Abstract
Agrobacterium tumefaciens and Agrobacterium rhizogenes transfer plasmid-encoded genes and virulence (Vir) proteins into plant cells. The transferred DNA (T-DNA) is stably inherited and expressed in plant cells, causing crown gall or hairy root disease. DNA transfer from A. tumefaciens into plant cells resembles plasmid conjugation; single-stranded DNA (ssDNA) is exported from the bacteria via a type IV secretion system comprised of VirB1 through VirB11 and VirD4. Bacteria also secrete certain Vir proteins into plant cells via this pore. One of these, VirE2, is an ssDNA-binding protein crucial for efficient T-DNA transfer and integration. VirE2 binds incoming ssT-DNA and helps target it into the nucleus. Some strains of A. rhizogenes lack VirE2, but they still transfer T-DNA efficiently. We isolated a novel gene from A. rhizogenes that restored pathogenicity to virE2 mutant A. tumefaciens. The GALLS gene was essential for pathogenicity of A. rhizogenes. Unlike VirE2, GALLS contains a nucleoside triphosphate binding motif similar to one in TraA, a strand transferase conjugation protein. Despite their lack of similarity, GALLS substituted for VirE2.
Agrobacterium rhizogenes root-inducing (Ri) plasmids show many similarities to Agrobacterium tumefaciens tumor-inducing (Ti) plasmids, including nearly identical organizations of the vir operons (6, 32, 35, 36, 44, 80). One notable exception to this rule is the absence of virE1 and virE2 from the Ri plasmid (and the rest of the genome) in some strains of A. rhizogenes (2, 6, 36, 44). This raises an important question which is the subject of our paper. How can A. rhizogenes transfer DNA into plant cells efficiently when two critical virulence proteins are missing: the single-stranded (ss) DNA-binding protein VirE2 (10-12, 16, 19, 28, 56, 76) and its secretory chaperone, VirE1 (17, 66, 67, 85)? Our work shows that the GALLS protein encoded by the Ri plasmid can replace VirE2 and VirE1 and that the GALLS gene is essential for the virulence of A. rhizogenes strains that lack virE1 and virE2.
A. tumefaciens secretes the ssDNA-binding protein VirE2 into plant cells via the secretion system comprised of VirB1 through VirB11 (VirB1-11) and VirD4 (3, 9, 27, 39, 60, 74, 75, 82, 86). VirE2 is required only in plant cells; transgenic plants that produce VirE2 are susceptible to virE2 mutant A. tumefaciens (13). Inside plant cells, VirE2 protects ssT-DNA (T strands) (64, 73) from nuclease attack and promotes their nuclear import (26, 50, 84, 88). A virE2 mutation drastically reduces the amount of T-strand DNA recovered from the cytoplasm of infected plant cells (84), even though T-strand levels in bacterial cells remain normal (26, 64, 73). Although virE2 null mutations severely reduce tumorigenesis, some transformation of plant cells occurs (24, 63). In the absence of VirE2, integrated T-DNAs are often truncated at their left ends (50), confirming that VirE2 protects T strands from nuclease attack. Thus, T strands are more susceptible to degradation in the absence of VirE2 (84).
The central region of VirE2 contains two nuclear localization signals (NLSs) (13). The NLSs overlap regions important for binding ssDNA and for cooperative interaction between VirE2 molecules (13, 19, 67). However, the NLSs remain accessible for nuclear targeting when VirE2 binds to DNA, despite the involvement of these regions in protein-DNA and protein-protein interactions (88). Fluorescently labeled ssDNA coated with VirE2 accumulates in nuclei upon microinjection of the complex into plant cells (88). Thus, VirE2 retains its nuclear localization capability when bound to ssDNA.
VirE2 can bind T strands from another bacterial cell. Mixed-infection experiments suggest that A. tumefaciens lacking T-DNA may transport VirE2 directly into plant cells. Tumors form readily when a single plant wound is inoculated with two nonpathogenic strains of A. tumefaciens, one lacking T-DNA and the second mutated in virE2 (10, 46, 66). Both VirE2 and T-strand donors must contain wild-type virB1-11 and virD4 genes and chromosomal loci (chvA, chvB, and exoC) necessary for binding to plant cells (5, 10, 78). Because both donors must be able to bind plant cells, VirE2 and T strands are probably exported directly, and independently, into plant cells.
A. rhizogenes 1724 lacks virE2, but the strain can still transfer T-DNA efficiently (44). The GALLS gene from A. rhizogenes 1724 restored pathogenicity when we introduced it into a virE2 mutant A. tumefaciens. In addition, GALLS protein supplied by mixed infection replaced VirE2 effectively. Different A. rhizogenes strains contained either the GALLS gene or virE2, but not both. A transposon insertion in the GALLS gene of A. rhizogenes K599 abolished its ability to induce hairy root disease. Although GALLS substituted for VirE2, the proteins lack obvious similarities. Instead, the amino terminus of GALLS resembles plasmid-encoded TraA (strand transferase) proteins from A. tumefaciens and Rhizobium meliloti (21).
MATERIALS AND METHODS
Bacterial strains.
Table 1 lists the A. rhizogenes, A. tumefaciens, and Agrobacterium vitis strains used. In gene construction experiments, Escherichia coli TOP10 competent cells [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG] (Invitrogen) were transformed with ligated plasmid DNA as recommended by the supplier.
TABLE 1.
Agrobacterium strains
| Hosta | Plasmid | Opine | GALLS geneb | virE2b | virD2b | Source/notes | Reference |
|---|---|---|---|---|---|---|---|
| A4 (no. 100) | pRiA4 | Agropine | + | − | + | S. Farrand | 80 |
| A4 (no. 117) | pRiA4 | Agropine | + | − | + | F. White via S. Farrand | 80 |
| A136 | None | None | − | − | − | E. Nester | 79 |
| A136 (R1000) | pRiA4 | Agropine | + | − | + | E. Nester | 80 |
| A136 (A348) | pTiA6 | Octopine | − | + | + | E. Nester | 24 |
| A136 (A856) | pTiAg162 | A. vitis | − | + | + | E. Nester | 38 |
| A136 (MX243) | pTiA6::Tn3 | Octopine | − | + | + | E. Nester/(virB1::Tn3) | 63 |
| A136 (MX328) | pTiA6::Tn3 | Octopine | − | + | + | E. Nester/(virD4::Tn3) | 63 |
| A136 (MX358) | pTiA6::Tn3 | Octopine | − | Tn3 | + | E. Nester/(virE2::Tn3) | 63 |
| A136 (MX368) | pTiA6::Tn3 | Octopine | − | + | + | E. Nester/(virB10::Tn3) | 63 |
| ATCC 15834 | pRi15834 | Agropine | + | − | + | F. White via S. Farrand | 80 |
| C58C1 | pRi1724::kan | Mikimopine | + | − | + | N. Tanaka via S. Farrand | 44 |
| C58C1 | pRiA4 | Agropine | + | − | + | Y. Dessaux via S. Farrand | 80 |
| C58C1RS | pArA4a | None | − | − | − | Y. Dessaux via S. Farrand | 80 |
| C58C1RS | pRiTR105 | Agropine | + | − | + | A. Kerr via S. Farrand | 80 |
| ICPB-TR7 | pRiTR7 | Mannopine | − | + | + | A. Kerr via S. Farrand | 51 |
| NCIB8196 | pRi8196 | Mannopine | − | + | + | A. Kerr via S. Farrand | 14 |
| NCPPB1855 | pRi1855 | Agropine | + | − | + | S. Farrand | 14 |
| NCPPB2655 | pRi2655 | Cucumopine | + | − | + | A. Kerr via S. Farrand | 51 |
| NCPPB2657 | pRi2657 | Cucumopine | + | − | + | A. Kerr via S. Farrand | 51 |
| NCPPB2659 (K599) | pRiK599 | Cucumopine | + | − | + | A. Kerr via S. Farrand | 51 |
| NT1 | pRiK599 | Cucumopine | + | − | + | S. Farrand | 51 |
| A136 (WR5000) | pTiA6ΔvirE2 | Octopine | − | ΔvirE2 | + | W. Ream/(ΔvirE2::nptII) | 19 |
Alternate strain names (or isolate numbers) are indicated in parentheses.
+, present; −, absent.
Media.
E. coli was selected on ampicillin (50 μg/ml), kanamycin (25 μg/ml), gentamicin (50 μg/ml), or nalidixic acid (30 μg/ml) in Luria (L) agar or L broth (10 g of tryptone/liter, 5 g of yeast extract/liter, 10 g of NaCl/liter, pH 7) (41). Drug-resistant A. tumefaciens was selected on carbenicillin (100 μg/ml), gentamicin (50 μg/ml), or kanamycin (100 μg/ml) in AB-glucose agar (15 g of Difco agar/liter, 5 g of glucose/liter, 3 g of K2HPO4/liter, 1 g of NaH2PO4/liter, 1 g of NH4Cl/liter, 0.3 g of MgSO4 · 7H2O/liter, 0.15 g of KCl/liter, 10 mg of CaCl2/liter, 2.5 mg of FeSO4 · 7H2O/liter), YEP broth (10 g of yeast extract/liter, 5 g of NaCl/liter, 10 g of peptone/liter), or YMA agar (15 g of Difco agar/liter, 0.4 g of yeast extract/liter, 10 g of mannitol/liter, 0.5 g of K2HPO4/liter, 0.2 g of MgSO4 · 7H2O/liter, 0.1 g of NaCl/liter, pH 6.8 to 7.0). Matings were performed on 1.5% nutrient agar (Difco).
