SUMMARY
The liver and pancreas arise from common endodermal progenitors. How these distinct cell fates are specified is poorly understood. Here, we describe prostaglandin E2 (PGE2) as a regulator of endodermal fate specification during development. Modulating PGE2 activity has opposing effects on liver-versus-pancreas specification in zebrafish embryos as well as mouse endodermal progenitors. The PGE2 synthetic enzyme cox2a and receptor ep2a are patterned such that cells closest to PGE2 synthesis acquire a liver fate whereas more distant cells acquire a pancreas fate. PGE2 interacts with the bmp2b pathway to regulate fate specification. At later stages of development, PGE2 acting via the ep4a receptor promotes outgrowth of both the liver and pancreas. PGE2 remains important for adult organ growth, as it modulates liver regeneration. This work provides in vivo evidence that PGE2 may act as a morphogen to regulate cell fate decisions and outgrowth of the embryonic endodermal anlagen.
INTRODUCTION
How multiple different cell types are generated from common progenitors is a central question in developmental biology. Understanding this process will have therapeutic significance for repair of adult organs. One such context is the development of distinct organs such as the liver, pancreas, lungs and intestine from the primordial gut endoderm. The specification and outgrowth of these organs is regulated by a dynamic array of signals including fibroblast growth factors (Fgfs), bone morphogenetic proteins (Bmps), retinoic acid, and sonic hedgehog (Zaret and Grompe, 2008). Here, we uncover the prostaglandin pathway as a regulator of the specification and outgrowth of the embryonic liver and pancreas.
While the adult organs are histologically and functionally distinct, the embryonic liver and pancreas are thought to arise from a common population of endodermal cells. Experimental support for this bipotential population comes from mouse explant studies as well as zebrafish cell-lineage studies. Endoderm explants from mouse embryos have the potential to give rise to either liver or pancreatic progenitors depending on extrinsic cues from adjacent mesoderm: Fgfs from the cardiac mesoderm (Deutsch et al., 2001) or Bmps from the septum transversum mesenchyme (Rossi et al., 2001) induce hepatic differentiation, while inhibition of these signals causes expression of the pancreatic marker Pdx1 (Deutsch et al., 2001; Rossi et al., 2001). Similarly, reciprocal effects on hepatic versus pancreatic progenitor populations have been observed in response to Wnt signaling during zebrafish development (Goessling et al., 2008). These reciprocal effects of Fgf, Bmp, and Wnt signals on liver and pancreas progenitor populations suggest that these signals act on common endodermal progenitors to specify one fate at the cost of the other. Indeed, a population of bipotential progenitors has been delineated by single-cell-lineage labeling in embryonic mouse (Miki et al., 2012; Spence et al., 2009; Tremblay and Zaret, 2005) and zebrafish (Chung et al., 2008). In mouse, single-cell labeling of anterior endoderm at early somite stages, prior to expression of organ-specific markers, identified bipotential progenitors as well as closely-interspersed ventral pancreas and liver progenitors (Miki et al., 2012; Tremblay and Zaret, 2005). While these studies map bipotential progenitors and the timing of their segregation into a liver or pancreas anlagen, it is thought that the fates of these segregated populations may be reversible in early somite stages such that modulation of extrinsic signals can shift one fate into another (Miki et al., 2012). In zebrafish, endodermal cells capable of both a liver and exocrine pancreas fate have been identified at the 6-8 somite stage (Chung et al., 2008). While these labeling studies localize bipotential progenitors, how this population spatially relates to multiple signals that regulate liver versus pancreas specification is poorly understood.
Prostaglandin molecules have long been recognized as lipid-derived cytokines that modulate diverse biologic processes including vasoregulation, inflammation, and pain (Funk, 2001). In addition, we recently described prostaglandin E2 (PGE2) as a conserved regulator of hematopoietic stem cell formation and function (North et al., 2007). However, a role for prostaglandins in developmental fate decisions and solid organ development has not been appreciated, in part because in vivo effects cannot be easily studied independent of maternal prostaglandins in mammalian models. Likewise, there is little knowledge of the spatio-temporal expression pattern of components of the prostaglandin pathway during development. In particular, despite a wide body of knowledge of prostaglandin function in the adult gastrointestinal tract, induction and function of components of the prostaglandin pathway in relation to organogenesis of solid endodermal tissues has not been described.
Here, we demonstrate a role for PGE2 in regulating the outgrowth of the liver and pancreas buds in vivo as they emerge from the zebrafish gut endoderm. Moreover, we uncover an earlier role for PGE2 in specification of endoderm into liver versus pancreas progenitors. PGE2 likewise promotes a liver fate at the cost of a pancreas fate in bipotential mouse embryonic endoderm, suggesting an evolutionarily conserved developmental role for PGE2. Similar to other canonical pathways regulating organ development, we uncover exquisite spatial and temporal patterning of PGE2 pathway components consistent with a role in specifying bipotential endodermal cells. Finally, we show that PGE2 modulates liver regeneration in adult zebrafish, demonstrating that the role of PGE2 in endodermal outgrowth may have implications for regeneration of adult endodermal derivatives. Together, this work reveals a previously unappreciated role for a lipid-derived signaling molecule with morphogenetic properties that regulate endodermal fate decisions and proliferation during development.
RESULTS
Prostaglandin Levels Affect Liver Development
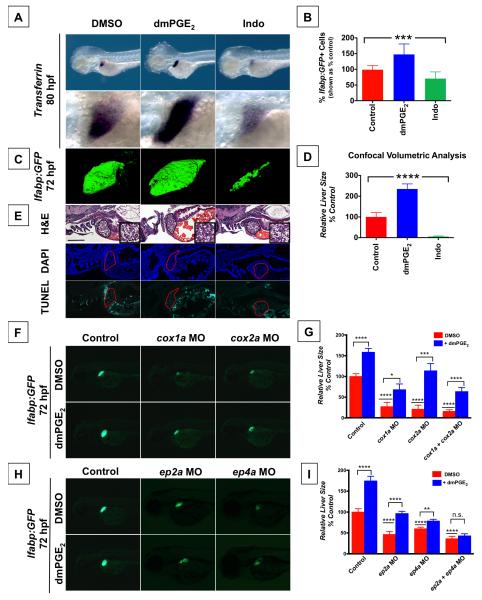
The prostaglandin pathway was identified as a modifier of endodermal organ development through a chemical genetic screen in zebrafish (Garnaas et al., 2012). Specifically, modulators of PGE2 synthesis and signaling affected embryonic liver size: a larger liver resulted from exposure to compounds that increase prostaglandin signaling activity, whereas a smaller liver size was caused by compounds that decrease prostaglandin signaling. To corroborate the screening results, zebrafish embryos were exposed from 48-80 hpf to a long acting derivative, 16,16-dimethyl-PGE2 (dmPGE2), or the non-selective Cox inhibitor Indomethacin (Indo), which has been shown to suppress PGE2 production in zebrafish by mass spectrometry (North et al., 2007). Exposure to dmPGE2 caused a striking increase in embryonic liver size (51.4% embryos with larger liver, n=18/35) as visualized by expression of transferrin at 80 hpf (Figure 1A). A larger embryonic liver developed in the presence of Cay10397, an inhibitor of 15-hydroxy prostaglandin dehydrogenase (pgdh) that deactivates prostaglandins in vivo and augments endogenous prostaglandin levels (Figure S1E). In contrast, exposure to Indo caused a dramatic decrease in embryonic liver size (76.5% embryos with smaller liver, n=26/34), indicating a role for endogenous prostaglandin activity in normal liver development (Figure 1A). Similarly, exposure to the selective Cox1 inhibitor SC-560 or the selective Cox2 inhibitor NS-398 both resulted in a smaller embryonic liver (Figure S1E). The same effects were observed by visualizing fluorescence after treatment of liver fatty acid binding protein:GFP (lfabp:GFP) liver reporter fish (Figure S1A, S1F), and these effects were statistically significant by volumetric analysis of confocal images (Figure 1C,D). FACS analysis of these embryos demonstrated statistically significant changes in GFP+ liver cell number, but no change in cell size or shape (Figure 1B). Hepatocyte number was increased without changes in cell density or tissue architecture following dmPGE2 exposure, as revealed by histological analysis (Figure 1E, S1B-D). Furthermore, visualization of apoptotic cells in treated embryos showed no significant differences in cell death that could explain effects on organ size (Figure 1E). Visualization of mitotic figures marked by phospho-histone H3 (pHH3+) in the liver bud of lfabp:GFP reporter fish demonstrated an increase in cell proliferation following exposure to dmPGE2, and a decrease in cell proliferation following exposure to Indo, suggesting that the effect of PGE2 is in part mediated by regulating proliferation (Figure S5D,E).
