Abstract
Background
Urinary tract infection (UTI) is commonly experienced by women of various age groups especially elderly ones. We planned to find out the prevalent microbial strains causing UTI in slum inhabitant adolescent and adult women in Dhaka City, Bangladesh.
Methods and materials
Urine sample was collected from 462 UTI suspected female subjects. Pathogenic bacteria were identified using standard microbiological tests, and antimicrobial sensitivity profiles of the pathogens were determined.
Results
Bacteriuria was present in 9% of the subjects. A higher incidence (16.8%) of UTI was noted among adult women aged above 19 years. Escherichia coli (69%), Streptococcus spp. (15%) and Pseudomonas aeruginosa (7%) were more frequently isolated from the urine samples compared to Enterococcus faecalis (3%), Staphylococcus aureus (2%), Klebsiella pneumoniae (2%) and Hafnia alvei (2%). The E. coli isolates showed complete resistance to commonly used drugs, and 58% of these isolates were multidrug resistant (MDR). Minimum Inhibitory Concentration (MIC) values for ciprofloxacin ranged between 64µg/ml and 512µg/ml, and the Minimum Bactericidal Concentration (MBC) values against the isolates were 128µg/ml or above. Isolated strains of E. coli exhibited equal extent of ciprofloxacin resistance irrespective of the presence or absence of plasmid in them.
Conclusion
The extent of drug resistance among the uropathogens if ignored may render them uncontrollable. This study suggests regular monitoring of drug resistance phenotype of the UTI pathogens to reduce the morbidity of female UTI patients and offer better treatment strategy in the healthcare sectors of Bangladesh.
Keywords: Urinary tract infection (UTI), Multidrug resistance (MDR), Adolescent women
Introduction
Acute UTI occurs in many women each year, and the annual costs of caring for those women are invariably very high in Bangladesh. Approximately, 60% of all women experience at least one UTI within their lifetime and roughly 20–30% women suffer from repeated infections (1, 2). Sexual activities of women have been considered as important risk factors for UTI infections and recurrences (3), and with as frequently early marriage occurs in the slum women in Bangladesh, UTI may be promoted. In post-menopausal women, UTI risk factors may also comprise urinary incontinence (4). Bacterial virulence properties may affect the risk of recurrence of infection as well(5).
UTI is commonly caused by Escherichia coli, Proteus, Klebsiella, Enterococcus, and Enterobacter spp. (6). However, Pseudomonas, Staphylococcus aureus, Group B Streptococcus are usually reported with increased rates in patients with urological disorders and following repetitive courses with antibiotic treatments (7). Uropathogenic E. coli utilize a number of virulence factors to adherence the uroepithelial cells; however, the strains fit to a limited number of serogroups mainly to O1, O2, O4, O6, O7, O14, O15, O18, O22 and O75 (8, 9). Anti-microbial resistance among uropathogenic E. coli may be increased with temporal and geographic fluctuations which may introduce multidrug resistant E. coli into the community (10). In recent years, an increased number of Extended-Spectrum-Beta-Lactamases (ESBL) producing pathogens have been observed in outpatient settings, especially related to urinary tract infections (UTI), narrowing the treatment option with antibiotics (11).
In addition to age, sex, marital and social status, other risk factors include nature and type of strains associated with UTI. For example, Shiga toxin-producing E. coli may contribute to hemolytic uremic syndrome, elevated white blood cell count and C-reactive protein levels in infected patients (12). Little is known about the pathogenesis, natural history, risk factors, and epidemiology of the multi-drug resistant UTI pathogens of slum adolescent and adult women origin in Bangladesh. Therefore, we designed our study to determine the prevalence, drug resistance phenotype and plasmid profile of the uropathogens.
Materials and Methods
Study population and sample: The study included UTI suspected female subjects living in slums located around the Dhaka City, Bangladesh. Urine sample was collected from February 2011 to December 2011. Individuals were requested to fill out a questionnaire regarding their consent, morbidity and recent history of medication. Subjects receiving antimicrobial treatment for existing complications were excluded from this study. One midstream-urine sample per female subject was collected and examined by standard quantitative culture methods (13). Positive culture was defined as the culture of a single microorganism at a concentration of >105 colony-forming units (CFU)/ml (14). In cases of delay in processing, the samples were stored at 4°C.
