Abstract
Mirror neurons are a specific type of visuomotor neuron that discharge both when a monkey executes a motor act and when it observes a similar motor act performed by another individual. In this article, we review first the basic properties of these neurons. We then describe visual features recently investigated which indicate that, besides encoding the goal of motor acts, mirror neurons are modulated by location in space of the observed motor acts, by the perspective from which the others’ motor acts are seen, and by the value associated with the object on which others’ motor acts are performed. In the last part of this article, we discuss the role of the mirror mechanism in planning actions and in understanding the intention underlying the others’ motor acts. We also review some human studies suggesting that motor intention in humans may rely, as in the monkey, on the mirror mechanism.
Keywords: motor act, space-selective mirror neurons, view-selective mirror neurons, subjective value, motor intention understanding
1. Introduction
Mirror neurons were described for the first time 20 years ago in the monkey ventral premotor area F5 [1–3], and subsequently in the monkey inferior parietal area PFG [4,5]. Their discovery was preceded by a prolonged anatomical and functional investigation of the premotor areas that enabled our group to highlight a series of unexpected functions of these areas [6,7]. Among these functions, were the coding of the goal of motor acts rather than movements that form them [7,8], the responsiveness to objects in area F5 ([9] and see [10]) and the coding of the peripersonal space in area F4 [11,12].
In area F5, besides purely motor neurons, two categories of visuomotor neurons were found. One, referred to as ‘canonical neurons’ [13], is responsive to the presentation of three-dimensional objects. The other, referred to as ‘mirror neurons’, is responsive to the observation of motor acts performed by others [2,3]. The main property of canonical neurons is to match the shape and size of the observed object with a specific type of prehension, whereas the main property of mirror neurons is that of matching observation of hand and mouth motor acts with the execution of the same or similar motor acts. This matching mechanism enables the observing individual to achieve an automatic understanding—i.e. an understanding without inferential processing of others’ goal-directed motor acts.
Subsequently, neurons with mirror properties were also described in cortical areas outside the parietofrontal circuit, such as the mesial frontal cortex [14] in non-human primates and the hippocampus [15] in humans. There is much evidence that an observation/execution mechanism, demonstrated with electrophysiological (electroencephalography (EEG), magnetoencephalography (MEG) and transcranial magnetic stimulation (TMS)) and brain imaging (positron emission tomography (PET) and functional magnetic resonance imaging (fMRI)) techniques, is present in humans in areas homologous to those containing mirror neurons in the monkey, plus the insula and the cingulate cortex (see [16–21]). This review focuses on recent data on the functional properties of parietal and premotor mirror neurons, including their role in intention understanding. In the last section, we discuss some human studies dealing with this latter issue.
2. Basic properties of monkey mirror neurons
(a). Visual and motor properties
Before describing the characteristics of visual responses of mirror neurons, it is important to stress the main properties of F5 motor neurons, including mirror neurons (figure 1). It is now well established that these neurons code the goal of the motor acts [28]. Evidence supporting this point is provided by neurons that discharge when the monkey grasps an object (e.g. food) with its right hand, left hand and the mouth [7]. It is clear that this type of neural behaviour cannot be explained in terms of movements. Additional evidence in favour of goal coding was provided by experiments in which the monkeys grasped the food with normal or inverted pliers (i.e. pliers that require the opening of the hand to close the pliers and therefore to grasp the object). These experiments showed that the neuron discharge correlated with the goal of the motor act, regardless of whether the hand closed or opened to achieve the goal [8]. Further studies demonstrated that the motor goal is also coded by motor neurons of the inferior parietal lobule (IPL) [5].
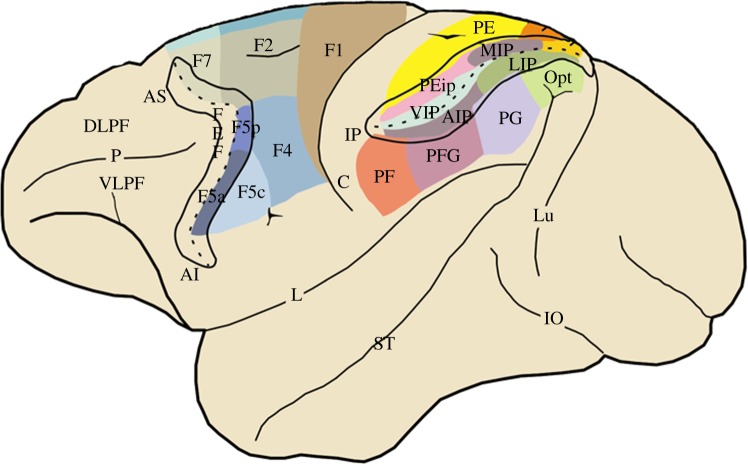
Figure 1.
Lateral view of the monkey brain showing the subdivisions of the agranular frontal and posterior parietal cortices. The intraparietal and arcuate sulci have been opened to show the areas buried inside them. Agranular frontal areas have been labelled according to Matelli et al. [22,23]. Note that area F5 is formed by three further subdivisions: F5c, F5p and F5a [24]. Posterior parietal areas are defined according to Pandya and Seltzer and Gregoriou et al. [25,26]. The areas buried inside the intraparietal sulcus are defined according to functional criteria (for references, see [27]). AI, inferior arcuate sulcus; AIP, anterior intraparietal area; AS, superior arcuate sulcus; C, central sulcus; DLPF, dorsolateral prefrontal cortex; FEF, frontal eye field; IO, inferior occipital sulcus; L, lateral fissure; LIP, lateral intraparietal area; Lu, lunate sulcus; MIP, medial intraparietal area; P, principal sulcus; ST, superior temporal sulcus; VIP, ventral intraparietal area; VLPF, ventrolateral prefrontal cortex.
The main feature of the visual properties of mirror neurons in both premotor and parietal cortices is the congruence between the executed and the observed motor act [2–5]. Some mirror neurons show a strict correspondence between the effective observed and executed motor act; others show a correspondence in the goal of the observed and executed motor act, but not in the precise movements necessary to achieve the goal. Although some other specific visual characteristics have been already observed in the early studies on mirror neurons, these characteristics have been recently re-investigated and will be dealt with below.
An important aspect of mirror neuron responses that must be stressed for understanding their function is to clarify what their discharge encodes. Single neuron extracellular recordings give information about neuron output. Because the output is the same both when the monkey executes a motor act and when it observes the same motor act made by another agent, it follows that because the neuron codes the motor act during active performance, it also codes the same motor act during observation.
Further evidence that mirror neurons code the goal of others’ motor acts is provided by the experiment in which auditory stimuli typical of certain motor acts were presented to the monkey [29]. The results showed that a large number of the recorded mirror neurons responded not only to the observation of specific motor acts (e.g. breaking a peanut), but also to their sound. By contrast, other types of auditory stimuli related to different motor acts or unspecific sound-like white noise, were not effective. This finding clearly supports the notion that mirror neuron responses code the goal of motor acts performed by others.
(b). The mirror neuron circuit
It has been known, for many years, that there are many neurons in the region of the superior temporal sulcus (STS) that discharge in response to the observation of actions made by others [30]. This region, as discussed below, represents the major input to the IPL, in turn, connected to ventral premotor cortex. In the rostral part of the ventral bank of STS, some neurons were described that fired during observation of hand grasping. However, neurons in STS do not discharge during active movements. This strongly suggests that STS is the likely origin of the higher-order visual input necessary for the genesis of mirror neurons in the parietofrontal circuit.
