Abstract
High-intensity focused ultrasound (HIFU) is a non-invasive technology, which can be used occlude blood vessels in the body. Both the theory underlying and practical process of blood vessel occlusion are still under development and relatively sparse in vivo experimental and therapeutic data exist. HIFU would however provide an alternative to surgery, particularly in circumstances where serious complications inherent to surgery outweigh the potential benefits. Accordingly, the HIFU technique would be of particular utility for fetal and placental interventions, where open or endoscopic surgery is fraught with difficulty and likelihood of complications including premature delivery. This assumes that HIFU could be shown to safely and effectively occlude blood vessels in utero. To understand these mechanisms more fully, we present a review of relevant cross-specialty literature on the topic of vascular HIFU and suggest an integrative mechanism taking into account clinical, physical and engineering considerations through which HIFU may produce vascular occlusion. This model may aid in the design of HIFU protocols to further develop this area, and might be adapted to provide a non-invasive therapy for conditions in fetal medicine where vascular occlusion is beneficial.
Keywords: high-intensity focused ultrasound, prenatal, blood vessels, pathophysiology
1. Introduction
Surgical techniques can be used to create permanent arterial or venous occlusion, either for therapeutic benefit or prior to ligation of an artery or vein, and require close proximity to or contact with the vessels to be occluded. High-intensity focused ultrasound (HIFU) is a non-invasive alternative method of occlusion which may avoid complications inherent to surgery. HIFU could be of particular use in fetal surgery, were it shown to occlude blood vessels safely and effectively. This would potentially offer an in-utero treatment for fetal diseases such as twin-to-twin transfusion syndrome (TTTS), twin-reversed arterial perfusion (TRAP) sequence, bronchopulmonary sequestration (BPS) or sacrococcygeal teratoma (SCT).
HIFU involves the production of alternating high positive (compression) and negative (rarefaction) pressures which cause tissue heating (with the potential to boil interstitial water) and acoustic cavitation bubbles to form, which can have extremely energetic locally destructive activity. In addition, ultrasound has an acoustic radiation force (non-ionizing), which in a high power focused beam can have a significant impact on exposed tissues. How each of these interacts with tissue, and in particular blood vessels, is not yet fully understood. In the current absence of specific experimental work designed to understand these mechanisms more fully, we present a review of relevant literature on the topic of vascular HIFU and suggest an integrative mechanism taking into account clinical, physical and engineering considerations through which HIFU may produce vascular occlusion.
2. High-intensity focused ultrasound: principles of action
HIFU is a therapeutic technique which uses a focused piezoelectric transducer outside the body to converge ultrasonic energy (usually 1–3 MHz for non-invasive applications) into a tissue target and produce localized destruction. The focal zone is the only position where the ultrasound intensity (energy per unit area) is high enough to produce change in the tissue, and create a lesion. Lesions are ellipsoidal, typically 1–2 mm diameter and 8–15 mm long (figure 1). The use of diagnostic imaging allows non-invasive identification of the target and monitoring of response.
Figure 1.
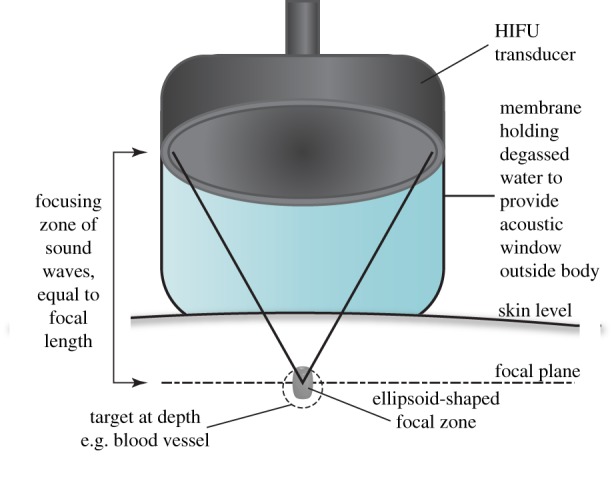
Non-invasive set-up of HIFU transducer to produce tissue lesions at depth. (Online version in colour.)
HIFU energy can pass through different tissue layers en route to the target. Providing regions of bone and air are avoided, relatively little energy will be lost at each tissue interface. The main energy loss is owing to attenuation (absorption and scatter) within the tissue layers. Alternative techniques such as radio frequency ablation (RFA), laser photocoagulation and microwave energy have poor tissue penetration so use invasive methods to destroy deep tissue.
HIFU exposure can be continuous (primarily used to induce heating) or pulsed, which may better exploit acoustic cavitation. Ultrasound can be converted to thermal energy mainly via a plethora of molecular relaxation processes, which occur following ultrasound excitation. When temperatures in excess of 56°C are maintained for more than or equal to 2 s [1,2], this causes coagulative necrosis owing to protein denaturation, cellular destruction [2] and tissue stiffening [3]. This is a common form of unplanned cell death in response to a cellular insult, where tissue architecture is characteristically preserved, cells retain their outline while their proteins coagulate, and metabolic activity ceases [4]. Histological studies show coagulative necrosis in response to thermal damage within HIFU lesions. In soft tissue, HIFU lesions are characterized by a necrotic centre and a rim of functionally impaired cells (lacking glycogen stores) which fade to ghost outlines leaving a sharp rim between affected and unaffected tissues 48 h after exposure, with the lesion encapsulated by granulation tissue [5]. Detachment of cells from their basement membrane and each other is also observed [6] with evidence of a strong acute inflammatory response and remodelling in a chronic inflammatory response, involving cellular regeneration, proliferation, migration and removal of debris lasting up to three months [7].
Applying pulses of HIFU can cause rapid changes in tissue pressure, described as the peak rarefaction pressure amplitude (PRPA). There is a threshold PRPA for each tissue at which acoustic cavitation occurs, where gas- or liquid-filled cavities form, usually at points of ‘weakness’, such as interfaces between different tissue layers or fluid-filled structures [8]. These bubbles oscillate and exert shear stresses on the surrounding tissue or may expand rapidly and collapse, destroying surrounding tissue structure, in a technique known as histotripsy. This mechanism does not rely on tissue heating to work [7]. Cavitation may be observed at lower PRPAs when ultrasound contrast agents (UCA) are present. These micrometre-sized gas bodies, used to enhance the echogenicity of blood, provide pre-existing gaseous cavities which can promote acoustic cavitation. Histology of tissue subjected to histotripsy reveals emulsification, and regions filled with acellular debris produced by mechanical fractionation of the tissue [9].
3. Development of techniques for high-intensity focused ultrasound-mediated vascular occlusion
A comprehensive history of HIFU is beyond the scope of this review. For our purposes, it is important to note that although HIFU was first described in 1942 [10] the availability of diagnostic ultrasound and MRI for guidance transformed the potential for HIFU as a non-invasive vascular occlusion therapy. The first specific attempts to produce HIFU-mediated vascular occlusion were in 1972 and relied upon visual targeting of surface vessels, the auricular artery in rabbits [11]. Since then the trend has been towards non-invasive targeting of non-surface vessels (table 1). HIFU has been demonstrated to produce a spectrum of vascular changes: vascular occlusion of non-bleeding arteries and veins [14,15,20–24,27,28,30,34–36], haemostasis of lacerated arteries and veins [16–19,26], thrombus generation [11,14,23,24,36], vascular spasm and reduction in blood flow [15,24,25,28,34,35,37,38], arterial rupture [15,20,21,34] or no observed functional impairment [13,33,39]. There is a single case report of successful treatment in human pregnancy: a TRAP sequence where HIFU was applied to the insertion of the umbilical cord of the acardiac fetus [30]. This study did not demonstrate whether selective vascular occlusion or localized soft tissue destruction at the cord insertion point (the method used by RFA) in the acardiac twin caused division of the fetal circulations. A summary of the different HIFU mechanisms discussed in this paper and their experimental results is provided (tables 1 and 2; figure 2).
Table 1.
Summary of mechanisms used and experimental outcomes in animal models and human studies examining continuous HIFU-mediated vascular occlusion. Key: o, vascular effect observed; x, vascular effect not observed; -, vascular effect not reported/investigated.