Preparation of Ri plasmid DNA.
Ri plasmid DNA was prepared as described by Currier and Nester (15). Supercoiled plasmid DNA was isolated by CsCl-ethidium bromide density gradient centrifugation, as described by Ream and Field (48).
Cosmid library preparation and screening.
CsCl-purified pRi1724::kan plasmid DNA was partially digested with SalI (New England Biolabs) and joined to SalI-cut pVK100 cosmid (37) DNA using T4 DNA ligase (Fermentas). The ends of the cut vector were dephosphorylated with calf intestinal phosphatase (Fermentas) prior to ligation in order to prevent recircularization of the vector. Ligated DNA was packaged (in vitro) into phage lambda particles using Gigapack III XL packaging extracts (Stratagene). The packaged DNAs were transfected into E. coli LE392 [hsdR514 supE44 supF58 lacY1 or Δ(lacIZY)6 galK2 galT22 glnV44 metB1 trpR55] (52), and the transfected cells were plated on L agar containing kanamycin. Resistant colonies were cultured in L broth containing kanamycin, and cosmid DNAs were prepared using an alkaline lysis method (Qiagen). Purified cosmid DNAs were transformed into the virE2 mutant A. tumefaciens MX358 by the freeze-thaw method (34), and transformants were selected on AB-glucose agar containing kanamycin and carbenicillin. Each transformant was inoculated onto leaves of Kalanchoe daigremontiana, where MX358 is avirulent (63). Cosmid-containing MX358 derivatives that caused tumors on K. daigremontiana leaves were also tested on carrot root slices. Cosmids that restored pathogenicity to MX358 were isolated using a Qiagen alkaline lysis DNA preparation kit. These DNAs were digested with SalI and examined by agarose gel electrophoresis. The exact endpoints of the pRi1724::kan sequences in each cosmid were determined by DNA sequence analysis using primer oligonucleotides that flank the SalI site of pVK100, which lies in the tetracycline resistance (tet) gene (forward primer, 5′-AATCTTGCTCGTCTCGCTGG-3′; reverse primer, 5′-TCCGCCCGATATAGAGAACC-3′). Davis Sequencing (Davis, Calif.) performed the DNA sequence analyses. One cosmid (pLH77) was selected for further study because it was the smallest cosmid that complemented mutations in virE2.
Transposon mutagenesis.
The cosmid pLH77, which contains the GALLS region of pRi1724, was mutagenized with Tn3-lac as described by Stachel et al. (61). The target cosmid (pLH77; a kanamycin-resistant IncP replicon) was transformed into E. coli HB101 (recA13 hsdR hsdM hsdS20 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 supE44) (7) containing the Tn3-lac transposon donor plasmid pHoHo1 (an ampicillin-resistant ColE1 replicon) and the transposase-producing plasmid pSShe (a chloramphenicol-resistant pACYC184 replicon) (61). The strain was mated on nutrient agar (Difco) at 28°C with a recipient (E. coli SF800; polA1 thy gyrA [Nalr]) (31) and HB101 containing pRK2013 (22), a kanamycin-resistant plasmid capable of mobilizing pLH77. This triparental mating mixture was plated on L agar containing ampicillin, kanamycin, and nalidixic acid to select SF800 transconjugants that contained pLH77::Tn3-lac cosmids. Mutant cosmid DNAs were prepared from SF800 transconjugants by an alkaline lysis method (Qiagen). Purified DNAs were transformed into E. coli TOP10, and transformants were selected on L agar containing ampicillin and kanamycin. Cosmid DNA was prepared from these transformants, digested with SalI, and analyzed by agarose gel electrophoresis. Because all cosmids that complemented mutations in virE2 shared a region comprised of two SalI restriction fragments of 13,478 and 3,953 bp, we screened for pLH77::Tn3-lac insertions that disrupted either of these SalI fragments. The locations of the insertions in these cosmids (Table 2) were determined by DNA sequence analysis using a primer complementary to sequences from 89 to 71 bp from the left end of the Tn3-lac element (5′-ATTCCGGTCATCTGAGACC-3′). Davis Sequencing performed the DNA sequence analyses.
TABLE 2.
Tn3-lac insertions, β-galactosidase activity, and virE2 complementation
| Allele | Locationa
|
Orientation | No. of β-galactosidase unitsb
|
Replaces virE2c | ||||
|---|---|---|---|---|---|---|---|---|
| ORF | Nucleotide | Coordinate | +AS | −AS | +AS/−AS | |||
| 306 | 55 | 869 | 67618 | Antisense | 3.5 ± 0.2 | 3.3 ± 0.6 | 1.1 | No |
| 335 | 55 | 1,417 | 67070 | Sensed | 11.4 ± 2.4 | 5.9 ± 1.1 | 1.9 | No |
| 312 | 55 | 1,544 | 66943 | Antisense | 7.1 ± 0.7 | 6.6 ± 0.3 | 1.1 | No |
| 301 | 55 | 2,677 | 65810 | Antisense | 3.0 ± 0.2 | 3.4 ± 0.2 | 0.9 | No |
| 315 | 55 | 3,050 | 65437 | Sense | 6.5 ± 0.7 | 4.5 ± 0.2 | 1.4 | No |
| 334 | 55 | 3,908 | 64579 | Sense | 6.4 ± 0.4 | 4.6 ± 0.4 | 1.4 | No |
| 321 | 55 | 4,871 | 63616 | Sense | 7.1 ± 0.1 | 5.1 ± 0.1 | 1.4 | Partial |
| 303 | 56 | 248 | 69584 | Antisense | 4.1 ± 0.2 | 4.2 ± 0.1 | 1.0 | Yes |
| 323 | 58/59 | NA | 72012 | (Sense)e | 7.8 ± 0.3 | 8.0 ± 0.4 | 1.0 | Yes |
| 313 | 59 | 657 | 72195 | Sense | 5.4 ± 0.8 | 5.2 ± 0.8 | 1.0 | Yes |
| 314 | 60 | 60 | 73488 | Antisense | ND | ND | ND | Yes |
| 310 | 61 | 605 | 75259 | Sense | 25.3 ± 2.2 | 25.3 ± 1.9 | 1.0 | Yes |
| 330 | 61 | 701 | 75355 | Sense | 25.3 ± 2.5 | 25.3 ± 3.3 | 1.0 | Yes |
| MX243 | virB1 | ND | NA | Sense | 12.2 ± 2.7 | 2.5 ± 0.4 | 4.9 | NA |
| A348f | None | NA | NA | No lacZ | 2.5 ± 0.3 | 2.7 ± 0.6 | 0.9 | NA |
ORF, ORF disrupted by the Tn3-lac insertion (ORF 55 is the GALLS gene); Nucleotide, distance (in base pairs) of the Tn3-lac transposon from the first base of the ORF; Coordinate, position of the insertion in the sequence of pRi1724 (accession no. AP002086); NA, not applicable; ND, the exact location of the Tn3-lac insertion in the virB1 gene of A. tumefaciens MX243 was not determined.
β-Galactosidase units are as defined by Miller (43) as follows: 1,000 × A420/A600 × min. +AS and −AS, presence or absence of acetosyringone in the medium; +AS/−AS, ratio of β-galactosidase units in acetosyringone-treated cultures to that in untreated cultures; ND, β-galactosidase activity was not determined.
Replaces virE2, ability of a cosmid that contains a particular Tn3-lac allele to restore virulence to the virE2 mutant A. tumefaciens MX358.
Allele 335 created a translational (in-frame) fusion between lacZ and the GALLS gene. All other sense-oriented Tn3-lac insertions (in ORFs 55, 59, and 61) are transcriptional (out-of-frame) fusions.
(Sense), insertion 323 lies between ORFs 58 and 59 with lacZ in the sense orientation relative to the flanking ORFs.
A. tumefaciens A348 does not contain a lacZ gene and was included to measure background levels of o-nitrophenyl-β-d-galactopyranoside conversion to o-nitrophenol. All pLH77::Tn3-lac derivatives were tested for β-galactosidase activity in the A348 background.
Homogenotization.
The mutant GALLS gene containing Tn3-lac-301 (Table 2) was introduced into the Ri plasmid of A. rhizogenes K599 by homologous recombination as described previously (25). We selected A. rhizogenes K599 for homogenotization because it contained an Ri plasmid with the GALLS gene in an A. rhizogenes chromosomal background (Table 1, NCPPB2659). In contrast, pRi1724::kan resided in the A. tumefaciens C58C1 chromosomal background (Table 1), and the parental A. rhizogenes strain was not available. Cosmid pLH77::Tn3-lac-301 was transformed into wild-type A. rhizogenes K599; transformants were selected on AB-glucose agar containing carbenicillin and kanamycin. After mating of an A. rhizogenes K599 transformant with E. coli 2174(pPH1JI), which encodes resistance to gentamicin (25), a homogenote (A. rhizogenes MX599) was selected on AB-glucose agar containing carbenicillin and gentamicin.
Construction of GALLS gene subclones.
