao et al., 1999; Saito et al., 2001). For example, ABCB1 expression in pre-term placentas is crucial in protecting the placental cells and fetus, both of which are more vulnerable in early pregnancy (Ni & Mao, 2011).
Table 1.
Substrates and inhibitors of ABCB1, ABCG2, ABCC2, OATP1B1, OATP1B3 (adapted from Sissung et al., 2010)(Sissung et al., 2010)
| ABCB1 | Substrates | Inhibitors |
| Antibiotics | actinomycin D erythromycin gramicidin D rifampin salinomycin sparfloxacin valinomycin |
|
| Anti-Cancer Drugs | bisantrene daunorubicin diflomotecan docetaxel doxorubicin epirubicin etoposide gefitinib imatinib irinotecan mitoxantrone paclitaxel romidepsin teniposide tipifarnib vinblastine vincristine |
sunitinib tamoxifen |
| Antifungals | itraconazole ketoconazole |
ketoconazole |
| Antihistamines | certirizine fexofenadine loratadine terfenadine |
|
| Antihypertensive Drugs | losartin talinolol |
nicardipine quinidine verapamil |
| CNS Drugs | chlorpromazine clozapine fluphenazine olanzapine quetiapine risperidone |
|
| Flavonoids | biochanin A genistein oroxylin A |
|
| Heart Medications | digoxin diltiazem ouabain quinidine verapamil |
gallopamil |
| HIV-1 Protease Inhibitors | abacavir amprenavir aquinavir darunavir indinavir lopinavir nelfinavir ritonavir saquinavir |
|
| Immunosuppressants | cyclosporin A dexamethasone D-penicillamine enkephalin FK 506 hydrocortisone prednisolone rapamycin tacrolimus triamcinolone |
cyclosporin A valspodar |
| Sedatives | midazolam | |
| Statins | atorvastatin cerivastatin lovastatin |
|
| Miscellaneous | asimadoline cimetidine colchicine domperidine eletriptan flesinoxan glabridin ivermectin loperamide ondansetron quinacrine ranitidine topiramate |
dexverapamil emopamil JAI-51 quinacrine tariquidar |
| ABCG2 | Substrates | Inhibitors |
| Antibiotics | ciprofloxacin erythromycin nitrofurantoin norfloxacin ofloxacin |
novobiocin rapamycin |
| Anti-Cancer Drugs | 9-aminocamptothecin bisantrene cladribine daunorubicin diflomotecan doxorubicin epirubicin erlotinib etoposide flavopiridol gefitinib gimatecan homocamptothecin imatinib methotrexate mitoxantrone SN-38 (irinotecan metabolite) teniposide tomudex topotecan |
biricodar diethylstilbestrol elacridar fumitremorgin gefitinib ginsenoside ortataxel sunitinib tamoxifen tryprostatin vandetanib |
| Antihypertensive Drugs | olmesartan | dihydropyridine dipyridamole reserpine |
| Anti-inflammatory Drugs | chrysin curcumin |
|
| Antiplatelets | dipyridamole | |
| Calcium Channel Blockers | azidopine dipyridamole nitrendipine |
nicardapine nimodipine nitrendipine |
| Flavonoids | seravastatin | acacetin apigenin genistein naringenin quercetin silymarin techochrysin |
| HIV-1 Protease Inhibitors | abacavir lamivudine nelfinavir zidovudine (AZT) |
abacavir amprenavir atazanavir delavirdine efavirenz lopinavir nelfinavir ritonavir saquinavir |
| Immunosuppressants | cyclosporin A lefunomide sirolimus sulfasalazine tacrolimus |
cyclosporin A sirolimus tacrolimus |
| Specific Inhibitors | GF120918 Ko143 tariquidar (XR9576) |
|
| Statins | pitavastatin posuvastatin seravastatin |
rosuvastatin |
| Miscellaneous | glyburide protoporphyrin |
pantoprazole |
| ABCC2 | Substrates | Inhibitors |
| Antibiotics | ampicillin azithromycin cefodizime ceftriaxone grepafloxacine |
azithromycin |
| Anti-Cancer Drugs | camptothecin cisplatin doxorubicin etoposide irinotecan methotrexate mitoxantrone vinblastine vincristine |
BTK lonafarnib |
| Antihypertensive Drugs | olmesartan | |
| Antiinflammatory Drugs | curcumin | |
| Blood-Glucose Lowering Drugs |
glibenclamide | |
| HIV-1 Protease Inhibitors | adefovir cidofovir indinavir lopinavir nelfinavir ritonavir saquinavir |
|
| Immunosuppressants | cyclosporin A | |
| Statins | pravastatin | |
| Miscellaneous | temocaprilate valproate |
MK-571 furosemide PAK-104P phenobarbital probenecid |
| OATP1B1 | Substrates | Inhibitors |
| Antibiotics | benzylpenicillin rifampin |
clarithromycin erythromycin hyperforin rapamycin rifampin rifamycin SV roxithromycin telithromycin |
| Anti-Cancer Drugs | ACU-154 atrasentan Bamet-R2 Bamet-UD2 demethylphalloin dihydromicrocystin-LR irinotecan methotrexate SN-38 |
antamanide ketoconazole paclitaxel PKI-166 SN-38 |
| Anti-Diabetics | glibenclamide pioglitazone rosiglitazone |
|
| Antifungals | caspofungin | clotrimazole |
| Antihistamines | fexofenadine | |
| Antihypertensive Drugs | bosentan enalapril olmesartan temocapril valsartan |
telmisartan |
| Antiinflammatory Drugs | valsartan D-penicillamine enkephalin troglitazone sulfate |
troglitazone troglitazone sulfate |
| Blood-Glucose Lowering Drugs |
repaglinide | |
| Fibrates | gemfibrozil gemfibrozil-1-Oglucuronide |
|
| Flavonoids | biochanin A | |
| Heart Medications | digoxin | |
| HIV-1 Protease Inhibitors | indinavir nelfinavir ritonavir saquinavir |
|
| Immunosuppressants | cyclosporin A tacrolimus |
|
| Statins | atorvastatin cerivastatin fluvastatin pitavastatin pravastatin rosuvastatin simvastatin acid |
atorvastatin BMS-241423 atorvastatin analogue) BMS-243887 atorvastatin analogue) lovastatin lovastatin acid lovastatin lactone pravastatin simvastatin simvastatin lactone |
| Miscellaneous | BQ-123 bromosulphophthalein |
carbamazepine glycyrrhizin metyrapone mifepristone sildenafil |
| OATP1B3 | Substrates | Inhibitors |
| Antibiotics | rifampin | clarithromycin erythromycin hyperforin rifampin rifamycin roxithromycin telithromycin |
| Anti-Cancer Drugs | demethylphalloin dihydromicrocystin-LR docetaxel imatinib irinotecan methotrexate paclitaxel SN-38 |
|
| Antihistamines | fexofenadine | |
| Antihypertensives | bosentan enalapril olmesartan telmisartan valsartan |
|
| Anti-inflammatory Drugs | D-penicillamine enkephalin | troglitazone sulfate |
| Blood-Glucose Lowering Drugs |
repaglinide | |
| Heart Medications | digoxin ouabain |
|
| Immunosuppressants | cyclosporin A | |
| Statins | fluvastatin pitavastatin pravastatin rosuvastatin |
pravastatin |
| Miscellaneous | BQ-123 bromosulphophthalein |
bromosulphophthalein glycyrrhizin |
ABCB1 contains at least 66 coding SNPs of which 24 are synonymous and 42 are non-synonymous (Wolf et al., 2011). Two of the synonymous SNPs and 12 non-synonymous SNPs are associated with altered function or expression of the ABCB1 protein. 1236C>T, 3435C>T, and 2677G>T/A have been well characterized. These SNPs may be associated with altered mRNA levels, mRNA stability (Cascorbi, 2006), protein folding (Kimchi-Sarfaty et al., 2007) and drug pharmacokinetics (Longo et al., 2010); however, others have found no association between these SNPs and ABCB1 function (Sissung et al., 2010).
Haplotype analysis revéals an additive effect of these SNPs on ABCB1 function. There are at least 64 ABCB1 haplotypes, including the common haplotype, ABCB1*13, that contains the three previously mentioned SNPs; thus, haplotype structure may denote larger scale linkage with other functional polymorphisms (Kroetz et al., 2003). Interestingly, functional changes in ABCB1 were only detected when 2677G>T and either 1236C>T or 3435C>T were present in combination, not 2677G>T alone. This change in function may be due to minor differences in the tertiary structure of the protein (Kimchi-Sarfaty et al., 2007).
Frequencies of ABCB1 SNPs and haplotypes vary across races. Kroetz et al. (2003) found 16 variants specific to African Americans, 8 to Caucasians, and 3 to Asian-Americans (Kroetz et al., 2003). Though variant allele frequencies are generally higher in African Americans (Wang et al., 2005), the three SNPs previously listed are twice as common in Caucasians as in African Americans. Likewise, frequency of ABCB1*1 was race-specific (Caucasians = 0.32, African Americans = 0.05, Asian-Americans = 0.266, Mexican-Americans = 0.35, Pacific Islanders = 0.333) (Kroetz et al., 2003). Therefore, ABCB1 substrates may be transported differently depending on racial backgrounds.
