Abstract
There is strong evidence that neonates imitate previously unseen behaviours. These behaviours are predominantly used in social interactions, demonstrating neonates' ability and motivation to engage with others. Research on neonatal imitation can provide a wealth of information about the early mirror neuron system (MNS), namely its functional characteristics, its plasticity from birth and its relation to skills later in development. Although numerous studies document the existence of neonatal imitation in the laboratory, little is known about its natural occurrence during parent–infant interactions and its plasticity as a consequence of experience. We review these critical aspects of imitation, which we argue are necessary for understanding the early action–perception system. We address common criticisms and misunderstandings about neonatal imitation and discuss methodological differences among studies. Recent work reveals that individual differences in neonatal imitation positively correlate with later social, cognitive and motor development. We propose that such variation in neonatal imitation could reflect important individual differences of the MNS. Although postnatal experience is not necessary for imitation, we present evidence that neonatal imitation is influenced by experience in the first week of life.
Keywords: neonatal imitation, newborn, social development, mother–infant interaction, mu suppression, sensorimotor
1. Introduction
In the past few decades, human and non-human primate research has brought great insights into our understanding of the brain mechanisms that connect action and perception, and such work has begun to illuminate the nature of how these mechanisms support important cognitive processes and behaviours [1,2]. In particular, parietal-frontal circuits support several functions, such as space and object coding, action recognition and imitation [3–5]. Neurophysiological experiments on mirror neurons in monkeys demonstrate that even at the single cell level, sensory information is processed and translated into a motor format, thus facilitating the coupling between sensory and motor codes. Such studies have contributed to our understanding of how social interactions depend on mirroring mechanisms embedded in parietal-premotor circuits. According to the mirror neuron hypothesis, observed actions are understood in terms of one's own action programmes. This action–perception system allows individuals to understand others' actions as if they were performing those same actions themselves. (It is necessarily the case that, in order for an individual to be capable of reproducing (imitating) an action, that action must be in the individual's motor repertoire.) In fact, several brain imaging experiments in human adults have revealed that the mirror neuron system (MNS) is activated during the observation and imitation of simple and complex actions [6–8].
These issues have also been explored in infant development using less invasive techniques, such as electroencephalography (EEG). EEG studies reveal that during the execution and observation of actions, specific frequency bands within the alpha range (9–13 Hz in the adult and 5–9 Hz in infants) desynchronize in newborns [9–12] and older infants [13–15]. This suppression, termed the mu rhythm, is associated with the activation of mirror neuron areas (i.e. inferior frontal gyrus, ventral premotor cortex, posterior parietal lobe) [16] and thus may be considered a marker for mirror neuron activity.
One research arena that is particularly well suited for investigating fundamental characteristics of the mirror mechanism is that of early imitation. Recent work has addressed this issue in an EEG study of newborn macaques [17]. This study revealed that the mu rhythm desynchronizes during the observation and imitation of facial gestures such as lipsmacking (LPS), an important communicative gesture in macaques. The mirror neuron mechanism, therefore, may be the basis for human and non-human primate infants’ capacities to respond appropriately to their mothers and to tune their own behaviour with that of their mothers’ through elaborate face-to-face communicative signals and matching behaviours. Indeed, infants recognize and respond to social signals from birth, and are born with the ability to engage in social interactions. Newborns’ early imitative capacities, insofar as they indicate a functioning MNS, can be informative about the early development of this system, including its innateness, plasticity and individual differences.
In this paper, we assess the current understanding of early sensorimotor development in human and non-human primate infants, focusing on the evidence for an action–perception and mirroring mechanism operating at birth [17–20], instantiated in neonatal imitation. Neonatal imitation refers to the ability of infants to match others’ actions in the first four weeks of life. We argue that complementary behavioural and neural studies are necessary for understanding the early functioning and developmental changes of the MNS. In this review, we examine the evidence for the phenomenon of neonatal imitation, in both experimental and natural contexts, addressing common criticisms and proposing best practice procedures for eliciting imitation in the laboratory. We examine whether early individual differences in experience (e.g. culture) influence infants’ imitation and whether individual differences in imitation are related to later developmental outcomes.
2. Historical and recent observations of neonatal imitation
Human infant imitation has been studied for almost a century [21–23]. Early reports were primarily anecdotal or uncontrolled observations [22,24,25]. Maratos found that one-month-olds imitated tongue protrusion (TP), mouth opening (MO) and head shaking [26,27]. Imitation in newborns was subsequently confirmed by Meltzoff & Moore [28,29], in their seminal, well-controlled experiments, and thereafter found in infants as young as 45 min after birth [29,30]. Importantly, Meltzoff and co-workers demonstrated that infants could identify the particular body part producing the modelled action as well as the particular action pattern of that body part [28,31,32]. In addition to facial imitation, neonates only 3–96 h old also appear to imitate finger movements [33,34]. These studies, and others (table 1), provide strong evidence that neonatal imitation is present from birth. This evidence suggests newborns are capable of perceptual–motor coordination and cross-modal matching (i.e. matching the visual perception of the model with the proprioceptive experience of performing the action themselves) as well as demonstrating that newborns already possess complex social and cognitive skills.
Table 1.
Published studies of neonatal imitation. Criteria for inclusion: tested primate infants under 28 days of age, used a structured paradigm (predetermined demonstration/response frequency/length), dynamic actions were visually demonstrated with a live model (sound imitation and imitation from videos were excluded), study is published in English (or an English translation is available), and the test was carried out with at least five infants (no case studies). Sample size refers to the number of infants who produced usable data for one or more conditions, and the number of infants excluded is in parentheses. NR, not reported (for this specific age group). Actions modelled by unfamiliar individuals, unless otherwise indicated. TP, tongue protrusion; MO, mouth opening; LPS, lipsmacking; SFM, sequential finger movement; IFP, index finger protrusion; ID, infant-determined (length varied across individuals); rounds, the number of times the demonstration period was presented. Results are as interpreted by the authors of each study; +/−, positive/ negative results. Studies are arranged by infant age (with younger infants at the top of the table) and species (humans listed first).
| study | sample size | age | actions | demonstration | response period (s) | rounds | results |
|---|---|---|---|---|---|---|---|
| Kugiumutzakis, studies I–III [30] | 121 (NR) | 10–45 min | TP, MOa | 3–19 s | 10 | 5 | + |
| Kugiumutzakis, study IV [30] (same data in [35]) | 49 (NR) | 14–42 min | TP, MO, eyes open/closea | 3–19 s | 10 | 5 | + |
| Reissland [36] | 12 (0) | <1 h | lips widening, lip pursinga | 35–155 s | none | 4–14 | + |
| Meltzoff & Moore [29] | 40 (67) | M = 32 h | MO, TPa | 20 s | 20 | 12 | + |
| Field et al. [37] | 96 (NR) | 35–42 h | happiness, sadness, surprisea | ID habituation | none | ≥1 (ID) | + |
| Field et al. [38] | 74 (NR) | M = 36 h | happiness, sadness, surprisea | ID habituation | none | ≥1 (ID) | + |
| Kaitz et al. [39] | 26 (58) | 10–51 h | TP, happiness, sadness, surprise | ID habituation | none | 1 | + for TP |
| Meltzoff & Moore [40] | 40 (53) | 13–67 h | TP, head movementa | 20 s | 20 | 2 | + |
| Nagy et al. [33,34] | 39 (4) | 3–96 h | IFP | length NR | M = 50 | 25 | + |
| Anisfeld et al. [41] | 83 (103) | 40 h | TP, MO | 20 s | 20 | 4 | + for TP |
| Vinter, study I [42] | 16 (NR) | 2–5 days | TP, hand opening/closinga | 15 s | 25 | 4 | + |
| Nagy et al. [43] | 115 (6) | 1–5 days | TP | length NR | ID; approx. 50 | ID | + |
| Heimann et al., study I [44,45] | 23 (9) | 2–3 days | TP, MO, LPS | ID; M = 38 s | 60 | 1 | + for TP |
| Koepke et al., study I [46] | 6 (5) | 14–16 days | TP, lip protrusion, MO, SFM | 15 s | 20 | 1 | − |
| Koepke et al., study II [46] | 14 (9) | 17–21 days | TP, MO | 15 s | 150 | 1 | − |
| Lewis & Sullivan [47] | 14 (6) | 2 wks | MO, TP, arm wave, SFM | 10 s | 10 | 3 | − |
| Hayes & Watson, study I [48] | 11 (32) | 17–20 days | TP, MO | 15 s | 150 | 1 | − |
| Hayes & Watson, study II [48] | 16 (39) | 17–22 days | TP, MO | ≥ 15 s | 150 | 1 | − |
| Fontaine [49] | 12 (NR) | 21–33 days | TP, MO, cheeks swelling, eyes open/close, hand open/close, IFP | 20 s | 30 | 2 | − |
| Heimann et al., study II [44,45] | 23 (9) | 3 weeks | TP, MO | ID; M = 38 s | 60 | 1 | + for TP |
| McKenzi & Over [50] | 14 (NR) | 9–30 days | MO, TP, hand to face, hand to midline | 15 s | 20 | 1 | − |
| Meltzoff & Moore, study I [28] | 6 (NR) | 12–17 days | TP, MO, lip protrusion, SFMa | 15 s | 20 | ≤3 | + |
| Meltzoff & Moore, study II [28] | 12 (NR) | 16–21 days | TP, MOa | 15 s | 150 | 1 | + |
| Heimann & Schalller [51] | 11 (17) | 14–21 days | mother modelled: MO, TP | 15–20 s | 60 | 1 | + for TP |
| Bard, study I [52]b | 5 (0) | 7–15 days | TP, MO | 20 s | 20 | 6 | + for MO |
| Ferrari et al. [18]c | 21 (0) | 1–14 days | MO, LPS, TP, hand open/close, eyes open/closea | 20 s | 20 | 1 | + for LPS & TP |
| Paukner et al. [53]c (includes some [54] data) | 60 (0) | 1–8 days | LPS, TP | 20 s | 20 | 3 | + for LPS |
| Ferrari et al. [54] (includes [18] data)c | 41 (NR) | 1–8 days | LPS, TP | 20 s | 20 | 3 | + |
aIndicates action-specificity, in which positive results indicate greater imitation in the modelled action relative to non-modelled/control action(s).