The GALLS-containing cosmid pLH77 was digested with BamHI and XhoI (New England Biolabs) to produce a 6,194-bp restriction fragment that contained the GALLS coding sequence and promoter. We inserted this fragment into pBluescript SK(−) (Stratagene) cut with BamHI and XhoI. The resulting plasmid, pLH337, was cleaved with XhoI and ligated to SalI-digested pVK100 (37), creating pLH338. To create an in-frame deletion near the 3′ end of the GALLS gene, we digested pLH337 with NcoI, which cuts the GALLS gene twice, and recircularized the truncated plasmid to form pLH341. This plasmid was cut with XhoI and inserted into SalI-digested pVK100 to create pLH344. To create a plasmid that contained only the 5′ end of the GALLS gene, we digested pLH337 with EcoRI and ligated the resulting 2,221-bp fragment to EcoRI-cut pBluscript SK(−) to create pLH342. This plasmid was digested with XhoI and ligated to SalI-cut pVK100 to create pLH345. Plasmids pLH338, pLH344, and pLH345 were transformed into the virE2 mutant A. tumefaciens MX358.
Virulence assays.
Tests for tumorigenesis were performed on carrot root slices and K. daigremontiana leaves as described previously (19). A. tumefaciens and A. rhizogenes were grown overnight at 28°C with aeration in YEP broth containing the appropriate antibiotics. Fresh carrots were washed with soap, rinsed with sterile distilled water and then with 70% ethanol, and submerged in 20% bleach-0.1% sodium dodecyl sulfate (SDS) for 20 min. After the bleach treatment, the carrots were rinsed with sterile distilled water and sliced into segments ∼5 mm thick; each slice was placed on water agar (1.5%) with the basal (upper) surface upward. The basal surface of each carrot root slice was inoculated with 25 μl (∼5 × 108 CFU) of bacterial culture. For mixed-infection experiments, equal volumes of cultures were mixed so that the inoculum contained ∼2.5 × 108 CFU of each strain.
β-Galactosidase assays.
We used the procedure developed by Miller (43) to measure expression of β-galactosidase in A. tumefaciens containing Tn3-lac insertions. Bacteria were grown in YEP broth (10 ml) at 28°C overnight with aeration. Cells were harvested by centrifugation, suspended in 10 ml of induction broth {18 g of glucose/liter, 60 mg of K2HPO4/liter, 20 mg of NaH2PO4/liter, 1 g of NH4Cl/liter, 0.3 g of MgSO4 · 7H2O/liter, 0.15 g of KCl/liter, 10 mg of CaCl2/liter, 2.5 mg of FeSO4 · 7H2O/liter, 4 mM MES [2-(N-morpholino)ethanesulfonic acid], pH 5.5}, and divided into two 5-ml cultures. For each strain, one 5-ml induction broth culture was supplemented with acetosyringone (100 μM) dissolved in sterile dimethyl sulfoxide, and both cultures were incubated at room temperature (20 to 22°C) for 6 h. Next, 1 ml of each culture was harvested by centrifugation, the cells were suspended in 0.55 ml of Z buffer (43), and β-galactosidase assays were performed (43). The optical density at 420 nm was measured in a Molecular Dynamics SpectraMax 250 microtiter plate reader; two aliquots were measured for each sample. The assays were repeated three times on separate days, and the results are expressed as mean values.
Genomic-DNA isolation and Southern blot analysis.
Genomic DNA was prepared from overnight broth cultures of A. rhizogenes and A. tumefaciens as described by Ream and Field (48). A. rhizogenes A4 (no. 100), A4 (no. 117), ATCC 15834, ICPB-TR7, NCIB8196, and NCPPB1855 were cultured in YMA broth; all other strains were grown in YEP broth. Approximately 10 μg of total nucleic acids (DNA and RNA) was mixed with restriction endonuclease buffer and incubated with BamHI (New England Biolabs) at 37°C for 2 h.
Southern blot analysis was performed as described previously (48). Probes were labeled with [α-32P]dCTP (10 mCi/ml; ICN) using a random-prime labeling system (Invitrogen). The GALLS gene probe was prepared from a 6,194-bp gel-purified XhoI-BamHI restriction fragment from pLH338. The virE2 probe was prepared from a 1,600-bp PCR product amplified from pEG202-virE2 (67) using the primers 5′VIRE2 (5′-GGCTGGAATTCCCGGGGATCC-3′) and 3′VIRE2 (5′-ACTCGAGCGGCCGCCATGG-3′). The virD2 probe was prepared from a 1,742-bp gel-purified BamHI fragment excised from pWR140, a pUC18 plasmid containing the restriction fragment which comprises the highly conserved endonuclease domain of virD2 (29, 58). Hybridizations were performed overnight at 42°C in 50% formamide-1% SDS-1 M NaCl-10% dextran sulfate-0.01% calf thymus DNA. After hybridization, filters were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 42°C, in 2× SSC plus 1% SDS at 65°C, and in 0.1× SSC plus 1% SDS at 42°C. The probed filters were exposed to X-ray film.
RESULTS
A protein encoded by A. rhizogenes plasmid pRi1724 substitutes for VirE2.
A. tumefaciens MX358 cannot induce tumors on carrot roots or K. daigremontiana leaves due to a mutation in virE2 (63), whereas a strain (C58C1) harboring the root-inducing plasmid pRi1724::kan (44) induced adventitious roots (but not tumors) on carrots, as expected. In pRi1724::kan, plasmid pHSG298::BamHI fragment 7 was integrated into pRi1724 by homologous recombination (44). This placed pHSG298, which encodes kanamycin resistance, between directly repeated copies of BamHI fragment 7 (coordinates 177472 to 185555 of pRi1724; accession no. AP002086). This insertion does not affect the ability of pRi1724::kan to induce hairy roots. C58C1 is an A. tumefaciens strain that lacks the Ti plasmid and consequently virE2 (30, 72). The nucleotide sequence of pRi1724::kan shows that it contains homologs of all the A. tumefaciens Ti plasmid-encoded vir genes except virE1 and virE2 (44). Thus, both C58C1(pRi1724::kan) and MX358 lack a functional virE2 gene. However, C58C1(pRi1724::kan) is proficient at T-DNA transmission (transfer and integration) to plant cells (44). These observations suggest that pRi1724::kan may encode another protein that can substitute for VirE2.
To test this idea, we transformed pRi1724::kan into the virE2 mutant A. tumefaciens MX358 and found that pRi1724::kan fully restored virulence (Fig. 1A). To identify the gene (or genes) necessary, we constructed a cosmid library from pRi1724::kan and identified seven cosmids that restored pathogenicity to A. tumefaciens MX358. These cosmids had a 17,437-bp region in common (coordinates 60824 to 78260 in accession no. AP002086). We selected one cosmid, pLH77, for further study. Nucleotide sequence analysis of this cosmid showed that it contained pRi1724::kan DNA extending from a SalI site at coordinate 54036 to another SalI site at coordinate 81573. MX358(pLH77) exhibited wild-type virulence on carrots (Fig. 1B).
FIG. 1.
(A to H) Carrots inoculated on the basal surface with derivatives of the virE2 mutant A. tumefaciens MX358 containing pRi1724::kan (A), pLH77 (the GALLS gene-positive cosmid) (B), pLH77-mutant GALLS gene containing Tn3-lac-301 (C), pLH77-mutant GALLS gene containing Tn3-lac-321 (D), pLH338 (the GALLS gene-positive plasmid) (E), pLH345 (mutant GALLS gene with deletion of EcoRI plasmid) (F), pLH344 (mutant GALLS gene with deletion of NcoI plasmid) (G), pVK100 (cosmid-plasmid vector) (H). (I to K) Mixed infections (of the basal surface) with C58C1(pRi1724::kan) plus MX358 (virE2 mutant) (I), MX234(pRi1724::kan) (virB1 mutant) plus MX358 (J), and MX368(pRi1724::kan) (virB10 mutant) plus MX358 (K). (L) Basal surface of a carrot inoculated with wild-type A. tumefaciens A348. (M to P) Uninoculated basal (M) and apical (N) surfaces and carrots inoculated on the apical surface with A. rhizogenes MX599 (O) and A. rhizogenes K599 (P). MX599 is a Tn3-lac-301-containing mutant GALLS gene derivative of wild-type A. rhizogenes K599 (Table 1).
To identify the gene on cosmid pLH77 that was responsible for functional complementation of the virE2 mutation in MX358, we performed transposon mutagenesis of the cosmid. Tn3-lac insertions in pLH77 identified a gene that was required to restore virulence to MX358. We characterized 13 insertion mutations in the pRi1724::kan sequences contained in pLH77 (Fig. 2). Six different insertions abolished the ability of the cosmid to restore pathogenicity to MX358 (e.g., Tn3-lac-301) (Table 2 and Fig. 1C). All of these insertions disrupted a large open reading frame (ORF) designated ORF 55 (1,769 codons), which we called the GALLS gene because that sequence occurs twice in the predicted amino acid sequence. A seventh insertion (no. 321 [Fig. 2]), located 145 codons upstream of the 3′ end of ORF 55 (the GALLS gene), diminished but did not eliminate the ability of pLH77 to restore virulence to MX358 (Fig. 1D). Insertions in other ORFs in pLH77 (e.g., ORFs 56, 59, 60, and 61) did not affect the ability of pLH77 to substitute for virE2.
FIG. 2.