|
in vivo models—continuous HIFU | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| references | model | vessel | animals (n) | exposures (n) | vessel diameter (mm) | non-invasive? | targeting | intensity (W cm−2) | frequency (MHz) | focal length (mm) | no. exposures | exposure time (s) |
| [11] | rabbit | auricular artery | 12 | 28 | 0.7–1.0 | Y | visual | 25–1500 | 1 | 125 | 1 | 0.5–720 |
| [12] | dog | unspec. vein | 19 | - | NR | Y | A-mode | NR | 3 | NR | NR | NR |
| man | varicose leg veins | 11 | - | NR | Y | visual | NR | 3 | NR | NR | NR | |
| [13] | rabbit | aorta, IVC | 24 | - | NR | N | visual | 1500 | 4 | NR | 20 | 5 |
| [14] | rat | femoral vein | 6 | - | 1.5 | N | A-mode | 167 | 7.31 | 20 | 4–7 | 3 |
| [15] | rabbit | femoral art., vein | 19 | 26 | 1.0–1.3 | Y | MRI | 4400–8800 | 1.49 | NR | 5–8 | 1 |
| [15] | rabbit | renal artery | 9 | - | 0.6 | Y | MRI | 6500 2800 | 1.5 | NR | 12–16 | 10 |
| [16] | rabbit | liver microvasculaturea | 8 | 27 | <0.5 | N | visual | 3000 | 3.3 | 40 | 1 | 1–2 |
| [17] | pig | muscular arteriesa | 5 | 41 | 3–10 | N | visual | 3100 | 3.5 | 55 | 1 | 10–20 |
| [18] | pig | muscular arteriesa | 3 | 89 | 3–10 | N | Doppler | 2000 | 3.5 | 55 | 1 | 5–20 |
| [19] | pig | muscular arteries | 4 | 76 | 3–10 | N | Doppler | 3000 | 3.5 | 55 | 1 | 17–25 |
| [20] | rat | femoral art., vein | 10 | - | 0.5–1.5 | N | visual | 4660 | 1.7 | 150 | 1 | 2 |
| [21] | rat | femoral art., vein | 16 | - | 0.5–1.5 | N | visual | 1690–4660 | 1.7 | 150 | 1 | 5 |
| [22] | rat | femoral artery | 18 | 36 | 0.5 | NR | NR | 800 | 1 | NR | 1 | 5–10 |
| 10 000 | 3 | 1 | 5–10 | |||||||||
| [23] | rabbit | auricular veina | 15 | - | 1.0 | Y | visual | 750 | 3.9 | 35 | 1–3 | 3 |
| [24] | rat | femoral artery | 23 | - | 0.5 | Y | Doppler | 530–2750 | 3.3 | NR | 1 | 5 |
| [25] | sheep | uterine artery | 7 | - | NR | N | Doppler | NR | 1.05 | 100 | 6–7 | 8–10 |
| [26] | rabbit | femoral artery | 25 | - | 1.5 | N | visual | 3000 | 9.6 | 25 | NR | 5–10 |
| [27] | rabbit | umbilical artery | 11 | - | 0.4–0.8 | Y | Doppler | 1400–5500 | 2.26 | 70 | 3–15 | 5 |
| [28] | rabbit | renal artery | 8 | - | 0.5 | Y | Doppler | 4000 | 2.2 | 60 | 2–10 | 5 |
| [29] | human (in utero) | umbilical artery | 1 | - | NR | Y | Doppler | 2300 | 1.71 | NR | NR | 10 |
| [30] | human (in utero) | umbilical artery | 1 | - | NR | Y | Doppler | 4600 | 1.71 | 60 | NR | 10 |
|
in vivo models—continuous HIFU | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| references | model | vessel | tissue temp (°C) | cavitation | vascular occlusion | haemostasis | thrombus | endothelial damage | vascular spasm | vascular rupture | vascular wall damage | length of follow-up |
| [11] | rabbit | auricular artery | 38.0–66.7 | NR | x | - | o | o | - | - | o | 72 hours |
| [12] | dog | unspec. vein | NR | NR | - | - | o | - | - | - | o | none |
| man | varicose leg veins | NR | NR | - | - | o | - | - | - | o | none | |
| [13] | rabbit | aorta, IVC | 68.0–80.1 | NR | x | - | - | - | x | x | o | six months |
| [14] | rat | femoral vein | <45 | x | o | - | o | o | - | - | - | 28 days |
| [15] | rabbit | femoral art., vein | NR | o | o | - | - | - | o | o | - | 14 days |
| [15] | rabbit | renal artery | 64 | o | o | - | - | - | o | o | - | 7 days |
| [16] | rabbit | liver microvasculaturea | 59–86 | o | o | o | x | o | - | - | o | none |
| [17] | pig | muscular arteriesa | NR | NR | x | o | o | o | o | - | o | none |
| [18] | pig | muscular arteriesa | NR | NR | x | o | - | - | - | o | - | none |
| [19] | pig | muscular arteries | NR | NR | o | o | o | o | - | - | o | none |
| [20] | rat | femoral art., vein | NR | NR | o | o | - | - | - | o | - | none |
| [21] | rat | femoral art., vein | NR | NR | o | - | - | - | - | o | - | none |
| [22] | rat | femoral artery | 46 | NR | x | - | - | - | - | - | - | none |
| 98 | NR | o | - | - | - | - | - | o | 12 days | |||
| [23] | rabbit | auricular veina | NR | NR | o | o | o | o | - | - | o | 28 days |
| [24] | rat | femoral artery | 98 | NR | o | - | o | o | o | - | o | 3 days |
| [25] | sheep | uterine artery | NR | o | x | - | - | o | o | - | o | NR |
| [26] | rabbit | femoral artery | NR | NR | o | o | o | o | - | - | o | 60 days |
| [27] | rabbit | umbilical artery | NR | NR | o | - | o | o | - | x | o | none |
| [28] | rabbit | renal artery | NR | NR | o | - | - | - | o | o | o | 7 days |
| [29] | human (in utero) | umbilical artery | NR | NR | x | - | - | - | - | - | - | three weeks |
| [30] | human (in utero) | umbilical artery | NR | NR | o | - | - | - | - | - | - | 20 weeks |
|
ex vivo experimental models—continuous HIFU | ||||||||||||
| references | model | vessel | animals (n) | exposures (n) | vessel diameter (mm) | non-invasive? | targeting | intensity (W cm−2) | frequency (MHz) | focal length (mm) | number of exposures | exposure time (s) |
| [31] | dog (ex vivo) | coronary artery | 6 | - | NR | N/A | visual | NR | NR | NR | 1 | 15 |
| [32] | rat (ex vivo) | femoral artery | 1 | - | 0.5 | N/A | visual | 230–1400 | 1.54 | 38 | 2 | 20 |
| [33] | pig (ex vivo) | hepatic art., portal vein | 20 | - | 4 | N/A | B-mode USS | NR | 0.94 | 140 | NR | 10 |
| references | model | vessel | tissue temp (°C) | cavitation | vascular occlusion | haemostasis | thrombus | endothelial damage | vascular spasm | vascular rupture | vascular wall damage | length of follow-up |
| [31] | dog (ex vivo) | coronary artery | NR | NR | N/A | N/A | N/A | o | N/A | N/A | - | N/A |
| [32] | rat (ex vivo) | femoral artery | NR | NR | N/A | N/A | N/A | - | N/A | N/A | o | N/A |
| [33] | pig (ex vivo) | hepatic art., portal vein | NR | NR | N/A | N/A | N/A | o | N/A | N/A | o | N/A |
aLacerated vessels.
Table 2.
Summary of mechanisms and experimental outcomes in animal models and human studies examining pulsed HIFU-mediated vascular occlusion. Key: o, vascular effect observed; X, vascular effect not observed; -, vascular effect not reported/investigated.
|
in vivo models—pulsed HIFU | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| reference | model | vessel | no. animals | no. exposures | vessel diameter (mm) | non-invasive? | targeting | intensity (W cm−2) | PRPA (MPa) | frequency (MHz) | PRF (Hz) | burst length (ms) | exposure time (s) |
| [34] | rabbit | auricular artery, vein | 22 | - | 1.0 | yes | visual | NR | 4–37 | 0.68–2.02 | 5–20 | 10 | 10–180 |
| [40] | rabbit | auricular vein | 16 | - | 1.0 | yes | visual | NR | 1–9 | 1.17 | 1 | NR | 1–120 |
| [37] | rat | aorta, femoral artery | 9 | - | 1 | no | Doppler | 578–1734 | NR | 1 | 1 | 50 | 6 |
| [35] | rabbit | auricular vein | 21 | - | 1 | yes | visual | 23 | 9 | 1.17 | NR | NR | 60 |
| reference | model | vessel | tissue temp (°C) | cavitation | vascular occlusion | haemostasis | thrombus | endothelial damage | vascular spasm | vascular rupture | vascular wall damage | length of follow-up | additional IV therapy |
| [34] | rabbit | auricular artery, vein | NR | o | o | - | - | - | o | o | o | 3 h | - |
| [40] | rabbit | auricular vein | NR | o | X | - | o | o | - | - | o | 1 h | UCA |
| [37] | rat | aorta, femoral artery | 29–30 | o | X | X | X | X | o | X | X | immediate sacrifice | UCA |
| [35] | rabbit | auricular vein | NR | o | o | - | o | o | - | X | X | 14 days | UCA + fibrinogen |
Figure 2.
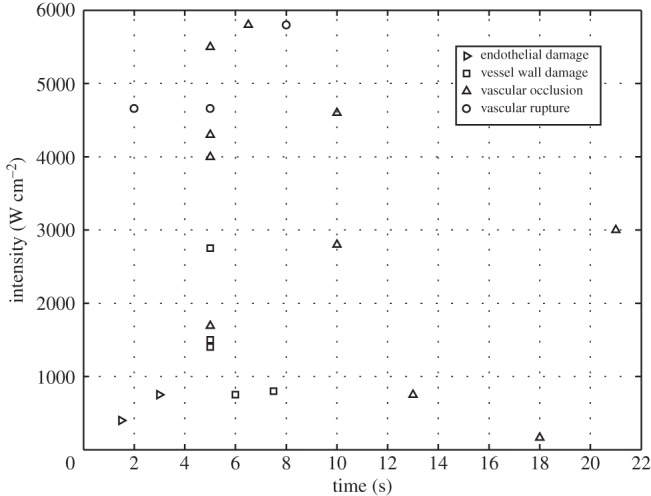
Summary of vascular responses for intensity against time for the exposure conditions listed in table 1. No differentiation has been made between intensities reported as spatial peak or spatial average.
The heterogeneity of these results demonstrates that describing the properties of delivered energy alone is not predictive of the outcome in blood vessels. In some studies, vascular occlusion has been successful at lower energy levels where only non-occlusive endothelial or vascular wall damage has been reported and at higher levels where vascular rupture would be expected. This may be explained by blood vessels forming part of a biological system, with multiple anatomical, physiological, mechanical, thrombotic and inflammatory responses to the absorption of ultrasound energy. How these biological responses may best interact with the properties of HIFU to produce vascular occlusion is the focus of this review.
Neither coagulative necrosis nor tissue emulsification alone are likely to be effective mechanisms of vascular occlusion. HIFU can destroy soft tissue microvasculature by coagulative necrosis even when not deliberately targeted at the vessels, occluding vessels up to a diameter of 0.5 mm [41], working with limited efficacy on lacerated vessels of 0.5–2.5 mm and being ineffective on vessels more than 2.5 mm [16]. However, both damaged [42] and undamaged [6] un-occluded blood vessels are often seen surrounding soft tissue HIFU lesions, resulting in circumferential haemorrhage [43], with greater haemorrhage in more vascular regions [44]. Changes suggestive of coagulative necrosis are also seen in vessel walls exposed to HIFU without loss of structure or function [13,16]. In emulsified tissue, there are also margins which demonstrate circumferential haemorrhage around the lesioned area [45]. Pulsed HIFU has been shown to produce oedema and disruption of the extracellular matrix and collagen fibrils of vessels, without occlusion [13,16,46], acoustic cavitation in tissue is associated with unintended vascular rupture [8,47] and may therefore be used to increase vascular permeability.
