Abstract
Objective
Although the epidemiology of typical endometrial carcinomas (grades 1–2 endometrioid or Type I) is well established, less is known regarding higher grade endometrioid or non-endometrioid carcinomas (Type II). Within a large Gynecologic Oncology Group trial (GOG-210), which included central pathology review, we investigated the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologic categories.
Methods
Based on epidemiologic questionnaire data, risk factor associations, expressed as odds ratios (OR) with 95% confidence intervals (CI), were estimated comparing grade 3 endometrioid and Type II cancers (including histologic subtypes) to grades 1–2 endometrioid cancers.
Results
Compared with 2,244 grades 1–2 endometrioid cancers, women with Type II cancers (321 serous, 141 carcinosarcomas, 77 clear cell, 42 mixed epithelial with serous or clear cell components) were older; more often non-white, multiparous, current smokers; and less often obese. Risk factors for grade 3 endometrioid carcinomas (n=354) were generally similar to those identified for Type II cancers, although patients with grade 3 endometrioid tumors more often had histories of breast cancer without tamoxifen exposure while those with Type II tumors were more frequently treated with tamoxifen. Patients with serous cancers and carcinosarcomas more frequently had breast cancer histories with tamoxifen treatment compared to patients with other tumors.
Conclusions
Risk factors for aggressive endometrial cancers, including grade 3 endometrioid and non-endometrioid tumors, appear to differ from lower grade endometrioid carcinomas. Our findings support etiologic differences between Type I and II endometrial cancers as well as additional heterogeneity within Type II cancers.
Keywords: endometrial cancer, Type II endometrial cancer, serous endometrial cancer, mixed malignant müllerian tumors, etiology, epidemiology
INTRODUCTION
In 1983, Bokhman proposed that endometrial cancers could be divided into two broad types based on fundamental differences in endocrine and metabolic functioning and accepted endometrial cancer risk factors [1]. Specifically, he proposed that the numerically predominant endometrioid form of endometrial cancer has a hormonally driven etiology (i.e., relative excess estrogen exposure), develops from endometrial hyperplasia, pathologically is more well-differentiated, and portends a favorable prognosis. In contrast, other endometrial cancers were viewed as unrelated to typical endometrial cancer risk factors, not associated with endometrial hyperplasia, and pathologically high grade, often resulting in death.
Following Bokhman's seminal contribution, clinicopathologic studies led to the view that the predominant form of endometrial cancer (referred to as Type I) corresponds histologically to endometrioid adenocarcinomas, whereas other forms (Type II) encompass most non-endometrioid histologic types, with serous carcinoma representing the prototype [2]. Consistent with this view, it is widely accepted that atypical endometrial hyperplasia is an immediate precursor of endometrioid adenocarcinoma, whereas serous carcinoma generally arises in an atrophic background [3], possibly as a result of malignant change in the endometrial surface epithelium [4]. Studies showing differences in molecular markers according to histology support that there may be at least two broad classes of endometrial carcinoma [5, 6].
Many pathologists and gynecologists have embraced the view that there are at least two main biological types of endometrial cancer (and possibly more), but most epidemiologic studies have assessed risk factors for endometrial cancer overall--which essentially represent the risks for the predominant Type I tumors, especially in largely Caucasian populations [7]. Registry data consistently have shown Type II cancers to more frequently occur among older and non-white women [8]. In addition, some epidemiologic investigations have found that Type II cancers are less strongly linked to classic Type I risk factors, such as obesity, nulliparity, and hormones [7, 9–11]. However, these studies have had relatively limited numbers of non-endometrioid cancers, incomplete collection of relevant risk factors and lacked centralized pathological review. In addition, in some investigations it has been difficult to distinguish effects related to different histologies from those associated with other correlated clinical parameters, such as stage and grade.
To address limitations of prior studies investigating etiological heterogeneity in endometrial cancer, we analyzed detailed epidemiologic questionnaire data collected in a large Gynecologic Oncology Group (GOG) molecular staging trial. Given that grades 1–2 endometrioid carcinomas are associated with generally better outcomes, this case-case comparison involving relatively large numbers of Type II cancers offered an opportunity to assess risk factors for more lethal forms of endometrial cancer using a nationwide sample with central pathology review.
METHODS
Study Population
Beginning in September 2003, endometrial cancer patients were approached for participation in GOG 210, a molecular and surgico-pathological staging study of endometrial carcinoma, conducted at 62 U.S. institutions. For the current investigation, patients were recruited from 53 institutions (131 treatment sites), and had to have had a histologic diagnosis of endometrial carcinoma or carcinosarcoma by an endometrial biopsy or dilation and curettage and completion of full surgical staging. All stages, grades, and histologic types were eligible.