Figure 1. PGE2 affects Embryonic Liver Outgrowth.
(A) In situ hybridization for transferrin, a liver-specific marker, reveals that embryonic liver size is regulated by PGE2. Exposure to dmPGE2 from 48-80 hpf dramatically increases embryonic liver size (51.4% embryos with an enlarged liver, n = 18 / 35) whereas exposure to Indomethacin (Indo) decreases embryonic liver size (76.5% embryos with a smaller liver, n = 26 / 34). Left lateral view, row below is magnified view.
(B) FACS quantification of GFP+ liver cells in lfabp:GFP embryos at 72 hpf following exposure to dmPGE2 or Indo. dmPGE2 increases the relative number of liver cells, whereas Indo decreases the relative number of liver cells. (Data are represented as mean ± SEM; ***significant across treatment groups, ANOVA, p=0.0009).
(C) Confocal microscopy visualization of the liver in lfabp:GFP embryos at 72 hpf following exposure to dmPGE2 or Indo. Exposure to dmPGE2 from 48-72 hpf enlarges the developing liver, whereas exposure to Indo severely abrogates liver development. Left lateral view. (Data are represented as mean ± SEM; n = 5 / treatment).
(D) Analysis of liver [volume] × [mean fluorescence intensity] visualized by confocal microscopy, corroborating the effect of dmPGE2 to increase embryonic liver size and Indo to diminish embryonic liver size. (Data are represented as mean ± SEM; n = 5 / treatment, ****significant across treatment groups, ANOVA, p<0.0001).
(E) Histological analysis reveals that modulation of PGE2 levels changes embryonic liver size, but does not alter liver morphology (n = 5 / treatment). TUNEL analysis shows no apoptosis following modulation of PGE2 activity (n = 5 / treatment). Scale bar = 100 μm.
(F-I) Morpholino-mediated knockdown of PGE2 synthetic enzymes (F,G) or PGE2 receptors ep2a and ep4a (H,I) alters liver development, as visualized by fluorescence in lfabp:GFP embryos. Left lateral view. Impact on liver size is quantified by [area] × [fluorescence intensity] in lfabp:GFP embryos as shown in (Figure 1G,I). (Data are represented as mean ± SEM; n ≥ 30 / condition; significant by t-test comparing each morpholino vs. control and each morpholino without vs. with dmPGE2, **** p<0.0001, * p<0.05, *** p<0.001, n.s. = not significant).
See also Figure S1.
The impact of PGE2 was confirmed by modulation of the prostaglandin synthetic pathway. Morpholino-mediated knockdown of PGE2 synthesis enzymes, cox1a, cox2a, and prostaglandin E synthase (pges), resulted in a smaller embryonic liver as quantified by measuring liver size in lfabp:GFP embryos at 72 hpf condition, and this effect was partially rescued by exposure to dmPGE2 (Figure 1F,G, S1G). Likewise, selective pharmacologic blockade of Cox1 and Cox2 by SC-560 and NS-398, respectively, both resulted in a smaller embryonic liver, and this effect was partially rescued by PGE2 (Figure S1E,F). In contrast, knockdown or pharmacologic blockade of pgdh led to a larger embryonic liver which was further augmented with exogenous dmPGE2 (Figure S1E-G).
The effects of PGE2 are mediated via the G-protein-coupled receptors EP2 and EP4 (Funk, 2001). We therefore tested whether knockdown of these receptors could generate the same phenotypic effects as reducing levels of PGE2. Indeed, morpholino-mediated knockdown of either ep2a or ep4a resulted in a smaller embryonic liver, and this effect was only partially rescued by exogenous addition of PGE2 (Figure 1H,I). Moreover, combined knockdown of both the ep2a and ep4a receptors more severely abrogated liver development (Figure 1I, S1G). Whereas the combined knockdown of both cox1a and cox2a synthetic enzymes could be partially rescued by exogenous dmPGE2 (Figure 1G, S1G), the combined knockdown of both ep2a and ep4a receptors could not be rescued by exogenous dmPGE2 (Figure 1I, S1G), consistent with PGE2 acting through these receptors to enlarge the developing liver and further confirming the role of PGE2 signaling. These data provide in vivo demonstration that PGE2 activity modulates embryonic liver development.
Signaling through EP2 and EP4 is thought to regulate phosphorylation by increasing intracellular levels of cAMP and activating the downstream effector kinase PKA (Funk, 2001). Therefore, to test if PGE2 acts through these downstream effectors to promote liver outgrowth, zebrafish embryos were exposed to forskolin, a cAMP activator, or H89, a PKA inhibitor, from 48-72 hpf. Augmenting cAMP activity resulted in a larger embryonic liver, whereas inhibiting PKA activity resulted in a smaller embryonic liver (Figure S1H). Furthermore, the effect of dmPGE2 to augment liver size was blunted by H89, whereas the effect of Indo to diminish liver size was countered by forskolin (Figure S1H). These data are consistent with PGE2 acting through downstream effectors cAMP and PKA to promote liver outgrowth.
