Abstract
Background and purpose
Management for in-field failures after thoracic radiation is poorly defined. We evaluated SBRT as an initial or second course of treatment re-irradiating in a prior high dose region.
Materials and methods
Thirty-three patients were treated with re-irradiation defined by the prior 30 Gy isodose line. Kaplan–Meier estimates were performed for local (LC), regional (RC), distant control (DC), and overall survival (OS). The plans when available were summed to evaluate doses to critical structures. Patient and treatment variables were analyzed on UVA for the impact on control and survival measures.
Results
Median follow-up was 17 months. Treatment for sequential courses was as follows: (course1:course2) EBRT:SBRT (24 patients), SBRT:SBRT (7 patients), and SBRT:EBRT (3 patients). Median re-irradiation dose and fractionation was 50 Gy and 10 fractions (fx), with a median of 18 months (6–61) between treatments. Median OS was 21 months and 2 year LC 67%, yet LC for >1 fraction was 88% (p = 0.006 for single vs. multiple). 10 patients suffered chronic grade 2–3 toxicity (6 chest wall pain, 3 dyspnea, 1 esophagitis) and 1 grade 5 toxicity with aorta-esophageal fistula after 54 Gy in 3 fx for a central tumor with an estimated EQD2 to the aorta of 200 Gy.
Conclusion
Tumor control can be established with re-irradiation using SBRT techniques for in-field thoracic failures at the cost of manageable toxicity.
Keywords: SBRT, Re-irradiation, Lung
Local control has improved with the use of concurrent chemotherapy in locally advanced lung cancers and in stage I lung cancer with the use of stereotactic body radiation therapy (SBRT) or accelerated hypofractionated radiotherapy (AHRT). Despite these advances, locoregional relapses occur in at least one-third of stage III patients and in up to one fifth of early-stage patients [1–3]. Similarly, new primary or metastatic second tumors can occur within previously irradiated regions [4,5].
Patients are frequently not candidates for surgery although this should be considered in selected patients [6,7]. Chemotherapy has historically been given, but generally not to provide permanently durable control. As patients progress onto 2nd or 3rd line chemotherapy, response rates and durability are even lower with response rates between 10% and 20% [2]. Therefore, re-irradiation has been explored employing a variety of techniques, doses, and fraction sizes over the past several decades. However, using traditional fractionated techniques with mostly palliative intent, outcomes have been poor with limited survival, tumor control, and at the cost of substantial side effects [3,8–11].
SBRT has demonstrated excellent results for early-stage, inoperable non-small cell lung cancer (NSCLC), however, the role of hypofractionated radiotherapy using SBRT techniques for patients who have previously received EBRT has been examined in only 5 small series [12–15]. Each suggests a relatively high likelihood of establishing local control at the expense of modest toxicity. A recent report has further evolved the understanding of re-irradiation employing dose modeling to better comprehend the additive dose from standard and large dose per fraction plans [16]. The current study describes our institutional experience treating patients with prior thoracic radiotherapy for in-field tumors in the context of these prior reports.
Materials and methods
Data acquisition
Clinical information on 373 patients treated with SBRT techniques between 2001 and 2012 was screened for a second course of radiotherapy to the thorax. Patients receiving SBRT as the initial course of treatment or with SBRT as treatment for failure or new disease after EBRT were included. Each patient was evaluated in a multidisciplinary setting by surgery, pulmonology, medical oncology, and radiation oncology. While no strict selection criteria were applied, in general if patients were medically fit and the tumors were surgically resectable, surgery was offered. In each case, the treatment was intended to provide durable control, and in non-metastatic patients, with curative intent. Furthermore, the treating physicians had to feel as though all visible disease could be targeted with a dose above more conventional palliative regimens. A total of 86 patients were indentified that received at least two courses of thoracic radiotherapy, of whom 33 received radiation for in-field disease. We categorized in-field disease as tumor within the high dose region (>30 Gy) as previously described in the literature [12]. For patients treated with single fraction radiosurgery as the initial course of treatment, the 50% isodose line defined the area of re-irradiation. Clinical outcome measures were determined using the patents’ electronic medical records including review of all relevant imaging studies and treatment planning data. This retrospective study was approved by the institutional review board.
