Abstract
The observation of actions executed by others results in desynchronization of electroencephalogram (EEG) in the alpha and beta frequency bands recorded from the central regions in humans. On the other hand, mirror neurons, which are thought to be responsible for this effect, have been studied only in macaque monkeys, using single-cell recordings. Here, as a first step in a research programme aimed at understanding the parallels between human and monkey mirror neuron systems (MNS), we recorded EEG from the scalp of two monkeys during action observation. The monkeys were trained to fixate on the face of a human agent and subsequently to fixate on a target upon which the agent performed a grasping action. We found that action observation produced desynchronization in the 19–25 Hz band that was strongest over anterior and central electrodes. These results are in line with human data showing that specific frequency bands within the power spectrum of the ongoing EEG may be modulated by observation of actions and therefore might be a specific marker of MNS activity.
Keywords: EEG, mu rhythm, motor system, mirror neurons, macaque
1. Introduction
The mu rhythm is an electroencephalogram (EEG) oscillation within the alpha band range of 8–13 Hz recorded over central scalp locations. Suppression of power in this frequency band over central sites is thought to occur during action execution and observation of action [1–5]. This rhythm desynchronizes (i.e. decreases in amplitude) over sensorimotor areas during preparation, execution or imagination of movement or during somatosensory stimulation [6–9]. Particular attention has recently been paid to the mu rhythm as it is thought to provide a non-invasive tool that could be used to tap into neural responses related to the putative mirror neuron system (MNS) in humans [4,10–13]. However, most of our knowledge about the MNS comes from neurophysiological studies conducted with macaque monkeys, using single-unit recording in the ventral premotor cortex (area F5) [14–16]. Subsequent studies in the posterior parietal cortex (area PFG), an area anatomically connected with F5, found visuomotor neurons endowed with similar mirror properties [17–19]. Only one study in humans, using patients with epilepsy, has recorded single-cell activity finding mirror properties in areas (mesial cortex, entorhinal cortex and the parahippocampal region) that are not considered to be part of the classical MNS [20]. Thus, the nature of mirror neurons in humans within the parietal and frontal regions remains an open question. Owing to the invasive nature of this recording technique, direct evidence of the existence of MNS in humans is still lacking.
Our understanding of the nature and properties of mirror neurons rests primarily on the adult macaque monkey model. In contrast to the large body of research in humans [6,7,10–13], scalp-EEG recordings are seldom performed with monkeys, and only a few research studies are available. Early reports on the characteristics of scalp-EEG in adult macaques, however, suggest that the baseline spontaneous dominant rhythm is around 10–12 Hz [21,22], a frequency comparable to that observed in adult humans [23].
The main evidence for oscillatory activity of the motor and somatosensory cortex has been derived from local field potentials (LFPs) recorded from electrodes inserted into the cortex of non-human primates. In an early study, Murthy & Fetz [24] described bursts of activity in the 25–35 Hz frequency band from the motor and somatosensory cortices that appeared to occur during movements in which the monkey relied on tactile and proprioceptive information during exploration to find a raisin. However, the experimental design lacked precise timing for the actions, and the correlation between the frequency bursts and the monkey behaviours was not conclusive.
Sanes & Donoghue [25] measured LFPs from the motor cortex of two monkeys trained on a motor task and maintaining precise timing of the animal's behaviour. They found that bursts of 15–30 Hz were most prominent while the monkey was waiting for the go-cue to perform the motor action and that the onset of the action resulted in a desynchronization in the 15–30 Hz activity that returned to baseline once the action was complete, and the monkey was still again. This study suggests that the 15–30 Hz frequency band may reflect a ‘resting’ state of the motor system [26] that is desynchronized during task performance.
In these studies [24–26], LFP activity was band-pass filtered from 10 to 100 Hz preventing the analysis of slower, alpha band, activity. One study examined the spectrum of cortical activity in baboons [27]. Recording electrocorticogram (ECoG) from the somatosensory and parietal cortices while the animals were able to move freely, the researchers identified two rhythms that were synchronized while the animals were still and desynchronized during movements. Consistent with the studies reported above [24–26], activity in 18–27 Hz measured over the motor cortex was most prominent, whereas power in the 10–15 Hz band in the inferior parietal lobe (IPL) was maximal during periods of inactivity. Interestingly, the location and activity of these rhythms mirrored recent findings by Ritter et al. [28], who recorded simultaneous EEG and functional magnetic resonance imaging while human adults performed movements of opening and closing of their hands. They found that desynchronization of the mu rhythm correlated with the blood oxygen level-dependent response in the posterior IPL and rolandic beta desynchronized in the posterior bank of the somatosensory cortex.