Tn3-lac insertions in the GALLS gene region. The map shows the 17,437-bp region shared by seven cosmids that complemented mutations in virE2. The arrows beneath the map indicate the locations and orientations of ORFs. The vertical arrows represent Tn3-lac insertions; allele numbers are above each arrow. The short vertical arrows indicate Tn3-lac insertions oriented with the lac operon in the sense orientation relative to the disrupted coding sequence; all of the insertions are transcriptional (out-of-frame) fusions of the ORF with the lac genes, except for allele 335, which formed a translational (in-frame) fusion with the GALLS gene. The long vertical arrows indicate Tn3-lac insertions in which the lac genes are oriented antisense relative to the coding sequence.
The GALLS gene can replace virE2.
To test whether the GALLS gene was the only gene required to restore virulence to MX358, we constructed a plasmid that contained the GALLS gene without other sequences from pRi1724::kan. Restriction sites for XhoI and BamHI lie 779 bp upstream and 111 bp downstream, respectively, of the GALLS coding sequence, but the gene itself does not contain cleavage sites for these enzymes (44). We used these sites to excise a 6,194-bp BamHI-XhoI restriction fragment from pLH77. This fragment contained the entire GALLS gene, including a putative promoter region, but it did not contain other ORFs. We inserted it into the broad-host-range plasmid pVK100. The resulting plasmid, pLH338, fully restored virulence to the virE2 mutant A. tumefaciens MX358 (Fig. 1E) and to a virE2 deletion mutant (WR5000; data not shown).
The GALLS gene is essential for pathogenicity of A. rhizogenes.
Previous studies did not address the function of GALLS or its role in T-DNA transmission from wild-type A. rhizogenes to plants (44, 45). As described above, we demonstrated that GALLS was sufficient to replace VirE2 in A. tumefaciens, where T-DNA transfer normally requires VirE2 (24, 63). However, this experiment did not test whether GALLS is important for T-DNA transmission from strains of A. rhizogenes that naturally lack VirE2. These bacteria must rely on an alternative means to protect and target T-DNA inside plant cells. To determine whether GALLS is essential for this process, we introduced the mutant GALLS gene containing Tn3-lac-301 (Table 2) into wild-type A. rhizogenes K599. The resulting strain (A. rhizogenes MX599) lacked an intact copy of the GALLS gene. We compared the abilities of MX599 and K599 to induce growth of adventitious roots (i.e., hairy root disease) on the apical surfaces of carrot root slices. Elevated auxin levels stimulated the growth of unorganized callus on the apical surfaces of uninoculated carrot root slices (Fig. 1N). In contrast, no response occurred on uninoculated basal surfaces (Fig. 1M). Certain strains of A. rhizogenes, including K599, induce growth of adventitious roots only on the apical surfaces of carrot root slices (Fig. 1P) (51). The mutant GALLS gene containing Tn3-lac-301 abolished the ability of A. rhizogenes MX599 to induce growth of adventitious roots on the apical surfaces of carrot roots (Fig. 1O). Thus, the GALLS gene was essential for T-DNA transmission from A. rhizogenes K599.
Bioinformatic analysis of GALLS protein.
Although the authors of the pRi1724 sequence selected the second AUG in ORF 55 as the start codon (44), we believe that the first AUG (seven codons upstream) is the true start; only this AUG is preceded by a properly situated “ideal” ribosome binding site (AGGAG) (57). The predicted GALLS amino acid sequence contains several distinct domains (Fig. 3). The N-terminal region (residues 160 to 555) shares eight short conserved motifs (9 to 20 residues) with TraA strand transferase-helicase conjugation proteins encoded by A. tumefaciens pTiC58 and A. rhizogenes pRi1724 (Fig. 4). These TraA proteins are related to TraI, encoded by the F plasmid of E. coli (8, 21). TraI has helicase activity and the ability to nick within the F origin of transfer (oriT) sequence (1, 68). TraA contains sequences related to the helicase domain of TraI (21). The GALLS protein contains three motifs (I, II, and III) found in the TraI/TraA helicase domains, including a nucleoside triphosphate (NTP)-binding motif (Fig. 4). Five additional blocks of highly conserved sequences (TraA-like motifs) are found only in TraA and GALLS. TraA also has a domain related to MobA (an oriT-nicking protein) encoded by the IncQ plasmid RSF1010 (21), but GALLS lacks MobA-related sequences.
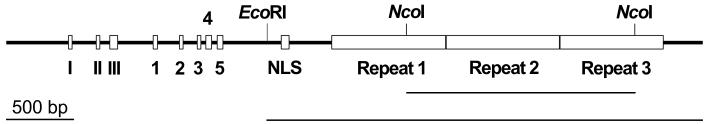
FIG. 3.
Domains in the GALLS protein. Conserved NTP-binding/helicase motifs (I, II, and III), TraA-like sequences (1 to 5), a putative NLS, and GALLS repeats are shown. NcoI, restriction sites used to make in-frame deletions within the GALLS repeats (short underlined region); EcoRI, restriction site used to remove the entire 3′ end of the GALLS gene, including the NLS, GALLS repeats, and C terminus (long underlined region).
FIG. 4.
The amino acid sequences of the helicase domains of TraA encoded by pTiC58 and pRi1724 were aligned with the corresponding regions of GALLS encoded by pRi1724 and pRiA4. We used the ClustalW program to align the sequences. ATP-binding domains identified by Farrand et al. (21) are underlined with a dashed line. The numbers indicate the locations of the adjacent amino acid in each protein. Solid boxes, amino acids that are identical in all four proteins; shaded boxes, similar amino acids. The groups of amino acids considered similar in this analysis were I, L, M, and V; A, G, and S; H, K, and R; D and E; N and Q; F, W, and Y; and S and T. Dashes indicate gaps placed in the sequences by the ClustalW program to maximize alignment.
The GALLS protein contains a putative NLS near the center of the protein (residues 705 to 724). Nuclear import of large proteins depends on NLSs, which contain short regions rich in basic amino acids (18, 59). Receptors, called NLS-binding proteins, recognize NLSs and direct NLS-containing proteins to nuclear pores, where transport into nuclei occurs (18, 59). The putative NLS (KRKRAAAKEEEIDSRKKMARH; basic amino acids are in boldface) lies downstream of the NTP-binding and helicase domains and other TraA-related sequences (Fig. 3).
A large portion of the C-terminal region of the GALLS protein does not show significant similarity to any protein sequence currently available. Much of this region (residues 832 to 1671) consists of very similar sequences repeated three times (Fig. 3). The first and third repeats contain the GALLS sequence, for which the protein is named; the second repeat contains SALLS instead. Repeats 1 and 2 each contain 289 residues, but repeat 3 is truncated at its C-terminal end and contains only 262 amino acids.
GALLS repeats are important for function.
The GALLS protein consists of at least three distinct functional domains: (i) NTP-binding and helicase motifs similar to TraA, (ii) a putative NLS, and (iii) the GALLS repeats. The obvious similarity of the N terminus of GALLS to TraA led us to ask whether this region of the GALLS protein was sufficient to substitute for VirE2. The GALLS coding sequence contains a single EcoRI restriction site at codons 674 to 675 between the TraA-like domain (codons 42 to 555) and the putative NLS (codons 705 to 724) (Fig. 3). Another EcoRI site lies 205 bp upstream of codon 1. We used these EcoRI sites to construct a plasmid (pLH345) that contained the putative promoter and the first 675 codons of the mutant GALLS gene. This plasmid encoded a truncated protein that contained the entire TraA-like region but lacked the putative NLS and the GALLS repeats; it was unable to restore virulence to MX358 (Fig. 1F).
Next, we asked whether all three GALLS repeats were necessary for the ability of the GALLS gene to substitute for virE2. We used in-frame NcoI restriction sites to delete sequences encoding two of the GALLS repeats (Fig. 3). This fused the N-terminal portion of repeat 1 to the C-terminal portion of repeat 3, which is missing 27 amino acids from its C-terminal end. The resulting plasmid (pLH344) encoded a protein that contained one truncated (262-residue) repeat instead of two full-length (289-residue) repeats and one truncated repeat. MX358(pLH344) induced tumors on carrots, although it exhibited reduced virulence compared to strains containing the wild-type GALLS gene (Fig. 1G).
The ability of the GALLS gene to compensate for the absence of virE2 did not depend on the presence of all three repeats. A Tn3-lac insertion in the third GALLS gene repeat (no. 321 [Fig. 2]) eliminated the last 47 codons of the third GALLS gene repeat, as well as the unique 98-codon sequence that comprises the 3′ end of the GALLS gene. MX358 harboring this mutant GALLS gene exhibited diminished virulence (Fig. 1D) compared to strains containing the wild-type GALLS gene (Fig. 1E).
The VirB1-11/VirD4 secretion system exports GALLS protein.
The VirB1-11/VirD4 secretion system appears to transport certain Vir proteins (e.g., VirE2 and VirF) into plant cells, even in the absence of T-DNA (3, 10, 42, 46, 49, 53-55, 60, 66, 74, 75). For example, tumors form when a single plant wound is inoculated with two nonpathogenic strains of A. tumefaciens, one lacking T-DNA and the second mutated in virE2 (10, 46, 66), provided both VirE2 and T-strand donors contain wild-type virB1-11 and virD4 genes (10). Because GALLS substitutes for VirE2, we asked whether GALLS protein is also secreted via the VirB1-11/VirD4 system. Mixed infection with C58C1(pRi1724::kan) restored tumorigenicity to the virE2 mutant A. tumefaciens MX358. Carrot roots (or wounds on K. daigremontiana leaves) infected with both strains formed unorganized tumors (and some roots) with normal efficiency (Fig. 1I). However, strains harboring the GALLS cosmid (pLH77) and mutations in either virB1 (MX243), virB10 (MX368), or virD4 (MX328) were unable to promote tumorigenesis when coinoculated with a virE2 mutant (MX358) (Fig. 1J and K). This suggests that GALLS was secreted via the VirB1-11/VirD4 type IV secretion system.
