Prescription opioid misuse and unintentional overdose deaths involving these analgesics are increasing public health concerns among all ages in the United States.1-4 According to the US Centers for Disease Control and Prevention, prescription opioid-related overdose deaths have become the leading cause of injury deaths, outpacing motor-vehicle crashes in 6 US states and Washington, DC, in 2006.5 The increases in illicit prescription opioid use and related deaths are associated with a marked increase in number of opioid prescriptions written.6,7
OxyContin (Purdue Pharma) is an extended-release oxycodone formulation approved by the US Food and Drug Administration (FDA) for treatment of moderate to severe pain. It is a Schedule II controlled substance in the United States with a significant history of aggressive commercial marketing as well as abuse and diversion.8,9 Pharmacokinetic studies of orally administered OxyContin report a mean terminal half-life (t1/2) of approximately 4.5 to 6.5 hours, mean time to maximum concentration (Tmax) of 2.5 to 5 hours, and oral bioavailability equivalent to immediate-release oxycodone (mean 60%, standard deviation [SD] 20%).10-12 Oxycodone is metabolized by N-demethylation via cytochrome P450 3A4 to noroxycodone, its primary metabolite. It is unlikely that noroxycodone contributes to the pharmacodynamic response in humans, because it has 4-fold lower affinity for the receptor than oxycodone and low μ-opioid receptor activation in GTPγS binding studies.13 However, oxycodone also is metabolized by O-demethylation via cytochrome P450 2D6 to oxymorphone,13 a μ-opioid agonist with analgesic potency approximately 10 times that of parenteral morphine.14
Immediate-release oxycodone products are prescribed approximately 5 times more frequently than extended-release oxycodone products; however, emergency department visits report the nonmedical use of extended-release oxycodone products approximately 4 times more often than immediate-release oxycodone products.15 OxyContin tablets can be easily crushed and snorted or injected to bypass the sustained-release feature, which likely contributes to its abuse liability. In 2008, 15% of publically funded substance abuse treatment admissions with prescription opioid abuse (eg, abuse defined as substance causing problems leading to the admission) reported snorting prescription opioids.16 However, there are no intranasal (IN) pharmacokinetic data demonstrating that OxyContin tablets have immediate-release characteristics when crushed, and the IN bioavailability of OxyContin is unknown despite its common misuse by this route. Thus, this study evaluated the pharmacokinetics of oxycodone and its key metabolites when administered as IN crushed OxyContin tablets compared with intravenous (IV) immediate-release oxycodone solution.
MATERIALS AND METHODS
Participants
Eight healthy adult volunteers (4 male, 4 female) with current recreational use (eg, use to get “high,” not for pain relief) of prescription opioids (confirmed by self-report and positive opioid urine drug test) and experience snorting opioids completed this inpatient study. Participants were 19 to 36 years old (mean = 26.3) and weighed 60.5 to 90.9 kg (mean = 71.8 kg). Self-reported race was 7 Caucasian and 1 Asian/Caucasian. Participants reported mean (SD) number of days within the last 30 days using the following: prescription opioids: 12.4 (5.5); cocaine, 6.8 (8.9); benzodiazepines, 2.8 (3.5); and alcohol 6.7, (7.7). Volunteers were determined to be in good health by history and physical examination, laboratory testing, and electrocardiography. Volunteers were excluded if they were pregnant or breastfeeding, seeking treatment for their substance use, taking daily prescribed medications, or physically dependent (ie, withdrawal) on opioids, alcohol, or sedatives/hypnotics. To ensure lack of physical dependence on opioids, volunteers were required to have a negative opioid urine specimen and simultaneously not display signs and symptoms of withdrawal. Volunteers with current medical or psychiatric illnesses (eg, diabetes, bipolar disorder) and abnormal nasal exams (eg, infections, erosions, malformations) were excluded. Participants gave written informed consent prior to participation. The University of Kentucky (UK) Institutional Review Board approved this study. Subjects were paid for participation.