Treatment
Patients were immobilized using a conventional wingboard during EBRT, and either the Elekta Bodyframe or BodyFix cradle (Elekta, Stockholm, Sweden) during SBRT. Abdominal compression was applied to dampen respiratory motion during the SBRT treatment course. Four-dimensional (4D) computed tomography (CT) scans were completed on all patients receiving EBRT or SBRT after 2007. Prior to 4D CT imaging, the GTV was defined on a free breathing CT scan for EBRT. For SBRT during the pre-4D CT imaging era, the GTV was the fusion of 2 separate CT datasets obtained after the patient was set-up and then repositioned within the stereotactic frame, meaning the GTV became a compromised ITV taking into account some degree of set-up uncertainty and tumor motion. After the implementation of 4D CT imaging, a standard ITV based on 10 phase image acquisition and MIP reconstruction was generated for both the SBRT and EBRT plans. No additional expansion was made for a clinical target volume. The planning target volume (PTV) for EBRT cases was a uniform 1 cm expansion in the pre-ITV era and for the SBRT plans during this same time-frame the PTV was a 1 cm expansion in the cranial caudal direction and 5 mm axially. Once the ITV technique was used, the PTV expansion was 5 mm for both the EBRT and SBRT plans. EBRT patients were treated with 4–6 beams using 3D conformal principles. The SBRT patients were treated with seven to ten beams using a combination of both coplanar and non-coplanar beams. Beam energies for all patients were 6 or 10 MV. For target localization, daily image guidance was accomplished with cone-beam CT beginning in February 2009, and employed for EBRT and SBRT patients. Prior to 2009, SBRT patients were treated based on stereotactic coordinates from the body frame with orthogonal MV portal imaging used for confirmation while the EBRT patients were imaged with weekly MV portal images. Dosimetric parameters including field arrangement, beam energy, and customized blocking to cover the tumor or spare nearby critical structures were based on the judgment of the radiation oncologist.
Composite plans were generated of current and prior RT courses when available (19 of the 33 cases). Dose volume histograms were analyzed with clinical judgment used when dose volume constraints were to be exceeded or unavailable in the retreatment setting. To compare against previous reports, the composite plans when available were generated as both an absolute summation of the two plans and with the treated doses converted to equivalent doses in 2 Gy fractions (EQD2) [16]. Rigid registrations were used to combine CT simulation scans and transfer dose onto the more recent scan. All doses were recalculated to an EQD2, with the formula: d/n/((d + a/β)/(2 + a/β)), with d the dose per fraction (Gy) and n the number of fractions. For tumor dose and acute side effects an a/β) value of 10 Gy was used and for late effects an a/β; value of 3 Gy. MIM® Software (v5.6, MIM Software Inc, Cleveland, OH) was used to convert dose to the EQD2, fuse simulation scans, and combine the dose files. Given the retrospective nature of this project spanning nearly a decade, there was significant heterogeneity among the dose and fractionation schemes.
Follow-up
In general, patients underwent a CT chest within 6–8 weeks of treatment completion. CT scans were then repeated at 3–4 month intervals for the first 2 years and repeated at 6 month intervals thereafter. PET scans were not routinely performed in the absence of concerning chest CT findings. At each follow-up, patients were screened for acute and late toxic effects. Toxicity was retrospectively scored according to Common Terminology Criteria for Adverse Effects version 4.0 (CTCAE v4.0).
Patients were considered to have failed locally after evidence of increased size of enhancing tumor in the treated region. PET scans or biopsy was employed to assist with differentiating radiation related lung changes with local or regional recurrence. Patients were screened for regional failures defined as nodal disease in the hilum or mediastinum, or distant failures.
Statistical analysis
Kaplan–Meier estimates were completed for overall survival, local and regional control. Time to distant failure and progression free survival were estimated in the non-metastatic patients. Time to event data was recorded from date of radiation completion for the retreatment course, either SBRT or EBRT. Treatment and patient related characteristics (tumor size, location (central vs. peripheral), dose, number of fractions, and retreatment interval) were described and evaluated for their impact on toxicity using the paired or unpaired t-test for continuous variables and the Chi Square or Fisher’s Exact test for categorical data. The Log-Rank statistic was used to compare patients treated with single vs. multiple fractions at retreatment. After assessing for proportional hazards assumptions, an unadjusted hazard ratio (HR) was reported using a Cox Regression Analysis for factors impacting the time to event of interest.
Results
Thirty-three patients were treated with repeat thoracic radiotherapy using SBRT or AHRT as a component of their treatment. Patient characteristics are detailed in Table 1. Median age was 66 years. The majority of patients (88%) were treated for primary lung cancer. Average tumor size at retreatment was 2.5 cm (0.6–5.4 cm). Prior lung resections (wedge or lobectomy) had been completed in 24% of patients. Central tumors, defined by RTOG 0813 criteria, were treated with SBRT techniques in 17 patients. These central tumors include disease within the hilum but mediastinal recurrences were not treated with SBRT techniques. Eight patients had oligo-metastatic disease at time of retreatment.