Prostaglandin Levels Affect Pancreas Development
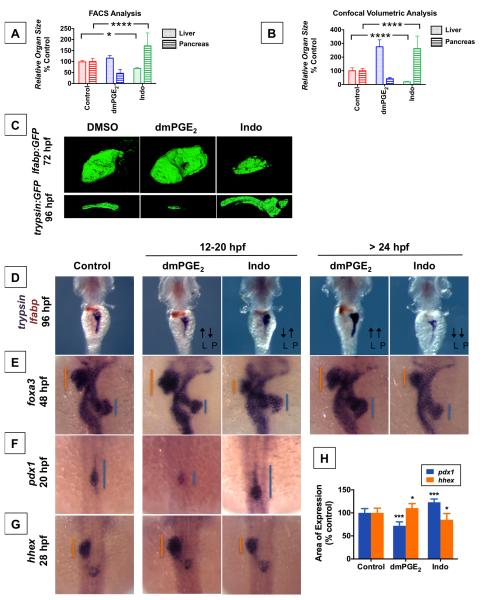
Given the shared origin of liver and pancreas from gut endoderm, we next examined the impact of PGE2 on pancreas development. Zebrafish embryos were exposed to dmPGE2 or Indo during outgrowth of the exocrine pancreas from 48-84 hpf and examined for markers of exocrine (trypsin, carboxypeptidase A) or endocrine (insulin) differentiation. dmPGE2 caused a dramatic increase in exocrine pancreas size, whereas Indo diminished exocrine pancreas size or completely abrogated exocrine marker expression (Figure 2A, S2A). A statistically significant effect was confirmed by volumetric analysis of the exocrine pancreas as visualized by confocal microscopy of transgenic trypsin:GFP fish (Figure 2D,E). FACS analysis of elastase:GFP reporter fish demonstrated statistically significant changes in GFP+ exocrine pancreas cell number, but no change in cell size or shape (Figure 2B). In contrast, modulation of PGE2 levels had no observable effects on endocrine pancreas formation as visualized by insulin expression (Figure 2C). No histological differences in the exocrine pancreas were detectable in all treated embryos (Figure 2F, S2C) nor discernable effects on cell death as assessed by TUNEL staining (Figure S2B).
Figure 2. PGE2 affects Embryonic Pancreas Outgrowth.
(A) In situ hybridization for trypsin, an exocrine pancreas-specific marker, following exposure to dmPGE2 or Indo reveals that embryonic pancreas size is regulated by PGE2. dmPGE2 increases exocrine pancreas size (57.6% embryos with an enlarged pancreas, n = 38 / 66), whereas Indo diminishes exocrine pancreas size (70.6% embryos with a smaller pancreas, n = 36 / 51). Dorsal view.
(B) FACS quantification of GFP+ exocrine pancreas cells in elastase:GFP embryos at 96 hpf following exposure to dmPGE2 or Indo. dmPGE2 increases and Indo decreases the relative number of exocrine pancreas cells. (Data are represented as mean ± SEM; ****significant across treatment groups, ANOVA, p < 0.0001).
(C) In situ hybridization for insulin following exposure to dmPGE2 or Indo. (n > 30 / condition). Dorsal view.
(D) Confocal microscopy visualization of exocrine pancreas in trypsin:GFP embryos at 96 hpf following exposure to dmPGE2 or Indo. Exposure to dmPGE2 enlarges and Indo severely diminishes pancreas development (n = 5 / condition). Right lateral view.
(E) Analysis of pancreas [volume] × [mean fluorescence intensity] visualized by confocal microscopy, corroborating the effect of dmPGE2 to increase and Indo to diminish embryonic pancreas size. (Data are represented as mean ± SEM; ****significant across treatment groups, ANOVA, p < 0.0001).
(F) Histological analysis reveals that modulating PGE2 levels changes embryonic pancreas size without discernible changes in pancreas cell morphology (n = 5 / treatment). Shown are exocrine pancreas cells with apical accumulation of secretory granules.
(G,H) Morpholino-mediated knockdown of PGE2 synthetic enzymes or PGE2 receptors ep2a and ep4a abrogates pancreas development, and this effect is partially rescued by addition of dmPGE2, as visualized by in situ hybridization for trypsin expression. Combined knockdown of both ep2a and ep4a receptors severely diminishes pancreas development and this cannot be rescued by addition of dmPGE2. Left lateral view. Impact on pancreas size quantified by [area] × [fluorescence intensity] in trypsin:GFP fish as shown in (Figure 2H). (Data are represented as mean ± SEM; n > 30 / condition; significant by t-test comparing each morpholino treatment vs. control and each morpholino treatment without vs. with dmPGE2, **** p<0.0001, *** p<0.001, n.s. = not significant).
See also Figure S2.
We next tested whether inhibition of PGE2 synthesis enzymes or receptors would similarly influence exocrine pancreas development. Knockdown of cox1a, cox2a, or pges or exposure to SC-560 or NS-398 abrogated development of the exocrine pancreas visualized by expression of trypsin at 84 hpf, and this effect was partially rescued by exogenous dmPGE2 (Figure 2G,H, S2E). Knockdown of either ep2a or ep4a resulted in a smaller exocrine pancreas, and this effect could only be partially rescued by exogenous dmPGE2 (Figure 2G,H). Again, combined knockdown of both receptors ep2a and ep4a caused severe diminution of the developing exocrine pancreas, and this effect could no longer be rescued by dmPGE2, consistent with PGE2 acting through these receptors to enlarge the developing pancreas (Figure 2G,H). Inhibition of PGE2 breakdown by the pgdh inhibitor Cay10397 augmented trypsin expression, demonstrating that similar to the liver bud, increasing endogenous PGE2 levels results in a larger exocrine pancreas (Figure S2D). These data provide in vivo evidence that levels of PGE2 modulate embryonic pancreas development. Further experiments using dmPGE2 and Indo and expression analysis of the intestinal marker intestinal fatty acid binding protein (ifabp) demonstrated an impact on intestinal development at 96 hpf (Figure S2F,G). These observations suggest that PGE2 activity may regulate the outgrowth of other endodermal derivatives in addition to the liver and pancreas.
Prostaglandin has Reciprocal Effects on Liver vs. Pancreas Endodermal Specification
We have shown that prostaglandin activity is required for normal development of the liver and pancreas, and augmenting prostaglandin levels enlarges both the developing liver and pancreas. These effects were observed when zebrafish embryos were treated after 48 hpf, when liver and pancreas populations have begun to express markers of mature differentiation. We next sought to elucidate whether PGE2 had a similar role before 24 hpf, a period when markers of specified liver and pancreas progenitors are first expressed but before differentiation and expansion of these progenitors into specialized populations. Zebrafish embryos were exposed to dmPGE2 at various timeframes before and after 24 hpf. Surprisingly, exposure between 12-20 hpf resulted in statistically significant opposing effects on the liver and pancreas, with dmPGE2 resulting in a larger liver but smaller pancreas, and Indo resulting in a smaller liver but larger pancreas, as quantified by FACS analysis of lfabp:GFP or elastase:GFP reporter fish (Figure 3A) and confocal volumetric analysis (Figure 3B,C). The reciprocal effect on liver vs. pancreas size when PGE2 levels are modulated between 12-20 hpf contrasts the effect observed after 24 hpf, when impact on the liver and pancreas size is in parallel.
Figure 3. PGE2 influences Liver vs. Pancreas Specification.
(A) FACS quantification of liver and pancreas GFP+ cells in lfabp:GFP or elastase:GFP fish, respectively, at 96 hpf following exposure to dmPGE2 or Indo from 12-20 hpf. (Data are represented as mean ± SEM; significant across treatment groups, ANOVA, **** p<0.0001, * p<0.05).
(B) Analysis of organ [volume] × [fluorescence intensity] of the liver or pancreas in lfabp:GFP at 72 hpf or trypsin:GFP fish at 96 hpf, respectively, visualized by confocal microscopy following treatment with dmPGE2 or Indo from 12-20 hpf. (Data are represented as mean ± SEM; significant across treatment groups, ANOVA, **** p<0.0001, n = 5 / condition).
(C) Confocal microscopy of the liver at 72 hpf in lfabp:GFP fish or pancreas at 96 hpf in trypsin:GFP fish following exposure to dmPGE2 or Indo from 12-20 hpf. Volume and fluorescence intensity are quantified in (Figure 3B).
