Abstract
Ethylene is a gaseous ripening phytohormone of fruits and plants. Presently, ethylene is primarily measured with stationary equipment in laboratories. Applying in situ measurement at the point of natural ethylene generation has been hampered by the lack of portable units designed to detect ethylene at necessary resolutions of a few parts per billion. Moreover, high humidity inside controlled atmosphere stores or containers complicates the realization of gas sensing systems that are sufficiently sensitive, reliable, robust and cost efficient. In particular, three measurement principles have shown promising potential for fruit supply chains and were used to develop independent mobile devices: non-dispersive infrared spectroscopy, miniaturized gas chromatography and electrochemical measurement. In this paper, the measurement systems for ethylene are compared with regard to the needs in fruit logistics; i.e. sensitivity, selectivity, long-term stability, facilitation of automated measurement and suitability for mobile application. Resolutions of 20–10 ppb can be achieved in mobile applications with state-of-the-art equipment, operating with the three methods described in the following. The prices of these systems are in a range below €10 000.
Keywords: ethylene, ethene, gas analytics, electrochemical sensors, gas chromatography, non-dispersive infrared
1. Introduction
The ambient conditions in the supply chain for perishable goods like climacteric fruits are of great importance. On the one hand, ambient conditions in a container or transport compartment are a prerequisite to maintain the freshness of the transported perishable cargo while, on the other hand, by controlling the conditions, a specific behaviour of the commodity—like reduced metabolism and hence respiration—can be ensured. Low temperature remains the key to maintain good fruit quality and it is mandatory to ensure that the cold chain is not interrupted during transport. A very good example of the consequences of changes in ambient conditions is the transport of strawberries. Just 1 h storage at increased temperatures of only a few degrees can reduce the shelf life or edibility of the fruit by a day. Besides the temperature, the composition of the ambient air has considerable influence on the eventual fruit quality and, consequently, on the shelf life of the transported perishable cargo. Modified and controlled atmosphere (MA/CA) is the key to extend the shelf life of fruits [1–3]. Besides CO2 and O2, ethylene is the most important gas to be monitored and controlled in the supply chain of fruits [4–6].
Less than 1 part per million (ppm) by volume of ethylene gas is sufficient to trigger the ripening process of climacteric fruit [7]. Hence, ethylene gas is considered a ‘phytohormone’. Thus, knowing the concentration of ethylene gas in storage rooms is important regarding two aspects. Firstly, the ethylene concentration indicates the stage of fruit ripening (figure 1), and countermeasures like venting fresh air can take place when the concentration is approaching a critical range, e.g. 1 ppm. Secondly, by knowing the ethylene concentration, a prediction of the remaining shelf life is possible. Therefore, it is necessary to analyse the rise of ethylene in the pre-climacteric stage of fruit development, when the ambient concentration is below 1 ppm. Hence, ethylene should be measured with a resolution of 50 ppb or better. As in this low concentration range many other organic substances will be present, any potential sensing method requires good sensitivity as well as good selectivity. Furthermore, the measurement system must be small as well as sufficiently robust for usage in a container or other transport compartment.
Figure 1.
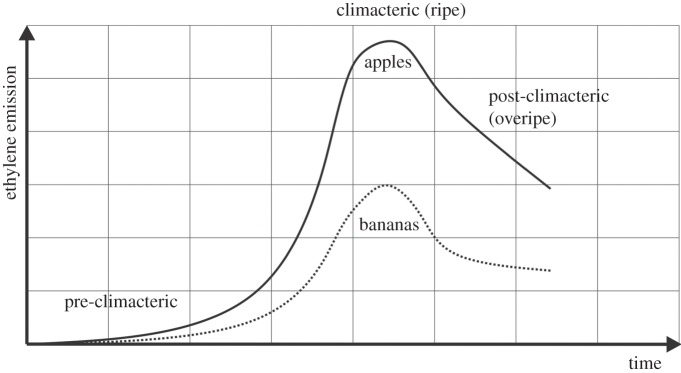
Typical ethylene efflux rate over time. Climacteric fruits have a distinctive peak during the ripening process. Whereas the peak concentration can be measured with several devices, the detection of the progress of biological processes during the pre-climacteric phase is still challenging. Concentrations of only few tens of ppb have to be measured (adapted from [8]).
Ethylene is an invisible, colourless and odourless gas, which has no known hazardous effect on humans at the concentrations encountered within the fruit supply chain. Ethylene is a naturally occurring gas, associated with plants under stress and fruit maturation in horticulture. The relatively small and simple ethylene molecule consists of two carbon atoms associated with four hydrogen atoms. The molecular weight of ethylene is 28.05 g mol−1.
Different amounts of ethylene are produced by fruits and vegetables as they ripen (figure 1) [9]. Normally, ethylene cannot be sensed by humans; only experienced people can sometimes smell large quantities, but this may also be contributed to by volatile organic compounds from the fruit [10]. As its specific weight (1.178 kg m−3 at 15°C) is similar to that of air (1.225 kg m−3 at 15°C), ethylene diffuses freely to any other adjacent fruit commodity on the shelves on a truck or a ship or in a store.
Autocatalytic ethylene synthesis is an irreversible physiological ripening process in climacteric fruit. The accumulation of the ripening gas ethylene may lead to premature ripening of fruits and vegetables or their decay (table 1) with few exceptions. In climacteric fruits, ethylene stimulates ripening, which is undesired along the food/fruit supply chain, whereas with non-climacteric fruits, such as citrus, ethylene promotes senescence often associated with the loss of green skin coloration: this chlorophyll degradation in the peel of citrus fruit, such as lemons and easy peelers, is a process commercially applied on the farm for the German and British markets. These fruits are shipped after such de-greening, e.g. from South Africa to Europe. Ethylene can also induce or enhance physical disorders and chilling injuries in both climacteric and non-climacteric fruit (table 1).
Table 1.
Physiological effects of exogenous ethylene on fruit and vegetables as dependent on their climacteric classification [10].
| physiological parameter | climacteric fruit | non-climacteric fruit |
|---|---|---|
| ethylene synthesis | enhanced | no effect |
| autocatalytic ethylene | enhanced | no effect |
| fruit metabolism and respiration | enhanced | no effect |
| fruit ripening | enhanced | no effect |
| loss of green skin colour | negligible effect | often enhanced |
| decay microorganisms | no clear effect | often enhanced |
| induction of disorders or chilling injury | possible | possible |
Climacteric fruit can hence be the first major source of ethylene in all compartments along the food chain from the ship hull, regional distribution centre, wholesale market, receiving area, storage and display shelf in the supermarket.
As ethylene diffuses freely, considerable residual ethylene levels may also remain in compartments where climacteric fruit had previously been stored, depending on the amount, stage of ripeness, type of fruit previously stored there and storage time.
While ethylene efflux from fruits is not necessarily associated with their ethylene sensitivity (table 2), ethylene efflux of ripening fruits, however, correlates directly with internal ethylene concentration in the fruit core. Ethylene efflux of ripening climacteric fruits and vegetables exceeds that of non-climacteric fruits by multiples (table 2) [11]. Moreover, the sensitivity of fruit to ethylene shows large variations (table 2). At 20°C, carrots produce less than 0.1 μl while kiwifruits produce 1 μl of C2H4 kg−1 h−1, but both react very sensitively to low ethylene levels. For instance, 30 ppb of ethylene may cause softening and decay in kiwifruit (F Bollen 2013, personal communication). Ethylene levels of more than 100 ppb may induce isocoumarin synthesis in carrots, causing bitterness and unpleasant taste [12].
Table 2.
Ethylene efflux and sensitivity of selected fruit and vegetables [11]. +++, very large ethylene efflux above 100 μl kg−1 h−1; +, large efflux of 10–100 kg−1 h−1; O, intermediate level of ethylene efflux of 1–10 kg−1 h−1; −, 0.1–1 kg−1 h−1; −−−, minimal ethylene efflux of less than 0.1 kg−1 h−1 fresh mass during ripening or which are typical for the of non-climacteric fruits with ethylene emission.