Species is human unless otherwise indicated (bchimpanzee; cmacaque).
Neonatal imitation has also been observed in non-human primates, including chimpanzees [52,55], and rhesus macaques [18]. In fact, the phenomenon appears very similar in humans and macaques [56]. In both species, neonatal imitation of facial gestures is elicited in the laboratory most easily in the first few weeks after birth (compared with later in development), and mothers imitate facial gestures of infants more than infants imitate mothers. Additionally, in both species, there are large individual differences in imitative skills, that is, some infants consistently imitate while others do not, which may be a reflection of infants’ social predispositions [57–59]. Although not yet tested in humans, recent work demonstrates that macaque newborns recognize when others imitate them [60], suggesting action observation and execution are intricately linked.
Laboratory-based experimental investigations are, of course, limited in their ecological validity, as they only show what infants are capable of imitating in a somewhat artificial environment. Experimental control of the model (e.g. producing a passive face, gesturing on a fixed schedule, displaying more than one action to be imitated) may reduce imitation rates, creating situations rather different from natural face-to-face carer–infant interactions [52,61]. After all, imitation is both a cognitive and a social phenomenon [27], so not exhibiting socially appropriate behaviours may decrease infants’ motivation to engage. Complementary approaches include observing infants in less structured neonatal imitation paradigms (e.g. allowing models to adjust the timing or type of response as a function of infants’ responses [52,61]), and observing infants in natural interaction settings, such as mother–infant face-to-face play. The latter in particular can shed light on what infants actually do during typical social interactions with carers [62–65], and reveals the types of behaviours infants naturally imitate, how often they do so, and how parents contribute to this skill.
Human mothers engage in complex, emotional, two-way face-to-face exchanges with their newborns, including mutual gaze and body contact (e.g. hand–body contact, kisses), and exaggerated maternal facial and vocal expressions [63,65,66]. There is a fundamental motivation on the part of both the parents and newborns to be in social engagement with each other, reflected in their preferential responses to faces and eye contact [67–73]. Even neonates show myriad facial expressions and gestures when in face-to-face contact. These include different facial expressions of emotion, lip and tongue movements, and active shaping of the mouth, which are unconnected to clearly internal ‘biological’ events (e.g. digestion; [74]). This expressiveness provides a rich corpus of behaviours that helps adults understand the nature of infant needs and experience. Mothers are sensitive to neonates’ rare moments of alertness, and although such times are infrequent (15–20% of time observed), mothers choose them to socially engage with infants, otherwise providing relatively little social stimulation [75]. Human mothers initiate active engagements with clear ‘greeting’ and ‘marking’ behaviours, and also imitate infants’ expressions, including vocal and facial expressions, immediately after birth and in the first months of life [76–78]. Similar mother–infant interactions also occur in rhesus macaques [79] and gelada baboons [80]. For example, macaque mothers direct LPS—an affiliative facial gesture—at their infants, often in an exaggerated fashion (similar to human motherese), and while doing so, mothers place themselves directly in front of the infant, often lowering themselves to infants’ eye level and engaging in bouts of head bobbing [79].
It is interesting to note, however, that very few reports have investigated the natural occurrence of neonatal imitation [81–83]. From these few studies, it seems that human neonates themselves only rarely spontaneously imitate during interactions with parents. This observation is not surprising considering that newborns spend most of their time sleeping and, when awake, face-to-face interaction episodes are brief. We should also consider that, during interpersonal exchanges, imitation represents only one of many ways newborns can express themselves [74]. Thus, it is not imitation by the neonate per se that is critical for communication and social understanding, but a more fundamental capacity that infants’ occasional imitation reveals: that is, the capacity to connect one's own and another's actions and experience [83].
3. Why some laboratories have not found neonatal imitation at the population level
Neonatal imitation is a difficult behaviour to observe in the laboratory, as evidenced by some inconsistent findings [84–86], consequently, the phenomenon is not unanimously accepted. Experimental tests of neonatal imitation in humans have used a variety of procedures, modelled actions, inclusion criteria and operational definitions of imitation (see reviews [32,43,87,88]) and, it is not, therefore, surprising that results have varied across studies. Although methodological differences may account for different results [51], there has been only one previous systematic report, to the best of our knowledge, comparing successful and unsuccessful methods, specifically focused on TP imitation [43]. Numerous factors influence imitation, including the position of the infant [43], the length of response period [29] and infants’ age [43]. Out of 29 published studies of imitation in the first month of life (table 1), seven failed to find evidence of imitation (from five laboratories), and 21 found evidence of imitation (from 11 laboratories). It is instructive to consider the differences between studies that found evidence of imitation and those that did not.
One common feature of several studies reporting null results for facial gesture imitation is that infants were prevented from gesturing concurrently with the adult model through the use of a pacifier [46,48]. Pacifiers were used to block infants’ immediate facial mimicry to test delayed imitation [28], to rule out perceptual–motor resonance as an explanation for imitation [89,90], or to prevent the model from unintentionally imitating the infant [28,49]. In fact, concurrent interaction synchrony plays an important role in early parent–infant interactions [91], and infants who do not experience these synchronous interactions—such as when prevented with pacifiers—may be less likely to match facial gestures during still face (i.e. response) periods. Actual imitation rates may also be underestimated due to a related issue: that is, in some studies, researchers did not measure infants’ gestures produced during the gesture/dynamic stimulus period [49]. We think this omission may have limited infants’ opportunities for imitation, given that much of infants’ matching behaviour may occur during this dynamic period.
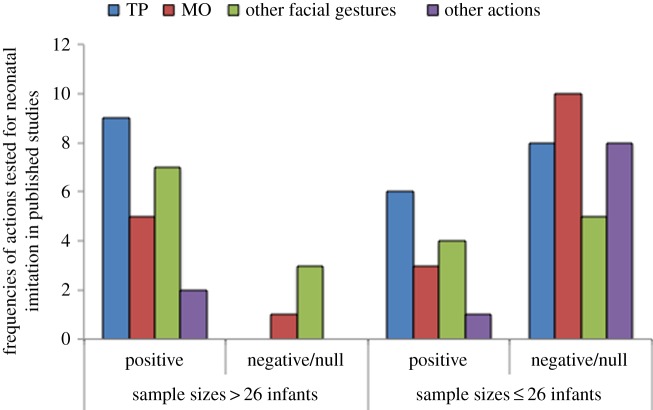
A second feature common among studies reporting null results is low statistical power resulting from small sample sizes (average number of usable participants: 12; range: 6–16 participants), relative to those reporting positive results (average number of usable participants: 43; range 6–121 participants), a point highlighted by others [29,43]. Of those studies with sample sizes larger than 26 infants (determined to be a necessary sample size, based on an a priori power analysis, reported below), the vast majority found positive results, whereas studies including 26 or fewer infants contribute the most to the ‘failures to replicate’, illustrated in figure 1. Thus, among the studies reported in table 1, over 85% of the behaviours examined in those with large sample sizes (ns ≥ 26) revealed positive results (i.e. evidence of neonatal imitation), whereas in studies with smaller sample sizes (ns < 26), 69% of behaviours tested failed to show any evidence of imitation. This result may explain why previous reviews, which did not consider sample size as a factor contributing to the reliability of a study's findings’ (e.g. see table 1 in [87]; see fig. 2 in [92] and see table 1 in [88]), have drawn different conclusions concerning the phenomenon of neonatal imitation. In the following, we discuss effect sizes found in neonatal imitation studies and suggest the sample sizes necessary to detect those effects.