4. Effect of heating on vessels and high-intensity focused ultrasound-mediated tissue fusion
Arteries and veins have a common basic structure (figure 3), comprising the tunica intima, an inner layer of endothelium on a basement membrane. The tunica media is an intermediate layer of smooth muscle, thicker in arteries than veins and absent in capillaries. The tunica adventitia is an external supportive layer and may be continuous with surrounding collagenous connective tissue, absent in capillaries. Arteries also contain elastin layers [48]. Capillaries, arterioles and venules (d ≤ 0.3 mm) comprise tissue microvasculature. Theoretical modelling of vascular thermal damage suggests that the first vessel changes are caused by thermal denaturation and shrinkage of collagen fibres while vessel integrity is maintained by elastic laminae, if present, and smooth muscle [49].
Figure 3.
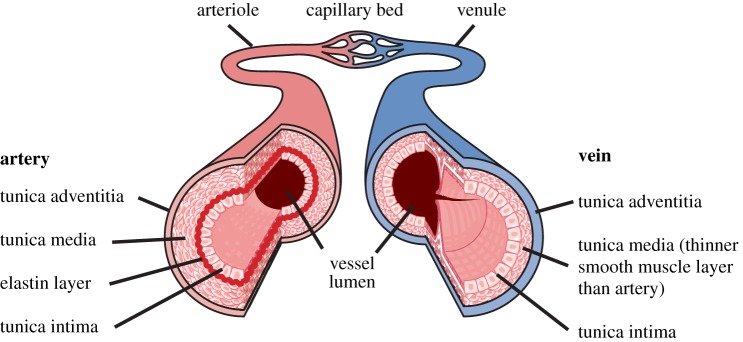
The structure of the arterial and venous vascular wall. (Online version in colour.)
Tissue fusion is a common method of therapeutic vascular occlusion. The vessel is mechanically compressed and occluded, while energy is converted to heat at the point of occlusion, which seals the vessel closed by tissue fusion. Tissue fusion requires partial denaturation of collagen (and elastin if present) such that the fractured structure is able to reform bonds outside the laminar structure (bridging), effectively fusing the many layers of the vessel wall together. Arteries and veins become more difficult to occlude by tissue fusion as their diameter, lumenal blood flow or pulse pressure increases. Collagen is a prominent feature of the tunica media and adventitia. Heating collagen to above its denaturation threshold of 62–67°C [50] unravels its rope-like structure. Collagen undergoes isovolumetric shrinkage of up to 60% of its length when heated above this threshold, the majority of which occurs within 1 s [51]. Such vessel shrinkage has been demonstrated ex vivo where the luminal cross-sectional area of a non-perfused vessel was reduced by up to 96% [32,52], although this effect is much less pronounced in perfused vessels in vivo [38]. Heating of collagen beyond this threshold leads to hyalinization and fracturing of its structure, which contributes to tissue stiffness, and is a possible explanation for why vessels subjected to multiple HIFU exposures stiffen and become prone to rupture [18]. During the initial phase of heating (less than 1 s), collagen is able to form bridging bonds to other partially denatured collagen fibres and fuse tissue [53]. However, the free ends of collagen must be physically adjacent to form bridging bonds, usually requiring a form of external compression. Capillaries, arterioles and venules have sufficiently narrow lumens that a combination of collagen shrinkage and cellular dehydration will result in vessel collapse and coagulation with minimal attendant cell damage or haemorrhage [54], the likely mechanism of HIFU-mediated microvascular destruction.
Vascular occlusion does not invariably result from heating a vessel. Heating to temperatures above 100°C with laser or diathermy, where the water in cells vaporizes and ruptures cellular membranes, produces a weak coagulum of denatured cells, collagen debris and carbonized tissue, unable to withstand physiological blood pressure [55], and can cause vascular rupture owing to vessel wall necrosis and elastin destruction [56]. A similar tissue vaporization is seen in HIFU tissue emulsification, although tissue carbonization is less likely to occur as desiccated tissue cannot propagate ultrasound, and further heating does not occur beyond this point. Successful vascular occlusion by collagen fusion occurs in temperature range 73–87°C [57], while temperatures below 54°C caused no structural changes in collagen [57].
The electrothermal bipolar vessel sealing system is approved for clinical occlusion of arteries (d ≤ 6 mm) and veins (d ≤ 12 mm) [58]. It uses mechanical compression combined with electrical heating to cause collagen bridging between vessel walls [59]. The resultant occlusion has a burst pressure well in excess of physiological pressures [60]. The system is computer controlled, monitoring electrical impedance to allow careful control of the energy supplied and prevent tissue under or overheating [59].
While there are no studies on HIFU-mediated vascular occlusion that investigate the presence of tissue fusion, several studies indirectly suggest that it occurs. Disruption of vascular and perivascular collagen has been observed with vascular occlusion using histology and electron microscopy [22,24,27]. The collagen changes can extend beyond the tunica media to the tunica adventitia and adjacent connective tissue, and shrinkage in these outer layers can result in lumenal constriction and occlusion [19,26]. In one study, vascular occlusion was an unintended side effect of HIFU, and fusion of the vessel via collagen was described, along with disruption of the elastic lamina [19]. Elastin fragmentation has been demonstrated in occluded vessels using van Gieson staining, changes which were not seen in non-occluded vessels [24].
Other studies have investigated the effects of HIFU specifically on the vascular collagen, using Masson's trichrome stain [12]. Although occlusion was not achieved, collagen in the vessel walls was fragmented and took on a corkscrew appearance, creating spaces within the tunica media which corresponded to the vacuolization seen in the smooth muscle when haemotoxylin and eosin staining was also performed. Similarly, collagen fibre swelling and vessel wall fracture in response to non-occlusive HIFU exposure [61], and the collapse of collagen in damaged microvasculature has been reported [42].
Successful HIFU-mediated vascular occlusion occurs within the temperature ranges discussed above. Occlusion has been reported in the range of 64–98°C [15,16,22,24], with non-occlusive vascular damage occurring 43–69°C [11,13,22] and no damage to the tunica media or adventitia at less than 43°C [11,14,37]. This agrees with theoretical modelling of temperature change in vessels which shows that collagen shrinkage would predominate in vessels before tissue necrosis at the temperatures typically achieved by HIFU [49,53]. The duration these temperatures were maintained varies between studies, affecting the overall amount of thermal energy supplied to the tissue, which may explain why the temperature ranges are wide and overlapping.
5. Effect of high-intensity focused ultrasound on thrombogenesis
Thrombogenesis may be an appropriate haemostatic response to vascular injury, or a non-haemostatic disease process. Virchow's triad suggests three predisposing factors which may result in non-haemostatic thrombus formation in vivo: endothelial damage, stasis of blood flow or hypercoagulability of blood [4]. With reference to HIFU-mediated vascular occlusion, all three mechanisms appear to be contributory to thrombus formation within vessels. The potential effect of ultrasound exposure and heating on blood constituents must also be considered.
Endothelial damage is an established pathophysiological mechanism by which vascular occlusion occurs. It leads to occlusive thrombus generation in the vessel lumen and permanent stenosis and fibrosis of the vessel in a chronic inflammatory process. This can be achieved by intravascular injection of sclerosing agents, mechanical damage to the tunica intima or can be iatrogenic. Endothelial damage results in exposure of subendothelial collagen, which is an activator of the coagulation cascade. Primary haemostasis is a sequence of activation, aggregation then adherence of platelets to areas of damaged endothelium. This leads to secondary haemostasis with deposition of fibrin around the adhered platelets and organization into stable thrombus. Widening of gaps between endothelial cells occurs allowing extravasation of plasma proteins and acute inflammatory response cells and the collection of subendothelial oedema. With more extreme damage, the endothelial cells die and cease their synthetic function, leading to a failure of vascular relaxation signalling, which may contribute to vascular spasm in addition to thrombogenesis.
Endothelial damage is often observed with vascular occlusion (table 1), as would be expected given that the endothelium is damaged at the lowest of reported exposures (figure 2). It is usually accompanied by thrombosis, which can produce an occlusive seal even where incomplete tissue fusion has occurred [55]. Transmural vascular damage, including endothelial damage and thrombosis has been demonstrated in studies using both scanning electron microscopy [23] and histology (PTAH staining) [24]. A sequence of changes leading to permanent obstruction has been described in rabbit veins, with transmural thermal injury occurring at day 0, followed by fibrin thrombus deposition, endothelial cell detachment and acute inflammatory response at day 2. By day 7, there was occlusive organized thrombus and chronic inflammatory response that persisted until day 28, by which time the vessel had been replaced by fibrous scar tissue without residual inflammatory reaction [23]. However, the question of whether HIFU can produce stable thrombus and vascular occlusion by endothelial damage alone remains.
Isolated endothelial damage has been produced in vivo by HIFU exposure without damage to the tunica media, adventitia or surrounding tissue [12,14,40], although this has not resulted in permanent vascular occlusion in the absence of acoustic cavitation or artificially elevated levels of fibrinogen, which makes the blood hypercoagulable and promotes formation of stable thrombus. It appears that HIFU exposures which do not produce inertial acoustic cavitation can damage the endothelium, but do not sufficiently expose subendothelial collagen to produce a clinically significant primary haemostatic response in vivo. This is supported by in vitro studies which show that although platelet aggregation occurs with HIFU exposure, adherence requires collagen substrate [62]. Where inertial cavitation is promoted by UCA, this results in a higher proportion of the endothelial surface area being damaged than at the same energy level without microbubbles, and in the generation of thrombus [40]. However, even with increased endothelial damage, elevated circulating levels of fibrinogen were required to produce occlusive thrombus [35].