A total of 3,838 cases were enrolled prior to September 24, 2007, when eligibility criteria changed from unrestricted enrollment to poor prognosis tumors and those occurring among non-obese and non-white patients. Prior to surgery, patients were asked to complete a questionnaire that asked about established endometrial cancer risk factors, including demographic factors, reproductive and medical history (including prior breast cancer), and family history. A total of 3,499 (91.2%) trial patients agreed to complete this questionnaire and had valid pathologic information (grade, stage and histology). The study was approved by institutional review boards at the NCI and participating study centers. All patients provided informed consent for completing the questionnaire. After completion, questionnaires were centrally scanned, and computer readable files created and checked for completeness and consistency of responses.
Of the patients from whom questionnaires were obtained, 11 were excluded because they never underwent surgery, 1 had a second primary, 24 had the wrong histology, 27 had the wrong primary site, 1 had inadequate pathologic material for review and 1 had improper surgery, leaving 3,434 patients for the present analyses.
Central Pathology Review
Pathologic diagnoses were made at participating GOG institutions and then reviewed by rotating teams of GOG pathologists. Stage information was determined post-operatively and coded according to FIGO 1988 criteria [12]. A review of data from approximately the first 800 cases indicated the need for a specialized review for tumors that did not show a high degree of diagnostic concordance between pathologists, notably carcinosarcomas and serous, mucinous and clear cell tumors; grade 3 endometrioid adenocarcinomas; and tumors involving the cervix or with non-nodal metastases. Specific classification criteria were determined by six GOG pathologists, and each case underwent specialized review by two pathologists. Cases for which there was disagreement between the two pathologists were reviewed by the remaining pathologists. According to FIGO criteria, nuclear grading was given precedence for clear cell, serous and squamous carcinomas, and almost all were judged to be grade 3 tumors.
Statistical Analyses
To assess risk factor associations, logistic regression was performed to estimate odds ratios (OR) with 95% confidence intervals (CIs) as a measure of association by histologic tumor type [13]. Given uncertainty of the significance of grade 3 endometrioid tumors in the literature, both the Type II and the grade 3 endometrioid cancers were compared to a referent group comprised of grades 1–2 endometrioid tumors (there was little variation in risk factors between the grades 1 and 2 tumors). ORs exceeding 1.0 indicated that the factor was more common amongpatients with Type II or grade 3 endometrioid tumors than among those with lower grade endometrioid tumors, while ORs less than 1.0 indicated less common exposures. However, given that this study comprised only endometrial cancer patients, ORs less than 1.0 could still imply increased risk compared to non-affected controls and ORs greater than 1.0 did not necessarily imply increased risk.
All regression models included age at diagnosis (continuous variable), year of enrollment and race (white, black, other, unknown). For serous carcinomas, the only non-endometrioid histological type for which we had sufficient numbers to allow detailed analyses, we conducted multivariable logistic regression to determine the independence of risk factor associations [13, 14]. In a separate analysis, we further adjusted for stage to rule out its independent effect on these associations.
Chi square testing was used to determine p values for differences across exposure categories and linear hypothesis testing was used to compare the equality of ORs between specific case groups (e.g., Type II or grade 3 vs. grades 1-2 endometrioid cancers; Type II vs. grade 3 endometrioid cancers; specific histologic groupings of Type II tumors vs. grades 1-2 endometrioid tumors). In these analyses, a p-value <0.05 was considered considered statistically significant [11]. All statistical analyses were conducted using SAS version 9.2 (Cary, NC).
RESULTS
Study Population
A total of 55.9% of the patients were 60 years of age or older, and 11.0% were non-white. The patients were generally educated and affluent, with 29.7% college graduates and 24.8% with annual household incomes of $70,000 or greater. A total of 65.4% were diagnosed with grades 1-2 endometrioid and 10.3% with grade 3 endometrioid cancers. The remaining patients were diagnosed with serous tumors (9.4%), carcinosarcomas (4.1%), clear cell cancers (2.2%), mixed epithelial tumors with serous or clear cell components (1.2%) and other malignant tumors (7.3%), the latter of which primarily consisted of mixed epithelial malignancies not otherwise specified. The majority of all graded and staged endometrial cancers were low grade (72.4% grades 1 or 2) or early stage (74.9% stage 1), reflective of the broad eligibility criteria during the initial trial years.
Patients with grade 3 endometrioid tumors, as well as those who we considered as having Type II tumors (comprised of serous, carcinosarcomas, and clear cell tumors) and those with tumors of other histologies (mixed epithelial, other malignant tumors), were diagnosed at significantly older ages than patients with grades 1-2 endometrioid cancers; the largest differences with grades 1-2 endometrioid cancers (median age 59.6 years) were for patients with serous cancers (67.4 years), carcinosarcomas (66.8 years), clear cell cancers (66.1 years) and mixed epithelial tumors with serous or clear cell compoents (66.6 years) (Table 1). Furthermore, grade 3 endometrioid and Type II patients were more often non-white than patients with grades 1-2 endometrioid tumors. Black patients were rarely diagnosed with low-grade endometrioid cancers, whereas larger percentages were observed for the other tumors. Type II tumors were more often diagnosed at high grades and stages.
Table 1.