| commodity | commodity classification | ethylene efflux | ethylene sensitivity |
|---|---|---|---|
| apple | climacteric | ++ | + |
| avocado | climacteric | + | + |
| banana | climacteric | O | + |
| carrot | non-climacteric | −−− | +++ |
| citrus | non-climacteric | −−− | O |
| kiwi | climacteric | − | +++ |
| pear | climacteric | + | + |
| passion fruit | climacteric | +++ | + |
| tomato | climacteric | O | + |
| onion | non-climacteric | −−− | − |
In recent years, three brands of vent control systems with real-time analysis of O2/CO2 have entered the market (eAutofresh by Carrier, AFAM+ by ThermoKing and AV+ by MCI). However, the monitoring of ethylene concentration is not facilitated regularly owing to the lack of suitable on-site instrumentation. In CA fruit stores, O2 and CO2 are routinely monitored. Ethylene is monitored daily, sometimes weekly, by taking gas samples, which are analysed by gas chromatographic measuring in external laboratories. The main demand during storage identified by the agro-food sector is ‘online’ monitoring of ethylene in the ppb range with low-cost devices in the storage chambers. Research and development are concentrated on the implementation of gas sensing systems for fruit monitoring, especially for CO2 measuring in containers. The first step is quality monitoring by recording respiration of climacteric fruit like bananas, using a CO2 sensor. More reliable predictions of the quality could be enabled by measuring the ethylene concentration inside a container. Latest work showed that a cost-efficient and sensitive system will be available in the near future and will give biologists the opportunity to create a shelf life prediction model that will be more accurate than those published in the past [13]. Several sensor technologies for the detection of specific gas concentrations are available but not all of these are equipped to withstand the rough conditions inside a container. Often, a mobile device with high sensitivity and well-customized selectivity to a specific gas compound like ethylene is not available, too expensive or not able to provide stable readings in the long term. The development of a mobile device for ethylene measuring is a difficult task. Small measuring devices for the detection of O2 and CO2 concentrations are available, but during field tests in a container they have not shown sufficient long-term stability owing to exposure to high humidity. The main goal of this research is to develop a high-sensitivity, small, mobile system that can be installed in containers and CA stores at a reasonable price, facilitating reliable continuous measuring of ethylene during the complete logistic chain. In the following sections, measurement principles and systems actually used in logistic processes will be discussed. Thereby, the focus will be on three measurement devices developed or used for ethylene detection by the authors. The systems themselves as well as the peripheral equipment potentially necessary for operation of such sensor systems in a container will be discussed, because there are some important issues with regard to practicability.
2. Ethylene measurement for fruit quality monitoring
In 2012, Cristescu et al. [14] described current methods for the detection of ethylene in plants. They give an overview on the possibilities for ethylene measurement under laboratory conditions. The highest sensitivity can be achieved by photoacoustic spectroscopy. We start our overview with a short summary of this technology. But the main focus of this paper is on measuring principles that are suitable to be applied along the cold chain of fresh fruits, i.e. inside moving trucks and containers, as well as during storage and post-harvest processing. In this case, factors other than the sensitivity become important as well, e.g. the suitability for mobile usage, long-term stability and the price. Non-dispersive infrared (NDIR) spectroscopy, miniaturized gas chromatography (GC) and electro-catalytic measurement are discussed in more detail owing to the latest developments and tests by the authors. However, for the sake of completeness, we summarize additionally measuring principles of electrochemical sensors.
(a). Photoacoustic spectroscopy
Photoacoustic spectroscopy provides the highest sensitivity values to ethylene gas. Figure 2 shows a general working principle of this measurement method. A laser beam (frequently used is a CO2 laser) is polarized and chopped in a specific frequency. Depending on the chemical structure, a specific gas compound absorbs the light at a specific wavelength—similar to NDIR spectroscopy. Owing to the absorption, the gas in the measurement chamber is periodically heated according to the copping frequency. The temperature changes induce pressure changes, which can be measured with a microphone. The photoacoustic amplitude rises with the concentration of the specific gas component.
Figure 2.
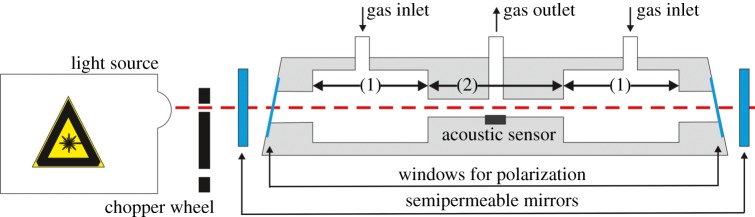
Schematic of a photoacoustic sensor: the test gas flows through two-buffer chambers (1) to the resonator section (2). (Online version in colour.)
Already in 1990 Harren et al. [15] described a measurement set-up in this context that was able to detect ethylene gas with a concentration of 6 parts per trillion (ppt). In 1996, the same group published the results of an optimization of a photoacoustic cell [16]. This system was able to detect ethylene down to 6 ppt with a time response of 2 s. The long-term stability of this device was 20 ppt at a gas flow of 6 l h−1. Parallel developments in this field are described in Gäbler et al. [17] and Stolik et al. [18]. They achieved a sensitivity of 120 ppt. First field tests on measuring ethylene from industrial sources in Houston, TX, USA were performed by De Gouw et al. [19] in 2009. For the measurement, they used a device by Sensor Sense Company. This indicates that a measurement system for mobile use is available. Other examples for research on this topic are described in Lima et al. [20], Mothe et al. [21], Schmitt et al. [22] and Nägele et al. [23].
Compared to other systems, the measuring devices based on photoacoustic spectroscopy provide the best sensitivity. Relatively small and mobile systems with a total size of 50×50×14 cm3 are available in the market. The main disadvantage is the price (ca € 30 000 for the Sensor Sense EDT-300 with a sensitivity of 0.3 ppb for ±2σ [19,24]; €20 000 for Gasera F10 with a sensitivity of only 800 ppb [25]). The measurement interval of the EDT-300 is 5 s with a response time of 30 s. The Gasera F10 has a response time of 30 s that can be adjusted to several minutes to improve the sensitivity. The lowest detection limit of 800 ppb can be achieved with a response time of 1 min.
The photoacoustic measurement principle has the disadvantage of an inherent sensitivity towards ambient noise and vibration. However, in the studies of de Gouw et al [19], the EDT-300 from Sensor Sense withstands the conditions in an airplane without being negatively influenced by the strong vibrations. But the harsh environment inside reefer containers on ocean vessels is even more challenging. Not only with regard to vibrations caused by the cooling unit and the vessel's engine, but also humidity, temperature and required long-term stability.
(b). Electrochemical sensors
A chemoresistive sensor device was described by Esser et al. [26,27] in 2012. In general, a chemoresistive sensor is a device that detects a gas through a change of the resistivity of an area or surface on the sensor induced by chemical bindings of the gas molecules. These active surfaces can be designed to be best fitted for the gas of interest.
In this particular sensor, a mixture of a copper complex and single-walled carbon nanotubes is placed between two gold electrodes. The copper complexes partly bind the ethylene and thereby the resistivity of the nanoparticles changes. A reaction selective to ethylene is based on the fact that the copper is a cofactor at the ethylene receptors and the fruit. The sensor was able to detect ethylene concentrations between 0.5 and 50 ppm. The system was tested with an ethylene nitrogen mixture. The flow rate of the sample gas was constant during the measurement.
Another attempt at research on electrochemical sensors was published in 2011 by Zevenberg et al. [28]. In that system, ethylene dissolves in a thin ionic liquid that covers a gold electrode. A voltage between this working electrode and a counter electrode induces oxidization of the ethylene at the working electrode. Owing to this oxidization, a small current (faradaic current) is induced. This current is measured to determine the ethylene concentration.
Also, the change of a capacitor caused by ethylene can be used as a detection method. Most common in this field is the use of SnO2 as active surface. One attempt in this field is presented by Agarwal et al. [29]. On the SnO2 surface, oxygen is ionized negatively and therewith the depletion is increased as well as the conduction region of the SnO2 surface. The negative oxygen ion reacts with the ethylene and brings the surface back to its original state.
In general, these sensors are optimized for ethylene detection but show high cross-selectivity to other gases and poor sensitivity. Therefore, these systems are not stand-alone systems. The system by Agar et al. [29] can be adapted to be used in GC that will be described in the following. The sensor described by Esser et al. [26] showed very good performances for such a relatively simple sensor. So far, only research on this topic is presented. Future work on this topic will be quite interesting to get a better and more detailed estimation of the capability of this device.
(c). Electro-catalytic sensor (ETH1010)
The measuring principle is based on ethylene oxidation on a gold Nafion anode with weak sulfuric acid used as the electrolyte. The characteristic double or ‘pi-bond’ is a prerequisite for this new nanotechnology. The inert gold does not react with most other compounds. Below a particle size of about 100 nm, the properties of nanoparticles of gold radically change and their extremely large surface area generates high reactivity (figure 3); nanoparticles catalyse particularly the oxidation of double carbon bonds [31].
Figure 3.
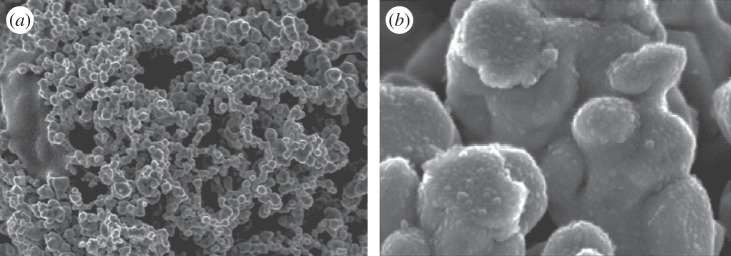
Scanning electron micrographs of gold nanoparticles: (a) magnification ×20k; (b) ×100k. Shekarriz & Allen [30].