(D) Double in situ hybridization for trypsin (purple) + lfabp (brown) at 96 hpf reveals reciprocal effects on organ size following 12-20 hpf exposure to dmPGE2 or Indo. Dorsal view.
(E) In situ hybridization for foxa3 at 48 hpf reveals reciprocal effects on the liver and pancreas buds emerging from the gut endoderm. The extent of the liver bud is indicated in orange, and of the pancreas bud in blue. Dorsal view.
(F,G) Early exposure to dmPGE2 or Indo results in a smaller (89.1% of embryos, n = 41 / 46) or larger (95.1% of embryos, n = 39 / 41) domain, respectively, of pancreas progenitor cells visualized by pdx1 at 20 hpf, and a larger (68.6% of embryos, n = 35 / 51) or smaller (72.7% of embryos, n = 32 / 44) domain, respectively, of liver progenitor cells marked by hhex at 28 hpf. Dorsal view.
(H) Quantification of the area of pancreas progenitors or liver progenitors following exposure to dmPGE2 or Indo during somitogenesis, shown as % control. (Data are represented as mean ± SEM; significant across treatment groups, ANOVA, ***p < 0.001 or *p = 0.013, n = 5 / treatment group).
See also Figure S3.
We further explored this reciprocal effect by simultaneously visualizing the emerging liver and pancreas buds marked by the pan-endodermal marker foxa3 at 48 hpf as well as markers of differentiated liver and pancreas cells at 96 hpf. Exposure to dmPGE2 or Indo after 24 hpf confirmed a parallel effect; in contrast, earlier exposure to these compounds at 12-20 hpf had opposing effects on the developing liver and pancreas buds (Figure 3D, S3A). Exposure to dmPGE2 from 12-20 hpf enlarges the liver bud but diminishes the pancreas bud at 48 hpf (Figure 3E), and larger liver but smaller pancreas organs develop by 96 hpf (Figure 3D). Conversely, Indo treatment from 12-20 hpf results in smaller liver and larger pancreas buds at 48 hpf (Figure 3E), and smaller liver and but larger pancreas organs are observed at 96 hpf (Figure 3D).
To investigate whether these reciprocal effects on the nascent liver and pancreas organs ensue from effects on their respective progenitor populations, we examined impact on endodermal progenitors specified to a liver or pancreas fate. dmPGE2 exposure from 12-20 hpf diminished the pancreas progenitor population marked by pdx1, but augments liver progenitors visualized with hhex (Figure 3F,G). Conversely, exposure to Indo from 12-20 hpf resulted in a larger pancreas progenitor population but diminished liver progenitor population (Figure 3F,G). Quantification of the area of pancreas progenitors marked by pdx1 or liver progenitors visualized by hhex expression confirmed a statistically significant change in response to modulating PGE2 activity (Figure 3H). To assess whether the observed effects of PGE2 activity on the size of the pancreas or liver progenitor populations at 20 hpf or 28 hpf, respectively, is mediated by differential expansion of progenitors, we quantified mitotic figures marked by pHH3 at 18 hpf in sox17:GFP endoderm reporter fish. Modulating PGE2 activity between 12-18 hpf results in no significant changes in the number of pHH3+ sox17:GFP+ cells (Figure S5A). These data suggest that the reciprocal effects of early PGE2 activity on liver versus pancreas development are caused by opposing effects on the specification of progenitor populations.
Put together, these data uncover a biphasic role for PGE2 levels in liver and pancreas development. At the earlier phase from 12-20 hpf before the liver and pancreas buds have emerged, PGE2 expands the liver progenitor population but diminishes the pancreas progenitor population. This earlier timeframe corresponds to the stage when bipotential progenitors capable of both liver and pancreas fates have been identified (Chung et al., 2008). Therefore, these reciprocal effects raise the possibility that PGE2 acts on bipotential endodermal progenitors to promote a liver fate at the expense of a pancreas fate.
Prostaglandin Affects Liver vs. Pancreas Specification of Murine Bipotential Endodermal Progenitors
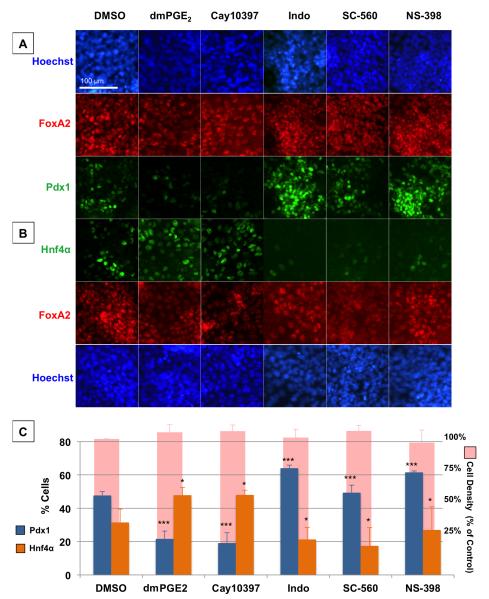
To directly test the hypothesis that PGE2 regulates the specification of bipotential endodermal progenitors towards a liver fate at the cost of a pancreas fate and suggest similar processes across species, we derived a population of bipotential endodermal progenitors through monolayer differentiation of mouse embryonic stem cells. These bipotential progenitors express the hepatopancreatic markers Hnf1β (Haumaitre et al., 2005; Lokmane et al., 2008), Prox1 (Burke and Oliver, 2002), and Onecut1 (Clotman et al., 2002; Jacquemin et al., 2003; Rausa et al., 1997) as well as the pan-endoderm marker FoxA2 (Figure S4). Upon further differentiation, subpopulations of these cells spontaneously express Hnf4α, a marker of hepatic specification, or Pdx1, a marker of pancreatic specification. Manipulation of Tgfβ and Fgf signaling allows enrichment of hepatic or pancreatic specification, whereas both subpopulations arise spontaneously in “neutral” conditions (Figure S4A-C). When levels of PGE2 were increased in “neutral” conditions by incubation with dmPGE2 or Cay10397, differentiation into Pdx1+ cells decreased whereas differentiation into Hnf4α+ cells increased significantly (Figure 4AC). In contrast, when levels of PGE2 were decreased by incubation with Indo, SC-560, or NS-398, the majority of the population was specified into a Pdx1+ pancreas fate, but specification into a liver fate was dramatically reduced (Figure 4A-C). These effects of PGE2 modulation occurred in as little as 16 hours, making it unlikely that differential proliferation could explain the changes in specification. Indeed, despite the statistically significant changes in cell differentiation, cell density as quantified by nuclear staining did not change with modulation of prostaglandin activity (Figure 4C). Additionally, we quantified proliferation by the percentage of FoxA2+ endoderm cells that were also pHH3+ after 18 hours of pharmacologic PGE2 modulation and found no statistically significant differences across the treatment conditions (Figure S5B,C). These results suggest that low levels or absence of PGE2 promotes pancreas specification, while PGE2 is necessary for liver specification. Furthermore, these results support the hypothesis that PGE2 regulates bipotential endoderm progenitor differentiation towards a liver versus pancreas fate across vertebrate species.
Figure 4. Liver vs. Pancreas Specification of Mouse Bipotential Endoderm Progenitors is Influenced by PGE2.