Histologic Classification of Tumors by Demographic and Clinical Characteristics, GOG-210 Trial Participants, 2003–2007, N=3,4341
| Type I | Type II | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Grade 1–2 Endometrioid | Grade 3 Endometrioid | Serous | Carcinosarcoma | Clear cell | Mixed epithelial with serous or clear cell components | Other malignant tumors | p-value4 | |
| (n=2,244) | (n=354) | (n=321) | (n=141) | (n=77) | (n=42)2 | (n=250)3 | ||
| % | % | % | % | % | % | |||
| Age, years | <0.001 | |||||||
| 54 or younger | 28.5 | 19.8 | 6.6 | 10.6 | 9.1 | 9.5 | 18.4 | |
| 55–59 | 23.0 | 22.9 | 11.2 | 12.1 | 13.0 | 19.0 | 17.2 | |
| 60–64 | 16.9 | 15.8 | 22.1 | 17.7 | 22.1 | 14.3 | 19.2 | |
| 65–69 | 12.9 | 13.8 | 19.3 | 22.0 | 19.5 | 23.8 | 15.2 | |
| 70 or older | 18.7 | 27.7 | 40.8 | 37.6 | 36.4 | 33.3 | 30.0 | |
| Median | 59.6 | 61.9 | 67.4 | 66.8 | 66.1 | 66.6 | 63.8 | |
| Race | <0.001 | |||||||
| White | 91.2 | 89.3 | 77.3 | 71.6 | 81.8 | 92.9 | 86.0 | |
| Black | 4.8 | 9.3 | 19.9 | 22.7 | 11.7 | 4.8 | 11.2 | |
| Other | 3.2 | 0.8 | 2.2 | 2.8 | 5.2 | 2.4 | 2.4 | |
| Unknown | 0.8 | 0.6 | 0.6 | 2.8 | 1.3 | 0.0 | 0.0 | |
| Grade | <0.001 | |||||||
| 1 | 59.2 | 0.0 | 0.0 | 0.7 | 0.0 | 2.4 | 18.8 | |
| 2 | 40.8 | 0.0 | 0.0 | 0.7 | 0.0 | 2.4 | 3.3 | |
| 3 | 0.0 | 100.0 | 100.0 | 4.3 | 100.0 | 16.7 | 36.7 | |
| Not graded | 0.0 | 0.0 | 0.0 | 94.3 | 0.0 | 78.6 | 40.0 | |
| Stage | <0.001 | |||||||
| I | 84.3 | 65.5 | 48.9 | 52.5 | 55.8 | 57.1 | 59.2 | |
| II | 6.3 | 7.3 | 6.2 | 7.8 | 18.2 | 9.5 | 10.4 | |
| III | 8.4 | 20.1 | 30.5 | 28.4 | 20.8 | 21.4 | 23.2 | |
| IV | 1.0 | 7.1 | 14.3 | 11.4 | 5.2 | 11.9 | 7.2 | |
Five endometrioid cases were not graded
Mixed epithelial include: mixed serous/endometrioid (n=26), mixed clear cell/endometrioid (n=11), mixed serous/clear cell (n=5)
Other malignant tumors include: mixed epithelial not otherwise specified (n=190), undiffferentiated carcinoma (n=20), mucinous adenocarcinoma (n=14), de-differentiated carcinoma (n=12), other carcinoma (n=12), squamous cell carcinoma (n=3), small cell carcinoma (n=2), unknown (n=2), granulosa cell tumor (n=1)
Chi-square p-value
Risk Factors for Type II Cancers Compared to Grades 1-2 Endometrioid Cancers
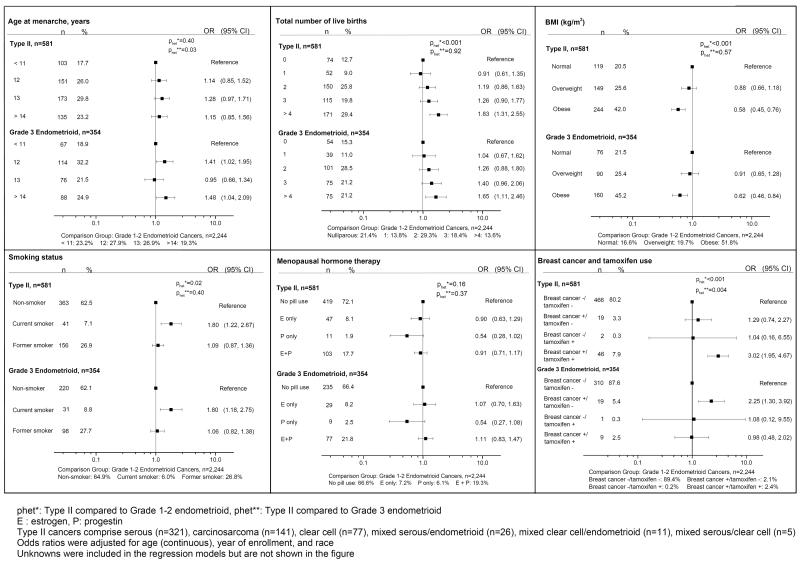
Even after adjusting for age differences, patients with Type II tumors had a higher odds of being postmenopausal than those with grades 1-2 endometrioid cancers (OR=1.39, 95% CI 0.99–2.02). After adjustment for age, enrollment year and race, patients with type II tumors had a higher odds of being multiparous, being current cigarette smokers, and having histories of breast cancer diagnoses that had been treated with tamoxifen (comparison with low-grade endometrioid tumors showed elevated ORs and significant p values for heterogeneity for all factors) (Figure 1). In contrast, obesity was less frequent among patients with Type II than Type I tumors, with decreasing ORs observed with increasing categories of BMI. Relationships for Type II vs. low-grade endometrioid tumors were not significantly different for age at menarche, use of menopausal hormones, menopause status, prior oophorectomy, history of infertility, or use of oral contraceptives (data not shown).