Study Setting
Subjects resided on an inpatient research unit at UK Chandler Medical Center for approximately 2½ weeks. Each study day, urine samples were tested for cocaine, amphetamine, methamphetamine, THC, methadone, opiates, phencyclidine, barbiturates, benzodiazepines, tricyclic antidepressants, and oxycodone to confirm the absence of unauthorized drugs. All results indicated abstinence from illicit drug use during study participation. Females had urine pregnancy tests weekly; all results were negative. Females did not take oral contraceptives. Subjects received a caffeine-free diet. No smoking was allowed 1 hour before or during study sessions. Acetaminophen, alumina/magnesia/simethicone, and ibuprofen were available to subjects as needed but were not given after midnight preceding study session days or during the 24-hour blood-sampling period.
Study Design and Procedure
This study used a within-subject, double-blind design nested within a larger gender comparison study evaluating the effects of pain on opioid abuse liability. This pharmacokinetic study evaluated blood samples from the no-pain control condition. Two IN drug conditions (crushed OxyContin 15 mg and 30 mg/70 kg) and one IV condition (oxycodone 5 mg/70 kg) were tested. Drug conditions were randomized; however, for safety, the lower IN dose always preceded the higher dose.
Drugs
Intranasal 15 mg/70 kg doses were prepared using 40-mg OxyContin tablets (Cardinal Health, Knoxville, Tennessee), and IN 30 mg/70 kg doses were prepared using OxyContin 80-mg tablets (Cardinal Health). The 40-mg and 80-mg tablets weigh approximately the same (ie, 133 mg), ensuring the same tablet/dose ratio (subject weight/volume snorted ratio) for each active drug condition. Tablets were rubbed with a 4 × 4 gauze pad wetted with normal saline to remove the outer coating and crushed prior to weighing. All IN doses were packaged in a 1-g glass vial (Health Care Logistics, Circleville, Ohio). Subjects poured the dose out onto a clean mirror, separated the dose into 2 similar-sized lines using a clean razor blade, and insufflated 1 line in each nostril through a clear 65-mm straw within 1 minute. For the IV condition, oxycodone powder (Spectrum Chemical Mfg Corp, Gardena, California) was weighed to produce a dose equivalent to 5 mg/70 mg. The powder was placed in clean class A graduated cylinders and dissolved in 0.9% sodium chloride for injection (Hospira, Lake Forest, Illinois) to 1 mL volume. The resulting solution was agitated using a vortex mixer to ensure complete dissolution. Final filter sterilization was performed using a 0.22-μm Millex GS 33 syringe-driven membrane filter (Millipore-Product Ref SLGS M33 SS). Injectables were prepared using aseptic technique and packaged in sterile syringes (Becton-Dickinson & Co, Franklin Lakes, New Jersey). Nursing staff infused the solution over 1 minute into an IV indwelling catheter in the forearm placed on the opposite arm from which blood samples were drawn.
Blood Sampling and Oxycodone and Oxycodone Metabolite Assays
Serial 5-mL blood samples were collected from an indwelling venous catheter into heparinized vacutainers 30 minutes before drug administration and 5, 10, 15, 30, and 45 minutes and 1, 1.5, 2, 4, 8, 12, and 24 hours after each IN and IV drug administration. An additional 2-minute postdrug administration sample was collected in the IV session. Samples were inverted 8 to 10 times, placed on ice, and centrifuged for 10 minutes at 4°C at 3000g. Plasma was aliquotted into #2, 2-mL DNA, DNase, RNase, pyrogen-free o-ring sealed screw-capped crytotubes and stored upright at −80°C until time of shipment to the University of Utah for analysis. Shipment was overnight on dry ice (surface temperature −78.5°C) according to Department of Transportation and International Air Transport Association guidelines.