Identification of the uropathogens: Nutrient agar plates were used for total bacterial count of the urine samples and uropathogens were isolated on Blood agar and MacConkey agar media. All the plates were incubated aerobically at 37°C for 24–48 hours and the colonies were enumerated. For confirmation of specific bacterial spp., standard biochemical tests were performed.
Serogrouping: The uropathogenic E. coli isolates were serotyped using different monovalent O-antisera (Eurobio, France) which have association with human infection. Strains that did not display agglutination with any of the 13 used O-antisera were defined as O non-typed (5, 8).
Antimicrobial susceptibility testing: Antimicrobial susceptibility testing of the isolated bacterial spp. was performed by disc diffusion method following the National Committee for Clinical Laboratory Standards (NCCLS) guidelines (15). Standard strains of Escherichia coli ATCC 25922, Enterococcus faecalis 29212, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923 were used for quality control. For this study, we presented susceptibility data for ampicillin (10µg), azithromycin (15µg), doxycycline (30µg), nalidixic acid (30µg), amoxicillin (10µg), tetracycline (30µg), cephalexin (30µg), ciprofloxacin (5µg), levofloxacin (5µg), imipenem (10µg), meropenem (10µg), amikacin (30µg), nitrofurantoin (300µg), netilmicine (30µg), gentamicin (10µg) and ceftriaxone (30µg).
Defining multidrug resistant (MDR) strains: MDR in Enterobacteriaceae is defined as resistance to at least one drug from three or more of the following antimicrobial categories: aminoglycosides (e.g., gentamicin, tobramycin, amikacin or netilmicin), carbapenems (imipenem, meropenem, ertapenem or doripenem), fluoroquinolones (e.g., ciprofloxacin), penicillins (e.g., ampicillin) and tetracyclines (e.g., tetracycline, doxycycline or minocycline) etc. (16).
Minimum inhibitory concentration (MIC): MIC is the lowest concentration of antimicrobial drug that inhibits visible growth of the test organism as outlined by the NCCLS (15). Minimum inhibitory concentrations (MICs) of the selected ciprofloxacin resistant isolates were determined in Mueller-Hinton broth by macro-broth dilution technique (17). The lowest concentration of antibiotic that killed 100% of the test organism on solid media was taken as minimum bactericidal concentration (MBC) for the bacterium.
Plasmid profiling: Alkaline lysis method was followed to extract plasmid DNA from the isolated pathogens (18) and the molecular weight of the unkwon plasmid was determined by agarose (0.8%) gel electrophoresis method comparing the pattern of migration with that of known plasmid markers.
Plasmid curing and plasmid profile analysis: Plasmid curing for the selected isolates was performed according to Tomeda, et al. (1968) (19), and then the isolates were transferred onto 30µg of ciprofloxacin containing plates. After overnight incubation, colonies which appeared on ciprofloxacin plates were assumed as strains bearing drug resistance genes in their chromosome. To confirm the absence of plasmid in the cured isolates, plasmid DNA extraction procedure was performed by the technique mentioned in the previous section.