Blood hypercoagulability has been described as the primary cause of ‘acoustic haemostasis’ and has also been suggested as a method of thrombogenesis [62,63]. This describes the expression of adhesion molecules on the surface of platelets (activation) in response to HIFU exposure and clumping (aggregation) in the absence of triggers from endothelial damage, occurring up to 50 s after initial HIFU exposure. It is known that platelets can be activated in the absence of tissue damage by shear stresses on their surface [64], and in the studies by Poliachik, aggregation occurred in response to acoustic cavitation. This is thought to be due to microstreaming causing shear stresses on the surface of the platelets which are sufficient to cause activation and aggregation but insufficient to cause disruption of the membrane [65]. However, adherence has not been observed without an artificial collagenous substrate or endothelial damage, meaning the resultant thrombus would be non-adherent in vivo. Given the nature of the continuous flow of the circulatory system, such aggregated platelets are more likely to result in distant emboli than occlusive thrombus.
Heating platelets has not been directly studied with reference to HIFU. It is known that platelets are optimally activated at body temperature, 36–38°C and are functionally impaired at temperatures more than 45°C, showing an inability to activate or aggregate [66]. Hence it is unlikely that platelets heated by HIFU have any role in vascular occlusion, other than as cellular debris in organized thrombus. Similarly, red blood cells haemolyse at more than 45°C [67].
Slowing of blood flow has been observed to contribute to HIFU-mediated vascular occlusion as unintended occlusion occurred more commonly at points of vessel compression in one study where the aim was to seal lacerated vessels [19]. Stasis of red blood cells is a known effect of low intensity unfocused ultrasound (intensities ≤ 12 W cm−2), with separation of blood into bands of cellular aggregates and plasma owing to the ultrasound standing waves [68], with normal circulation restored after exposure [69]. To form a standing wave, strong reflection of the plane of the wave is required, which is unlikely to occur in vivo. To date, this effect has not been observed in vessels exposed to HIFU. This remains an area for further investigation in HIFU.
This evidence suggests that endothelial damage and physiological primary haemostasis are the key features in thrombus generation resulting from HIFU exposure of blood vessels. However, there is minimal evidence that this mechanism alone can produce permanent vascular occlusion. It is more likely that thrombosis has a contributory role in maintaining and organizing HIFU-mediated vascular occlusion.
6. Effect of high-intensity focused ultrasound on blood flow
Vascular spasm is a recognized response of vessels to injury. The smooth muscle of the vessel contracts, as a physiological method of reducing blood flow, improving the success of primary haemostasis and ultimately reducing blood loss [70]. It is seen in vessels exposed to HIFU, both in the presence and the absence of vascular damage, and can be monitored non-invasively by colour Doppler measurements of peak systolic velocity (PSV). Induction of vascular spasm by HIFU to produce temporary cessation of blood flow has been shown [15] although the intensities used were above the threshold for acoustic cavitation causing vascular rupture. Regardless, this represents a means by which lumenal narrowing can occur.
Vascular spasm has been seen in vivo at HIFU intensities which produce transmural vascular damage [15,25,28,34] but also in exposures which do not produce vascular damage [24,52] and is not reliant on the occurrence of tissue heating [37]. Although the effect is described as transient, it persists for sufficient time post HIFU exposure to allow repeat PSV measurements taken by Doppler ultrasonography even in the absence of evidence of vascular damage on histology [37].
Vascular spasm is likely augmented by the loss of vascular relaxation in response to endothelial damage and cessation of endothelial cell synthetic function. In a series of ex vivo experiments in canine coronary arteries, vascular relaxation was lost as a result of endothelial damage and disruption of the nitric oxide signalling pathways, but mechanisms of relaxation remain intact in the vascular smooth muscle and can be activated by the administration of exogenous nitric oxide donors, isolating the cause as endothelial damage [31].
The radiation force of high-intensity sound waves can create localized streaming of liquids away from the focal point [71], even overcoming the physiological pressure gradient normally controlling blood flow in vessels. This has been observed in practice, where reversal of the arterial jet and pulsatile flow was observed in lacerated femoral, axillary and carotid arteries in pigs independent of mechanical compression exerted by the transducer being pressed against the vessel, which alone was insufficient to obstruct flow even temporarily [17]. The lumens of arteries are maintained patent by the pressure gradient of blood flow through them, with a typical mean arterial pressure (MAP) in the range of 85–125 mmHg in humans. Arteries such as the femoral, axillary and carotid artery will lose lumenal patency when the MAP ≤ 50 mmHg [72]. This suggests the possibility that acoustic streaming could disrupt the local pressure gradient in the artery sufficiently to cause it to collapse. Veins and the microvasculature are much more easily collapsible and so would also be expected to collapse by this mechanism.
A final hypothesized mechanical effect by which HIFU could occlude blood flow is by temporary compression of the vessel owing to its acoustic radiation force. When exposed to HIFU, tissue cannot respond fast enough to the changes between positive and negative pressures, meaning its motion becomes out of phase with the acoustic wave and energy is transferred to the tissue. This transfer of momentum to the tissue, in the direction of wave propagation, results in tissue displacement. The amount of pressure delivered will vary with both the intensity of the HIFU source and the attenuation of the overlying tissue, but will be magnified by the small area over which it is delivered.
Although much speculated upon, acoustic radiation force has not been observed to collapse blood vessels in vivo, however soft tissue deformation and displacement of 1–3 mm has been observed in vivo [73]. Given the recognized difficulties in making non-invasive in situ pressure measurements [52], it is not unreasonable that in vivo effects of acoustic radiation force have not been fully demonstrated. Synchronized systems to enable use of diagnostic Doppler ultrasound during HIFU exposure have been proposed [74,75], but these would only demonstrate ‘no flow’ in a vessel, not a definitive cause, and B-mode ultrasound imaging would likely lack the spatial resolution to determine reliable changes in diameter of vessels. In an experimental system to study isolated blood vessels in vitro, acoustic radiation force was sufficient to displace vessels out of the field of view of the microscope intended to monitor changes in vessel diameter [52].
Greater deformation and displacement of the proximal wall of the blood vessel compared to the distal wall would probably be expected, promoting collapse, as the blood within the vessel is much less stiff than surrounding soft tissues. Even without this difference, unidirectional pressure can occlude arterial blood flow at normal pulse pressures (e.g. pressing on a pulse point) and can be likened to the effect of momentum in the direction of HIFU wave propagation. Blood vessels are also maintained patent by the pulse pressure of blood flow, as previously discussed, and if this pressure has been reduced by acoustic streaming as observed experimentally [17], then lower pressures would be needed to produce such collapse or deformation. This is an area which would benefit from further experimental evaluation as whether compression and deformation of blood vessels by acoustic radiation force occurs is of relevance to the design of HIFU systems.
7. Contribution of the inflammatory response and repair mechanisms
There has been minimal work specifically on the contribution of inflammatory responses to vascular occlusion. The harmonic scalpel, an invasive system for producing vascular occlusion which uses ultrasound to create vibrational forces in the tissue, creates a more pronounced acute inflammatory response than electrocautery, despite resulting in a smaller area of injured tissue [59]. HIFU produces extensive oedema, disruption to tissue, inflammatory cell infiltration and activation of the cytokine cascade. Inertial cavitation induced damage produces a comparable activation of the acute inflammatory response, though this response does not convert into a chronic inflammatory response in the absence of tissue damage [7]. Extracellular oedema is known to reduce blood flow, especially in smaller vessels, and perivascular oedema resulting from HIFU exposure has been cited as contributing to lumenal constriction [32].
The role of the inflammatory response in controlling and organizing the physiological response to vascular damage cannot be discounted. Collateral circulation was seen to arise quickly following vascular occlusion [20], so while a distributing artery may be blocked the area is not automatically devascularized. Ultimately, the nature of the chronic inflammatory response will determine whether the vessel becomes patent again or remains permanently occluded and fibrosed [14,23].
8. An integrated mechanism of action
Consideration of the theoretical and experimental evidence of the responses of vessels exposed to HIFU demonstrates that there are many potential mechanisms through which the physical properties of HIFU may interact with a living system, and that contribute to vascular occlusion. Vascular occlusion cannot be considered simply as a side effect of vessel wall cellular destruction, and protocols for HIFU-mediated vascular occlusion should be designed to maximize this plethora of effects.
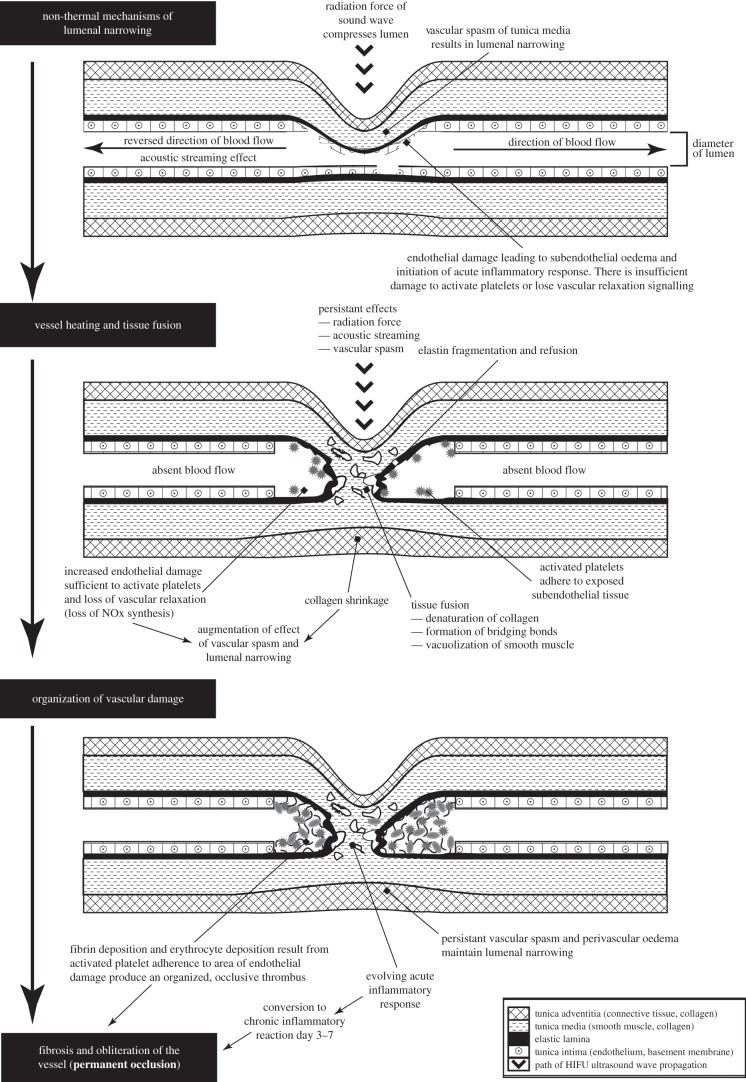
There are various ways in which the effects described above could be combined but as time and temperature appear to be important factors in distinguishing acute from more delayed vascular occlusion, a multi-stage process can be envisioned taking place along a typical heating–time curve of HIFU (figure 4). Working out the exact timescale and thermal doses to which these stages may correspond is a challenge for further experimental work.