ABCC2/MRP2
ABCC2, also known as MRP2, is expressed in several tissues, including the liver, intestine, kidney, BBB, and placenta and is localized to the apical membrane of epithelial cell. It actively exports anionic drug conjugates as well as many unconjugated substances and thus is an important part of drug detoxification (Table 1). Additionally, ABCC2 plays a major part in the transport of anticancer drugs (Cascorbi, 2006). For example, in vitro studies indicate that ABCC2 is expressed at higher levels in tamoxifen-resistant breast cancer cells, suggesting a role for ABCC2 in transporting the active metabolites of tamoxifen (Kiyotani et al., 2010).
Most ABCC2 polymorphisms are quite rare in the general population, however, −24C>T in the 5’-UTR, 1249G>A, and 3972C>T are all relatively common in healthy individuals. Their respective frequencies in Japanese were 0.18, 0.12, and 0.21 (Ito et al., 2001), versus 0.18, 0.21, and 0.34, in healthy German volunteers (Haenisch et al., 2008). The −24C>T and 3972C>T variants are also in linkage disequilibrium (Itoda et al., 2002; Suzuki & Sugiyama, 2002).
The functional importance of ABCC2 polymorphisms remains unclear. Cascorbi (2006) argued that ABCC2 SNPs are only of minor importance for drug availability (Cascorbi, 2006). Likewise, Hirouchi et al. (2004) found that 1249G>A did not affect ABCC2 transport function (Hirouchi et al., 2004).
We did not cover ABCC1 because few studies assessed consequences of polymorphisms in this gene and those that have failed to find any functionally significant effects (Cascorbi, 2006; Letourneau et al., 2005).
ABCG2/BCRP/MXR
Similar to ABCB1, ABCG2 (BCRP, MXR) is highly expressed in the placenta, the central nervous system (brain and BBB), liver, adrenal gland, testes, large and small intestine where it effluxes substrates across the apical membrane. In the gastrointestinal tract, ABCG2 limits the intestinal uptake of certain substrates, including antibiotics, quercetin, sulfasalazine, and dietary carcinogens (Robey et al., 2009). In the kidney and liver, ABCG2 is involved in both renal drug excretion and biliary excretion, respectively.
Several SNPs and at least one insertion-deletion variant have been identified (Choudhuri & Klaassen, 2006). The association between these SNPs and ABCG2 expression levels, cellular localization, and pharmacokinetics is unclear. The most commonly implicated SNPs are Q141K and V12M. The ABCG2 421C>A (Q141K) SNP has been linked to a reduction in ABCG2 transport activity while the V12M (G34A) SNP is related to aberrant transporter membrane localization (Generaux et al., 2011). However, others failed to confirm these findings (Honjo et al., 2002; Porcelli et al., 2009).
OATP1B1/SLCO1B1
OATP1B1 is a hepatic influx transporter (Niemi et al., 2011). To date, over 40 nonsynonymous SNPs have been indentified on SLCO1B1 (encoding OATP1B1) (Boivin et al., 2010; Niemi et al., 2011). Several of these SNPs have been associated with functional changes in the transporter. Some variations result in changes in the structure of the transmembrane-spanning domains (217T>C, 245T>C, 521T>C, and 1085T>C), while others alter the extracellular loop 5 (1294A>G, 1385A>G, and 1463A>C) (Tirona et al., 2001). The 521T>C variant was associated with reduced membrane expression of OATP1B1, lower levels of transport activity, and changes in the maximum transport velocity of OATP1B1 substrates (Niemi et al., 2011).
Functional analysis of OATP1B1 haplotypes showed that transport activity was significantly lower in variants *2, *3, *5, *6, *9, *10, *12, and *13 compared with the reference allele (Tirona et al., 2001). However, these functional changes did not coincide with changes in protein expression, except for the variant *2 in which expression was reduced. The reduction in transport activity may instead be associated with reduction in plasma membrane expression of OATP1B1 haplotypes. In addition, some haplotypes were associated with changes in pharmacokinetics (Tirona et al., 2001). There are contradictory findings regarding the effects of the four 388A>G haplotypes (*1A, *1B, *5, and *15) on transport activity which may be due to substrate-specificity (Niemi et al., 2011).
The most common SNP across populations was 388A>G, though the frequencies varied considerably between races (sub-Saharan Africa = 79%, East Asia = 74%, Oceania = 66%, America = 63%) (Pasanen et al., 2008; Tirona et al., 2001). Some SNPs, however, were race-specific. The variant 10499A>C was found primarily in Europeans and 1086C>T and 1463G>C were specific to sub-Saharan African populations (Pasanen et al., 2008). Other relatively common variants were 2000A>G (0.34) and 1463G>C (0.09) in African Americans and 463C>A (0.16) and 521T>C (0.14) in European Americans (Tirona et al., 2001).
Frequencies of OATP1B1 haplotypes also varied by geographical region (Pasanen et al., 2008). For example, the *1B haplotype was the most common in North Africa, sub-Saharan Africa, East Asia, Oceania and America, whereas the *1A haplotype was the most common in the Middle East, South/Central Asia and Europe. The frequency of the *15 haplotype was 24% in American, 16% in North African and European, 15% in Middle Eastern, 12% in Asian, 9% in South/Central Asian, and 2% in sub-Saharan African populations. It was not observed in Oceania. The *5 variant was more race-specific and only found in the Middle East (5%), North Africa (2%), and Europe (2%) (Pasanen et al., 2008).
OATP1B3/SLCO1B3
Like OATP1B1, OATP1B3 is a liver-specific influx transporter that moves substrates across the basolateral membrane into the hepatocytes for metabolism and elimination (Svoboda et al., 2011). Forty-one SLCO1B3 (encoding OATP1B3) polymorphisms, most of which are located in regulatory regions, have been indentified along with a variety of haplotypes (Boivin et al., 2010). Two clusters of deletions at −7 to −4 and −28 to −1 in the 5’-UTR that may result in reduced mRNA stability or problems with initiation of translation have been reported (Boivin et al., 2010). Fourteen SNPs, six of which are nonsynonymous, have been found in coding regions of OATP1B3 (Schwarz et al., 2011). Functional analysis indicates that variants 699G>A, 1559A>C,1679T>C, and the haplotype 334T>G and 699G>A alter transport levels and kinetics of OATP1B3; however, the change appears to be substrate-specific (Schwarz et al., 2011). Interestingly, 767G>C, 1559A>C, and 1679T>C were associated with lower total OATP1B3 protein expression. However, 669G>A expression was not different from that of wild-type and 334T>G and 439A>G variants had slightly increased expression. The SNPs 1559A>C and 1679T>C displayed lower levels of cell surface expression than wild-type (Schwarz et al., 2011). SNPs at 334T>G, 1564G>A, and the rare variant 1564G>T were associated with changes in cellular localization and transport properties of OATP1B3 (Letschert et al., 2004). Structural modeling suggests that 1679T>C faces the putative central pore of the transport protein that is thought to be necessary for the translocation of substrates (Schwarz et al., 2011).
The most common OATP1B3 polymorphisms in individuals of all racial backgrounds were 699G>A (Hispanic-Americans=0.833, Chinese-American=0.795, Caucasian-Americans=0.779, African Americans=0.478) and 334T>G (Hispanic-Americans=0.711, Chinese-Americans=0.620, Caucasian-Americans=0.523, African Americans=0.386). 1679T>C and 439A>G were specific to African-Americans (3.6% and 1.1% respectively) (Schwarz et al., 2011).
OCT1 and OCT2
The organic cation transporter (OCT) family of transporters includes but is not limited to OCT1, encoded by SLC22A1, and OCT2, encoded by SLC22A2. OCT1 and OCT2 are both influx transporters isolated on the the basolateral membrane. OCT1 is expressed in hepatocytes, whereas OCT2 expression is generally limited to kidney proximal tubules (Choi & Song, 2008). To date, 19 non-synonymous SLC22A1 and 10 non-synonymous SLC22A2 SNPs have been identified. In OCT1, 41C>T, 181C>T, 292T>C, 566C>T, 659G>T, 848C>T, 859C>G, 1022C>T, 1201G>A, 1256delATG, and 1393G>A have all been associated with functional changes which result in alterations in pharmacokinetics of its substrates (Choi & Song, 2008; Ciarimboli, 2011). Six OCT2 SNPs, 495G>A, 596C>T, 602C>T, 808G>T, 1198C>T, and 1294A>C were associated with phenotypic variation.
Among OCT1 polymorphisms, 4 were specific to Caucasians, 5 to African-Americans, and 3 to Asians (Choi & Song, 2008). In OCT2, 2 SNPs were found exclusively in Caucasians, 4 in African-Americans, and three in Asians. Some SNPs were common across races, but their frequencies varied. For example, in OCT1, the frequency of the variant 1022C>T was 8.2% in African Americans and 16% in Asians. In OCT2, the 808G>T variant allele frequency was 15.7% in Caucasians, 11% in African Americans, and 15% in Asians.
Expression and function of transporters in excretory organs and biological barriers
While a diverse set of organs and tissues are regulated by transporters, there are some similarities in how transporters function. Efflux transporters expressed on the basolateral side of epithelia extrude drugs from organs back into plasma, while basolateral influx transporters move drugs from the blood into the epithelia (Figures 1–3). Apical efflux transporters extrude drugs into the excrete (i.e., bile or urine) or into tissues, while apical influx transporters bring drugs from the lumen of the excretory organs into the epithelia or out of tissues into the epithelia. In this way, both influx and efflux transporters influence ADME and disposition of endogenous substrates (Leslie et al., 2005). Endothelial and hematopoietic transporters also act as gatekeepers that regulate the tissue concentrations of drugs and primarily serve a protective function by limiting tissue exposure to certain substrates.