Table 1.
Patient and tumor characteristics.
| Patient characteristics | |
| Patients | 33 |
| Median Age (Range) | 66 (45–80) |
| Gender | No. (%) |
| Female | 14 (42) |
| Male | 19 (58) |
| ECOG performance status | |
| ECOG 0 | 7 (21) |
| ECOG 1 | 19 (58) |
| ECOG 2 | 6 (18) |
| ECOG 3 | 0 |
| Stage at diagnosis* | No. (% of lung primary) |
| IA | 5 (17) |
| IB | 4 (14) |
| IIA | 2 (7) |
| IIB | 2 (7) |
| IIIA | 8 (28) |
| IIIB | 5 (17) |
| IV | 3 (10) |
| Prior lung surgery (lobectomy/wedge) | No. (%) |
| Yes | 8 (24) |
| No | 25 (76) |
| Tumor characteristics | |
| Histology in lung primary tumors | No. (% of all patients) |
| Adenocarcinoma | 12 (36) |
| Squamous cell carcinoma | 11 (33) |
| Small cell lung cancer | 4 (12) |
| Non-small cell lung cancer | 1 (3) |
| Adenocarcinoma and squamous cell carcinoma | 1 (3) |
| Non-lung primary tumors | No. (% of all patients) |
| Laryngeal | 2 (6) |
| Parotid | 1 (3) |
| Esophageal | 1 (3) |
| Tumor size† | |
| Average | 2.5 cm |
| Range | 0.6–5.4 cm |
| Location of lesion treated with SBRT‡ | No. (%) |
| Central | 17 (52) |
| Peripheral | 16 (48) |
Stage for the 29 lung primary tumors.
Size of lesions retreated with AHRT, excludes EBRT retreatment.
Central versus peripheral as defined by RTOG 0813 criteria.
Treatment details are outlined in Table 2. Treatment courses were as follows: 23 patients had EBRT followed by SBRT, 7 patients had SBRT then a second course of SBRT, and 3 patients had SBRT followed by EBRT. Three courses of thoracic radiation were administered to 4 patients and one patient was treated with 4 courses of definitive chest radiotherapy to different areas in the chest. Details for this patient’s treatment are outlined later in this report. The median interval between courses was 18 months with a range 6–61 months.
Table 2.
Treatment details.
| Treatment courses | n (%) |
| EBRT then SBRT | 23 (70) |
| SBRT then SBRT | 7 (21) |
| SBRT then EBRT | 3 (9) |
| Median interval between courses (range) | 18 months (6–61) |
| Data for first XRT course | |
| All patients (n = 33) | |
| Median dose all patients (range) | 60 Gy (22.5–80.5 Gy) |
| Median number of fractions (range) | 30 (1–37) |
| Patients treated initially with SBRT (n = 10, 30%) | |
| Median dose (range) | 50 Gy (22.5–60 Gy) |
| Median number of fractions (range) | 5 (1–5) |
| Fractionation schemes (dose/fraction) | n (%) |
| 54 Gy in 3 fractions (18 Gy) | 3 (30) |
| 50 Gy in 5 fractions (10 Gy) | 3 (30) |
| 40 Gy in 5 fractions (8 Gy) | 1 (10) |
| 60 Gy in 3 fractions (20 Gy) | 1 (10) |
| 22.5 Gy in a single fraction | 2 (20) |
| Patients treated initially with EBRT (n = 23, 70%) | |
| Median dose (range) | 66 Gy (45–80.5 Gy) |
| Median number of fractions (range) | 33 (28–37) |
| Chemotherapy | 16 (70% of EBRT group) |
| Concurrent | 14 (61% of EBRT group |
| Sequential | 2 (29% of EBRT group) |
| Data for retreatment course* | |
| All patients (n = 33) | |
| Median dose all patients (range) | 50 Gy (20–70.2 Gy) |
| Median number of fractions (range) | 10 (1–35) |
| Patients retreated with SBRT (n = 30, 91%) | |
| Median dose (range) | 50 Gy (20–54 Gy) |
| Median number of fractions (range) | 5 (1–10) |
| Fractionation schemes (dose/fraction) | n (% of SBRT patients) |
| 50 Gy in 10 fractions (5 Gy) | 14 (47) |
| 40 Gy in 5 fractions (8 Gy) | 3 (10) |
| 54 Gy in 3 fractions (18 Gy) | 2 (7) |
| 50 Gy in 5 fractions (10 Gy) | 2 (7) |
| 36 Gy in 2 fractions (18 Gy) | 2 (7) |
| 35 Gy in 5 fractions (7 Gy) | 1 (3) |
| 26 Gy in 2 fractions (13 Gy) | 1 (3) |
| 20–22.5 Gy in a single fraction | 5 (17) |
| Patients retreated with EBRT (n = 3, 9%) | |
| Doses for all 3 patients | 66 Gy – 70 Gy – 70.2 Gy |
| Number of fractions | 33 – 35 – 26 |
Includes 5 patients treated with >2 courses of XRT.