This point has been recently investigated using fMRI complemented by neuroanatomical tracing techniques in the monkey [31]. In the fMRI experiments, video-clips of grasping actions performed by a human actor were presented. The results showed activation in three nodes (figure 2a): STS, IPL and the arcuate region. A subsequent region of interest (ROI) analysis was carried out. For the parietal lobe, the chosen regions were the cytoarchitectonic areas PF, PFG, PG and anterior intraparietal (AIP), whereas for the STS region, they were the middle temporal area (MT) and its satellites, superior temporal polysensory middle (STPm) and two areas located in the rostral part of dorsal and ventral STS. The statistical analysis, based on the contrast between grasping action and a variety of static stimuli, showed that in the parietal lobe action-related increase in discharge was present in areas PFG and AIP. No action-specific activation was found in the other parietal areas. Action-related stimuli evoked stronger activation than static stimuli in all STS areas.
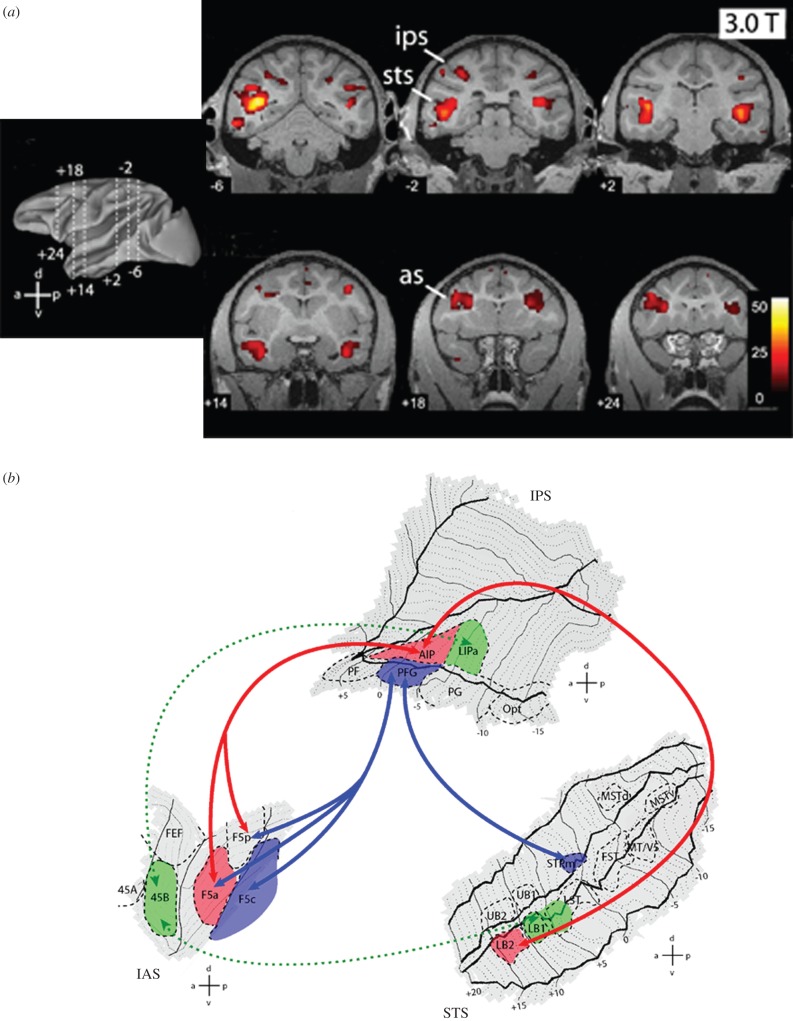
Figure 2.
(a) Overview of MR brain activations (recorded with 3T fMRI) during the observation of grasping acts. Leftmost image: lateral view of reconstructed left hemisphere indicating the six different antero-posterior levels at which coronal slices shown on the right have been taken. Right: statistical parametric maps activation for the contrast: hand action versus static control. The data are from a single monkey, overlaid onto its coronal anatomical sections. Numbers on each slice indicate y-coordinate (antero-posterior from interaural plane). as, arcuate sulcus; ips, intraparietal sulcus; sts, superior temporal sulcus. (b) Temporo-parieto-premotor grasping observation pathways in the monkey brain. Flattened representation of STS, IPS/IPL and IAS with ROIs indicated. Arrows and areas coloured in red and blue indicate, respectively, the STPm–PFG–F5c pathway and the LB2–AIP–F5a/p pathway. For the definition of areas PF, PFG, PG and Opt, see figure 1. Areas 45a and 45b are defined according to Gerbella et al. [32]. AIP, anterior intraparietal area; FEF, frontal eye fields; F5c, F5 convexity; F5p, F5 (bank) posterior; F5a, F5 (bank) anterior; FST, fundus of the STS; IAS, inferior arcuate sulcus; LIPa, anterior part of the lateral intraparietal area; MT/V5, middle temporal area; MSTd, middle superior temporal area, dorsal part; MSTv, middle superior temporal area, ventral part.
In order to elucidate which of these areas are connected with the parietal areas with mirror properties, tracers were injected in the PFG and AIP areas. The results showed a different pattern of connection of the two parietal areas with STS. Broadly speaking, area PFG was mostly connected with the upper bank of STS, and in particular with STPm, whereas AIP was mostly connected with the lower bank of STS, and in particular with the rostralmost subdivisions. Figure 2b shows the details of these connections, together with the connections of the inferior parietal areas and of STS areas to the frontal lobe. Note that the connection to AIP starts not only from STS areas, but also from a part of the cortex that is considered functionally and anatomically part of the inferotemporal cortex (see also [33]). Because inferotemporal cortex is considered to encode the meaning of the objects, these connections suggest that the mirror circuit also receives information concerning objects semantics. These data, together with previous neuroanatomical findings [33,34], indicate that there are two temporo-parieto-premotor streams that process information about the actions of others. One connects the upper bank of STS/STPm with PFG and then with premotor areas F5c and F4. The other connects the lower bank of rostral STS/inferotemporal cortex with AIP and then with F5p and F5a.
3. New findings on visual properties of mirror neurons
(a). Mirror properties of corticospinal tract neurons
Kraskov et al. [35] investigated the activity of corticospinal neurons located in F5. They first identified the pyramidal tract neurons (PTNs) using antidromic stimulation. Then, they tested these neurons for their mirror properties. About half of these neurons responded when monkeys observed the experimenter grasping an object. An interesting observation was that about 25% of these PTNs showed complete suppression of discharge during grasping observation, whereas they strongly discharged during grasping execution. The authors suggested that this PTN suppression might be involved in the inhibition of the observer's movement during action observation.
In a following study, they applied the same paradigm to PTNs recorded from primary motor cortex (F1) [36]. About half of these neurons were modulated during action observation. Of them, the majority increased their discharge during action observation (‘facilitation-type’ mirror neurons), whereas others showed reduced discharge or stopped firing (‘suppression-type’ mirror neurons). A comparison of the discharge of the facilitation-type neurons between grasping observation and execution showed that they discharged much less for action observation than for action execution. By comparing the properties of F1 mirror PTNs with those of F5 mirror PTNs, they observed that the visual response in F1 was much weaker than in F5. Thus, although many PTN F1 output neurons are active during action observation, their direct input to spinal circuitry may not be sufficient to produce overt muscle activity.
These data indicate that the understanding of motor goals is not only a function of F5 mirror neurons, but rather of the activation of a neural network that includes facilitatory corticospinal tract neurons.
(b). New visual properties of mirror neurons
Originally, the study of mirror neurons was focused on the match between visual and motor responses. This matching is, indeed, fundamental for the hypothesis that these neurons play a role in understanding the goal of motor acts. However, already in the early studies, some subcategories of mirror neurons, based on their specific visual properties, were identified. These subcategories included neurons showing selectivity for specific space positions, right or left hand, and for specific directions [3]. These previous observations have been recently extended, as will be described below.
(i). Space-sensitive mirror neurons
One of the issues addressed in these new studies is that of the possible influence on mirror neuron discharge of the spatial location of the observed actions. Caggiano et al. [37] performed a study in which they tested mirror neurons recorded from area F5, while the experimenter grasped objects in either the space around the monkey (peripersonal space) or in the space outside its reach (extrapersonal space; figure 3a). All neurons were also tested during active grasping by the monkey.