Plasma oxycodone, noroxycodone, and oxymorphone concentrations were determined using a validated liquid chromatography–electrospray ionization–tandem mass spectrometric (LC-ESI-MS/MS) method.17 In brief, deuterated analogs of oxycodone, oxymorphone, and noroxycodone were added to plasma as the internal standards. The pH of the plasma was made basic (>10) by addition of ammonium hydroxide, and plasma was extracted using liquid/liquid extraction with n-butylchloride/acetonitrile (4:1). The organic layer was collected, evaporated, and reconstituted with 0.1% formic acid in water and analyzed by LC-ESI-MS/MS. The mass spectrometer was operated in the selected reaction-monitoring mode. Quadrupole 1 was set to pass only the ions at m/z 316, 322, 302, 305, 302, and 305 that correspond to the MH+ ions of oxycodone, oxycodone-d6, oxymorphone, oxymorphone-d3, noroxycodone, and noroxycodone-d3, respectively. The MH+ ions were caused to undergo collision-induced dissociation in quadrupole 2 that produce product ions at m/z 298, 304, 284, 287, 284, and 287, respectively, that were then monitored selectively by quadrupole 3.
Oxymorphone and noroxycodone both have the same parent and product ion masses; their separation is achieved by liquid chromatography. Concentrations of oxycodone, oxymorphone, and noroxycodone were determined from the peak area ratios of the analyte to its internal standard and from comparison of the ratio with the calibration curve (0.2-250 ng/mL) that was generated from the analysis of human plasma fortified with known concentrations of the analytes and their internal standards. Lower limit of quantitation (LLOQ) was 0.2 ng/mL for 1 mL of plasma.
Pharmacokinetic and Bioavailability Analysis
The elimination rate constant (λz) was estimated by linear regression from a natural log-linear plot of the last 4 plasma concentration data points (time points 4, 8, 12, and 24 hours) during the terminal postdistribution phase. Concentrations below the LLOQ were excluded from the λz calculation such that oxycodone λz was based on the last 3 time points (ie, 4, 8, and 12 hours) in 6 subjects for the IV condition, 2 subjects for IN 15 mg/70 kg, and 1 subject for IN 30 mg/70 kg. Noroxycodone λz was based on the last 3 time points in 5 subjects for the IV condition and in 1 for 30 mg/70 kg. The terminal half-life (t1/2) was calculated as 0.693/λz.
Plasma area under the concentration–time curve (AUC) from time zero to infinity (AUC0-inf) was calculated as AUC from time zero to 24 hours (AUC0-24h) + concentration at 24 hours/λz. AUC0-24h was calculated by the linear trapezoidal rule. Cmax and Tmax are the observed maximum plasma concentrations and time to maximum concentrations, respectively. Observed absolute bioavailability (Fobs) of IN OxyContin was determined according to the following formula: Fobs = (AUC0-inf IN/AUC0-inf IV) × (dose IV/dose IN). Values below the LLOQ were scored as zero for determination of mean concentrations and to calculate AUCs. The data were analyzed for normality (skewness and kurtosis); all data were normally distributed except for Tmax. Thus, means ± SDs are presented. One-factor (drug condition) analysis of variance was conducted (Proc Mixed in SAS 9.1 for Windows) for each pharmacokinetic parameter except Tmax to determine differences among the drug conditions. Post hoc Tukey comparisons were completed between each drug condition when the analysis of variance was significant. Nonparametric Friedman tests with Wilcoxon signed rank comparisons using a Bonferroni correction were conducted for Tmax. Statistical significance was set at P < .05 (P < .0167 for Bonferroni corrections). Paired t tests were conducted when only 2 drug conditions were compared.
