Abstract
Postharvest technologies have allowed horticultural industries to meet the global demands of local and large-scale production and intercontinental distribution of fresh produce that have high nutritional and sensory quality. Harvested products are metabolically active, undergoing ripening and senescence processes that must be controlled to prolong postharvest quality. Inadequate management of these processes can result in major losses in nutritional and quality attributes, outbreaks of foodborne pathogens and financial loss for all players along the supply chain, from growers to consumers. Optimal postharvest treatments for fresh produce seek to slow down physiological processes of senescence and maturation, reduce/inhibit development of physiological disorders and minimize the risk of microbial growth and contamination. In addition to basic postharvest technologies of temperature management, an array of others have been developed including various physical (heat, irradiation and edible coatings), chemical (antimicrobials, antioxidants and anti-browning) and gaseous treatments. This article examines the current status on postharvest treatments of fresh produce and emerging technologies, such as plasma and ozone, that can be used to maintain quality, reduce losses and waste of fresh produce. It also highlights further research needed to increase our understanding of the dynamic response of fresh produce to various postharvest treatments.
Keywords: fresh produce, postharvest quality, ethylene, heat treatment, packaging, storage
1. Introduction
Fresh fruit and vegetables (FFV) are a major source of essential vitamins and minerals, such as vitamin A, vitamin C and potassium, needed for human wellbeing. They are, however, perishable living products that require coordinated activity by growers, storage operators, processors and retailers to maintain quality and reduce food loss and waste. The extent of coordination can vary greatly from loose in the case of local food supplies to complex for global supply chains. The Food and Agriculture Organization estimated that 32% (weight basis) of all food produced in the world was lost or wasted in 2009 [1]. When converted into calories, global losses represent approximately 24% of all food produced. Reducing the loss and waste of FFVs is important because these foods provide essential nutrients and represent sources of domestic and international revenue.
Fresh produce attributes (appearance, texture, flavour and nutritional value) have been traditional quality criteria, but increasingly safety (chemical, toxicological and microbial) and traceability are important for all the role players along the supply chain, from the farm to consumers. Fresh produce is often eaten raw or after minimal processing and food pathogen contamination can present risk of outbreaks of foodborne illnesses [2]. Listeria monocytogenes, Salmonella enteritidis phage, and Escherichia coli O157:H7 and O104:H4 are major pathogens contributing to outbreaks of foodborne illness with fresh produce as vectors for these pathogens [3]. Owing to multiple uncertainties along the supply chain, microbial contamination leading to spoilage and postharvest losses can occur at any of the stages in the continuum from farm to consumer. Therefore, postharvest treatments are essential to minimize microbial spoilage and reduce the risk of pathogen contamination for FFV [4].
Various postharvest physical, chemical and gaseous treatments may be applied to maintain fresh-like quality with high nutritional value and meet safety standards of fresh produce. These postharvest treatments are typically combined with appropriate management of storage temperatures. This article reviews the current status of postharvest treatments and emerging technologies that can be used to maintain quality and reduce wastage of fresh produce. An Overview of such technologies is presented in table 1.
Table 1.
Overview of postharvest treatments of fresh produce.
| treatment | benefits | limitations | commercial example application |
|---|---|---|---|
| heat treatment | reduction of chilling injury, delay of ripening, killing of critical insect contaminants and controls decay | high-energy costs and added labour | potato, tomato, carrot, strawberry, asparagus, broccoli, beans, kiwi, celery, lettuce, melon, grape, plum, peach, spinach and rocket leaves [5–11] |
| edible coating | provides a partial barrier, minimizes moisture loss; establishes modified atmosphere; preserves colour and texture; retains natural aroma | cost of scaling up, lack of edible materials with desired properties, regulatory challenges | apples, pears, carrots, celery, strawberry and mushrooms [12–15] |
| irradiation | inhibits sprouting of tubers, bulbs and roots, meets quarantine requirements for export trade and recognized as a safe process | capital intensive, lack of harmonization of regulations, slow consumer acceptance owing to perceived association with radioactivity | potato, onion, strawberry and mango [16–19] |
| antimicrobial and anti-browning agents | retards browning, deterioration of texture and microbial growth | inaccessible sites for treatments within fresh produce such as calyx and wax area | apple, strawberry, lettuce, melon, orange, prune, tomato, grapes and fresh-cut produce [20–29] |
| NO | inhibits ethylene biosynthesis, reduces respiration rate, water loss, browning, and lower incidence of postharvest diseases | commercial application depends upon the development of a smart carrier/controlled release system for NO | apple, banana, kiwifruit, mango, peach, pear, plum, strawberry, tomato, papaya, loquat, jujube fruit and bayberry [30–35] |
| sulfur dioxide | prevents postharvest decay | higher concentration may induce injuries and sulfite residues pose a health risk | grapes, litchi, fig, banana, lemon, apple and blueberries [36–39] |
| ozone | easily incorporated into existing cold storage, washing system, better efficacy than chlorine | does not penetrate natural openings, further research is needed to improve application | apples, cherries, carrots, garlic, kiwi, onions, peaches, plums, potatoes and table grapes [40–44] |
| ethylene | triggers ripening process thereby improves fruit colour and quality | need of optimum ethylene concentration, storage conditions for faster and more uniform ripening | banana, avocado, persimmon, tomato, kiwifruit, mango and citrus fruits [45–58] |
| 1-MCP | maintains fruit cell wall integrity and peel colour, and develop aroma and flavour | it can increase susceptibility to CO2 injury and chilling disorders. Additional exposure time is required for fruit to recover its ability to ripen normally | apple, avocado, banana, broccoli, cucumber, date, kiwifruit, mango, melon, nectarine, papaya, peach, pear, pepper, persimmon, pineapple, plantain, plum, squash and tomato [59–72] |
| CA storage | retards senescence, associated biochemical and physiological changes, reduction in decay severity | capital intensive, fruit volumes must be high and extended storage periods are needed to make investment economical | apple, pear, avocado, strawberry, cherry, cabbages, kiwifruit, avocados, persimmon, pomegranate, asparagus, banana, broccoli, cranberry, mango, melon, nectarine, peaches and plums [73–83] |
| MAP | delay in respiration, senescence, and slows down rate of deterioration | condensation inside the package resulting in microbial growth and decay of produce | strawberry, banana, cherries, carrots, fresh-cut fruits, salad mix and leafy green vegetables [84–89] |
2. Physical treatments
(a). Heat treatment
Heat treatment has been studied as an alternative to chemical treatments for harvested FFV. Treatments include hot water dip (HWD), saturated water vapour heat, hot dry air and hot water rinse (HWR) with brushing [5]. Beneficial effects of these heat treatments are linked (i) through changes in physiological processes such as a reduction in chilling injury and delay of ripening processes by heat inactivation of degradative enzymes [6], (ii) by killing of critical insect contaminations, and (iii) by controlling the onset of fungal decay [5]. Heat treatments can be of short- (up to 1 h) or long-term duration (up to 4 days). Heat treatments have been applied to firm potatoes, tomatoes, carrots and strawberries; to preserve the colour of asparagus, broccoli, green beans, kiwi fruits, celery and lettuce; to prevent development of overripe flavours in cantaloupe and other melons; and to generally add to the longevity of grapes, plums, bean sprouts and peaches, among others [5–7].
It has been demonstrated that heat shock by using hot water washing at temperatures ranging from 37 to 55°C for a duration of 30 s to 3 min can improve the postharvest quality of spinach, rocket leaves, apples and mandarin fruit [7–10]. A clear mode of action of any water treatment is to wash-off the spores from the fruit surface [5]. Hot water is a better vector of energy than air and has provided comparable reductions in fungal decay. Blue mould on grapefruit caused by Penicillium sp. has been controlled by dipping fruit in hot water for 2 min at 50°C [5]. Improvements in the quality of bell pepper, apples, melons, sweet corn, kumquat and grapefruit have been reported with cold water cleaning in combination with brushing and a short HWR [7]. Hot water treatments also influence the structure and composition of epicuticular waxes. Covering of cracks and wounds and the formation of anti-fungal substances in the wax after heating are thought to be possible modes of action [9].