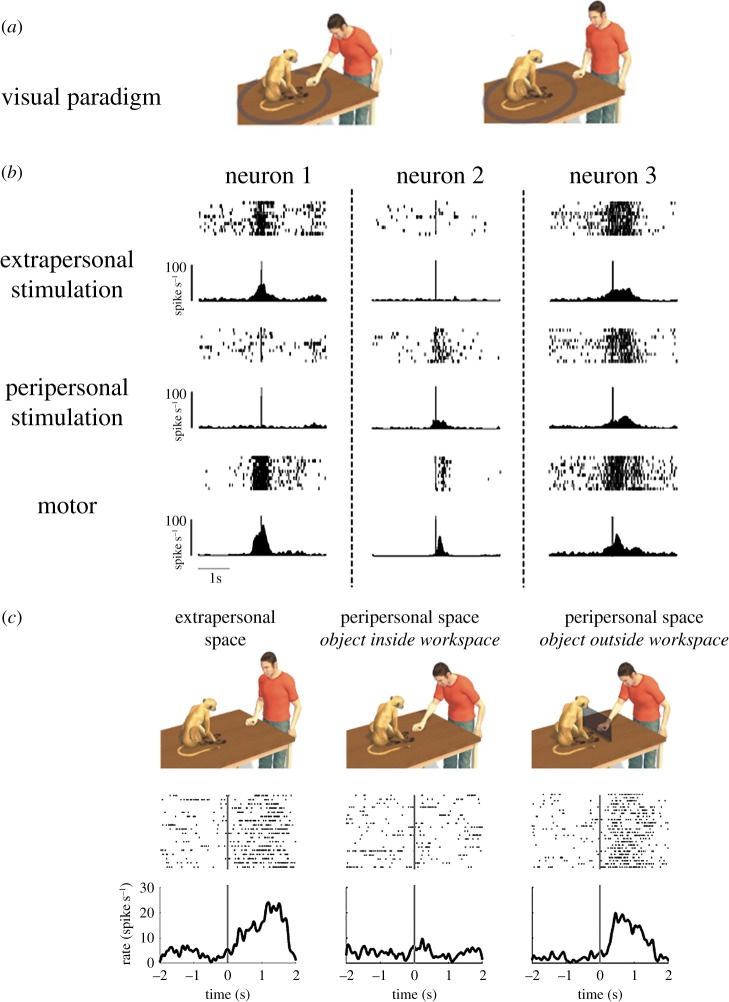
Figure 3.
Space-selective mirror neurons. (a) Schematic view of the visual conditions of the experimental paradigm: the monkey observes an experimenter executing a grasping act in the peripersonal (left) and extrapersonal (right) space of the monkey. (b) Examples of the responses of three mirror neurons during observation of grasping acts executed in the monkey's peri- and extrapersonal space and during monkey execution. Each panel shows the raster plot (top) and the cumulative histogram (bottom) of the neuron responses. Raster plots and histograms are aligned with the time of contact of the experimenter's or monkey hand with the object. Neuron 1 responds more strongly when the observed grasping is performed in the extrapersonal space, while neuron 2 presents a stronger discharge during observation of grasping performed in the peripersonal space. Neuron 3 does not show any space-selective visual response. All neurons discharge during grasping execution. (c) Operational encoding of the monkey peri- and extrapersonal space. The top part shows the experimental conditions: the experimenter grasps an object in the extrapersonal (left) or in the peripersonal space without (centre) or with (right) a frontal panel impairing the monkey's reach into its peripersonal space. Note that in this latter condition, the object (and the act performed by the experimenter) is metrically in the monkey's peripersonal space, but operationally outside it. The lower panels show the visual responses of a mirror neuron in the three conditions. The vertical lines mark the time of contact between the experimenter's hand and the object. Before closure with the frontal panel, the neuron was activated only during observation of grasping in the monkey's extrapersonal space. However, after closure, the neuron also responded to observation of grasping performed close to the monkey's body.
The results showed that the visual response of half of the recorded mirror neurons was influenced by the location in space of the observed motor acts, whereas the others did not show a differential modulation. Of those sensitive to action location in space, about half preferred the monkey's peripersonal space, the other half the extrapersonal space. Examples of the two types of space-selective neurons and one unselective neuron are shown in figure 3b.
Subsequently, the authors investigated whether space-selective neurons encode space in a ‘metric’ or in an ‘operational’ format. Metric format means that there is a fixed boundary between the two spaces, whereas operational format means that the space is dynamic and depends on the monkey's possibility to reach the objects. To study this issue, monkeys were tested with motor acts performed by the experimenter in the peripersonal and extrapersonal space. When neurons discharging differentially in the two space sectors were identified, a frontal transparent barrier was introduced that prevented the monkey from reaching for objects close to his body. About half of the tested space-selective mirror neurons were found to be influenced by the experimental manipulation (operational mirror neurons). More specifically, after closure of the transparent barrier, these neurons, when selective for the extrapersonal space, also started to respond in the peripersonal space, whereas when selective for the peripersonal space, they ceased to respond (figure 3c). In other words, the introduction of the barrier blocked the monkey's possibility to act in its reaching space, thus shrinking its space representation. As a consequence, an observed action performed in its peripersonal space was now located in the new extrapersonal space. The other half of neurons code space in a metric way (Cartesian mirror neurons) and were not influenced by the introduction of the panel.
A possible functional role of space-sensitive mirror neurons is that they may set which is the most appropriate behavioural reaction according to the location of the observed action in space. Peripersonal space suggests a possible immediate interaction with the action agent, whereas extrapersonal space implies a more complex behavioural pattern in order to interact with the acting individual. This interaction could be cooperative or competitive.
An interesting issue is which mechanism it is that may determine the spatial properties of these neurons. The most likely candidate is area F4. Neurons in this area respond to tactile, visual and more rarely, auditory stimuli [11,12,38,39]. Their visual receptive fields (RFs) are located around the tactile ones and extend into the monkey peripersonal space. Most of these RFs are ‘operational’, in the sense that their depth extent is modulated by the speed of an approaching stimulus [12]. Because information coming from F4 reaches the adjacent area F5 [40], one might postulate that this input gates the visual responses of ‘operational’ mirror neurons.
(ii). View-dependent mirror neurons
Recently, it was investigated whether mirror neurons can also provide information concerning the perspective from which the motor acts of others are observed [41]. Preliminary to this study was an investigation aimed to establish the capacity of mirror neurons to respond not only to actions performed by an experimenter, as done in earlier studies, but also to video-clips representing hand actions. The results showed that, although the responses to video-clips were typically weaker than to live actions, nevertheless they were consistent and strong enough to be studied. Only a minority of F5 mirror neurons did not respond to video-clip presentation. These results were fundamental for studying the responsiveness of neurons to different perspectives because it allowed a standardized presentation of the stimuli.
After this preliminary investigation, mirror neurons of monkey area F5 were tested while presenting movies showing grasping motor acts from different visual perspectives (figure 4a). The three tested perspectives were subjective (0°), side (90°) and frontal (180°). The results showed that the majority of the tested mirror neurons (74%) were view-dependent, with responses tuned to one or, more frequently, to two specific points of view. Among the neurons specific for only one view, there was a slight, although not significant, preference for the subjective view. Finally, a minority of the tested mirror neurons (26%) exhibited view-independent responses. Examples of the four categories of neurons are shown in figure 4b.
Figure 4.
