Abstract
Communication of symptoms is integral to quality patient care in any setting. However, communication between patients and nurses in the Intensive Care Unit (ICU) is complicated by oral or endotracheal intubation and fluctuating neurocognitive status or delirium. We report the 1) prevalence of delirium and delirium subtypes in a group of non-vocal, mechanically ventilated, critically ill patients; 2) impact of age on delirium presentation; and 3) influence of delirium and age on symptom communication over time. Interactions between mechanically ventilated, critically ill adults (N=89) and their nurses (N=30) were video recorded and analyzed for evidence of patient communication about symptoms at 4 time points across two consecutive days. Delirium was measured at study enrollment and immediately following video recording sessions for a total of 5 time points. Delirium prevalence on study enrollment was 23.6% and for observational time point was 28.7%. Participants aged 60 and older were more likely to be delirious on enrollment and during the videorecorded sessions. Older age (≥ 60 years) was associated with self-report of pain, drowsiness and cold. Delirium was associated with self-reported dry mouth.
Keywords: Older adults, critical illness, mechanical ventilation, communication, delirium, symptom management
Accurate symptom identification during critical illness is affected by patient communication difficulties in ICU1. Symptom management, an essential component of quality patient care, begins with the assessment and identification of symptoms from the patient’s perspective. Problems in symptom communication arise from structural obstacles to speech related to endotracheal intubation and mechanical ventilation. In addition, communication difficulties can develop as a result of cognitive changes that affect perception, attention or level of consciousness, which are all hallmark characteristics of delirium.
Delirium , a condition that frequently accompanies episodes of critical illness, 2, 3. represents a significant public health problem with profound impact on patient outcomes such as mortality4-7, length of hospital stay8, 9, duration of mechanical ventilation 10, and increased hospital costs 8, 11 . Generally, delirium is reversible, however, persistent long-term cognitive dysfunction is associated with delirium in critical illness12-18. Older adults are at greater risk for developing delirium during acute and critical illness19, 20. Delirium experienced by older adults is associated with increased mortality21, post-surgical complications 22, increased duration of mechanical ventilation and hospital length of stay23, increased care requirements at discharge 21-24, and decline in post-discharge physical function22.
Delirium is distinguished by symptoms of inattention with acute onset, fluctuating severity and changes in level of consciousness25, 26. Delirium can be further examined by motoric subtype20. Delirium presentation with increased motor movement is classified as hyperactive and delirium presentation with decreased motor movement is classified as hypoactive. Hypoactive delirium is most often misidentified because patients are quiet and less responsive27, 28. When examined longitudinally, critically ill patients most often experience a mixed type of delirium20 although older adults experience hypoactive delirium most often20. Delirium may further compromise patients’ ability to communicate their symptoms or symptom distress.
The effect of age and delirium on patients’ ability to communicate their symptom experience has not been studied. Furthermore, the impact of delirium on day-to-day care in the ICU is unknown. This secondary analysis of the Study of Patient-Nurse Effectiveness with Assisted Communication Strategies (SPEACS) study data provided an opportunity to explore the role age and delirium have on symptom communication over a two-day period.
For this study, we posed the following research questions;
What is the prevalence of delirium and delirium subtypes in a sample of intubated ICU patients across 5 time-points?
How does age impact delirium occurrence and/or presentation in this sample over time?
Is there a difference in symptom identification and initiation of symptom discussion by patients in delirious states than when patients are not delirious?
Is there a difference in frequency of symptoms identified and rate of initiation of symptom discussion between patients who are under age 60 and those age 60 and over?
METHODS
Design
We conducted a secondary analysis of data from a quasi-experimental sequential cohort clinical trial29. The SPEACS trial measured the effect of a multi-level -intervention (nurse education, communication materials and specialist support) to improve communication performance between critical care nurses and non-vocal ICU patients. SPEACS study design and preliminary results are detailed elsewhere 29-32.The University of Pittsburgh Institutional Review Board approved this sub-study.
Setting and Participants
Briefly, we recruited 89 intubated critically ill adults from a 32-bed medical ICU (MICU) and a 22-bed cardiovascular ICU (CTICU). Patients with previous history of cognitive, speech, hearing or language impairment were excluded. Participants were over 21 years of age, able to understand English, scored ≥ 13 on the Glasgow Coma Scale (GCS), nonspeaking due to endotracheal intubation and likely to remain intubated for at least 48 hours. 33. We obtained informed consent from participants or their proxy.