RESULTS
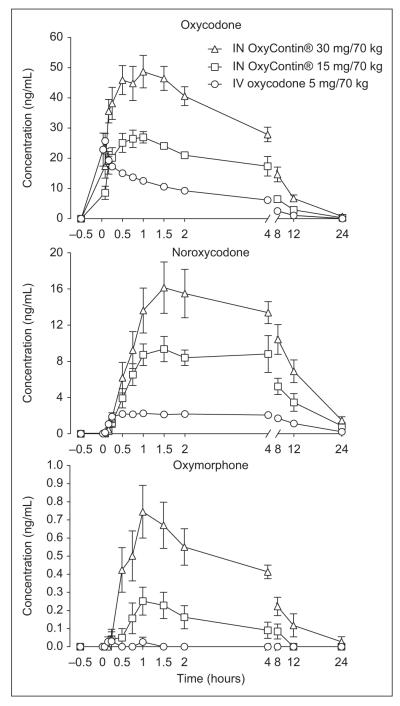
All drug doses were well tolerated by subjects without clinically significant adverse events (eg, no respiratory depression or vital sign abnormalities requiring medical intervention; no nasal infections). Oxycodone was detected in plasma at the first postdrug administration time point (5 minutes for IN doses and 2 minutes for the IV dose) for all subjects. Figure 1 shows the mean plasma concentrations of oxycodone, noroxycodone, and oxymorphone for each drug condition. Oxymorphone concentrations were below the LLOQ in 2 subjects after the IN 15 mg/70 kg dose and in 6 subjects after the IV dose. Therefore, oxymorphone pharmacokinetic results are not reported for the IV condition. Noroxycodone was identified in every subject after IN and IV drug administration.
Figure 1.
Mean plasma concentrations (n = 8) of oxycodone, noroxycodone, and oxymorphone after intranasal crushed OxyContin and IV oxycodone administration. Bars represent ±1 standard error. Bars are not present for all data points because they are too small to depict.
Compared with IV oxycodone, the mean IN bioavailability of crushed OxyContin was 78.2% ± 17.8% and 75.0% ± 15.5% in the 15 mg/70 kg and 30 mg/70 kg conditions, respectively; these 2 values did not differ significantly. The range across both IN doses was from 54% to 101%.
Results for Tmax, Cmax, AUC0-inf, metabolite/oxycodone AUC0-inf ratios, and t1/2 for all doses are shown in Table I. Mean oxycodone Tmax occurred later for both IN doses (65 and 52 minutes for the low and high dose, respectively) compared with the IV dose (7 minutes) as expected. Tmax differed significantly between the IV and the IN 15 mg/70 kg doses, but a trend was only observed between the IV and the IN 30 mg/70 kg doses (P = .0176 with Bonferroni correction). A similar pattern, but not statistically significant, was observed for noroxycodone Tmax, whereby the IV condition had a shorter Tmax than the IN conditions. There was a trend toward a noroxycodone Tmax difference between the 30 mg/70 kg IN dose and IV dose (P = .0171 with Bonferroni correction). Oxymorphone Tmax did not significantly differ between the IN doses.
Table I.
Pharmacokinetics of Oxycodone and Its Metabolites After Intranasal (IN) OxyContin and Intravenous (IV) Oxycodone Administration
| IV Oxycodone 5 mg/70 kg |
IN OxyContin 15 mg/70 kg |
IN OxyContin 30 mg/70 kg |
|
|---|---|---|---|
| Tmax, min | |||
| Oxycodonea | 7 ± 5 | 65 ± 73 | 52 ± 31 |
| 2-15 | 10-240 | 10-90 | |
| Noroxycodone | 111 ± 152 | 148 ± 148 | 206 ± 177 |
| 15-480 | 45-480 | 60-480 | |
| Oxymorphone | — | 98 ± 72 | 146 ± 147 |
| — | 45-240 | 60-480 | |
| Cmax, ng/mL | |||
| Oxycodoneb,c | 29.7 ± 11.3 | 32.8 ± 5.2 | 54.1 ± 12.4 |
| 17.8-52.7 | 25.7-40.0 | 39.9-76.7 | |
| Noroxycodonea,b,c | 2.5 ± 0.8 | 11.8± 5.1 | 19.5 ± 5.3 |
| 1.3-3.7 | 6.0-22.7 | 12.9-26.6 | |
| Oxymorphonec | — | 0.3 ± 0.2 | 0.8 ± 0.4 |
| — | 0.0-0.6 | 0.3-1.5 | |
| AUC0-inf | |||