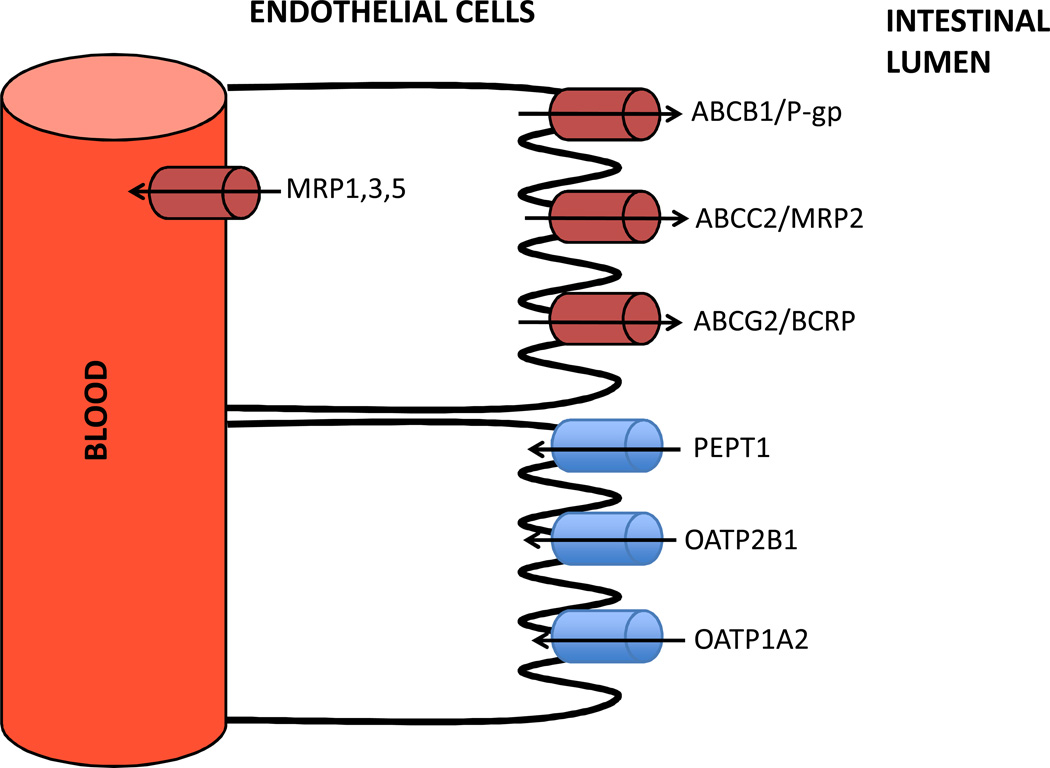
Figure 1.
Select transporters expressed in the gastrointestinal (GI) tract. Efflux transporters (red) efflux substrates back into the intestinal lumen, while influx transporters (blue) influx substrates from the intestinal lumen into the blood. PEPT: peptide transporter.
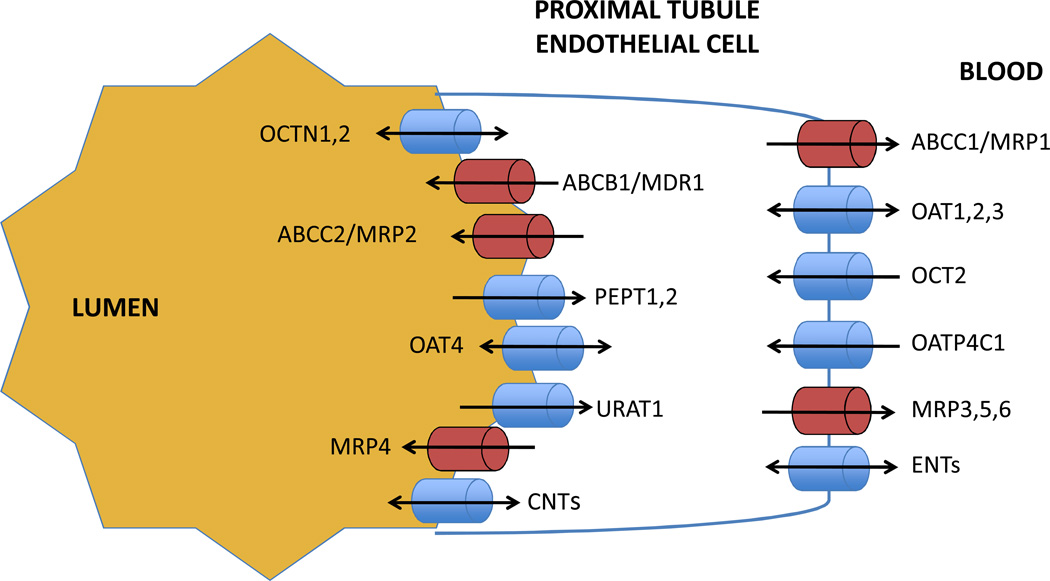
Figure 3.
Select transporters expressed in the renal proximal tubule. Influx transporters on the basolateral membrane (blue) influx substrates from the blood into the renal epithelial cells, while efflux transporters (red) transport substrates back into the blood. On the lumenal membrane, efflux transporters efflux substrates into the lumen, while influx transporters remove drugs from the lumen or act as bidirectional pumps. ENTs: equilibrative nucleoside transporters; CNTs: concentrative nucleoside transporters.
Gut
The gut wall is composed of several transporters that are expressed on the luminal membrane of enterocytes, including efflux transporters (e.g. ABCB1, ABCG2, ABCC2) and influx transporters (e.g. PEPT1, OATP2B1, OATP1A2) although uptake transporters are rather poorly characterized (Figure 1). Efflux transporters limit the absorption of various toxins in the gut lumen while influx transporters increase absorption of certain drugs. The interplay between these transporters, however, is complex. For instance, a decrease in fexofenadine exposure was observed when orally administered fexofenadine was administered with grapefruit juice (Cvetkovic et al., 1999; Dresser et al., 2005). This observation was unexpected given that fexofenadine is a substrate of ABCB1 and inhibition of ABCB1 with grapefruit juice was thought to increase fexofenadine exposure by blocking its efflux into the lumen of the gut. It was later determined, however, that grapefruit juice also blocks the influx transporter OATP1A2 thereby decreasing the oral absorption of this drug. However, little is known about how genetic variation in SLCO1A2 (encoding OATP1A2) affects the pharmacokinetics and therapeutic effects of its substrates (Franke et al., 2009).
Drug substrates can also induce ABCB1 expression in the gut. For instance, long-term levothyroxine treatment induces enterocellular ABCB1 expression. Consequently, patients with hyperthyroidism may require larger-than-normal doses of multiple drugs, including (but not limited to): digoxin (Frye & Mathews, 1987), cyclosporine A (Jin et al., 2005), and propanalol (Wells et al., 1983); albeit, mulitiple transporters and enzymes in multiple organs may also be involved. Transporter expression in the gut is therefore based on pharmacotherapy and may influence relationships between polymorphisms and inter-individual variation in drug disposition and outcomes.
Genetic variation in transporters also affects the systemic exposure of a variety of drugs by altering the influx or efflux of drugs between the intestinal epithelial barrier and the gut lumen thereby influencing intestinal absorption. ABCG2 limits the oral bioavailability of its substrates by effluxing drugs back into the intestinal lumen. In vitro studies have shown that in ABCG2-deficient mice, the intestinal uptake, and thus oral bioavailability of quercetin and dietary carcinogens such as aflatoxin, was increased (Robey et al., 2009). Additionally, the ABCG2 421C>A SNP is associated with decreased plasma membrane expression, decreased transport, and a profound increase in oral bioavailability and plasma levels of sulfasalazine. The activity of sulfasalazine in ulcerative colitis (UC) depends on minimal absorption in the upper GI tract but decreased ABCG2 activity increases sulfasalazine absorption in the upper GI tract (Urquhart et al., 2008).