All of these studies measured motor and somatosensory cortical activity during the execution of movements, but none measured LFPs or ECoG from monkeys observing actions. These studies suggest a remarkable correspondence in the neural activity (both in the frequency bands and the desynchronization during movement) between non-human primates and humans.
As a first step in bridging the knowledge gap between EEG during action observation that is recorded from the human scalp and the extensively studied MNS in macaques, we sought to determine whether an analogue of human EEG is recordable on the scalp of the adult rhesus macaque, and whether it was possible to modulate macaque EEG response through action observation.
2. Materials and methods
(a). Animals and surgical procedures
Two captive-born and individually housed adult female rhesus macaques (Macaca mulatta) served as subjects (M1 and M2). All experimental protocols were approved by the Ethical Committee for Animal Research of the University of Parma and by the Superior Institute for Health (last appraisal no. 2783, 26 January 2010). The authorization for conducting our experiments was confirmed by the Animal Health and Veterinary Medication Division of the Department of Public Veterinary Health, Nutrition and Food Safety of the Italian Ministry of Health (permit by ministerial decree no. 6/99-A, 29 January 1999; last renewals nos. 54/2010-B, 55/2010-C, 18 March 2010). The monkeys were housed and handled in strict accordance with the recommendations of the Weatherall Report about good animal practice. The well-being and health conditions of the animals were constantly monitored by the institutional veterinary doctor of the University of Parma.
A titanium head post (Crist Instrument, Hagerstown, MD) was surgically implanted on the skull using titanium screws. For this procedure, each animal was deeply anaesthetized with ketamine hydrochloride (5 mg kg−1 i.m.) and medetomidine hydrochloride (0.1 mg kg−1 i.m.) and its heart rate, temperature and respiration were carefully monitored and kept within physiological range. Pain medication was routinely given after surgery.
(b). Behavioural procedures
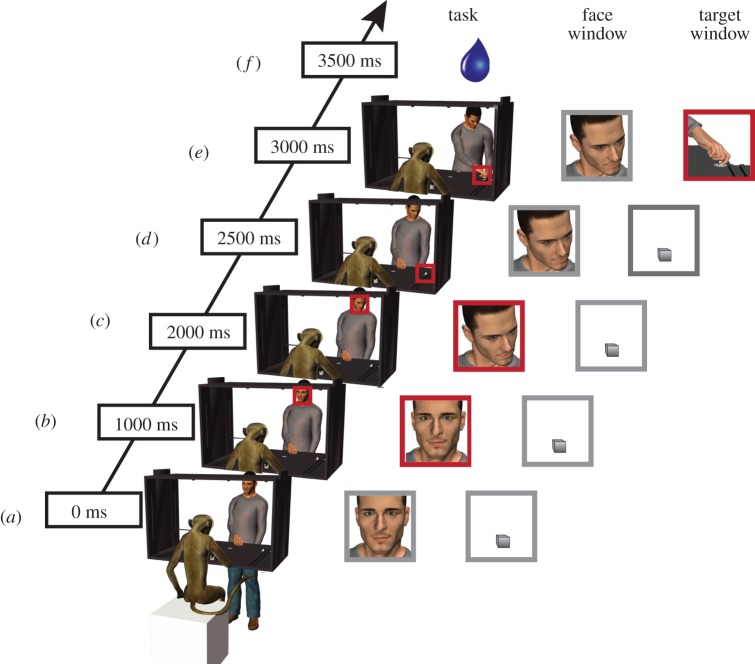
Each monkey was seated facing a table (60 × 60 cm) onto which two small objects (two metallic cubes that served as the target objects) were placed out of reach; one on the right, and one on the left side. The experimenter (hereafter called agent) sat at the other end of the table in front of the monkey with his right hand resting on a central platform located on the table between the two targets. The monkeys were previously trained to orient their gaze across two different fixation windows. The first window (15° × 15°) was located on the agent's face (face window) and the second (22° × 22°) on either the left or right target object (target window). The experimental set-up and the task are illustrated in figure 1. The monkey had to keep her hand on a handle embedded in the table during the whole task trial, including the baseline. A task trial began only if the monkey's hand was in contact with the handle for at least 1000 ms (figure 1a), at which point an LED instructed her to fixate the first window (face window; figure 1b). After 500 ms, the agent on cue shifted his head/gaze towards either the left or the right target object location (figure 1c). After 500 ms, a laser point instructed the monkey to shift her gaze to the same target object location (the target window) and to maintain her fixation in that second window for 1000 ms (figure 1d). While the monkey was fixating the target object, the agent was instructed to grasp the target object with his right hand (figure 1e). A juice reward was delivered after 200–300 ms if the monkey correctly fixated the windows for the established period of time. In order to keep artefacts, owing to hand movements to a minimum, the monkey was required to keep the hand, in a resting position, on the handle throughout the entire trial to get the reward. The release of the handle resulted in the trial being automatically aborted.