| Oxycodonea,b,c | 74.32 ± 13.89 | 172.02 ± 41.88 | 330.01 ± 65.45 |
| 58.58-102.80 | 122.15-247.74 | 237.49-405.08 | |
| Noroxycodonea,b,c | 32.69 ± 21.80 | 111.58 ± 49.39 | 197.34 ± 59.31 |
| 16.36-80.44 | 60.58-217.81 | 113.20-289.46 | |
| Oxymorphonec | — | 1.08 ± 1.11 | 5.76 ± 4.35 |
| — | 0.00-2.79 | 2.12-15.64 | |
| t1/2, h | |||
| Oxycodone | 3.3 ± 0.8 | 3.5 ± 0.9 | 3.6 ± 0.5 |
| 2.3-4.4 | 1.6-4.6 | 3.0-4.5 | |
| Noroxycodoneb | 7.1 ± 2.7 | 6.2 ± 2.3 | 5.8 ± 1.7 |
| 4.6-11.4 | 3.5-9.9 | 4.4-9.1 | |
| Noroxycodone AUC0-inf/Oxycodone AUC0-infa,b | 0.42 ± 0.19 | 0.64 ± 0.16 | 0.61 ± 0.18 |
| 0.23-0.78 | 0.36-0.88 | 0.40-0.88 | |
| Oxymorphone AUC0-inf/Oxycodone AUC0-infc | — | 0.01-0.01 | 0.02 ± 0.01 |
| — | 0.00-0.02 | 0.01-0.04 |
Results are the mean + standard deviation and ranges; all results based on n = 8 sample except oxymorphone (OM) Tmax results based on sample size of n = 6 for the IN 15 mg/70 kg dose. OM results not analyzed for the IV oxycodone dose because only 2 subjects had detectable OM levels.
Significant difference between IV and low IN dose (bottom row).
Significant difference between IV and high IN dose (top row).
Significant difference between IN doses.
Oxycodone, noroxycodone, and oxymorphone Cmax values were significantly different among drug conditions. The 30 mg/70 kg IN dose produced significantly higher Cmax compared with the lower IN dose for all 3 analytes and the IV dose for oxycodone and noroxycodone. The 15 mg/70 kg dose produced a significantly higher noroxycodone Cmax than the IV dose.
Oxycodone and noroxycodone AUC0-inf significantly differed among all drug conditions. The 30 mg/70 kg IN dose produced the highest values and the IV dose the lowest. Dose proportionality was evident between the 2 IN doses; oxycodone AUC0-inf was 1.92 times greater for the 30 mg/70 kg dose compared with the 15 mg/70 kg dose. Oxymorphone AUC0-inf was significantly higher in the 30 mg/70 kg IN dose condition compared with the 15 mg/70 kg IN condition, although overall, oxymorphone AUC values were low in comparison to AUC values for noroxycodone and oxycodone.
Oxycodone t1/2 was not significantly different among the 3 drug conditions, with mean values ranging from 3.3 to 3.6 hours. Noroxycodone t1/2 was not significantly different between the 2 IN doses, but the IV dose had a significantly longer t1/2 of 7.1 hours compared with the 30 mg/70 kg IN dose t1/2 of 5.8 hours. Oxymorphone t1/2 was not calculated because of frequent concentrations below the LLOQ.
Ratios of noroxycodone to oxycodone AUC0-inf were significantly higher in both IN conditions compared with the IV condition. No difference was found between IN doses. The ratio of oxymorphone to oxycodone AUC0-inf was significantly higher in the high IN dose compared with low IN dose condition. Semi-log plots of oxycodone, noroxycodone, and oxymorphone plasma concentrations are available as supplemental data at http://jcp.sagepub.com/supplemental/.