Results
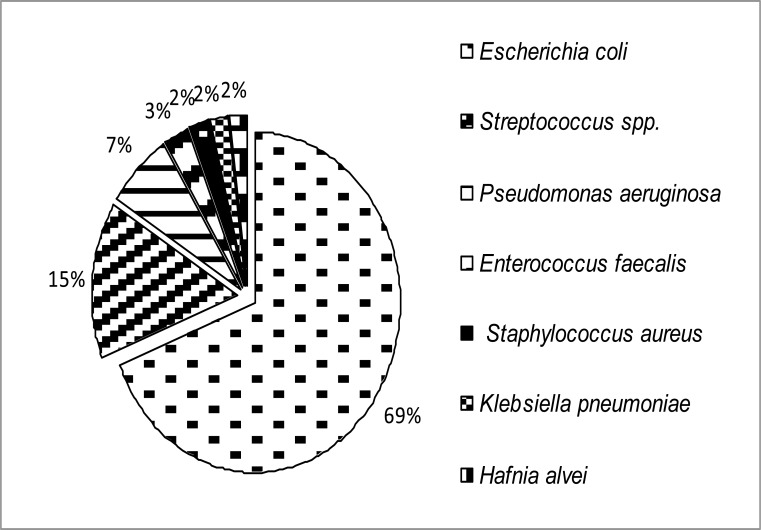
A total of 462 female subjects suspected of having urinary tract infection were included in this study; of them 42 (9%) had significant bacteriuria. Among the 42 positive cases, 29 were adult women (aged >19 years), 11 were adolescents (aged between 10–19 years) and the remaining two were children (aged <10 years). UTI prevalence in adult subjects (n=173) was 16.8%, but 6.6% in adolescents (n=167). Therefore, UTI prevalence between adult and adolescent women was significantly different (p=0.001). A complex microbial population as shown in figure 1 was identified from the urine sample. Escherichia coli (69%), Streptococcus spp. (15%) and Pseudomonas aeruginosa (7%) were most common, while Enterococcus faecalis (3%), Staphylococcus aureus (2%), Klebsiella pneumoniae (2%) and Hafnia alvei (2%) were less frequent in the urine samples.
Figure 1.
Distribution of uropathogens (n=42) among the slum women in Dhaka city, Bangladesh
All the 29 E. coli isolates were grouped with O-antisera where six strains (20.69%) were non-typable. Serogroup O6 was most frequently identified (24.14%) that was followed by serogroup O20 (13.79%) and O18 (10.34%) (Table 1). In total, these three serogroups (O6, O20 and O18) consisted of 48.28% of all E. coli isolates.
Table 1.
Frequency of E. coli serogroups in UTI patients living in the slum of Dhaka city, Bangladesh
| Serogroup | Number | Percent |
| O1 | 2 | 6.90 |
| O6 | 7 | 24.14 |
| O8 | 2 | 6.90 |
| O15 | 2 | 6.90 |
| O18 | 3 | 10.34 |
| O20 | 4 | 13.79 |
| O25 | 1 | 3.45 |
| O55 | 1 | 3.45 |
| O78 | 0 | 0.00 |
| O86 | 0 | 0.00 |
| O111 | 0 | 0.00 |
| O119 | 0 | 0.00 |
| O125 | 1 | 3.45 |
| Non-typed | 6 | 20.69 |
| Total | 29 | 100.00 |
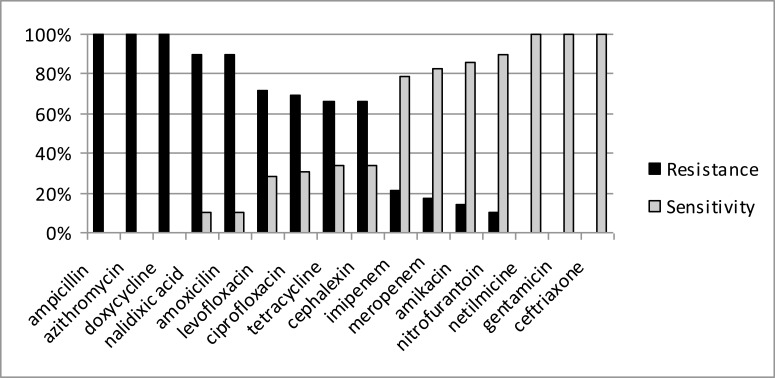
The E. coli isolates of this study, as represented in figure 2, were 100% resistant to ampicillin, azithromycin, doxycycline, 90% to nalidixic acid and amoxicillin, 70% to levofloxacin, 68% to ciprofloxacin and 65% to tetracycline and cephalexin. Conversely, lower resistance was recorded for imipenem (20%), meropenem (15%), amikacin (12%), and nitrofurantoin (10%). The E. coli isolates were completely (100%) sensitive to netilmycine, gentamicin and ceftriaxone.
Figure 2.
Antibiotic resistance/sensitivity pattern of the Escherichia coli isolates (n = 29)
Most of the isolated E. coli was found to be resistant to more than four test drugs (Table 2). Among the E. coli isolates, 17 (58%) were Multidrug Resistant, exhibiting their insensitiveness against ampicillin, doxycycline and ciprofloxacin, each belonging to different antibiotic groups. However, 11 (37.93%) of the E. coli isolates were resistant to five while two (6.89%) of them were resistant to seven different groups of antibiotic.