Figure 1.
Task and fixation windows. (a) The monkey and the agent are holding their hand on the table. (b) The monkey receives the instruction to fixate on a space window around the agent's face (face window). (c) The agent shifts his gaze towards the target object that could either be on the left or the right side. (d) The monkey receives the instruction to fixate on a space window around the target object (target window). (e) The agent grasps the target object. (f) The monkey receives a liquid reward if she successfully oriented her gaze in the correct windows throughout the task. The red squares indicate in which fixation window (face window or target window); the monkey was required to orient her gaze.
Although other stimuli have been presented to the monkeys, using the above-described experimental protocol, in this study, we are presenting only the most relevant data for the purpose of the study: observation of grasping.
(c). Data recording
A custom lycra EEG cap (Electro-Cap International, USA) was made and fitted with 14 tin electrodes. The electrode layout is illustrated in figure 2. Impedances were below 20 kΩ. EEG was band-pass filtered from 0.1 to 100 Hz prior to recording (James Long Company, USA) and digitized at a rate of 40 kHz (and down sampled to 1000 Hz offline) recorded using an Omniplex recording system (Plexon, USA). Eye movements were monitored throughout the experiment using a customized eye tracker (University of Tuebingen, Germany) equipped with a charge-coupled device camera (model ZC-F11CH4; Ganz, USA). The pupil was lit with infrared LEDs. The fixation task, including window size, duration, reward, etc., was computer-controlled though a customized LabView program (National Instruments, USA).
Figure 2.
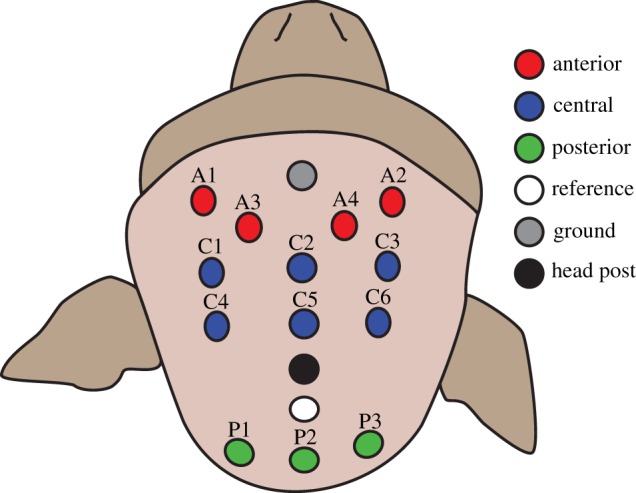
Electrode position on the scalp. Thirteen electrodes were placed approximately above the frontal, central and occipital cortices and were labelled anterior, central and posterior, respectively.
(d). Data pre-processing and artefact editing
All pre-processing and subsequent analyses were carried out in Matlab (R2013A; Mathworks, USA). Continuous EEG data from each day of recording (four total days for each monkey) were first baseline corrected, and forward/reverse Butterworth filtered (2–40 Hz pass band, 0.1–50 Hz stop band, 10 dB attenuation and 3 dB maximum ripple). An additional 49–51 Hz notch filter with 20 dB attenuation was also applied in order to remove any residual 50 Hz line noise present in the data. For each monkey, a list of channels containing spurious data (owing to electrical/mechanical failure during recording) was identified, and removed from further analysis. Continuous data from the remaining channels were then average referenced and segmented into epochs containing each individual trial. Each trial epoch was chosen to retain the interval beginning 700 ms prior to the end of the baseline, and ending 500 ms after the event of interest, which corresponded to the moment of contact between the experimenter's hand and the target object.
In order to remove trials with significant movement-related artefact, a threshold of 650 μV was chosen for both monkeys. Trials in which more than one channel exceeded this threshold were dismissed. Excluded from this criterion were the two most anterior channels positioned closest to the eyes. Because the eye movements of the monkey were built into the experimental design, and thus present on every trial, we could not exclude trials based on the presence of eye-movement artefact (we chose, instead, to remove this artefact via independent components analysis (ICA), as described below). For M1, this threshold procedure resulted in a pool of usable data consisting of 82 total trials containing more than 50% of the original data. For M2, we retained 74.66% of the original data, resulting in a usable pool of 79 trials.