Gold nanoparticles strongly absorb (carbon) double bonds and catalyse the oxidation of ethylene with oxygen to CO2 and water [30]:
Sampling air passes over the anode, which comprises a Nafion membrane, plated with gold nanoparticles. The oxidation of ethylene releases two protons, which travel through the membrane and through the electrolyte, a weak sulfuric acid solution, on the other side of the membrane to reach the counter electrode where they combine with the two released electrons to complete the electric circuit. The current that is generated in the process is a function of the oxidation rate or proportional to the ethylene concentration in air (figure 4). The low maintenance design requires occasional refilling of up to 50 ml distilled water for the electrolyte and return from long-haul shipment for annual service depending on usage, ambient temperature and humidity.
Figure 4.
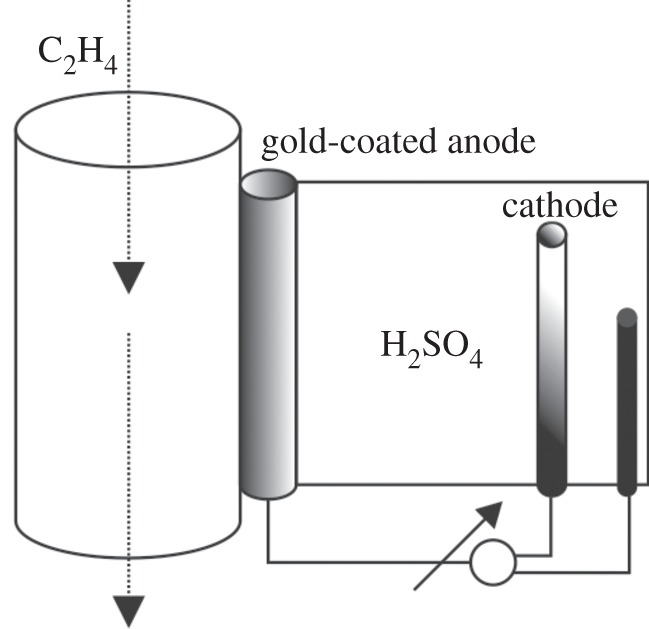
Schematic of the measuring principle with the gold nanoparticle-plated anode, cathode and sulfuric acid as electrolyte.
Calibration is done with gas from a standard gas cylinder or bag with, for example, 1–10 ppm C2H4 for the highest accuracy. Although the overall calibration is kept after switch off, the highest accuracy is achieved using calibration before use and leaving the unit switched on. An auto-zero function provides a fresh zero automatically when necessary, using the built-in potassium permanganate column [31]. The system includes a suction pump for ca 100 ml min−1 and provides real time and continuous measurements (figure 5a). It requires neither a pre-concentrator nor a catalytic converter for clean air during measurement, unlike some other systems, e.g. photoacoustic and miniaturized GC (§2d).
Figure 5.
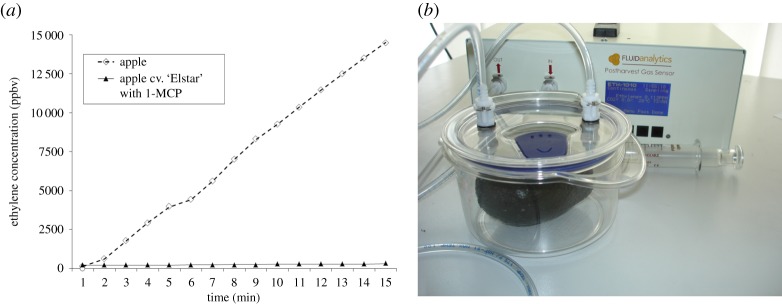
Measurement of ethylene efflux (a) from individual apple fruit without 1-MCP (top line) and apple cv. ‘Elstar’ with 1-MCP (bottom line) during shelf life with an immediate response of the ETH1010 within a minute, measured (b) with individual fruit in a respiration chamber in a closed gas circuit. (Online version in colour.)
Ethylene concentrations were measured in situ in the apple fruit supply chain to explore whether this new technology is suitable for portable use. While ethylene was essentially absent with values less than 11 ppb C2H4 at the CA store with long-term fruit storage and an effective ethylene scrubber, it had accumulated in the RA (regular atmosphere) cold store with apples (Elstar) over four months to about 5 ppm. In the grading facility with sorting line, ethylene accumulated to 0.5 ppm C2H4, while the concentration in the wooden bins with side vents was 72 ppb (figure 6a).
Figure 6.
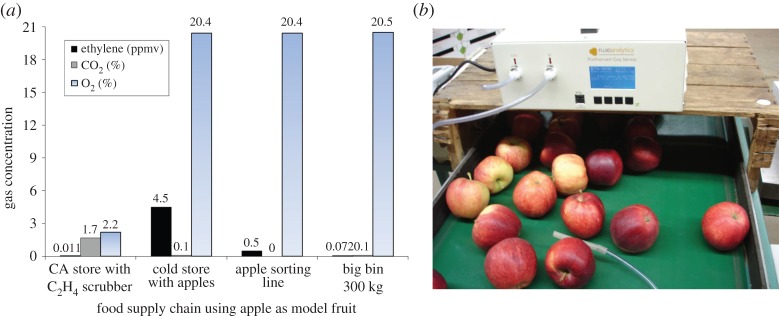
(a) Ethylene concentrations along the (apple) fruit supply chain, measured in situ in an open gas system with a portable ethylene unit and (b) example of sampling on the sorting belt [31]. (Online version in colour.)
In this study, the accuracy was between 96 and 98% of 0.05–0.15 ppm C2H4 and a variation coefficient of 0.5–2%, when the calibration gas of 8 ppm C2H4 was measured after calibration. The measuring range of the device was 0–50 ppm C2H4 with an accuracy of ±5% of reading and displayed a resolution of 1 ppb. The reproducibility of the C2H4 values was 93% with three subsequent measurements of a variety of fruits. Presently commercially available versions using nanotechnology (EASI-1; Felix, ICA 541 [32]) are equipped with a rechargeable battery for up to ca 8 h of operation.
In some climacteric fruit like apples, the application of 1-MCP (1-methylcyclopropene) at harvest, blocking the ethylene receptor, is a possibility to reduce subsequent ethylene efflux, postpone ripening and extend storability [31]. The striking similarity of the MCP and ethylene molecules also enables 1-MCP detection with the same technology; this could be realized, for example, by a dual channel unit with one channel for ethylene and the other for 1-MCP. R & D plans will go ahead with the ETH1010 in 2014. Fruit and vegetables treated with 1-MCP show dramatically reduced ethylene production rates (figure 5).
The ETH1010 is a very small and very sensitive ethylene measurement tool, which showed good long-term stability during field tests in CA stores. With an accuracy of 10 ppb, a reproducibility of 10 ppb and a response time between 30 s and 2 min the system is well suited for the use in the fruit supply chain. The response time depends on the difference between two measured concentrations—if the values are similar, the response time is short. A comparison of the measurement results from a GC system and the ETH1010 showed that the selectivity of this system is very good. The system is on the verge of debuting in the market. The price of ca €7000 (OEM device without display) includes measurement potential for temperature and three other gases relevant to the fruit supply chain.
(d). Gas chromatography
GC is a common measurement principle to evaluate the concentration of one or more gases in an air sample. Measurement systems based on the GC principle are normally fairly large and expensive and therefore not suitable for mobile application in containers or in CA stores. Latest GC systems have a 19-inch rack (Compact GC from Axel Semrau). The main parts of a gas chromatograph are an injection unit, a GC column and a non-selective gas sensor (figure 7).
Figure 7.
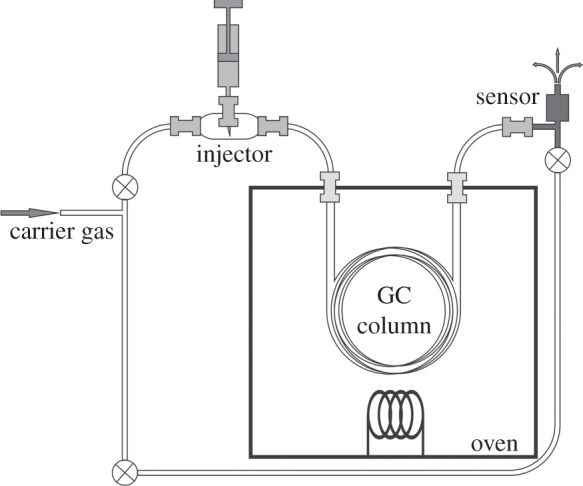
Schematic of a GC system. The sensor can be a semiconductor gas sensor for example; but more common is a flame ionization detector.
A carrier gas called mobile phase flows with a fixed flow rate through the system. The flow rate is specific for every column. There are two types of columns, a packed and a capillary, that are filled with a stationary phase. Depending on the kind of GC column the stationary phase can be thin film on the wall (capillary column) or it can be small particles filled into the column (packed column). A sample gas mixture is injected in the system ahead of the column. An inert gas like nitrogen or helium is used to avoid interactions or pollution of the sample by the carrier gas.