Figure 1.
Among published studies of neonatal imitation in humans, across a variety of facial and other actions (shown here: tongue protrusion, TP; mouth opening, MO; other facial gestures or other actions), sample size is a good predictor of whether the study found positive results (i.e. evidence of imitation) or negative/null results. We carried out an a priori power analysis to determine the sample size necessary for power = 0.80 (f = 0.40; α = 0.05) to detect this effect and determined a sample size of 26 is needed. The ‘frequencies of actions’ axis label refers to the number of modelled actions that were tested, both within and between studies. For example, nine studies with samples sizes >26 tested TP and found positive results, whereas six studies tested MO and, of these, five found positive results. (Online version in colour.)
4. Core questions and misunderstandings about neonatal imitation
(a). Is neonatal imitation a reflex?
It has been suggested that neonatal imitation is not actually imitation, but instead may be an automatic and involuntary reflex-like phenomenon, driven by subcortical mechanisms, a fixed action pattern or an innate releasing mechanism [39,46,48,50,93,94]. According to this view, matching should occur for only a few evolutionarily privileged gestures, that is, gestures that are, putatively, fixed and stereotypic, and produce a matching response that is time-locked to the modelled ‘trigger’ action [95]. This prediction, however, has been tested and has not been supported: infants produce a range of gestures which are not stereotyped, actions which have never been seen before are matched, corrections are made to initial attempts, and responses are not time-locked to modelled actions [31,32,40]. In addition, infants produce gestures without prompt after a delay, suggesting they are initiating social interaction rather than simply copying actions [96]. In humans, so-called deferred imitation is present (after a 24 h delay) from at least six weeks of life [31,97], and in some macaque infants, it is present (after a 60 s delay) in the first week of life [53], which indicates that these gestures are communicative and under voluntary control rather than reflexive fixed action patterns.
(b). Is neonatal imitation due to arousal?
Infants might be aroused when they view facial gestures and consequently increase their activity (e.g. produce more facial gestures themselves [98,99]). However, even if this point is accepted, infants’ capacity to match specific gestures goes beyond this general arousal response, reflecting additional neurophysiological and cognitive mechanisms. Numerous neonatal imitation tests have measured infants’ imitation of more than one action, and in these cases, arousal alone cannot account for infants’ imitation of specific actions [28,40]. Nagy et al. [43] also recently performed a thorough review of neonatal imitation of TP gestures (the gesture most commonly assumed to be produced by arousal) by assessing the specificity of the imitative response and measuring infants’ states [100] as well as other indicators of arousal, and concluded that TP imitation is not simply an arousal effect. In addition, newborns’ heart rates accelerate when imitating gestures and decelerate when performing unprompted gestures [96], suggesting that different mechanisms underlie imitative and exploratory spontaneous behaviours.
(c). Does imitation decline after the first month of life?
Given reports that imitation appears strong in the first month of life, but then declines in the following months [27,49,35,44,93], it has been suggested that early imitation may be a phenomenon quite distinct from imitation occurring later [58]. Neonatal imitation has been proposed to be a ‘transient ontogenetic adaptation’, important for survival in early infancy but then disappearing when no longer necessary [101, p. 89]. While it is true that the form and characteristics of imitation undergo changes throughout infancy, this particular characterization is misleading. Instead, careful testing has revealed that imitation does not decline after the first month of life, but depends on the type of action being presented. For example, facial imitation (e.g. TP, MO, emotional facial expressions) largely disappears by three months of age [49,93,94,102], whereas other actions (e.g. sounds, vocalizations, hand and finger movements) increase in frequency and accuracy [103,104], in line with the infants’ wider development (e.g. improvements in vision at a distance and manipulation skills). Interestingly, behaviours reliably imitated earlier in development can also be elicited later on if the social context is altered, for example, if presented in the context of games or playful interactions, or if the actions form part of a sequence requiring novel combinations [105]. Apparent declines in imitation in the laboratory setting may be due, therefore, to these wider changes in infants’ expectations and motivations during social interactions [97,106].
(d). Does neonatal imitation depend on learning?
Infants may learn to associate their own movements with those of others, and thus acquire the capacity to imitate through a process of associative learning [87,107]. While experience, including associative processes, undoubtedly plays a role in developing the corpus of behaviours that infants imitate (see §7), an associative learning account of the fundamental capacity to imitate is incompatible with the evidence on two fronts. First, only minutes to hours after birth, human infants imitate opening and closing of eyes [30,35], head movements [40], the /a/ sound [30,35], index finger protrusion [33,34], facial gestures (e.g. MO, TP; [29,40]) and emotional facial expressions (e.g. happiness, sadness, surprise [38]) prior to having opportunities to form strong associative links between action observation and imitative responses. Similarly, macaque infants reared in a nursery from birth imitate before they have experienced any contingent facial interactions with carers [18,53,108], and they additionally show specific electroencephalogram changes (i.e. mu suppression), evidence of a functioning MNS, on the day of birth [17,109]. These results fail to support an associative learning account of neonatal imitation [110,111].
Even setting aside such evidence, the associative learning account is problematic on a second front, because, for the proposed learned associations to be forged, it would require the neonate to experience high levels of contingent responses from social partners that are almost exclusively imitative. In fact, while parents do indeed provide imitative feedback during social interactions with their infants, the rate is typically quite low (e.g. one per 2–3 min [62]) and, moreover, such feedback occurs in the context of a wealth of parental behaviours that are non-imitative (e.g. affirmative marking, or even negating of infant expressions [112]). On a rigorous calculation of contingency [113], parents’ imitative responses are, therefore, relatively non-salient for the infant. According to the associative learning account, this situation then leaves infants with the challenge of identifying which particular adult gestures or expressions among this plethora match their own, a task that may be cognitively equivalent to that of the production of imitative acts themselves. In short, an associative learning account does not so much solve the problem of imitation, as raise a set of further questions concerning the basis of infant capacities for identifying the equivalence between their own and others’ actions.
5. Methodological differences across neonatal imitation studies
Standardizing the methodology for neonatal imitation tests would allow experimenters to more easily compare imitation across groups (e.g. species, cultures, special populations). We therefore propose a set of ‘best practices’ for testing neonatal imitation, which serves to facilitate the elicitation of the phenomenon.
(a). Sensitivity to infants’ states
Sensitivity to infants’ states is critical for maximizing the likelihood of neonatal imitation. Ideally, the test room should be quiet with few distractions (such as sounds or bright visual displays). Very young newborns or infants waking after sleeping may need time to adjust to the lighting of the room. Infants should be adequately fed and relatively awake before testing commences. In addition, infants should be seated or laying, and may need to be adjusted to maximize their comfort [30]. Infants should be attentive (i.e. looking at the model) for at least part of the time the model is performing the gestures. Infants who insist on sucking their thumbs may be excluded when facial gestures are modelled, or, ideally, thumb sucking could be coded and included in the analysis to determine whether it confounds or moderates imitation. If the attention criterion is not met, then infants should be excluded from data analysis, although, obviously, the number of infants and reason for exclusion should be clearly reported.
(b). Appropriately modelled actions
For standardization purposes, models should be unfamiliar to the infant (unless specific effects of the mother or caretaker are being investigated; [51,61,114]) and should avoid interacting with the infant before testing [29]. Models should be positioned at an appropriate distance, taking into account newborns’ reduced visual acuity, and should make continuous eye contact with infants for the duration of the test. Non-verbal cues such as eye contact set-up an expectation of a social exchange, and may direct infants’ attention towards the adults’ modelled actions [115]. There is disagreement about what constitutes adequate speed, rhythm and repetition of action presentation, so these aspects should be clearly documented. One critical aspect of the procedure is the length of time the gesture is modelled. In a review of TP studies, modelling the gesture for 60 s or longer resulted in evidence of imitation in all reported studies, whereas modelling the gesture for 40 s or less resulted in only 31% of studies finding evidence of imitation [84]. Therefore, we recommend a minimum of 60 s of presenting modelled gestures. Modelled behaviours should be age-appropriate, prominent in the infant's expressive repertoire and structured at a predetermined frequency and speed, so all infants view the same actions. We also recommend modelling actions in a ‘burst–pause’ procedure, whereby the model alternates between static and dynamic periods, as this procedure—compared with modelling only dynamic actions—results in higher frequencies of imitation [29].