After enrollment, participants were assigned to be cared for by a study nurse for two consecutive days. Nurse participants were employed with permanent assignment to one of the study ICUs, had at least one year critical care experience, and regularly worked two consecutive day shifts. Nurses were excluded from study participation if they had a diagnosed hearing or language problem. All study nurses provided informed consent. Since the focus of this study was on patients, all references to “participants” relate to patient participants.
Data collection
Measures
Patient Participant level variables
We collected participant information that related to communication performance and clinical demographics such as age, presence of delirium, and sedation status. We measured delirium using the Confusion Assessment Method – ICU (CAM-ICU) 25, 26, and sedation level using Richmond Agitation and Sedation Scale (RASS) 34. The CAM-ICU is a well-accepted and most frequently used measure of delirium in the ICU demonstrating high sensitivity and specificity when compared with criteria from the American Psychiatric Association. The CAM-ICU has high inter-rater reliability and predictive validity 25, 26 . Administration is simple and requires minimal time. The Richmond Agitation and Sedation Scale is a valid and reliable measure of depth and quality of sedation in the ICU patient. It has demonstrated high degree of inter-rater reliability and is highly correlated with physiologic measures of sedation level such as EEG and bi-spectral index (BIS)34 . We defined delirium subtypes as per Peterson, et al 20, i.e., delirium is hypoactive when the corresponding RASS score is 0 or lower; delirium is hyperactive when corresponding RASS is 1 or greater.
All demographic data, (i.e., age in years, gender, race/ethnicity) were collected from the electronic medical record and entered on TELEform (Version 10, Cardiff, Inc., Vista, CA) that were optically scanned into an Oracle (version 10g, Oracle Corp., Redwood Shores, CA) database.
Identification of symptom communication
Trained coders reviewed each video-recording to determine symptom communication. To best quantify the participant’s symptom communication, full video recorded sessions (range = 3 - 30 minutes, ~ 10 minutes on average) were analyzed assuming that nurses had relatively equal opportunities to elicit symptoms from participants across all time points. Nurses’ notes in the electronic medical record were reviewed for additional information about the participant’s symptom communication.
Data were collected via the investigator-developed ICU Symptom Management Record (SMR), which is a modification of chart abstraction tools originally developed and tested for pain assessment and treatment with critically ill intubated participants35. This modification expanded the tool to address other major symptoms experienced by intubated participants in the ICU and delineated participant self-report from nurse assessment and interpretation. The SMR includes the checklist of 15 symptoms from the C-MSAS which coders evaluated as present or absent in the video recorded dialogue between nurse and participant and in EMR nursing documentation.
To achieve consistency between raters, definitions were developed for each symptom from an extensive review of the literature. Definitions were refined during expert review and pilot testing. Additional symptoms, such as hot/cold, discomfort, frustrated, confused, scared, and an “other” category were added after preliminary use and pilot testing. The final SMR checklist contained 21 symptom categories and their descriptions. For the purposes of this study, when a symptom was identified as ‘present’, its identification was further explained by description of how the symptom was reported and by whom: (a) Participant self-report in video (e.g., Participant nods affirmatively when nurse asks if he is having pain), (b) Participant self-report documented in EMR (e.g., nursing notes read “patient complained of /nods ‘yes’ to/dyspnea”), and (c) Who (nurse or participant) initiated the symptom discussion.
Medical record abstraction was conducted to determine nurses’ documentation of patient reports of symptom communication. Nurses noted participant communication about a symptom using phrases such as “patient states…”; “patient complains of…”; “patient treated for complaint of…”. Nurse description s of treatments without a symptom label or phrases that were equivocal such as “patient appears …” or “patient has …” were excluded. Instances of participant initiated discussions or requests about symptoms were recorded from video evidence if participants summoned the nurse and communicated a symptom successfully to the nurse. Participant initiated discussions are important indicators of a higher level of participant communication ability and independence 36.
Presence or absence of participant self-report of symptoms from either the video recorded sessions or from the nurses notes were recorded onto a Teleform™ and optically scanned into the database. Raters achieved > 80% agreement on the presence/ absence of symptoms and treatment. Kappa (κ ) values for symptom identification ranged from .504 – 1.00, moderate to perfect agreement37. Several symptoms (nausea, worry, shortness of breath- not weaning, difficulty sleeping, confused, discomfort) were not able to be evaluated due to low occurrence/ low variability in the interrater reliability sample. Most (n=8) had κ values above .74 indicating substantial agreement37, 38.