Figure 1.
Odds ratios for Type II and Grade 3 Endometrioid Endometrial Cancers (as compared to Grade 1–2 endometrioid cancers), N=3,179
Figure 1 also demonstrates ORs for the grade 3 endometrioid tumors as compared to grades 1-2 endometrioid tumors. These tumors for the most part demonstrated ORs similar to those calculated for Type II tumors. However, women with grade 3 endometrioid tumors had somewhat later ages at menarche and higher rates of prior breast cancer without tamoxifen treatment as compared to those with grades 1-2 endometrioid tumors.
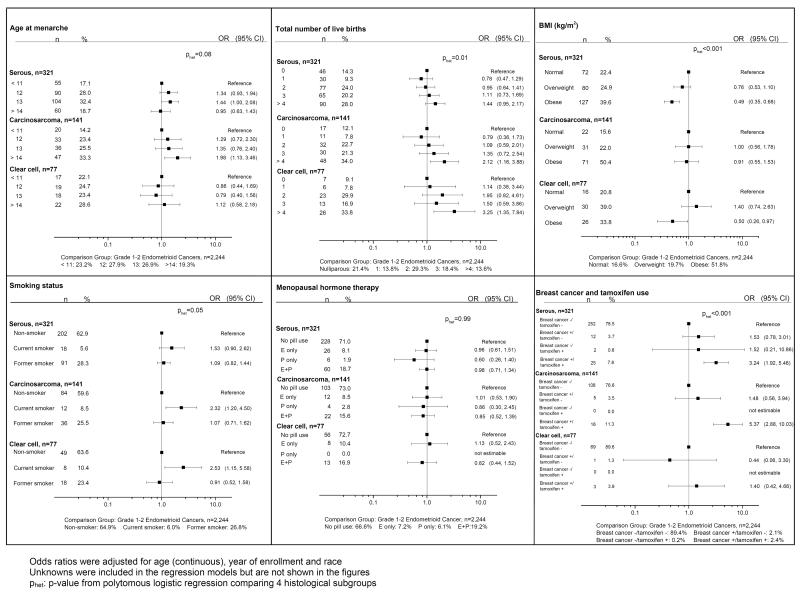
In further analyses, we compared risk factors for the three most specific histologic subgroups of Type II tumors (serous, carcinosarcoma, clear cell), using as a comparison grades 1-2 endometrioid cancers (Figure 2). There were some distinctive differences in risk factor patterns within the histologic subgroups. Later ages at menarche, multiparity, and current cigarette smoking were more commonly reported by women with carcinosarcomas. Multiparity and current smoking also were more common among women with clear cell cancers. In contrast, obesity was less common among women with either serous or clear cell cancers. Histories of breast cancer treated with tamoxifen were especially represented among women with either serous cancers or carcinosarcomas; in contrast, such a history did not appear related to the odds of clear cell cancers.
Figure 2.
Odds ratios for Histological Subgroups of Type II Endometrial Cancers (as compared to Grade 1–2 Endometrioid Cancers), N=2,783
Risk Factors for Serous Carcinomas Compared to Grades 1-2 Endometrioid Cancers
We conducted multi-variable logistic regression to identify factors significantly and independently related to serous cancers, the type II cancer where we had sufficient power to conduct such analyses (Table 2). Associations persisted for multiparity, BMI and the combined parameter of breast cancer and tamoxifen treatment. The association with a history of breast cancer without tamoxifen exposure was not statistically significant (OR=1.57, 0.78–3.14), whereas breast cancer with tamoxifen treatment was significantly elevated (3.20, 1.87–5.50). We considered how risk estimates were affected by additional adjustment for stage, given that previous studies have shown risk factors to vary by this clinical parameter; however, this had marginal impact on the magnitude of the associations (data not shown).
Table 2.