(c). Time frame for recording responses
At times, infants will imitate quickly [39], or even concurrently with the models’ actions [116], and these instances of imitation should be recorded as such. On other occasions, imitation may be delayed, and thus, after the modelled actions, the model should be still and wait for a predetermined period, allowing the infant to produce or finish producing a response. A microanalysis of infants’ imitation revealed that infants can take some time before they start to respond (e.g. 20–60 s [45]), and they may gradually refine and correct their responses (e.g. during a 2.5 min response period [31]), so sufficient time must be provided for infants to initiate, refine and complete their response. In addition, it is important that the length of this response period be predetermined and not based on infants’ behaviours [35], as this may introduce a bias for gestures produced spontaneously [48].
(d). More than one action to show specificity of response
More than one behaviour should be presented in order to show that the imitative response is not due to an infant's preference for a certain action (e.g. facial gesture) or a more general response to a moving social stimulus, and to decrease the probability of false-positives. The frequency of matched actions produced in the matching action condition should be higher than those in the non-matching (i.e. social control) action condition. For example, the frequency of infants’ TP when TP is modelled should be higher than the frequency of infants’ TP when MO is modelled, and vice versa [28]. Because some studies have suggested that infants may associate specific individuals with specific facial gestures [31], ideally, each action should be modelled by a different individual, and each action's test session should be separated by a break period in order to avoid carry-over effects across sessions.
(e). Testing for individual differences
For certain purposes, it may be useful to categorize infants based on whether or not they consistently and successfully imitate. In such cases, the definition of imitator should include consideration of imitation across test sessions. Ideally, infants should be tested multiple times within the same day (in different test sessions to avoid carry-over effects) or across days with the same gestures; infants should consistently imitate (i.e. imitate in the majority of sessions) to be defined as imitators.
(f). Sufficient power
We calculated effect sizes for neonatal imitation studies that have given sufficient detail necessary for such calculations [29,30,35,36,40–42,51], and found that among those actions analysed with parametric tests (10 actions), Cohen's d ranged from 0.34 (small) to 0.58 (medium), with a median of 0.40, and for studies that used non-parametric tests for analysis (nine actions), effect sizes (r) ranged from 0.37 (medium) to 3.75 (large), with a median of 0.64 (large). Using the most conservative estimate of effect size (d = 0.34), we carried out an a priori power analysis to determine the sample size necessary for power = 0.80 (f = 0.40; α = 0.05) to detect this effect and determined a sample size of 26 is needed [117]. Thus, like any study with infants, a relatively large sample is required to allow for small-to-medium effect sizes and potentially high dropout rates. Although it may be unnecessary for infants to complete all trials to be included, we think, at the very least, the number or proportion of unusable trials should be reported, along with reasons for excluding trials.
(g). Optional additional control conditions (static non-social baseline period and non-social comparison)
Infants’ actions produced after seeing the modelled gestures can additionally be compared to both a no-stimulation or static social baseline period (e.g. still face) and a non-social static and dynamic control condition (e.g. disc with both still and rotating periods), to guard against the possibility that the action in question may happen by chance or as a result of non-specific arousal. The non-social control stimulus should be matched to the social stimulus in its static and dynamic nature. To be classified as imitation, the model behaviour should increase in frequency relative to the baseline level, and should be more frequent in the test condition than in the non-social control condition. For example, in one study with five- to eight-week-old infants, TP and MO gestures were produced only when a social model (human face) produced the gestures, but not when inanimate objects produced similar movement patterns [118]. It is worth noting that the vast majority of studies fail to include this condition. Although its inclusion is not a necessary requirement for demonstrating neonatal imitation, it can increase the sensitivity of the test by allowing a subtraction of baseline rates across a more diverse collection of control conditions. This can be particularly useful for studies examining individual differences in imitative skills, as it offers a more sensitive test of imitation-specific action reproduction.
6. Neonatal imitation as a predictor of later developmental outcomes
A number of possibilities have been suggested for why some neonates imitate and others do not. Variability in recorded imitative performance may be due to error variance, methodological differences (as we described), or, perhaps most intriguingly, it may reflect genuine individual differences among infants. As we explain below, we think it may be useful to consider the extent to which these individual differences predict, or are related to, other behavioural outcomes. In particular, if some infants imitate because they possess a more responsive facial MNS, then other abilities that also rely on mirror neuron circuits (e.g. reaching–grasping, understanding goal-directed actions, emotion recognition) may be systematically related to early imitation. Indeed, many researchers argue that it is important to examine whether neonatal imitation is predictive of later social and cognitive development [44,45,58,103,119,120] because it could be an early marker of later deficits in social skills [57]. Previous studies suggest that in both humans and macaque monkeys, only about 50% of neonates consistently engage in imitation of facial gestures [53,54,121]. Only one study examined neonatal imitation predictively in human infants: imitation at three ages—2 to 3 days, three weeks and three months of age—predicted visual attention at three months of age. In particular, neonatal imitators had fewer looks away during a face-to-face interaction at three months of age compared with non-imitators [44,45]. In another recent study, female infants were found to imitate finger movements more than male infants [34], consistent with adult studies that demonstrate females have greater mu suppression when viewing actions [122,123].
Although correlational evidence should clearly be interpreted with caution, we have evidence that neonatal imitation skills in macaques are related to behaviours both within and outside of the neonatal imitation task. During neonatal imitation, macaque LPS imitators show increased visual attention to the faces of human social partners [108], are better at recognizing human social partners [59], and are better at remembering gestures and initiating social interactions after a delay (i.e. deferred imitation [53]). We also found that individual differences in neonatal imitation in macaques are positively correlated with later motor and social development. Specifically, infants who consistently imitate in the first week of life, compared with those who do not, show superior reaching–grasping abilities [54] and greater visual attention to the eyes between 10 and 28 days of age [57], suggesting links between neonatal imitation, intentional movements and general social attention capacities. By contrast, other individual characteristics of nursery macaques do not appear to be related to imitative skills, including infants’ body weight, gross motor maturity (e.g. muscle tone, response speed), the capacity to attend to visual stimuli or emotionality [54]. Together, these lines of evidence suggest that imitators may be advantaged in their voluntary motor and social-cognitive skills, compared with their non-imitative peers.
With regard to the wider implications of individual differences in imitation, although much can be learned from studying typically developing populations, as described above, the study of neonatal imitation in special populations may be particularly informative, especially in those with conditions associated with social deficits. For example, studies with human children have shown that imitation is impaired in children with autism spectrum disorders (ASDs), including oral–facial imitation [124,125] as well as immediate and deferred imitation of a variety of other actions [126,127]. We know of no work that has examined infants at high-risk for social deficits, such as siblings of children with ASD (who are therefore at higher risk for developing ASD), to see whether they exhibit neonatal imitation at the same levels as low-risk infants, or if failure to show neonatal imitation is associated with higher risk of a future diagnosis of ASD. We think that such high-risk infants, including siblings of children with an ASD diagnosis, would be particularly useful to study in this context, because it has been suggested that MNS dysfunction may be implicated in ASD [128], and information about the developmental emergence of this disorder could provide valuable insights. Notably, there is some work that suggests that these high-risk infants display lower levels of coherence in measures of mother–infant synchrony compared with low-risk infants at four months of age [129], which may be indicative of decreased social sensitivity and responsiveness at an early age prior to a clinical diagnosis.
7. Plasticity of neonatal imitation
Even though postnatal experience is not necessary for facial gesture imitation, neonatal imitation may nonetheless be influenced by experiences in the first weeks of life. Here, we describe studies that provide evidence of environmental influences on neonatal imitation, with nursery-reared newborn macaques, and discuss how, in humans, unique cultural influences may influence the types and frequencies of imitation.