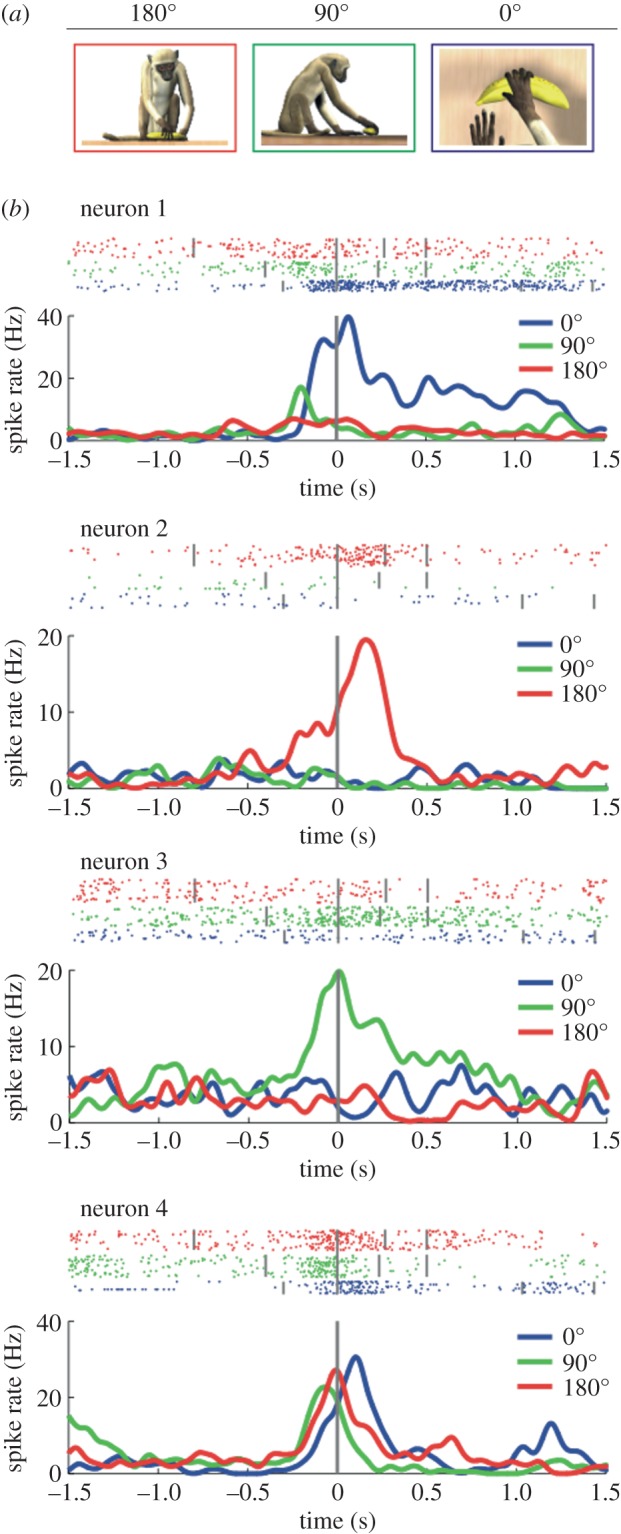
Responses of mirror neurons to grasping observed from three visual perspectives. (a) Experimental conditions (subjective view: 0°; side view: 90°; frontal view: 180°). (b) Examples of the responses of four mirror neurons during observation of grasping from the three perspectives. Rasters and histograms are temporally aligned (vertical grey line) with the moment at which the observed hand touches the object. Neuron 1 is selective for the subjective view, neuron 2 for the frontal view, neuron 3 for the side view. The activity of neuron 4 discharged equally well for all points of view.
A classical view in visual physiology is that view-independent neurons are generated by convergence of several lower-order neurons with view-dependent properties. In particular, this was postulated for STS neurons responding to biological stimuli, such as different head perspectives [42]. Thus, one may suggest that view-dependent mirror neurons in F5 represent an intermediate step leading to the formation of the view-independent ones. However, one must remember that mirror neurons are motor neurons, and their output target is always the same, regardless of what is the input triggering it. The message conveyed to other centres does not depend, therefore, on how a given neuron was triggered and whether the stimulus was subjective, side or frontal. Thus, both view-independent and view-dependent mirror neurons encode action goals, irrespective of the details of the observed motor acts.
What may be therefore the functional meaning of the view-dependent mirror neurons? An interesting possibility is that view-dependent mirror neurons play a role in the perception of the visual perspective of the observed actions. There is anatomical evidence that, in addition to feed-forward projections, the nodal areas of the mirror circuit plus STS are also connected by backward projections [33,34]. Hence, signals from view-dependent mirror neurons might contribute to action perception by modulating the activity of visual representations coded by STS neurons, reinforcing the processing of visual patterns that are associated with the different views of a grasping act. This hypothesis suggests that the understanding of action goal is carried out by mirror neurons, whereas the perception of specific details of the observed action is provided by higher-order visual neurons, following facilitation coming from mirror neurons (for a similar view in visual perception, see Hochstein & Ahissar [43]).
(iii). Mirror neurons sensitive to the value of an observed action
Objects grasped by an agent have a value not only for the individual performing that act, but also for the individual observing it. A recent study addressed the problem of whether F5 mirror neurons are involved in representing the value of the grasped object [44]. Three experiments were carried out.
In the first experiment, the responses of mirror neurons to the observation of grasping food were compared with those to the observation of grasping objects devoid of any value for the monkey. The results showed that about 70% of the studied mirror neurons were influenced by the type of the observed grasped object. More specifically, the large majority of these neurons were more strongly activated when grasping was directed towards food than when it was directed towards the object devoid of value.
These findings were confirmed in a second experiment, where the responses of mirror neurons were studied in response to the observation of grasping motor acts on objects associated with reward or on similar objects, but not rewarded. The data showed that about half of the tested mirror neurons exhibited stronger visual responses when the observed motor acts were performed on rewarded objects. Only a small percentage of neurons showed a stronger activity for non-rewarded objects. Finally, about 40% of neurons were not modulated by the presence or absence of reward associated with grasped objects.
In the third experiment, it was tested whether mirror neurons reflect differences in subjective value in a continuous rather than a categorical manner. In order to address this issue, the experiment was carried out by presenting to the monkey a series of blocks of grasping trials in which the experimenter grasped a non-food object. On a given block, the motor act directed to the object could be associated with one out of three types of consequences for the observing monkey: (i) the delivery of highly palatable food, (ii) the delivery of less palatable food or (iii) no reward at all. The results showed that about 50% of the tested neurons exhibited a significant effect of the stimulus subjective value. Among these value-sensitive mirror neurons, a large number (35%) showed selectivity for the favourite reward condition, whereas about half did not show any preference for the type of reward. Finally, a few neurons (about 15%) discharged stronger in response to the less favourite reward or even to the absence of a reward.
At first glance, this influence of the subjective value on mirror neuron responses may sound surprising. The traditional view on mirror neuron activity was that they were activated by the type of motor act, but not by the semantics of the object on which the motor act was performed. However, there is evidence from both fMRI and connectivity studies that one of the nodes of the mirror system (parietal area AIP) receives information not only from the lower bank of STS, but also from the inferotemporal lobe. Thus, in the case of food versus neutral object, it is possible to propose that the acquired value of food determines automatically the value-related response of mirror neurons. More difficult to describe is the anatomical circuit and the functional mechanism that may explain how neutral stimuli can acquire a value and influence the mirror neuron activity. There is rich evidence that in many parts of the brain, reward may influence the neuronal discharge during goal-directed motor acts [45]. As far as the frontal lobe is concerned, it has been demonstrated that neurons in both orbitofrontal cortex and cingulate sulcus have stronger activity when the monkey anticipates a larger reward [46–48]. This has been considered as the criterion for attributing to these areas the role of representing the reward value. Thus, we propose that these areas associate object and reward and their output concerning the reward value is pivotal in determining the value-related responses of mirror neurons. Note that the value that the observer attributes to the object that is grasped can be fundamental for selecting a possible behavioural response.