Focusing on the 23 patients treated initially with EBRT and retreated with SBRT, the median initial dose and number of fractions was 66 Gy and 33 fractions. Chemotherapy was delivered in 16 patients either concurrently (14 patients) or sequentially (2 patients). Four of the 7 patients not treated with chemotherapy had Stage I lung cancers, while 2 others had metastatic disease with consolidative chest radiotherapy after having stable disease following chemotherapy.
For the 10 patients treated initially with SBRT, the median dose and number of fractions was 50 Gy and 5 fractions. 54 Gy in 3 fractions and 50 Gy in 5 fractions were most often selected as the SBRT treatments. Two patients were treated initially with 22.5 Gy in a single fraction as part of an institutional dose escalation protocol for Stage I lung cancer.
For the 30 patients retreated with SBRT, 50 Gy in 10 fractions was most frequently applied and used in 14 of the 25 patients (56%). Five patients were retreated with single fraction radiosurgery of 20–22.5 Gy. This single fraction strategy was employed from 2003–2006 when a contemporaneous trial of single fraction SBRT was underway and subsequently abandoned due to suboptimal control rates [17]. Total dose and fraction for the remaining patients are outlined in Table 2. Only 3 patients were treated with EBRT after SBRT. Among the 3 patients, 2 had suffered mediastinal recurrences and were treated with conventionally fractionated radiation to the central chest. The final patient was treated with AHRT for a central lung and hilar failure, 70.2 Gy in 26 fractions.
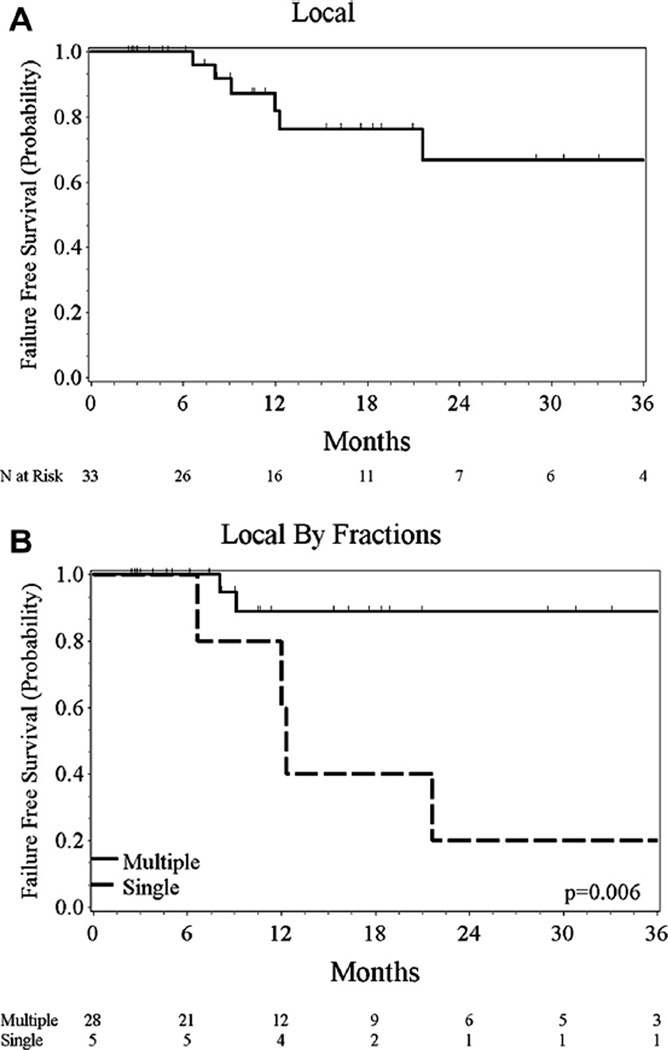
Six patients suffered a local failure after re-irradiation with a median follow-up of 17 months. Local control on Kaplan–Meier analysis was 67% at 2 years (95% CI 38–85%) (Fig. 1). All 6 local failures were noted in the patients who received SBRT at retreatment. Four of five patients treated with a single fraction of 20–22.5 Gy had local recurrence, while only 2/28 patients failed with >1 fraction (HR 7.5, 95% CI 1.4–41.2, p = 0.02). Tumor size analyzed as a continuous variable increased the hazard for local failure (HR 2.1, 95% CI 1.1–4.1, p = 0.03). Treatment interval was not found to affect local control (p = 0.47).