To determine the influence of early face-to-face interactions on imitation, we randomly assigned nursery-reared macaque newborns to either receive exposure to facial gestures (n = 12), extra handling (n = 12) or standard rearing (n = 15). The exposure to facial gestures consisted of human carers engaging in face-to-face communicative exchanges using LPS gestures directed at infants in 5 min long sessions, four times a day, starting from the first day of life. In each session, a human carer directed LPS gestures at the infant for 5 s, followed by 10 s of eye contact, then a 15 s break period. This sequence was repeated 10 times in the 5 min session. Infants in the extra-handling group were held at the same times and for the same durations as the exposure group, but did not receive the face-to-face interactions (caretakers’ faces were covered so infants could not see them). Infants in the standard rearing group did not see facial gestures and did not receive any handling beyond basic care and other (non-related) experimental procedures. On day 7 or 8, infants were tested for neonatal imitation with two gestures—LPS and TP—that were compared with a non-social control condition, a rotating disc with orthogonal stripes (for methodological details, see [53,54]). We found that only infants who were exposed to facial gestures showed increased LPS in the LPS condition (baseline: M = 2.00, s.d. = 2.41; stimulus: M = 9.83, s.d. = 8.09), t11 = 4.03, p = 0.002, but not in the other two conditions (TP or control disc), ps > 0.05, which suggests that early social experience—such as being held, mutual gaze and/or early communicative exchanges—may improve imitation. In addition, our results with macaques are consistent with a number of findings in human infants concerning the role of experience. For example, infants improve their matching precision across days [29,31] and across trials [33,130], and human infants exposed to TP every day from six to 14 weeks of life show stronger TP imitation at 14 weeks [94]. Although speculative, we think evidence of plasticity in neonatal imitation, as documented here, suggests plasticity of action–perception mechanisms, likely mediated by the MNS. Further tests using measures of mu rhythm as a function of experiences in the first weeks of life are necessary to more directly measure changes in the MNS.
In addition to controlled manipulations of infants’ early experiences, some work has examined imitation in relation to the cultural variability in newborns’ environments. Despite the universality of key features of parent–infant interactions, there is also notable variation in the extent and manner of parental responsiveness to infant behaviours. This variation is particularly apparent when comparing cultures that differ in the conditions and value systems accompanying child care [131]. Some, such as the USA and many north European countries, place great value on infant individuation and independence; and parents tend to use high levels of facial and vocal expressiveness to respond to as well as imitate infant signals in face-to-face play. In turn, this style of responsiveness predicts earlier emergence of infant self-awareness (i.e. mirror recognition) [132]. Others cultures (e.g. Japanese and certain rural African societies) place more value on infant affiliation and compliance, and on sharing and cohesiveness within the society. These parents, although similarly responsive to their infants, pick-up on different infant cues, and are more likely to use close physical contact to respond to their infants (e.g. kissing, or rhythmical patting), and parents show far less vocal and facial imitation [133,134]. Correspondingly, infant behaviour during interactions in these diverse cultures develops in different ways. Thus, a study comparing Nso mothers and infants (a rural society in the Cameroon) with those in Germany found most German infants to increasingly imitate maternal smiles during face-to-face interactions over the first three months, a pattern that did not occur in Nso infants [134]. Such findings indicate that, based on infants’ fundamental capacities to identify correspondences between their own and others’ actions, particular forms of infant expressive behaviours emerge in the development of different cultural styles of social communication. We believe that cross-cultural examinations of neonatal imitation and its developmental consequences would be a particularly fruitful direction for future research.
8. Conclusion
We believe the study of neonatal behaviour and its plasticity are critical for understanding the developmental emergence of the MNS, and the development of action–perception more generally. Despite some reviews that conclude that neonatal imitation is not a genuine phenomenon [87,99,107], when full account is taken of procedural factors and considerations of statistical power, the evidence that imitation is present from birth is compelling.
The formation of an action–perception mechanism has been debated in the recent literature and some scholars propose that it is unlikely that a rudimentary mechanism that matches observed facial gestures with the internal motor representation could be operative from birth. Instead, it is proposed that general sensorimotor connections link temporal regions that visually code for others’ actions with parietal regions that are involved in executing actions. Further, in this account, these connections are refined through Hebbian learning processes, and become tuned, so that visual and motor information become matched in the course of development [92]. The evidence on neonatal imitation reviewed here, however, does not support this proposal, as it clearly shows that, prior to any experience, there is a link between seeing facial gestures and the motor programmes activating the same motor representations. Nevertheless, learning is not irrelevant to this process; indeed, it is likely to play an important role in shaping and refining such connections and, based on the surrounding social input, regulate the development of brain regions involved in early facial motor control and sensorimotor matching. Recent work using EEG to measure brain responses to facial gestures in newborn monkeys shows that despite their limited social experience (i.e. monkeys have been reared in a nursery from the day of birth), there is specific cortical desynchronization within the alpha band, i.e. mu rhythm, during the observation and imitation of facial gestures [17]. The mu rhythm has been hypothesized to be an important indirect index of the mirror mechanism [109]. The existence of the mu rhythm in newborn macaques responding during observed and executed facial gestures supports the hypothesis that a mirror mechanism operates at birth, and it may sustain early imitative responses. Variation in neonatal imitation may reflect individual differences in the MNS, aiding in the early detection of social deficits [57]. Together, these findings highlight the value of neonatal imitation as a behavioural measure of the MNS, providing a window into the early development of the action–perception system.
Acknowledgements
We thank Peter Cooper and Krisztina Varga Jakobsen for helpful comments on an earlier draft of this manuscript. Special thanks to all who helped collect the macaque neonatal imitation data presented in this paper, especially Valentina Sclafani.
Funding statement
This research was supported by the Division of Intramural Research, NICHD and NICHD P01HD064653.
References
- 1.Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. ( 10.1146/annurev.neuro.27.070203.144230) [DOI] [PubMed] [Google Scholar]
- 2.Ferrari PF, Bonini L, Fogassi L. 2009. From monkey mirror neurons to primate behaviours: possible ‘direct’ and ‘indirect’ pathways. Phil. Trans. R. Soc. B 364, 2311–2323. ( 10.1098/rstb.2009.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzolatti G, Luppino G. 2001. The cortical motor system. Neuron 31, 889–901. ( 10.1016/S0896-6273(01)00423-8) [DOI] [PubMed] [Google Scholar]
- 4.Rizzolatti G, Fogassi L, Gallese V. 2001. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2, 661–670. ( 10.1038/35090060) [DOI] [PubMed] [Google Scholar]
- 5.Iacoboni M. 2009. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 60, 653–670. ( 10.1146/annurev.psych.60.110707.163604) [DOI] [PubMed] [Google Scholar]