Figure 4.
A suggested multi-stage integrated mechanism for HIFU-mediated vascular occlusion, presented as progression through time and temperature changes.
In the initial stage, we consider the effects taking place before temperatures reach the 64–98°C range where tissue fusion occurs, which for HIFU could mean from less than 1 s up to approximately 20 s. These effects are temporary, reversible or repairable, and alone have not been shown to produce permanent vascular occlusion. This stage relies on lumenal narrowing achieved through combination of the mechanical effects of ultrasound (tissue displacement and acoustic streaming), with tissue response such as vascular spasm, cell shrinkage, endothelial damage and accumulation of subendothelial oedema. This stage does not necessarily represent lumenal collapse but features processes through which vessel wall apposition could be produced.
In the next stage, temperatures will reach a range in which tissue fusion can occur, and the energy deposition will be sufficient to produce irreversible damage, with denaturation and shrinkage of collagen and fracturing of elastic laminae. There is also the persistent effect of vascular spasm, obstructed blood flow and the increasing endothelial destruction leads to a loss of vascular relaxation responses and activates platelets.
The final stage is dominated by tissue response that persists beyond the end of the HIFU heating. The mechanical effects of HIFU are removed and so perivascular oedema is the only potential method of weak external compression. In this stage, the strength of tissue fusion and/or thrombotic occlusion is tested by the return of physiological blood pressure. The vessel may be fused in a fully or partially occluded position, or not at all. Activation of secondary haemostatic mechanisms form occlusive, stable thrombus that can withstand the physiological flow pressures involved. Progression from acute to chronic inflammatory responses remodel the damaged vessel, which ultimately determines whether permanent occlusion of the vessel occurs over the following period, up to 28 days.
9. Potential application to fetal medicine
There are several diseases in fetal medicine where prenatal occlusion of abnormal blood vessel(s) is either curative or confers improvement in prognosis. Such treatments are invasive, requiring entry to the intrauterine cavity and amniotic sac and have inherent risks of preterm pre-labour rupture of membranes (pPROM), preterm labour (PTL) and extreme prematurity, chorioamonitis, chorio-amniotic membrane separation and maternal operative risks [76]. Hence, despite identification through antenatal screening, the mainstay of management for these conditions is expectant, monitoring for severe fetal compromise, a tipping point where the associated risks of invasive treatment become less than the potential benefit. Using such therapies as ‘salvage methods’ reduces their success rate. Development of non-invasive alternatives, such as HIFU, could not only reduce the risks associated with salvage therapies but may provide a therapeutic modality for less severely compromised fetuses in utero, or even represent a potential preventative treatment.
10. Twin-reversed arterial perfusion sequence
This affects 1% of monochorionic twin pregnancies [77]. The heart of one twin either fails to develop or is rudimentary, and the heart of the healthy ‘pump’ twin perfuses both bodies through placental arterio-arterial anastomoses or a direct connection between the umbilical cords. This can result in cardiac failure and death in the healthy twin, with an untreated mortality rate of 55% [77] and spontaneous resolution in less than or equal to 21% of cases [78]. The aim of treatment is to divide the circulations by umbilical cord occlusion of the acardiac twin. Bipolar diathermy, fetoscopic laser and RFA have all been used. Bipolar diathermy cord occlusion has been superseded by minimally invasive techniques, as the risks of pPROM and PTL were elevated (27%, 22%, respectively, survival 85%) [76]. Fetoscopic laser reports a 80% survival with pPROM in 18% and PTL in 3% within 28 days of procedure [79]; RFA reports a 70–92% survival rate [76,80] with a pPROM and preterm labour rate of 14% and 7%, respectively [76]. Hence, minimally invasive techniques reduce but do not remove the risks, without changing survival rates. Although TRAP may be diagnosed from 11 to 13 weeks gestation, treatment is delayed until after 16 weeks gestation to allow time for fusion of the amnion and chorion, by which time intrauterine death of the pump twin occurs in up to one-third of cases [78]. Non-invasive HIFU has been used to successfully produce umbilical cord occlusion from 13 weeks gestation [30] in one of the two reported cases without complications [29,30]. Although the authors report technical challenges related to achieving sufficient focal depth, HIFU is possible therapy for TRAP.
11. Twin-to-twin transfusion syndrome
TTTS affects 9–15% of monochorionic diamniotic twins [81,82], or 1 : 2500 livebirths, and is the most important cause of death and handicap in monochorionic twins [83]. It results from unequal sharing of blood supply between twins owing to placental vascular anastomoses, present in 95% monochorionic pregnancies [84,85]. Anastomoses allow ‘vascular steal’ or imbalance between circulations, a relative over and under-perfusion of the recipient and donor twin, respectively [86]. There is grading of disease progression from mild to severe (Quintero stages 1–4) [87] and while mild disease may not require treatment, severe disease left untreated has approaching 90% mortality [88]. In severe cases, the gold standard treatment is fetoscopic laser ablation of vascular anastomoses [89], either by selective ablation as they cross between the two ‘halves’ of the placenta, or by ablating the entire equator between the two halves of the placenta to create a functionally dichorionic placenta [90]. Success rates are quoted at single twin survival of 76–87% and double twin survival of 50–60% with a 5–25% double fetal loss rate [91] and an 11% rate of neurological impairment in survivors [92]. Fetal losses are attributed to either the complications of the procedure or recurrent disease, which occurs in up to 16% of cases and is associated with lower rates of survival and neurologically intact infants [93]. Five per cent to 30% of anastomoses can remain post ablation [94,95] and are recognized as causing recurrent disease [96] or a related condition, twin anaemia polycythaemia syndrome (TAPS). Iatrogenic TAPS is more common after ablation than spontaneously (13 versus 5%) [81,97]. HIFU may be an alternative to invasive therapy in TTTS. The energy levels needed to ablate placental vasculature [98] and the focal depths required are within the HIFU range of operation, and the reduction in complication rate may allow treatment of less severe disease. Either selective vascular ablation or equatorial division of the placenta could be performed, as points or confluent planes of ablation can be achieved with HIFU [20]. HIFU may reduce rates of recurrent TTTS and TAPS, as residual anastomoses would be identified by Doppler ultrasound [99] integrated into the HIFU system.
12. Bronchopulmonary sequestration
BPS is rare, characterized by a non-functional lung mass with an abnormal systemic arterial rather than pulmonary blood supply, typically diagnosed in the second trimester and reaching peak size by 28–30 weeks gestation. Masses are variable: some spontaneously regress, while others increase in size, causing pulmonary hypoplasia, mediastinal shift, cardiac failure, pleural effusions and hydrops [100]. The presence of pleural effusion and hydrops are poor prognostic features with untreated perinatal mortality of 95% [101]. Various therapies are used, and the more successful focus on ablation of the abnormal blood supply.
The sequestration can be removed by open fetal surgery, with 54% survival [102]. It carries additional maternal surgical risks and creates a uterine scar. Doppler ultrasound guided fetoscopic laser vessel ablation is a minimally invasive alternative, with survival rates in excess of 87.5% [101,103]; sclerosants injected into the aberrant vessel have shown high success rates, but trials have small numbers [104,105]. Complication rates are not quoted. Thoraco-amniotic shunting to drain pleural effusions is complicated by shunt blockage [106] and chest wall deformity in 77% cases [107]. Again, the energy levels, focal depth and suitable targeting method for BPS vasculature are well within the scope of a HIFU system integrated with Doppler ultrasound. In one animal model, the targeting of intra-thoracic structures led to skin burns in the fetus owing to reflection of the ultrasonic energy by the calcified rib cage [43], however in our experience it is possible to identify the aberrant vasculature using a sagittal view which would avoid transmission of ultrasonic energy through the rib cage.
13. Sacrococcygeal teratoma
SCT is the most common neoplasm in the fetus, occurring in 1 : 22 000 livebirths [108]. SCT is typically benign, though requiring removal postnatally to avoid malignant transformation [109]. They are attached into the coccyx and may extend into the pelvis and abdomen. They are identified and monitored prenatally by ultrasound and Doppler can assess vascularity. The antenatal course is usually uncomplicated, but highly vascular or rapidly growing tumours can cause high output cardiac failure, anaemia and hydrops. These are poor prognostic features [110]. Without cardiac compromise or hydrops, 88% survival is quoted [111]. Perinatal mortality results from tumour avulsion, rupture or haemorrhage at delivery [112,113]. With cardiac compromise or hydrops mortality approaches 100% [111]. Reducing tumour vascularity can slow its growth and the impact of ‘vascular steal’ effect. Open fetal surgery and resection of the teratoma is possible [114] though it has a higher risk of haemorrhage than other fetal surgery [115]. Minimally invasive devascularization of the tumour via percutaneous laser ablation [116–118] is possible although only superficial vessels can be occluded as the maximum penetration of laser is 2–3 mm. Both methods report mixed results. RFA has a 50% survival rate with significant morbidity reported owing to spread of thermal energy into adjacent soft tissues [119,120]. Alcohol sclerosis has been attempted but specific results are not reported [116].