Statistical analysis
All analyses were conducted using SAS (version 9.2, SAS Institute, Inc. Cary, NC) and EXCEL 2007 (Microsoft Corp., Redmond, WA). The level of significance was set at .05 for two-sided hypothesis testing. Age was dichotomized into two categories (<60 and ≥ 60 years). Participant self-reported symptoms were dichotomized (present or absent from either video or EMR source). Descriptive statistics (frequencies and percentages) were computed to summarize categorical variables (e.g., gender, age, CAM-ICU, delirium motoric subtypes, symptoms and participant initiation of symptom communication). Categories of delirium trajectories were defined as present for all five time points (enrollment and four study sessions), absent for all five time points and mixed or having at least one measurement where delirium was present and one where delirium was absent. Descriptive statistics (means, standard deviations and range) were computed for age as a continuous variable for description of the sample only.
Chi-square statistic was used to determine the relationship of delirium at enrollment and age category.
We employed marginal modeling with generalized estimating equations (GEE) and Wald statistics to assess the relationship of delirium presence with participant self-report of symptoms communicated and participant initiation of symptom discussion. We assumed a binomial response using cumulative logits, respectively. This approach was appropriate because of the repeated nature of the data, and the likelihood that symptoms or self-report might be correlated between individual participant sessions. We analyzed the effect of delirium and age category univariately and as a contrasted model.
RESULTS
Participant Characteristics
The average age in this sample was 56.81 years, with an age range or 24 -87 years and 40 (45%) participants were aged 60 or older. The sample included 44 (49.4%) male and 10 (11.2%) non-white participants.
Delirium
At enrollment, delirium was present in 21 (23.6%) participants. Older participants (≥age 60) were significantly more likely to test positive for delirium on enrollment (OR: 3.23, 95% CI:1.15-9.06 p=.02). Four participants (4.5%) had delirium at enrollment only.
There were missing data from delirium measures during 21 video observation sessions. These missing were at random. Two participants had delirium measures missing for all four sessions and 6 other participants had delirium measures missing from 1-3 sessions. Some of these missing data were a result of participant refusal to complete the CAM-ICU; participant availability, i.e., rapid participant transport off the ICU; or participant physiologic or psychological state, i.e., fatigue, decreased level of consciousness, or acute changes in physiologic state. These missing data were excluded from the analysis and construction of delirium trajectories.
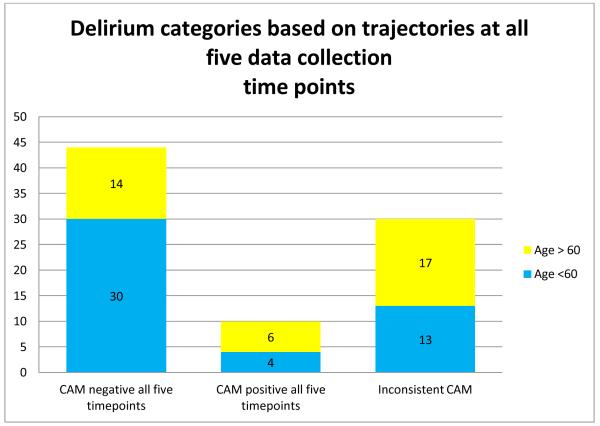
About half of all participants (44, 49.4%) were not delirious at any time during this study. Of the 356 observation sessions, 38 participants (42.7%) tested CAM positive (delirious) during at least one session. Ten (10) participants (12%) were delirious at all 5 data collection time points and 44 participants (49.4%) were delirium-free at all 5 time points. Age 60 and older was not significantly associated with delirium seen consistently over the five data collection time points (p=.07). (Figure 1)
Figure 1. Delirium over 5 timepoints.