The different components of the gas sample have different pass-through times or retention times through the column. Owing to these different retention times the parts of the gas sample are separated from each other and therefore they arrive at the sensor at different times. Knowing the specific retention time, every peak in the output signal can be assigned to a specific gas component. The intensity and the time at which the output signal appears indicate the concentration of the gas compound.
The ‘heart’ of a GC system is the separation column. As mentioned, there are two types of columns available. The first column is a capillary column that is typically used. They can be from five up to 100 m long. The diameters of such devices are between 50 μm and a few hundred micrometres. In a capillary column, the walls of the channels are coated with kinds of polysilicone, but also the use of alumina (Al2O3) is possible. The second type of column is the packed column that is in comparison to the capillary column relatively short—systems with 50 cm were discussed in the literature—and instead of the surface of the walls being the stationary phase, particles are the stationary phase. Typically, the particles are made of carbon and Al2O3. Besides the column length and the stationary phase, the temperature is also an important issue. For every column, a specific temperature and flow rate of the carrier gas are needed to get the best separation.
One of the latest developments on this subject was made by the Institute of Microsensor and Actuator Systems (IMSAS) [33–35] (figure 8). A mobile system was developed which is small, robust and sufficiently sensitive for use inside a container during transport. So far, this system has been tested in the laboratory. A field test for banana transports is planned for the near future.
Figure 8.
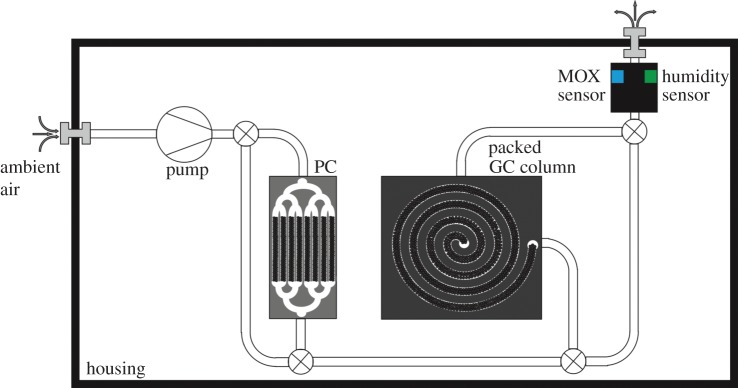
Schematic of miniaturized GC system with micro GC column (μ-column) and pre-concentrator (μPC). (Online version in colour.)
The challenge in developing this system was the miniaturization of the gas chromatographic column (μ-column) and the pre-concentrator (μPC; figure 9). The first attempt at developing a μ-column of silicon was described in 1979 in Terry et al. [36]. Several attempts have been reported since 1979 (e.g. [37]). One of the latest developments is presented in Sun et al. [38]. In this publication, a microfabricated GC system for the determination of trichloroethylene vapour is described. These attempts were not designed for ethylene detection but show promising results in the miniaturization of GC systems. The challenge in the measurement of ethylene is that it is a very small molecule and shows similar retention times to vaporized water for several stationary phases. A separation of these gas components can usually only be done with a very long column. The fact that the column must be heated to get the best separation behaviour is also a problem in mobile applications. The μ-column developed at the IMSAS with a length of 50 cm and a chip size of 40×40×1 mm3 is fairly small. The heating of the device is facilitated by a platinum heater at the bottom of the μ-column. Carbosieve SII, a very porous material based on carbon, is used as stationary phase, because it showed the best separation capabilities for ethylene and vaporized water.
Figure 9.
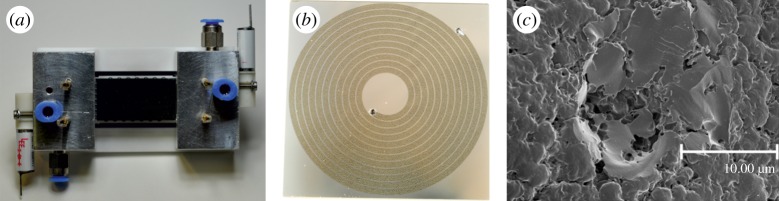
(a) μPC device in its housing; (b) μ-column with stationary phase; (c) porous surface of Carbosieve SII. (Online version in colour.)
The system described in this paper uses a pre-concentrator device (μPC) to increase the sensitivity of the system (figure 9). Moreover, the μPC replaces the injection and the sampling units that are normally used in GC systems. In Dow et al. [39], a system is described, which was designed for ethylene detection, but the sensitivity improvement with this μPC could not reach the necessary ppb level. In Lin et al. [40] and in Tian et al. [41], miniaturized μPC devices are described as well. These past attempts were not suitable for ethylene measurement. Both systems differ completely from the μPC device used in this research; however, showing very good results for the areas they were designed for.
The measurement cycle is as follows. In the first step, the gas from the container is pumped through the μPC and bypasses the μ-column. After about 25 min, owing to the adsorption process the maximum amount of ethylene is trapped in the μPC, but only a relatively small amount of water. In the second step of the measurement, the μPC is heated up to 220°C leading to desorption. After 5 min of heating the μPC is connected to the μ-column to inject the gas sample. After about 800 s, the ethylene is reaching the non-selective gas sensor, causing an output signal. The system operates with a metal oxide (MOX) sensor (SnO2 sensor, AS-MLK by Applied Sensors) instead of a common flame ionization detector (FID). Owing to the cross-selectivity to water an additional humidity sensor is applied for the possibility to certainly identify the water peak at the MOX sensor (figure 10).
Figure 10.
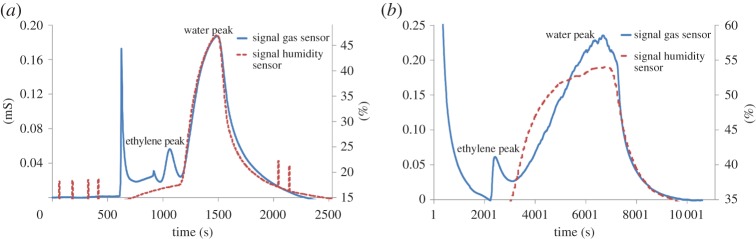
Output signal from the gas sensor (SnO2, solid line in millisiemens (mS)) and the humidity (dashed line in %) for (a) initial ethylene concentration of 400 ppb and (b) about 170 ppb. The two peaks after the injection peak were assigned according to the measurements of the humidity sensor. (Online version in colour.)
With the realization of the so-called large-capacity-on-chip pre-concentrator device, the detection limit of ethylene measurement was increased strongly in comparison to earlier devices. The adsorption material is Carbosieve SII (CSII). With the dimensions of 40.0×2.0×0.9 mm3 for each of the eight channels the μPC contains 191 mg of CSII. The heating of the CSII for the desorption process occurs directly on the CSII material using the walls between the channels as the heater.
Using this new μPC, a small GC system was able to measure about 170 ppb for the first time, which was provided by mixing the smallest concentration available at gas distributors (400 ppb) with synthetic air. The standard deviation σ of the signal corresponds to an ethylene concentration of 1.3 ppb. The noise limit is defined as ±3σ, which is 3.9 ppb. From this, we conclude that a detection limit of 10 ppb should be possible. One problem at the moment is caused by the heating of the μPC, because the heat of the housing creates a drift in the output signals. This problem must be solved before the detection limit of this device can reach this lower level. Therefore, at the moment the detection limit is approximated 50 ppb.
However, with the enlargement of the μPC the influence of the vaporized water owing to the cross-selectivity of the non-selective sensor increases as well. This was a huge problem in the past, because as mentioned earlier, for several stationary phases vaporized water and ethylene show similar retention times. However, from figure 10a,b, the separation of the water and the ethylene peak could be observed. This was done by using CSII as the stationary phase.
There are several differences between standard GC systems and this miniaturized system, making it suitable for use in logistic supply chains. First of all, this system will be more cost efficient. A cost estimate shows that the system might be available for under €2000 (instead of €18 000 or more for laboratory GC suitable for ethylene measurement).
The second important difference from conventional laboratory GC systems is that no specific carrier gas is needed. To avoid the necessity of an additional gas support, the system was designed to use synthetic air instead of nitrogen or helium as carrier gas. Synthetic air as mobile phase has some disadvantages related to the performance of the system, as high humidity causes an increased baseline signal. However, tests with humidified synthetic air showed that the impact of this effect is not very significant to the measurement. In this context, the disadvantage that the used sensor only detects ethylene in higher ppm concentrations is turning into an advantage, as ethylene concentrations in ambient air only arise in lower ppb levels and therefore will not cause any output signals by the sensor.
The size of the system is 33×27×15 cm3. First tests were performed for the communication between the computation unit of the so-called ‘Intelligent Container’ [42] and the sensor device. Owing to adsorption and desorption processes and the retention times, only one measurement per hour is possible. This is sufficient for measurement in a container or CA store.