Hong et al. [10] suggested that the combination of Bacillus amyloliquefaciens HF-01, sodium bicarbonate and hot water could be a promising method for the control of postharvest decay on citrus while maintaining fruit quality after harvest. To date, commercial applications of heat treatments are limited. Heat treatment provides an alternative to fungicide applications, and in Germany HWD has been used in the storage of organic apples. Treatment of fruit after a few days of cold storage or immediately after the opening of a long-term controlled atmosphere (CA) storage room provides new options for prolonging their subsequent storage life [11], although acceptance of this technology by fruit growers has been hampered by high-energy costs and also the need for added labour at the peak work period during harvest time.
(b). Edible coating
Edible coatings are thin layers of external coatings applied to the surface of fresh produce to enhance the waxy cuticle or as replacements for natural barriers where the produce cuticle has been removed [12,13]. The application of edible coatings on fresh produce provides a partial barrier to the movement of moisture on the surface of fresh produce, thereby minimizing moisture loss during postharvest storage; a gas barrier, thereby establishing a modified atmosphere around the product, which slows down respiration, senescence and enzymatic oxidation and preserves colour and texture; helps to retain volatile compounds contributing to produce a natural aroma and restrict foreign odours; maintains fresh produce structural integrity, and protects against mechanical damages; and serves as carriers of functional or active compounds, such as nutraceuticals, flavouring and colouring agents, antioxidants and antimicrobials, that will maintain/improve product quality and safety [13–15]. Edible coatings are composed of hydrophobic groups, such as lipid-based waxes; hydrocolloid/hydrophilic groups, such as polysaccharide or protein-based materials; or an integration of both groups in order to improve the functionality of the coating [12]. Within the last decade, there has been a considerable amount of research and innovations focused on the development of edible coatings from natural or synthetic sources in order to control physiological and pathological challenges of fresh produce (table 2).
Table 2.
| coating material | purpose of coating |
|---|---|
| guar gum; pea/potato starch ± potassium sorbate | antimicrobial |
| candelilla wax-based | antimicrobial; antioxidant; quality |
| soya bean gum; jojoba wax; glycerol and arabic gum | overall quality |
| Shellac ± Aloe vera gel | keeping quality |
| soy protein; carboxymethyl cellulose | antioxidant; H2O barrier |
| chitosan; zein | antioxidant; H2O barrier |
| beeswax; coconut and sunflower oil | antimicrobial; antioxidant; quality |
| pectin base; alginate; carboxymethyl cellulose | antioxidant; H2O barrier |
| chitosan; methyl cellulose | antimicrobial; antioxidant; O2/CO2/H2O barrier |
| soy protein; carboxymethyl cellulose | antioxidant; H2O barrier |
| pectin base | overall quality |
| Aloe vera gel | overall quality |
| agar; chitosan; acetic acid (combined) | antimicrobial; O2/CO2 barrier |
| whey protein; rice bran oil | H2O barrier; overall quality |
| chitosan | overall quality |
| sucrose-polyester based | H2O barrier; antioxidant activity |
| alginate and gellan based | O2/CO2/H2O barrier |
Several edible coatings including chitosan, Aloe vera, polyvinyl acetate, mineral oils, cellulose and protein based have shown desirable attributes on fresh produce with good barrier properties, without residual odour or taste and efficient antimicrobial activity [13]. However, more research is required to enhance moisture barrier properties of hydrophilic edible coatings, improve coating adhesion and durability during storage. To maximize the benefits of edible coatings for fresh produce, it is important to understand the effect of storage conditions on the desired functions and the adverse effect on fresh produce quality. The main limitation for the application of edible coating at the industrial level is the cost of scaling up research concepts or investment for new installation of film production and coating equipment, the lack of edible materials with desired physical and functional properties as well as the challenges of regulatory status for the different coating materials. Furthermore, process parameters, such as the method of coating and the amount of additives, can affect the film barrier properties and overall quality of the food product. One of the commercial coating products is Natureseal, which maintains colour, texture and shelf life of a number of fresh-cut fruits e.g. apples, pears, carrots, celery, etc., has recorded a good success. However, further research development is required to investigate the influence of edible coatings on individual cultivars of fresh-cuts in order to understand the variation in shelf life.
(c). Irradiation
Irradiation exposes food to radiant energy from γ rays and e-beam (high-energy electrons) that penetrate objects and break molecular bonds, including the DNA of living organisms. Ionizing radiation from cobalt-60 or caesium-137, or machine generated electron beams are used as a source of irradiation for extending shelf life of fresh produce [16]. By inhibiting cellular reproduction, irradiation can neutralize pest and food safety problems. The effect depends on the doses, measured in kilograys (kGy). Low doses of irradiation (less than 1 kGy) only disrupt cellular activity enough to inhibit sprouting of tubers, bulbs and roots and delay senescence. Medium doses (1–10 kGy) reduce microbial loads while high doses (more than 10 kGy) kill a broad spectrum of fungi and bacteria spp. and pests [17]. Most medium- and high-level doses are not appropriate for fresh produce because they can cause sensory defects (visual, texture and flavour) and/or accelerated senescence due to the irreparable damage to DNA and proteins. Irradiation presents an effective postharvest treatment for destroying bacteria, moulds and yeasts, which cause food spoilage, and also control insect and parasite infestation resulting in reduced storage losses, extended shelf life and improved parasitological and microbiological safety of foods [16]. Irradiation has been commercialized for control of potato and onion sprouting, and strawberry decay [17]. Low-dose γ-irradiation on mango (0.3–0.7 kGy) resulted in delay in ripening and extension of shelf life by a minimum of 3–4 days [18]. Recently, Pandey et al. [19] reported an irradiation dose of 1 kGy to be the only effective dose in which enhanced shelf life was achieved without any deterioration of various quality attributes of litchi fruit.
While much of the focus of irradiation use on FFV has been for extending shelf life and reducing decay, it has been known for many decades that irradiation is effective at killing, sterilizing or preventing further development of a wide variety of insect pests of quarantine importance on perishable FFV. Despite some misconceptions, exposing food to irradiation does not make the food itself radioactive. The irradiation process produces very little chemical change in food and does not change the nutritional value of food. Extensive research and testing has demonstrated that irradiated food is safe and wholesome [17].
3. Chemical treatments
(a). Antimicrobial and anti-browning agents
Over the past decade, the increasing number of reported outbreaks of foodborne illnesses has heightened the concern of regulatory agencies, producers and the consumers about the microbial safety of FFV. Outbreaks have been associated with vegetables such as cabbage, celery, cucumber, leeks, watercress, lettuce and sprouts [2,3]. Antimicrobial and anti-browning agents offer the possibility to maintain safety and can be grouped into chemical- and natural/bio-based agents [20]. Chemical-based agents include chlorine-based solutions, peroxyacetic acid (PAA), organic acids, hydrogen peroxide (H2O2) and electrolysed water [20]. A chlorine-based solution such as NaClO has been one of the commonly used disinfectants for fresh produce, owing to its very potent oxidizing properties and cost effectiveness [20]. However, its efficacy as an antimicrobial agent is dependent on the levels of chlorine and at high levels may cause taste and odour defects on treated products. Additionally, chlorine-based compounds have been reported to have limited effectiveness in the reductions of microbial load on fresh produce [21]. Surfactants, detergents and solvents, alone or coupled with physical manipulation such as brushing, may be used to reduce hydrophobic nature of the waxy cuticle or remove part of the wax to increase exposure of microorganisms to chlorine. However, chlorine has been associated with the possible formation of carcinogenic chlorinated compounds and this may lead to new regulatory restrictions in the EU [20].
PAA is a very strong oxidizing agent, with no harmful by-products [22]. PAA has been reported to be effective in controlling E. coli O157:H7 and L. monocytogenes on apples, strawberries, lettuce and cantaloupe [23]. A 5 log reduction in Enterobacter sakazakii was reported for lettuce when treated with PAA [24]. Landfeld et al. [25] reported that decontamination treatment of fresh-cut carrot with PAA reduced the initial load of aerobic mesophilic bacteria by about 4 log units and yeasts and moulds by 3.5 log units and no further microbial growth was observed during storage.