Fig. 1.
Local control for all patients (A) and patients stratified by multiple and single fraction (B).
Four patients suffered a regional failure with a 2 year regional control rate of 83% (95% CI 59–93). One of these patients received 4 courses of radiotherapy. They were originally treated with chemotherapy and 70 Gy EBRT to the right lung and hilum. Nine months later, a right lower lobe tumor was treated with SBRT. An additional 10 months later, the patient progressed in the lower mediastinum and right hilum requiring re-irradiation, 66 Gy in 33 fractions. Finally, seven months later, the upper mediastinum and right supraclavicular region was treated to 66 Gy in 33 fractions but this required no additional overlap. Progressive dyspnea ensued as a result of his therapy as he became oxygen dependent.
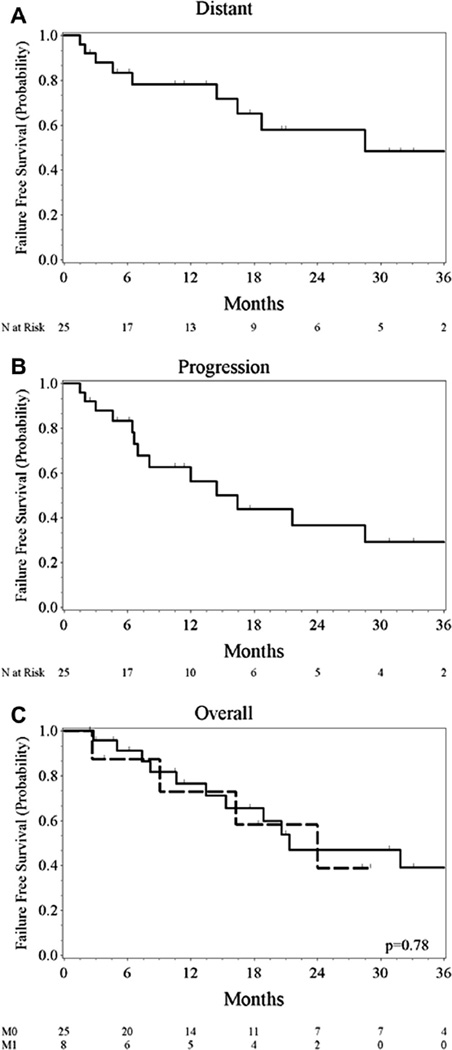
Excluding the eight patients with oligo-metastatic disease, distant failures were noted in 9 of the remaining 25 patients. Distant metastatic free survival was 58% at 2 years (95% CI 32–77).
At the time of this analysis, 12 of the 25 patients without metastatic disease had not progressed. Median PFS for these patients was 16 months (95% CI 6.6–NR). Among the entire group, 17 of the 33 patients have died. Median overall survival was 21 months (95% CI 15–51 months). One and 2 year overall survival was 76% and 45%. Cause of death was related to cancer in 14 of the 17 deaths with distant disease noted at time of death in 13 of 17 patients (Fig. 2).
Fig. 2.
Distant failure free survival (A) and progression free survival (B) for non-metastatic patients (n = 25) and overall survival for all patients stratified by presence (M1, n = 8), or absence (M0, n = 25) of metastases (C).
When analyzed for effects on PFS and OS, retreatment interval did not impact PFS although the hazard for death was diminished when retreatment occurred beyond 2 years (HR 0.285, 95% CI 0.08–0.99, p = 0.05).
Composite dosimetry was available in 19 of the 33 cases. The summed composite median max dose was 103 Gy and had a range of 74–130 Gy. After converting to an EQD2, the median and range for the max dose was 209 Gy and 118–507 Gy. The median lung volume receiving 20 Gy was 26% (5–40%), median max dose to the heart 62 Gy (1–229 Gy), median max dose to the trachea 60 Gy (15–126 Gy, and dose to 30 cc of chest wall 75 Gy (40–153 Gy). The median max and mean esophageal dose was 69 Gy (11–129 Gy) and 21 Gy (3–45 Gy) respectively.