4. Motor intention and mirror neurons
It is important at the outset of this section to clarify some terminological aspects. We consider ‘movement’, ‘motor act’ and ‘action’ as terms describing different aspects of motor behaviour. We define as movement sensu stricto a simple joint displacement or orofacial twitches. For example, a finger flexion elicited by electrical or magnetic stimulation represents a typical movement. Motor act, we define as a series of movements organized in such a way as to achieve a specific motor goal. For instance, shaping of the hand in such a way as to grasp an object represents a typical motor act. Finally, we define an action as a sequence of fluently linked motor acts that, as its final outcome, determines the achievement of a behavioural goal. For example, reaching, grasping and bringing a piece of food to the mouth for eating represent a typical action.
Another important distinction is that between motor goal and motor intention. We define as motor goal the outcome of a motor act, whereas we define as motor intention the final outcome of an action that leads to positive or negative reinforcement. Although this distinction is somehow implicit in the definition of motor act and action, it is important to stress it because, frequently, the two terms are confused with each other.
(a). Coding of motor sequences in the parietal and premotor cortex
The same motor act may be part of different actions having different final behavioural goals. In order to assess the neural basis of this motor organization, single neurons were recorded from the IPL and tested in different actions [4].
Neurons discharging during the execution of grasping motor acts were tested in two main conditions. In the first, the monkey had to reach and grasp a piece of food and bring it to the mouth; in the second, the monkey had to reach and grasp an object and place it into a container (figure 5a, left). In the first condition, the monkey was allowed to eat the grasped food; in the second condition, it was rewarded with a piece of food given to it by the experimenter.
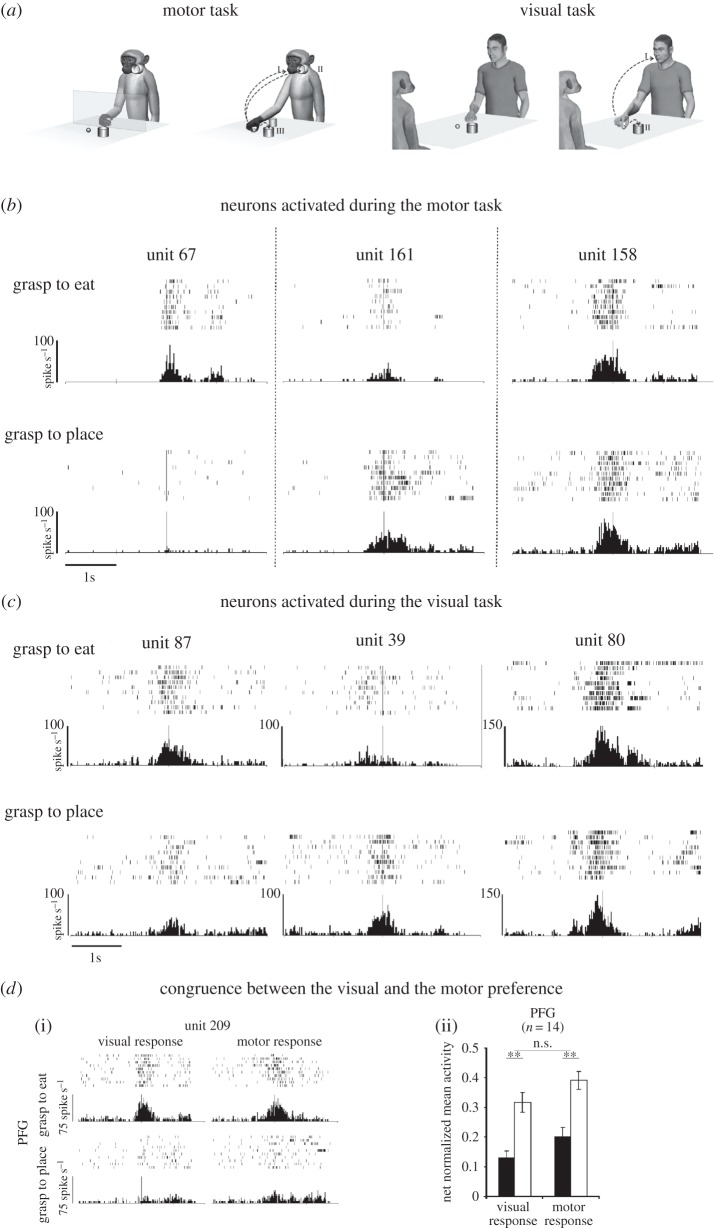
Figure 5.
(a) Motor task. The monkey, starting with its hand from a fixed position (left), reaches and grasps a piece of food (or an object), then it brings the food to the mouth and eats it (grasp-to-eat condition I) or places it (or the object) into a container (grasp-to-place condition) located near the mouth (II) or near the target (III). Visual task. The experimenter, starting with his hand from a fixed position (left), reaches and grasps a piece of food or an object (right), then he brings the food to the mouth and eats it (grasp-to-eat condition I) or places it (or the object) into a container located near the target (grasp-to-place condition II). (b) Examples of the discharge of three IPL neurons during the motor task. Neuron 67 is selective for grasping to eat, neuron 161 shows the opposite behaviour, while the response of neuron 158 is not affected by the action goal. (c) Examples of the discharge of three IPL neurons during the visual task. Neuron 87 discharges stronger during observation of grasping to eat, neuron 39, on the contrary, during observation of grasping to place, while neuron 80 discharges equally well in both conditions. In both (b) and (c), rasters and histograms are aligned (vertical bar) with the moment when the monkey or the experimenter, respectively, touched the food/object. (d) Congruence between the visual and the motor response of mirror neurons encoding action goal in area PFG. (i) Example of a neuron discharging stronger during grasping for eating than during grasping for placing, both when the action is executed and when it is observed. Conventions as in (b) and (c). (ii) Population-averaged responses, showing the same pattern of differential activity between the preferred (white bar) and not preferred (black bar) action during motor and visual tasks. Depending on the neuron, the preferred action could be grasp-to-eat or grasp-to-place.
The results showed that the discharge of the large majority of the recorded neurons was modulated depending on the action in which the grasping act was embedded. Examples are shown in figure 5b. Unit 67 discharged during grasping, when it was followed by bringing the food to the mouth. By contrast, the neuron was virtually silent when grasping was followed by placing the object into the container. Unit 161 is an example of a neuron discharging preferentially during grasping-to-place. Finally, unit 154 discharged in the same way in the two conditions.
In total, about two-thirds of neurons discharged preferentially when grasping was embedded into a specific action. Of them, the great majority (73%) preferred grasping for eating. In order to control that the differential discharge of neurons during the same motor act performed in the two conditions was not due to a mere difference in the stimuli to be grasped, monkeys were trained to grasp the same piece of food in both conditions. The results showed that neuron selectivity remained the same, not depending on the stimulus used.
Human studies have shown that the first component of an action is influenced by subsequent components of that action [49–53]. In other words, programming of a motor act takes into account the constraints posed by the target of the subsequent motor act. This point was tested in the two conditions of the experiment described above, measuring the kinematics of the reach-to-grasp motor act. It was found that this motor act, when followed by arm flexion (bringing the food to the mouth) was faster than the same motor act when followed by arm abduction (placing the food into the container). A third experimental condition was therefore introduced to render the reaching-to-grasp movement for placing kinematically similar to that for eating. In this new condition, the monkey had to grasp a piece of food and place it into a container located near the monkey mouth, so that the animal was required, as in the bringing to the mouth condition, to flex the arm. The results showed that the arm velocity in the new placing condition was faster both than in the original placing condition as well as than in the grasping-to-eat condition. These findings indicate that the differential discharge during grasping in the two actions did not depend on kinematic parameters.
In a subsequent study, neurons were recorded from both F5 and the IPL (area PFG) in order to compare the activity of F5 and PFG in motor sequence organization [54]. The same conditions as in the study described above were used. The results confirmed that a large number of F5 neurons code grasping according to the goal of the action in which it is embedded. The same effect was found also in PFG. A comparison of the neuronal properties of the two areas showed that there is a larger percentage of grasping neurons in area PFG that are modulated by the action in which they are embedded and that they are more strongly influenced by the action goal than neurons in F5.