While it is unlikely that the entire tumour could be ablated by HIFU exposure to the soft tissue, especially as larger tumours are more likely to require treatment, it has been demonstrated that destruction of tumour vasculature increases the volume of tissue destroyed for the amount of energy supplied [121,122]. The vasculature of the SCT could be pre-mapped by colour Doppler ultrasound or MR angiography and used to target HIFU. Ultrasound energy is recognized to have a lower ‘thermal spread’ than other applications [59] so may produce less thermal injury in adjacent soft tissues. Reduction in vascularity may also reduce risks associated with haemorrhage at time of delivery. Animal studies have demonstrated that HIFU lesions in the fetal pelvis can be made despite the restrictions of the bony pelvis on the acoustic window [123], and that survival is compatible with the generation of necrotic tissue within the fetus [43,44]. HIFU represents a potential non-invasive therapy for SCT in the antenatal period, especially of fetuses with significant compromise in utero.
14. Physics and engineering requirements
HIFU treatment comprises a number of components—targeting, energy delivery and monitoring of its effects. Targeting is most usually achieved by either ultrasound or magnetic resonance imaging (MRI). Therapy ultrasound beams are usually generated from a transducer that is focused using either a bowl configuration, a plane transducer/lens combination or a multi-element array with which focusing is achieved by appropriate application of phase and amplitude to each element. Where ultrasound is used to monitor HIFU ablation of soft tissue, a successful treatment is assessed in terms of the appearance of a bright echo, whereas with MRI thermometry sequences are used to calculate thermal dose [124]. If the spatial precision of HIFU damage is to be harnessed to its fullest extent, it is important to account for tissue motion when placing the lesions. This presents a major challenge to successful targeting and treatment delivery and may be achieved, for example, by synchronizing the HIFU exposure with cardiac or respiratory motion, and specifically for fetal medicine, accounting for movement of the fetus and umbilical cord within the amniotic sac.
In obstetrics, ultrasound image guidance is likely to be the method of choice for reasons of safety, clinical and patient familiarity, targeting efficacy and real-time responsiveness to fetal movement. To successfully occlude a selected blood vessel it needs to be identified clearly for targeting, currently best done using Doppler ultrasound techniques. Vessels of more than 1 mm diameter are routinely identified in clinical practice by Doppler ultrasound [125]. The main disadvantage of diagnostic ultrasound is that, unlike MRI, it does not permit the measurement of absolute temperature rise during exposure, both owing to interference of the therapy beam with the diagnostic information and to a lack of consistent sound speed dependence on temperature. Should accurate thermal dosimetry become a key factor then MRI may be more suitable.
The choice of appropriate HIFU transducer geometry and exposure frequency depends on the clinical application and, more specifically, the depth of vessel to be occluded. In choosing the frequency to be used, a trade-off is necessary between the amount of energy absorbed within the target volume (which increases with frequency) and the ability to get sufficient energy to the target depth (which decreases with increasing frequency owing to attenuation by the overlying tissues). It has been shown that a ‘rule of thumb’ for determining the optimum frequency is to allow the total attenuation in the overlying tissue to be around 10 dB [126]. For typical soft tissues with an attenuation of 0.7 dB cm−1 MHz−1, this would imply an optimum frequency of around 2.6 MHz for a target depth of 5 cm and 1.4 MHz for 10 cm. It should also be remembered that the focal region is smaller at higher frequencies, if all other source geometries remain the same. The inclusion of a central aperture in the HIFU transducer design allows an US imaging probe to be inserted into the treatment head, thus ensuring a fixed geometry between targeting and treatment components, most efficiently with coincident acoustic axes. This also facilitates the monitoring of a treatment immediately after cessation of the HIFU exposure.
15. Undesirable effects of high-intensity focused ultrasound applied to tissue and vessels
The main specific complication of vessel exposure to HIFU is rupture and haemorrhage. Exposure to higher intensities in the experimental range has been associated with rupture of vessels, potentially owing to acoustic cavitation [15,20,21] as has multiple exposures of the vessel wall which cause overheating and tissue stiffening by a cumulative effect [19,34,38]. This effectively represents an upper exposure limit above which the application of HIFU becomes counterproductive. Therefore, HIFU appears to differ from several other occlusive techniques, particularly laser and electrocautery, which rely on multiple exposures of blood vessels to ensure permanent and stable occlusion. It is possible that it would be unsafe to use HIFU in this manner, although varying the exposure site in larger vessels may mitigate this effect.
Paradoxically, the non-invasive nature of HIFU also represents a risk of complications. Invasive methods have greater control over where the energy (thermal and mechanical) they supply is delivered as they are visually targeted and controlled. Excessive soft tissue destruction or thermal spread to adjacent structures may cause unintended damage and therefore complications. This is of particular significance where the targets are small, or hard to locate, and the margin of error is limited. Injuries to bowel [15,44], nerves [20] or adjacent vessels [14] have been reported in fetal and vascular HIFU experiments, with or without associated vascular occlusion. Mistargeting is also an issue as not only does it fail to occlude the target vessels, but also repeat exposure is likely to be precluded until such time as tissue healing has been allowed to occur. However, accuracy of vascular targeting has been shown to be improved with the use of colour flow Doppler ultrasound over even surgical exposure and visual targeting in the application of HIFU [18].
If an inappropriate acoustic window is used, or there are elements in the acoustic window which scatter or reflect, rather than transmitting, the ultrasonic energy, heating to unpredictable locations may occur, causing burns, typically on the skin because this has a higher absorption coefficient compared with most other soft tissues. In some animal experiments, skin burns were also caused by poor acoustic coupling to the animal's skin owing to trapped air pockets in the animal's fur. The incidence of this is reduced with more extensive shaving of animal models and application of depilatory creams, and should not be a significant issue in humans.
16. Conclusion
We present an integrated multi-stage mechanism through which HIFU-mediated vascular occlusion could occur. If the integrated technique described here is to be implemented, it is important to optimize treatment delivery, and more detailed experimental research must be carried out to validate it and establish the exposure parameters that will provide the most efficient vascular occlusion for the clinical applications being considered. This will involve optimization of transducer design as well as exposure regimes. We propose that should this optimization occur, HIFU has the potential to be adapted to provide a non-invasive therapy for conditions in fetal medicine where vascular occlusion is required.
Funding statement
The authors acknowledge funding from Action Medical Research grant no. GN2052, the Isaac Newton Trust and the Genesis Research Trust. C.C.L. is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. G.R.tH. and I.H.R. are funded by the Engineering and Physical Sciences Research Council (EPSRC) grant no. EP/F0-25750.
References
- 1.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. 1992. MR-guided focused ultrasound surgery. J. Comput. Assist. Tomogr. 16, 956–965. ( 10.1097/00004728-199211000-00024) [DOI] [PubMed] [Google Scholar]
- 2.Clarke RL, ter Haar GR. 1997. Temperature rise recorded during lesion formation by high-intensity focused ultrasound. Ultrasound Med. Biol. 23, 299–306. ( 10.1016/S0301-5629(96)00198-6) [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Martin RW, Rouseff D, Vaezy S, Crum LA. 1999. Detection of high-intensity focused ultrasound liver lesions using dynamic elastometry. Ultrason. Imaging 21, 107–126. ( 10.1177/016173469902100203) [DOI] [PubMed] [Google Scholar]
- 4.Underwood JCEBR. 2004. General and systematic pathology, 4th edn New York, NY: Churchill Livingstone. [Google Scholar]
- 5.ter Haar GR, Robertson D. 1993. Tissue destruction with focused ultrasound in vivo. Eur. Urol. 23(Suppl. 1), 8–11. [DOI] [PubMed] [Google Scholar]