- 6.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. 1999. Cortical mechanisms of human imitation. Science 286, 2526–2528. ( 10.1126/science.286.5449.2526) [DOI] [PubMed] [Google Scholar]
- 7.Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. 2004. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 42, 323–334. ( 10.1016/S0896-6273(04)00181-3) [DOI] [PubMed] [Google Scholar]
- 8.Jackson PL, Meltzoff AN, Decety J. 2006. Neural circuits involved in imitation and perspective-taking. Neuroimage 31, 429–439. ( 10.1016/j.neuroimage.2005.11.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler K, Biermann-Ruben K, Jonas M, Roman SH, Bäumer T, Münchau A, Schnitzler A. 2006. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. Neuroimage 33, 227–238. ( 10.1016/j.neuroimage.2006.06.014) [DOI] [PubMed] [Google Scholar]
- 10.Lepage JF, Théoret H. 2006. EEG evidence for the presence of an action observation–execution matching system in children. Eur. J. Neurosci. 23, 2505–2510. ( 10.1111/j.1460-9568.2006.04769.x) [DOI] [PubMed] [Google Scholar]
- 11.Muthukumaraswamy SD, Johnson BW. 2004. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology 41, 152–156. ( 10.1046/j.1469-8986.2003.00129.x) [DOI] [PubMed] [Google Scholar]
- 12.Muthukumaraswamy SD, Johnson BW. 2004. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clin. Neurophysiol. 115, 1760–1766. ( 10.1016/j.clinph.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 13.Marshall PJ, Young T, Meltzoff AN. 2011. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Dev. Sci. 14, 474–480. ( 10.1111/j.1467-7687.2010.00991.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saby JN, Marshall PJ, Meltzoff AN. 2012. Neural correlates of being imitated: an EEG study in preverbal infants. Soc. Neurosci. 7, 650–661. ( 10.1080/17470919.2012.691429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall PJ, Meltzoff AN. 2011. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Dev. Cogn. Neurosci. 1, 110–123. ( 10.1016/j.dcn.2010.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. 2011. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 31, 14 243–14 249. ( 10.1523/JNEUROSCI.0963-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari PF, Vanderwert RE, Paukner A, Bower S, Suomi SJ, Fox NA. 2012. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cogn. Neurosci. 24, 1165–1172. ( 10.1162/jocn_a_00198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. 2006. Neonatal imitation in rhesus macaques. PLoS Biol. 4, e302 ( 10.1371/journal.pbio.0040302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casile A, Caggiano V, Ferrari PF. 2011. The mirror neuron system: a fresh view. Neuroscientist 17, 524–538. ( 10.1177/1073858410392239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepage JF, Théoret H. 2007. The mirror neuron system: grasping others’ actions from birth? Dev. Sci. 10, 513–523. ( 10.1111/j.1467-7687.2007.00631.x) [DOI] [PubMed] [Google Scholar]
- 21.Guillaume P. 1926. L'imitation chez l'enfant. Paris, France: Libr. Felix Alcan. [Engl. transl. 1971 Imitation in children. Chicago, IL: University of Chicago Press.] [Google Scholar]
- 22.Valentine CW. 1930. The psychology of imitation with special reference to early childhood. Brit. J. Psychol. Gen. Sect. 21, 105–132. ( 10.1111/j.2044-8295.1930.tb00580.x) [DOI] [Google Scholar]
- 23.Piaget J. 1935. Les théories de l'imitation. Cahiers pédagogie expérimentale et de psychologie de l'enfant 6, 1–13. [Google Scholar]
- 24.Gardner J, Gardner H. 1970. A note on selective imitation by a six-week-old infant. Child Dev. 41, 1209–1213. ( 10.2307/1127349) [DOI] [PubMed] [Google Scholar]
- 25.Zazzo R. 1957. Le problème de l'imitation chez le nouveau-né. Enfance 10, 135–142. ( 10.3406/enfan.1957.1350) [DOI] [Google Scholar]
- 26.Maratos O. 1973. The origin and development of imitation in the first six months of life. In Paper presented at the Annual Meeting of the British Psychological Society, April 1973, Liverpool Abstract available at http://files.eric.ed.gov/fulltext/ED096001.pdf. [Google Scholar]
- 27.Maratos O. 1982. Trends in the development of imitation in the first six months of life. In Intersubjective communication and emotion in ontogeny (ed. Bever TG.), pp. 81–101. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 28.Meltzoff AN, Moore MK. 1977. Imitation of facial and manual gestures by human neonates. Science 198, 75–78. ( 10.1126/science.198.4312.75) [DOI] [PubMed] [Google Scholar]
- 29.Meltzoff AN, Moore MK. 1983. Newborn infants imitate adult facial gestures. Child Dev. 54, 702–709. ( 10.2307/1130058) [DOI] [PubMed] [Google Scholar]
- 30.Kugiumutzakis G. 1998. Neonatal imitation in the intersubjective companion space. In Intersubjective communication and emotion in early ontogeny (ed. Braten S.), pp. 63–88. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Meltzoff AN, Moore MK. 1994. Imitation, memory, and the representation of persons. Infant Behav. Dev. 17, 83–99. ( 10.1016/0163-6383(94)90024-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meltzoff AN, Moore MK. 1997. Explaining facial imitation: a theoretical model. Early Dev. Parent. 6, 179–192. () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy E, Kompagne H, Orvos H, Pal A, Molnar P, Janszky I, Bardos G. 2005. Index finger movement imitation by human neonates: motivation, learning, and left-hand preference. Pediatr. Res. 58, 749–753. ( 10.1203/01.PDR.0000180570.28111.D9) [DOI] [PubMed] [Google Scholar]
- 34.Nagy E, Kompagne H, Orvos H, Pal A. 2007. Gender-related differences in neonatal imitation. Infant Child Dev. 16, 267–276. ( 10.1002/icd.497) [DOI] [Google Scholar]
- 35.Kugiumutzakis J. 1999. Genesis and development of early infant mimesis to facial and vocal models. In Imitation in infancy (eds Nadel J, Butterworth G.), pp. 36–59. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Reissland N. 1988. Neonatal imitation in the first hour of life: observation in rural Nepal. Dev. Psychol. 24, 464–469. ( 10.1037/0012-1649.24.4.464) [DOI] [Google Scholar]
- 37.Field TM, Woodson R, Cohen D, Greenberg R, Garcia R, Collins K. 1983. Discrimination and imitation of facial expressions by term and preterm neonates. Infant Behav. Dev. 6, 485–489. ( 10.1016/S0163-6383(83)90316-8) [DOI] [Google Scholar]
- 38.Field TM, Woodson R, Greenberg R, Cohen D. 1982. Discrimination and imitation of facial expressions by neonates. Science 418, 179–181. ( 10.1126/science.7123230) [DOI] [PubMed] [Google Scholar]
- 39.Kaitz M, Meschulach-Sarfaty O, Auerbach J, Eidelman A. 1988. A reexamination of newborns’ ability to imitate facial expressions. Dev. Psychol. 24, 3–7. ( 10.1037/0012-1649.24.1.3) [DOI] [Google Scholar]
- 40.Meltzoff AN, Moore KM. 1989. Imitation in newborn infants: exploring the range of gestures imitated and the underlying mechanisms. Dev. Psychol. 25, 954–962. ( 10.1037/0012-1649.25.6.954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anisfeld M, Turkewitz G, Rose SA, Rosenberg FR, Sheiber FJ, Couturier-Fagan DA, Sommer I. 2001. No compelling evidence that newborns imitate oral gestures. Infancy 2, 111–122. ( 10.1207/S15327078IN0201_7) [DOI] [PubMed] [Google Scholar]
- 42.Vinter A. 1986. The role of movement in eliciting early imitations. Child Dev. 57, 66–71. ( 10.2307/1130638) [DOI] [Google Scholar]
- 43.Nagy E, Pilling K, Orvos H, Molnar P. 2012. Imitation of tongue protrusion in human neonates: specificity of the response in a large sample. Dev. Psychol. 49, 1628–1638. ( 10.1037/a0031127) [DOI] [PubMed] [Google Scholar]
- 44.Heimann M, Nelson KE, Schaller J. 1989. Neonatal imitation of tongue protrusion and mouth opening: methodological aspects and evidence of early individual differences. Scand. J. Psychol. 30, 90–101. ( 10.1111/j.1467-9450.1989.tb01072.x) [DOI] [PubMed] [Google Scholar]
- 45.Heimann M. 1989. Neonatal imitation, gaze aversion, and mother–infant interaction. Infant Behav. Dev. 12, 493–503. ( 10.1016/0163-6383(89)90029-5) [DOI] [Google Scholar]
- 46.Koepke JE, Hamm M, Legerstee M, Russell M. 1983. Neonatal imitation: two failures to replicate. Infant Behav. Dev. 6, 97–102. ( 10.1016/S0163-6383(83)80012-5) [DOI] [Google Scholar]
- 47.Lewis M, Sullivan MW. 1985. Imitation in the first six months of life. Merrill-Palmer Q. 31, 315–333. [Google Scholar]
- 48.Hayes LA, Watson JS. 1981. Neonatal imitation: fact or artifact? Dev. Psychol. 17, 655–660. ( 10.1037/0012-1649.17.5.655) [DOI] [Google Scholar]
- 49.Fontaine R. 1984. Imitative skills between birth and six months. Infant Behav. Dev. 7, 323–333. ( 10.1016/S0163-6383(84)80047-8) [DOI] [Google Scholar]