Liver
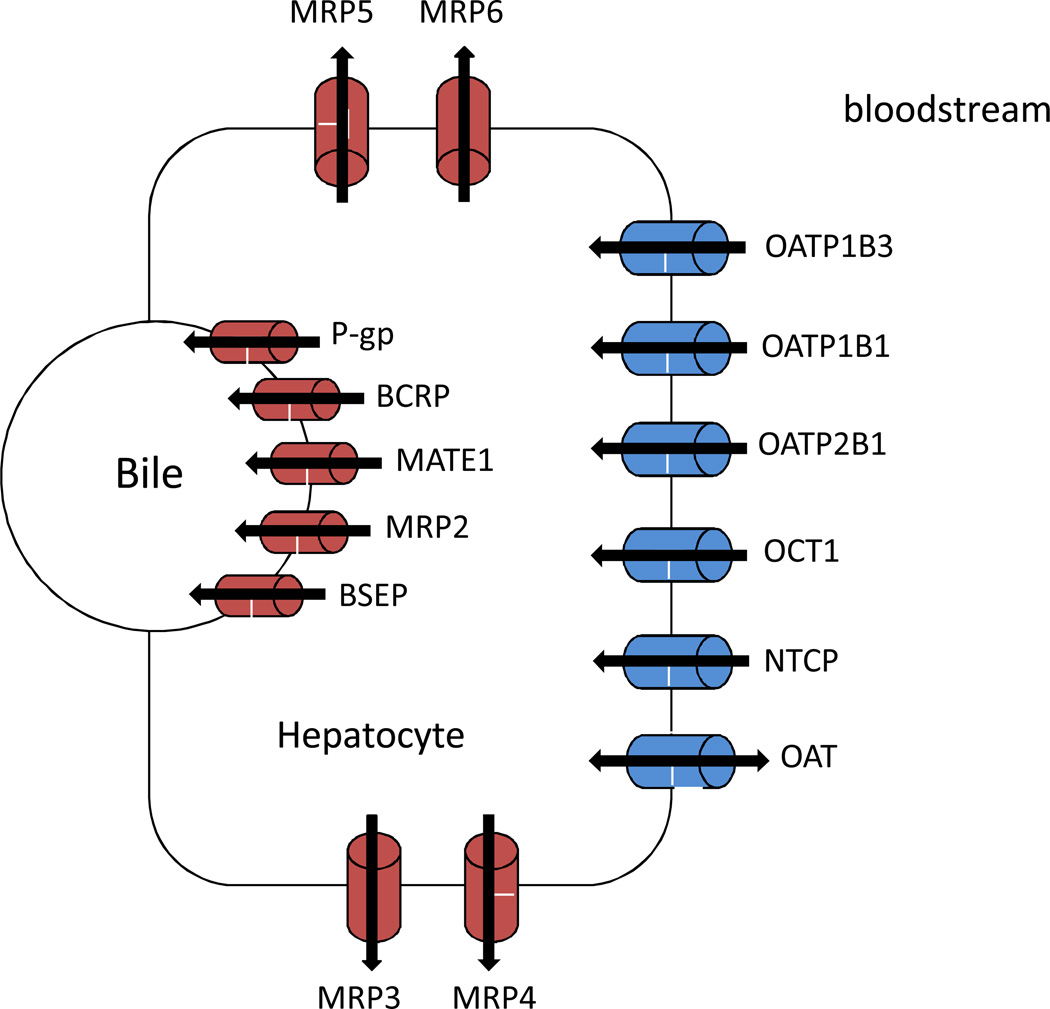
In the liver, influx transporters of the SLC family act to uptake drugs into hepatocytes where metabolism occurs. Efflux transporters, primarily ATP-dependent transporters that are located at the canalicular or sinusoidal membrane of hepatocytes, then act to export these drugs and their metabolites back into the blood or into the bile (Muller & Fromm, 2011) (Figure 2). Both influx and efflux transporters play a critical role in drug metabolism and clearance. For instance, statin metabolism and elimination is largely governed by how rapidly the liver is able to uptake, metabolize, and then clear these drugs (Rodrigues, 2010).
Figure 2.
Select transporters expressed on the canalicular and sinusoidal membrane of hepatocytes. Influx transporters (blue) transport drugs from the blood into hepatocytes where they are metabolized. Efflux transporters (red) then efflux drugs and their metabolites into bile or back into the blood. MATE1: multidrug and toxin extrusion 1 protein; MRP: multidrug resistance protein; NTCP: Sodium/taurocholate cotransporting polypeptide; OCT: Organic cation transporter.
Genetic variation in these transporters is a source of inter-individual variability of liver processing of drugs. For example, it was previously shown that in ABCG2-deficient mice, biliary excretion of various drugs, such as the antibiotics nitrofurantonin, ciprofloxacin, grepafloxacin, and ofloxacin was very low. Expression of transporters in the hepatocyte and other excretory organs can also influence ADME. For instance, individuals with certain ABCG2 SNPs experience more extensive reductions in low-density lipoprotein cholesterol (LDL-C) after treatment with rosuvastatin because they have reduced capacity to remove statins from the liver and have increased GI-uptake due to reductions in ABCG2 activities (Generaux et al., 2011). As a second example, individuals with certain SLCO1B1 polymorphism have a reduced rate of statin uptake resulting in slower metabolism and elimination of certain statins (Niemi et al., 2004). Therefore, hepatocellular transport of drugs is a key facilitator of the metabolism and elimination of drugs.
Kidney
Proximal tubule epithelia express tight junctions that limit substrate diffusion; thus transporters are essential mediators of renal elimination pathways. Influx transporters, including OATPs, OATs, and OCTs transport substrates into the renal epithelial cell while various MRPs transport substrates back into the bloodstream (Figure 3). Equilibrative nucleoside transporters (ENTs) function as bidirectional receptors at the basolateral membrane. Lumenal efflux transporters, such as ABCs, MRPS, and MATEs, efflux substrates into the lumen. Other transporters, such as OATPs, OATs, OCTNs, CNTs, PEPTs and others, either pump drugs into the lumen, remove drugs from the lumen, or function as bidirectional pumps.
Transporter SNPs modulate the renal elimination of drugs. For example, OCT1 and OCT2 polymorphisms are related to altered uptake of metformin in the proximal-renal tubule (Shu et al., 2001; Takane et al., 2008; Wang et al., 2008). More recent findings suggest that MATE1 is related to the elimination of metformin into the lumen, and polymorphisms in MATE contribute to the coordinate function of both transporters (Meyer zu Schwabedissen et al., 2010).
Transporter SNPs can also influence organ-specific toxicities. For instance, cisplatin-induced nephrotoxicity is caused by increased uptake of cisplatin by OCT2 into the proximal-renal tubular cells that are unable to eliminate cisplatin into the urine. A polymorphism in OCT2 that decreases renal uptake, 808G>T, is associated with lessened renal toxicity because less cisplatin is sequestered in the proximal tubule epithelia (Filipski et al., 2009).
Endothelial barriers
There are multiple blood-tissue barriers that shelter various tissues from circulating blood (Poduslo et al., 1994). Perhaps the best-known barrier is the BBB, which significantly limits the penetration of myriad substances into the CNS (Miller, 2010). The BBB is quite impermeable to the penetration of drugs, due to the presence of tight junctions between cells and the high expression of efflux transporters (i.e. P-glycoprotein) on the luminal membrane of the endothelial cells. Other blood-tissue barriers, however, can be more readily penetrated by various drugs. For instance, the blood-nerve-barrier (BNB) is composed of the endoneurial microvasculature and the innermost layers of the perineurium. Peripheral nerve microvascular endothelial cells (PnMECs) constitute the main interface between the peripheral nerves and the blood. PnMECs express P-glycoprotein, however, alterations to P-glycoprotein activity greatly increase drug penetration into the nerve, whereas the brain is less affected by decreased P-glycoprotein activity and remains highly impermeable to drugs (Saito et al., 2001). Similarly, P-glycoprotein is expressed in the endothelium of the heart and other tissues that are more permeable toward circulating ABCB1 substrates (Meissner et al., 2004; Meissner et al., 2002).
Genetic polymorphisms in transporters influence the degree to which the nervous system is exposed to various compounds. Few studies have characterized ABCB1 expression in endothelial cells based on genetic variation, but two evaluated ABCB1 SNP effects on expression in cardiac endothelium (Meissner et al., 2002). Contrary to most other tissues, patients carrying variant alleles at 2677G>T and 3435C>T actually express higher levels of ABCB1 in the endocardium and are more protected from cardiotoxic drugs (Meissner et al., 2004; Sissung et al., 2011). We previously demonstrated that patients carrying ABCB1 variants have a greater propensity to distribute drugs into extrahepatic tissues if they carry variant alleles (i.e., less ABCB1 function) (Sissung et al., 2008). While, it is currently unclear how ABCB1 variants influence endothelial expression of ABCB1, ABCB1 does protect various tissues from toxic substances, and ABCB1 alleles influence the degree to which these substances enter tissues (Sissung et al., 2008).
Hematopoietic cells
Nearly all hematopoietic cells express drug transporters that regulate various endobiotic processes, (Table 2) alter paracrine signaling, and can confer resistance to therapeutics in normal and diseased (e.g., HIV-infected or cancerous) cells (Kock et al., 2007). In normal lymphocytes ABCB1 expression ranges from 20–80% of B-cells and 30–100% of T-cells (Kock et al., 2007), whereas approximately 40–65% of leukocytes express ABCB1 (Chaudhary et al., 1992). Monocytic expression of ABCB1 has been problematic for HIV therapeutics that are substrates of ABCB1; this is especially true for antiretrovirals that induce ABCB1 expression in target cells (Kock et al., 2007). ABCB1 overexpression is also a problem in the treatment of leukemia. Some estimate that 45% of patients with newly diagnosed AML overexpress ABCB1, while 65% of patients with refractory AML overexpress ABCB1 (Robey et al., 2009). ABCB1 effluxes multiple therapeutics used to treat AML (Kock et al., 2007). However, ABCB1 also protects hematopoietic cells from cytotoxicity of certain anticancer therapies. For instance, taxanes can cause severe neutropenia, and higher ABCB1 expression appears to have a protective effect (Sissung et al., 2008; Sissung et al., 2006).
Table 2.