The GALLS gene is expressed constitutively and induced modestly by acetosyringone.
The vir genes of A. tumefaciens belong to a regulon controlled by VirA, a sensor-kinase protein located in the inner membrane, and VirG, a transcriptional activator protein (63, 65). This two-component regulatory system responds to phenolic compounds (e.g., acetosyringone) and sugars released by wounded plants, and it stimulates transcription of the other vir operons, which are not expressed constitutively (62, 81).
To monitor the expression of the GALLS gene (and other ORFs), we measured β-galactosidase activity in derivatives of wild-type A. tumefaciens A348 harboring pLH77 with Tn3-lac insertions. The lac operon was oriented in the same direction as the GALLS coding sequence (sense orientation) in four of these mutants (Fig. 2). Three of the insertions formed transcriptional (out-of-frame) fusions between the mutant GALLS gene and lacZ (alleles 315, 321, and 334). In the absence of acetosyringone, these strains produced 4.5 to 5.1 U of β-galactosidase (Table 2). Addition of acetosyringone to the growth medium increased β-galactosidase levels 40% (Table 2). One allele (335) created a translational (in-frame) fusion between lacZ and the first 471 codons of the GALLS gene. This strain produced 5.9 U of β-galactosidase constitutively, and in the presence of acetosyringone, β-galactosidase activity increased 1.9-fold (Table 2). For comparison, we tested a strain (MX243) with a Tn3-lac insertion in virB1 (63), a gene in the virAG regulon. MX243 constitutively produced only 2.5 U of β-galactosidase, but addition of acetosyringone stimulated expression 4.9-fold (Table 2).
Different A. rhizogenes strains contain either the GALLS gene or virE2, but not both.
The GALLS gene is present in the mikimopine-type plasmid pRi1724 and in the agropine-type plasmid pRiA4 (accession no. AP002086 and AB050904), both of which lack virE2 (6, 36, 44). To learn whether some strains of A. rhizogenes contain virE2 instead of the GALLS gene, we examined cucumopine- and mannopine-type A. rhizogenes isolates for the presence of virE2, the GALLS gene, and virD2. Our analysis also included A. vitis A856, octopine-type A. tumefaciens A348, the mikimopine-type plasmid pRi1724::kan, and seven agropine-type A. rhizogenes isolates. In addition, we tested a plasmid (pArA4a) from agropine-type A. rhizogenes A4 that is not required for pathogenesis. We isolated genomic DNA from each bacterial strain, digested each DNA sample with BamHI, and separated the restriction fragments by agarose gel electrophoresis. We prepared Southern blots from each of three gels and probed them with radiolabeled GALLS gene, virE2, or virD2 sequences.
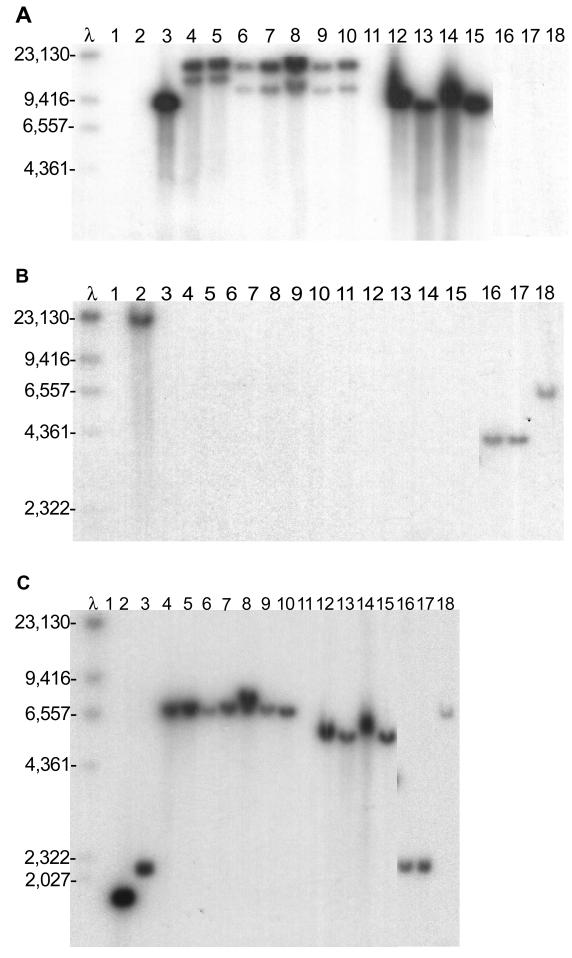
Mikimopine-, cucumopine-, and agropine-type A. rhizogenes contained the GALLS gene (Fig. 5A, lanes 3 to 15) and virD2 (Fig. 5C, lanes 3 to 15), but not virE2 (Fig. 5B, lanes 3 to 15). As predicted from the nucleotide sequence (44) (accession no. AP002086), pRi1724 contained the GALLS gene on an 8,946-bp BamHI fragment (Fig. 5A, lane 3), and virD2 was on a 2,069-bp BamHI fragment (Fig. 5C, lane 3). The GALLS gene probe hybridized to one ∼8.9-kb BamHI fragment in all four cucumopine-type strains (Fig. 5A, lanes 12 to 15), and the virD2 probe hybridized to an ∼5.6-kb BamHI fragment (Fig. 5C, lanes 12 to 15). Agropine-type strains produced two distinct restriction patterns when probed with GALLS gene sequences. Two strains [R1000 and C58C1(pRiA4)] with plasmid pRiA4 in the A. tumefaciens C58 chromosomal background yielded GALLS gene-specific BamHI fragments of ∼17 kb and 12,369 bp (accession no. AB050904) (Fig. 5A, lanes 4 and 5). However, five other agropine-type A. rhizogenes strains, including two isolates of A. rhizogenes A4, yielded GALLS gene-specific BamHI fragments of ∼17 and 11 kb (Fig. 5A, lanes 6 to 10). In contrast, no restriction fragment length polymorphisms were seen among agropine-type A. rhizogenes DNAs probed with virD2 sequences: each strain yielded a 6,320-bp BamHI fragment (accession no. ARVIRCD) (Fig. 5C, lanes 4 to 10). A. rhizogenes A4 contains another large plasmid (pArA4a) which is not required for pathogenesis (80); as expected, this plasmid did not contain the GALLS gene, virD2, or virE2 (Fig. 5A, B, and C, lanes 11).
FIG. 5.
Southern blot analysis of the GALLS, virE2, and virD2 genes in various A. rhizogenes, A. tumefaciens, and A. vitis strains. The blots were probed with radiolabeled GALLS gene (A), virE2 (B), or virD2 (C) sequences. λ, phage λ DNA digested with HindIII. The size of each band in base pairs is indicated. Each panel contained DNA from the following strains: lanes 1, A136 (no Ti plasmid); lanes 2, A348 (octopine-type pTiA6 in A136); lanes 3, C58C1(pRi1724::kan) (mikimopine-type Ri plasmid); lanes 4, A136(pRiA4) (agropine-type Ri plasmid); lanes 5, C58C1(pRiA4) (agropine-type Ri plasmid); lanes 6, ATCC 15834 (agropine-type A. rhizogenes); lanes 7, A4 (no. 117) (agropine-type A. rhizogenes A4; isolate 117); lanes 8, A4 (no. 100) (agropine-type A. rhizogenes A4; isolate 100); lanes 9, NCPPB1855 (agropine-type A. rhizogenes); lanes 10, C58C1RS(pRiTR105) (agropine-type Ri plasmid); lanes 11, C58C1RS(pArA4a) (large plasmid [not associated with virulence] from A. rhizogenes A4); lanes 12, NCPPB2655 (cucumopine-type A. rhizogenes); lanes 13, NCPPB2657 (cucumopine-type A. rhizogenes); lanes 14, NCPPB2659 (cucumopine-type A. rhizogenes K599); lanes 15, NT1(pRiK599) (cucumopine-type Ri plasmid); lanes 16, NCIB8196 (mannopine-type A. rhizogenes); lanes 17, ICPB-TR7 (mannopine-type A. rhizogenes); lanes 18, A. vitis A856 (limited-host-range grape-specific strain).
A. tumefaciens A348, A. vitis A856, and mannopine-type A. rhizogenes did not hybridize with the GALLS gene probe (Fig. 5A, lanes 2 and 16 to 18), but these strains contained sequences homologous to virE2 (Fig. 5B) and virD2 (Fig. 5C). Both mannopine-type A. rhizogenes strains produced BamHI fragments of ∼4.1 kb when probed with virE2 (Fig. 5B, lanes 16 and 17) and ∼2.3 kb when probed with virD2 (Fig. 5C, lanes 16 and 17). A. vitis A856 contained virE2 on a BamHI fragment of ∼6.4 kb (Fig. 5B, lane 18) and virD2 on a BamHI fragment of ∼7.1 kb (Fig. 5C, lane 18). In A. tumefaciens A348, virE2 is on a 19,285-bp BamHI fragment (accession no. AF242881) (Fig. 5B, lane 2), and the 5′ end of virD2 (which was used as the probe) lies on a 1,742-bp BamHI fragment (83) (Fig. 5C, lane 2). The GALLS gene was present in agropine-, cucumopine-, and mikimopine-type A. rhizogenes strains, whereas A. tumefaciens, A. vitis, and mannopine-type A. rhizogenes strains contained virE2.