(A and B) Immunohistochemistry analysis of differentiating mouse endodermal progenitors following 24hr exposure to various modulators of PGE2 activity. Panendodermal cells marked by FoxA2 (red) are capable of either pancreas specification marked by Pdx1 (green in row A) or liver specification marked by Hnf4α (green in row B). Scale bar = 100 μm.
(C) The percentage of FoxA2+ cells that become Pdx1+ (blue) or Hnf4α+ (orange) following exposure to various modulators of PGE2 activity. (Data are represented as mean ± SEM; significant compared to DMSO by ANOVA test, ***p < 0.001, *p < 0.05, 3 independent experiments). Shown also is cell density as % of control (pink), and differences are not significant by ANOVA test (p = 0.854).
Prostaglandin Pathway Genes are Patterned to Regulate Liver vs. Pancreas Specification of Endoderm
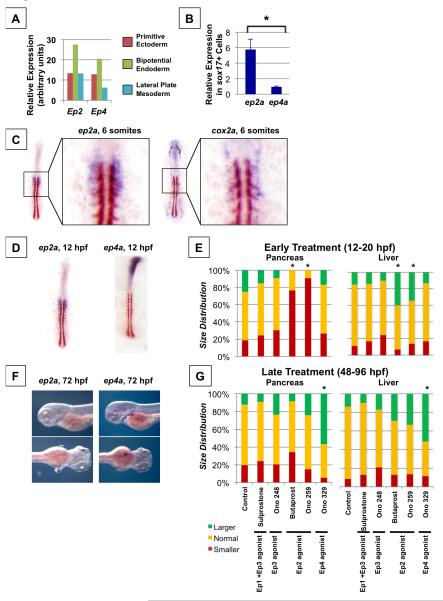
Having demonstrated in vivo and in the context of mouse bipotential endoderm that PGE2 activity influences liver versus pancreas specification, we next examined expression of components of the prostaglandin pathway in relation to endodermal progenitors. We first examined mammalian expression by microarray analysis in mouse embryonic stem cells at various stages of differentiation, and confirmed that Ep2 and Ep4 expression is enriched in a population of bipotential mouse endoderm relative to primitive ectoderm or lateral plate mesoderm (Figure 5A). We further confirmed conserved enrichment of the PGE2 receptors ep2a and ep4a in the zebrafish endoderm monolayer by analysis of GFP+ cells sorted from a primitive endoderm reporter fish, sox17:GFP, at the 6-8 somite stage. We find that ep2a is highly enriched in the zebrafish endoderm, consistent with responsiveness to PGE2 in this population (Figure 5B). A population of bipotential liver and exocrine pancreas progenitors has been delineated by single-cell labeling in the endoderm monolayer of zebrafish (Chung et al., 2008), located between somites 1 to 3 at the 6-8 somites stage. We therefore examined expression of components of the prostaglandin pathway in relation to this population of bipotential endodermal cells. At 10 hpf, when somitogenesis is beginning, ep2a is expressed in a population of cells adjacent to the notochord, spanning 4 cells from the midline and localized along the A-P axis in the region where the first somites will form (Figure S6A). Remarkably, at the 6-somite stage (12 hpf), the PGE2 receptor ep2a is localized to endoderm between somites 1 to 3 in a population of cells spanning 4 cells from the midline (Figure 5C, S6C,D), co-localized with the population of bipotential progenitors (Chung et al., 2008). We next examined expression of PGE2 synthesis components: expression of cox2a was found in a population of cells on the lateral edge of the population expressing ep2a (Figure 5C, S6A). Expression of cox1a was found in a broader population of cells even more lateral to the ep2a-expressing cells (Figure S6B). Expression of other PGE2 pathway genes including the receptor ep4a and the degrading enzyme pgdh did not appear to be specifically patterned in relation to the population of bipotential progenitors during early somitogenesis (Figure 5D, S6B). As cox2a is known to be an inducible and rate-limiting enzyme in PGE2 synthesis, this patterning of cox2a expression is consistent with highest levels of PGE2 occurring at the most lateral side of the ep2a-expressing cells, and lower levels of PGE2 occurring at the most medial aspect of the ep2a-expressing cells.
Figure 5. Prostaglandin Pathway Genes are Patterned to Regulate Endoderm Specification.
(A) Microarray analysis reveals enrichment of Ep2 and Ep4 in bipotential endoderm progenitors compared to primitive ectoderm or lateral plate mesoderm derived from mouse embryonic stem cells, shown in arbitrary units.
(B) qPCR analysis of sox17+ endodermal cells sorted from sox17:gfp zebrafish embryos reveals enrichment of ep2a compared to ep4a. (t-test, * p < 0.05, n = 3).
(C) Double in situ hybridization for ep2a (purple) or cox2a (purple) and myod (brown) in zebrafish embryos at the 6 somite stage.
(D) Double in situ hybridization for ep2a (purple) or ep4a (purple) and myod (brown) in zebrafish embryos at the 6 somite stage.
(E) Size distribution of pancreas or liver progenitor domain following exposure to pharmacologic agonists or antagonists of various PGE2 receptors from 12-20 hpf. Impact on pancreas progenitors was assessed at 20 hpf by in situ hybridization to pdx1, and impact on liver progenitors was assessed at 28 hpf by in situ hybridization to hhex. The following pharmacologic agents were used: Sulprostone (Ep1 + Ep3 agonist), Ono 248 (Ep3 agonist), Butaprost (Ep2 agonist), Ono 259 (Ep2 agonist), and Ono 329 (Ep4 agonist). (*significant vs. Control by Chi-square test, p < 0.05, n > 30 for each condition).
(F) Expression of ep2a and ep4a at 72 hpf visualized by in situ hybridization. Ep4a but not ep2a expression at 72 hpf corresponds to the emerging liver bud.
(G) Size distribution of pancreas or liver organs following exposure to pharmacologic agonists or antagonists of various PGE2 receptors from 48-96 hpf. Impact on pancreas outgrowth was assessed at 96 hpf by in situ hybridization to trypsin, whereas impact on liver outgrowth was assessed at 96 hpf by in situ hybridization to lfabp. (*significant vs. Control by Chi-square test, p < 0.05, n > 30 for each condition).
See also Figure S6.
Given the finding that the PGE2 receptors ep2a and ep4a are differentially patterned during embryogenesis, we exposed zebrafish embryos to receptor-selective agonists in order to delineate the role of specific receptors at different stages of development. Exposure to Ep1 or Ep3 agonists Sulprostone or Ono248, respectively, had no significant impact on developing pancreas or liver size (Figure 5E). In contrast, exposure to selective Ep2 agonists Butaprost or Ono259 during somitogenesis 12-20 hpf results in a smaller pancreas but larger liver (Figure 5E), similar to dmPGE2. Moreover, exposure to a selective Ep4 agonist, Ono329, during somitogenesis has no significant impact on developing pancreas or liver size (Figure 5E), consistent with expression of ep2a but not ep4a corresponding to the population of bipotential endodermal progenitors. At later time points during organ outgrowth, we have shown that exposure to dmPGE2 results in a larger pancreas and liver (Figure 3A-D). Exposure to the Ep4 agonist Ono329 at 48-96 hpf results in a larger pancreas and liver organ (Figure 5G). In contrast, no significant effects on developing pancreas or liver size was observed following exposure to selective Ep2 agonists Butaprost or Ono259 (Figure 5G). Consistent with these receptor-specific effects, we find that ep4a but not ep2a is expressed in the developing liver bud during liver outgrowth (Figure 5F). Put together, these expression patterns and receptor-specific functional data suggest that the specificity and changing roles of PGE2 through development are dictated by receptor localization.