As noted above, the presence of eye-movement-related artefact was a potential confound which was addressed via an ICA-based approach using the FastICA algorithm developed for Matlab by Hyvärinen [29]. For each day of recording, the pool of trials that remained after thresholding was concatenated and input into the algorithm. Of the resultant ICA components for each day, two were identified which contained the eye movements. This identification was made possible because of the precise timing of eye movements at the end of the first fixation interval (face window) on each trial, which was built into the experimental design. Time series for each of these components contained a high-amplitude/low-frequency (saccade-like) waveform that peaked within the 300 ms following the end of the fixation interval, during which the monkey made a saccade towards the target object, on approximately half of the trials. The first component was weighted most heavily at the left anterior-most anterior channel, whereas the second was weighted most heavily at the right anterior-most anterior channel, and indeed, we confirmed that the first of these contained the saccades which the monkey made to the left, whereas the other contained the saccades made to the right. Additionally, on most trials, each component contained a saccade-like waveform at the end of the baseline interval corresponding to the onset of fixation, as well as an eye movement in the interval following the observation of the grasp/object–contact. Thus, the removal of these components allowed us to reduce the effects of the monkeys’ eye movements in the baseline, post-fixation and grasp intervals of our data.
(e). Fast Fourier transform-based event-related desynchronization analysis
In order to test for differences in event-related desynchronization (ERD) across scalp locations, we computed ERD in each of three frequency bands (7–13, 13–19 and 19–25 Hz) for each trial/channel. The ERD compared spectral power in the 500 ms interval centred on the event of interest (the contact between the experimenter's hand and the target object), to power in the first 500 ms of the baseline interval. For each trial, EEG data during the intervals to be compared were segmented, and Fourier coefficients for each interval were obtained via Fast Fourier transform. Our choice to compare 500 ms intervals resulted in frequency bins with a bandwidth of 2 Hz. ERD at each resultant frequency bin was computed in dB units, i.e. ten times the log (log10) ratio of power in the grasp interval and power in the baseline. Thus, large negative ERD scores reflect strong desynchronization with respect to baseline, whereas strong positive ERS scores reflect relative synchronization. ERD was then averaged over frequency bins contained in our specified frequency bands (7–13, 13–19 and 19–25 Hz). In total, this procedure resulted in grasp versus baseline ERD scores in each band, for each channel on each trial.
In order to assess differences in ERD topography, ERD in each band was averaged within three disjoint sets of channels (figure 2) corresponding to anterior (A1, A2, A3, A4), central (C1, C2, C3, C4, C5 and C6) and posterior groups (P1, P2, P3). These channel-group-averaged ERDs were then compared across grasping observation trials via balanced ANOVA for each band using the Matlab statistical toolbox.
3. Results
(a). Event-related desynchronization topography
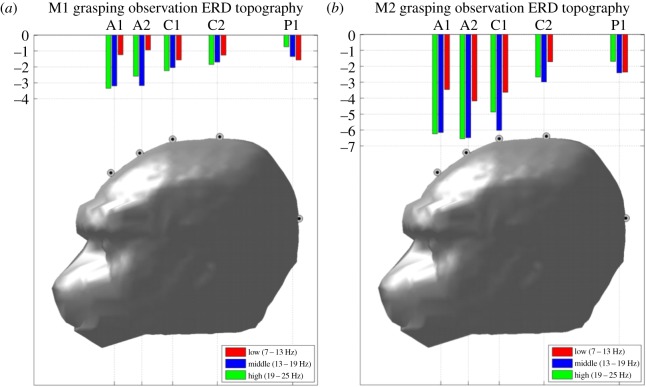
We compared ERD magnitudes for grasping observation trials across anterior, central and posterior electrodes separately for each frequency band via one-way balanced ANOVA. For M1 (figure 3a), the comparison of mean ERD for the three groups of electrodes shows significant differences (F2,243 = 5.14, p < 0.01) only for the 19–25 Hz frequency band. Post hoc comparisons revealed that for this band, anterior ERDs (mean = 3.04, s.e. = 0.54) are stronger than the posterior ERDs (mean = 0.69, s.e. = 0.52; Tukey's HSD, p < 0.05). For M2 (figure 3b), the three groups of electrodes show significant differences for the 7–13 Hz (F2,234 = 6.17, p < 0.01), the 13–19 Hz (F2,234 = 15.66, p < 0.01) and the 19–25 Hz frequency bands (F2,234 = 28.84, p < 0.01). Follow-up comparisons showed that the anterior ERDs for 19–25 Hz frequency band (mean = −6.5, s.e. = 0.54) are stronger than the central (mean = −3.73, s.e. = 0.41), which, in turn, are stronger than the posterior (mean = −1.36, s.e. = 0.48; Tukey's HSD, p < 0.05). Likewise, in the 13–19 Hz band, we observed anterior ERD (mean = −6.3, s.e. = 0.5) stronger than central ERD (mean = −4.19, s.e. = 0.48), which, in turn, are stronger than posterior ERD (mean = −2.3, s.e. = 0.53; Tukey's HSD, p < 0.05). Finally, in the 7–13 Hz band, we observed anterior ERD (mean = −4.17, s.e. = 0.53) stronger than posterior (mean = −1.72, s.e. = 0.48).