*5 Cases with Missing Delirium Data – Unable to classify
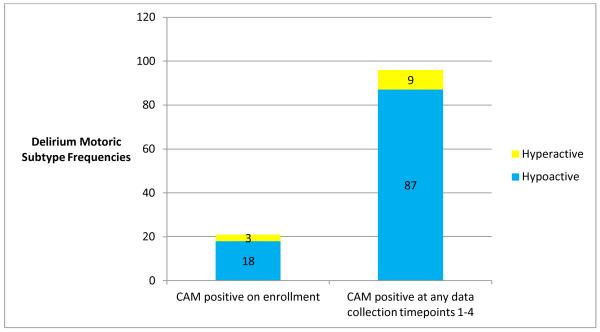
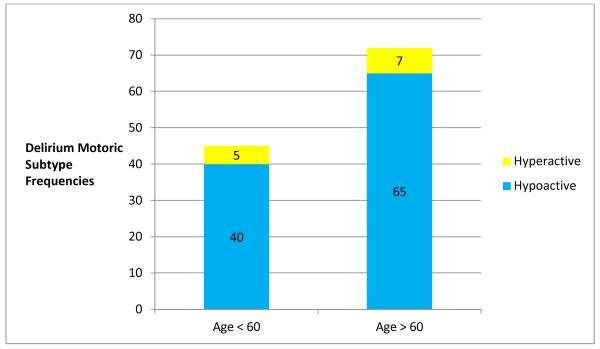
Delirium was present in 96 (28.7%) videorecorded sessions. Of these sessions, older adults were represented with a significantly greater frequency (58/96 sessions) than those participants who were younger (OR: 2.62, 95% CI: 1.19-5.77, p=.016). Hypoactive delirium was seen most frequently (87/96 sessions, 90.6%) in the entire group. When examined longitudinally, most participants who had delirium had the hypoactive subtype (n=31, 81.5%) and 7 participants had a mixed subtype of delirium, meaning that during some observation sessions, they were hypoactive (RASS ≤ 0) and, during other sessions, they were hyperactive (RASS >1). None of the participants had the hyperactive subtype across all observations of delirium. (Figure 2)
Figure 2.
Delirium motoric subtypes at enrollment and at during data collection over 2 days.
Symptoms
All 21 symptoms in our schema were reported at least once, however several symptoms (difficulty sleeping, difficulty communicating, confusion, sadness) were reported by participants with very low frequency. Symptom discussion by participants occurred in (260/356; 73.0%) of the observation sessions. The range of different symptoms discussed was 1-7 per session.
There was a significant difference in patient reports of dry mouth (OR: 3.60,95% CI: 1.1-11.83; p=0.03) when delirium was present (See Table 1). All other symptoms reported by participants did not differ significantly when delirium was present. All reports of confusion (n=3) were from patients who tested positive for delirium. Other symptoms (worry, nausea, difficulty communicating) were not reported at all by participants who were delirious.
Table 1.
Symptom frequency associated with the presence of delirium
| Symptom | Number (%) of sessions symptom is present |
Number (%) of sessions where delirium present and symptom is present* |
Odds Ratio | 95% CI | p-value** |
|---|---|---|---|---|---|
| Pain | 172 (48.3) | 39 (24.5) | .88 | 0.57-1.37 | 0.57 |
| Discomfort | 48 (13.5) | 15 (31.9) | 1.16 | 0.61- 2.20 | 0.65 |
| Lack of Energy | 41 (11.5) | 9 (23.7) | 0.78 | 0.31-1.92 | 0.59 |
| Shortness of Breath during weaning | 35 (9.8) | 7 (21.2) | 0.62 | 0.26- 1.45 | 0.27 |
| Nervous Anxious |
32 (9.0) | 5 (17.8) | 0.56 | 0.20- 1.59 | 0.28 |
| Hot | 26 (7.3) | 9 (34.6) | 1.25 | 0.50- 3.13 | 0.63 |
| Drowsy | 23 (6.5) | 8 (36.3) | 1.44 | 0.56- 3.69 | 0.44 |
| Thirst | 18 (5.1) | 4 (22.2) | 0.71 | 0.16- 3.13 | 0.65 |
| Cold | 17 (4.8) | 6 (35.3) | 1.42 | 0.50- 4.01 | 0.50 |
| Frustrated | 16 (4.5) | 2 (13.3) | 0.37 | 0.08-1.63 | 0.19 |
| Dry Mouth | 15 (4.2) | 8 (57) | 3.60 | 1.10- 11.83 | .035 |
| Shortness of Breath not during weaning | 16 (4.5) | 3(23.1) | 0.56 | 0.16- 1.93 | 0.36 |
| Scared | 14 (3.9) | 2 (20) | 0.69 | 0.20- 2.39 | 0.56 |
Delirium measures absent in 21 sessions.
p-values generated from marginal models using generalized estimating to model probability of symptom present with presence of delirium. Models also included fixed effect for session.
Low Frequency Symptoms (n<5 sessions) were omitted from the model: Hunger, Restless, Nausea, Sad, Difficulty Communicating, Confused, Difficulty Sleeping
Frequencies of symptoms reported by participants aged 60 and older were significantly different from younger participants in the following symptoms: Pain (OR: 2.12, 95% CI: 1.12-4.00, p=0.02); drowsy (OR: 0.41, CI 95%: 0.17-0.98, p=0.04); and cold (OR: 0.31,95% CI: 0.11-0.88, p=0.03). (Table 2) When delirium was introduced into the model, age ≥ 60 was associated with participant report of pain (OR: 0.44, 95% CI: 0.22-0.85,p=0.01). The association between age and participant report of drowsiness remained significant when controlling for delirium (OR: 4.71, 95% CI: 1.40-15.89, p=0.01). Presence of delirium and older age were associated with self-reported drowsiness (OR: 0.13, 95% CI:0.02-0.88, p=.04) and feeling hot (OR:0.013, 95% CI: 0.02-0.75, p=.04) although this association was incrementally quite small. (Table 3)
Table 2.