Twelve patients suffered toxicity from the retreatment. Grade 2 toxicity was seen in 10 patients while 1 patient suffered grade 3 toxicity requiring supplemental oxygen. Chest wall pain was the most common treatment related toxicity experienced in the entire cohort and included 6 patients. Nearly all of these patients were treated with oral pain medications and resolution of symptoms was seen at their subsequent follow-up in 2 of the 6 patients. Median time to development of toxicity was 7 months (95% CI 2–16 months) with a range of 2–20 months.
One patient suffered grade 5 toxicity with exsanguination from an aorta-esophageal fistula 6 months after retreatment with 54 Gy in 3 fractions for a central tumor. The patient had been treated 1 year earlier with chemotherapy and radiation to 74 Gy. Unfortunately, the dosimetry file was not available for either the original or repeat course of radiation for summation. However, the DVHs from each plan were available for an estimation of the composite doses. The maximum dose to the esophagus was 20.5 Gy in 3 fractions or 6.8 Gy per fraction for the SBRT plan or an EQD2 of 28.6 Gy. The esophagus received a maximum dose of 66 Gy from the fractionated radiation given with concurrent chemotherapy. The retreatment was over the primary centrally located tumor where the combined maximum esophageal dose would have been approximately 94.6 Gy which is not the highest among the composite plans available. However, the aorta was within the 100% iso-dose lines for both the SBRT and EBRT plans equating to a summed dose of 128 Gy or a maximum summed EQD2 of 200 Gy.
Composite dosimetry was available in 19 of the 33 cases. MIM® Software (v5.6, MIM Software Inc, Cleveland, OH) was used to convert dose to the EQD2 and combine the dose files. The summed composite median max dose was 103 Gy and range of 74–130 Gy. After converting to an EQD2, the median and range for max dose was 209 Gy and 118–507 Gy. The median lung volume receiving 20 Gy was 26% (5–40%), median max dose to the heart 62 Gy (1–229 Gy), median max dose to the trachea 60 Gy (15–126 Gy, and dose to 30 cc of chest wall 75 Gy (40–153 Gy). The median max and mean esophageal dose was 69 Gy (11–129 Gy) and 21 Gy (3–45 Gy) respectively.
Discussion
The idea of retreating lung tumors with radiation is not new. A recent comprehensive review of the literature identified 2 prospective and 9 retrospective studies from 1982 to 2009 [18]. Both prospective studies accrued nearly a decade ago. Wu et al. conducted a 23 patient phase I–II study of conventional thoracic re-irradiation revealing a median survival of 14 months and a 2 year locoregional PFS rate of 42%. No grade ≥3 acute toxicity was reported although 2 patients had symptomatic late grade 3 toxicity. Kramer et al. evaluated the efficacy of palliative re-irradiation with two 8 Gy fractions given a week apart in 28 patients. Median survival was only 5.6 months, but temporary palliative improvements were noted in a substantial proportion of patients. These two studies underscore the difference in intent of retreatment, i.e. whether treatment intent is definitive tumor control, solely for relief of symptoms, or perhaps both.
Our experience as part of a growing body of literature suggests re-irradiation of focal targets within the thorax using SBRT techniques is both feasible and rationale. To the best of our knowledge, our series of 33 patients is the largest specifically addressing re-irradiation to a previously treated lesion using stereotactic techniques and definitive doses. At MDACC, Kelly et al. reported outcomes of 36 patients with prior thoracic radiation, however only 11 patients had received SBRT for what was defined as in-field relapses [12]. An updated report increased this number to 19 [19]. In their experience, a much larger number of patients were treated elsewhere in the thorax which likely represents a different clinical scenario and risk profile with toxicity related to cumulative large volumes of irradiated lung. For this reason, we have excluded patients with out of field relapse from our series.
Two year LC in our present series was 67% compared to 92% in the MDACC series. The difference can in part be attributed to the inclusion of retreatment for out of field relapses (80% of patients in the MDACC series) where practitioners were presumably more likely to deliver higher dose per fraction and feel less compelled to sacrifice tumor coverage to avoid toxicity. As a result, a higher biological equivalent dose (BED) was delivered in the MDACC study where 72% of patients received 50 Gy in 4 fractions. However, the better local control in the MDACC series was offset by higher rates of toxicity. Grade 3 toxicity occurred in 33% of patients and more than 50% experienced grade 2 or higher pneumonitis.