Odds Ratios for Serous Endometrial Cancers (as Compared to Grade 1–2 EM Endometrial Cancers Among GOG-210 Participants, 2003–2007, N=2,565
| Grade 1–2 endometrioid (n=2,244) | Serous (n=321) | Odds Ratio1 | 95% CI | P | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Total number of live births | 0.02 | |||||||
| Nulliparous | 481 | 21.4 | 46 | 14.3 | 1.00 | reference | ||
| 1 | 309 | 13.8 | 30 | 9.3 | 0.80 | 0.47 | 1.32 | |
| 2 | 658 | 29.3 | 77 | 24.0 | 0.96 | 0.63 | 1.44 | |
| 3 | 412 | 18.4 | 65 | 20.2 | 1.16 | 0.75 | 1.79 | |
| ≥4 | 305 | 13.6 | 90 | 28.0 | 1.64 | 1.07 | 2.52 | |
| BMI, kg/m2 | <0.001 | |||||||
| Underweight/normal | 372 | 16.6 | 72 | 22.4 | 1.00 | reference | ||
| Overweight | 441 | 19.7 | 80 | 24.9 | 0.69 | 0.48 | 1.01 | |
| Obese | 1,163 | 51.8 | 127 | 39.6 | 0.46 | 0.32 | 0.65 | |
| Smoking status | 0.53 | |||||||
| Non-smoker | 1,456 | 64.9 | 202 | 62.9 | 1.00 | reference | ||
| Current smoker | 135 | 6.0 | 18 | 5.6 | 1.49 | 0.86 | 2.57 | |
| Former smoker | 602 | 26.8 | 91 | 28.3 | 1.09 | 0.82 | 1.45 | |
| Menopausal hormone therapy | 0.64 | |||||||
| No pill use | 1,494 | 66.6 | 228 | 71.0 | 1.00 | reference | ||
| Estrogen only use | 162 | 7.2 | 26 | 8.1 | 0.96 | 0.60 | 1.52 | |
| Progesterone only use | 136 | 6.1 | 6 | 1.9 | 0.64 | 0.27 | 1.53 | |
| Estrogen plus progestin | 432 | 19.3 | 60 | 18.7 | 0.91 | 0.65 | 1.27 | |
| Breast cancer and Tamoxifen use | <0.001 | |||||||
| Breast cancer−/Tamoxifen− | 2,006 | 89.4 | 252 | 78.5 | 1.00 | reference | ||
| Breast cancer+/Tamoxifen− | 48 | 2.1 | 12 | 3.7 | 1.57 | 0.78 | 3.14 | |
| Breast cancer−/Tamoxifen+ | 5 | 0.2 | 2 | 0.6 | 1.64 | 0.24 | 11.04 | |
| Breast cancer+/Tamoxifen+ | 53 | 2.4 | 25 | 7.8 | 3.20 | 1.87 | 5.50 | |
Odds ratios were adjusted for age (continuous), year of enrollment, race, current menstrual status, parity, smoking status, BMI, menopausal hormone therapy, breast cancer and tamoxifen use
Unknowns were included in the regressions models but are not shown in the table
DISCUSSION
Our analysis of epidemiological data for endometrial cancers, classified centrally by a pathology panel, showed that relationships of several established endometrial cancer risk factors - obesity, parity, and smoking - differ significantly for Type II vs. grades 1-2 endometrioid cancers. In support of extensive prior clinical and incidence surveys, we also found that Type II tumors are diagnosed more often among older and non-white women [8, 15–20]. These differences in risk factor relationships, in combination with the more frequent occurrence of Type II cancers after menopause, provide some of the strongest epidemiologic support that endometrial cancers are etiologically heterogeneous.
Varying definitions of Type I and II endometrial cancers, including inconsistent classification of grade 3 endometrioid tumors, have complicated the interpretation of results from previous investigations. Some grade 3 endometrioid carcinomas show concomitant grade 1 patterns, suggesting that the former represents tumor progression of the latter and a shared etiology, whereas others may arise from atrophic endometrium. Our central pathology review was based on a limited number of slides per patient, precluding our evaluating whether grade 3 endometrioid carcinomas were associated with lower grade patterns of carcinoma or arose from hyperplastic or inactive benign endometrium. Our findings, however, suggested that grade 3 endometrioid tumors more closely resembled Type II than grades 1-2 endometrioid tumors, arguing for the inclusion of grade 3 endometrioid carcinomas as Type II cancers [21, 22]. Future molecular analyses comparing the profiles of grade 3 endometrioid carcinomas to lower grade tumors, as has been done between low-grade endometrioid and serous carcinomas (23), may provide insights as to their origins.
The importance of excess estrogen in the etiology of Type I cancers is firmly established by data linking risk to unopposed estrogen use [23–25]. The strong links between postmenopausal obesity and increased circulating estrogen levels and between obesity and endometrial cancer risk are consistent with these observations [26, 27]. Thus, the weaker link between obesity and Type II cancers in this and prior analyses [7, 10] provides evidence for etiological differences between tumor types, and a less hormonally dependent etiology of Type II cancers. However, given that we did not have unaffected women in our study, we could not eliminate some effect of obesity on Type II tumors. Other investigations have noted obesity related to risk of serous carcinomas, albeit to a lesser extent than for type I cancers [28–30].