H2O2 possesses a bactericidal, sporicidal and inhibitory ability, owing to its property as an oxidant and being able to generate other cytotoxic oxidizing species, such as hydroxyl radicals [20]. Treatment with H2O2 can extend the shelf life and reduce natural and pathogenic microbial populations in melons, oranges, apples, prunes, tomatoes, whole grapes and fresh-cut produce [26]. However, H2O2 treatment requires a long duration of application and can cause injury on some produce. Also, it is accepted as a generally recognized as safe for some food applications but not yet approved as an antimicrobial agent [23,26]. However, a recent study by Lopez-Galvez et al. [27] found that the newly developed H2O2-based sanitizers provoked a significant increase in the respiration rate and the electrolyte leakage of fresh-cut iceberg lettuce compared with tap water washing.
Organic acid, ascorbic acid and calcium-based solutions have been applied largely to slow down enzymatic and non-enzymatic browning, deterioration of texture and microbial growth on fresh produce. Treatment of fresh-cut melon dipped in 0.52 mM citric acid for 30 s prior to modified atmosphere packaging (MAP) maintained microbial safety and prevented translucency and discoloration [28]. Inhibitory effects of organic acids (acetic, lactic and malic acids) combined with MAP on foodborne pathogens, including E. coli O157:H7, S. Typhimurium and L. monocytogenes, on cabbage was reported by Bae et al. [29]. However, there are factors limiting the efficacy of antimicrobial and anti-browning agents such as internalization of bacteria and inaccessible sites within fresh produce such as the calyx. These limitations highlight the need for novel means of applying of antimicrobial and anti-browning agents.
(b). Nitric oxide
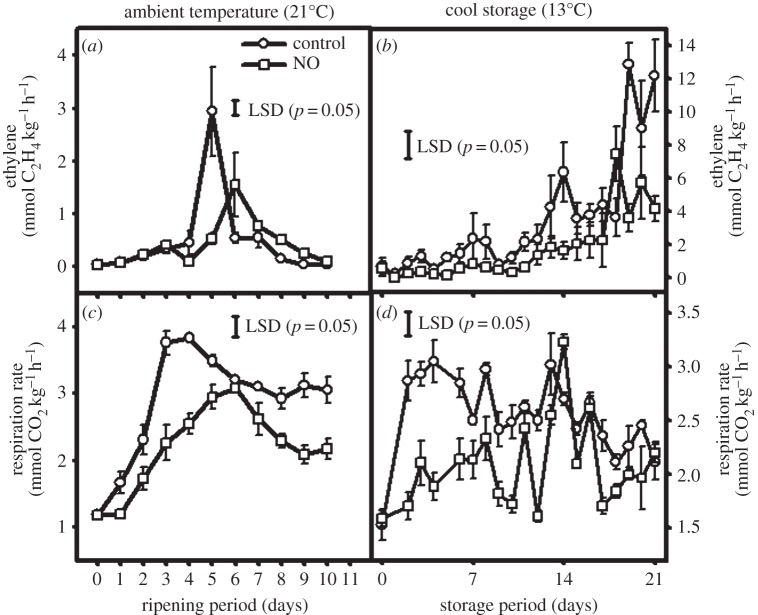
Nitric oxide (NO) is a highly reactive free radical gas and acts as a multifunctional signalling molecule in various plant physiological processes, such as fruit ripening and senescence of FFV [30]. Endogenous NO concentrations decrease with maturation and senescence in FFV, thereby offering an opportunity for modulation of their levels with exogenous application to exert the opposite effects [31]. Optimum NO levels delay the climacteric phase of many tropical fruits, prolong the postharvest storage life by impeding ripening and senescence, suppress biosynthesis of ethylene, reduce ethylene production and, consequently, delay in fruit ripening [31]. NO gas is applied as a fumigant or released from compounds such as sodium nitroprusside, S-nitrosothiols and also diazeniumdiolates used as a dipping treatment. Reduced ethylene production during ripening in NO-fumigated fruit has been claimed owing to binding of NO with 1-aminocyclopropane-1-carboxylic acid (ACC) and ACC oxidase to form a stable ternary complex, thus limiting ethylene production (figure 1a,b) [32]. Other mechanism of NO action include the inhibition of ethylene biosynthesis, cross-communication with other phytohormones, regulation of gene expression [33] and amelioration of oxidative postharvest stress [34].
Figure 1.
Ethylene production (a,b) and respiration rate (c,d) of mango as influenced by NO fumigation, ripening period and storage temperature. Reprinted from Zaharah & Singh [32] with permission from Elsevier.
Successful application of NO has been reported for apple, banana, kiwifruit, mango, peach, pear, plum, strawberry, tomato, papaya, loquat, Chinese winter jujube fruit and Chinese bayberry [33]. NO treatments reduce the rate of respiration (figure 1c,d), water loss and inhibit browning, as well as reducing the incidence of postharvest diseases. NO fumigation in combination with cold storage has a synergistic effect in extending storage life of fruit such as plum and mango [32,34]. Similarly, NO combined with modified atmosphere conditions extended the postharvest life of green beans, broccoli and bok choy [35]. Commercial application of NO in FFV depends upon the development of a smart carrier/controlled release system for NO.
(c). Sulfur dioxide
Sulfur dioxide (SO2) is widely used on table grapes to prevent decay during storage, by either initial fumigation of fruit from the field followed by weekly fumigation of storage rooms or slow release from in-package pads containing sodium metabisulfite [36]. SO2 technology has also been tested for control of postharvest decay on other fruits such as litchi, fig, banana, lemon or apple [37]. Cantín et al. [38] reported that SO2 fumigation followed by CA storage (3%O2+6 or 12%CO2) is a promising postharvest strategy for fresh blueberries to reduce decay, extend market life and maintain high nutritional value. Rivera et al. [39] demonstrated that SO2 is an effective and practical technology for reducing the risk of blueberry grey mould decay during storage and it could be used for the export market. There are disadvantages to SO2 use; the SO2 concentration necessary to inhibit fungal growth may induce injuries in grape fruits and stems, and sulfite residues pose a health risk for some individuals [36–38], as well as firming of the texture of some fruit species (pomaces), incomplete de-sulphiting and incomplete re-colouring of red fruits. Nevertheless, SO2 treatment is a widespread process because of its advantages of universal antiseptic action and economic application.
4. Gaseous treatments
(a). Ozone
Recent research and commercial applications have verified that ozone can replace traditional sanitizing agents [40,41]. Ozone is a very pungent, naturally occurring gas with strong highly reactive oxidizing properties. Ozone is reported to have 1.5 times the oxidizing potential of chlorine and 3000 times the potential of hypochlorous acid. Contact times for antimicrobial action are typically four to five times less than that for chlorine. Ozone rapidly attacks bacterial cell walls and is more effective than chlorine against the thick-walled spores of plant pathogens and animal parasites, at practical and safe concentrations [42]. Ali [41] reported that the fruit exposed to 2.5 ppm ozone had higher levels of total soluble solids, ascorbic acid content, β-carotene content, lycopene content, and antioxidant activity and also reduced weight loss at day 10 compared with untreated fruit. The sensory attributes of papaya of ozone-treated fruit was also superior in sweetness and overall acceptability endorsing ozone as a non-thermal and safe food preservation technique for FFV. Ozone can be employed in cold storage, washing system or process water sterilization. Huyskens-Keil et al. [42] reported that irradiation and washing with ozonated water slightly reduced respiration in white asparagus spears, but increased spear tissue toughness. However, neither washing the asparagus spears with ozonated water (3 or 4.5 ppm) nor treating them with radiation (1 kJ m−2) systematically and significantly affected their microbial loads during storage [43]. Some commercial use has occurred with commodities such as apples, cherries, carrots, garlic, kiwi, onions, peaches, plums, potatoes and table grapes [44]. However, ozone does not penetrate natural openings or wounds efficiently. Additional research is needed to define the potential and limits of the effective use of ozone for postharvest treatments for the quality and safety of FFV.
(b). Ethylene
Endogenous ethylene production and its exogenous application exhibit both beneficial and deleterious effects on horticultural fresh produce. Beneficial effects of exogenously applied ethylene includes triggering ripening, improving fruit colour and quality in some crops, such as bananas and avocados, kiwifruit, persimmon, tomato, mangoes, de-greening of citrus fruit [45,46]. The deleterious effects of ethylene in postharvest phase horticultural commodities has also been documented, such as shorter storage life, promotion of senescence, fruit softening, discoloration (browning) and russet spotting in lettuce, yellowing of leafy vegetables and cucumbers and increased susceptibility of FFV to decay [47,48]. Therefore, ethylene management plays a pivotal role in maintaining postharvest life and quality of climacteric and non-climacteric horticultural produce. Most commercial strategies for maintaining horticultural commodities involve storing at low temperatures, blocking ethylene biosynthesis and its action, minimizing exposure produce to ethylene during ripening, harvest, storage and transport by controlling temperature and atmospheric gas composition [49]. Newly developed ethylene measurement devices will enable to detect critical concentrations during storage and transportation [50].