Figure 3.
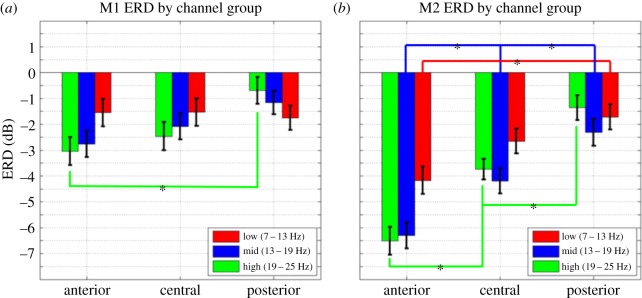
Mean ERD for the anterior, central and posterior groups of electrodes for (a) M1 and (b) M2. Only the three most sensitive frequency bands are shown. *p < 0.05, Tukey's HSD.
The results described above demonstrate that grasping observation ERD is distributed along a clear antero-posterior gradient in which the anterior and central electrodes are the most sensitive, especially for the 13–19 and 19–25 Hz bands. To further visualize this topographic specificity of EEG suppression, ERD is plotted across five groups of electrodes defined according to their scalp position along the antero-posterior axis (figure 4a,b). The ERD topography obtained for these groups illustrates the fact that desynchronization for each band is clearly not evenly distributed on the scalp, but rather, falls off from anterior to posterior scalp locations.
Figure 4.
Topographic view of ERD for grasping observation along the antero-posterior axis for (a) M1 and (b) M2. A1–A2, C1–C2 and P1 indicate the anterior, central and posterior groups of electrodes along this axis, respectively. The 7–13, 13–19 and 19–25 Hz bands are all shown.
Our experimental protocol required the monkeys to keep their right hand on a handle throughout the whole EEG recording trials (§2a). To verify that the ERDs obtained during action observation were not confounded by clenching the handle or doing other putative hand movements, we recorded the electromyogram (EMG) activity of the flexor digitorum superficialis muscle during a control session using the same experimental protocol described above. The results shown in the electronic supplementary material, figure S1, indicate that no hand movements were made during the EEG recordings.
4. Discussion
To the best of our knowledge, this work is the first to report action observation-induced ERD in adult monkey-scalp-recorded EEG. We found ERD at the 19–25 Hz frequency band sensitive to the observation of actions. This effect was stronger in anterior-central regions compared with the posterior ones. This band resembles both the spectral and topographical characteristics of the beta rhythms (around 20 Hz) identified in humans which have demonstrated sensitivity to the observation of others’ actions [3,11,13,30–33].
Our interpretation of these data is enhanced by the experimental design making it unlikely that the findings are a function of attentional differences, or the result of overt motor responses. Throughout the experiment, the monkeys were required to keep their arms and bodies still by keeping their hand in a rest position over a handle located on the table in front of them. By recording the EMG of the hand flexor muscles (flexor digitorum superficialis), we confirmed that during the observation of the task the monkeys were not involved in any active hand movement. In addition, careful eye tracking of the monkeys’ gaze ensured that fixation on the target was maintained and consistent across conditions. If the animals shifted their gaze away from the stimuli or moved their hand during a trial, then they were not rewarded, and the trial was excluded from analysis. Thus, the differential suppression of the signal recorded in the 19–25 Hz bands is probably due to the goal-directed movement of the hand, suggesting that these frequency bands are sensitive to the observation of biologically meaningful events and may reflect the activation of specific cortical networks. Similar to the suppression recorded with EEG in humans, the specific ERD described here in the beta bands might reflect processing of information related to others’ actions [4,11,30], which is known from monkey single-cell recording to involve premotor and parietal areas [14–19].
(a). Event-related desynchronization topography
Our data revealed stronger ERD over the anterior and central electrodes compared with the posterior ones. For both monkeys, the anterior and central electrodes were the most sensitive to EEG activity modulation during action observation in the 13–19 and 19–25 Hz bands. This topographic differential sensitivity of ERD has its parallel in the human data where both the alpha and the beta bands desynchronize during the observation of others’ actions. In particular, action observation affects the rolandic rhythms recorded in central electrodes in humans [3,10,11,13,30–34]. Within the rolandic rhythm there are, at least, two components whose spectral peaks are described around 10 and 20 Hz [35,36]. This pattern of activation in humans, generally acquired via magnetoencephalography, is interpreted as reflecting a frontoparietal network of sensorimotor rather than visual processing of an occipital network [1].