We also found further support for etiologic heterogeneity of endometrial cancer through higher exposure rates of multiparity and current cigarette smoking among patients with Type II than grades 1-2 endometrioid tumors. Both risk factors have consistently been associated with reduced risks of endometrial (mainly endometrioid) cancers [31, 32]. In comparison to grades 1-2 endometrioid cancers, both of these exposures were more prevalent for clear cell carcinomas and carcinosarcomas, implying a less protective role for these latter tumors. Although the mechanisms that account for the effects of parity on endometrial cancer risk are undefined, it is likely that a hormonal mechanism is involved with the apparent protective effect of cigarette smoking [33].
Our failure to observe large differences between grades 1-2 endometrioid and Type II tumors with respect to exogenous hormone use may have reflected a limitation of our questionnaire and/or inability to distinguish effects of long-term use of unopposed estrogens from combined estrogen plus progestin therapy, which is controversial with respect to its relationship to endometrioid cancers. Further, the interpretation may have been complicated by modifying effects of body mass index, given that exogenous hormone use primarily affects risk among thin women [25]. A recent meta-analysis concluded that evidence argues against significant variations in hormone usage by histologic subtypes, but further investigations appear warranted [34].
Although it is well recognized that women with a history of breast cancer are at an increased risk of developing subsequent endometrial cancer [35], the histologic specificity of these subsequent cancers has not been well elaborated. A number of previous studies have noted the development of uterine serous cancers among women with breast cancer [7, 36–39], but our results expand on these findings by showing that breast cancer predisposes to serous cancers as well as carcinosarcomas and grade 3 endometrioid tumors. Several hypotheses have been proposed for the excess of endometrial cancers among women with prior breast cancer, including similar risk profiles, radiation treatment of proximate organs, manifestations of rare inherited cancer syndromes (e.g., Li-Fraumeni and Lynch syndromes), or the result of mutations in cancer predisposing genes [40, 41]. Some of these factors might explain the increased risk that we observed for high-grade endometrioid tumors. Further, although we could not fully account for effects of all treatments these women experienced, those who developed serous cancers or carcinosarcomas in our study had unusually high rates of exposure to tamoxifen. This relationship is consistent with previous observations supporting an important role of tamoxifen in the etiology of Type II cancers [37, 38, 42–46]. The association with tamoxifen might be unexpected given that it is a weak estrogen, but data also suggest that it can form DNA adducts, which could produce cancers via non-hormonal effects. Tamoxifen has also been associated with the development of benign endometrial polyps, which may undergo malignant transformation, as suggested from several small clinical series of both serous carcinomas and carcinosarcomas [47, 48].
Although our study had a number of strengths, including large numbers of carefully clinico-pathologically characterized rare cancers and extensive information on postulated endometrial cancer risk factors, there were also some limitations. Cases were recruited into the trial at multiple sites and there could have been associated selection biases. The lack of a control group might also be viewed as a limitation, although the validity of our approach is reinforced by the commonality of results with those derived from a recent cohort study [10] that involved a smaller number of cases but a comparison group of non-diseased study subjects. Further, our risk factor questionnaire was self-completed by patients, and there could have been some errors in completion; the amount of unknown responses, however, indicated good comprehension. Finally, although the study was quite large, we still had relatively small numbers of the rare cancers, including clear cell cancers, limiting our ability to fully define risk predictors.
Despite these limitations, this investigation offered an unprecedented opportunity for epidemiologically testing the hypothesis that there are distinctive etiologic differences between Type I and II endometrial cancers. Our findings demonstrated that risk factors for aggressive endometrial cancer, including grade 3 endometrioid and non-endometrioid tumors, differ from lower grade endometrioid carcinomas. Furthermore, we demonstrated that within Type II tumors that there could be additional etiologic heterogeneity based on tumor histology. These findings may have value for improving cancer surveillance and support the need for molecular profiling of endometrial cancers.
Highlights
-
➢
Substantial differences in epidemiologic risk factors were seen between patients with Type I and II endometrial cancers.
-
➢
Risk factors for grade 3 endometrioid cancers were generally similar to Type II cancers.
-
➢
There was evidence of etiologic heterogeneity within Type II cancers, e.g., higher rates of tamoxifen exposure among serous and carcinosarcoma patients.
Acknowledgments
Role of the funding source This study was supported in part by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). In addition, this research was supported in part by funds provided by the intramural research program of the National Cancer Institute, National Institutes of Health.
Other acknowledgements The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Northwestern University, University of Mississippi, University of Colorado-Anschutz Cancer Pavilion, University of California at Los Angeles, Fred Hutchinson Cancer Research Center, Penn State Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center, Indiana University Medical Center, Wake Forest University Health Sciences, University of California Medical Center at Irvine – Orange Campus, Magee Women's Hospital – University of Pittsburgh Medical Center, University of New Mexico, Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council/Ohio State, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, Women's Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago, Mayo Clinic, Case Western Reserve University, Moffitt Cancer Center and Research Institute, Yale University, University of Wisconsin Hospital, Women and Infants' Hospital, The Hospital of Central Connecticut at New Britain General, GYN Oncology of West Michigan, PLLC and Community Clinical Oncology Program,
Footnotes
Author contributions SM, WTC, DM, DEC, JLW, RGM, LSD, RAS and RZ were involved with recruiting patients to the clinical trial and/or reviewing clinical and pathologic characteristics of their malignancies. LAB, MES and ASF contributed to the design and concept of the analyses undertaken for the epidemiologic component of the study. All authors reviewed and commented upon a draft of the paper and approved it for submission.