DISCUSSION
The GALLS gene from A. rhizogenes 1724 can substitute for virE2.
The root-inducing plasmid pRi1724::kan fully restored virulence to a virE2 mutant strain of A. tumefaciens, indicating that the Ri plasmid contained one or more genes that could substitute for virE2. Several lines of evidence support our conclusion that a single gene, the GALLS gene, is sufficient to confer this phenotype. First, seven cosmids derived from pRi1724::kan were able to substitute for virE2, and all of these cosmids shared a region that includes the GALLS gene. Furthermore, Tn3-lac insertions in the GALLS gene abolished the ability of these cosmids to replace virE2, whereas insertions elsewhere did not. Finally, a plasmid containing only the GALLS gene was able to restore virulence to a virE2 mutant A. tumefaciens strain, and deletions in this ORF abolished or severely reduced virulence.
GALLS protein is exported via the VirB1-11/VirD4 secretion system.
Nonpathogenic strains that contained the GALLS gene restored virulence to a virE2 mutant A. tumefaciens strain upon mixed infection of wounded plant tissue with both strains. This “complementation” (by mixed infection) required the virB operon and virD4 in the GALLS donor, which suggests that the GALLS protein is secreted via the VirB1-11/VirD4 type IV secretion system. Similarly, VirE2 protein can be supplied by mixed infection, and this process also requires virB1-11 and virD4 in the VirE2 donor (10). Thus, both VirE2 and its alternate, GALLS, are secreted from bacterial cells via the type IV secretion system.
GALLS and VirE2 show another similarity: the presence of NLSs. The VirE2 protein is important for T-DNA transmission, but it is required only in plant cells (13). Plants that express the VirE2 protein are susceptible to transformation by a virE2 mutant A. tumefaciens strain (13, 47). VirE2 contains two NLSs and binds to a host protein (VIP1) involved in nuclear targeting (13, 69-71, 76, 77). The GALLS protein contains a putative NLS that strongly resembles the NLS in VirD2, which binds a different nuclear targeting protein (AtKAPα) (4, 69, 76, 87). The ability of GALLS to substitute for VirE2 suggests that it probably functions inside the plant cell. The observations that both proteins (i) contain NLSs, (ii) are secreted via the VirB1-11/VirD4 system, and (iii) can “complement” a virE2 mutation by mixed infection suggest that GALLS, like VirE2, may be secreted into plant cells.
GALLS protein contains several distinct domains.
The N-terminal region of GALLS contains sequences, including NTP-binding motifs, found in strand transfer helicases (e.g., TraA of pTiC58 and TraI of F) and other helicases (e.g., the RecBCD enzyme) (21, 33; A. E. Gorbalenya, E. V. Koonin, A. P. Donchenko, and V. M. Blinov, Letter, Nature 333:22, 1988). This TraA-like region was not sufficient to complement mutations in virE2. Deletion of the remainder of the protein abolished its ability to replace VirE2. However, the TraA-like region of GALLS was highly conserved between the mikimopine-type plasmid pRi1724 and the agropine-type plasmid pRiA4 (compare sequences for accession no. AP002086 and AB050904), suggesting that it may be important for T-DNA transfer from A. rhizogenes.
The TraA-like region of GALLS probably interacts with T-DNA and promotes its transfer, but the molecular function of GALLS may differ from that of TraI and TraA. They possess an oriT-nicking domain (21), which appears to be absent from GALLS. These strand transferase enzymes may be anchored in the bacterial membrane, allowing their helicase activities to translocate one strand of a nicked plasmid DNA into a recipient cell (20, 23). Recently, Llosa et al. proposed an alternative model in which a molecule of the nickase-helicase protein is secreted into the recipient cell after nicking at oriT (40). In this model, a second molecule of the nickase-helicase remains bound to the plasmid DNA in the donor, where the helicase activity displaces the transferred DNA strand. In contrast, GALLS appears to be secreted from the bacterial cell and may perform its role in the recipient plant cell. Thus, the intriguing similarities to other proteins that mediate DNA transfer do not tell the whole story.
A. tumefaciens secretes at least four virulence proteins into plant cells via the VirB1-11/VirD4 secretion system: VirD2, VirE2, VirE3, and VirF. Secretion signals are located near the C termini of these proteins; the sequence RPR may be an important component of these secretion signals (3, 53, 60, 74, 75). The last 11 residues of GALLS contain a similar sequence (RIRVR), which may permit its secretion via the VirB1-11/VirD4 system. The C terminus of GALLS is important, although not essential, for its ability to substitute for VirE2. The Tn3-lac-321-containing mutant GALLS gene mutation replaced the last 145 codons of the GALLS gene with 11 codons encoding GSDAQWNENSR, which do not resemble type IV secretion signals in other Vir proteins. This mutation severely reduced functional complementation of virE2, perhaps due to loss of the putative secretion signal. The last 11 residues in the TraA-like region of GALLS contain another possible secretion signal (RHRSR). This sequence may permit some secretion of GALLS in the absence of its normal C terminus. Alternatively, loss of the C terminus may partially destabilize the mutant protein or affect another activity of GALLS.
The NLSs in VirE2 are crucial for its function (13), and the putative NLS in GALLS is likely important too. The NLS in GALLS is highly conserved between pRi1724 and pRiA4, even though the flanking regions are not conserved (compare sequences for accession no. AP002086 and AB050904). If this NLS functions in plant cells, GALLS may help target T strands to the nucleus, as VirE2 does (13, 88), or GALLS may perform a different function inside the nucleus that compensates for the absence of VirE2.
The three GALLS repeats were important for the ability of the protein to replace VirE2, and they are highly conserved between pRi1724 and pRiA4 (compare sequences for accession no. AP002086 and AB050904). Nevertheless, a mutant protein with a single truncated copy of the repeat retained partial activity. Also, a transposon insertion that disrupted the third repeat did not abolish the ability of the GALLS gene to complement mutations in virE2. In contrast to the three GALLS repeats, most of the unique C terminus (downstream of the third repeat) is poorly conserved between pRi1724 and pRiA4, which has novel 20- and 5-codon insertions in this region (compare sequences for accession no. AP002086 and AB050904). However, the putative C-terminal secretion signals are highly conserved. The GALLS repeats may play a direct role in the activity that allows GALLS to substitute for VirE2, or loss of these regions may simply destabilize the other domains of the protein. In summary, the GALLS protein appears to work best when all three repeats are intact, but it can tolerate some disruption of this region.
A. rhizogenes strains contain either the GALLS gene or virE2, but not both.
VirE2 and GALLS perform complementary functions despite their complete lack of sequence similarity. Efficient T-DNA transmission requires only one of these proteins, not both. All agropine-, cucumopine-, and mikimopine-type A. rhizogenes strains that we examined contained the GALLS gene but not virE2, and the GALLS gene was essential for the pathogenicity of cucumopine-type A. rhizogenes K599. Conversely, two mannopine-type strains contained virE2 but not the GALLS gene; the same is true of the A. tumefaciens and A. vitis isolates that have been examined. Thus, all of the A. rhizogenes isolates that we tested contained the genes necessary to transmit T-DNA efficiently, albeit using different proteins to protect T strands and target them to the nucleus.
In Ti and Ri plasmids, the vir genes occupy a contiguous region. The operons that encode proteins involved directly in T-DNA transmission (virB1-11, virC1-2, virD1-5, and virE1-3) and the genes that regulate vir operon expression (virA and virG) are arranged in the same order and orientation in different Ti and Ri plasmids (Fig. 6). Thus, the vir regulon comprises a discrete unit that is highly conserved. However, the GALLS genes in pRi1724 and pRiA4 are not located with the other vir operons. For example, the essential portion of the vir regulon in pRi1724 begins with virA at coordinate 189552 and extends (through coordinates 217594/1) to the end of virE3 at coordinate 132; the GALLS gene (ORF 55) lies ∼63 kb from the vir regulon (at coordinates 68466 to 63178; accession no. AP002086) (44). The GALLS (TraA-like) gene in pRiA4 adjoins the traG, traD, oriT, and traA loci, which mediate conjugal transfer of the Ri plasmid to other bacteria (accession no. AB050904). Although the GALLS gene lies just 338 bp from traG in pRiA4, almost 69 kb separate these genes in pRi1724 (44). Thus, the GALLS gene is not near the vir regulon in either Ri plasmid, and it lies in a different context in each plasmid.
FIG. 6.
Arrangement of virulence (vir) operons in octopine-type (pTiA6) (A), nopaline-type (pTiC58) (B), and mikimopine-type (pRi1724) (C) plasmids.
The GALLS gene is not a typical member of the vir regulon.