We have previously demonstrated the importance of developmental signaling pathways for liver regeneration (Goessling et al., 2008). Having shown a significant role for PGE2 during embryonic liver outgrowth, we examined whether PGE2 impacts adult liver regeneration. Partial hepatectomies were performed in adult fish and regrowth was quantified after exposure to PGE2 modifiers. We found that PGE2 regulates adult liver regeneration, and similar to embryonic liver outgrowth, this role is predominantly mediated via the ep4a receptor (Figure S6E,F). Similar to the developmental context of liver bud outgrowth, PGE2 regulates cell proliferation in the regenerating liver (Figure S6G). These data suggest that the developmental role for PGE2 in liver outgrowth may be recapitulated in the context of adult liver regeneration.
Prostaglandin and Bmp Signaling Pathways Interact to Regulate Liver vs. Pancreas Specification
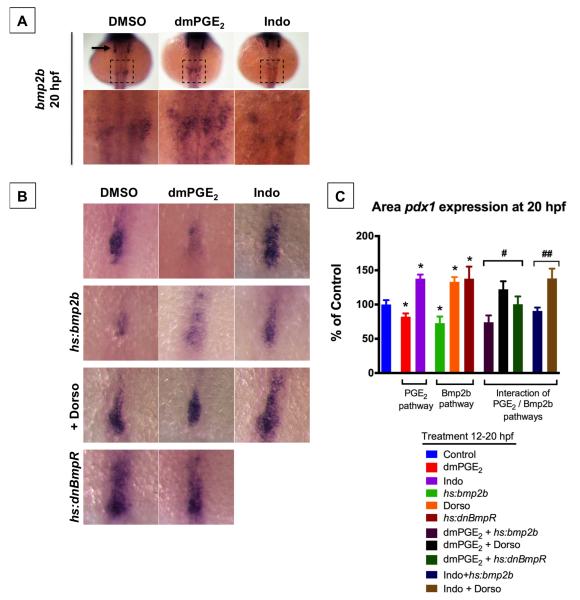
Much like the impact of prostaglandin described here, Bmp signaling has been shown to promote hepatic versus pancreatic fate in both mouse (Rossi et al., 2001) and zebrafish (Chung et al., 2008) embryonic endoderm. Moreover, similar to cox2a at the 6-8 somite stage, bmp2b expression is spatially-regulated in 2 bilateral stripes at the 10 somite stage and affects liver versus pancreas specification (Chung et al., 2008). Given these parallels in both timing and pattern of expression, we explored the possibility that these pathways interact to regulate liver versus pancreas specification. Exposure to Indo from 12-20 hpf diminishes bmp2b expression in the lateral plate mesoderm at 20 hpf, while exposure to dmPGE2 increases expression of bmp2b (Figure 6A). This finding demonstrates that PGE2 activity can regulate bmp2b expression, and therefore suggests that the prostaglandin pathway may be acting via bmp2b signaling to regulate liver versus pancreas specification. To further test this possibility, we modulated both prostaglandin activity as well as bmp2b activity and assayed the impact on pancreas specification marked by pdx1. As previously reported, activation of bmp2b in heat-shock-inducible hs:bmp2b transgenic fish diminishes the pancreas progenitor population marked by pdx1 (Chung et al., 2008; Figure 6B,C). In contrast, inhibition of bmp signaling by exposure to dorsomorphin, an inhibitor of bmp receptors alk 2, 3, and 6, or by activation of a dominant-negative bmp receptor in heat-shock-inducible hs:dnBmpR transgenic fish dramatically promotes the pancreas progenitor population (Figure 6B,C). In support of a signaling cascade in which bmp2b acts downstream of the prostaglandin pathway, the effect of dmPGE2 to promote pancreas progenitors is blunted by bmp receptor blockade with dorsomorphin or heat-shock induction of dnBmpR (Figure 6B,C). Conversely, the effect of Indo to promote pancreas progenitors is blunted following activation of bmp2b (Figure 6B,C). These data support a model in which the prostaglandin and bmp2b pathways interact to regulate liver vs. pancreas specification, and that PGE2 acts at least in part by positively regulating bmp2b expression.
Figure 6. PGE2 Interacts with bmp2b Pathway to Regulate Liver vs. Pancreas Specification.
(A) Modulation of PGE2 levels from 12-20 hpf impacts expression of bmp2b in the LPM as shown by in situ hybridization at 20 hpf. dmPGE2 increases bmp2b expression (n = 15 / 19 embryos), whereas Indo decreases bmp2b expression (n = 14 / 21 embryos). Lower row is magnified view of area in dashed square. bmp2b is also expressed in the otic vesicles (arrow).
(B,C) Size of pancreas-specified population marked by pdx1 is impacted by modulation of PGE2 or bmp2b pathways. Representative views are shown in (B), and quantification is shown in (C). dmPGE2 or heat-shock activation of bmp2b diminishes whereas Indo, dorsomorphin (Dorso), or heat-shock activation of dnBmpR promotes the pancreas progenitor population. The impact of dmPGE2 is blunted by Dorso or dnBmpR, and the impact of Indo is blunted by heat-shock activation of bmp2b. (Data are represented as mean ± SEM; n ≥ 10 per condition; t-test comparisons: * p < 0.05 comparing treatment vs. control; # no significant difference comparing with vs. without dmPGE2; ## no significant difference comparing with vs. without Indo).
DISCUSSION
In this study we uncover evidence of a previously uncharacterized role for PGE2 in embryonic development. Through genetic and chemical approaches in zebrafish embryos, we provide in vivo demonstration that PGE2 activity has a critical role in the specification and outgrowth of the liver and pancreas. During early somitogenesis, we reveal that PGE2 activity regulates specification of endoderm into a liver versus pancreas fate. We further provide evidence suggesting mammalian conservation of this role in the context of mouse embryonic endoderm. Later in embryonic development, we uncover a role for PGE2 activity in regulating outgrowth of the liver and pancreas buds. In line with this role in regulating embryonic liver outgrowth, PGE2 activity influences liver regeneration in the adult zebrafish.
PGE2 Regulates the Fate Specification of Bipotential Endoderm
We show that PGE2 activity promotes a liver progenitor population at the cost of a pancreas progenitor population. These findings are consistent with a role for PGE2 in regulating cell fate decisions in a population of bipotential endodermal progenitors. An alternative interpretation is that PGE2 activity may differentially influence the proliferation or maintenance of endodermal cells already specified to a liver or pancreas fate. In support of the first model, the reciprocal effects of prostaglandin activity occur at a stage when bipotential endodermal cells have been delineated (Chung et al., 2008). Moreover, we examine a population of mouse endodermal cells that can be specified into both liver and pancreas fates, and show that modulating prostaglandin activity can shift this population predominantly into one fate or the other. We show that in both the in vivo context of zebrafish endoderm as well as the in vitro context of mouse bipotential endodermal cells, modulating prostaglandin activity does not differentially alter cell proliferation. Put together, these observations argue that PGE2 acts on bipotential endodermal cells to guide specification towards a liver fate versus a pancreas fate.