Selected ABC transporters expressed in peripheral blood cells (adapted from Kock et al., 2007) (Kock et al., 2007)
| Peripheral Blood Cell | Transporters Expressed |
|---|---|
| Natural Killer (NK) Cell | P-gp, MRP1, Mini-P-gp |
| T Cell | P-gp, MRP1, MRP2 |
| B Cell | P-gp, MRP1 |
| Erythrocyte | MRP1, MRP4, MRP5, BCRP, P-gp (?) |
| Monocyte | P-gp |
| Neutrophil G | P-gp |
| Eosinophil G | P-gp |
| Basophil G | P-gp |
| Platelet | MRP4, MRP1 |
Polymorphisms in ABCB1 and ABCG2 have been linked to differential transport of substrates in hematopoietic cells (Hitzl et al., 2001). These studies consistently found that carriers of ABCB1 variant alleles have lower expression and function of ABCB1 in multiple cell populations (Hitzl et al., 2001). Studies in mice lacking various ABC transporters demonstrated that normal physiological processes were interrupted while certain hematopoietic cells were more susceptible to drug-induced toxicity (Kock et al., 2007). Therefore, ABCB1 variants alter the physiology, disease, and treatment with agents that target or cause undesirable toxicity in hematopoietic cells.
Transporter pharmacogenetics in normal physiology and pathophysiology
Transporter genetic variation is responsible for a variety of effects of pharmacotherapy on different organ systems and tissues. However, the ultimate measure of the complexities of transporters is how an individual patient responds to drug therapy. Transporter genetics can influence disease etiology, disease progression, and the disposition towards endogenous substrates, drug clearance, drug distribution, and drug resistance; all of these factors then influence the response to treatment. Therefore, transporter pharmacogenetics is very complex with a single variant potentially influencing a variety of processes involved in diseases and treatments. Consequently, there is considerable heterogeneity in the literature and few true pharmacogenetic tests are currently available that inform therapy decisions.
Transporter genetics affect physiological substrates
Studies are emerging suggesting that commonly inherited transporter polymorphisms can affect inter-individual variation in normal physiology. For instance, polymorphisms in transporters are related to sterol homeostasis, lipid metabolism, uric acid elimination, conjugated bilirubin extrusion into the bile, and others (Ueda, 2011). For example, the ABCG2 421C>A SNP that encode Q141K is strongly associated with hyperuricemia (i.e., gout) such that ~10% of gout cases in whites are attributable to this SNP (Dehghan et al., 2008; Dresser et al., 2005; Woodward et al., 2009). ABCG2 is localized to the brush border of the proximal tubule where it plays a role in renal filtration of urate, and Q141K results in a 53% reduction in the rate of urate efflux (Woodward et al., 2009).
Transporter expression is also affected by physiologic substrates that interact with various nuclear receptors (i.e., PXR, CAR, MR, GR, FXR, VDR, etc.). PXR is involved in the expression of a wide array of transporters and metabolizing enzymes (Tirona, 2011). PXR is activated by myriad substrates such as xenobiotics, glucocorticoids, antibiotics, and bile acids; these substrates bind to PXR resulting in its nuclear localization and transcription of its target genes (Tirona, 2011).
PXR expression was very low in endoscopic biopsies of patients with ulcerative colitis (UC), resulting in reduced expression of PXR-induced detoxification enzymes and drug transporters (Langmann et al., 2004; Ufer et al., 2009). Several investigators have studied ABCB1 and PXR genetic variation in UC. Four studies investigated the role of PXR SNPs in adult UC so far with three confirming a relationship between PXR SNPs and UC (Andersen et al.; Dring et al., 2006; Martinez et al., 2007), and one not confirming the relationship (Choudhuri & Klaassen, 2006), though in the latter study, SNP frequency differed in the control population, not the patient population. Multiple studies (Ardizzone et al., 2007; Fiedler et al., 2007; Ho et al., 2005; Ho et al., 2006; Huebner et al., 2009; Juyal et al., 2009; Martinez et al., 2007; Ostergaard et al., 2009; Osuga et al., 2006; Tahara et al., 2008; Urcelay et al., 2006) and two meta-analyses (Annese et al., 2006; Onnie et al., 2006) have demonstrated that ABCB1 variants are related to the risk of UC, with few demonstrating no relationship (Fischer et al., 2007; Oostenbrug et al., 2006; Palmieri et al., 2005). Martinez et al. ((Martinez et al., 2007) detected significant interaction between ABCB1 and PXR in UC. This suggested an epistatic interaction whereby PXR And ABCB1 allelic variation influences ABCB1 expression in the UC gut, and therefore both alleles contribute to disease susceptibility by altering ABCB1 expression. Moreover, the 3435C>T SNP has recently been related to UC therapy with tacrolimus due to local uptake deficiencies (Herrlinger et al., 2011). Therefore, it appears that breakdown of the gut detoxification system, merely by altering the expression of the ABCB1 transporter, is involved in UC etiology and treatment.
Disease biology can also influence transporter interactions with physiologic substrates. For example, we identified a testosterone-transporting function of OATP1B3 in which commonly inherited polymorphisms (i.e. S112A and M233I) influenced the degree to which testosterone was imported into cells (Hamada et al., 2008). Although OATP1B3 was originally considered a liver-specific transporter, we also showed that OATP1B3 was aberrantly expressed in a variety of tumors, including prostate cancer (Hamada et al., 2008; Pressler et al., 2011). We then determined that polymorphisms in SLCO1B3 were associated in the duration of the response to androgen-deprivation therapy (ADT), and overall survival from diagnosis in men with prostate cancer (Hamada et al., 2008; Sharifi et al., 2008; Yang et al., 2011). This has since been confirmed by others (Wright et al., 2011). It remains to be determined if prostate tumors are more susceptible to certain OATP1B3 substrate drugs based on their increased expression at the site of the tumor.
The physiological and pathophysiological role of transporter polymorphisms is poorly studied in comparison to their role in drug disposition. It is therefore crucial that future investigations focus on how inherited genetic variation in transporters influences the etiology, disease-state, and treatment of different illnesses that are treated with pharmacological substances that are themselves substrates of drug transporters. Such research will make the application of transporter pharmacogenetics in personalized medicine more likely.
Transporter pharmacogenetics in clinical pharmacology
Transporter genetics in drug pharmacokinetics
Transporter genetic variants are heavily linked to inter-individual variation in drug pharmacokinetics. Initial reports indicated that the ABCB1 3435C>T polymorphism was related to lowered ABCB1 expression and higher digoxin concentrations (Hoffmeyer et al., 2000). In addition, the ABCB1 2677G>T/A and 3435C>T haplotype was more strongly related to digoxin pharmacokinetics (i.e. variants had high AUC) (Johne et al., 2002; Verstuyft et al., 2003). Other studies of the relationship between ABCB1 SNPs and drug pharmacokinetics have been largely inconsistent (reviewed in (Cascorbi, 2006; Eichelbaum et al., 2004; Haufroid, 2011; Sakaeda, 2005)).
This inconsistency is largely due to the multiple functions of ABCB1. For example, ABCB1 also regulates absorption of drugs through the gut wall (oral drugs), limits drug biodistribution into a variety of extrahepatic tissues, transports drugs into the cerebro-spinal fluid, eliminates drugs through both the renal and hepatobilliary routes, and mediates enterohepatic recirculation. Moreover, a single variant in ABCB1 can produce opposite effects on transporter expression in different tissues (Hoffmeyer et al., 2000; Meissner et al., 2004) and there are a number of synergistic compensatory transport pathways for certain drugs (Zhou et al., 2009). As such, the contribution of ABCB1 pharmacogenetics towards drug ADME is extremely complex and is largely dependent upon the properties of each individual drug. Adding to the complexity of these studies is the heterogeneity of patient populations undergoing a variety of therapies and cotherapies.
As an example, multiple studies have showed that docetaxel pharmacokinetics is related to ABCB1 alleles, whereas other studies failed to confirm these findings (Baker et al., 2009; Bosch et al., 2006; Chew et al., 2011; Fajac et al., 2010; Sissung et al., 2008; Tran et al., 2006). This is likely because intravenous docetaxel is biodistributed differently in the extrahepatic tissues of patients carrying different genetic variants, is eliminated by ABCB1 to a different extent, and undergoes enterohepatic recirculation. Each of these events can alter the plasma pharmcokinetic profile of docetaxel and each event is likely influenced by ABCB1 SNPs differently. In addition, docetaxel is heavily transported by ABCB1, and ABCC2 may compensate for deficiencies in ABCB1-mediated transport. Therefore, it is exceedingly difficult to ascertain a true mechanism behind the contribution of ABCB1 polymorphisms to changes in drug pharmacokinetics.
In summary, ABCB1 is poorly understood for the following reasons: (i) high genetic variation in different populations (i.e. different haplotypes) that are poorly understood; (ii) variability in a given SNPs influences on expression/function in different organ systems; (iii) compensatory transporters; (iv) expression in multiple organs influences several biological processes involved in absorption, distribution, elimination, and enterohepatic recirculation; (v) ABCB1 transports myriad substrates; and (vi) physiological and disease influences on drug transport.
SNPs in other transporters clearly influence drug therapy. These transporters tend to be expressed in fewer tissues and regulate fewer biological processes. For example, the OATP1B1 is almost exclusively expressed in hepatocytes where it is involved in the uptake of bile acids, eicosanoids, DHEA, estrogens, and other endogenous compounds (Niemi et al., 2011). Thus, OATP1B1 is primarily involved in influxing compounds into the liver where they are subsequently metabolized, not in multiple other biological pathways relating to drug pharmacokinetics. OATP1B1 is known to transport all statins in current clinical use, and the SLCO1B1 (encoding OATP1B1) 521C>T SNP was initially related to increased AUC of pravastatin (Niemi et al., 2004) and the unfavorable plasma pharmacokinetics of cholesterol synthesis biomarkers (Niemi et al., 2005). A comparison of all statins demonstrated that simvastatin pharmacokinetics was heavily influenced by SLCO1B1 521C>T (3.2-fold increase in AUC in 521CC carriers) followed by atorvastatin, and pravastatin or rosuvastatin. Fluvastatin AUC was not affected by SLCO1B1 521C>T presumably because fluvastatin is influxed into liver by several other uptake transporters (Niemi et al., 2011).