The sources of these two spectral peaks have been suggested to be different. The alpha component has its generator in the somatosensory cortex, whereas the beta in the motor cortex [35,36]. In our study, we found that the 20–25 Hz band is the most sensitive in both monkeys, and the magnitude of suppression is more robust in the anterior and central electrodes. Other work in humans has shown that during action observation, frequency bands within this range show similar desynchronization [3,11,30,34,37] in central electrodes while subjects are observing others’ behaviour. It is possible that this band is analogous to the human beta band and that activity in this band may reflect the activation of the motor cortex occurring while observing actions performed by others. This result is also compatible with the idea that the observation of hand grasping actions recruits mirror neuron populations in the posterior parietal lobe, the ventral premotor cortex and as recently demonstrated, in the primary motor cortex [17,38,39]. Therefore, under the current experimental conditions, this frequency band could be considered an indirect correlate tapping the activity of the mirror mechanism.
Recent work in newborn monkeys has shown that lower frequency bands recorded over frontal electrodes are suppressed during the observation and execution of facial gestures [40]. However, the frequencies sensitive to this set of stimuli were within the 5–6 Hz band; similar to the human alpha in infancy and consistent with developmental findings of the human infant mu rhythm. Clearly, further analysis is warranted to assess possible longitudinal changes in EEG frequency bands in the monkey.
While the data between the two monkeys are congruent, there are also differences. One monkey (M1) had greater suppression only over the central electrodes, whereas the other (M2) had significant suppression in the same frequency bands in both the anterior and the central electrodes. Furthermore, in M2, the 13–19 Hz band also has significant desynchronization, whereas this is not present in M1.
Single-cell studies in monkeys showed that neurons in F5 and PFG can code different aspects of an action: the type of grip and the overall goal of the action, transcending the motor specifics [17,41–45]. Such responses have been interpreted in terms of how the motor cortex is hierarchically organized in order to allow an agent to visually guide movements in space to reach [19,46–48]. The capacity of mirror neurons to code the goal of an action suggests that in the parietal–frontal cortical networks, actions (both executed or observed) can be coded at a more abstract level, independently from the specific dynamics and kinematics of the movements. The current findings indicate that the 19–25 Hz band is sensitive to movement directed at a target when it is performed with a biological effector.
Future experiments are required in order to clarify important issues, which are critical in mirror neurons research. It is important to determine if the moving hand alone, miming the action (but with no target to grasp) is a sufficient stimulus to elicit EEG desynchronization. It is known from single-cell studies that mirror neurons do not respond to mimed actions, although a weaker response can be often present. It is possible that with EEG recordings, the observation of biological movements devoid of the target–object, which is known to involve temporoparietal–premotor networks, could produce EEG changes similar to those reported during observation of goal-directed movements. The work in humans has shown that meaningful and meaningless movements can induce desynchronization of the alpha rhythm [3,8,10,37]. Related to this point, it would be interesting to investigate whether actions with a tool, or objects moving with a biological kinematics towards a goal, are capable of eliciting a similar desynchronization.
5. Conclusion
The relation between the scalp-EEG signals we present here and the underlying activity of the mirror neuron system should be interpreted with caution. First, a key component of the mirror neuron system is its activation during the production of goal-directed actions. In our study, we were unable to analyse the data during action execution owing to excessive artefact contamination. Therefore, we cannot compare the ERD found in this experiment during action observation with the activation of the motor cortex. Second, the position of the head-post prevented placement of electrodes over the parietal cortex resulting in limited scalp coverage. In humans, the rolandic rhythms are observed in central and parietal electrodes, and the greater anterior desynchronization in the macaque may represent a different pattern of scalp activity in response to mirror neuron system activation.
Finally, the ability to measure scalp-EEG from an adult macaque while they observe goal-directed actions is only the first step in bridging the relationship between the firing of mirror neurons and the activity observed at the scalp. Future studies are needed to link the two signals through simultaneous recordings of single-cell and scalp-EEG.
All experimental protocols were approved by the Ethical Committee for Animal Research of the University of Parma and by the Superior Institute for Health (last appraisal no. 2783, 26 January 2010).
Funding statement
This research was supported by the Division of Intramural Research, NICHD and NIH P01HD064653.