DISCUSSION
This study evaluated the pharmacokinetic profile of IN OxyContin in comparison to IV oxycodone. The IN drug administration method used in this study is clinically relevant because OxyContin was administered in the manner by which it is commonly abused—by snorting crushed tablets. There are several important study results. First, crushed OxyContin was rapidly absorbed by the IN route and was reliably detected in plasma within 5 minutes of dosing. Second, OxyContin had high intranasal bioavailability: 78% and 75% after 15 mg/70 kg and 30 mg/70 kg, respectively. Third, the t1/2 of IN OxyContin was approximately 3.5 hours and was not statistically different than the t1/2 of 3.3 hours for IV oxycodone. These data demonstrate that crushing and snorting OxyContin tablets is a highly efficient drug delivery method that clearly bypasses the extended-release Acrocontin (Purdue Pharma) drug delivery matrix.
The major metabolite for both IN and IV conditions was noroxycodone. Oxymorphone was present in low concentrations, often below the LLOQ, consistent with other studies administering oral and parenteral oxycodone.13,18 Previous research has shown that oxycodone AUC rather than oxymorphone AUC is more closely associated with overall subjective ratings of oxycodone drug effects,19 and blockade of oxymorphone production through inhibition of CYP2D6 does not attenuate the psychomotor or subjective drug effects of oxycodone.18 Therefore, oxymorphone is unlikely to be a significant contributor to the pharmacodynamic effects of oxycodone. Other oxidative and reduced oxycodone metabolites have been identified in the last decade20; however, there are limited data regarding their contribution to the pharmacodynamic effects of oxycodone.
Intranasal bioavailability of crushed OxyContin tablets in this study (~77%) exceeds its oral bioavailability estimated at approximately 60%.13,14 Intranasal drug administration is typically associated with greater drug delivery than oral administration because the nasalcavity has a porous endothelial basement membrane, generally lower enzymatic activity than the gastrointestinal tract, and a large surface area with rich venous vasculature that bypasses presystemic gastrointestinal (GI) and hepatic first-pass metabolism.21,22 However, the present bioavailability results exceed a previous report of 47% IN bioavailability of oxycodone solution administered by nasal spray,23 perhaps the only other study to date to examine the bioavailability of IN oxycodone. The lower bioavailability in that study may be attributable to solution volumes exceeding the 0.3- to 0.4-mL nasal mucosa limit for absorption.22 Thus, excess solution likely entered the nasopharynx and esophagus, becoming subjected to GI absorption and metabolism. Likewise, although IN bioavailability was high in the current study, making it unlikely that significant amounts of drug entered the GI system, it is still possible that some amount was absorbed there. This could help explain why there was more variability in Tmax and noroxycodone/oxycodone AUC ratios for the IN doses compared with the IV dose.
Moreover, IN drug absorption has been shown to be more efficacious with powder compared with liquid formulations in animals,24,25 although it can also produce highly variable results because of unpredictable disposition within the nose. This latter point may help explain the wide range of bioavailability reported here (54%-101%).
OxyContin tablets contain excipients that may enhance IN drug delivery. These include talc, lactose, triacetin, ammonio methacrylate copolymer, magnesium stearate, povidone, and stearyl alcohol (oral communication with Purdue Pharma, September 11, 2006). The direct effects of these excipients on IN oxycodone drug absorption are unknown, but polymer interactions with nasal mucous can enhance mucoadhesion and are used to optimize human IN drug delivery.22 Lactose also has been shown in rabbits to be a rapid release excipient, producing maximum apomorphine concentrations more quickly by the IN route compared with subcutaneous injections without lactose.26 However, excipients may also have adverse medical effects when administered by routes for which they were not intended. For instance, pulmonary granulomatosis has been reported when pills containing talc are crushed and injected,27 and nasal fungal infections, septal and palatal perforation, and erosion of turbinates have been reported after snorting crushed opioid analgesics.28-30
This study has limitations. It examined only 2 IN doses, so linear pharmacokinetics cannot be determined. Given constraints on study duration and allowable blood draw volumes, IN bioavailability comparisons with other opioids commonly misused by this route (eg, hydrocodone) and oral OxyContin were not included, and these comparisons will be important to pursue in future work.