The vir genes of A. tumefaciens belong to a regulon controlled by VirA and VirG (65). This two-component regulatory system responds to phenolic compounds (e.g., acetosyringone) and sugars released by wounded plants (62, 81), and it stimulates transcription of the other vir operons, which are not expressed constitutively (63, 65). For example, A. tumefaciens MX243, which contains a Tn3-lac insertion in virB1 (on the Ti plasmid), constitutively produced only background levels of β-galactosidase, but addition of acetosyringone induced expression approximately fivefold. In contrast, Tn3-lac insertions in the GALLS gene produced significant constitutive levels of β-galactosidase, but acetosyringone stimulated expression only 40%, despite the elevated copy number of the mutant GALLS gene-Tn3-lac fusion, which was present on a multicopy IncP replicon (pVK100). Thus, the expression pattern of the GALLS gene differs from those of genuine members of the virAG regulon.
Conclusion. The GALLS gene, a large gene from A. rhizogenes, is able to restore pathogenicity to a virE2 A. tumefaciens mutant. Although GALLS can substitute for VirE2, the proteins do not resemble each other. Instead, GALLS contains regions of strong similarity to TraA, a strand transferase-helicase crucial for plasmid conjugation. Conserved sequences shared by GALLS and TraA include a nucleoside triphosphate-binding motif, which is absent from VirE2. Both GALLS and VirE2 contain NLSs, although the NLS in GALLS resembles the one in VirD2 instead of the NLSs in VirE2. The GALLS protein also contains three C-terminal repeats that are important for its function. Both VirE2 and GALLS appear to be secreted from A. tumefaciens via the VirB1-11/VirD4 secretion system, and either protein can restore virulence to a virE2 mutant when supplied in trans by mixed infection. Although the biochemical functions of GALLS are unknown, it seems likely that GALLS is secreted from A. rhizogenes into plant cells. There, GALLS may localize T strands to the nucleus and protect them from nuclease attack, as VirE2 does.
Acknowledgments
We thank Stephen K. Farrand for apprising us that pRi1724 lacks virE1 and virE2, for suggesting that we look for a gene that could substitute for virE2, and for providing bacterial cultures, advice, and encouragement. Sami Murakami performed the initial mixed-infection experiments, and Eric Coultas prepared several cosmid DNAs. Jodi Humann prepared Fig. 2, 3, and 6 and assisted with the others. Jennifer Pitrak and Jodi Humann provided critiques of the manuscript.
This work was supported by grant 2002-35319-11555 from the USDA.
REFERENCES
- 1.Abdel-Monem, M., G. Taucher-Scholz, and M. Q. Klinkert. 1983. Identification of Escherichia coli helicase I as the traI gene product of the F sex factor. Proc. Natl. Acad. Sci. USA 80:4659-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyama, T., M. Takanami, and A. Oka. 1989. Signal structure for transcriptional activation in the upstream regions of virulence genes on the hairy-root-inducing plasmid A4. Nucleic Acids Res. 17:8711-8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmakuri, K., Z. Ding, and P. J. Christie. 2003. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol. 49:1699-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas, N., and V. Citovsky. 1997. Nuclear localization signal binding protein from Arabidopsis mediates nuclear import of Agrobacterium VirD2 protein. Proc. Natl. Acad. Sci. USA 94:10723-10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binns, A. N., C. E. Beaupre, and E. M. Dale. 1995. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J. Bacteriol. 177:4890-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birot, A. M., and F. Casse-Delbart. 1988. Map location on Agrobacterium root-inducing plasmids of homologies with the virulence region of tumor-inducing plasmids. Plasmid 19:189-202. [DOI] [PubMed] [Google Scholar]
- 7.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw, H. D., B. A. Traxler, E. G. Minkley, E. W. Nester, and M. P. Gordon. 1990. Nucleotide sequence of the traI (helicase I) gene from the sex factor F. J. Bacteriol. 172:4127-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie, P. J. 1997. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J. Bacteriol. 179:3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie, P. J., J. E. Ward, S. C. Winans, and E. W. Nester. 1988. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J. Bacteriol. 170:2659-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citovsky, V., G. De Vos, and P. Zambryski. 1988. Single-stranded DNA binding protein encoded by the virE locus of Agrobacterium tumefaciens. Science 240:501-504. [DOI] [PubMed] [Google Scholar]
- 12.Citovsky, V., M. L. Wong, and P. Zambryski. 1989. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc. Natl. Acad. Sci. USA 86:1193-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Citovsky, V., J. Zupan, D. Warnick, and P. Zambryski. 1992. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science 256:1802-1805. [DOI] [PubMed] [Google Scholar]
- 14.Costantino, P., M. L. Mauro, G. Micheli, G. Risuleo, P. J. J. Hooykaas, and R. A. Schilperoort. 1981. Fingerprinting and sequence homology of plasmids from different virulent strains of Agrobacterium rhizogenes. Plasmid 5:170-182. [DOI] [PubMed] [Google Scholar]
- 15.Currier, T. C., and E. W. Nester. 1976. Evidence for diverse types of large plasmids in tumor-inducing Agrobacterium. J. Bacteriol. 126:157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das, A. 1988. Agrobacterium tumefaciens virE operon encodes a single-stranded DNA-binding protein. Proc. Natl. Acad. Sci. USA 85:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, W., L. Chen, W. T. Peng, X. Liang, S. Sekiguchi, M. P. Gordon, L. Comai, and E. W. Nester. 1999. VirE1 is a specific molecular chaperone for the exported single-stranded-DNA-binding protein VirE2 in Agrobacterium. Mol. Microbiol. 31:1795-1807. [DOI] [PubMed] [Google Scholar]
- 18.Dingwall, C., and R. A. Laskey. 1986. Protein import into the cell nucleus. Annu. Rev. Cell Biol. 2:367-390. [DOI] [PubMed] [Google Scholar]
- 19.Dombek, P., and W. Ream. 1997. Functional domains of Agrobacterium tumefaciens single-stranded DNA-binding protein VirE2. J. Bacteriol. 179:1165-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreiseikelmann, B. 1994. Translocation of DNA across bacterial membranes. Microbiol. Rev. 58:293-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrand, S. K., I. Hwang, and D. M. Cook. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 178:4233-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth, N., K. Ippen-Ihler, and R. A. Skurray. 1996. Structure and function of the F factor and mechanism of conjugation, p. 2377-2401. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. C. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 24.Garfinkel, D. J., and E. W. Nester. 1980. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J. Bacteriol. 144:732-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garfinkel, D. J., R. B. Simpson, L. W. Ream, F. F. White, M. P. Gordon, and E. W. Nester. 1981. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143-153. [DOI] [PubMed] [Google Scholar]
- 26.Gelvin, S. B. 1998. Agrobacterium VirE2 proteins can form a complex with T strands in the plant cytoplasm. J. Bacteriol. 180:4300-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelvin, S. B. 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene jockeying” tool. Microbiol. Mol. Biol. Rev. 67:16-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gietl, C., Z. Koukolikova-Nicola, and B. Hohn. 1987. Mobilization of T-DNA from Agrobacterium to plant cells involves a protein that binds single-stranded DNA. Proc. Natl. Acad. Sci. USA 84:9006-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas, J. H., L. W. Moore, W. Ream, and S. Manulis. 1995. Universal PCR primers for detection of phytopathogenic Agrobacterium strains. Appl. Environ. Microbiol. 61:2879-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton, C. M., H. Lee, P. L. Li, D. M. Cook, K. R. Piper, S. Beck von Bodman, E. Lanka, W. Ream, and S. K. Farrand. 2000. TraG from RP4 and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 182:1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffron, F., P. Bedinger, J. J. Champoux, and S. Falkow. 1977. Deletions affecting the transposition of an antibiotic resistance gene. Proc. Natl. Acad. Sci. USA 74:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirayama, T., T. Muranaka, H. Ohkawa, and A. Oka. 1988. Organization and characterization of the virCD genes from Agrobacterium rhizogenes. Mol. Gen. Genet. 213:229-237. [DOI] [PubMed] [Google Scholar]
- 33.Hodgman, T. C. 1988. A new superfamily of replicative proteins. Nature 333:22-23. (Erratum, 333:578.) [DOI] [PubMed] [Google Scholar]
- 34.Holsters, M., D. deWaele, A. Depicker, E. Messens, M. Van Montagu, and J. Schell. 1978. Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 163:181-187. [DOI] [PubMed] [Google Scholar]
- 35.Huffman, G. A., F. F. White, M. P. Gordon, and E. W. Nester. 1984. Hairy root inducing plasmid: physical map and homology to tumor-inducing plasmids. J. Bacteriol. 157:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouanin, L. 1984. Restriction map of an agropine-type Ri plasmid and its homologies with Ti plasmids. Plasmid 12:91-102. [DOI] [PubMed] [Google Scholar]
- 37.Knauf, V. C., and E. W. Nester. 1982. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45-54. [DOI] [PubMed] [Google Scholar]
- 38.Knauf, V. C., C. G. Panagopoulos, and E. W. Nester. 1982. Genetic factors controlling the host range of Agrobacterium tumefaciens. Phytopathology 72:1545-1549. [Google Scholar]
- 39.Kuldau, G. A., G. De Vos, J. Owen, G. McCaffrey, and P. Zambryski. 1990. The virB operon of Agrobacterium tumefaciens pTiC58 encodes 11 open reading frames. Mol. Gen. Genet. 221:256-266. [DOI] [PubMed] [Google Scholar]
- 40.Llosa, M., F. X. Gomis-Ruth, M. D. Collins, and F. de la Cruz. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 41.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Melchers, L. S., M. J. Maroney, A. D. Dulk-Ras, D. V. Thompson, H. A. J. van Vuuren, R. A. Schilperoort, and P. J. J. Hooykaas. 1990. Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence: molecular characterization of the virF locus. Plant Mol. Biol. 14:249-259. [DOI] [PubMed] [Google Scholar]
- 43.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 44.Moriguchi, K., Y. Maeda, M. Satou, N. S. Hardayani, M. Kataoka, N. Tanaka, and K. Yoshida. 2001. The complete nucleotide sequence of a plant root-inducing (Ri) plasmid indicates its chimeric structure and evolutionary relationship between tumor-inducing (Ti) and symbiotic (Sym) plasmids in Rhizobiaceae. J. Mol. Biol. 307:771-784. [DOI] [PubMed] [Google Scholar]
- 45.Moriguchi, K., Y. Maeda, M. Satou, M. Kataoka, N. Tanaka, and K. Yoshida. 2000. Analysis of unique variable region of a plant root inducing plasmid, pRi1724, by the construction of its physical map and library. DNA Res. 7:157-163. [DOI] [PubMed] [Google Scholar]
- 46.Otten, L., H. DeGreve, J. Leemans, R. Hain, P. J. J. Hooykaas, and J. Schell. 1984. Restoration of virulence of vir region mutants of Agrobacterium tumefaciens strain B653 by coinfection with normal and mutant Agrobacterium strains. Mol. Gen. Genet. 175:159-163. [Google Scholar]
- 47.Ream, W. 1998. Import of Agrobacterium tumefaciens virulence proteins and transferred DNA into plant cell nuclei. Subcell. Biochem. 29:365-384. [DOI] [PubMed] [Google Scholar]
- 48.Ream, W., and K. G. Field. 1999. Molecular biology techniques: an intensive laboratory course. Academic Press, San Diego, Calif.