Beneficial effects of ethylene biosynthesis inhibitors such as aminoethoxyvinylglycine alone on postharvest quality have been demonstrated in apples and stone fruits [51,52] and in combination with controlled atmosphere (CA) storage [53,54]. Treating FFV with inhibitors of ethylene action, such as 1-methylcyclopropene (1-MCP) or NO alone or in combination with MAP or CA storage, also impedes ethylene production and action consequently extends storage life and maintains quality of FFV (§§3b and 4c).
Commercialization and limitations of different ethylene inhibitors are discussed in detail by Martínez-Romero et al. [55]. A recent development has been palladium-promoted zeolite materials, which can be effective ethylene scavengers to prolong the shelf life of climacteric fresh produce, such as bananas and avocados [56,57]. The material has the potential to be used commercially, as an alternative and/or supplemental treatment to 1-MCP. Martínez-Romero et al. [58] developed a carbon-heat hybrid ethylene scrubber for fresh horticultural produce storage purposes. The device comprised a cartridge heater tightly joined to the activated carbon–1% palladium. Application of heat pulses leads to an increase in ethylene oxidation and to auto-regeneration of the activated carbon.
(c). 1-Methylcyclopropene
The discovery and patenting of cyclopropenes as inhibitors of ethylene perception represents a major breakthrough in controlling ethylene responses of horticultural products [59]. The process of discovery of the effects of cyclopropenes, and their proposed method of action, has been described [60,61]. Of the cyclopropenes, 1-MCP proved to be extremely active, but unstable in the liquid phase. However, 1-MCP can be complexed with α-cyclodextrin to maintain its stability; this development represented a major step towards its commercialization as it was then possible to release 1-MCP from the complex to expose to the horticultural products. Regulatory approval for use of 1-MCP has been obtained in more than 50 countries, and approval for use of the technology continues to occur around the world. 1-MCP is registered for use on a wide variety of FFV including apple, avocado, banana, broccoli, cucumber, date, kiwifruit, mango, melon, nectarine, papaya, peach, pear, pepper, persimmon, pineapple, plantain, plum, squash and tomato. 1-MCP affects many ripening and senescence processes [62,63], including pigment changes, softening and cell wall metabolism, flavour and aroma, and nutritional properties, but to varying degrees in both non-climacteric and climacteric products. While aqueous 1-MCP shows similar responses as those treated with gaseous 1-MCP, ripening actors such as activity of cell wall-associated enzymes, e.g. lycopene, antioxidant and volatiles of avocado, are delayed but recover to reach levels similar to those of untreated fruit [64]. The range of responses reflects the enormous diversity of these crops in terms of both inherent diversity and morphological derivation [65]. Several generalizations can be made about responses of crops to 1-MCP [65,66]:
— genotype, cultivar and maturity effects can be highly variable;
— climacteric fruit are affected by 1-MCP treatment, but the capacity to interrupt ripening once initiated varies by fruit and attributes studied;
— non-climacteric fruit also respond to 1-MCP, providing insights into ethylene-dependent and -independent events during ripening;
— treated fruits are firmer, slower to soften, slower to change peel colour, and develop aroma and flavour. If 1-MCP concentrations and exposure periods for each fruit must be appropriate to allow ripening to occur, so the final quality of the treated fruit is similar to that of the untreated product; and
— the effect of 1-MCP on physiological disorders is dependent on the role of ethylene. 1-MCP decreases senescent-related and ethylene-induced disorders (senescent breakdown of apples and water soaking of watermelons). 1-MCP can increase susceptibility of apple fruit to CA storage-related disorders (CO2 injury). Some chilling-related disorders (woolliness and internal breakdown of peaches and nectarines, chilling injury of citrus and bananas) can be increased by inhibition of ethylene production. Other chilling-related disorders (superficial scald of apples and pears and internal flesh browning of avocados and pineapples) are decreased by inhibition of ethylene production.
Registration of 1-MCP for FFV has focused on major or specialty products important to specific countries. The apple has been an excellent crop for use of 1-MCP, and the technology is used extensively around the world to maintain quality through the whole marketing chain from storage to consumer [66]. Success of 1-MCP technology for apples is largely associated with a fruit where maintenance of ‘at harvest’ quality and only moderate softening to a crisp fracturable texture is desirable. Watkins et al. [67] found that rapid treatment of fruit with 1-MCP after harvest can afford storage operators more freedom to delay CA storage application, but attention to cultivar, fruit maturity and susceptibility of fruit to storage disorders must be considered. Challenges exist for the effective use of 1-MCP for fruit that ripen uniformly to a melting texture and/or have major colour, flavour and aroma changes that are expected by the consumer. Failure to ripen normally has been shown in avocado, banana, pear and tomato, where fruits were treated at an early ripening stage or where applied 1-MCP concentrations were too high [68–71]. Despite the challenges, successful commercialization of SmartFresh for treatment of avocados, bananas, melons, persimmons and tomatoes has resulted from careful attenuation of 1-MCP concentrations and/or ripening stage at harvest.
Recently, 1-MCP formulations have been approved by the Environmental Protection Agency and other regulatory authorities for preharvest applications, and these are marketed as Harvista for FFV. Semi-commercial trials have been carried out in several US locations, Argentina, Brazil, Canada, Chile, New Zealand and South Africa. Harvista has useful effects on delaying fruit drop, slowing fruit maturation and ripening, and in maintaining postharvest quality, including improving the effects of SmartFresh [71,72].
(d). Controlled atmosphere storage
CA storage refers to the monitoring and adjustment of the CO2 and O2 levels within gas tight stores at optimum storage temperature. Thus, the atmosphere is controlled rather than established passively as in the case of MAP outlined in §4e, though the effects of altered atmospheres on metabolism of FFV are essentially the same. In most cases, the concentrations of CO2 are higher and those of O2 are lower, optimum concentrations depending on the specific product and the purpose of the CA storage conditions. Reduced O2 and elevated CO2 levels affect both primary (glycolysis, fermentation and aerobic respiration) and secondary (e.g. processes involved in ethylene production and action, pigments, phenolics and volatiles) metabolism [73].
Each FFV has an optimal range of O2 and CO2 for maintaining quality and extending shelf life [74], and these can differ for whole and fresh-cut products of the same fruit or vegetable. Benefits of CA as well as detrimental effects resulting from exposure of FFV to atmospheres outside of the safe range were summarized by Kader [75]. The beneficial effects of CA include:
— retardation of senescence and associated biochemical and physiological changes, e.g. slowing down rates of respiration, ethylene production and softening;
— reduction in sensitivity to ethylene action at O2 levels less than 8% and/or CO2 levels more than 1%;
— alleviation of certain physiological disorders, e.g. chilling injury of avocado and superficial scald of apples and pears;
— direct or indirect effect on postharvest pathogens (bacteria and fungi) and consequently decay incidence and severity e.g. CO2 at 10–15% inhibits development of Botrytis rot on strawberries and cherries; and
— low O2 (less than 1%) and/or elevated CO2 (40–60%) can be a useful tool for insect control in some fresh and dried fruits, flowers and vegetables; and dried nuts and grains.
The detrimental effects of CA include:
— initiation and/or aggravation of certain physiological disorders, such as internal browning in apples and pears, brown stain of lettuce and chilling injury of some commodities;
— irregular ripening of fruits, such as banana, mango, pear and tomato, can result from exposure to O2 levels below 2% and/or CO2 levels above 5% for more than one month;
— development of off-flavours and off-odours at very low O2 concentrations (as a result of anaerobic respiration) and very high CO2 levels (as a result of fermentative metabolism); and
— increased susceptibility to decay when the fruit is physiologically injured by insufficient O2 or too-high CO2 concentrations.