It may sound surprising that most IPL neurons discharge differently for the same motor act depending on the final action goal. This arrangement might seem highly inefficient, because a large number of neurons with similar properties are required for executing different types of actions. There is, however, another aspect of motor organization that must be taken into consideration: the fluidity with which one motor act follows another. This fluidity is necessary, because action execution should occur without any gap. The results of the above-described studies suggest that neurons encoding specific motor acts within an action form pre-wired intentional chains, in which a neuron encoding a motor act is facilitated by the neuron encoding the previous one.
This type of action organization raises two questions. The first one is whether more complex actions present the same type of chain organization. The second one is how this high-level, goal-centred organization is translated into actual movement organization, in which kinematic parameters must be taken into consideration.
In order to answer the first question, an experiment was carried out in which monkeys were trained to perform complex action sequences [55]. Specifically, the monkey had to grasp the lid of a container and subsequently grasp the food or the object inside it in order to eat the food (first condition, eating) or to place the object into another container (second condition, placing). Neurons were recorded from both area PFG and area F5. Confirming the previous studies, a large number of PFG and F5 neurons displayed action-goal selectivity during the second grasping act, when the vision of the target allowed the monkey to perform the last part of the action (bringing to the mouth or placing). Notably, a small percentage of PFG neurons, but not neurons in F5, reflected the final goal from the early phase of action unfolding, when only memory-driven information was available. On the other hand, when monkeys could not see the target before action onset, neurons lost their early selectivity. These findings suggest that a higher-order chain organization does exist in PFG, but it is likely that other cortical areas, such as prefrontal cortex, play a crucial role in this organization.
As far as the second question is concerned, we have no empirical data to show how goal-related organization is translated into a kinematic-related organization. We know, however, as it will be described later, that a chain organization similar to that described at the neuronal level in the monkey also exists in humans and is reflected in the movement kinematics [56].
(b). Coding of observed motor sequences in the parietal and premotor cortex
As already mentioned, in the IPL, there are a considerable percentage of mirror neurons discharging both during grasping observation and grasping execution. In the same study in which the motor properties of IPL neurons were investigated during execution of action sequences, grasping mirror neurons were tested in a visual task in which the experimenter performed, in front of the monkey, the same actions that the monkey performed in the motor task, for example, grasping to eat and grasping to place [4]. The monkey had simply to observe the performed actions (figure 5a, right).
The results showed that while the discharge of some neurons was not influenced by the motor act following the observed grasping, the majority (75%) of mirror neurons discharged differently according to whether the grasping made by the experimenter was followed by bringing to the mouth or by placing. Examples are shown in figure 5c. Neurons responding to the observation of grasping for eating were the most commonly encountered.
In a subsequent experiment, grasping mirror neurons were recorded from area F5 as well as from area PFG, with the same experimental paradigm used in the study described above [54]. It was found that, as in PFG, also in F5, most grasping mirror neurons discharged differently during observation of grasping when this act was embedded into different actions. In both F5 and PFG, the large majority of neurons discharged more strongly during the eating condition than during the placing condition, but the prevalence of ‘eating-related’ mirror neurons was larger (90% versus 80%) in F5 than in PFG.
In a group of F5 and PFG mirror neurons, visual and motor selectivity for eating or placing was compared. The results showed that the great majority of the tested neurons showed the same specificity during grasping observation and grasping execution (figure 5d).
The most accepted interpretation of the role of mirror neurons is that they allow one to understand the goal of an observed motor act (see for review [57]). The above-described findings point to a new mechanism, based on mirror neurons, that allows one to encode differently two identical observed grasping motor acts according to the action in which these acts are embedded. This ability, based on context or on the past history (e.g. blocked design), enables the individual to understand others’ intentions. More specifically, the selection of a specific population of grasping neurons activates the same motor chain that the observers would activate when performing the observed action themselves. As discussed before concerning action execution, the activation of a given chain corresponds with a specific intention. Thus, the observation of a motor act, which triggers a given chain, would provide the observer with the capacity to understand the agent's specific intention. This mechanism allows an automatic, not inferential understanding of the motor intentions of others.
5. Coding motor intention in humans
In comparison with the enormous amount of studies devoted to action observation (see for review [16–21]), only a limited number of papers addressed the issue of understanding motor intention in humans. An early attempt to investigate the relation between intention coding and the mirror mechanism was done by Iacoboni et al. [58]. This fMRI study was formed by three experimental conditions. In the first, referred to as ‘context’, the subjects were instructed to observe a scene composed of objects arranged either as for the beginning of a breakfast or as for the end of it. In the second, referred to as ‘action’, the subjects had to observe a hand grasping a mug on an empty background. In the third condition, referred to as ‘intention’, the subjects had to observe the same hand motor act embedded in one of the two contexts of the ‘context’ condition. The context suggested the agent's intention, and specifically the scene representing breakfast beginning suggested that the intention of the agent was to grasp the mug for drinking, whereas the scene representing breakfast end suggested that the intention of the agent was to grasp it for cleaning the table. The results showed that areas belonging to the cortical mirror system were activated in both ‘action’ and ‘intention’ conditions. However, the contrast between ‘intention’ and ‘action’ conditions showed activation in the right caudal inferior frontal gyrus.
The conclusion that the right hemisphere is involved in understanding the intention of others has been also reached by Ortigue et al. [59] using high-density EEG study. They investigated the temporal dynamics of brain activations produced by the observation of motor acts of subjects instructed to understand the agent's intention. The results revealed that after an initial bilateral activation of occipital and temporo-parietal areas, there was a selective activation of the right temporo-parietal region. This late activation was interpreted by the authors as related to the understanding of intentions of others.
In a study prompted by the findings of Fogassi et al. [4] in the monkey (see above), Cattaneo et al. [56] asked typically developing children to grasp a piece of food for eating it or to grasp a piece of paper for placing it into a container. In a subsequent experimental condition, the same children had to observe an experimenter performing the same actions. In both conditions, the electromyography activity of a muscle involved in mouth opening (mylohyoid muscle, MH) was recorded. It was found that during execution there was an increase of MH activity already during the phase in which the hand was approaching the object, about 800 ms before actual grasping. A precocious activation of MH was also found during the observation of the eating action. By contrast, no MH activity increase was found during execution and observation of placing. These findings suggest that humans are endowed with a chain organization similar to that of the monkey (see above), allowing, on the one hand, the execution of fluent motor actions, and on the other hand, most interestingly, the understanding of motor intentions of others.
The studies discussed above indicate that the mirror mechanism might explain some instances of intention understanding. However, intention understanding encompasses different levels, ranging from motor intention comprehension to intention understanding based on propositional attitudes such as beliefs or desires. Thus, other mechanisms, beside that based on the mirror mechanism, are likely to be involved.
Recent fMRI experiments support these theoretical considerations. Brass et al. [60] asked subjects to observe several types of unusual actions in plausible and implausible contexts. An activation of the mirror network was present in both context conditions. However, when comparing actions performed in an implausible context versus actions performed in a plausible context, there were additional activations of the STS region and of the anterior mesial frontal cortex (see also [61] for similar observations). The activated areas are considered to be part of the so-called mentalizing network [62], that is that network that becomes active when an observer predicts someone else's behaviour based on his/her belief state.
We mentioned that there are different levels of intention understanding. They should correspond to different mechanisms. As discussed in this section, understanding action intention relies on the mirror mechanism and the motor chain organization of the cortical motor system. By contrast, understanding the reasons underlying a given action appears to be localized in areas, such as the anterior mesial frontal cortex and the right temporo-parietal junction, which do not seem to have mirror properties. Several attempts have been made to integrate these two kinds of intention understanding (see [62]). However, it is important to stress that there are currently no neurophysiological data that can account for the mechanisms underlying the ‘mentalizing network’.