- 6.Susani M, Madersbacher S, Kratzik C, Vingers L, Marberger M. 1993. Morphology of tissue destruction induced by focused ultrasound. Eur. Urol. 23(Suppl. 1), 34–38. [DOI] [PubMed] [Google Scholar]
- 7.Burks SR, Ziadloo A, Hancock HA, Chaudhry A, Dean DD, Lewis BK, Frenkel V, Frank JA. 2011. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS ONE 6, e24730 ( 10.1371/journal.pone.0024730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry FJ, Kossoff G, Eggleton RC, Dunn F. 1970. Threshold ultrasonic dosages for structural changes in the mammalian brain. J. Acoust. Soc. Am. 48(Suppl. 2), 1413 ( 10.1121/1.1912301) [DOI] [PubMed] [Google Scholar]
- 9.Winterroth F, Xu Z, Wang TY, Wilkinson JE, Fowlkes JB, Roberts WW, Cain CA. 2011. Examining and analyzing subcellular morphology of renal tissue treated by histotripsy. Ultrasound Med. Biol. 37, 78–86. ( 10.1016/j.ultrasmedbio.2010.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynn JG, Zwemer RL, Chick AJ, Miller AE. 1942. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 26, 179–193. ( 10.1085/jgp.26.2.179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallon JT, Stehbens WE, Eggleton RC. 1972. Effect of ultrasound on arteries. Arch. Pathol. 94, 380–388. [PubMed] [Google Scholar]
- 12.Schultz-Haakh H, Li JK, Welkowitz W, Rosenberg N. 1989. Ultrasonic treatment of varicose veins. Angiology 40, 129–137. ( 10.1177/000331978904000208) [DOI] [PubMed] [Google Scholar]
- 13.Yang R, et al. 1992. Feasibility of using high intensity focused ultrasound for treatment of unresectable retroperitoneal malignancies. J. Ultrasound Med. 11, 1.1740826 [Google Scholar]
- 14.Delon-Martin C, Vogt C, Chignier E, Guers C, Chapelon JY, Cathignol D. 1995. Venous thrombosis generation by means of high-intensity focused ultrasound. Ultrasound Med. Biol. 21, 113–119. ( 10.1016/0301-5629(94)00095-6) [DOI] [PubMed] [Google Scholar]
- 15.Hynynen K, Colucci V, Chung A, Jolesz F. 1996. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med. Biol. 22, 1071–1077. ( 10.1016/S0301-5629(96)00143-3) [DOI] [PubMed] [Google Scholar]
- 16.Vaezy S, et al. 1997. Liver hemostasis using high-intensity focused ultrasound. Ultrasound Med. Biol. 23, 1413–1420. ( 10.1016/S0301-5629(97)00143-9) [DOI] [PubMed] [Google Scholar]
- 17.Vaezy S, et al. 1998. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med. Biol. 24, 903–910. ( 10.1016/S0301-5629(98)00050-7) [DOI] [PubMed] [Google Scholar]
- 18.Martin RW, Vaezy S, Kaczkowski P, Keilman G, Carter S, Caps M, Beach K, Plett M, Crum L. 1999. Hemostasis of punctured vessels using Doppler-guided high-intensity ultrasound. Ultrasound Med. Biol. 25, 985–990. ( 10.1016/S0301-5629(99)00027-7) [DOI] [PubMed] [Google Scholar]
- 19.Vaezy S, et al. 1999. Use of high-intensity focused ultrasound to control bleeding. J. Vasc. Surg. 29, 533–542. ( 10.1016/S0741-5214(99)70282-X) [DOI] [PubMed] [Google Scholar]
- 20.Rivens IH, Rowland IJ, Denbow M, Fisk NM, ter Haar GR, Leach MO. 1999. Vascular occlusion using focused ultrasound surgery for use in fetal medicine. Eur. J. Ultrasound 9, 89–97. ( 10.1016/S0929-8266(99)00008-7) [DOI] [PubMed] [Google Scholar]
- 21.Denbow ML, Rivens IH, Rowland IJ, Leach MO, Fisk NM, ter Haar GR. 2000. Preclinical development of noninvasive vascular occlusion with focused ultrasonic surgery for fetal therapy. Am. J. Obstet. Gynecol. 182, 387–392. ( 10.1016/S0002-9378(00)70229-8) [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara R, Sasaki K, Ishikawa T, Suzuki M, Umemura S-I, Kushima M, Okai T. 2002. Arterial blood flow occlusion by high intensity focused ultrasound and histologic evaluation of its effect on arteries and surrounding tissues. J. Med. Ultrasonics 29, 85–90. ( 10.1007/BF02481229) [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH, Vaezy S, Martin RW, Cho MY, Noble ML, Crum LA, Kimmey MB. 2003. High-intensity focused US: a potential new treatment for GI bleeding. Gastrointest. Endosc. 58, 111–115. ( 10.1067/mge.2003.322) [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Okai T, Sasaki K, Umemura S, Fujiwara R, Kushima M, Ichihara M, Ichizuka K. 2003. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med. Biol. 29, 1471–1477. ( 10.1016/S0301-5629(03)00951-7) [DOI] [PubMed] [Google Scholar]
- 25.Nizard J, Pessel M, De Keersmaecker B, Barbet JP, Ville Y. 2004. High-intensity focused ultrasound in the treatment of postpartum hemorrhage: an animal model. Ultrasound Obstet. Gynecol. 23, 262–266. ( 10.1002/uog.1007) [DOI] [PubMed] [Google Scholar]
- 26.Zderic V, Keshavarzi A, Noble ML, Paun M, Sharar SR, Crum LA, Martin RW, Vaezy S. 2006. Hemorrhage control in arteries using high-intensity focused ultrasound: a survival study. Ultrasonics 44, 46–53. ( 10.1016/j.ultras.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 27.Ichizuka K, et al. 2007. Application of high-intensity focused ultrasound for umbilical artery occlusion in a rabbit model. Ultrasound Obstet. Gynecol. 30, 47–51. ( 10.1002/uog.4008) [DOI] [PubMed] [Google Scholar]
- 28.Ichihara M, Sasaki K, Umemura S, Kushima M, Okai T. 2007. Blood flow occlusion via ultrasound image-guided high-intensity focused ultrasound and its effect on tissue perfusion. Ultrasound Med. Biol. 33, 452–459. ( 10.1016/j.ultrasmedbio.2006.08.016) [DOI] [PubMed] [Google Scholar]
- 29.Ichizuka K, Hasegawa J, Nakamura M, Matsuoka R, Sekizawa A, Okai T, Umemura S. 2012. High-intensity focused ultrasound treatment for twin reversed arterial perfusion sequence. Ultrasound Obstet. Gynecol. 40, 476–478. ( 10.1002/uog.11114) [DOI] [PubMed] [Google Scholar]
- 30.Okai T, Ichizuka K, Hasegawa J, Matsuoka R, Nakamura M, Shimodaira K, Sekizawa A, Kushima M, Umemura S. 2013. First successful case of non-invasive in-utero treatment of twin reversed arterial perfusion sequence by high-intensity focused ultrasound. Ultrasound Obstet. Gynecol. 42, 112–114. ( 10.1002/uog.12466) [DOI] [PubMed] [Google Scholar]
- 31.Discigil B, King RM, Pearson PJ, Capellini VK, Rodrigues AJ, Schaff HV, Evora PRB. 2008. High-frequency ultrasonic waves cause endothelial dysfunction on canine epicardial coronary arteries. Rev. Bras. Cir. Cardiovasc. 23, 190–196. ( 10.1590/S0102-76382008000200007) [DOI] [PubMed] [Google Scholar]
- 32.Henderson PW, Lewis GK, Shaikh N, Sohn A, Weinstein AL, Olbricht WL, Spector JA. 2010. A portable high-intensity focused ultrasound device for noninvasive venous ablation. J. Vasc. Surg. 51, 707–711. ( 10.1016/j.jvs.2009.10.049) [DOI] [PubMed] [Google Scholar]
- 33.Jiao J, Wu F, Zou J, Li F, Liu F, Zhao X, Wang Q. 2013. Effect of ablations by pulsed versus continuous high-intensity focused ultrasound on isolated perfused porcine liver. Nan Fang Yi Ke Da Xue Xue Bao. 33, 230–234. [PubMed] [Google Scholar]
- 34.Mahoney K, Martin H, Hynynen K. 2000. Focused ultrasound effects on blood vessels in vivo-limits for vascular interventions. In Ultrasonics Symposium, 2000 IEEE, 22 Oct 2000–25 Oct 2000, San Juan, Puerto Rico, vol. 2, pp. 1405–1408. ( 10.1109/ULTSYM.2000.921585) [DOI] [Google Scholar]
- 35.Hwang JH, Zhou Y, Warren C, Brayman AA, Crum LA. 2010. Targeted venous occlusion using pulsed high-intensity focused ultrasound. IEEE Trans. Biomed. Eng. 57, 37–40. ( 10.1109/TBME.2009.2029865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue Y, Chen W, Wang Z. 2010. The impact of microbubbles-mediated intermitten HIFU on blood flow in femoral artery of rabbit. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 27, 58–61. [PubMed] [Google Scholar]
- 37.Yang FY, Chiu WH, Liu SH, Lin GL, Ho FM. 2009. Functional changes in arteries induced by pulsed high-intensity focused ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 56, 2643–2649. ( 10.1109/TUFFC.2009.1355) [DOI] [PubMed] [Google Scholar]
- 38.Hynynen K, Chung AH, Colucci V, Jolesz FA. 1996. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med. Biol. 22, 193–201. ( 10.1016/0301-5629(95)02044-6) [DOI] [PubMed] [Google Scholar]
- 39.Koruth JS, et al. 2011. Safety and efficacy of high-intensity focused ultrasound atop coronary arteries during epicardial catheter ablation. J. Cardiovasc. Electrophysiol. 22, 1274–1280. ( 10.1111/j.1540-8167.2011.02084.x) [DOI] [PubMed] [Google Scholar]
- 40.Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. 2006. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound Med. Biol. 32, 1611–1619. ( 10.1016/j.ultrasmedbio.2006.07.016) [DOI] [PubMed] [Google Scholar]
- 41.Yang R, et al. 1991. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg. 126, 1002–1009 (discussion 9–10) ( 10.1001/archsurg.1991.01410320088012) [DOI] [PubMed] [Google Scholar]
- 42.Wu F, Chen W-Z, Bai J, Zou J-Z, Wang Z-L, Zhu H, Wang ZB. 2001. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrsound. Med. Biol. 27, 1099–1106. ( 10.1016/S0301-5629(01)00389-1) [DOI] [PubMed] [Google Scholar]
- 43.Paek BW, et al. 2003. Tissue ablation using high-intensity focused ultrasound in the fetal sheep model: potential for fetal treatment. Am. J. Obstet. Gynecol. 189, 702–705. ( 10.1067/S0002-9378(03)00664-1) [DOI] [PubMed] [Google Scholar]