References
- 1.Pineda JA. 2005. The functional significance of mu rhythms: translating ‘seeing’ and ‘hearing’ into ‘doing’. Brain Res. Brain Res. Rev. 50, 57–68. ( 10.1016/j.brainresrev.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Vanderwert RE, Fox NA, Ferrari PF. 2013. The mirror mechanism and mu rhythm in social development. Neurosci. Lett. 540, 15–20. ( 10.1016/j.neulet.2012.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti DV, Rossini PM. 2002. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage 17, 559–572. ( 10.1006/nimg.2002.1192) [DOI] [PubMed] [Google Scholar]
- 4.Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. 2011. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 31, 14 243–14 249. ( 10.1523/JNEUROSCI.0963-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepage J-F, Théoret H. 2006. EEG evidence for the presence of an action observation-execution matching system in children. Eur. J. Neurosci. 23, 2505–2510. ( 10.1111/j.1460-9568.2006.04769.x) [DOI] [PubMed] [Google Scholar]
- 6.Pfurtscheller G, Neuper C, Andrew C, Edlinger G. 1997. Foot and hand area mu rhythms. Int. J. Psychophysiol. 26, 121–135. ( 10.1016/S0167-8760(97)00760-5) [DOI] [PubMed] [Google Scholar]
- 7.Neuper C, Wörtz M, Pfurtscheller G. 2006. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 159, 211–222. ( 10.1016/S0079-6123(06)59014-4) [DOI] [PubMed] [Google Scholar]
- 8.Pfurtscheller G, Berghold A. 1989. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr. Clin. Neurophysiol. 72, 250–258. ( 10.1016/0013-4694(89)90250-2) [DOI] [PubMed] [Google Scholar]
- 9.Arroyo S, Lesser RP, Gordon B, Uematsu S, Jackson D, Webber R. 1993. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalogr. Clin. Neurophysiol. 87, 76–87. ( 10.1016/0013-4694(93)90114-B) [DOI] [PubMed] [Google Scholar]
- 10.Muthukumaraswamy SD, Johnson BW. 2004. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology 41, 152–156. ( 10.1046/j.1469-8986.2003.00129.x) [DOI] [PubMed] [Google Scholar]
- 11.Cochin S, Barthelemy C, Roux S, Martineau J. 1999. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur. J. Neurosci. 11, 1839–1842. ( 10.1046/j.1460-9568.1999.00598.x) [DOI] [PubMed] [Google Scholar]
- 12.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. 2005. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res. Cogn. Brain Res. 24, 190–198. ( 10.1016/j.cogbrainres.2005.01.014) [DOI] [PubMed] [Google Scholar]
- 13.Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. 1998. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc. Natl Acad. Sci. USA 95, 15 061–15 065. ( 10.1073/pnas.95.25.15061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180. ( 10.1007/BF00230027) [DOI] [PubMed] [Google Scholar]
- 15.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. 1996. Premotor cortex and the recognition of motor actions. Brain Res. 3, 131–141. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. 2003. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 17, 1703–1714. ( 10.1046/j.1460-9568.2003.02601.x) [DOI] [PubMed] [Google Scholar]
- 17.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science 308, 662–667. ( 10.1126/science.1106138) [DOI] [PubMed] [Google Scholar]
- 18.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. 2002. Action representation and the inferior parietal lobule. In Attention performance XIX common mechanisms in perception and action (eds Prinz W, Hommel B.), pp. 247–266. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. 2010. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb. Cortex 20, 1372–1385. ( 10.1093/cercor/bhp200) [DOI] [PubMed] [Google Scholar]
- 20.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. 2010. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 20, 750–756. ( 10.1016/j.cub.2010.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurko MF, Andy OJ. 1967. Comparative EEG frequencies in rhesus, stumptail, and cynomolgus monkeys. Electroencephalogr. Clin. Neurophysiol. 23, 270–272. ( 10.1016/0013-4694(67)90124-1) [DOI] [PubMed] [Google Scholar]
- 22.Schmid MC, Oeltermann A, Juchem C, Logothetis NK, Smirnakis SM. 2006. Simultaneous EEG and fMRI in the macaque monkey at 4.7 Tesla. Magn. Reson. Imaging 24, 335–342. ( 10.1016/j.mri.2005.12.024) [DOI] [PubMed] [Google Scholar]
- 23.Lindsay D. 1936. Brain potentials in children and adults. Science 84, 354 ( 10.1126/science.84.2181.354) [DOI] [PubMed] [Google Scholar]
- 24.Murthy VN, Fetz EE. 1992. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc. Natl Acad. Sci. USA 89, 5670–5674. ( 10.1073/pnas.89.12.5670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanes J, Donoghue JP. 1993. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc. Natl Acad. Sci. USA 90, 4470–4474. ( 10.1073/pnas.90.10.4470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker SN, Olivier E, Lemon RN. 1997. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J. Physiol. 501, 225–241. ( 10.1111/j.1469-7793.1997.225bo.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rougeul A, Bouyer J, Dedet L, Debray O. 1979. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogr. Clin. Neurophysiol. 46, 310–319. ( 10.1016/0013-4694(79)90205-0) [DOI] [PubMed] [Google Scholar]