Use of CA technology is limited to relatively few FFV, the major crop being apples. Other FFV include cabbages, sweet onions, kiwifruits, avocados, persimmons, pomegranates, nuts and dried fruits, and vegetables [74]. Atmospheric modification during long-distance transport is used on apples, asparagus, avocados, bananas, broccoli, cranberries, cherries, figs, kiwifruits, mangos, melons, nectarines, peaches, pears, plums and strawberries. Limited use of CA for many FFV is related to the high levels of capital investment required for high-quality storage rooms and the maintenance and monitoring of atmospheres; therefore, fruit volumes must be high enough to fill rooms, and extended storage periods are needed to make investments economical. For shipping containers, limitations include maintaining container identity for those with equipment. Specific CA storage technologies that are used are a function of the FFV, growing region and size of the industry. Where used, static systems are still used for the majority of FFV CA storage, ranging from simple controlled ventilation systems to conventional CA (more than 2 kPa O2). The use of low O2 (LO) and ultra low O2 (ULO) CA storage, where O2 levels are as low as 1.5–2.0 and 0.8–1.2 kPa, respectively [76], is increasingly becoming common. Chong et al. [77] proposed a hollow fibre membrane module for generating nitrogen-enriched air stream that controls the concentration of both O2 and CO2 in the CA storage.
Other developments include dynamic CA (DCA), initial low O2 stress (ILOS) and hypobaric storage. The principle of DCA is that responses of fruit to decreasing O2 in the storage atmosphere are monitored. As O2 levels reach the anaerobic compensation point, stress responses by the fruit can be detected and the O2 increased to slightly higher levels. The most common commercial DCA system is based on chlorophyll fluorescence (DCA-DF; [78]), but others include measurement of ethanol in the fruit (dynamic controlled systems, DCS; [79]) and the respiration quotient (DCS-RQ; [80]). Most research has been carried out with apples and pears, a major impetus for research being the loss of diphenylamine, the antioxidant used widely for control of the physiological disorder superficial scald in Europe. However, DCA has been tested with avocado [81] and should work with any chlorophyll-containing product [82]. ILOS involves the use of low partial pressures of O2, e.g. 0.4 kPa, that result in ethanol accumulation for short periods of time that result in delay of fruit ripening [83]. Hypobaric or low-pressure storage results in very low O2 around the product and delay ripening and senescence [76] but has yet to find commercial acceptance because of factors such as cost and safety. These issues may be addressed in the future.
(e). Modified atmosphere packaging
MAP generally involves the packaging of a whole or fresh-cut product in plastic film bags, and can be either passive or active. In passive MAP, the equilibrium concentrations of O2 and CO2 are a function of the product weight and its respiration rate, which is affected by temperature and the surface area, perforations, thickness and permeability to gases of films used in packaging. In active MAP, the desired atmosphere is introduced in the package headspace before heat sealing, but the final atmosphere will eventually be a function of the same factors that affect passive MAP. Correct equilibrium atmosphere can delay respiration, senescence, and slow down the rate of deterioration, thereby extending product storage life [3]. More recently, active MAP also includes technologies to adsorb substances, such as O2, ethylene, moisture, CO2, flavours/odours, and release substances such as CO2, antimicrobial agents, antioxidants and flavours [84].
An extensive number of models have been developed to predict respiration rates of FFV under MAP conditions [3]. An example of prediction models is that of Mahajan et al. [85], where the Pack-in-MAP software for optimum packaging solutions for FFV was developed. Behind the software is an extensive database on product respiration rate, optimum temperature, optimum ranges of O2 and CO2 and gas permeability of packaging materials commonly used in MAP (figure 2). The software is based on a series of mathematical algorithms to simulate the evolution of internal gas composition in the packaging as a result of food respiration and mass transfer through the packaging material and, when used in a reverse manner, to identify the window of gas permeability that satisfy food requirements, size and number of micro-perforations, if needed [86].
Figure 2.
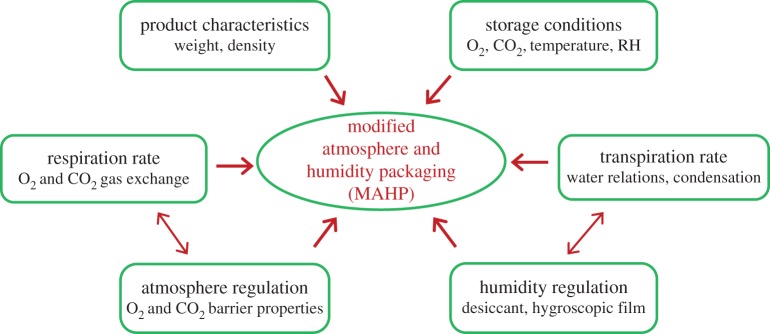
Factors involved in designing MAHP for FFV. (Online version in colour.)
Current MAP design considers the respiration rate of product as the only important parameter for deciding target gas barrier properties required to achieve an equilibrium-modified atmosphere. However, besides in-package gas composition it is also important to take into consideration the in-package level of humidity, in order to avoid condensation and/or mould and bacterial development in MAP systems [87]. It is well known that the in-package humidity is influenced by respiration and transpiration of the fresh produce as well as the water vapour permeability of the packaging material (figure 2). However, most polymeric materials (polyethylene, polypropylene or polyvinyl chloride) used in MAP have lower water vapour permeability relative to transpiration rates of fresh produce; therefore, most water molecules evaporated from the produce do not escape through the film and remain within the package, enhancing the water vapour pressure in the package microenvironment. Under these near-saturation conditions, even minor temperature fluctuation may result in condensation inside the package resulting in produce sliminess and enhancement of microbial growth and decay of produce [88]. Therefore, the major challenge of modified atmosphere and humidity packaging (MAHP) is finding a solution for creating optimal atmosphere and reducing the risk of water condensation in the package while still maintaining produce weight loss as low as possible. Recent developments on high water vapour permeable films such as Xtend, NatureFlex or biodegradable films or use of hygroscopic additives located within the package headspace or directly integrated into the packaging material. However, the hygroscopic additives should not be used for fresh produce, which have high water activity, in order to avoid excessive weight loss of the packed food. Recently, Singh et al. [89] reported that the humidity absorption of the trays with NaCl improved the quality and shelf life of fresh mushrooms. In this study, different percentage of NaCl was incorporated in the polymer matrix of a film from which trays were produced. The results indicate that the amount of water vapour absorbed by the tray is directly proportional to the percentage of salt incorporated in the trays, which enhanced the total appearance of the package. The maximal capacity and the rate of moisture absorption by the humidity regulating trays need to be studied further in order to confirm whether the trays have enough moisture absorption capacity to prevent condensation for the selected horticultural crops. Additionally, questions about sustainability and consumer expectations of such humidity regulating trays have to be taken into consideration.
5. Emerging technologies
Plasma is an emerging technique for decontaminating FFV. Plasma is composed of ionized gas molecules, which have been dissociated via an energy input. Depending on the mode of particles activation and the excitation energy, they can generate high or low temperatures, referred to as thermal or cold plasma, respectively [90]. Cold plasma at atmospheric pressure can be generated by transforming argon gas into plasma at radio frequency of 27 MHz [91] or by electric discharge between two electrodes separated by dielectric barriers [92]. Three basic mechanisms have been suggested for the inactivation microbial spores in plasma environments, including the erosion of microbial spore surface atom by atom through adsorption of reactive free radicals ‘etching’; direct destruction of DNA via UV irradiation and volatilization of compounds from the spore surface by UV photons through intrinsic photo-desorption. Fernández et al. [93] revealed that at the optimal operating conditions of cold gas plasma treatment about 15 min treatment time was required to achieve 2.72, 1.76 and 0.94 log-reductions in viable cells of S. enteric sv. Typhimurium on lettuce, strawberry surfaces and potato tissue, respectively. Recent study by Baier et al. [91] on fresh corn salad leaves showed that the plasma treatment at 20 W for 1 min successfully inactivated E. coli by 4 log-cycles. However, more research is required for a complete understanding of the role of microbial cell structure, physiology and stress resistance mechanisms involved in plasma resistance. Also, the effect of plasma treatment on food enzymes and postharvest quality attributes of FFV requires more detailed study. Safety of gases, consumer perception and the translation of laboratory scale to large commercial scale, also requires further investigation.