Overall, these results should be of significant interest to health providers, toxicologists, and public health officials as prescription opioid overdose deaths have increased. OxyContin was easily crushed to bypass the sustained-release mechanism and, when snorted, had high IN bioavailability with rapid delivery into circulating blood. These data, combined with availability of high-dose/low-volume tablets (eg, 80-mg tablets) and research demonstrating that immediate-release oxycodone has abuse potential (eg, produces drug liking and high),31 clarify that OxyContin lacks abuse-deterrent properties and has significant abuse liability. These qualities, unfortunately, are not unique to many currently FDA-approved opioid pain relievers. It is hoped that the FDA’s new requirements for risk evaluation and mitigation strategies for new drug applications with abuse liability combined with technological advances will allow new opioid pain relievers with abuse-deterrent properties to replace older drug delivery systems that are easily exploited and have contributed to the growing number of unintentional prescription opioid overdose deaths and current epidemic of prescription opioid use disorders.
Supplementary Material
Acknowledgments
We thank Becky Freeman, Victoria Casselton, Marie Bate, RN, Chris Riegert, RN, Stacy Miller, RN, and the GCRC/CR-DOC nursing staff for their support of this project.
Financial disclosure: This study was supported by NIDA R01 DA016718, K12 DA14040, the UK General Clinical Research Center M01-RR02602, and the UK Clinical Research Development and Operations Center (CR-DOC).
Footnotes
None of the authors have conflicts of interest to disclose.
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
Supplementary data for this article are available at http://jcp.sagepub.com/supplemental/.
REFERENCES
- 1.Office of Applied Studies . Results from the 2008 National Survey on Drug Use and Health (NSDUH): National Findings. Office of Applied Studies; Rockville, MD: 2009. (Substance Abuse and Mental Health Service Administration NSDUH Series H-36 SMA 09-4434). [Google Scholar]
- 2.Lofwall MR, Schuster A, Strain EC. Changing profile of abused substances by older persons entering treatment. J Nerv Ment Dis. 2008;196:898–905. doi: 10.1097/NMD.0b013e31818ec7ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 4.Sung HE, Richter L, Vaughan R, Johnson PB, Thom B. Non-medical use of prescription opioids among teenagers in the United States: trends and correlates. J Adolesc Health. 2005;37:44–51. doi: 10.1016/j.jadohealth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Overdose deaths involving prescription opioids among Medicaid enrollees—Washington, 2004-2007. MMWR Morb Mortal Wkly Rep. 2009;58:1171–1175. [PubMed] [Google Scholar]
- 6.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–511. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 8.Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99:221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. General Accounting Office (GAO) Prescription Drugs: OxyContin Abuse and Diversion and Efforts to Address the Problem. United States Government General Accounting Office; Washington D.C.: 2003. Report to Congressional Requesters; pp. 1–57. GAO 04-110. [Google Scholar]
- 10.Benziger DP, Kaiko RF, Miotto JB, Fitzmartin RD, Reder RF, Chasin M. Differential effects of food on the bioavailability of controlled-release oxycodone tablets and immediate-release oxycodone solution. J Pharm Sci. 1996;85:407–410. doi: 10.1021/js950403a. [DOI] [PubMed] [Google Scholar]
- 11.Benziger DP, Miotto J, Grandy RP, Thomas GB, Swanton RE, Fitzmartin RD. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J Pain Symptom Manage. 1997;13:75–82. doi: 10.1016/s0885-3924(96)00300-4. [DOI] [PubMed] [Google Scholar]
- 12.Poyhia R, Seppala T, Olkkola KT, Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J clin Pharmacol. 1992;33:617–621. doi: 10.1111/j.1365-2125.1992.tb04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79:461–479. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Beaver WT, Wallenstein SL, Houde RW, Rogers A. Comparisons of the analgesic effects of oral and intramuscular oxymorphone and of intramuscular oxymorphone and morphine in patients with cancer. J clin Pharmacol. 1977;17:186–198. doi: 10.1177/009127007701700402. [DOI] [PubMed] [Google Scholar]
- 15.Worthy Kendra, Governale Laura. [Accessed October 13, 2010];FDA Anesthetic and Life Support Drugs and Drug Safety Risk Management Advisory Committee Meeting on May 5, 2008. Summary of Drug Abuse “Rates” in the United States; presentation by Catherine Dormitzer, Division of Epidemiology and Sales distribution data (IMS Health) Year 2007. http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4356s1-00-index.htm.