- 49.Regensburg-Tuink, A. J. G., and P. J. J. Hooykaas. 1993. Transgenic N. glauca plants expressing bacterial virulence gene virF are converted into hosts for nopaline strains of A. tumefaciens. Nature 363:69-71. [DOI] [PubMed] [Google Scholar]
- 50.Rossi, L., B. Hohn, and B. Tinland. 1996. Integration of complete transferred DNA units is dependent on the activity of virulence E2 protein of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 93:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryder, M. H., M. E. Tate, and A. Kerr. 1985. Virulence properties of strains of Agrobacterium on the apical and basal surfaces of carrot root disks. Plant Physiol. 77:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schrammeijer, B., A. den Dulk-Ras, A. C. Vergunst, E. J. Jacome, and P. J. J. Hooykaas. 2003. Analysis of Vir protein translocation from Agrobacterium tumefaciens using Saccharomyces cerevisiae as a model: evidence for transport of a novel effector protein VirE3. Nucleic Acids Res. 31:860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrammeijer, B., J. Hemelaar, and P. J. J. Hooykaas. 1998. The presence and characterization of a virF gene on Agrobacterium vitis Ti plasmids. Mol. Plant-Microbe Interact. 11:429-433. [DOI] [PubMed] [Google Scholar]
- 55.Schrammeijer, B., E. Risseeuw, W. Pansegrau, T. J. Regensburg-Tuink, W. L. Crosby, and P. J. J. Hooykaas. 2001. Interaction of the virulence protein VirF of Agrobacterium tumefaciens with plant homologs of the yeast Skp1 protein. Curr. Biol. 11:258-262. [DOI] [PubMed] [Google Scholar]
- 56.Sen, P., G. J. Pazour, D. Anderson, and A. Das. 1989. Cooperative binding of the VirE2 protein to single-stranded DNA. J. Bacteriol. 171:2573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity of nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shurvinton, C. E., L. Hodges, and W. Ream. 1992. A nuclear localization signal and the C-terminal omega sequence in the Agrobacterium tumefaciens VirD2 endonuclease are important for tumor formation. Proc. Natl. Acad. Sci. USA 89:11837-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silver, P. A. 1991. How proteins enter the nucleus. Cell 64:489-497. [DOI] [PubMed] [Google Scholar]
- 60.Simone, M., C. A. McCullen, L. E. Stahl, and A. N. Binns. 2001. The carboxy-terminus of VirE2 from Agrobacterium tumefaciens is required for its transport to host cells by the virB-encoded type IV transport system. Mol. Microbiol. 41:1283-1293. [DOI] [PubMed] [Google Scholar]
- 61.Stachel, S. E., G. An, C. Flores, and E. W. Nester. 1985. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 4:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stachel, S. E., E. Messens, M. Van Montagu, and P. Zambryski. 1985. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624-629. [Google Scholar]
- 63.Stachel, S. E., and E. W. Nester. 1986. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 5:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stachel, S. E., B. Timmerman, and P. Zambryski. 1987. Activation of Agrobacterium tumefaciens vir gene expression generates multiple single-stranded T-strand molecules from the pTiA6 T-region: requirement of 5′ virD gene products. EMBO J. 6:857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stachel, S. E., and P. Zambryski. 1986. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell 46:325-333. [DOI] [PubMed] [Google Scholar]
- 66.Sundberg, C., L. Meek, K. Carroll, A. Das, and W. Ream. 1996. VirE1 protein mediates export of the single-stranded DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J. Bacteriol. 178:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sundberg, C. D., and W. Ream. 1999. The Agrobacterium tumefaciens chaperone-like protein, VirE1, interacts with VirE2 at domains required for single-stranded DNA binding and cooperative interaction. J. Bacteriol. 181:6850-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Traxler, B. A., and E. G. Minkley. 1988. Evidence that DNA helicase I and ortT site-specific nicking are both functions of the F TraI protein. J. Mol. Biol. 204:205-209. [DOI] [PubMed] [Google Scholar]
- 69.Tzfira, T., and V. Citovsky. 2002. Partners-in-infection: host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell Biol. 12:121-129. [DOI] [PubMed] [Google Scholar]
- 70.Tzfira, T., M. Vaidya, and V. Citovsky. 2001. VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 20:3596-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tzfira, T., M. Vaidya, and V. Citovsky. 2002. Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis nuclear protein VIP1. Proc. Natl. Acad. Sci. USA 99:10435-10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Larebeke, N., C. Genetello, J. Schell, R. A. Schilperoort, A. K. Hermans, J. P. Hernalsteens, and M. Van Montagu. 1975. Acquisition of tumor-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature 255:742-743. [DOI] [PubMed] [Google Scholar]
- 73.Veluthambi, K., W. Ream, and S. B. Gelvin. 1988. Virulence genes, borders, and overdrive generate single-stranded T-DNA molecules from the A6 Ti plasmid of Agrobacterium tumefaciens. J. Bacteriol. 170:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. T. de Vlaam, T. J. G. Regensburg-Tuink, and P. J. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290:979-982. [DOI] [PubMed] [Google Scholar]
- 75.Vergunst, A. C., M. C. M. van Lier, A. den Dulk-Ras, and P. J. J. Hooykaas. 2003. Recognition of the Agrobacterium tumefaciens VirE2 translocation signal by the VirB/D4 transport system does not require VirE1. Plant Physiol. 133:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ward, D. V., and P. Zambryski. 2001. The six functions of Agrobacterium VirE2. Proc. Natl. Acad. Sci. USA 98:385-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward, D. V., J. R. Zupan, and P. C. Zambryski. 2002. Agrobacterium VirE2 gets the VIP1 treatment in plant nuclear import. Trends Plant Sci. 7:1-3. [DOI] [PubMed] [Google Scholar]
- 78.Ward, J. E., E. M. Dale, and A. N. Binns. 1991. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc. Natl. Acad. Sci. USA 88:9350-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watson, B., T. C. Currier, M. P. Gordon, M. D. Chilton, and E. W. Nester. 1975. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 123:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White, F. F., and E. W. Nester. 1980. Relationship of plasmids responsible for hairy root and crown gall tumorigenicity. J. Bacteriol. 144:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winans, S. C. 1992. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol. Rev. 56:12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winans, S. C., D. L. Burns, and P. J. Christie. 1996. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 4:64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yanofsky, M. F., S. G. Porter, C. Young, L. M. Albright, M. P. Gordon, and E. W. Nester. 1986. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell 47:471-477. [DOI] [PubMed] [Google Scholar]
- 84.Yusibov, V. M., T. R. Steck, V. Gupta, and S. B. Gelvin. 1994. Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc. Natl. Acad. Sci. USA 91:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou, X. R., and P. J. Christie. 1999. Mutagenesis of the Agrobacterium VirE2 single-stranded DNA-binding protein identifies regions required for self-association and interaction with VirE1 and a permissive site for hybrid protein construction. J. Bacteriol. 181:4342-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu, J., P. M. Oger, B. Schrammeijer, P. J. J. Hooykaas, S. K. Farrand, and S. C. Winans. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 182:3885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ziemienowicz, A., T. Merkle, F. Schoumacher, B. Hohn, and L. Rossi. 2001. Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins. Plant Cell 13:369-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zupan, J. R., V. Citovsky, and P. Zambryski. 1996. Agrobacterium VirE2 protein mediates nuclear uptake of single-stranded DNA in plant cells. Proc. Natl. Acad. Sci. USA 93:2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]