In conjunction with our functional data, we reveal exquisite patterning of genes in the prostaglandin signaling pathway consistent with a key role in endodermal specification. The prostaglandin receptor ep2a is expressed in a population of endoderm corresponding to the previously-characterized bipotential progenitor population (Chung et al., 2008). This pattern raises the intriguing possibility that ep2a may serve as an early marker of the liver-pancreas bipotential progenitors. While studies in zebrafish and mouse embryos have characterized the signals directing the specification of this population, a marker for this population has not been described. Our microarray data show enrichment of Ep2 in mouse bipotential endoderm and suggest that the patterning of ep2a in zebrafish may be conserved.
Understanding PGE2 as a Morphogen
Concomitant with patterning of ep2a, the rate-limiting enzyme in PGE2 synthesis cox2a is patterned in 2 bilateral stripes such that endodermal cells closest to this source of prostaglandin are specified to become liver, whereas endodermal cells farther from this source of prostaglandin are specified to become exocrine pancreas. These findings lay groundwork for understanding prostaglandin activity as a morphogen whereby a gradient of prostaglandin activity may pattern a field of endoderm (Figure 7). This model makes several predictions. First, while there is no direct way to visualize PGE2 in vivo as has been achieved with fluorescent fusion proteins (Muller et al., 2012), we speculate that there may be a graded distribution of the readily diffusible PGE2 molecule acting on the population of ep2a-expressing cells based on the distinct spatial restriction of cox2a expression. A morphogen gradient of another non-peptidic signaling molecule, retinoic acid, has long been functionally recognized but only recently was visually proven (Shimozono et al., 2013). Prostaglandins are widely assumed to act as paracrine hormones exerting effects by interacting with extracellular receptors. However, while prostaglandin transport proteins have been identified in the cell membrane, the mechanisms regulating prostaglandin exit from cells and how far they act is poorly understood (Schuster, 2002). Second, a prevailing view of morphogen gradients postulates that uniform clearance across the exposed tissue generates a decaying concentration gradient of the morphogen (Rogers and Schier, 2011). Consistent with this notion, expression of the PGE2-degrading enzyme pdgh is uniform. Moreover, we show that targeted knockdown or pharmacologic inhibition of this enzyme activity has phenotypic consequences similar to augmentation of PGE2 levels. Third, consistent with a morphogen model, our observations in mouse bipotential endoderm suggest that this cell population has different genetic responses to different levels of PGE2. High levels of PGE2 promote a liver specification program, whereas lower levels or absence of PGE2 promote a pancreas specification program. A similar activity gradient regulating liver versus pancreas specification has been postulated of bmp2b (Chung et al., 2008), and indeed we find evidence for an interaction between the PGE2 and bmp2b pathways. How different levels of PGE2 are transduced into a liver versus pancreas cell fate, and how this activity gradient interacts with other signals known to regulate liver and pancreas specification, remains to be elucidated.
Figure 7. Model for the Role of PGE2 Activity in Liver vs. Pancreas Endoderm Specification.
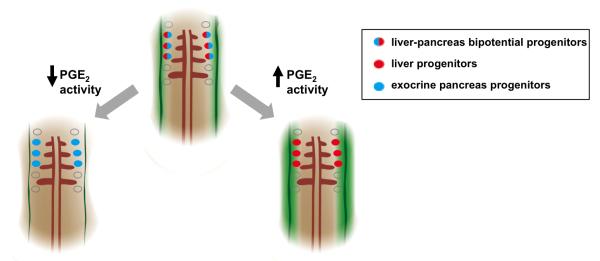
A model for PGE2 regulation of endodermal specification into liver or pancreas progenitors. Bipotential endodermal cells capable of both a liver or exocrine pancreas fate (red/blue cells) have been found between somites 1-3. Levels of PGE2 (green gradient) may form a gradient with highest activity closest to the 2 bilateral stripes of cox2a (solid green bands), the rate-limiting enzyme in PGE2 synthesis. Cells closest to the highest levels of PGE2 are more likely to acquire a liver fate (red cells), whereas cells farther from the source of PGE2 acquire an exocrine pancreas fate (blue cells). Augmenting levels of PGE2 promotes a liver fate (red cells) at the cost of a pancreas fate (blue cells), whereas decreasing levels of PGE2 promotes a pancreas fate (blue cells) at the cost of a liver fate (red cells).
Dynamic Roles for PGE2 in Different Developmental Contexts
Our findings demonstrate that the role of PGE2 activity in the context of liver and pancreas development changes over time. This is a fundamental property of other highly-conserved signaling pathways that regulate liver and pancreas development. Shifting roles in liver and pancreas development have been demonstrated for Wnt, Shh, and Bmp signaling pathways, and these changes can occur in just a few hours (Goessling et al., 2008; Wandzioch and Zaret, 2009; Zaret and Grompe, 2008). Similarly, in this study, we find a timeframe during early somitogenesis in which PGE2 activity regulates liver versus pancreas specification of gut endoderm and a later phase in which PGE2 activity promotes the expansion and outgrowth of both liver and pancreas buds. A changing role for PGE2 may in part be governed by exquisite spatial and temporal regulation of receptor expression in different contexts. We find that ep2a and ep4a are differently patterned in different developmental contexts. Moreover, consistent with their distinct expression, we demonstrate receptor-specific effects of blocking ep2a or ep4a, and find that ep2a has a critical role in regulating liver versus pancreas fate decisions, whereas ep4a has a role in regulating outgrowth of the liver and pancreas buds. In conjunction with carefully regulated production, the regulation of receptor expression to dictate shifting roles of secreted factors may be a common theme in development. Delineating the role of prostaglandins at specific stages of liver and pancreas organogenesis may help inform how we tailor stem cell differentiation for therapy.
The Role of PGE2 in Gastrointestinal Regeneration and Cancer
A second fundamental property of signaling pathways that regulate solid organ development is their importance in the context of regeneration and cancer (Goessling et al., 2008; Rhim and Stanger, 2010). Indeed, in this study, we show that modulating prostaglandin activity influences liver regeneration after adult hepatectomy. These findings parallel our observations that genetically or chemically diminishing prostaglandin activity abrogates outgrowth of the embryonic liver bud. Furthermore, consistent with our findings that excess prostaglandin activity promotes growth of the embryonic pancreas bud and primordial intestine marked by ifabp, dysregulation of the prostaglandin pathway is a key feature of both pancreatic and colon cancer, and inhibitors of COX synthetic enzymes have long been known to reduce the occurrence and death from cancers of the colon, lung, and other endodermal derivatives (Rothwell et al., 2011; Wang and Dubois, 2010). Therefore, while prostaglandins have traditionally been thought to promote cancer by augmenting inflammation and angiogenesis, the findings presented in this study provide an ontogenic context for investigating the role of prostaglandin activity in gastrointestinal cancer and regeneration.