Symptom frequency associated with age ≥ 60 years
| Symptom | Symptom present for those age ≥ 60 |
Odds Ratio | 95% CI | p-value** |
|---|---|---|---|---|
| Pain | 61(35.4) | 2.12 | 1.12-4.00 | .02 |
| Discomfort | 24(50) | 0.81 | 0.37-1.75 | 0.59 |
| Lack of Energy | 23(56.1) | 0.58 | 0.29 - 1.17 | 0.13 |
| Shortness of Breath during weaning* |
18 (51.4) | 0.74 | 0.32- 2.10 | 0.48 |
| Nervous Anxious |
16 (50) | 0.82 | 0.32- 2.10 | 0.67 |
| Hot | 15(57.7) | 0.57 | 0.24- 1.38 | 0.21 |
| Drowsy* | 15 (65.2) | 0.41 | 0.17- 0.98 | 0.04 |
| Thirst | 7 (38.9) | 0.42 | 0.13- 1.35 | 0.14 |
| Cold | 12(70.59) | 0.31 | 0.11- 0.88 | 0.03 |
| Frustrated | 9(56.25) | 0.60 | 0.19- 1.87 | 0.38 |
| Dry Mouth | 10(66.7) | 0.39 | 0.13- 1.18 | .091 |
| Shortness of Breath not during weaning |
9(56.25) | 0.62 | 0.17- 2.27 | 0.47 |
| Scared | 8(57.1) | 0.59 | 0.17- 2.12 | 0.42 |
p-values generated from marginal models using generalized estimating to model probability of symptom present with age ≥ 60 years. Models also included fixed effect for session.
Low Frequency Symptoms (n<5 sessions)
Hunger, Restless, Nausea, Sad, Difficulty Communicating, Confused, Difficulty Sleeping
Table 3.
Symptom frequency associated with delirium and age ≥ 60 years
| Symptom | Odds Ratio |
Delirium 95% CI |
p- value** |
Odds Ratio |
Age > 60 95% CI |
p-value** | Odds Ratio | Delirium * Age > 60 95% CI |
p- value** |
|---|---|---|---|---|---|---|---|---|---|
| Pain | 0.91 | 0.48-1.72 | 0.76 | 0.44 | 0.22-0.85 | 0.01 | 1.07 | 0.45-2.57 | 0.87 |
| Discomfort | 0.62 | 0.23-1.68 | .35 | 1.22 | 0.53-2.83 | 0.64 | 1.48 | 0.48-4.57 | 0.50 |
| Lack of Energy |
0.77 | 0.19-3.07 | 0.71 | 1.66 | 0.76-3.64 | 0.20 | 0.85 | 0.14-5.28 | 0.86 |
| Shortness of Breath during weaning |
0.53 | 0.13-2.13 | .37 | 1.39 | 0.54-3.60 | 0.49 | 1.08 | 0.18-6.21 | 0.93 |
| Nervous Anxious |
0.25 | 0.06-1.12 | 0.07 | 0.52 | 0.34-2.61 | 0.90 | 2.30 | 0.30-17.85 | 0.42 |
| Hot | 3.98 | 1.05-15.01 | 0.04 | 3.59 | 1.14- 11.26 |
0.03 | 0.013 | 0.02-0.75 | .04 |
| Drowsy | 4.30 | 1.02-18.13 | 0.05 | 4.71 | 1.40- 15.89 |
0.01 | 0.13 | 0.02-0.88 | .04 |
| Cold | 0.10 | 0.10-10.27 | 0.10 | 3.32 | 0.9-12.06 | 0.07 | 1.03 | 0.08-13.62 | 0.98 |
| Frustrated | 0.38 | 0.04-3.81 | 0.41 | 2.96 | 0.84- 10.42 |
0.09 | 0.38 | 0.01-10.16 | 0.56 |
| Dry Mouth | 2.3099 | 0.38-14.10 | 0.16 | 1.75 | 0.38-7.99 | 0.47 | 1.29 | 0.12-4.92 | 0.83 |
| Shortness of Breath not during weaning |
0.46 | 0.04-5.46 | 0.47 | 1.45 | 0.32-6.59 | 0.63 | 1.26 | 0.07-13.37 | 0.87 |
| Scared | 1.60 | 0.24- 10.54 |
0.36 | 2.38 | 0.47- 12.09 |
0.30 | 0.26 | 0.02-3.99 | 0.33 |
p-values generated from marginal models using generalized estimating to model probability of symptom present with delirium present and age ≥ 60 years. Models also included fixed effect for session.