- 50.McKenzie BE, Over R. 1983. Young infants fail to imitate facial and manual gestures. Infant Behav. Dev. 6, 85–95. ( 10.1016/S0163-6383(83)80011-3) [DOI] [Google Scholar]
- 51.Heimann M, Schaller J. 1985. Imitative reactions among 14–21 day old infants. Infant Mental Health J. 6, 31–39. () [DOI] [Google Scholar]
- 52.Bard KA. 2007. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Anim. Cogn. 10, 233–242. ( 10.1007/s10071-006-0062-3) [DOI] [PubMed] [Google Scholar]
- 53.Paukner A, Ferrari PF, Suomi SJ. 2011. Delayed imitation of lipsmacking gestures by infant rhesus macaques (Macaca mulatta). PLoS ONE 6, e28848 ( 10.1371/journal.pone.0028848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrari PF, Paukner A, Ruggiero A, Darcey L, Unbehagen S, Suomi SJ. 2009. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 80, 1057–1068. ( 10.1111/j.1467-8624.2009.01316.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. 2004. Imitation in neonatal chimpanzees (Pan troglodytes). Dev. Sci. 7, 437–442. ( 10.1111/j.1467-7687.2004.00364.x) [DOI] [PubMed] [Google Scholar]
- 56.Paukner A, Ferrari PF, Suomi SJ. 2013. A comparison of neonatal imitation abilities in human and macaque infants. In Navigating the social world: what infants, children, and other species can teach us (eds Banaji MR, Gelman SA.), pp. 133–138. New York, NY: Oxford University Press. [Google Scholar]
- 57.Paukner A, Simpson EA, Ferrari P, Mrozek T, Suomi SJ. Submitted. Neonatal imitation predicts how infants engage with faces. Dev. Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suddendorf T, Oostenbroek J, Nielsen M, Slaughter V. 2012. Is newborn imitation developmentally homologous to later social-cognitive skills? Dev. Psychobiol. 55, 54–58. ( 10.1002/dev.21005) [DOI] [PubMed] [Google Scholar]
- 59.Simpson EA, Paukner A, Sclafani V, Suomi SJ, Ferrari PF. 2014. Person recognition during neonatal imitation in rhesus macaques. PLoS ONE 4, e302 ( 10.1371/journal.pbio.0040302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sclafani V, Paukner A, Suomi SJ, Ferrari PF. Submitted. Imitation promotes affiliation in infant macaques at risk for impaired social behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ullstadius E. 1998. Neonatal imitation in a mother–infant setting. Early Dev. Parent 7, 1–8. () [DOI] [Google Scholar]
- 62.Pawlby SF. 1977. Imitative interaction. In Studies in mother–infant interaction (ed. Schaffer HR.), pp. 203–224. New York, NY: Academic Press. [Google Scholar]
- 63.Stern DN. 1985. The interpersonal world of the infant: a view from psychoanalysis and developmental psychology. New York, NY: Basic Books. [Google Scholar]
- 64.Trevarthen C, Aitken KJ. 2001. Infant intersubjectivity: research, theory, and clinical applications. J. Child Psychol. Psychiatr. 42, 3–48. ( 10.1111/1469-7610.00701) [DOI] [PubMed] [Google Scholar]
- 65.Tronick EZ. 1989. Emotions and emotional communication in infants. Am. Psychol. 44, 112–119. ( 10.1037/0003-066X.44.2.112) [DOI] [PubMed] [Google Scholar]
- 66.Trevarthen C. 1974. Conversation with a two-month-old. New Sci. 2, 230–235. [Google Scholar]
- 67.Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, Stein A. 2008. A specific and rapid neural signature for parental instinct. PLoS ONE 3, e1664 ( 10.1371/journal.pone.0001664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caria A, de Falco S, Venuti P, Lee S, Esposito G, Rigo P, Bornstein MH. 2012. Species-specific response to human infant faces in the premotor cortex. Neuroimage 60, 884–893. ( 10.1016/j.neuroimage.2011.12.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swain JE. 2008. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent–infant attachment. Psychiatry 5, 28–36. [PMC free article] [PubMed] [Google Scholar]
- 70.Goren CC, Sarty M, Wu PY. 1975. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 56, 544–549. [PubMed] [Google Scholar]
- 71.Johnson MH, Morton J. 1991. Biology and cognitive development: the case of face recognition. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 72.Batki A, Baron-Cohen S, Wheelwright S, Connellan J, Ahluwalia J. 2000. Is there an innate gaze module? Evidence from human neonates. Infant Behav. Dev. 23, 223–229. ( 10.1016/S0163-6383(01)00037-6) [DOI] [Google Scholar]
- 73.Farroni T, Csibra G, Simion F, Johnson MH. 2002. Eye contact detection in humans from birth. Proc. Natl Acad. Sci. USA 99, 9602–9605. ( 10.1073/pnas.152159999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trevarthen C. 1979. Communication and cooperation in early infancy: a description of primary intersubjectivity. In Before speech (ed. Bullowa M.), pp. 321–347. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 75.Feldman R, Eidelman AI. 2007. Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Dev. Psychobiol. 49, 290–302. ( 10.1002/dev.20220) [DOI] [PubMed] [Google Scholar]
- 76.MacFarlane A. 1977. The psychology of childbirth. London, UK: Fontana. [Google Scholar]
- 77.Papoušek H, Papoušek M. 1977. Mothering and the cognitive head-start: psychobiological considerations. In Studies in mother–infant interaction (ed. Shaffer HR.), pp. 63–85. London, UK: Academic Press. [Google Scholar]
- 78.Trevarthen C. 1977. Descriptive studies in infant behavior. In Studies in mother–infant interaction (ed. Schaffer HR.), pp. 27–270. London, UK: Academic Press. [Google Scholar]
- 79.Ferrari PF, Paukner A, Ionica C, Suomi SJ. 2009. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 19, 1768–1772. ( 10.1016/j.cub.2009.08.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mancini G, Ferrari PF, Palagi E. 2013. Rapid facial mimicry in geladas. Sci. Rep. 3, 1527 ( 10.1038/srep01527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavelli M, Fogel A. 2002. Developmental changes in mother-infant face-to-face communication: birth to 3 months. Dev. Psychol. 38, 288–305. ( 10.1037/0012-1649.38.2.288) [DOI] [PubMed] [Google Scholar]
- 82.Legerstee M, Pomerleau A, Malcuit G, Feider H. 1987. The development of infants’ responses to people and a doll: implications for research in communication. Infant Behav. Dev. 10, 81–95. ( 10.1016/0163-6383(87)90008-7) [DOI] [Google Scholar]
- 83.Murray L. 2014. The psychology of babies: how relationships support development from birth to two. London, UK: Constable & Robinson. [DOI] [PubMed] [Google Scholar]
- 84.Anisfeld M. 1991. Neonatal imitation. Dev. Rev. 11, 60–97. ( 10.1016/0273-2297(91)90003-7) [DOI] [Google Scholar]
- 85.Meltzoff AN, Gopnik A. 1993. The role of imitation in understanding persons and developing a theory of mind. In Understanding other people's minds (eds Baron-Cohen S, Tager-Flushberg H, Cohen DJ.), pp. 335–366. Oxford, UK: University Press. [Google Scholar]
- 86.Uzgiris IC. 1991. The social context of infant imitation. In Social influences and socialization in infancy. (eds Lewis M, Feinman S.), pp. 215–251. New York, NY: Plenum Press. [Google Scholar]
- 87.Ray E, Heyes C. 2011. Imitation in infancy: the wealth of the stimulus. Dev. Sci. 14, 92–105. ( 10.1111/j.1467-7687.2010.00961.x) [DOI] [PubMed] [Google Scholar]
- 88.Oostenbroek J, Slaughter V, Nielsen M, Suddendorf T. 2013. Why the confusion around neonatal imitation? A review. J. Reprod. Infant Psychol. 31, 328–341. ( 10.1080/02646838.2013.832180) [DOI] [Google Scholar]
- 89.Gibson JJ. 1966. The senses considered as perceptual systems. Boston, MA: Houghton Mifflin. [Google Scholar]
- 90.Gibson JJ. 1979. The ecological approach to visual perception. Boston, MA: Houghton Mifflin. [Google Scholar]
- 91.Feldman R. 2007. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatr. 48, 329–354. ( 10.1111/j.1469-7610.2006.01701.x) [DOI] [PubMed] [Google Scholar]
- 92.Cook R, Bird G, Catmur C, Press C, Heyes C. In press Mirror neurons: from origin to function. Behav. Brain Sci. [DOI] [PubMed] [Google Scholar]
- 93.Abravanel E, Sigafoos AD. 1984. Exploring the presence of imitation during early infancy. Child Dev. 55, 381–392. ( 10.2307/1129950) [DOI] [PubMed] [Google Scholar]
- 94.Jacobson SW. 1979. Matching behavior in the young infant. Child Dev. 50, 425–430. ( 10.2307/1129418) [DOI] [PubMed] [Google Scholar]
- 95.Meltzoff AN, Moore MK. 1999. Resolving the debate about early imitation. In The Blackwell reader in developmental psychology (eds Slater A, Muir D.), pp. 151–155. Oxford, UK: Blackwell. [Google Scholar]