Table 2.
Drug resistant phenotypes of selected E. coli in UTI patients living in the slum of Dhaka city, Bangladesh
| Percentage (%) of resistant isolates |
Resistant phenotype† |
| 100% | AMP + DO |
| 58.62% | AMP + DO + CIP |
| 37.93% | AMP + DO + CIP + TET + CL |
| 6.89% | AMP + DO + CIP + TET + CL + IM + AMK |
AMP=Ampicillin; DO=Doxycycline; CIP=Ciprofloxacin; TET= Tetracycline; CL= Cephalexin; IM= Imipenem; AMK= Amikacin
Among the multidrug resistant isolates, ten highly resistant strains were chosen for Minimum Inhibitory Concentration (MIC) determination against ciprofloxacin (Table 3). MIC values were lower with the range of 64–128 µg/ml. The Minimum Bactericidal Concentration (MBC) values against the isolates were 128 µg/ml or more.
Table 3.
MIC and MBC values for ciprofloxacin resistant E. coli in UTI patients living in the slum of Dhaka city, Bangladesh
| Isolate number |
MIC (µg/ml) | MBC (µg/ml) |
| 3 | 64 | 256 |
| 5 | 128 | 256 |
| 8 | 128 | 256 |
| 9 | 64 | >128 |
| 11 | 128 | 256 |
| 16 | 64 | 128 |
| 18 | 128 | 256 |
| 21 | 64 | 128 |
| 23 | 64 | >128 |
| 27 | 128 | 256 |
Plasmid profile of highly ciprofloxacin resistant E. coli isolates showed plasmids with variable size ranging from 140 to < 2 MDa (Figure 3), among them, four isolates were found to pose large plasmid of 140 MDa size.
Figure 3.
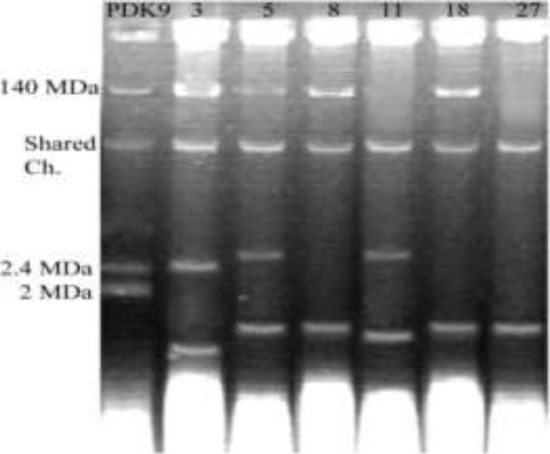
Electrophoretic patterns of plasmid DNA from uropathogenic isolates of E. coli electrophoresed in 0.8% agarose gel; first lane showing Marker plasmid pDK9 and rests indicating isolate number.
Plasmid was cured from the six selected isolates (isolate no. 3, 5, 8, 11, 18 and 27) and was grown on nutrient agar. These isolates showed no bands on agarose gel when plasmid DNA extraction procedure was performed. It emphasizes that the resistance of genes might be carried by the chromosomal DNA of the isolates.
Discussion
In Bangladesh, slums are population-dense residences with poor facilities of the basic requirements of life. Slum inhabitants reside in a highly polluted environment comprising of numerous pathogenic microorganisms. Thus, the lives of slum populations are always at risk of getting infected with various pathogenic microbes. Urinary tract infections (UTIs) are common in women, often associated with significant morbidity and mortality (20), and may affect women of all age groups especially sexually active ones (21). We studied 462 urine samples and found UTI in about 9% cases where a higher incidence (16.8%) of UTI was noticed for adult women aged over 19 years. The frequency is close to the incidence reported by Ahmed and Avasarala (2008), i.e. 12.7% (22), but is higher than the study of Singh MM et al. (2001) (23) who reported 4.2% UTI in a community based study. In Bangladesh, Begum et al. (2006) (24) reported 16.4% UTI in the female garments workers of Dhaka City. Patients in Bangladesh usually see a doctor after experiencing severe health complications for a particular disease condition. Therefore, Bashar et al. (2009) and Rahman et al. (2009) reported higher frequency of UTI i.e., 27% and 24.14% respectively in hospital or clinic based study (25, 26).