Participants initiated discussions about symptoms in 110 (30.9%) sessions. Discussions about pain (n=43) and discomfort (n=13) were the most frequently initiated by participants. Participants who initiated discussions about symptoms were delirious in only 18 of the 110 (16.4%) sessions. Delirium significantly affected patient initiated symptom discussion (OR: 1.91, CI: 1.06-3.43:p=0.03).
DISCUSSION
This is the first study to explore the prevalence of delirium and delirium subtypes based on age. Our findings confirm previous research showing that delirium is a common occurrence during critical illness with greater incidence and risk among patients over 60 years of age19, 20. Some patients (10/89, 11.2%) in this study were delirious at all time points over the course of three days but the difference was not significant between groups based on age. Hypoactive delirium was seen more often than hyperactive delirium, a finding consistent with others27 and notable because of its contribution to underrecognition of delirium in the ICU. Although all patient reports of confusion were from patients experiencing delirium (n=3), there were few differences in symptoms identified by patients during delirium compared to when patients were delirium-free.
This study is also the first to examine the effects of delirium and age on symptom expression among nonvocal intubated patients in the ICU using direct observation. Our findings differ from Puntillo and colleagues’ checklist survey of ICU patients at high risk of dying in which patients who tested positive for delirium were significantly more likely to endorse feeling confused (43% vs. 22%,p = .004) and sad (46% vs. 31%, p = .04) and less likely to endorse being tired (57% vs. 77%, p = .006) than delirium-free patients39. Symptoms reported by participants with delirium were only different from those without for dry mouth and this association was not significant when we controlled for age over 60. Puntillo and colleagues did not explore difference in symptom report based on age.
Our sample differs from the Puntillo et al study in that all participants in our study were intubated; whereas significantly fewer participants in the Puntillo study were able to report their symptoms if they were mechanically ventilated39. Our methods also differed from the Puntillo et al study by examining naturally occurring symptom communication between participants and their nurses rather than requesting participant response to a list of 10 common symptoms39. Thirst and communication difficulty are symptoms shown in previous research with ICU patients as most common and most distressful39-41, however, these symptoms were not identified often by patients in our study. This is likely a reflection of the methodological differences. Nurses drive and control communication with patients in the ICU42 . Most of the symptom communication was nurse-initiated with only 17% initiated by participants.
Our findings are similar to those found in a study of communication capacity and delirium in terminally ill cancer patients43. Using the Communication Capacity Scale and Memorial Delirium Assessment Scale, those patients who were delirious in their final week of life had more difficulty participating in complex conversations43. Delirium imposes significant restrictions on communication with families and care providers and had a negative impact on symptom assessment and patient participation in decision making in terminally ill cancer patients. Decreased communication participation may be a marker of delirium and improved communication might be a marker of resolving delirium in critical illness. It is unknown whether facilitation of communication during critical illness may have a protective effect on delirium.
Limitations of this study include the use of videorecorded data to ascertain symptom discussions by patients. These discussions might have occurred outside the videotaped sessions at other times during the day. Use of electronic medical records to identify patient-reported symptoms could also introduce bias because symptoms recorded in the medical record by nurses are filtered by the nurse. Some symptoms may be omitted and those recorded by the nurse may or may not be a priority for patients. Further, we enrolled participants at various times during their ICU admission with a median time to enrollment of 23 days. Delirium incidence and experience might be different after this length of stay. Finally, we examined the occurrence of symptoms as single entities. Additional work is needed to validate symptom clusters in critically ill patients and the impact of age and delirium on symptom clusters44.
We excluded participants with pre-existing cognitive impairment. Given the high prevalence (89%) of hospitalized older adults with delirium superimposed on dementia, our sample might not be representative of hospitalized older adults45. Conversely, dementia increases the risk of developing delirium46. Often older adults with dementia arrive at the hospital without a definitive diagnosis or early in their dementia illness trajectory when cognitive impairment is under recognized or dismissed by family members. Our sample might have included patients with dementia that was yet unrecognized.