- 28.Ritter P, Moosmann M, Villringer A. 2009. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum. Brain Mapp. 30, 1168–1187. ( 10.1002/hbm.20585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyvärinen A. 1999. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 10, 626–634. ( 10.1109/72.761722) [DOI] [PubMed] [Google Scholar]
- 30.Cochin S, Barthelemy C, Lejeune B. 1998. Perception of motion and qEEG activity in human adults. Electroencephalogr. Clin. Neurophysiol. 107, 287–295. ( 10.1016/S0013-4694(98)00071-6) [DOI] [PubMed] [Google Scholar]
- 31.Rossi S, Tecchio F, Pasqualetti P, Ulivelli M, Pizzella V, Romani GL, Passero S, Battistini N, Rossini PM. 2002. Somatosensory processing during movement observation in humans. Clin. Neurophysiol. 113, 16–24. ( 10.1016/S1388-2457(01)00725-8) [DOI] [PubMed] [Google Scholar]
- 32.Järveläinen J, Schürmann M, Avikainen S, Hari R. 2001. Stronger reactivity of the human primary motor cortex during observation of live rather than video motor acts. Neuroreport 12, 3493–3495. ( 10.1097/00001756-200111160-00024) [DOI] [PubMed] [Google Scholar]
- 33.Cheyne D, Gaetz W, Garnero L, Lachaux J-P, Ducorps A, Schwartz D, Varela FJ. 2003. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Res. Cogn. Brain Res. 17, 599–611. ( 10.1016/S0926-6410(03)00173-3) [DOI] [PubMed] [Google Scholar]
- 34.Muthukumaraswamy SD, Johnson BW. 2004. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clin. Neurophysiol. 115, 1760–1766. ( 10.1016/j.clinph.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 35.Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. 2005. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage 26, 347–355. ( 10.1016/j.neuroimage.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 36.Hari R, Salmelin R. 1997. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 20, 44–49. ( 10.1016/S0166-2236(96)10065-5) [DOI] [PubMed] [Google Scholar]
- 37.Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. 2012. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: an EEG study. PLoS ONE 7, e37534 ( 10.1371/journal.pone.0037534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. 1996. Action recognition in the premotor cortex. Brain 119, 593–609. ( 10.1093/brain/119.2.593) [DOI] [PubMed] [Google Scholar]
- 39.Vigneswaran G, Philipp R, Lemon RN, Kraskov A. 2013. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol. 23, 236–243. ( 10.1016/j.cub.2012.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrari PF, Vanderwert RE, Paukner A, Bower S, Suomi SJ, Fox NA. 2012. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cogn. Neurosci. 24, 1165–1172. ( 10.1162/jocn_a_00198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umiltà MA, Escola L, Intskirveli I, Grammont F, Rochat M, Caruana F, Jezzini A, Gallese V, Rizzolatti G. 2008. When pliers become fingers in the monkey motor system. Proc. Natl Acad. Sci. USA 105, 2209–2213. ( 10.1073/pnas.0705985105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamagata T, Nakayama Y, Tanji J, Hoshi E. 2012. Distinct information representation and processing for goal-directed behavior in the dorsolateral and ventrolateral prefrontal cortex and the dorsal premotor cortex. J. Neurosci. 32, 12 934–12 949. ( 10.1523/JNEUROSCI.2398-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama Y, Yamagata T, Tanji J, Hoshi E. 2008. Transformation of a virtual action plan into a motor plan in the premotor cortex. J. Neurosci. 28, 10 287–10 297. ( 10.1523/JNEUROSCI.2372-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari PF, Bonini L, Fogassi L. 2009. From monkey mirror neurons to primate behaviours: possible ‘direct’ and ‘indirect’ pathways. Phil. Trans. R. Soc. B 364, 2311–2323. ( 10.1098/rstb.2009.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonini L, Ugolotti Serventi F, Bruni S, Maranesi M, Bimbi M, Simone L, Rozzi S, Ferrari PF, Fogassi L. 2012. Selectivity for grip type and action goal in macaque inferior parietal and ventral premotor grasping neurons. J. Neurophysiol. 108, 1607–1619. ( 10.1152/jn.01158.2011) [DOI] [PubMed] [Google Scholar]
- 46.Bonini L, Serventi FU, Simone L, Rozzi S, Ferrari PF, Fogassi L. 2011. Grasping neurons of monkey parietal and premotor cortices encode action goals at distinct levels of abstraction during complex action sequences. J. Neurosci. 31, 5876–5886. ( 10.1523/JNEUROSCI.5186-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner EP, et al. 2007. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J. Neurophysiol. 97, 387–406. ( 10.1152/jn.00558.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizzolatti G, Luppino G. 2000. The cortical motor system. Neuron 31, 889–901. ( 10.1016/S0896-6273(01)00423-8) [DOI] [PubMed] [Google Scholar]