This study reports association of Escherichia coli (69%), Streptococcus spp. (15%), Pseudomonas aeruginosa (7%), Enterococcus faecalis (3%), Staphylococcus aureus (2%), Klebsiella pneumoniae (2%) and Hafnia alvei (2%) in UTI. Other investigators (Basar et al. 2009, Saber et al. 2010, and Jan et al. (2009) also reported higher association of E. coli (66.67%, 77.8% and 52.65% cases respectively) in UTI patients (25, 27–28). A major part of O-groupable E. coli strains mainly O1, O2, O4, O6, O7, O18 and O75 have been reported to account for UTI in different parts of the world (29). The uropathogenic E. coli isolates of this study mainly belong to one of 3 serogroups O6, O15 and O18. Strains belonging to these serogroups have been reported to pose specific virulence factors for their invasive ability (29). The finding of this study nearly supports this theory.
Antibiotic resistance among uropathogens has become a public health concern in Bangladesh (30). Under individual predisposing conditions, E. coli can multiply rapidly in the urinary tract of an UTI patient. However, if the patient doesn't maintain antibiotic dose regimen properly, the organism may emerge as drug resistant variant and eventually result in both community-and nosocomially acquired UTIs (31, 32). The present study found the E. coli isolates to be completely resistant to the commonly used drugs such as penicillin group and also to a higher extent to broad spectrum quinolone group. A similar frequency among the UTI related E. coli isolates was recorded in some developing countries (33–34). However, a lower frequency for the same pathogen was reported fromthe isolates of developed countries (35). Antibiotic abuse and practicing incomplete antibiotic regimen has considerably promoted the dissemination of multidrug resistant bacteria (36–37). This study reports lower resistance for less commonly used drugs like imipenem, meropenem, amikacin and nitrofurantoin, and complete sensitivity to netilmycine, gentamicin and ceftriaxone among the E. coli isolates. This finding is supported by the study of Sharmin et al. (2009) which reported a good sensitivity for imipenem, ceftazidime and amikacin against UTI-isolates of E. coli in Bangladesh (38). Encarnacion AR (2012) also reported sensitivity of the UTI-isolates of E. coli for amikacin in Philippines (39). This finding suggests the use of drugs that are less commonly prescribed by practitioners for arresting the pathogens in UTI patients may be beneficial.
Multidrug resistance among the pathogenic microbes is a common problem which may be either plasmid-borne or chromosomal or sometimes both (40, 41). At least 17 of all the Escherichia coli isolates examined in this study exhibited multidrug resistance. Such multidrug resistance complicates empiric treatment of E. coli infections and may contribute plasmid mediated multidrug resistance transfer (42). The present study demonstrated chromosome-borne resistance for ciprofloxacin since the E. coli isolates were able to colonize on the ciprofloxacin containing plates even after curing off their plasmids. A similar mode of resistance has been studied in detail by Lindgren et al. (2003) (43). On the other hand, Paiva et al. (2012) (44) studied ciprofloxacin resistance gene in plasmid DNA of uropathogenic E. coli. The evolution of fluoroquinolone resistance has been found to be involved in the accumulation of multiple mutations in several genes, and it is ultimately reflected in the phenotype of the resistant organism. Therefore, the use of ciprofloxacin to treat patients should be restricted. Healthcare practitioners must think critically before prescribing the commonly used drugs to a patient. They should suggest a drug only after having the antibiogram of the associated pathogens during a disease.
Acknowledgements
The authors acknowledge the Ministry of Science and Information & Communication Technology Government of the People's Republic of Bangladesh for funding this research.