Therefore, transporter SNPs are important determinants of inter-individual variation in the pharamcokinetic profiles of a multitude of agents. However, plasma pharmacokinetics is typically derived from plasma concentration vs. time profiles that are insufficient to detect the multiple contributions of transporters to bodily functions that affect the various aspects of pharmacokinetics. Therefore, multivariate pharmacokinetic models must incorporate well-studied genetic variants to better represent the contribution of transporters to the ADME properties of drugs.
Drug efficacy
Multiple studies have investigated how transporter polymorphisms are related to the benefit of various agents (Sissung et al., 2010). For instance, evidence suggests that the anti-platelet activity of clopidogrel is linked to ABCB1 3435C>T (reviewed in (Ellis et al., 2009; Johnson et al., 2011)). An initial study determined that patients carrying two variant alleles at ABCB1 3435C>T had a higher event rate at 1 year than those carrying wild-type alleles. Those carrying variant alleles in both CYP2C19 And ABCB1 had the highest risk of events (HR(95%CI)=5.31(2.13–13.20)) (Simon et al., 2009). This finding was not replicated in a GWAS study of the healthy Amish population, although it is unclear why the two studies present different findings (Shuldiner et al., 2009). In a larger study of patients with acute coronary syndromes, ABCB1 3435C>T was again strongly related to increased cardiovascular death, MI, or stroke during clopidogrel therapy (HR(95%CI) = 1.72(1.22–2.44)), but did not have increased risk of reduced prasugrel efficacy (Mega et al., 2010). This same study demonstrated that healthy volunteers receiving clopidogrel had a 7.3% reduction in maximum platelet aggregation if they carried 3435TT genotypes. A more recent study conflicted with these results (Wallentin et al., 2010).
Drug toxicity
Statin-induced myopathy is directly related to the AUC of circulating active statin metabolites. As such, the SLCO1B1 SNPs that influence the exposure to active statin metabolites are also associated with statin-induced myopathy (Niemi, 2010). However, not all pharmacogenetic relationships with toxicity are due to plasma exposure-related differences. For instance, the aforementioned blood-endothelial barriers polymorphically exclude certain drugs from different tissues. For instance, we previously showed that taxane-induced neutropenia and peripheral neuropathy were associated with SNPs in ABCB1; however, these same SNPs were not related to overall exposure to docetaxel in the plasma (Baker et al., 2009; Bosch et al., 2006; Chew et al., 2011; Fajac et al., 2010; Sissung et al., 2008; Tran et al., 2006). We have also shown that intracardiac romidepsin exposure and QT-interval were increased in mice lacking ABCB1, although the plasma pharmacokinetics was unchanged (Sissung et al., 2011). Humans have polymorphic expression of ABCB1 in the cardiac endothelium (Meissner et al., 2004), and those carrying alleles associated with low intracardiac ABCB1 expression had the highest QT-interval prolongation following romidepsin (Sissung et al., 2011). Therefore, since ABCB1 expression in hematopoietic cells and endothelial blood-tissue barriers varies with inherited ABCB1 polymorphisms, these polymorphisms also alter the exposure of certain tissues independently of the plasma.
Drug-drug interactions
Many drugs are substrates of multiple transporters and are also metabolized by similar cytochrome P450. This has complicated the evaluation of drug-drug interactions with transporters because genetic variation in a single transporter is unlikely to alter the ADME properties of a given drug consistently among a large group of patients with various comorbidities and cotherapies. Nonetheless, inhibition of polymorphic transporters clearly influences several drug-drug interactions. For instance, certain drugs (e.g., gemfibrozil) inhibit multiple transporters and cytochrome P450s in the liver and elsewhere (Muller & Fromm, 2011). The hepatocyte is the site for the pharmacological action of statin drugs while the plasma exposure to statins and their metabolites is responsible for statin-induced myotoxicity (Rodrigues, 2010). Since OATP1B1 is mainly a liver influx transporter, inhibition of OATP1B1 increases the myotoxic effects of statins while reducing their activity as HMG-CoA reductase inhibitors.
CONCLUSION
Transporter pharmacogenetics is an important source of inter-individual variation in organ function, physiology, disease, and drug treatment. Despite the large number of studies devoted to transporter pharmacogenetics and transporters in general, there has been relatively little progress in using germline genetic markers in transporters in the clinic. Future studies must be broadened in order to capture the intricacies of how a single variant can influence the entire spectrum of bodily functions and drug disposition/activity.
Acknowledgments
Financial Support
Support provided by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Bethesda, Md.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
REFERENCES
- Andersen V, Christensen J, Ernst A, Jacobsen BA, Tjonneland A, Krarup HB, Vogel U. Polymorphisms in NF-kappaB, PXR, LXR, PPARgamma and risk of inflammatory bowel disease. World J Gastroenterol. 17(2):197–206. doi: 10.3748/wjg.v17.i2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol. 2006;12(23):3636–3644. doi: 10.3748/wjg.v12.i23.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzone S, Maconi G, Bianchi V, Russo A, Colombo E, Cassinotti A, Penati C, Tenchini ML, Bianchi Porro G. Multidrug resistance 1 gene polymorphism and susceptibility to inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(5):516–523. doi: 10.1002/ibd.20108. [DOI] [PubMed] [Google Scholar]
- Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, Messersmith WA, Wolff AC, Carducci MA, Sparreboom A. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85(2):155–163. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin AA, Cardinal H, Barama A, Pichette V, Hebert MJ, Roger M. Organic anion transporting polypeptide 1B1 (OATP1B1) and OATP1B3: genetic variability and haplotype analysis in white Canadians. Drug Metab Pharmacokinet. 2010;25(5):508–515. doi: 10.2133/dmpk.dmpk-10-sh-046. [DOI] [PubMed] [Google Scholar]
- Bosch TM, Huitema AD, Doodeman VD, Jansen R, Witteveen E, Smit WM, Jansen RL, van Herpen CM, Soesan M, Beijnen JH, Schellens JH. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res. 2006;12(19):5786–5793. doi: 10.1158/1078-0432.CCR-05-2649. [DOI] [PubMed] [Google Scholar]
- Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112(2):457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80(11):2735–2739. [PubMed] [Google Scholar]
- Chew SC, Singh O, Chen X, Ramasamy RD, Kulkarni T, Lee EJ, Tan EH, Lim WT, Chowbay B. The effects of CYP3A4, CYP3A5, ABCB1, ABCC2, ABCG2 and SLCO1B3 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of docetaxel in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol. 2011;67(6):1471–1478. doi: 10.1007/s00280-011-1625-9. [DOI] [PubMed] [Google Scholar]
- Choi MK, Song IS. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet. 2008;23(4):243–253. doi: 10.2133/dmpk.23.243. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25(4):231–259. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7(2):159–174. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27(8):866–871. [PubMed] [Google Scholar]
- Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77(3):170–177. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O'Donoghue D, O'Sullivan M, O'Morain C, Mahmud N, Wikstrom AC, Kelleher D, McManus R. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006;130(2):341–348. doi: 10.1053/j.gastro.2005.12.008. quiz 592. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M, Fromm MF, Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit. 2004;26(2):180–185. doi: 10.1097/00007691-200404000-00017. [DOI] [PubMed] [Google Scholar]
- Ellis KJ, Stouffer GA, McLeod HL, Lee CR. Clopidogrel pharmacogenomics and risk of inadequate platelet inhibition: US FDA recommendations. Pharmacogenomics. 2009;10(11):1799–1817. doi: 10.2217/pgs.09.143. [DOI] [PubMed] [Google Scholar]
- Fajac A, Gligorov J, Rezai K, Levy P, Levy E, Selle F, Beerblock K, Avenin D, Saintigny P, Hugonin S, Bernaudin JF, Lokiec F. Effect of ABCB1 C3435T polymorphism on docetaxel pharmacokinetics according to menopausal status in breast cancer patients. Br J Cancer. 2010;103(4):560–566. doi: 10.1038/sj.bjc.6605789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler T, Buning C, Reuter W, Pitre G, Gentz E, Schmidt HH, Buttner J, Ockenga J, Gerloff T, Meisel C, Lochs H, Roots I, Kopke K, Johne A. Possible role of MDR1 two-locus genotypes for young-age onset ulcerative colitis but not Crohn's disease. Eur J Clin Pharmacol. 2007;63(10):917–925. doi: 10.1007/s00228-007-0334-0. [DOI] [PubMed] [Google Scholar]