The system can be designed for multiple gas detection. An example is the system CompactGC by Axel Semrau. Having a larger scale and price, this system is optimized for mobile application.
The μGC system will be further improved in the future. For the μ-column, a capillary column is under development that will have a better performance and thus will shorten the measurement period. The presently used sensor is one designed to detect methane, as no other simple MOX sensor was available. Agarwal et al. [29] described a new sensor that can be used to improve the μGC further. Here, a SnO2 capacitive sensor is described, which is quite similar to the sensor that is used in the above-mentioned μGC system but is optimized for ethylene detection. Such a sensor is not a stand-alone sensor device, owing to high cross-selectivity and poor sensitivity.
The designed μGC system showed that such systems can be very small and sensitive enough to be used in a container. Moreover, this system needs no additional support of carrier gas, as it is designed to use ambient air. Therefore, a tube is needed to get air from the ambient into the container. In a container, no real-time measurement is necessary and thus this system can be suitable for use inside containers. At present, only a prototype is available.
A commercial solution for a mobile GC system is the Photovac Explorer [43]. It operates with a battery (8 h life time) and a refillable carrier gas cylinder. The sensitivity to ethylene is 100 ppbv. Its size is 39×27×15 cm3. The price for this system is ca €19 000. It is designed as a multi-gas analyser using three columns. At the moment, the price and necessity of a specific carrier gas prevent this system from being suitable for use on a larger scale in the fruit supply chain.
(e). Non-dispersive infrared spectroscopy
NDIR absorption spectroscopy provides a relatively simple and robust solution for the measurement of IR-active gases. The gas molecules absorb IR radiation at a specific wavelength. The attenuation of IR radiation is measured and the gas concentration can be calculated according to the Lambert–Beer law.
As ethylene is IR-active mainly around 10.6 μm, this absorption can be used to measure also small concentrations. Yet, available IR sources for this wavelength range are either expensive or have a comparably low intensity. This can be partly compensated by choosing optimal length of the optical path. The absorption spectrum of 100% ethylene for an optical path length of 1 m at normal conditions is shown in figure 11a.
Figure 11.
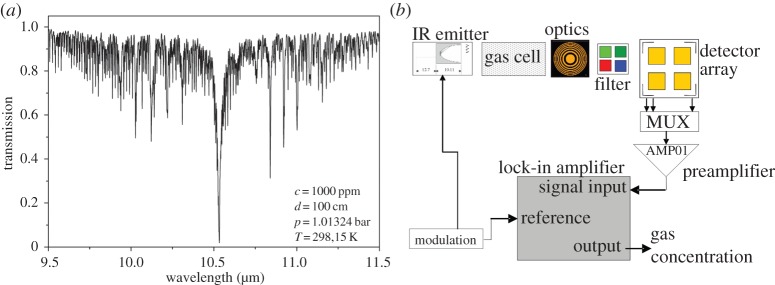
(a) Infrared transmission spectrum of ethylene between 9.5 and 11.5 μm (100%, 1 m optical path length). Source: HITRAN [44]. (b) Scheme of the measurement system consisting of a microstructured IR emitter, a miniaturized multi-reflection cell, a thermopile array with integrated optical filters and microstructured optics (Fresnel lenses) and signal-processing electronics. (Online version in colour.)
We developed a filter spectrometer for ethylene monitoring in fruit applications. The aim is a small and low-cost optical system for ethylene detection in the mid-infrared at a wavelength of 10.6 μm. In fruit storage applications, other gases appear and can disturb the optical measurement of ethylene. Numerous hydrocarbons in ppb concentrations are negligible for optical measurement, but ammonia, ethanol and acetaldehyde can occur in concentration ranges of several ppm, and thus have to be considered as interfering in the IR measurements of ethylene. While ethanol and acetaldehyde are the result of fruit stress, ammonia interference may be caused by the leakage of the cooling system. Therefore, the absorption not only at 10.6 μm, but also at other wavelengths has to be determined, i.e. a multi-spectral measurement has to be performed. A scheme of the measurement system is shown in figure 11b. The modulated radiation of a thermal emitter is coupled into a long-path gas cell and is detected by a multi-sensor array, pre-amplified and processed by a lock-in technique. The IR emitter, the compact long path absorption cell, the detection module and system electronics were all integrated into a compact system.
A miniaturized White cell has been developed to reach the required optical path length for the detection of low ethylene concentrations occurring in fruit storages. The principle of a White cell is based on multiple reflections between three spherical concave mirrors, which all have the same radius of curvature. The optical set-up provides a high light transmission where radiation losses are caused only by absorption and scattering on the reflecting surfaces. The optical path, i.e. the number of reflections, is dependent on the adjustment of the mirrors, but limited by the diameter of the active area of the source. Here, an optical path length of 1.6 m is implemented in a volume of 11×5×6 cm3. The body of the cell is made of aluminium. The mirrors are gold-coated convex glass lenses and are glued into the White cell [45,46].
For multi-spectral measurements, a thermopile detector array with integrated optical filters and microstructured Si Fresnel lenses has been developed. It consists of a substrate chip with an array of 2×2 thermopile elements based on silicon technology that is attached to the base of the package. Four different optical filters are flip-chipped onto the thermopile elements. Attached on the top of the package is a Fresnel multi-lens, intended to divide the total IR radiation transmitted by the absorption cell into four and focus into the corresponding absorption zone of the thermopiles.
A detailed description of the processing of the thermopiles can be found in [47]; the optical characterization of the thermopiles was shown in [48]. The parameters of the optical filters were estimated from the absorption spectra of the relevant gases, i.e. ethylene, ammonia, ethanol and acetaldehyde [49]. All gases show interfering absorptions at 10.6 μm, i.e. they can cause interferences in the IR measurement of ethylene. Filter wavelengths for ethylene at 10.6 μm, ethanol at 3.46 μm, ammonia at 9.7 μm and a reference channel at 3.95 μm have been chosen for the four-element thermopile array.
The set-up of the optical system with miniaturized absorption cell, IR emitter, optical chopper, coupling optics and detector module is shown in figure 12a. The IR emitter is mounted in a TO8 housing. For mechanical modulation of the radiation, a motor driving a chopper blade and a light barrier is used. Different set-ups of coupling optics for the White cell have been tested. In addition to spherical mirrors, a lens made of zinc selenite (ZnSe), parabolic mirrors and an ellipsoid mirror were compared. The ZnSe lens showed the best results with regard to coupling efficiency and compactness and has been used in the final set-up. To avoid radiation losses at an additional window, the ZnSe lens is mounted on a tube and closes the White cell at the input focus. The distance between the output focus of the White cell and the detection module depends on the tolerances of the focal length of the Fresnel multi-lens. To protect the detection module from humidity a thin calcium fluoride (CaF2) window seals the White cell.
Figure 12.
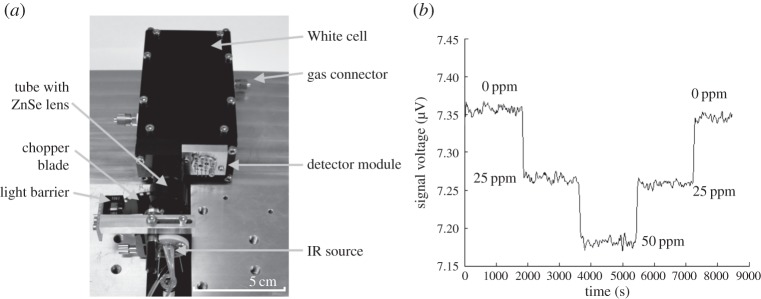
(a) Optical system with miniaturized White cell, IR source, optical chopper, ZnSe lens and detector module. (b) Detector signal during measurement of different probes with 0, 25 and 50 ppm ethylene concentrations.
The fourfold thermopile array provides three information channels and one reference channel. To acquire the information from the three channels, a multiplexer is used. Next to a pre-amplification stage, a synchronous detection (lock-in amplifier) is used in order to increase the signal-to-noise ratio. The emitter is mechanically modulated at 8 Hz. The modulation signal of the infrared source is also used as reference signal for the lock-in amplifier. The lock-in amplifier has been designed and digitally implemented on a PSoC 5 microcontroller (from Cypress). By using an electrically modulated IR emitter instead of an optical chopper, the system has been integrated on a European standard PCB card. The size of the whole measurement system is 10×12×16.6 cm3.
The optical set-up has been tested with and without the pre-amplification board. Figure 12b shows the chopped signal without pre-amplification. The White cell was exposed to different concentrations of ethylene at 0, 25, 50, 25 and 0 ppm again with a duration of 30 min for each step. The detection limit (3σ) of the optical set-up with and without pre-amplification board is below 20 ppm for a measurement time (integration time) of 2 s. The detection limit can be enhanced to 5 ppm (3σ, 2 s integration time) by using a dual detector array (two-channel approach), owing to a lower noise on the sensor signal. By using a dual detector array instead of fourfold detector array, the available optical power of signal is distributed on a much smaller detector area, which results in a much better signal-to-noise ratio.