- 96.Nagy E, Molnar P. 2004. Homo imitans or homo provocans? Human imprinting model of neonatal imitation. Infant Behav. Dev. 27, 54–63. ( 10.1016/j.infbeh.2003.06.004) [DOI] [Google Scholar]
- 97.Meltzoff AN, Moore MK. 1992. Early imitation within a functional framework: the importance of person identity, movement, and development. Infant Behav. Dev. 15, 479–505. ( 10.1016/0163-6383(92)80015-M) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones SS. 2006. Exploration or imitation? The effect of music on 4-week-old infants’ tongue protrusions. Infant Behav. Dev. 29, 126–130. ( 10.1016/j.infbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 99.Jones SS. 1996. Imitation or exploration? Young infants’ matching of adults’ oral gestures. Child Dev. 67, 1952–1969. ( 10.1111/j.1467-8624.1996.tb01837.x) [DOI] [PubMed] [Google Scholar]
- 100.Prechtl HFR. 1974. The behavioural states of the newborn infant. Brain Res. 76, 185–212. ( 10.1016/0006-8993(74)90454-5) [DOI] [PubMed] [Google Scholar]
- 101.Bjorklund DF. 1987. A note on neonatal imitation. Dev. Rev. 7, 86–92. ( 10.1016/0273-2297(87)90006-2) [DOI] [Google Scholar]
- 102.Field T, Goldstein S, Vega-Lahr N, Porter K. 1986. Changes in imitative behavior during early infancy. Infant Behav. Dev. 9, 415–421. ( 10.1016/0163-6383(86)90015-9) [DOI] [Google Scholar]
- 103.Maratos O. 1998. Neonatal, early and later imitation: same order phenomena? In The development of sensory, motor and cognitive capacities in early infancy: from perception to cognition (eds Simion F, Butterworth G.), pp. 145–160. Hove, UK: Psychology Press Ltd. [Google Scholar]
- 104.Kuhl PK, Meltzoff AN. 1996. Infant vocalizations in response to speech: vocal imitation and developmental change. J. Acoust. Soc. Am. 100, 2425 ( 10.1121/1.417951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanna E, Meltzoff A. 1993. Peer imitation by toddlers in laboratory, home and day-care contexts: implications for social learning and memory. Dev. Psychol. 29, 701–710. ( 10.1037/0012-1649.29.4.701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaplan F, Oudeyer PY. 2007. The progress-drive hypothesis: an interpretation of early imitation. In Models and mechanisms of imitation and social learning: behavioural, social and communication dimensions (eds Dautenhahn K, Nehaniv C.), pp. 361–377. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 107.Heyes C. 2001. Causes and consequences of imitation. Trends Cogn. Sci. 5, 253–261. ( 10.1016/S1364-6613(00)01661-2) [DOI] [PubMed] [Google Scholar]
- 108.Simpson EA, Paukner A, Sclafani V, Suomi SJ, Ferrari PF. In press Visual attention during neonatal imitation in newborn macaque monkeys. Dev. Psychobiol. ( 10.1002/dev.21146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanderwert RE, Fox NA, Ferrari PF. 2012. The mirror mechanism and mu rhythm in social development. Neurosci. Lett. 540, 15–20. ( 10.1016/j.neulet.2012.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrari PF, Tramacere A, Simpson EA, Iriki A. 2013. Mirror neurons through the lens of epigenetics. Trends Cogn. Sci. 17, 450–457. ( 10.1016/j.tics.2013.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simpson EA, Fox NA, Tramacere A, Ferrari PF. In press. Neonatal imitation and an epigenetic account of mirror neuron development. Behav. Brain Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray L, Fiori-Cowley A, Hooper R, Cooper PJ. 1996. The impact of postnatal depression and associated adversity on early mother–infant interactions and later infant outcome. Child Dev. 67, 2512–2526. ( 10.2307/1131637) [DOI] [PubMed] [Google Scholar]
- 113.Watson JS. 1979. Perception of contingency as a determinant of social responsiveness. In Origins of the infant's social responsiveness. (ed. Thoman EB.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- 114.Ullstadius E. 2000. Variability in judgment of neonatal imitation. J. Reprod. Infant Psychol. 18, 239–247. ( 10.1080/713683038) [DOI] [Google Scholar]
- 115.Csibra G, Gergely G. 2006. Social learning and social cognition: the case for pedagogy. In Processes of change in brain and cognitive development: attention and performance XXI (eds Munakata Y, Johnson MH.), pp. 249–274. New York, NY: Oxford University Press. [Google Scholar]
- 116.Nagy E. 2006. From imitation to conversation: the first dialogues with human neonates. Infant Child Dev. 15, 223–232. ( 10.1002/icd.460) [DOI] [Google Scholar]
- 117.Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 118.Legerstee M. 1991. The role of person and object in eliciting early imitation. J. Exp. Child Psychol. 51, 423–433. ( 10.1016/0022-0965(91)90086-8) [DOI] [PubMed] [Google Scholar]
- 119.Heimann M. 2001. Neonatal imitation: a fuzzy phenomenon? In Emerging cognitive abilities in early infancy (eds Lacerda F, von Hofsten C, Heimann M.), pp. 231–246. London, UK: Erlbaum. [Google Scholar]
- 120.Siller M, Sigman M. 2004. From neonatal imitation to social cognition: social and cognitive pathways to developmental continuity. In Social and moral development: emerging evidence on the toddler years (eds Leavitt LA, Hall DMB.), pp. 143–164. New Brunswick, NJ: Johnson & Johnson Pediatric Institute. [Google Scholar]
- 121.Heimann M. 2002. Notes on individual differences and the assumed elusiveness of neonatal imitation. In The imitative mind: development, evolution, and brain bases. (eds Meltzoff AN, Prinz W.), pp. 74–84. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 122.Cheng Y, Chou KH, Decety J, Chen IY, Hung D, Tzeng OL, Lin CP. 2009. Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience 158, 713–720. ( 10.1016/j.neuroscience.2008.10.026) [DOI] [PubMed] [Google Scholar]
- 123.Silas J, Levy JP, Nielsen MK, Slade L, Holmes A. 2010. Sex and individual differences in induced and evoked EEG measures of action observation. Neuropsychologia 48, 2417–2426. ( 10.1016/j.neuropsychologia.2010.03.004) [DOI] [PubMed] [Google Scholar]
- 124.Page J, Boucher J. 1998. Motor impairments in children with autistic disorder. Child Lang. Teach. Ther. 14, 233–259. ( 10.1177/026565909801400301) [DOI] [Google Scholar]
- 125.Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. 2003. Imitation performance in toddlers with autism and those with other developmental disorders. J. Child Psychol. Psychiatr. 44, 763–781. ( 10.1111/1469-7610.00162) [DOI] [PubMed] [Google Scholar]
- 126.Dawson G, Meltzoff AN, Osterling J, Rinaldi J. 1998. Neuropsychological correlates of early symptoms of autism. Child Dev. 69, 1276–1285. ( 10.1111/j.1467-8624.1998.tb06211.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Munson J, Faja S, Meltzoff A, Abbott R, Dawson G. 2008. Neurocognitive predictors of social and communicative developmental trajectories in pre-schoolers with autism spectrum disorders. J. Int. Neuropsychol. Soc. 14, 956 (doi:10.10170S1355617708081393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Williams JH, Whiten A, Suddendorf T, Perrett DI. 2001. Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev. 25, 287–295. ( 10.1016/S0149-7634(01)00014-8) [DOI] [PubMed] [Google Scholar]
- 129.Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. 2006. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. J. Child Psychol. Psychiatr. 47, 511–523. ( 10.1111/j.1469-7610.2005.01528.x) [DOI] [PubMed] [Google Scholar]
- 130.Soussignan R, Courtial A, Canet P, Danon-Apter G, Nadel J. 2011. Human newborns match tongue protrusion of disembodied human and robotic mouths. Dev. Sci. 14, 385–394. ( 10.1111/j.1467-7687.2010.00984.x) [DOI] [PubMed] [Google Scholar]
- 131.Tronick E. 2007. The neurobehavioral and social-emotional development of infants and children. New York, NY: Norton. [Google Scholar]
- 132.Keller H, Kärtner J, Borke J, Yovsi R, Kleis A. 2005. Parenting styles and the development of the categorical self: a longitudinal study on mirror self-recognition in Cameroonian Nso and German families. Int. J. Behav. Dev. 29, 496–504. ( 10.1080/01650250500147485) [DOI] [Google Scholar]
- 133.Fogel A, Toda S, Kawai M. 1988. Mother-infant face-to-face interaction in Japan and the United States: a laboratory comparison using 3-month-old infants. Dev. Psychol. 24, 398–406. ( 10.1037/0012-1649.24.3.398) [DOI] [Google Scholar]
- 134.Kärtner J, Keller H, Yovsi RD. 2010. Mother–infant interaction during the first 3 months: the emergence of culture-specific contingency patterns. Child Dev. 81, 540–554. ( 10.1111/j.1467-8624.2009.01414.x) [DOI] [PubMed] [Google Scholar]