References
- 1.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 2.Patton JP, Nash DB, Abrutyn E. Urinary tract infection: economic considerations. Med Clin North Am. 1991;75(2):495–513. doi: 10.1016/s0025-7125(16)30466-7. [DOI] [PubMed] [Google Scholar]
- 3.Kelsey MC, Mead MG, Gruneberg RN, Oriel JD. Relationship between sexual intercourse and urinary-tract infection in women attending a clinic for sexually transmitted diseases. J Med Microbiol. 1979;12(4):511–512. doi: 10.1099/00222615-12-4-511. [DOI] [PubMed] [Google Scholar]
- 4.Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30(1):152–156. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 5.Foxman B, Zhang L, Tallman P, et al. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172(6):1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 6.Guentzel MN. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. Barron's Medical Microbiology. 4th ed. Univ of Texas Medical Branch; 1996. [PubMed] [Google Scholar]
- 7.Bell LE, Mattoo TK. Update on childhood urinary tract infection and vesicoureteral reflux. Semin Nephrol. 2009;29(4):349–359. doi: 10.1016/j.semnephrol.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Vranes J, Schonwald S, Kuzmanovic SN, Ivanicic B. Low virulence of E. coli strains causing exacerbation of chronic pyelonephritis. Acta Clin Crout. 2001;40:165–170. [Google Scholar]
- 9.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis. 2001;33(1):89–94. doi: 10.1086/320880. [DOI] [PubMed] [Google Scholar]
- 11.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14(4):933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura N, Yamazaki T, Tamai H. Risk factors for the development of Escherichia coli O157:H7 associated with hemolytic uremic syndrome. Pediatr Int. 1999;41(2):218–222. doi: 10.1046/j.1442-200x.1999.4121040.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarridge JE, Pezzlo MT, Vosti KL, Weissfeld AS. Laboratory Diagnosis of Urinary Tract Infections. Washington DC: American Society for Microbiology; 1987. [Google Scholar]
- 14.Burnett RW, Haber MH, Hackel E, Hanson CA, Keren DF, Lee-Lewandrowski E. Clinical Laboratory Medicine. Philadelphia: Williams & Wilkins; 1994. pp. 1113–1120. [Google Scholar]
- 15.NCCLS, author. Performance Standards for Antimicrobial Susceptibility Testing, 11th Informational Supplement, NCCLS document M100-S11. Wayne, Pennsylvania: National Committee for Clinical Laboratory Standards. (NCCLS); 2001. [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.NCCLS, author. Methods for dilution in antimicrobial susceptibility test, Approved Standard M2–M5. Wayne, Pennsylvania: National Committee for Clinical Laboratory Standards (NCCLS); 1993. [Google Scholar]
- 18.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomeda M, Inuzuka M, Kubo N, Nakamura S. Effective elimination of drug resistance in sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968;95:1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Foxman B, Zhang L, Tallman P, et al. Transmission of uropathogens between sex partners. J Infect Dis. 1997;175(4):989–992. doi: 10.1086/514007. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed SM, Avasarala AK. Urinary tract infections (UTI) among adolescent girls in rural Karimnagar district, AP - K.A.P. STUDY. Indian J Prev Soc Med. 2008;39(1−2):67–70. [Google Scholar]
- 23.Singh MM, Devi R, Garg S, Mehra M. Effectiveness of syndromic approach in management of reproductive tract infections in women. Indian J Med Sci. 2001;55(4):209–214. [PubMed] [Google Scholar]
- 24.Begum N, Mamoon ABA, Hossain M, Begum N, Chowdhury SA, Rahman MF. UTI among female workers in a selected garment industry of Dhaka city: A cross sectional study. The ORION Medical Journal. 2006;23:325–327. [Google Scholar]
- 25.Bashar MA, Ahmed MF, Rahman SR, Gomes DJ. Distribution and Resistance Trends of Escherichia coli from Urinary Tract Infections Isolated in Dhaka City. Ban J Med Sci. 2009;15(2):93–98. [Google Scholar]