To the best of our knowledge, there is only one infrared gas sensor for monitoring fruit ripening processes commercially available [50,51]. The detection limit (3σ) of the SmartGAS sensor is 20 ppm for a measurement time (integration time) of 15 s. The SmartGAS system uses a two-channel approach, one channel for the ethylene detection and the other channel for CO2 measurements. Other IR optical ethylene measurement systems on the market are combustible gas detectors for monitoring the lower explosion limit with a detection limit in the range of 100 ppm or more [52].
The NDIR-based systems are quite small, relatively cost efficient and can be designed for multiple gas detection. At present, however, the available systems are not sensitive enough for quality prediction during transport. First tests showed that this can be further improved by combining the system and a pre-concentrator. First tests of combining an NDIR system with a pre-concentrator are described in Sklorz et al. [53,54]. The results show promising opportunities for this method to improve sensitivity.
In ripening facilities, this system would be an attractive alternative, as the achieved sensitivity is sufficient there. The advantage of measuring CO2 at the same time is quite important during ripening.
A new, improved SmartGAS NDIR system will be available soon. This system will also be tested in a banana container. The sensitivity of this new device will be in the lower ppm range. The combination with a pre-concentrator shows great potential to overcome the sensitivity issue of such devices. It appears feasible to achieve measuring in the respective ppb ranges, and thus the development of shelf life models using ethylene as a parameter will be a potential option.
3. Comparison of methods for ethylene determination
Several measuring methods for the detection of ethylene have been investigated in the past. Current developments were highlighted in the previous section in addition to the earlier overview by Cristescu [14]. In terms of sensitivity, some available systems can detect ethylene at very low concentrations of only a few ppt (§2), which are suitable for use in laboratory-based biological research on climacteric fruits (table 3).
Table 3.
Examples for commercial ethylene measuring equipment. Calibration is possible for all devices.
| detection method | model | in situ measurement | carrier gas | net price in €000 | resolution in ppb (=10−9) |
|---|---|---|---|---|---|
| gas chromatograph (FID) | various stationary equipment | not available | required | 18–20 | 10–100 |
| Photovac Explorer [41] | not available | required | 19 | 100 | |
| catalytic sensor | ETH1010 [31] | available | not required | 7 | 10 |
| Absoger [33] | available | not required | ca 7 | 10 | |
| photoacoustic (PA) | Sensor Sense EDT-300 [24] | available | not required | 30 | 0.3 |
| Gasera F10 [25] | available | not required | ca 20 | 800 | |
| NDIR | SmartGas [50] | available | not required | more than 1.5 | 20 000 |
For the mobile usage of such devices in the supply chain of fruits, the sensitivity is not the only factor to be regarded, because in this case other issues play an equally important role. Most of these highly sensitive devices, like gas chromatographs, are not suitable for mobile application, because of the necessity of specific components like a carrier gas bottle or their relatively large size. However, the Photovac Explorer has a small size but is quite expensive and the system is not designed for usage in container application. This might be possible in the future. In comparison, the system with the best sensitivity to ethylene is the photoacoustic sensor, however, with the disadvantage of high price and high sensitivity to both noise and vibrations. The EDT-300 is a device for research, which showed good performance in an airplane. Use in a container might be more challenging for the device. Investigations and field tests according to container transportations should be conducted.
Three other developments for ethylene measurement are on the verge of being used in the fruit supply chain: the NDIR device, the μGC system and the electro-catalytic sensor ETH1010 (table 4). We depicted these three systems, because they are showing good overall performances and the prices are in a reasonable range. In the following, we compare these three systems in detail according to the advantages and disadvantages, like all measurement systems have. This comparison together with the information about the other depicted devices and measurement tools give an idea of the important things that have to be kept in mind to find a system that fulfils the requirements of each part of the logistic chain.
Table 4.
Comparison of the three depicted ethylene measurement systems.
| criterion | μGC system | NDIR | ETH1010 |
|---|---|---|---|
| sensitivity | less than 200 ppb | more than 20 ppm | 10 ppb |
| selectivity | good, depending on the column | good, except for some gases, e.g. CO2 | high selectivity due to measurement principle |
| humidity | influence to baseline | condensation can cause problems | no negative influence |
| price | about €2000 | more than €1500 | €7000 |
| power consumption | 12 V/0.5 A (1.5 A for 7 min per measurement) | 12 V/24 V 0.8 A | 12 V/0.1 A |
| autonomous measurement possible | yes | yes | yes |
| size | approx. 33×27×15 cm3 | approx. 35×11×7 cm3 (SmartGAS) approx. 10×12×16.6 cm3 (IPM) | approx. 20×20×10 cm3 (in the future about 50% smaller) |
| measurement time | about 1 per hour | continuous/in situ measurement | continuous/in situ measurement |
| carrier gas | in future: ambient air, now: synthetic air | not required | not required |
| long-term stability | not yet tested, risk of sensor drift, accuracy depends on pump and mass flow controller | not yet tested in a container, in ripening facilities the IR source showed no drift over a longer period | not yet tested in a container, field tests in CA stores: standard variation of 0.5% |
| calibration | 1 test gas required | 1 test gas required | 1 test gas required |
| automated base line correction | offset compensated by second chamber | clean air provided by permanganate filter | |
| analysis of other gases | possible for almost every gas | possible for IR-active gases (e.g. O2, CO2) | integrated CO2 and optional O2 sensor, option for 1-MCP in two chamber system |
| maintenance | pumps, valves and filter need maintenance, specific lifetime of sensor | pump and IR source (specific lifetime) need maintenance | refill of water to chemical cell, replacement of permanganate |
| time-to-market | prototype available | system available (20 ppm, SmartGAS) | available 2014 (Fluid Analytics) |
| future options | new MOX sensor, capillary column for better performance | new SmartGAS system (less than 10 ppm), combination with a pre-concentrator | 1-MCP measurement |
| key advantage | good accuracy at low price | fast measurement at low price | best resolution for mid-priced device, no sensitivity towards humidity |
With the current sensitivity of 170 ppb (μGC), 20 ppm (NDIR) and 10 ppb (ETH1010), these systems do not show the best sensitivity of all systems, but they are still good enough for use in the fruit supply chain. Presently, in this context the ETH1010 is the best-suited system for monitoring the quality of climacteric fruits. The sensitivity of μGC system, however, is also suited for such measuring. At present, with regard to sensitivity, the NDIR is only suited for use in ripening facilities.
The required selectivity is achieved by different techniques. The ETH1010 is highly selective owing to the electrochemical properties of gold nanoparticles. NDIR devices use a filter specific to the absorption wavelength of ethylene. However, cross-sensitivities cannot be avoided and must be compensated by reference measurements especially for CO2, if ethylene concentrations below 10 ppm should be measured. The column of a GC must be specially adapted to the target gas to achieve best accuracy, but the device can be adapted to several gases.
The potentially very high humidity (80–98%) inside a container, CA store, or transport compartment, e.g. of a reefer ship, has to be considered in several respects. Firstly, the device must withstand the humidity conditions in a container. CO2 sensors tested during field tests broke owing to very high humidity in the container. Secondly, condensation in the measurement chamber or on the IR source, for example, can affect NDIR devices. Therefore, an in-built heater of the chamber is required. Thirdly, cross-sensitivities have to be considered. The GC needs compensation by a reference measurement, as the response time of water is close to that of ethylene.
The electrochemical cell of the ETH1010 uses water as a reactant and has therefore lower sensitivity towards changing humidity; conversely, if the sensor is left in very low humidity and dry ambient conditions, the sensor cell will require constant monitoring and replenishment of the water in the electrolyte reservoir.
The price of such a device is always important, especially if application on a larger scale is considered. One of the key advantages of the μGC is its price that is estimated to be €2000 in a midscale range production. At €7000 the ETH1010 is in the medium-priced range and therefore the use inside fruit containers on a larger scale seems to be difficult at present. However, for application in CA stores and on board a ship this technology could be the preferable measurement method.
The power consumption of all three devices is in a reasonable range. Only the μGC needs relatively high current supply at 1.5 A, but only for a short period during the measurement of approximately 7 min.
Should the system be applied in an intelligent logistic process, an autonomous measurement is absolutely mandatory. However, all three methods are providing this feature.
Compared to other devices described in the literature or commercial measurement devices with a good sensitivity, all three systems are of fairly small size and it is possible to install them inside a container in which the space is limited.
With a measurement time of about 1 h, the μGC device is by far the slowest measurement system. With the other devices, continuous in situ measurement is possible. For monitoring the stage of ripening, a measurement time of 1 h is also sufficient, as the transportation of the fruit takes 10–14 days. During this time, the fruits remain in the same container, and a change in the ethylene concentration arises only very slowly. Another disadvantage of the μGC system compared to the other two systems is the fact that a carrier gas is needed. The system was tested with humidified synthetic air instead of common carrier gas (N2, He). According to the tests with synthetic air, the use of ambient air as carrier gas should be possible in the future and therefore no extra gas supply will be needed.