The authors declare that there are no conflicts of interest.
Reference List
- [1].Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- [2].Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- [3].Ellenson LH, Ronnett BM, Kurman RJ. Precursor lesions of endometrial carcinoma. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein's Pathology of the Female Genital Tract. Springer; 2012. pp. 359–92. [Google Scholar]
- [4].Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- [5].Arafa M, Somja J, Dehan P, Kridelka F, Goffin F, Boniver J, et al. Current concepts in the pathology and epigenetics of endometrial carcinoma. Pathology. 2010;42:613–7. doi: 10.3109/00313025.2010.520307. [DOI] [PubMed] [Google Scholar]
- [6].Lim D, Oliva E. Nonendometrioid endometrial carcinomas. Semin Diagn Pathol. 2010;27:241–60. doi: 10.1053/j.semdp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [7].Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Womens Health (Larchmt) 2011;20:1157–63. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- [9].Sherman ME, Sturgeon S, Brinton LA, Potischman N, Kurman RJ, Berman ML, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10:963–8. [PubMed] [Google Scholar]
- [10].Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ, et al. Endometrial cancer risk factors by two main histologic subtypes in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2012 doi: 10.1093/aje/kws200. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zelmanowicz A, Hildesheim A, Sherman ME, Sturgeon SR, Kurman RJ, Barrett RJ, et al. Evidence for a common etiology for endometrial carcinomas and malignant mixed mullerian tumors. Gynecol Oncol. 1998;69:253–7. doi: 10.1006/gyno.1998.4941. [DOI] [PubMed] [Google Scholar]
- [12].Zaino RJ. FIGO staging of endometrial adenocarcinoma: a critical review and proposal. Int J Gynecol Pathol. 2009;28:1–9. doi: 10.1097/PGP.0b013e3181846c6d. [DOI] [PubMed] [Google Scholar]
- [13].Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons, Inc.; New York, NY: 2000. [Google Scholar]
- [14].Martinez ME, Cruz GI, Brewster AM, Bondy ML, Thompson PA. What can we learn about disease etiology from case-case analyses? Lessons from breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2710–4. doi: 10.1158/1055-9965.EPI-10-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boruta DM, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115:142–53. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- [16].Fader AN, Starks D, Gehrig PA, Secord AA, Frasure HE, O'Malley DM, et al. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2009;115:244–8. doi: 10.1016/j.ygyno.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [17].Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control. 2009;16:46–52. doi: 10.1177/107327480901600107. [DOI] [PubMed] [Google Scholar]
- [18].Sabatino SA, Stewart SL, Wilson RJ. Racial and ethnic variations in the incidence of cancers of the uterine corpus, United States, 2001–2003. J Womens Health (Larchmt) 2009;18:285–94. doi: 10.1089/jwh.2008.1171. [DOI] [PubMed] [Google Scholar]
- [19].Sagr ER, Denschlag D, Kerim-Dikeni A, Stanimir G, Gitsch G, Gilbert L. Prognostic factors and treatment-related outcome in patients with uterine papillary serous carcinoma. Anticancer Res. 2007;27:1213–7. [PubMed] [Google Scholar]
- [20].Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. 2003;98:176–86. doi: 10.1002/cncr.11484. [DOI] [PubMed] [Google Scholar]
- [21].Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124:15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- [22].Zannoni GF, Vellone VG, Arena V, Prisco MG, Scambia G, Carbone A, et al. Does high-grade endometrioid carcinoma (grade 3 FIGO) belong to type I or type II endometrial cancer? A clinical-pathological and immunohistochemical study. Virchows Arch. 2010;457:27–34. doi: 10.1007/s00428-010-0939-z. [DOI] [PubMed] [Google Scholar]
- [23].Doherty JA, Cushing-Haugen KL, Saltzman BS, Voigt LF, Hill DA, Beresford SA, et al. Long-term use of postmenopausal estrogen and progestin hormone therapies and the risk of endometrial cancer. Am J Obstet Gynecol. 2007;197:139–7. doi: 10.1016/j.ajog.2007.01.019. [DOI] [PubMed] [Google Scholar]
- [24].Lacey JV, Jr., Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724–31. doi: 10.1158/1055-9965.EPI-05-0111. [DOI] [PubMed] [Google Scholar]