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86(4):396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Lakatos PL, Lakatos L, Kovacs A, Molnar T, Altorjay I, Papp M, Szilvasi A, Tulassay Z, Osztovits J, Papp J, Demeter P, Schwab R, Tordai A, Andrikovics H. ATP-binding cassette transporter ABCG2 (BCRP) and ABCB1 (MDR1) variants are not associated with disease susceptibility, disease phenotype response to medical therapy or need for surgeryin Hungarian patients with inflammatory bowel diseases. Scand J Gastroenterol. 2007;42(6):726–733. doi: 10.1080/00365520601101559. [DOI] [PubMed] [Google Scholar]
- Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Scherkenbach LA, Sparreboom A. Pharmacogenetics of the organic anion transporting polypeptide 1A2. Pharmacogenomics. 2009;10(3):339–344. doi: 10.2217/14622416.10.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25(8):423–429. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Frye R, Mathews SE. Effect of digoxin-like immunoreactive factor on the TDx digoxin II assay. Clin Chem. 1987;33(4):629–630. [PubMed] [Google Scholar]
- Generaux GT, Bonomo FM, Johnson M, Doan KM. Impact of SLCO1B1 (OATP1B1) and ABCG2 (BCRP) genetic polymorphisms and inhibition on LDL-C lowering and myopathy of statins. Xenobiotica. 2011;41(8):639–651. doi: 10.3109/00498254.2011.562566. [DOI] [PubMed] [Google Scholar]
- Haenisch S, May K, Wegner D, Caliebe A, Cascorbi I, Siegmund W. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genomics. 2008;18(4):357–365. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]
- Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, Mitsuya H, Dahut WL, Figg WD. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14(11):3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufroid V. Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCC2 and their impact on drug disposition. Curr Drug Targets. 2011;12(5):631–646. doi: 10.2174/138945011795378487. [DOI] [PubMed] [Google Scholar]
- Herrlinger KR, Koc H, Winter S, Teml A, Stange EF, Fellermann K, Fritz P, Schwab M, Schaeffeler E. ABCB1 single-nucleotide polymorphisms determine tacrolimus response in patients with ulcerative colitis. Clin Pharmacol Ther. 2011;89(3):422–428. doi: 10.1038/clpt.2010.348. [DOI] [PubMed] [Google Scholar]
- Hirouchi M, Suzuki H, Itoda M, Ozawa S, Sawada J, Ieiri I, Ohtsubo K, Sugiyama Y. Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm Res. 2004;21(5):742–748. doi: 10.1023/b:pham.0000026422.06207.33. [DOI] [PubMed] [Google Scholar]
- Hitzl M, Drescher S, van der Kuip H, Schaffeler E, Fischer J, Schwab M, Eichelbaum M, Fromm MF. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11(4):293–298. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, Arnott ID, Satsangi J. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128(2):288–296. doi: 10.1053/j.gastro.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Ho GT, Soranzo N, Nimmo ER, Tenesa A, Goldstein DB, Satsangi J. ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: discrimination of critical variants using a gene-wide haplotype tagging approach. Hum Mol Genet. 2006;15(5):797–805. doi: 10.1093/hmg/ddi494. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, Bates SE. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1) Cancer Biol Ther. 2002;1(6):696–702. doi: 10.4161/cbt.322. [DOI] [PubMed] [Google Scholar]
- Huebner C, Browning BL, Petermann I, Han DY, Philpott M, Barclay M, Gearry R, McCulloch A, Demmers P, Ferguson LR. Genetic analysis of MDR1 and inflammatory bowel disease reveals protective effect of heterozygous variants for ulcerative colitis. Inflamm Bowel Dis. 2009;15(12):1784–1793. doi: 10.1002/ibd.21019. [DOI] [PubMed] [Google Scholar]
- Ito K, Oleschuk CJ, Westlake C, Vasa MZ, Deeley RG, Cole SP. Mutation of Trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. J Biol Chem. 2001;276(41):38108–38114. doi: 10.1074/jbc.M105160200. [DOI] [PubMed] [Google Scholar]
- Itoda M, Saito Y, Soyama A, Saeki M, Murayama N, Ishida S, Sai K, Nagano M, Suzuki H, Sugiyama Y, Ozawa S, Sawada J., Ji Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: an association between single nucleotide polymorphisms in the 5'-untranslated region and exon 28. Drug Metab Dispos. 2002;30(4):363–364. doi: 10.1124/dmd.30.4.363. [DOI] [PubMed] [Google Scholar]
- Jin M, Shimada T, Shintani M, Yokogawa K, Nomura M, Miyamoto K. Long-term levothyroxine treatment decreases the oral bioavailability of cyclosporin A by inducing P-glycoprotein in small intestine. Drug Metab Pharmacokinet. 2005;20(5):324–330. doi: 10.2133/dmpk.20.324. [DOI] [PubMed] [Google Scholar]
- Johne A, Kopke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, Eichelbaum M, Brockmoller J, Cascorbi I, Roots I. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72(5):584–594. doi: 10.1067/mcp.2002.129196. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Cavallari LH, Beitelshees AL, Lewis JP, Shuldiner AR, Roden DM. Pharmacogenomics: application to the management of cardiovascular disease. Clin Pharmacol Ther. 2011;90(4):519–531. doi: 10.1038/clpt.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juyal G, Midha V, Amre D, Sood A, Seidman E, Thelma BK. Associations between common variants in the MDR1 (ABCB1) gene and ulcerative colitis among North Indians. Pharmacogenet Genomics. 2009;19(1):77–85. doi: 10.1097/FPC.0b013e32831a9abe. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M, Tanigawara Y, Flockhart DA, Desta Z, Skaar TC, Aki F, Hirata K, Takatsuka Y, Okazaki M, Ohsumi S, Yamakawa T, Sasa M, Nakamura Y, Zembutsu H. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28(8):1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock K, Grube M, Jedlitschky G, Oevermann L, Siegmund W, Ritter CA, Kroemer HK. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: relevance for physiology and pharmacotherapy. Clin Pharmacokinet. 2007;46(6):449–470. doi: 10.2165/00003088-200746060-00001. [DOI] [PubMed] [Google Scholar]
- Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13(8):481–494. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127(1):26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Letourneau IJ, Deeley RG, Cole SP. Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1) Pharmacogenet Genomics. 2005;15(9):647–657. doi: 10.1097/01.fpc.0000173484.51807.48. [DOI] [PubMed] [Google Scholar]
- Letschert K, Keppler D, Konig J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8) Pharmacogenetics. 2004;14(7):441–452. doi: 10.1097/01.fpc.0000114744.08559.92. [DOI] [PubMed] [Google Scholar]
- Longo R, D'Andrea M, Sarmiento R, Gasparini G. Pharmacogenetics in breast cancer: focus on hormone therapy, taxanes, trastuzumab and bevacizumab. Expert Opin Investig Drugs. 2010;19(Suppl 1):S41–S50. doi: 10.1517/13543781003732701. [DOI] [PubMed] [Google Scholar]
- Martinez A, Marquez A, Mendoza J, Taxonera C, Fernandez-Arquero M, Diaz-Rubio M, de la Concha EG, Urcelay E. Role of the PXR gene locus in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1484–1487. doi: 10.1002/ibd.20252. [DOI] [PubMed] [Google Scholar]
- Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Jedlitschky G, Meyer zu Schwabedissen H, Dazert P, Eckel L, Vogelgesang S, Warzok RW, Bohm M, Lehmann C, Wendt M, Cascorbi I, Kroemer HK. Modulation of multidrug resistance P-glycoprotein 1 (ABCB1) expression in human heart by hereditary polymorphisms. Pharmacogenetics. 2004;14(6):381–385. doi: 10.1097/00008571-200406000-00007. [DOI] [PubMed] [Google Scholar]
- Meissner K, Sperker B, Karsten C, Meyer Zu Schwabedissen H, Seeland U, Bohm M, Bien S, Dazert P, Kunert-Keil C, Vogelgesang S, Warzok R, Siegmund W, Cascorbi I, Wendt M, Kroemer HK. Expression and localization of P-glycoprotein in human heart: effects of cardiomyopathy. J Histochem Cytochem. 2002;50(10):1351–1356. doi: 10.1177/002215540205001008. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol. 2010;298(4):F997–F1005. doi: 10.1152/ajprenal.00431.2009. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31(6):246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Fromm MF. Transporter-mediated drug-drug interactions. Pharmacogenomics. 2011;12(7):1017–1037. doi: 10.2217/pgs.11.44. [DOI] [PubMed] [Google Scholar]
- Ni Z, Mao Q. ATP-binding cassette efflux transporters in human placenta. Curr Pharm Biotechnol. 2011;12(4):674–685. doi: 10.2174/138920111795164057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87(1):130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- Niemi M, Neuvonen PJ, Hofmann U, Backman JT, Schwab M, Lutjohann D, von Bergmann K, Eichelbaum M, Kivisto KT. Acute effects of pravastatin on cholesterol synthesis are associated with SLCO1B1 (encoding OATP1B1) haplotype *17. Pharmacogenet Genomics. 2005;15(5):303–309. doi: 10.1097/01213011-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ, Eichelbaum M, Kivisto KT. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14(7):429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, Mathew CG. Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis. 2006;12(4):263–271. doi: 10.1097/01.MIB.0000209791.98866.ba. [DOI] [PubMed] [Google Scholar]
- Oostenbrug LE, Dijkstra G, Nolte IM, van Dullemen HM, Oosterom E, Faber KN, de Jong DJ, van der Linde K, te Meerman GJ, van der Steege G, Kleibeuker JH, Jansen PL. Absence of association between the multidrug resistance (MDR1) gene and inflammatory bowel disease. Scand J Gastroenterol. 2006;41(10):1174–1182. doi: 10.1080/00365520600575746. [DOI] [PubMed] [Google Scholar]