Clinical Implications
Delirium presents a difficult challenge for nursing practice. Patients with delirium have increased need for nursing attention and time47. Several studies have reported high levels of burden and distress for nurses when they care for patients with delirium outside the ICU48, 49. Effective symptom management improves patient distress and can have a positive impact on health-related quality of life. The ability of critical care nurses to discern and manage symptoms in nonvocal critically ill patients is an integral part of nursing care quality and safe patient care42. Conversely, misinterpretation or omission of symptom communication can lead to serious consequences or delayed treatment in this physiologically fragile population.
Delirium assessment should be part of postacute care. Gerontological nurses responsible for post-ICU care should be aware of the potential that delirium associated with critical illness might be present well after the patient’s ICU stay50. The potential for delirium superimposed on dementia is an additional condition that warrants careful assessment and management45. Hypoactive delirium is under-recognized by clinicians so assumptions that quiet patients are sleeping or simply well-behaved should be avoided. In addition, symptom identification should be carefully explored in older adults with or without delirium.
Research Implications
Multiple stakeholders, such as the American Association of Critical Care Nursing, National Quality Forum51 and the National Institute of Nursing Research52 have given priority to research in symptom management. While nurses embrace the strong relationship of symptom management to quality care, there have been few studies of symptom management in the ICU. Studies of symptom management have been devoted largely to pain and pain management in the critically ill patient.
Furthermore, there are no studies to our knowledge of the symptom experience of critically ill older adults or studies that describe the impact of delirium on symptom expression on adults of any age. Evidence is needed to support consistent and efficient symptom identification and to identify and test appropriate and effective strategies to manage symptoms.
Figure 3.
Sessions (enrollment and videotaped) where delirium was present. Delirium motoric subtypes by age < 60 or ≥ 60.
Acknowledgments
Funding: National Institute of Nursing Research (K24-NR010244) and the National Institute of Child Health and Human Development (5R01-HD 043988) (PI: Happ). National Institute of Mental Health, T-32 (MH19986) (PI: Reynolds), Tate. John A. Hartford Foundation ‘Building Academic Geriatric Nursing Capacity’ Pre-Doctoral Scholarship (Nilsen and Campbell) and the National Institute of Nursing Research (F31 NR012856; Nilsen) and (F31 NR011561; Campbell).
Footnotes
Dr Tate had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Puntillo KA, Smith D, Arai S, Stotts N. Critical care nurses provide their perspectives of patients’ symptoms in intensive care units. Heart & Lung. 2008;37(6):466–75. doi: 10.1016/j.hrtlng.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of icu delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 3.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: Occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–8. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–9. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 5.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. J Am Med Assoc. 2004;291(14):1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 7.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–8. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: A prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9(4):R375–81. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37(6):1898–905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 11.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 12.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–34. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 14.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, et al. Delirium accelerates cognitive decline in alzheimer disease. Neurology. 2009;72(18):1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130(3):869–78. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–6. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 17.Morandi A, Rogers BP, Gunther ML, Merkle K, Pandharipande P, Girard TD, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The visions prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2182–9. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167(15):1629–34. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–84. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 21.McAvay GJ, Van Ness PH, Bogardus ST, Jr., Zhang Y, Leslie DL, Leo-Summers LS, et al. Older adults discharged from the hospital with delirium: 1-year outcomes. J Am Geriatr Soc. 2006;54(8):1245–50. doi: 10.1111/j.1532-5415.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 22.Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes associated with delirium in older patients in surgical icus. Chest. 2009;135(1):18–25. doi: 10.1378/chest.08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman CM, Calcutt RA, Robinson BR, Branson RD, Chris B, Athota KP, et al. Delirium in the critically ill geriatric surgical patient. J Am Coll Surg. 2009;209(3, Supplement):S54–S5. [Google Scholar]