6. Conclusion
The mirror mechanism has raised great interest over the past two decades, because it allows one to unify action production and action observation. This motor-based action and intention understanding appears to represent the primary way for interindividual interactions. This is corroborated by its presence not only in humans and monkeys, but also in evolutionarily distant vertebrate classes, such as birds [63,64]. It would be of great interest to learn whether the mirror mechanism also exists in other mammalian species. Its demonstration in rodents would be particularly interesting. Besides its theoretical interest, this would allow the characterization of the mirror mechanism at a neurochemical level.
Another extremely interesting issue to be addressed is the relationship between the basic mirror circuit and other circuits that, in the monkey, can exert a top-down control. The data from Buccino et al. [65] in humans suggest that during imitation learning, the prefrontal lobe (area [46]) is playing a fundamental role in the reorganization of the basic information about the motor acts of others captured by the mirror system. A similar kind of ability could be studied in the monkey to understand in depth how the interaction between prefrontal lobe and the parieto-premotor mirror circuit takes place. In a similar vein, it will be of great interest to assess in more controlled conditions the findings of Mukamel et al. [15] in humans showing the activation of hippocampal cells during action observation, and in particular whether this hippocampal activation is related to episodic memory.
Finally, the link between the mirror mechanism and mentalizing will be achieved only if it becomes possible to record single neurons from awake human beings during such processes. Currently, this technology has been used only in some laboratories in epileptic patients, exploiting special electrodes placed intracortically in deep mesial structures [15,66]. It is likely that in the near future modifications of classical macroelectrodes for intracortical EEG recordings will also allow the recording of single neurons from other neural structures, thus enabling the investigation of neuronal responses during cognitive tasks, in particular those requiring reasoning about the behaviour of others.
Funding statement
This study was supported by the European Grant ‘Cogsystems’, by the Italian institute of technology (IIT), and by Italian PRIN to L.F.
References
- 1.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180. ( 10.1007/BF00230027) [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. 1996. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 3, 131–141. ( 10.1016/0926-6410(95)00038-0) [DOI] [PubMed] [Google Scholar]
- 3.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. 1996. Action recognition in the premotor cortex. Brain 119, 593–609. ( 10.1093/brain/119.2.593) [DOI] [PubMed] [Google Scholar]
- 4.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science 308, 662–667. ( 10.1126/science.1106138) [DOI] [PubMed] [Google Scholar]
- 5.Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. 2008. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur. J. Neurosci. 28, 1569–1588. ( 10.1111/j.1460-9568.2008.06395.x) [DOI] [PubMed] [Google Scholar]
- 6.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. 1988. Functional organization of inferior area 6 in the macaque monkey: I. Somatotopy and the control of proximal movements. Exp. Brain Res. 71, 475–490. ( 10.1007/BF00248741) [DOI] [PubMed] [Google Scholar]
- 7.Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. 1988. Functional organization of inferior area 6 in the macaque monkey: II. Area F5 and the control of distal movements. Exp. Brain Res. 71, 491–507. ( 10.1007/BF00248742) [DOI] [PubMed] [Google Scholar]
- 8.Umiltà MA, Escola L, Intskirveli I, Grammont F, Rochat M, Caruana F, Jezzini A, Gallese V, Rizzolatti G. 2008. How pliers become fingers in the monkey motor system. Proc. Natl Acad. Sci. USA 105, 2209–2213. ( 10.1073/pnas.0705985105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. 1997. Object representation in the ventral premotor cortex (area F5) of the monkey. J. Neurophysiol. 78, 2226–2230. [DOI] [PubMed] [Google Scholar]
- 10.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. 1995. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 18, 314–320. ( 10.1016/0166-2236(95)93921-J) [DOI] [PubMed] [Google Scholar]
- 11.Gentilucci M, Scandolara C, Pigarev IN, Rizzolatti G. 1983. Visual responses in the postarcuate cortex (area 6) of the monkey that are independent of eye position. Exp. Brain Res. 50, 464–468. [DOI] [PubMed] [Google Scholar]
- 12.Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. 1996. Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 76, 141–157. [DOI] [PubMed] [Google Scholar]
- 13.Rizzolatti G, Fadiga L. 1998. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5). Novartis Found Symp. 218, 81–95. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K, Saito N, Iriki A, Isoda M. 2011. Representation of others’ action by neurons in monkey medial frontal cortex. Curr. Biol. 21, 249–253. ( 10.1016/j.cub.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 15.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. 2010. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 20, 750–756. ( 10.1016/j.cub.2010.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. 2008. The human inferior parietal lobule in stereotaxic space. Brain Struct. Funct. 212, 481–495. ( 10.1007/s00429-008-0195-z) [DOI] [PubMed] [Google Scholar]
- 17.Cattaneo L, Rizzolatti G. 2009. The mirror neuron system. Arch. Neurol. 66, 557–560. ( 10.1001/archneurol.2009.41) [DOI] [PubMed] [Google Scholar]
- 18.Fabbri-Destro M, Rizzolatti G. 2008. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda) 23, 171–179. ( 10.1152/physiol.00004.2008) [DOI] [PubMed] [Google Scholar]
- 19.Van Overwalle F, Baetens K. 2009. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48, 564–584. ( 10.1016/j.neuroimage.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 20.Molenberghs P, Cunnington R, Mattingley JB. 2012. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 36, 341–349. ( 10.1016/j.neubiorev.2011.07.004) [DOI] [PubMed] [Google Scholar]
- 21.Fogassi L, Simone L. 2013. The mirror system in monkeys and humans and its possible motor-based functions. Adv. Exp. Med. Biol. 782, 87–110. ( 10.1007/978-1-4614-5465-6_5) [DOI] [PubMed] [Google Scholar]
- 22.Matelli M, Luppino G, Rizzolatti G. 1985. Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav. Brain Res. 18, 125–136. ( 10.1016/0166-4328(85)90068-3) [DOI] [PubMed] [Google Scholar]
- 23.Matelli M, Luppino G, Rizzolatti G. 1991. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J. Comp. Neurol. 311, 445–462. ( 10.1002/cne.903110402) [DOI] [PubMed] [Google Scholar]
- 24.Belmalih A, Borra E, Contini M, Gerbella M, Rozzi S, Luppino G. 2009. Multimodal architectonic subdivision of the rostral part (area F5) of the macaque ventral premotor cortex. J. Comp. Neurol. 512, 183–217. ( 10.1002/cne.21892) [DOI] [PubMed] [Google Scholar]
- 25.Pandya DN, Seltzer B. 1982. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 204, 196–210. ( 10.1002/cne.902040208) [DOI] [PubMed] [Google Scholar]
- 26.Gregoriou GG, Borra E, Matelli M, Luppino G. 2006. Architectonic organization of the inferior parietal convexity of the macaque monkey. J. Comp. Neurol. 496, 422–451. ( 10.1002/cne.20933) [DOI] [PubMed] [Google Scholar]
- 27.Rizzolatti G, Luppino G, Matelli M. 1998. The organization of the cortical motor system: new concepts. Electroencephalogr. Clin. Neurophysiol. 106, 283–296. ( 10.1016/S0013-4694(98)00022-4) [DOI] [PubMed] [Google Scholar]