- Ostergaard M, Ernst A, Labouriau R, Dagiliene E, Krarup HB, Christensen M, Thorsgaard N, Jacobsen BA, Tage-Jensen U, Overvad K, Autrup H, Andersen V. Cyclooxygenase-2, multidrug resistance 1, and breast cancer resistance protein gene polymorphisms and inflammatory bowel disease in the Danish population. Scand J Gastroenterol. 2009;44(1):65–73. doi: 10.1080/00365520802400826. [DOI] [PubMed] [Google Scholar]
- Osuga T, Sakaeda T, Nakamura T, Yamada T, Koyama T, Tamura T, Aoyama N, Okamura N, Kasuga M, Okumura K. MDR1 C3435T polymorphism is predictive of later onset of ulcerative colitis in Japanese. Biol Pharm Bull. 2006;29(2):324–329. doi: 10.1248/bpb.29.324. [DOI] [PubMed] [Google Scholar]
- Palmieri O, Latiano A, Valvano R, D'Inca R, Vecchi M, Sturniolo GC, Saibeni S, Bossa F, Latiano T, Devoto M, Andriulli A, Annese V. Multidrug resistance 1 gene polymorphisms are not associated with inflammatory bowel disease and response to therapy in Italian patients. Aliment Pharmacol Ther. 2005;22(11–12):1129–1138. doi: 10.1111/j.1365-2036.2005.02701.x. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Neuvonen PJ, Niemi M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics. 2008;9(1):19–33. doi: 10.2217/14622416.9.1.19. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci U S A. 1994;91(12):5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli L, Lemos C, Peters GJ, Paradiso A, Azzariti A. Intracellular trafficking of MDR transporters and relevance of SNPs. Curr Top Med Chem. 2009;9(2):197–208. doi: 10.2174/156802609787521562. [DOI] [PubMed] [Google Scholar]
- Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. Expression of OATP family members in hormone-related cancers: potential markers of progression. PLoS One. 6(5):e20372. doi: 10.1371/journal.pone.0020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999;96(7):3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Massey PR, Amiri-Kordestani L, Bates SE. ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem. 2010;10(8):625–633. doi: 10.2174/187152010794473957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61(1):3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AC. Efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol. 2010;6(5):621–632. doi: 10.1517/17425251003713519. [DOI] [PubMed] [Google Scholar]
- Saito T, Zhang ZJ, Ohtsubo T, Noda I, Shibamori Y, Yamamoto T, Saito H. Homozygous disruption of the mdrla P-glycoprotein gene affects blood-nerve barrier function in mice administered with neurotoxic drugs. Acta Otolaryngol. 2001;121(6):735–742. doi: 10.1080/00016480152583683. [DOI] [PubMed] [Google Scholar]
- Sakaeda T. MDR1 genotype-related pharmacokinetics: fact or fiction? Drug Metab Pharmacokinet. 2005;20(6):391–414. doi: 10.2133/dmpk.20.391. [DOI] [PubMed] [Google Scholar]
- Schellens JH, Malingre MM, Kruijtzer CM, Bardelmeijer HA, van Tellingen O, Schinkel AH, Beijnen JH. Modulation of oral bioavailability of anticancer drugs: from mouse to man. Eur J Pharm Sci. 2000;12(2):103–110. doi: 10.1016/s0928-0987(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, Zijlmans JM, Fibbe WE, Borst P. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94(8):4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz UI, Meyer zu Schwabedissen HE, Tirona RG, Suzuki A, Leake BF, Mokrab Y, Mizuguchi K, Ho RH, Kim RB. Identification of novel functional organic anion-transporting polypeptide 1B3 polymorphisms and assessment of substrate specificity. Pharmacogenet Genomics. 2011;21(3):103–114. doi: 10.1097/FPC.0b013e328342f5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Hamada A, Sissung T, Danesi R, Venzon D, Baum C, Gulley JL, Price DK, Dahut WL, Figg WD. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102(5):617–621. doi: 10.1111/j.1464-410X.2008.07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50(1):161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J Pharmacol Exp Ther. 2001;299(1):392–398. [PubMed] [Google Scholar]
- Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- Sissung TM, Baum CE, Deeken J, Price DK, Aragon-Ching J, Steinberg SM, Dahut W, Sparreboom A, Figg WD. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14(14):4543–4549. doi: 10.1158/1078-0432.CCR-07-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44(2):152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissung TM, Gardner ER, Piekarz RL, Howden R, Chen X, Woo S, Franke R, Clark JA, Miller-DeGraff L, Steinberg SM, Venzon D, Liewehr D, Kleeberger SR, Bates SE, Price DK, Rosing DR, Cabell C, Sparreboom A, Figg WD. Impact of ABCB1 allelic variants on QTc interval prolongation. Clin Cancer Res. 2011;17(4):937–946. doi: 10.1158/1078-0432.CCR-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, Mielke S. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42(17):2893–2896. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Adv Drug Deliv Rev. 2002;54(10):1311–1331. doi: 10.1016/s0169-409x(02)00075-3. [DOI] [PubMed] [Google Scholar]
- Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab. 2011;12(2):139–153. doi: 10.2174/138920011795016863. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kagawa Y, Takaai M, Taguchi M, Hashimoto Y. Directional transcellular transport of bisoprolol in P-glycoprotein-expressed LLC-GA5-COL150 cells, but not in renal epithelial LLC-PK1 Cells. Drug Metab Pharmacokinet. 2008;23(5):340–346. doi: 10.2133/dmpk.23.340. [DOI] [PubMed] [Google Scholar]
- Takane H, Shikata E, Otsubo K, Higuchi S, Ieiri I. Polymorphism in human organic cation transporters and metformin action. Pharmacogenomics. 2008;9(4):415–422. doi: 10.2217/14622416.9.4.415. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirona RG. Molecular mechanisms of drug transporter regulation. Handb Exp Pharmacol. 2011;(201):373–402. doi: 10.1007/978-3-642-14541-4_10. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276(38):35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, Dieras V, Pons G, Goldwasser F, Treluyer JM. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79(6):570–580. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ueda K. ABC proteins protect the human body and maintain optimal health. Biosci Biotechnol Biochem. 2011;75(3):401–409. doi: 10.1271/bbb.100816. [DOI] [PubMed] [Google Scholar]
- Ufer M, Hasler R, Jacobs G, Haenisch S, Lachelt S, Faltraco F, Sina C, Rosenstiel P, Nikolaus S, Schreiber S, Cascorbi I. Decreased sigmoidal ABCB1 (P-glycoprotein) expression in ulcerative colitis is associated with disease activity. Pharmacogenomics. 2009;10(12):1941–1953. doi: 10.2217/pgs.09.128. [DOI] [PubMed] [Google Scholar]
- Urcelay E, Mendoza JL, Martin MC, Mas A, Martinez A, Taxonera C, Fernandez-Arquero M, Diaz-Rubio M, de la Concha EG. MDR1 gene: susceptibility in Spanish Crohn's disease and ulcerative colitis patients. Inflamm Bowel Dis. 2006;12(1):33–37. doi: 10.1097/01.mib.0000194184.92671.78. [DOI] [PubMed] [Google Scholar]
- Urquhart BL, Ware JA, Tirona RG, Ho RH, Leake BF, Schwarz UI, Zaher H, Palandra J, Gregor JC, Dresser GK, Kim RB. Breast cancer resistance protein (ABCG2) and drug disposition: intestinal expression, polymorphisms and sulfasalazine as an in vivo probe. Pharmacogenet Genomics. 2008;18(5):439–448. doi: 10.1097/FPC.0b013e3282f974dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstuyft C, Schwab M, Schaeffeler E, Kerb R, Brinkmann U, Jaillon P, Funck-Brentano C, Becquemont L. Digoxin pharmacokinetics and MDR1 genetic polymorphisms. Eur J Clin Pharmacol. 2003;58(12):809–812. doi: 10.1007/s00228-003-0567-5. [DOI] [PubMed] [Google Scholar]
- Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(9749):1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang B, Tang K, Lee EJ, Chong SS, Lee CG. A functional polymorphism within the MRP1 gene locus identified through its genomic signature of positive selection. Hum Mol Genet. 2005;14(14):2075–2087. doi: 10.1093/hmg/ddi212. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18(7):637–645. doi: 10.1097/FPC.0b013e328302cd41. [DOI] [PubMed] [Google Scholar]
- Wells PG, Feely J, Wilkinson GR, Wood AJ. Effect of thyrotoxicosis on liver blood flow and propranolol disposition after long-term dosing. Clin Pharmacol Ther. 1983;33(5):603–608. doi: 10.1038/clpt.1983.81. [DOI] [PubMed] [Google Scholar]
- Wolf SJ, Bachtiar M, Wang J, Sim TS, Chong SS, Lee CG. An update on ABCB1 pharmacogenetics: insights from a 3D model into the location and evolutionary conservation of residues corresponding to SNPs associated with drug pharmacokinetics. Pharmacogenomics J. 2011;11(5):315–325. doi: 10.1038/tpj.2011.16. [DOI] [PubMed] [Google Scholar]
- Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 20(4):619–627. doi: 10.1158/1055-9965.EPI-10-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, Pomerantz M, Freedman M, Ross R, Regan M, Sharifi N, Figg WD, Balk S, Brown M, Taplin ME, Oh WK, Lee GS, Kantoff PW. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 29(18):2565–2573. doi: 10.1200/JCO.2010.31.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos. 2009;37(5):946–955. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]