6. Conclusion and future prospects
A wide range of physical and chemical treatments exist to maintain and extend shelf life FFV. Specific treatments may only be applicable to certain types of product and spoilage conditions and the effectiveness of existing treatments on emerging quality issues need to be assessed. Postharvest treatments, such as CA and MAP, in combination with appropriate temperature control are the basis for maintaining physical, nutritional and sensory attributes, and by reducing decay incidence. These can be supplemented by chlorine, SO2, irradiation, hot water, hot air, antimicrobial agents and edible coatings as appropriate for the specific product. Newer technologies include ULO and postharvest technologies based on ethylene oxidation, inhibitors of ethylene action and modulators of ripening, such as NO. Research on these technologies is continuing on a range of FFV, including climacteric and non-climacteric types. Research with DCA represents a new era where the technology is applied in a dynamic fashion, recognizing that the metabolism of the product changes in response to the applied storage conditions. To date, use of this technology is limited but the introduction of nanotechnology to the postharvest arena may open new opportunities. For example, the development of nanocomposite packaging materials with tuneable architecture can act as a smart carrier/controlled release system for 1-MCP, NO or antimicrobial agents. Future research in development of delivery systems will not only improve efficacy of postharvest systems but may also address the safety issues.
Funding statement
This work is partly based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation and also partly supported by the project ReguPack (project no. IGF-N04261/12) funded by the German Ministry of Economy and Technology.
References
- 1.Lipinski B, Hanson C, Lomax J, Kitinoja L, Waite R, Searchinger T. 2013. Reducing Food Loss and Waste. Working Paper, Installment 2 of Creating a Sustainable Food Future. Washington, DC, USA. [Google Scholar]
- 2.Warriner K, Huber A, Namvar A, Fan W, Dunfield K. 2009. Recent advances in the microbial safety of fresh fruits and vegetables. Adv. Food Nutr. Res. 57, 155–208. [DOI] [PubMed] [Google Scholar]
- 3.Caleb OJ, Mahajan PV, Al-Said FA, Opara UL. 2013. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences: a review. Food Bioprocess Technol. 6, 303–329. ( 10.1007/s11947-012-0932-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olaimat AN, Holley RA. 2012. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. 32, 1–19. ( 10.1016/j.fm.2012.04.016) [DOI] [PubMed] [Google Scholar]
- 5.Schirra M, D'Hallewin G, Ben-Yehoshua S, Fallik E. 2000. Host–pathogen interactions modulated by heat treatment. Postharvest Biol. Technol. 21, 71–85. ( 10.1016/S0925-5214(00)00166-6) [DOI] [Google Scholar]
- 6.Lurie S. 1998. Postharvest heat treatments. Postharvest Biol. Technol. 14, 257–269. ( 10.1016/S0925-5214(98)00045-3) [DOI] [Google Scholar]
- 7.Fallik E. 2004. Pre-storage hot water treatments (immersion, rinsing and brushing). Postharvest Biol. Technol. 32, 125–134. ( 10.1016/j.postharvbio.2003.10.005) [DOI] [Google Scholar]
- 8.Glowacz M, Mogren LM, Reade JPH, Cobb AH, Monaghan JM. 2013. Can hot water treatments enhance or maintain postharvest quality of spinach leaves?. Postharvest Biol. Technol. 81, 23–28. [Google Scholar]
- 9.Tahir II, Johansson E, Olsson ME. 2009. Improvement of apple quality and storability by a combination of heat treatment and controlled atmosphere storage. HortScience 44, 1648–1654. [Google Scholar]
- 10.Hong P, Hao W, Luo J, Chen S, Hu M, Zhong G. 2014. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 88, 96–102. ( 10.1016/j.postharvbio.2013.10.004) [DOI] [Google Scholar]
- 11.Maxin P, Weber RWS, Pedersen H, Williams M. 2012. Control of a wide range of storage rots in naturally infected apples by hot-water dipping and rinsing. Postharvest Biol. Technol. 70, 25–31. ( 10.1016/j.postharvbio.2012.04.001) [DOI] [Google Scholar]
- 12.Gol NB, Patel PR, Rao TVR. 2013. Improvement of quality and shelf life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 85, 185–195. ( 10.1016/j.postharvbio.2013.06.008) [DOI] [Google Scholar]
- 13.Dhall RK. 2013. Advances in edible coatings for fresh fruits and vegetables: a review. Crit. Rev. Food Sci. Nutr. 53, 435–450. ( 10.1080/10408398.2010.541568) [DOI] [PubMed] [Google Scholar]
- 14.Mohebbi M, Ansarifar E, Hasanpour N, Amiryousefi MR. 2012. Suitability of Aloe vera and gum tragacanth as edible coatings for extending the shelf life of button mushroom. Food Bioprocess Technol. 5, 3193–3202. ( 10.1007/s11947-011-0709-1) [DOI] [Google Scholar]
- 15.Ghasemnezhad M, Zareh S, Rassa M, Sajedi RH. 2013. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J. Sci. Food Agric. 93, 368–374. ( 10.1002/jsfa.5770) [DOI] [PubMed] [Google Scholar]
- 16.Farkas J. 2014. Food technologies: food irradiation. Encycl. Food Safety 3, 178–186. [Google Scholar]
- 17.Ferrier P. 2010. Irradiation as a quarantine treatment. Food Policy 35, 548–555. ( 10.1016/j.foodpol.2010.06.001) [DOI] [Google Scholar]
- 18.Mahto R, Das M. 2013. Effect of gamma irradiation on the physico-chemical and visual properties of mango (Mangifera indica L.), cv. Dushehri and Fazli stored at 20°C. Postharvest Biol. Technol. 86, 447–455. ( 10.1016/j.postharvbio.2013.07.018) [DOI] [Google Scholar]
- 19.Pandey N, Joshi SK, Singh CP, Rajput S, Kumar S, Khandal RK. 2013. Enhancing shelf life of litchi (Litchi chinensis) fruit through integrated approach of surface coating and gamma irradiation. Radiat. Phys. Chem. 85, 197–203. ( 10.1016/j.radphyschem.2012.11.003) [DOI] [Google Scholar]
- 20.Artés F, Gómez P, Aguayo E, Escalona V, Artés-Hernández F. 2009. Sustainable sanitation techniques for keeping quality and safety of fresh-cut. Postharvest Biol. Technol. 51, 287–296. ( 10.1016/j.postharvbio.2008.10.003) [DOI] [Google Scholar]
- 21.Baskaran SA, Upadhyay A, Kollanoor-Johny A, Upadhyaya I, Mooyottu S, Amalaradjou MAR, Schreiber D, Venkitanarayanan K. 2013. Efficacy of plant-derived antimicrobials as antimicrobial wash treatments for reducing enterohemorrhagic Escherichia coli O157:H7 on apples. J. Food Sci. 78, 1399 ( 10.1111/1750-3841.12174) [DOI] [PubMed] [Google Scholar]
- 22.Carrasco G, Urrestarazu M. 2010. Green chemistry in protected horticulture: the use of peroxyacetic acid as a sustainable strategy. Int. J. Mol. Sci. 11, 1999–2009. ( 10.3390/ijms11051999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodgers S, Cash J, Siddiq M, Ryser E. 2004. A comparison of different chemical sanitizers for inactivating Escherichia coli O157:H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. J. Food Prot. 67, 721–731. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Ryu J, Beuchat L. 2006. Survival of Enterobacter sakazakii on fresh produce as affected by temperature, and effectiveness of sanitizers for its elimination. Int. J. Food Microbiol. 111, 134–143. ( 10.1016/j.ijfoodmicro.2006.05.021) [DOI] [PubMed] [Google Scholar]
- 25.Landfeld A, Erban V, KováŖíková E, Houška M, Kýhos K, Pruchová J, Novotná P. 2010. Decontamination of cut carrot by persteril® agent based on the action of peroxyacetic acid. Czech J. Food Sci. 28, 564–571. [Google Scholar]