- 26.Rahman F, Chowdhury S, Rahman MM, Ahmed D, Hossain A. Antimicrobial Resistance Pattern of Gram-negative Bacteria Causing Urinary Tract Infection. S J Pharm Sci. 2009;2(1):44–50. [Google Scholar]
- 27.Jan N, Meshram SU, Kulkarni A. Plasmid profile analysis of multidrug resistant E. coli isolated from UTI patients of Nagpur City, India. Romanian Biotechnological Letters. 2009;14(5):4635–4640. [Google Scholar]
- 28.Saber MH, Barai L, J Haq A, Jilani MSA, Begum MJ. The Pattern of Organism Causing Urinary Tract Infection in Diabetic and Non Diabetic Patients in Bangladesh. Bangladesh J Med Microbiol. 2010;04(01):6–8. [Google Scholar]
- 29.Blanco M, Blanco JE, Alonso MP, et al. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Res Microbiol. 1997;148(9):745–755. doi: 10.1016/s0923-2508(97)82450-3. [DOI] [PubMed] [Google Scholar]
- 30.Rahman MM, Haq JA, Hossain MA, Sultana R, Islam F, Islam AH. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an urban hospital in Dhaka, Bangladesh. Int J Antimicrob Agents. 2004;24(5):508–510. doi: 10.1016/j.ijantimicag.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Gales AC, Gordon KA, Wilke WW, Pfaller MA, Jones RN. Occurrence of single-point gyrA mutations among ciprofloxacinsusceptible Escherichia coli isolates causing urinary tract infections in Latin America. Diagn Microbiol Infect Dis. 2000;36(1):61–64. doi: 10.1016/s0732-8893(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 32.Gupta K, Stamm WE. Pathogenesis and management of recurrent urinary tract infections in women. World J Urol. 1999;17(6):415–420. doi: 10.1007/s003450050168. [DOI] [PubMed] [Google Scholar]
- 33.Matute AJ, Hak E, Schurink CA, et al. Resistance of uropathogens in symptomatic urinary tract infections in Leon, Nicaragua. Int J Antimicrob Agents. 2004;23(5):506–509. doi: 10.1016/j.ijantimicag.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Pieboji JG, Koulla-Shiro S, Ngassam P, Adiogo D, Njine T, Ndumbe P. Antimicrobial resistance of Gram-negative bacilli isolates from inpatients and outpatients at Yaounde Central Hospital, Cameroon. Int J Infect Dis. 2004;8(3):147–154. doi: 10.1016/j.ijid.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46(8):2540–2545. doi: 10.1128/AAC.46.8.2540-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MG, Henry GL. Drug availability in Jamaica. West Indian Med J. 1989;38(2):105–109. [PubMed] [Google Scholar]
- 37.Hossain MM, Glass RI, Khan MR. Antibiotic use in a rural community in Bangladesh. Int J Epidemiol. 1982;11:402–405. doi: 10.1093/ije/11.4.402. [DOI] [PubMed] [Google Scholar]
- 38.Sharmin S, Alamgir F, Fahmida, Saleh AA. Antimicrobial sensitivity pattern of uropathogens in children. Bangladesh J Med Microbiol. 2009;03(01):18–22. 2009. [Google Scholar]
- 39.Encarnacion AR. Pathogens Causing Urinary Tract Infection and Their Resistance Patterns among Pediatric Patients in Chong Hua Hospital (January 2003 to June 2005) PIDSP Journal. 2012;13(1):37–43. [Google Scholar]
- 40.Wang M, Sahm DF, Jacoby GA, Hooper DC. Emerging plasmid-mediated quinolone resistance associated with the qnr gene in Klebsiella pneumoniae clinical isolates in the United States. Antimicrob Agents Chemother. 2004;48(4):1295–1299. doi: 10.1128/AAC.48.4.1295-1299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin Microbiol Infect. 2007;13(1):5–18. doi: 10.1111/j.1469-0691.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 42.Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob Agents Chemother. 2006;50(6):2251–2254. doi: 10.1128/AAC.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komp LP, Karlsson A, Hughes D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob Agents Chemother. 2003;47(10):3222–3232. doi: 10.1128/AAC.47.10.3222-3232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paiva MC, Nascimento AM, Camargo IL, Lima-Bittencourt CI, Nardi RM. The first report of the qnrB19, qnrS1 and aac(6)-Ib-cr genes in urinary isolates of ciprofloxacin-resistant Escherichia coli in Brazil. Mem Inst Oswaldo Cruz. 2012;107(5):687–689. doi: 10.1590/s0074-02762012000500018. [DOI] [PubMed] [Google Scholar]