- 28.Rizzolatti G, Fogassi L, Gallese V. 2009. The mirror neuron system: a motor-based mechanism for action and intention understanding. In The cognitive neuroscience IV (ed. Gazzaniga M.), pp. 625–640. Cambridge, MA: MIT Press. [Google Scholar]
- 29.Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. 2002. Hearing sounds, understanding actions: action representation in mirror neurons. Science 297, 846–848. ( 10.1126/science.1070311) [DOI] [PubMed] [Google Scholar]
- 30.Perrett DI, Harries MH, Bevan R, Thomas S, Benson PJ, Mistlin AJ, Chitty AK, Hietanen JK, Ortega JE. 1989. Frameworks of analysis for the neural representation of animate objects and actions. J. Exp. Biol. 146, 87–113. [DOI] [PubMed] [Google Scholar]
- 31.Nelissen K, Borra E, Gerbella M, Rozzi S, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. 2011. Action observation circuits in the macaque monkey cortex. J. Neurosci. 31, 3743–3756. ( 10.1523/JNEUROSCI.4803-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. 2010. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb. Cortex 20, 141–168. ( 10.1093/cercor/bhp087) [DOI] [PubMed] [Google Scholar]
- 33.Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. 2008. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex 18, 1094–1111. ( 10.1093/cercor/bhm146) [DOI] [PubMed] [Google Scholar]
- 34.Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. 2006. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex 16, 1389–1417. ( 10.1093/cercor/bhj076) [DOI] [PubMed] [Google Scholar]
- 35.Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. 2009. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron 64, 922–930. ( 10.1016/j.neuron.2009.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigneswaran G, Philipp R, Lemon RN, Kraskov A. 2013. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol. 23, 236–243. ( 10.1016/j.cub.2012.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caggiano V, Fogassi L, Rizzolatti G, Their P, Casile A. 2009. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science 324, 403–406. ( 10.1126/science.1166818) [DOI] [PubMed] [Google Scholar]
- 38.Graziano MS, Yap GS, Gross G. 1994. Coding the visual space by premotor neurons. Science 266, 1054–1057. ( 10.1126/science.7973661) [DOI] [PubMed] [Google Scholar]
- 39.Graziano MS, Hu XT, Gross CG. 1997. Visuospatial properties of ventral premotor cortex. J. Neurophysiol. 77, 2268–2292. [DOI] [PubMed] [Google Scholar]
- 40.Matelli M, Camarda R, Glickstein M, Rizzolatti G. 1986. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J. Comp. Neurol. 251, 281–298. ( 10.1002/cne.902510302) [DOI] [PubMed] [Google Scholar]
- 41.Caggiano V, Fogassi L, Rizzolatti G, Pomper JK, Their P, Giese MA, Casile A. 2011. View-based encoding of actions in mirror neurons of area F5 in macaque premotor cortex. Curr. Biol. 21, 144–148. ( 10.1016/j.cub.2010.12.022) [DOI] [PubMed] [Google Scholar]
- 42.Perrett DI, Mistlin AJ, Chitty AJ. 1987. Visual cells responsive to faces. Trends Neurosci. 9, 358–364. ( 10.1016/0166-2236(87)90071-3) [DOI] [Google Scholar]
- 43.Hochstein S, Ahissar M. 2002. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron 36, 791–804. ( 10.1016/S0896-6273(02)01091-7) [DOI] [PubMed] [Google Scholar]
- 44.Caggiano V, Fogassi L, Rizzolatti G, Casile A, Giese MA, Thier P. 2012. Mirror neurons encode the subjective value of an observed action. Proc. Natl Acad. Sci. USA 109, 11 848–11 853. ( 10.1073/pnas.1205553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz W. 2000. Multiple reward signals in the brain. Nat. Rev. Neurosci. 1, 199–207. ( 10.1038/35044563) [DOI] [PubMed] [Google Scholar]
- 46.Roesch MR, Olson CR. 2003. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J. Neurophysiol. 90, 1766–1789. ( 10.1152/jn.00019.2003) [DOI] [PubMed] [Google Scholar]
- 47.Roesch MR, Olson CR. 2007. Neuronal activity related to anticipated reward in frontal cortex: does it represent value or reflect motivation? Ann. N.Y. Acad. Sci. 1121, 431–446. ( 10.1196/annals.1401.004) [DOI] [PubMed] [Google Scholar]
- 48.Maunsell JH. 2004. Neuronal representations of cognitive state: reward or attention? Trends Cogn. Sci. 8, 261–265. ( 10.1016/j.tics.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 49.Marteniuk RG, MacKenzie CL, Jeannerod M, Athenes S, Dugas C. 1987. Constraints on human arm movement trajectories. Can. J. Psychol. 41, 365–378. ( 10.1037/h0084157) [DOI] [PubMed] [Google Scholar]
- 50.Jeannerod M. 1988. The neural and behavioural organization of goal-directed movements. New York, NY: Clarendon Press/Oxford University Press. [Google Scholar]
- 51.Johnson-Frey SH, McCarty M, Keen R. 2004. Reaching beyond spatial perception: effects of intended future actions on visually-guided prehension. Vis. Cogn. 11, 371–399. ( 10.1080/13506280344000329) [DOI] [Google Scholar]
- 52.Rosenbaum DA, Cohen RG, Jax SA, Weiss DJ, van der Wel R. 2007. The problem of serial order in behavior: Lashley's legacy. Hum. Mov. Sci. 26, 525–554. ( 10.1016/j.humov.2007.04.001) [DOI] [PubMed] [Google Scholar]
- 53.Fabbri-Destro M, Cattaneo L, Boria S, Rizzolatti G. 2009. Planning actions in autism. Exp. Brain Res. 192, 521–525. ( 10.1007/s00221-008-1578-3) [DOI] [PubMed] [Google Scholar]
- 54.Bonini L, Rozzi S, Ugolotti SF, Simone L, Ferrari PF, Fogassi L. 2010. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb. Cortex 20, 1372–1385. ( 10.1093/cercor/bhp200) [DOI] [PubMed] [Google Scholar]
- 55.Bonini L, Ugolotti SF, Simone L, Rozzi S, Ferrari PF, Fogassi L. 2011. Grasping neurons of monkey parietal and premotor cortices encode action goals at distinct levels of abstraction during complex action sequences. J. Neurosci. 31, 5876–5887. ( 10.1523/JNEUROSCI.5186-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, Rizzolatti G. 2007. Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl Acad. Sci. USA 104, 17 825–17 830. ( 10.1073/pnas.0706273104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzolatti G, Sinigaglia C. 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 11, 264–274. ( 10.1038/nrn2805) [DOI] [PubMed] [Google Scholar]
- 58.Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. 2005. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 3, e79 ( 10.1371/journal.pbio.0030079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortigue S, Sinigaglia C, Rizzolatti G, Grafton ST. 2010. Understanding actions of others: the electrodynamics of the left and right hemispheres. A high-density EEG neuroimaging study. PLoS ONE 5, e12160 ( 10.1371/journal.pone.0012160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brass M, Schmitt RM, Spengler S, Gergely G. 2007. Investigating action understanding: inferential processes versus action simulation. Curr. Biol. 17, 2117–2121. ( 10.1016/j.cub.2007.11.057) [DOI] [PubMed] [Google Scholar]
- 61.Liepelt R, Von Cramon DY, Brass M. 2008. How do we infer other's goals from non stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage 43, 784–792. ( 10.1016/j.neuroimage.2008.08.007) [DOI] [PubMed] [Google Scholar]
- 62.Frith CD. 2007. The social brain? Phil. Trans. R. Soc. B 362, 671–678. ( 10.1098/rstb.2006.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prather JF, Peters S, Nowicki S, Mooney R. 2008. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451, 249–250. ( 10.1038/nature06492) [DOI] [PubMed] [Google Scholar]
- 64.Keller GB, Hahnloser RH. 2009. Neural processing of auditory feedback during vocal practice in a songbird. Nature 457, 187–190. ( 10.1038/nature07467) [DOI] [PubMed] [Google Scholar]
- 65.Buccino G, Vogt S, Ritzl A, Fink G, Zilles K, Freund H, Rizzolatti G. 2004. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 42, 323–333. ( 10.1016/S0896-6273(04)00181-3) [DOI] [PubMed] [Google Scholar]
- 66.Kreiman G, Koch C, Fried I. 2000. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat. Neurosci. 3, 946–953. ( 10.1038/78868) [DOI] [PubMed] [Google Scholar]