- 26.Cengiz MF, Certel M. 2013. Effects of chlorine, hydrogen peroxide, and ozone on the reduction of mancozeb residues on tomatoes. Turk. J. Agric. For. 38, 1–6. ( 10.3906/tar-1307-14) [DOI] [Google Scholar]
- 27.Lopez-Galvez F, Ragaert P, Palermo LA, Eriksson M, Devlieghere F. 2013. Effect of new sanitizing formulations on quality of fresh-cut iceberg lettuce. Postharvest Biol. Technol. 85, 102–108. ( 10.1016/j.postharvbio.2013.05.005) [DOI] [Google Scholar]
- 28.Aguayo E, Allende A, Artés F. 2003. Keeping quality and safety of minimally fresh processed melon. Eur. Food Res. Technol. 216, 494–499. [Google Scholar]
- 29.Bae YM, Choi NY, Heu S, Kang DH, Lee SY. 2011. Inhibitory effects of organic acids combined with modified atmosphere packaging on foodborne pathogens on cabbage. J. Korean Soc. Appl. Biol. Chem. 54, 993–997. ( 10.1007/BF03253191) [DOI] [Google Scholar]
- 30.Wendehenne D, Durner J, Klessig DF. 2004. Nitric oxide: a new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 7, 449–455. ( 10.1016/j.pbi.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 31.Singh Z, Khan AS, Zhu S, Payne AD. 2013. Nitric oxide in the regulation of fruit ripening: challenges and thrusts. Stewart Postharvest Rev. 4, 3. [Google Scholar]
- 32.Zaharah SS, Singh Z. 2011. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 62, 258–266. ( 10.1016/j.postharvbio.2011.06.007) [DOI] [Google Scholar]
- 33.Manjunatha G, Gupta KJ, Lokesh V, Mur LAJ, Neelwarne B. 2012. Nitric oxide counters ethylene effects on ripening fruits. Plant Signal. Behav. 7, 476–483. ( 10.4161/psb.19523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SP, Singh Z, Swinny EE. 2009. Post-harvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 53, 101–108. ( 10.1016/j.postharvbio.2009.04.007) [DOI] [Google Scholar]
- 35.Soegiarto L, Wills RBH. 2004. Short term fumigation with nitric oxide gas in air to extend the postharvest life of broccoli, green bean, and bok choy. Hortic. Tech. 14, 538–540. [Google Scholar]
- 36.Palou L, Serrano M, Martinez-Romero D, Valero D. 2010. New approaches for postharvest quality retention of table grapes. Fresh Produce 4, 103–110. [Google Scholar]
- 37.Sivakumar D, Terry LA, Korsten L. 2010. An overview on litchi fruit quality and alternative postharvest treatments to replace sulfur dioxide fumigation. Food Rev. Int. 26, 162–188. ( 10.1080/87559121003590516) [DOI] [Google Scholar]
- 38.Cantín CM, Minasa IS, Goulas V, Jiménez M, Manganaris GA, Michailides TJ, Crisosto CH. 2012. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 67, 84–91. ( 10.1016/j.postharvbio.2011.12.006) [DOI] [Google Scholar]
- 39.Rivera SA, Zoffoli JP, Latorre BA. 2013. Determination of optimal sulfur dioxide time and concentration product for postharvest control of gray mold of blueberry fruit. Postharvest Biol. Technol. 83, 40–46. ( 10.1016/j.postharvbio.2013.03.007) [DOI] [Google Scholar]
- 40.Horvitz S, Cantalejo MJ. 2014. Application of ozone for the postharvest treatment of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 54, 312–339. ( 10.1080/10408398.2011.584353) [DOI] [PubMed] [Google Scholar]
- 41.Ali A, Ong MK, Forney CF. 2014. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 142, 19–26. ( 10.1016/j.foodchem.2013.07.039) [DOI] [PubMed] [Google Scholar]
- 42.Huyskens-Keil S, Hassenberg K, Herpich WB. 2011. Impact of postharvest UV-C and ozone treatment on textural properties of white asparagus (Asparagus officinalis L.). J. Appl. Bot. Food Qual. 84, 229–234. [Google Scholar]
- 43.Hassenberg K, Huyskens-Keil S, Herpich WB. 2012. Impact of postharvest UV-C and ozone treatments on microbiological properties of white asparagus (Asparagus officinalis L.). J. Appl. Bot. Food Qual. 85, 174–181. [Google Scholar]
- 44.Suslow TV. 2004. Ozone applications for postharvest disinfection of edible horticultural crops. Publication 8133. Oakland, CA: University of California, Division of Agriculture and Natural Resources. [Google Scholar]
- 45.Wills R, McGlasson B, Graham D, Joyce D. 2007. Postharvest: an introduction to the physiology and handling of fruit, vegetables and ornamentals. Boston, MA: CABI Publishing. [Google Scholar]
- 46.Singh SP, Singh Z. 2012. Postharvest oxidative behaviour of 1-methylcyclopropene treated Japanese plums (Prunus salicina Lindell) during storage under controlled and modified atmospheres. Postharvest Biol. Technol. 74, 26–35. ( 10.1016/j.postharvbio.2012.06.012) [DOI] [Google Scholar]
- 47.Saltveit ME. 1999. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 15, 279–292. ( 10.1016/S0925-5214(98)00091-X) [DOI] [Google Scholar]
- 48.Wills R. 2005. Minimizing the harmful effects of ethylene on the quality of fruit and vegetables. Environmentally friendly technologies for agricultural produce quality (ed. Ben-Yehoshua S.), pp. 133–148. London, UK: Taylor and Francis. [Google Scholar]
- 49.Watkins CB. 2002. Ethylene synthesis, mode of action, consequences and control. In Fruit quality and its biological basis (ed. Knee M.), pp. 180–222. Sheffield, UK: Sheffield Academic Press. [Google Scholar]
- 50.Janssen S, Schmitt K, Blanke M, Wöllenstein ML, Bauersfeld J, Lang W. 2014. Ethylene detection in fruit supply chains. Phil. Trans. R. Soc. A 372, 20130311 ( 10.1098/rsta.2013.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lluís P, Carlos HC. 2003. Postharvest treatments to reduce the harmful effects of ethylene on apricots. Acta Hortic. 599, 31–38. [Google Scholar]
- 52.Silverman EP, Petracek PD, Noll MR, Warrior P. 2004. Aminoethoxyvinylglycine effects on late-season apple fruit maturation. Plant Growth Regul. 43, 153–161. ( 10.1023/B:GROW.0000040113.05826.d2) [DOI] [Google Scholar]
- 53.Garner D, Crisosto CH, Otieza E. 2001. Controlled atmosphere storage and aminoethoxy-vinylglycine postharvest dip delay post cold storage softening of ‘Snow King’ peach. HortTechnology 11, 598–601. [Google Scholar]
- 54.Stover E, Fargione MJ, Watkins CB, Iungerman KA. 2003. Harvest management of Marshall ‘McIntosh’ apples: effects of AVG, NAA, ethephon, and summer pruning on preharvest drop and fruit quality. HortScience 38, 1093–1099. [Google Scholar]
- 55.Martínez-Romero D, Bailén G, Serrano M, Guillén F, Valverde JM, Zapata P, Castillo SD. 2007. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Crit. Rev. Food Sci. Nutr. 47, 543–560. ( 10.1080/10408390600846390) [DOI] [PubMed] [Google Scholar]
- 56.Terry LA, Ilkenhans T, Poulston S, Rowsell L, Smith AWJ. 2007. Development of new palladium-promoted ethylene scavenger. Postharvest Biol. Technol. 45, 214–220. ( 10.1016/j.postharvbio.2006.11.020) [DOI] [Google Scholar]
- 57.Smith AWJ, Poulston S, Rowsell L, Terry LA, Anderson JA. 2009. A new palladium-based ethylene scavenger to control ethylene-induced ripening of climacteric fruit. Platinum Metals Rev. 53, 112–122. ( 10.1595/147106709X462742) [DOI] [Google Scholar]
- 58.Martínez-Romero D, Guillén F, Castillo S, Zapata PJ, Serrano M, Valero D. 2009. Development of a carbon-heat hybrid ethylene scrubber for fresh horticultural produce storage purposes. Postharvest Biol. Technol. 51, 200–205. ( 10.1016/j.postharvbio.2008.07.013) [DOI] [Google Scholar]
- 59.Blankenship SM, Dole JM. 2003. 1-Methylcyclopropene: a review. Postharvest Biol. Technol. 28, 1–25. ( 10.1016/S0925-5214(02)00246-6) [DOI] [Google Scholar]