The long-term stability especially in a container is not yet tested for all three systems. Vibration, humidity and temperature (approx. 13°C) could cause problems in this context. Nevertheless, some parts influence the long-term stability and also are important according to maintenance. Mechanical parts like pumps (used in μGC and SmartGAS) can show fatigue and thereby cause a drift of the measured values or a complete failure. Also, the MOX sensor of the GC can drift, as the active surface corrodes over time. The IR source in the NDIR device can show ageing effects and must be replaced according to the hours of use. The lifespan of the MOX sensor and the IR source must be investigated in long-term field tests.
Calibration is very important if the highest accuracy of the measurement is needed. A typical calibration procedure requires test/reference gases with at least two different concentrations to compensate for drifts in zero offset and sensitivity. The number of required test gases can be reduced to one for the presented systems owing to inherent features of the measurement principle and built-in mechanism.
The ETH1010 comes with a permanganate filter, which provides clean air for automatic zero calibration. This filter needs to be replaced according to its use.
The sensitivity of NDIR devices has to be recalibrated frequently owing to ageing effects of the IR source. Zero offsets can be automatically compensated by the second reference detector with a different wavelength.
The baseline of the GC is adjusted to zero with the ambient air after the start of each measurement and thereby compensates drifts caused by humidity. Because the ethylene concentration of the output of the μPC is much higher than that of ambient air, the effect of ambient air to a shift of the baseline can be neglected.
The analyses of other gases can be quite important as well. Aside from its sensitivity, the key advantage of the ETH1010 in this context is the potential to detect 1-MCP in the second chamber. The system itself is equipped with integrated CO2 and O2 sensor based on other measurement methods. The NDIR system can be designed to detect every other IR-active gas. In the context of intelligent logistics, the measurement of CO2 is the most important feature. In this case, a simultaneous measurement of the most important gases for ripening and transportation of climacteric fruits can be done. A GC system can be adjusted to any kind of gas. Therefore, the measurement of several gases is possible.
At present, only the SmartGAS system is available on the market. The ETH1010 will follow this year. The μGC is only available as a prototype. More investigation and field tests are needed before this system will be available for commercial use.
In the future, all three systems can be further improved. As indicated above, a new sensor and a novel capillary column for the GC system will be explored to improve the sensitivity and the measurement time. A potential combination of the NDIR system with a pre-concentrator may be employed to overcome the relatively poor sensitivity of the NDIR system. First tests showed the feasibility of such combination and promising improvements of sensitivity [48,49]. Further tests with the SmartGAS system indicated that the required ppb levels can be achieved, but more research in this field is necessary. As mentioned earlier, the ETH1010 can be extended with the second chamber for 1-MCP measurement.
4. Conclusion and outlook
The monitoring of the freshness of perishable goods like climacteric fruits is very complex. One of the most important and difficult tasks is the measurement of ethylene gas. In the past, the parameter ethylene was often neglected in the prediction of the remaining shelf life during transport, as no suitable measurement system was available for the detection of ethylene changes in the ppb range. In the past few years, the technology has improved and small, robust systems are on the verge of deployment for use in quality prediction of climacteric fruits in containers. In this paper, three measurement systems are described in particular, as besides their improved sensitivity and selectivity they will be small, robust and cost efficient enough for use in a container or CA store. In the future, all three depicted systems will be present in the fruit supply chain. At present, the μGC seems to be the best-suited device for use in a container, as the sensitivity of the NDIR device and the price of the ETH1010 prevent those systems from being used on a larger scale. Nevertheless, the NDIR system is already on the market and an improved system will be available in 2014. Also, the potential to improve the sensitivity with a μPC will be explored in the near future. The ETH1010 is on the verge of becoming a commercial product. Potential use on a larger scale and optimization of the production line will lead to a decrease in price for the system, making the ETH1010 an interesting solution—besides CA stores and on board a ship—for container applications.
Acknowledgements
Further information about the project can be found at www.intelligentcontainer.com. We also thank Dole Fresh Fruit Europe for provision of test facilities. We are grateful to Dr Reza Shekarriz, Fluid Analytics, USA for supplying valuable information and insights into the nanotechnology of the electro-catalytic sensor and Dr David Cooke, UK for revising the first draft of a part (M.B.) of this manuscript.
Funding statement
The research project ‘The Intelligent Container’ is supported by the Federal Ministry of Education and Research, Germany, under reference no. 01IA10001. We thank the European Commission for funding part of the research in this article in the frame of the projects ‘GoodFood’ and ‘FoodMicroSystems’.
References
- 1.Mahajan PV, Caleb OJ, Singh Z, Watkins CB, Geyer M. 2014. Postharvest treatments of fresh produce. Phil. Trans. R. Soc. A 372, 20130309 ( 10.1098/rsta.2013.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson AK. 2010. Controlled atmosphere storage of fruits and vegetables. Wallingford, UK: CABI. [Google Scholar]
- 3.Voesenek LA, Vriezen WH, Smekens MJ, Huitink FH, Bogemann GM, Blom CW. 1997. Ethylene sensitivity and response sensor expression in petioles of Rumex species at low O2 and high CO2 concentrations. Plant Physiol. 114, 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.do Nascimento JRO, Júnior AV, Bassinello PZ, Cordenunsi BR, Mainardi JA, Purgatto E, Lajolo FM. 2006. Beta-amylase expression and starch degradation during banana ripening. Postharvest Biol. Technol. 40, 41–47. ( 10.1016/j.postharvbio.2005.11.008) [DOI] [Google Scholar]
- 5.Jedermann R, Geyer M, Praeger U, Lang W. 2012. Sea transport of bananas in containers—parameter identification for a temperature model. J. Food Eng. 115, 330–338. ( 10.1016/j.jfoodeng.2012.10.039) [DOI] [Google Scholar]
- 6.Jedermann R, Moehrke A, Lang W. Supervision of banana transport by the intelligent container. In 4th Int.Workshop on Cold Chain Management, Bonn, Germany, 27–28 September 2010 (ed. Kreyenschmidt J.), pp. 75–84. [Google Scholar]
- 7.Inaba A, Nakamura R. 1988. Numerical expression for estimating the minimum ethylene exposure time necessary to induce ripening in banana fruit. J. Am. Soc. Hort. Sci. 113, 561–564. [Google Scholar]
- 8.Abeles FB, Morgan PW, Saltveit ME., Jr 1992. Ethylene in plant biology. New York, NY: Academic Press. [Google Scholar]
- 9.Shiomi S, Wamocho LS, Agong SG. 1996. Ripening characteristics of purple passion fruit on and off the vine. Postharvest Biol. Technol. 7, 161–170. ( 10.1016/0925-5214(95)00023-2) [DOI] [Google Scholar]
- 10.Blanke M. 2008. Tragbares Ethylenmessgerät mit hoher Auflösung durch neue Sensortechnologie. Erwerbs-Obstbau 50, 77–84. ( 10.1007/s10341-008-0064-1) [DOI] [Google Scholar]
- 11.Saltveit ME. 1999. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 15, 279–292. ( 10.1016/S0925-5214(98)00091-X) [DOI] [Google Scholar]
- 12.Lafuente MT, López-Gálvez G, Cantwell M, Yang SF. 1996. Factors influencing ethylene-induced isocoumarin formation and increased respiration in carrots. J. Am. Soc. Hort. Sci. 121, 537–542. [Google Scholar]