In a series of 15 patients, Trakul et al. noted a similar control rate to our own. About half of the re-irradiation patients received single fraction radiosurgery while the remaining patients received 3–5 fractions with total doses ranging from 30 to 50 Gy. Local control at one year was 65.5% with an improved control rate noted for longer retreatment intervals [14].
For the 25 patients in our series retreated with hypofractionated radiation, the most common dose and fractionation was 50 Gy and 10 fractions in 47% of patients. The 2 yr LC rate was 88% vs. 20% for multiple vs. single fraction retreatments (Log Rank, p = 0.006). This is a more comparable result to the MDACC experience [12].
Highlighting the potential for toxicity, a 29 patient series outlined in-field SBRT retreatment in an even more heterogeneous group of patients and doses. Greater than 50% overlap of the PTVs defined in-field retreatment [13]. Local control was achieved in nearly 50% of patients. However, grade 5 toxicity from massive bleeding occurred in 3 patients with central tumors. Their treatment approach highlights the difficult balance between tumor control and toxicity.
Toxicity in our series was general reasonable. Unfortunately, one patient suffered grade 5 toxicity with aorta-esophageal fistula developing after 54 Gy in 3 fractions for a central tumor. The patient exsanguinated 6 months after retreatment. The treatment occurred during the first years of our retreatment experience using a dose and fractionation not contemplated in our current practice. The retreatment was over the primary centrally located tumor where the combined maximum esophageal dose would have been approximately 94.6 Gy, but the aorta received a summed dose of 128 Gy or a maximum summed EQD2 of 200 Gy. Substantial toxicity for SBRT treatments in central tumors has subsequently been defined with significant risk of Grade 5 toxicity [20]. We therefore now use great caution in treating central tumors and this has likewise influenced our current dose selection of 50 Gy in 10 fractions.
Six of 33 patients (18%) experienced chest wall pain requiring narcotics [12]. Pain developed at a median of 7 months although it was seen as late as 20 months. This outcome is not unexpected as chest wall pain and rib fractures have been correlated with the volume of chest wall receiving radiation [21]. One-third of these patients experienced resolution of their pain at subsequent follow-up generally 3 months later. Toxicity outcomes comparing patients in the 4 largest series are outlined in Table 3.
Table 3.
Incidence of relevant toxicity in published series of re-irradiation with SBRT.
| Toxicity | MDACC series [12] | Karolinska Univ series [13] | Stanford series [14] | Current study |
|---|---|---|---|---|
| Patients with in-field recurrence or second primary | n = 11 | n = 29 | n = 15 | n = 33 |
| n (%) | n (%) | n (%) | n (%) | |
| Chest wall pain requiring narcotics | 3 (27) | 5 (17) | 1 (7) | 6 (18) |
| Pneumonitis | ||||
| Grade 2 | 5 (45) | 3 (10) | 0 | 2 (6) |
| Grade 3 | 0 | 1 (3) | 0 | 1 (3) |
| Esophageal injury | ||||
| Esophagitis* | 0 | 0 | 1 (7) | 0 |
| Stricture leading to dilatation | 1 (9) | 0 | 0 | 0 |
| Aorta-esophageal fistula resulting in Grade 5 toxicity | 0 | 0 | 0 | 1 (3)† |
| Vascular injury and death | 0 | 3(10%) | 0 | 1(3) † |
A single patient suffered esophagitis with no Grade defined.
A single patient in the current series died of massive hemoptysis and was scored as both vascular and esophageal injury.
Most recently Meijneke et al. reported outcomes with re-irradiation of 20 patients using dose summation and highlighted the lack of toxicity (no Grade 3–5 events) despite the median max dose converted to an EQD2 of 363 Gy. Our data corroborate their findings with similar max dose ranges of 123–590 Gy compared to our own of 118–507 Gy. Despite the high max doses in the lung, with the rapid fall off using SBRT, doses to surrounding organs at risk are not uniformly high as illustrated by the low lung V20 values of 15% and 26% within the Dutch analysis and our own respectively [16]. These reports should help providers better understand the summation of dose in the context of variable doses per fraction.
Consistent with the most common dose in our series, we have settled on 50 Gy in 10 fractions balancing the goal of local control while acknowledging the predominant pattern of failure is distant and the risk of toxicity with more intensive fractionation schemes is real.