- [25].Razavi P, Pike MC, Horn-Ross PL, Templeman C, Bernstein L, Ursin G. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:475–83. doi: 10.1158/1055-9965.EPI-09-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–77. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- [27].Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bjorge T, Engeland A, Tretli S, Weiderpass E. Body size in relation to cancer of the uterine corpus in 1 million Norwegian women. Int J Cancer. 2010;120:378–383. doi: 10.1002/ijc.22260. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- [29].Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. The impact of BMI on subgroups of uterine cancer. Br J Cancer. 2009;101:534–6. doi: 10.1038/sj.bjc.6605158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCullough ML, Patel AV, Patel R, Rodriguez C, Feigelson HS, Bandera EV, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17:73–9. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- [31].Pocobelli G, Doherty JA, Voigt LF, Beresford SA, Hill DA, Chen C, et al. Pregnancy history and risk of endometrial cancer. Epidemiology. 2011;22:638–45. doi: 10.1097/EDE.0b013e3182263018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang HP, Brinton LA, Platz EA, Lissowska J, Lacey JV, Jr., Sherman ME, et al. Active and passive cigarette smoking and the risk of endometrial cancer in Poland. Eur J Cancer. 2010;46:690–6. doi: 10.1016/j.ejca.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sowers MR, Crawford S, McConnell DS, Randolph JF, Jr., Gold EB, Wilkin MK, et al. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136:1588–95. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- [34].Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010 doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- [35].Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat. 2007;105:195–207. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- [36].Chan JK, Manuel MR, Cheung MK, Osann K, Husain A, Teng NN, et al. Breast cancer followed by corpus cancer: is there a higher risk for aggressive histologic subtypes? Gynecol Oncol. 2006;102:508–12. doi: 10.1016/j.ygyno.2006.01.014. [DOI] [PubMed] [Google Scholar]
- [37].Gehrig PA, Bae-Jump VL, Boggess JF, Groben PA, Fowler WC, Jr., Van LL. Association between uterine serous carcinoma and breast cancer. Gynecol Oncol. 2004;94:208–11. doi: 10.1016/j.ygyno.2004.04.009. [DOI] [PubMed] [Google Scholar]
- [38].Liang SX, Pearl M, Liang S, Xiang L, Jia L, Yang B, et al. Personal history of breast cancer as a significant risk factor for endometrial serous carcinoma in women aged 55 years old or younger. Int J Cancer. 2010 doi: 10.1002/ijc.25395. [DOI] [PubMed] [Google Scholar]
- [39].Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003;91:463–9. doi: 10.1016/j.ygyno.2003.08.018. [DOI] [PubMed] [Google Scholar]
- [40].Lavie O, Ben-Arie A, Segev Y, Faro J, Barak F, Haya N, et al. BRCA germline mutations in women with uterine serous carcinoma--still a debate. Int J Gynecol Cancer. 2010;20:1531–4. doi: 10.1111/IGC.0b013e3181cd242f. [DOI] [PubMed] [Google Scholar]
- [41].Pennington KP, Walsh T, Lee M, Pennil C, Novetsky AP, Agnew KJ, et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer. 2012 doi: 10.1002/cncr.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Barakat RR, Wong G, Curtin JP, Vlamis V, Hoskins WJ. Tamoxifen use in breast cancer patients who subsequently develop corpus cancer is not associated with a higher incidence of adverse histologic features. Gynecol Oncol. 1994;55:164–8. doi: 10.1006/gyno.1994.1271. [DOI] [PubMed] [Google Scholar]
- [43].Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356:881–7. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- [44].Bland AE, Calingaert B, Secord AA, Lee PS, Valea FA, Berchuck A, et al. Relationship between tamoxifen use and high risk endometrial cancer histologic types. Gynecol Oncol. 2009;112:150–4. doi: 10.1016/j.ygyno.2008.08.035. [DOI] [PubMed] [Google Scholar]
- [45].Curtis RE, Freedman DM, Sherman ME, Fraumeni JF., Jr. Risk of malignant mixed mullerian tumors after tamoxifen therapy for breast cancer. J Natl Cancer Inst. 2004;96:70–4. doi: 10.1093/jnci/djh007. [DOI] [PubMed] [Google Scholar]
- [46].Hoogendoorn WE, Hollema H, van Boven HH, Bergman E, de Leeuw-Mantel G, Platteel I, et al. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat. 2008;112:99–108. doi: 10.1007/s10549-007-9823-1. [DOI] [PubMed] [Google Scholar]
- [47].Fotiou S, Hatjieleftheriou G, Kyrousis G, Kokka F, Apostolikas N. Long-term tamoxifen treatment: a possible aetiological factor in the development of uterine carcinosarcoma: two case-reports and review of the literature. Anticancer Res. 2000;20:2015–20. [PubMed] [Google Scholar]
- [48].McCluggage WG, Sumathi VP, McManus DT. Uterine serous carcinoma and endometrial intraepithelial carcinoma arising in endometrial polyps: report of 5 cases, including 2 associated with tamoxifen therapy. Hum Pathol. 2003;34:939–43. doi: 10.1016/s0046-8177(03)00335-6. [DOI] [PubMed] [Google Scholar]