- 13.Jedermann R, Praeger U, Geyer M, Lang W. 2014. Remote quality monitoring in the banana chain. Phil. Trans. R. Soc. A 372, 20130303 ( 10.1098/rsta.2013.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cristescu SM, Mandon J, Arslanov D, De Pessemier J, Hermans C, Harren FJ. 2013. Current methods for detecting ethylene in plants. Ann. Bot. 111, 347–360. ( 10.1093/aob/mcs259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harren F, Bijnen F, Reuss J, Voesenek L, Blom C. 1990. Sensitive intracavity photoacoustic measurements with a CO2 waveguide laser. Appl. Phys. B 50, 137–144. ( 10.1007/BF00331909) [DOI] [Google Scholar]
- 16.Bijnen F, Reuss J, Harren F. 1996. Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Rev. Sci. Instrum. 67, 2914–2923. ( 10.1063/1.1147072) [DOI] [Google Scholar]
- 17.Gaebler R. 1998. Aufbau eines transportablen photoakustischen Spektrometers für den empfindlichen und kontinuierlichen Ethylennachweis in der Pfanzenphysiologie. Dissertation, University of Bonn, Bonn, Germany. [Google Scholar]
- 18.Stolik S, Ramon-Gallegos E, Pacheco M, Tomas SA, Cruz-Orea A, Perez-Zapata AJ, Gaebler R, Sanchez-Sinencio F. 2001. Photoacoustic measurement of ethylene as a real time biomarker of lipid peroxidation processes in mice. Anal. Sci. 17, 365–367. ( 10.2116/analsci.17.365) [DOI] [PubMed] [Google Scholar]
- 19.De Gouw JA, et al. 2009. Airborne measurements of ethene from industrial sources using laser photo-acoustic spectroscopy. Environ. Sci. Technol. 43, 2437–2442. ( 10.1021/es802701a) [DOI] [PubMed] [Google Scholar]
- 20.Lima G, Sthel M, Da Silva M, Schramm D, De Castro M, Vargas H. 2011. Photoacoustic spectroscopy of CO2 laser in the detection of gaseous molecules. J. Phys. Conf. Ser. 274, 012086 ( 10.1088/1742-6596/274/1/012086) [DOI] [Google Scholar]
- 21.Mothé G, Castro M, Sthel M, Lima G, Brasil L, Campos L, Rocha A, Vargas H. 2010. Detection of greenhouse gas precursors from diesel engines using electrochemical and photoacoustic sensors. Sensors 10, 9726–9741. ( 10.3390/s101109726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt K, Müller A, Huber J, Busch S, Wöllenstein J. 2011. Compact photoacoustic gas sensor based on broadband IR source. Procedia Eng. 25, 1081–1084. ( 10.1016/j.proeng.2011.12.266) [DOI] [Google Scholar]
- 23.Nägele M, Sigrist M. 2000. Mobile laser spectrometer with novel resonant multipass photoacoustic cell for trace-gas sensing. Appl. Phys. B 70, 895–901. ( 10.1007/PL00021151) [DOI] [Google Scholar]
- 24.Sensor Sense. 2014. Solutions for trace gas detection. See http://www.sensor-sense.nl/images/stories/downloads/datasheet%20etd-300.pdf
- 25.Gasera Ltd. 2014. F10—Photo acoustic multi-gas analyzer, datasheet. Turkun, Finland. See http://www.gasera.fi/uploads/Brochures/gasera_brochure_F10_4pages_13032013_web.pdf
- 26.Esser B, Schnorr JM, Swager TM. 2012. selective detection of ethylene gas using carbon nanotube-based devices: utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 51, 5752–5756. ( 10.1002/anie.201201042) [DOI] [PubMed] [Google Scholar]
- 27.Swager TM, Esser B, Schnorr JM. 2013. Ethylene sensor. US Patent Application 13/832,430. [Google Scholar]
- 28.Zevenbergen MA, Wouters D, Dam V-AT, Brongersma SH, Crego-Calama M. 2011. Electrochemical sensing of ethylene employing a thin ionic-liquid layer. Anal. Chem. 83, 6300–6307. ( 10.1021/ac2009756) [DOI] [PubMed] [Google Scholar]
- 29.Agarwal M, Balachandran MD, Shrestha S, Varahramyan K. 2012. SnO2 nanoparticle-based passive capacitive sensor for ethylene detection. J. Nanomater. 2012, 145406 ( 10.1155/2012/145406) [DOI] [Google Scholar]
- 30.Shekarriz R, Allen W. 2008. Nanoporous gold electrocatalysis for ethylene monitoring and control. Eur. J. Hort. Sci. 73, 171–176. [Google Scholar]
- 31.Blanke MM, Shekarriz R. 2012. gold nanoparticles and sensor technology for sensitive ethylene detection. Acta Hort. (ISHS) 934, 255–262. [Google Scholar]
- 32.ABSOGER. Ethylene analyser. Les Barthes, France. See http://www.absoger-controlled-atmosphere-nitrogen-generator.com/fruit/fruit_contact.php?lang=1.
- 33.Sklorz A, Janssen S, Lang W. 2013. Application of a miniaturised packed gas chromatography column and a SnO2 gas detector for analysis of low molecular weight hydrocarbons with focus on ethylene detection. Sens. Actuators B 180, 43–49. ( 10.1016/j.snb.2011.12.110) [DOI] [Google Scholar]
- 34.Janssen S, Tessmann T, Niessen M, Sklorz A, Lang W. 2013. Large-capacity-on-chip preconcentrator device for selective ethylene measurement below 400 PPBV. In 17th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (Transducers and Eurosensors XXVII) 2771–2774. ( 10.1109/Transducers.2013.6627380) [DOI] [Google Scholar]
- 35.Janssen S, Lang W. In press High sensitive and selective ethylene measurement by using a large-capacity-on-chip preconcentrator device. Sens. Actuators B ( 10.1016/j.snb.2014.02.001) [DOI] [Google Scholar]
- 36.Terry SC, Jerman JH, Angell JB. 1979. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Dev. 26, 1880–1886. ( 10.1109/T-ED.1979.19791) [DOI] [Google Scholar]
- 37.Kim SK, Chang H, Zellers ET. 2011. Microfabricated gas chromatograph for the selective determination of trichloroethylene vapor at sub-parts-per-billion concentrations in complex mixtures. Anal. Chem. 83, 7198–7206. ( 10.1021/ac201788q) [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Cui D, Chen X, Zhang L, Cai H, Li H. 2013. Fabrication and characterization of MEMS-based gas chromatography column with embedded micro-posts for separation of environmental carcinogens. J. Chromatogr. A 1291, 122–128. ( 10.1016/j.chroma.2013.03.022) [DOI] [PubMed] [Google Scholar]
- 39.Dow ABA, Sklorz A, Lang W. 2011. A microfluidic preconcentrator for enhanced monitoring of ethylene gas. Sens. Actuators A 167, 226–230. ( 10.1016/j.sna.2011.01.019) [DOI] [Google Scholar]
- 40.Lin Y-S, Kuo C-Y, Tian W-C, Wu T-H, Sheen H-J, Kuo H-Y, Lu C-J. 2013. Batch fabrication of micro preconcentrator with thin film microheater using Tollen's reaction. In 17th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (Transducers and Eurosensors XXVII) 2025–2028. ( 10.1109/Transducers.2013.6627195) [DOI] [Google Scholar]
- 41.Tian W-C, Chan HK, Lu C-J, Pang SW, Zellers ET. 2005. Multiple-stage microfabricated preconcentrator-focuser for micro gas chromatography system. Microelectromech. Syst. J. 14, 498–507. ( 10.1109/JMEMS.2005.844842) [DOI] [Google Scholar]
- 42.Jedermann R, Pötsch T, Lloyd C. 2014. Communication techniques and challenges for wireless food quality monitoring. Phil. Trans. R. Soc. A 372, 20130304 ( 10.1098/rsta.2013.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airmet Scientific. The Explorer portable gas chromatograph, datasheet, Melbourne, Autralia. See http://www.airmet.com.au/Product/The-Explorer-Portable-Gas-Chromatograph.aspx.
- 44.HITRAN. 2014. Database. See http://www.cfa.harvard.edu/hitran/.
- 45.Fonollosa J, Halford B, Fonseca L, Santander J, Udina S, Moreno M, Hildenbrand J, Wöllenstein J, Marco S. 2009. Ethylene optical spectrometer for apple ripening monitoring in controlled atmosphere store-houses. Sens. Actuators B 136, 546–554. ( 10.1016/j.snb.2008.12.015) [DOI] [Google Scholar]
- 46.Hildenbrand J, et al. 2008. A compact optical multichannel system for ethylene monitoring. Microsyst. Technol. 14, 637–644. ( 10.1007/s00542-007-0475-1) [DOI] [Google Scholar]
- 47.Fonseca L, et al. 2004. Feasibility of a flip-chip approach to integrate an IR filter and an IR detector in a future gas detection cell. Microsyst. Technol. 10, 382–386. ( 10.1007/BF02637108) [DOI] [Google Scholar]
- 48.Hartwig S, et al. 2005. A highly sensitive IR-optical sensor for ethylene-monitoring. In Microtechnologies for the New Millennium 2005, Seville, Spain, 9–11 May 2005, pp. 452–460. International Society for Optics and Photonics. [Google Scholar]
- 49.Pouchert CJ. 1997. The Aldrich library of FT-IR spectra. Milwaukee, WI: Aldrich. [Google Scholar]
- 50.SmartGAS. 2012. Datasheet smart MODUL for ethylene. See www.smartgas.eu. [Google Scholar]
- 51.SmartGAS. 2013. Silarex, the revolution in gas measurement by SmartGAS. See http://www.silarex.com/index.php.
- 52.General Monitors. 2013. Datasheet IR400 combustible ethylene gas detector. See http://s7d9.scene7.com/is/content/minesafetyappliances/IR400%20Data%20Sheet
- 53.Sklorz A, Miyashita N, Schafer A, Lang W. 2010. Low level ethylene detection using preconcentrator/sensor combinations. In Proc. IEEE Sensors 2010 2494–2499. ( 10.1109/ICSENS.2010.5690105) [DOI] [Google Scholar]
- 54.Sklorz A, Schafer A, Lang W. 2012. Merging ethylene NDIR gas sensors with preconcentrator-devices for sensitivity enhancement. Sens. Actuators B 170, 21–27. ( 10.1016/j.snb.2010.11.049) [DOI] [Google Scholar]