Re-irradiation after EBRT with SBRT for patients with local recurrences or tumors in the high dose region of prior treatment offers an option for patients not candidates for surgical resection, and offers control rates far greater than systemic chemotherapy. Similarly for initial treatment of central lesions, SBRT for retreatment in central tumors carries substantial risk of high grade toxicity. Clinical trials exploring the optimal dose and comparisons to other salvage options are sorely needed.
Footnotes
Conflict of interest statement
None to declare for all contributing authors.
References
- 1.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 2.Noble J, Ellis PM, Mackay JA, Evans WK, Lung C. Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2006;1:1042–1058. [PubMed] [Google Scholar]
- 3.Wu KL, Jiang GL, Qian H, Wang LJ, Yang HJ, Fu XL, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I–II clinical trial. Int J Radiat Oncol Biol Phys. 2003;57:1345–1350. doi: 10.1016/s0360-3016(03)00768-5. [DOI] [PubMed] [Google Scholar]
- 4.Jeremic B, Shibamoto Y, Acimovic L, Nikolic N, Dagovic A, Aleksandrovic J, et al. Second cancers occurring in patients with early stage non-small-cell lung cancer treated with chest radiation therapy alone. J Clin Oncol. 2001;19:1056–1063. doi: 10.1200/JCO.2001.19.4.1056. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 6.Neri S, Takahashi Y, Terashi T, Hamakawa H, Tomii K, Katakami N, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol. 2010;5:2003–2007. doi: 10.1097/JTO.0b013e3181f8a785. [DOI] [PubMed] [Google Scholar]
- 7.Bauman JE, Mulligan MS, Martins RG, Kurland BF, Eaton KD, Wood DE. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg. 2008;86:1632–1638. doi: 10.1016/j.athoracsur.2008.07.042. discussion 1638–9. [DOI] [PubMed] [Google Scholar]
- 8.Green N, Melbye RW. Lung cancer: retreatment of local recurrence after definitive irradiation. Cancer. 1982;49:865–868. doi: 10.1002/1097-0142(19820301)49:5<865::aid-cncr2820490507>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Montebello JF, Aron BS, Manatunga AK, Horvath JL, Peyton FW. The reirradiation of recurrent bronchogenic carcinoma with external beam irradiation. Am J Clin Oncol. 1993;16:482–488. doi: 10.1097/00000421-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y, Murakami M, Yoden E, Sasaki R, Okuno Y, Nakajima T, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2002;52:390–396. doi: 10.1016/s0360-3016(01)02644-x. [DOI] [PubMed] [Google Scholar]
- 11.Tada T, Fukuda H, Matsui K, Hirashima T, Hosono M, Takada Y, et al. Non-small-cell lung cancer: reirradiation for loco-regional relapse previously treated with radiation therapy. Int J Clin Oncol. 2005;10:247–250. doi: 10.1007/s10147-005-0501-1. [DOI] [PubMed] [Google Scholar]
- 12.Kelly P, Balter PA, Rebueno N, Sharp HJ, Liao Z, Komaki R, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. 2010;78:1387–1393. doi: 10.1016/j.ijrobp.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peulen H, Karlsson K, Lindberg K, Tullgren O, Baumann P, Lax I, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol. 2011;101:260–266. doi: 10.1016/j.radonc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Trakul N, Harris JP, Le QT, Hara WY, Maxim PG, Loo BW, Jr, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol. 2012;7:1462–1465. doi: 10.1097/JTO.0b013e31825f22ce. [DOI] [PubMed] [Google Scholar]
- 15.Seung SK, Solhjem M. Salvage SBRT for previously irradiated lung cancer. J Cancer Ther. 2011;2:190–195. [Google Scholar]
- 16.Meijneke TR, Petit SF, Wentzler D, Hoogeman M, Nuyttens JJ. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol. 2013;107:423–427. doi: 10.1016/j.radonc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Urbanic JJ, McMullen KP, Stieber VW, Hinson WH, Kearns WT, Hampton CJ, et al. A phase I/II dose-escalation/efficacy study of single fraction stereotactic body radiotherapy (SF-SBRT) for metastatic cancer. American Society for Therapeutic Radiology and Oncology 50th annual meeting; Boston, MA. 2008. [Google Scholar]
- 18.Jeremic B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: a review. Int J Radiat Oncol Biol Phys. 2011;80:969–977. doi: 10.1016/j.ijrobp.2011.01.069. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Zhang X, Vinogradskiy YY, Swisher SG, Komaki R, Chang JY. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:1017–1023. doi: 10.1016/j.ijrobp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 21.Dunlap NE, Cai J, Biedermann GB, Yang W, Benedict SH, Sheng K, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]