- 24.Rudolph JL, Harrington MB, Lucatorto MA, Chester JG, Francis J, Shay KJ. Validation of a medical record-based delirium risk assessment. J Am Geriatr Soc. 2011;59(Suppl 2):S289–94. doi: 10.1111/j.1532-5415.2011.03677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: Validation of the confusion assessment method for the intensive care unit (cam-icu) Critical Care Medicine. 2001;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (cam-icu) Journal of Amercan Medical Association. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 27.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–31. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 28.Meagher DJ, O’Hanlon D, O’Mahony E, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12(1):51–6. doi: 10.1176/jnp.12.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Happ MB, Sereika S, Garrett K, Tate J. Use of the quasi-experimental sequential cohort design in the study of patient-nurse effectiveness with assisted communication strategies (speacs) Contemp Clin Trials. 2008;29(5):801–8. doi: 10.1016/j.cct.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radtke JV, Tate JA, Happ MB. Nurses’ perceptions of communication training in the icu. Intensive & Critical Care Nursing. 2012;28(1):16–25. doi: 10.1016/j.iccn.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Happ MB, Garrett K, Thomas DD, Tate J, George E, Houze M, et al. Nurse-patient communication interactions in the intensive care unit. Am J Crit Care. 2011;20(2):e28–40. doi: 10.4037/ajcc2011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsen ML, Sereika S, Happ MB. Nurse and patient characteristics associated with duration of nurse talk during patient encounters in icu. Heart & Lung: The Journal of Acute and Critical Care. 2013;42(1):5–12. doi: 10.1016/j.hrtlng.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teasdale G, Gentleman D. The description of ‘conscious level’: A case for the glasgow coma scale. Scott Med J. 1982;27(1):7–9. doi: 10.1177/003693308202700103. [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in icu patients: Reliability and validity of the richmond agitation-sedation scale (rass) JAMA. 2003;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 35.Gelinas C, Fortier M, Viens C, Fillion L, Puntillo K. Pain assessment and management in critically ill intubated patients: A retrospective study. Am J Crit Care. 2004;13(2):126–35. [PubMed] [Google Scholar]
- 36.Light J, Dattilo J, English J, Gutierrez L, Hartz J. Instructing facilitators to support the communication of people who use augmentative communication systems. J Speech Hear Res. 1992;35(4):865–75. doi: 10.1044/jshr.3504.865. [DOI] [PubMed] [Google Scholar]
- 37.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 38.Banerjee M, Capozzoli M, McSweeney L, Sinha D. Beyond kappa: A review of interrater agreement measures. The Canadian Journal of Statistics / La Revue Canadienne de Statistique. 1999;27(1):3–23. [Google Scholar]
- 39.Puntillo KA, Arai S, Cohen NH, Gropper MA, Neuhaus J, Paul SM, et al. Symptoms experienced by intensive care unit patients at high risk of dying*. Critical Care Medicine. 2010;38(11):2155–60. doi: 10.1097/CCM.0b013e3181f267ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Critical Care Medicine. 2004;32(7):1527–34. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 41.Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Critical Care Medicine. 2001;29(2):277–82. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Finke EH, Light J, Kitko L. A systematic review of the effectiveness of nurse communication with patients with complex communication needs with a focus on the use of augmentative and alternative communication. Journal of Clinical Nursing. 2008;17(16):2102–15. doi: 10.1111/j.1365-2702.2008.02373.x. [DOI] [PubMed] [Google Scholar]
- 43.Morita T, Tei Y, Inoue S. Impaired communication capacity and agitated delirium in the final week of terminally ill cancer patients: Prevalence and identification of research focus. J Pain Symptom Manage. 2003;26(3):827–34. doi: 10.1016/s0885-3924(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 44.Sousa KH, McDermott CM, Weiss J. The importance of assessing symptoms and symptom clusters in critical care units. AACN Advanced Critical Care. 2011;22(3):275–82. doi: 10.1097/NCI.0b013e3182209279. 10.1097/NCI.0b013e3182209279. [DOI] [PubMed] [Google Scholar]
- 45.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. Journal of the American Geriatrics Society. 2002;50(10):1723–32. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 46.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Archives of Internal Medicine. 2007;167(15):1629–34. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 47.Pretto M, Spirig R, Milisen K, DeGeest S, Regazzoni P, Hasemann W. Effects of an interdisciplinary nurse-led delirium prevention and management program (dpmp) on nursing workload: A pilot study. International Journal of Nursing Studies. 2009;46(6):804–12. doi: 10.1016/j.ijnurstu.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Mc Donnell S, Timmins F. A quantitative exploration of the subjective burden experienced by nurses when caring for patients with delirium. Journal of Clinical Nursing. 2012;21(17-18):2488–98. doi: 10.1111/j.1365-2702.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- 49.Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43(3):183–94. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 50.Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: Asystematic review of frequency and prognosis. Age and Ageing. 2009;38(1):19–26. doi: 10.1093/ageing/afn253. [DOI] [PubMed] [Google Scholar]
- 51.Forum NQ. National voluntary consensus standards for nursing-sensitive care: An initial performance measure set. National Quality Forum; Washington, DC: 2004. [Google Scholar]
- 52.Buerhaus PI. A discussion with patricia a. Grady on the 20th anniversary of the national institute of nursing research. Journal of Nursing Scholarship. 2006;38(3):208–12. doi: 10.1111/j.1547-5069.2006.00104.x. [DOI] [PubMed] [Google Scholar]