- 60.Sisler EC. 2006. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol. Adv. 24, 357–367. ( 10.1016/j.biotechadv.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 61.Sisler EC, Serek M. 2003. Compounds interacting with the ethylene receptor in plants. Plant Biol. 5, 473–480. ( 10.1055/s-2003-44782) [DOI] [Google Scholar]
- 62.Watkins CB. 2006. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotech. Adv. 24, 389–409. ( 10.1016/j.biotechadv.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 63.Serek M, Woltering EJ, Sisler EC, Frello S, Sriskandarajah S. 2006. Controlling ethylene responses in flowers at the receptor level. Biotechnol. Adv. 24, 368–381. ( 10.1016/j.biotechadv.2006.01.007) [DOI] [PubMed] [Google Scholar]
- 64.Zhang ZK, Huber DJ, Rao JP. 2013. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 76, 58–64. ( 10.1016/j.postharvbio.2012.09.003) [DOI] [Google Scholar]
- 65.Huber DJ. 2008. Suppression of ethylene responses through application of 1-methylcyclopropene: a powerful tool for elucidating ripening and senescence mechanisms in climacteric and nonclimacteric fruits and vegetables. HortScience 43, 106–111. [Google Scholar]
- 66.Watkins CB. 2008. Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 43, 86–94. [Google Scholar]
- 67.Watkins CB, Nock JF. 2012. Rapid 1-methylcyclopropene (1-MCP) treatment and delayed controlled atmosphere storage of apples. Postharvest Biol. Technol. 69, 24–31. ( 10.1016/j.postharvbio.2012.02.010) [DOI] [Google Scholar]
- 68.Bai J, Mattheis JP, Reed N. 2006. Re-initiating softening ability of 1-methylcyclopropene-treated ‘Bartlett’ and ‘d'Anjou’ pears after regular air or controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 81, 959–964. [Google Scholar]
- 69.Golding JB, Shearer D, Wyllie SG, McGlasson WB. 1998. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87–98. ( 10.1016/S0925-5214(98)00032-5) [DOI] [Google Scholar]
- 70.Mir N, Canoles M, Beaudry R, Baldwin E, Mehla CP. 2004. Inhibiting tomato ripening with 1-methylcyclopropene. J. Am. Soc. Hortic. Sci. 129, 112–120. [Google Scholar]
- 71.McArtney SJ, Obermiller JD, Schupp JR, Parker ML, Edgington TB. 2008. Preharvest 1-methylcyclopropene delays fruit maturity and reduces softening and superficial scald of apples during long-term storage. HortScience 43, 366–371. [Google Scholar]
- 72.Watkins CB, James H, Nock JF, Reed N, Oakes RL. 2010. Preharvest application of 1-methylcyclopropene (1-MCP) to control fruit drop of apples, and its effects on postharvest quality. Acta Hortic. 877, 365–374. [Google Scholar]
- 73.Beaudry RM. 1999. Effect of O2 and CO2 partial pressure on selected phenomena affecting fruit and vegetable quality. Postharvest Biol. Technol. 15, 293–303. ( 10.1016/S0925-5214(98)00092-1) [DOI] [Google Scholar]
- 74.Saltveit ME. 2003. Is it possible to find an optimal controlled atmosphere? Postharvest Biol. Technol. 27, 3–13. ( 10.1016/S0925-5214(02)00184-9) [DOI] [Google Scholar]
- 75.Kader A. 2004. Controlled atmosphere storage. In USDA Handbook 66. See www.ba.ars.usda.gov/hb66/013ca.pdf. [Google Scholar]
- 76.Hoehn E, Prange RK, Vigneault C. 2009. Storage technology and applications, pp. 17–50. Boca Raton, FL: CRC Press, Taylor and Francis Group. [Google Scholar]
- 77.Chong KL, Peng N, Yin H, Lipscomb G, Chung TS. 2013. Food sustainability by designing and modelling a membrane controlled atmosphere storage system. J. Food Eng. 114, 361–374. ( 10.1016/j.jfoodeng.2012.08.027) [DOI] [Google Scholar]
- 78.Prange RK, Wright AH, DeLong JM, Zanella A. 2013. History, current situation and future prospects for dynamic controlled atmosphere (DCA) storage of fruits and vegetables, using chlorophyll fluorescence. Acta Hortic. 1012, 905–915. [Google Scholar]
- 79.Veltman RH, Verschoor JA, van Dugteren JHR. 2003. Dynamic control system (DCS) for apples (Malus domestica Borkh. cv ‘Elstar’): optimal quality through storage based on product response. Postharvest Biol. Technol. 27, 79–86. ( 10.1016/S0925-5214(02)00186-2) [DOI] [Google Scholar]
- 80.Gasser F, Eppler T, Naunheim W, Gabioud S, Nising AB. 2010. Dynamic CA storage of apples: monitoring of the critical oxygen concentration and adjustment of optimum conditions during oxygen reduction. Acta Hortic. 876, 39–46. [Google Scholar]
- 81.Burdon J, Lallu N, Haynes G, McDermott K, Billing D. 2008. The effect of delays in establishment of a static or dynamic controlled atmosphere on the quality of olor modifcado fruit. Postharvest Biol. Technol. 49, 61–68. ( 10.1016/j.postharvbio.2008.01.002) [DOI] [Google Scholar]
- 82.Prange RK, DeLong JM, Harrison PA, Leyte JC, McLean SD. 2003. Oxygen concentration affects chlorophyll fluorescence in chlorophyll-containing fruit and vegetables. J. Am. Soc. Hortic. Sci. 128, 603–607. [Google Scholar]
- 83.Zanella A. 2003. Control of apple superficial scald and ripening: a comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra low oxygen storage. Postharvest Biol. Technol. 27, 69–78. ( 10.1016/S0925-5214(02)00187-4) [DOI] [Google Scholar]
- 84.Rodriguez-Lafuente A, Nerin C, Batlle R. 2010. Active paraffin-based paper packaging for extending the shelf life of cherry tomatoes. J. Agric. Food Chem. 58, 6780–6786. ( 10.1021/jf100728n) [DOI] [PubMed] [Google Scholar]
- 85.Mahajan PV, Oliveira FAR, Montanez JC, Frias J. 2007. Development of user-friendly software for design of modified atmosphere packaging for fresh and fresh-cut produce. Innov. Food Sci. Emerg. Technol. 8, 84–92. ( 10.1016/j.ifset.2006.07.005) [DOI] [Google Scholar]
- 86.Sousa-Gallagher MJ, Mahajan PV. 2013. Integrative mathematical modeling for MAP design of fresh-produce: theoretical analysis and experimental validation. Food Control 29, 444–450. ( 10.1016/j.foodcont.2012.05.072) [DOI] [Google Scholar]
- 87.Sousa-Gallagher MJ, Mahajan PV, Mezdad T. 2013. Engineering packaging design accounting for transpiration rate: Model development and validation with strawberries. J. Food Eng. 119, 370–376. ( 10.1016/j.jfoodeng.2013.05.041) [DOI] [Google Scholar]
- 88.Linke M, Geyer M. 2013. Condensation dynamics in plastic film packaging for fruit and vegetables. J. Food Eng. 116, 144–154. ( 10.1016/j.jfoodeng.2012.11.026) [DOI] [Google Scholar]
- 89.Singh P, Saengerlaub S, Stramm C, Langowski HC. 2010. Humidity regulating packages containing sodium chloride as active substance for packing of fresh raw Agaricus mushrooms. In Proc. 4th Int. Workshop Cold Chain Management (ed. Kreyenschmidt J.). Bonn, Germany: University of Bonn. [Google Scholar]
- 90.Niemira BA. 2012. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 3, 125–142. ( 10.1146/annurev-food-022811-101132) [DOI] [PubMed] [Google Scholar]
- 91.Baier M, Foerster J, Schnabel U, Knorr D, Ehlbeck J, Herppich WB, Schlüter O. 2013. Direct non-thermal plasma treatment for the sanitation of fresh corn salad leaves: Evaluation of physical and physiological effects and antimicrobial efficacy. Postharvest Biol. Technol. 84, 81–87. ( 10.1016/j.postharvbio.2013.03.022) [DOI] [Google Scholar]
- 92.Pankaj SK, Misra NN, Cullen P. 2013. Kinetics of tomato peroxidase inactivation by atmosphere pressure cold plasma based on dielectric discharge. Innov. Food Sci. Emerg. Technol. 19, 153–157. ( 10.1016/j.ifset.2013.03.001) [DOI] [Google Scholar]
- 93.Fernández A, Noriega E, Thompson A. 2013. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 33, 24–29. ( 10.1016/j.fm.2012.08.007) [DOI] [PubMed] [Google Scholar]