A New Understanding of PGE2 Function
A dynamical array of protein signaling factors has been implicated in regulating specification of gut endoderm (Zaret and Grompe, 2008). Our findings place PGE2 among these canonical signaling pathways, and thereby challenge longstanding notions of prostaglandin function. We demonstrate that, much like canonical protein signaling pathways, the PGE2 pathway exhibits exquisite spatial and temporal patterning of its synthetic enzymes and receptors, changing roles at different times in organogenesis, and a role in adult organ homeostasis. From an evolutionary perspective, prostaglandins have been found in prokaryotes and plants, and thus predate the protein signaling pathways that regulate development of metazoans (Richards and Degnan, 2009). We postulate that prostaglandins offer unique ancestral advantages compared to protein signaling pathways. Prostaglandins are synthesized de novo from metabolism of membrane-derived arachidonic acid, and therefore do not have the storage and energy requirements of protein signaling pathways. Additionally, prostaglandins are thought to have an evanescent half-life (Funk, 2001), and therefore may allow more temporally and spatially accurate signaling between cells. The specification of endoderm into liver or pancreas fates, in which regulatory signals must act over the span of a few cells and in a small time window, is exemplary of such a sensitive and critical developmental process. Our findings open the door to discovery of additional developmental programs regulated by prostaglandins and may offer new therapeutic avenues for tissue repair and cancer treatment.
EXPERIMENTAL PROCEDURES
Zebrafish Husbandry
Zebrafish were maintained according to IACUC protocols. Transgenic lines lfabp:GFP, lf:dsRed;elastase:GFP, gut:GFP, trypsin:GFP, hs:bmp2b, and hs:dnBmpR were described previously (Chung et al., 2008; Goessling et al., 2008; Korzh et al., 2008). Heat-shock of hs:bmp2b fish or hs:dnBmpR fish at 12 hpf was performed at 37°C or 40°C for 30 minutes as previously described (Chung et al., 2008).
Embryonic Zebrafish Experiments
Embryos were exposed to pharmacologic agents at the timepoints described at a concentration of 10 uM except for: dorsomorphin 30 uM, H89 0.5 uM, Forskolin 0.5 uM. DMSO carrier content was 0.1%. Embryos were processed for in situ hybridization using standard procotols (http://zfin.org/ZFIN/Methods/ThisseProtocol.html). Morpholino (GeneTools) knockdown was performed as previously described (North et al., 2007). Embryos were processed for whole mount immunohistochemistry using standard protocols and antibodies against pHH3 (Millipore, titer 1:500) and GFP (Millipore, titer 1:300). For section immunohistochemistry, hematoxylin / eosin staining was performed on alternate sections using standard techniques as previously described (Goessling et al., 2008). Antibodies to PCNA (Anaspec, titer 1:200) were visualized by DAB and counterstained with methyl green. Apoptosis was visualized using the ApopTag kit (Chemicon).
Adult Zebrafish Experiments
Liver resection was performed and regeneration evaluated as previously described (Goessling et al., 2008). Adult zebrafish were fixed with paraformaldehyde, paraffin embedded and cut in 10 μm sections for histological analysis. Hematoxylin / eosin staining was performed on alternate sections using standard techniques as previously described (Goessling et al., 2008).
Quantification of Liver or Pancreas Size
Liver or pancreas size was quantified by FACS of GFP+ cells in fluorescent reporter fish, confocal microscopy and volumetric analysis, and measurement of area of marker expression visualized by in situ hybridization using ImageJ. Volumetric analysis incorporated volume and average fluorescence intensity of 3D confocal stacks using ImageJ.
Flow cytometry analysis
Whole fluorescent embryos were manually dissociated in 0.25% trypsin for 20 minutes and then manually dissociated and analyzed for %GFP fluorescence by flow cytometry. At least 100,000 cells were analyzed per sample (n=10-20 / treatment).
qPCR
qPCR was performed using primer sets for pdx1, ep2a, and ep4a. RNA was extracted and RT-PCR was performed as previously described (Goessling et al., 2009).
Mouse ES Cell Differentiation, Treatment and Immunohistochemistry
Mouse ES cell culture and stepwise differentiation into endoderm and then bipotential hepatopancreatic progenitors was performed according to previously published protocols (Sherwood et al., 2011) as schematized in Figure S4 and detailed in Supplemental Methods.
Microarray
Bipotential hepatopancreatic progenitors were derived from mES cells as described, processed for microarray using the Illumina TotalPrep RNA Amplification Kit (Ambion), and hybridized to Mouse Ref8 v2.0 chips (Illumina). Microarray values were compared with previously obtained microarray data (Sherwood R, personal communication) using GenomeStudio software (Illumina).
Statistical Analysis
Pooled data were calculated as mean ± S.E., with number of repeats as indicated. Pairwise comparison was performed by t test, multiple comparisons by ANOVA, and distribution comparisons by Chi-square test.
Supplementary Material
HIGHLIGHTS.
PGE2 acts as a morphogen to regulate liver-versus-pancreas specification of endoderm
Expression of PGE2 pathway genes is patterned to regulate endoderm specification
PGE2 interacts with bmp2b signaling to promote a liver fate over a pancreas fate
PGE2 promotes organ growth later in development and liver regeneration in adults
Prostaglandins have long been recognized to modulate processes such as vasoregulation and inflammation. Here, Nissim et al. find evidence for prostaglandin E2 (PGE2) in regulating the specification of endoderm into liver or pancreas the context of embryonic development.
ACKNOWLEDGEMENTS
This work was supported by the National Pancreas Foundation and NIH NIDDK T32 (S.N.) and by NIH NIDDK R03DK085445, R01DK090311, Harvard Stem Cell Institute and the Pew Charitable Trusts (W.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Developmental cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Garnaas MK, Cutting CC, Meyers A, Kelsey PB, Jr., Harris JM, North TE, Goessling W. Rargb regulates organ laterality in a zebrafish model of right atrial isomerism. Dev Biol. 2012;372:178–189. doi: 10.1016/j.ydbio.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol. 2003;258:105–116. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Korzh S, Pan X, Garcia-Lecea M, Winata CL, Wohland T, Korzh V, Gong Z. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. 2008;8:84. doi: 10.1186/1471-213X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokmane L, Haumaitre C, Garcia-Villalba P, Anselme I, Schneider-Maunoury S, Cereghini S. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008;135:2777–2786. doi: 10.1242/dev.023010. [DOI] [PubMed] [Google Scholar]
- Miki R, Yoshida T, Murata K, Oki S, Kume K, Kume S. Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech Dev. 2012;128:597–609. doi: 10.1016/j.mod.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336:721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausa F, Samadani U, Ye H, Lim L, Fletcher CF, Jenkins NA, Copeland NG, Costa RH. The cut-homeodomain transcriptional activator HNF-6 is coexpressed with its target gene HNF-3 beta in the developing murine liver and pancreas. Dev Biol. 1997;192:228–246. doi: 10.1006/dbio.1997.8744. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Stanger BZ. Molecular biology of pancreatic ductal adenocarcinoma progression: aberrant activation of developmental pathways. Prog Mol Biol Transl Sci. 2010;97:41–78. doi: 10.1016/B978-0-12-385233-5.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GS, Degnan BM. The dawn of developmental signaling in the metazoa. Cold Spring Harbor symposia on quantitative biology. 2009;74:81–90. doi: 10.1101/sqb.2009.74.028. [DOI] [PubMed] [Google Scholar]
- Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annual review of cell and developmental biology. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68-69:633–647. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Maehr R, Mazzoni EO, Melton DA. Wnt signaling specifies and patterns intestinal endoderm. Mech Dev. 2011;128:387–400. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozono S, Iimura T, Kitaguchi T, Higashijima S, Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Developmental cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Eicosanoids and cancer. Nature reviews Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.