- 16.United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies . Treatment Episode Data Set—Admissions (TEDSA), 2008 [computer file]. ICPSR27241-v2. Inter-university Consortium for Political and Social Research [distributor]; Ann Arbor, MI: 2010. doi:10.3886/ICPSR27241. Electronic file accessed October 13, 2010. [Google Scholar]
- 17.Fang WB, Moody DE. Determination of oxycodone and metabolites by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Paper presented at: the Society of Forensic Toxicologists Annual Meeting; Durham, NC. October 15-19, 2007.2007. [Google Scholar]
- 18.Heiskanen T, Olkkola KT, Kalso E. Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacol Ther. 1998;64:603–611. doi: 10.1016/S0009-9236(98)90051-0. [DOI] [PubMed] [Google Scholar]
- 19.Poyhia R, Olkkola KT, Seppala T, Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Br J clin Pharmacol. 1991;32:516–518. doi: 10.1111/j.1365-2125.1991.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldacci A, Caslavska J, Wey AB, Thormann W. Identification of new oxycodone metabolites in human urine by capillary electrophoresis-multiple-stage ion-trap mass spectrometry. J Chromatogr A. 2004;1051:273–282. [PubMed] [Google Scholar]
- 21.Shelley K, Paech MJ. The clinical applications of intranasal opioids. Curr Drug Deliv. 2008;5:55–58. doi: 10.2174/156720108783330989. [DOI] [PubMed] [Google Scholar]
- 22.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Takala A, Kaasalainen V, Seppala T, Kalso E, Olkkola KT. Pharmacokinetic comparison of intravenous and intranasal administration of oxycodone. Acta Anaesthesiol Scand. 1997;41:309–312. doi: 10.1111/j.1399-6576.1997.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama T, Morita T, Horikiri Y, Yamahara H, Yoshino H. Influence of fillers in powder formulations containing N-acetyl-L-cysteine on nasal peptide absorption. J Control Release. 2007;120:88–94. doi: 10.1016/j.jconrel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Schipper NG, Romeijn SG, Verhoef JC, Merkus FW. Nasal insulin delivery with dimethyl-beta-cyclodextrin as an absorption enhancer in rabbits: powder more effective than liquid formulations. Pharm Res. 1993;10:682–686. doi: 10.1023/a:1018999414088. [DOI] [PubMed] [Google Scholar]
- 26.Ugwoke MI, Exaud S, Van Den Mooter G, Verbeke N, Kinget R. Bioavailability of apomorphine following intranasal administration of mucoadhesive drug delivery systems in rabbits. Eur J Pharm Sci. 1999;9:213–219. doi: 10.1016/s0928-0987(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 27.Waller BF, Brownlee WJ, Roberts WC. Self-induced pulmonary granulomatosis: a consequence of intravenous injection of drugs intended for oral use. Chest. 1980;78:90–94. doi: 10.1378/chest.78.1.90. [DOI] [PubMed] [Google Scholar]
- 28.Greene D. Total necrosis of the intranasal structures and soft palate as a result of nasal inhalation of crushed OxyContin. Ear Nose Throat J. 2005;84:512, 514, 516. [PubMed] [Google Scholar]
- 29.Jewers WM, Rawal YB, Allen CM, et al. Palatal perforation associated with intranasal prescription narcotic abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:594–597. doi: 10.1016/j.tripleo.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Yewell J, Haydon R, Archer S, Manaligod JM. Complications of intranasal prescription narcotic abuse. Ann Otol Rhinol Laryngol. 2002;111:174–177. doi: 10.1177/000348940211100212. [DOI] [PubMed] [Google Scholar]
- 31.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:181–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.