- 44.Kim Y, et al. 2011. Non-invasive pulsed cavitational ultrasound for fetal tissue ablation: feasibility study in a fetal sheep model. Ultrasound Obstet. Gynecol. 37, 450–457. ( 10.1002/uog.8880) [DOI] [PubMed] [Google Scholar]
- 45.Owens GE, Miller RM, Owens ST, Swanson SD, Ives K, Ensing G, Gordon D, Xu Z. 2012. Intermediate-term effects of intracardiac communications created noninvasively by therapeutic ultrasound (histotripsy) in a porcine model. Pediatr. Cardiol. 33, 83–89. ( 10.1007/s00246-011-0094-6) [DOI] [PubMed] [Google Scholar]
- 46.Hancock HA, Smith LH, Cuesta J, Durrani AK, Angstadt M, Palmeri ML, Kimmel E, Frenkel V. 2009. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med. Biol. 35, 1722–1736. ( 10.1016/j.ultrasmedbio.2009.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lele PP. 1967. Production of deep focal lesions by focused ultrasound—current status. Ultrasonics 5, 105–112. ( 10.1016/S0041-624X(67)80011-8) [DOI] [PubMed] [Google Scholar]
- 48.Burkitt HGYBHJWWPR. 1993. Wheater's functional histology: a text and colour atlas. Edinburgh, UK: Churchill Livingstone. [Google Scholar]
- 49.Agah R, Pearce JA, Welch AJ, Motamedi M. 1994. Rate process model for arterial tissue thermal damage: implications on vessel photocoagulation. Lasers Surg. Med. 15, 176–184. ( 10.1002/lsm.1900150205) [DOI] [PubMed] [Google Scholar]
- 50.Florkin MSEH. 1971. Comprehensive biochemistry. 26C: extracellular and supporting structures. Amsterdam: Elsevier. [Google Scholar]
- 51.Chen SS, Wright NT, Humphrey JD. 1997. Heat-induced changes in the mechanics of a collagenous tissue: isothermal free shrinkage. J. Biomech. Engg. 119, 372–378. ( 10.1115/1.2798281) [DOI] [PubMed] [Google Scholar]
- 52.Tokarczyk A, Rivens I, van Bavel E, Symonds-Tayler R, ter Haar G. 2013. An experimental system for the study of ultrasound exposure of isolated blood. Phys. Med. Biol. 58, 2281–2304. ( 10.1088/0031-9155/58/7/2281) [DOI] [PubMed] [Google Scholar]
- 53.Moros EG. 2013. Physics of thermal therapy: fundamentals and clinical applications. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- 54.DeLia JE, Rogers JG, Dixon JA. 1985. Treatment of placental vasculature with a neodymium–yttrium–aluminum–garnet laser via fetoscopy. Am. J. Obstet. Gynecol. 151, 1126–1127. ( 10.1016/0002-9378(85)90395-3) [DOI] [PubMed] [Google Scholar]
- 55.White RA, Kopchok G, Peng SK, Fujitani R, White G, Klein S, Uitto J. 1987. Laser vascular welding—how does it work? Ann. Vasc. Surg. 1, 461–464. [DOI] [PubMed] [Google Scholar]
- 56.Banga I. 1966. Structure and function of elastin and collagen. Budapest: Akadémiai Kiadó. [Google Scholar]
- 57.Martinot VL, Mordon SR, Mitchell VA, Pellerin PN, Brunetaud JM. 1994. Determination of efficient parameters for argon laser-assisted anastomoses in rats: macroscopic, thermal, and histological evaluation. Lasers Surg. Med. 15, 168–175. ( 10.1002/lsm.1900150204) [DOI] [PubMed] [Google Scholar]
- 58.Landman J, Kerbl K, Rehman J, Andreoni C, Humphrey PA, Collyer W, Olweny E, Sundaram C, Clayman RV. 2003. Evaluation of a vessel sealing system, bipolar electrosurgery, harmonic scalpel, titanium clips, endoscopic gastrointestinal anastomosis vascular staples and sutures for arterial and venous ligation in a porcine model. J. Urol. 169, 697–700. ( 10.1016/S0022-5347(05)63995-X) [DOI] [PubMed] [Google Scholar]
- 59.Manouras A, Markogiannakis HE, Kekis PB, Lagoudianakis EE, Fleming B. 2008. Novel hemostatic devices in thyroid surgery: electrothermal bipolar vessel sealing system and harmonic scalpel. Expert Rev. Med. Devices 5, 447–466. ( 10.1586/17434440.5.4.447) [DOI] [PubMed] [Google Scholar]
- 60.Harold KL, Pollinger H, Matthews BD, Kercher KW, Sing RF, Heniford BT. 2003. Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the hemostasis of small-, medium-, and large-sized arteries. Surg. Endosc. 17, 1228–1230. ( 10.1007/s00464-002-8833-7) [DOI] [PubMed] [Google Scholar]
- 61.Jiang F, He M, Liu YJ, Wang ZB, Zhang L, Bai J. 2013. High intensity focused ultrasound ablation of goat liver in vivo: pathologic changes of portal vein and the ‘heat-sink’ effect. Ultrasonics 53, 77–83. ( 10.1016/j.ultras.2012.04.001) [DOI] [PubMed] [Google Scholar]
- 62.Poliachik SL, Chandler WL, Mourad PD, Ollos RJ, Crum LA. 2001. Activation, aggregation and adhesion of platelets exposed to high-intensity focused ultrasound. Ultrasound Med. Biol. 27, 1567–1576. ( 10.1016/S0301-5629(01)00444-6) [DOI] [PubMed] [Google Scholar]
- 63.Poliachik SL, Chandler WL, Ollos RJ, Bailey MR, Crum LA. 2004. The relation between cavitation and platelet aggregation during exposure to high-intensity focused ultrasound. Ultrasound Med. Biol. 30, 261–269. ( 10.1016/j.ultrasmedbio.2003.10.012) [DOI] [PubMed] [Google Scholar]
- 64.Sakariassen KS, Holme PA, Orvim U, Barstad RM, Solum NO, Brosstad FR. 1998. Shear-induced platelet activation and platelet microparticle formation in native human blood. Thromb. Res. 92, S33–S41. ( 10.1016/S0049-3848(98)00158-3) [DOI] [PubMed] [Google Scholar]
- 65.Williams AR, O'Brien WD, Jr, Coller BS. 1976. Exposure to ultrasound decreases the recalcification time of platelet rich plasma. Ultrasound Med. Biol. 2, 113–118. ( 10.1016/0301-5629(76)90019-3) [DOI] [PubMed] [Google Scholar]
- 66.Rao GH, Smith CM, 2nd, Escolar G, White JG. 1993. Influence of heat on platelet biochemistry, structure, and function. J. Lab. Clin. Med. 122, 455–464. [PubMed] [Google Scholar]
- 67.Gershfeld NL, Murayama M. 1988. Thermal instability of red blood cell membrane bilayers: temperature dependence of hemolysis. J. Membr. Biol. 101, 67–72. ( 10.1007/BF01872821) [DOI] [PubMed] [Google Scholar]
- 68.Dyson M, Pond JB, Woodward B, Broadbent J. 1974. The production of blood cell stasis and endothelial damage in the blood vessels of chick embryos treated with ultrasound in a stationary wave field. Ultrasound Med. Biol. 1, 133–148. ( 10.1016/0301-5629(74)90003-9) [DOI] [PubMed] [Google Scholar]
- 69.ter Haar G, Dyson M, Smith SP. 1979. Ultrastructural changes in the mouse uterus brought about by ultrasonic irradiation at therapeutic intensities in standing wave fields. Ultrasound Med. Biol. 5, 167–179. ( 10.1016/0301-5629(79)90085-1) [DOI] [PubMed] [Google Scholar]
- 70.Quick AJ. 1969. Hemostasis in surgical procedures. Surg. Gynecol. Obstet. 128, 523–532. [PubMed] [Google Scholar]
- 71.Nyborg WLM, Warren PM. 1965. 11—Acoustic streaming. Properties of polymers and nonlinear acoustics, vol. 2, pp. 265–331. New York, NY: Academic Press. [Google Scholar]
- 72.American College of Surgeons CoT. 2008. ATLS, advanced trauma life support for doctors. Chicago, IL: American College of Surgeons. [Google Scholar]
- 73.Laughner JI, Sulkin MS, Wu Z, Deng CX, Efimov IR. 2012. Three potential mechanisms for failure of high intensity focused ultrasound ablation in cardiac tissue. Circ. Arrhythmia Electrophysiol. 5, 409–416. ( 10.1161/CIRCEP.111.967216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen WS, et al. 2011. Single-element ultrasound transducer for combined vessel localization and ablation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 766–775. ( 10.1109/TUFFC.2011.1869) [DOI] [PubMed] [Google Scholar]
- 75.Vaezy S, Shi X, Martin RW, Chi E, Nelson PI, Bailey MR, Crum LA. 2001. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med. Biol. 27, 33–42. ( 10.1016/S0301-5629(00)00279-9) [DOI] [PubMed] [Google Scholar]
- 76.Bebbington MW, Danzer E, Moldenhauer J, Khalek N, Johnson MP. 2012. Radiofrequency ablation vs bipolar umbilical cord coagulation in the management of complicated monochorionic pregnancies. Ultrasound Obstet. Gynecol. 40, 319–324. ( 10.1002/uog.11122) [DOI] [PubMed] [Google Scholar]
- 77.Moore TR, Gale S, Benirschke K. 1990. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am. J. Obstet. Gynecol. 163, 907–912. ( 10.1016/0002-9378(90)91094-S) [DOI] [PubMed] [Google Scholar]
- 78.Lewi L, Valencia C, Gonzalez E, Deprest J, Nicolaides KH. 2010. The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am. J. Obstet. Gynecol. 203, 213.e1–213.e4. ( 10.1016/j.ajog.2010.04.018) [DOI] [PubMed] [Google Scholar]
- 79.Hecher K, Lewi L, Gratacos E, Huber A, Ville Y, Deprest J. 2006. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet. Gynecol. 28, 688–691. ( 10.1002/uog.3816) [DOI] [PubMed] [Google Scholar]
- 80.Lee H, Wagner AJ, Sy E, Ball R, Feldstein VA, Goldstein RB, Farmer DL. 2007. Efficacy of radiofrequency ablation for twin-reversed arterial perfusion sequence. Am. J. Obstet. Gynecol. 196, 459.e1–459.e4. [DOI] [PubMed] [Google Scholar]
- 81.Lewi L, et al. 2008. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am. J. Obstet. Gynecol. 199, 514.e1–514.e8. [DOI] [PubMed] [Google Scholar]
- 82.Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. 1997. The hidden mortality of monochorionic twin pregnancies. Br. J. Obstet. Gynaecol. 104, 1203–1207. ( 10.1111/j.1471-0528.1997.tb10948.x) [DOI] [PubMed] [Google Scholar]
- 83.Mahieu-Caputo D, et al. 2005. Paradoxic activation of the renin–angiotensin system in twin–twin transfusion syndrome: an explanation for cardiovascular disturbances in the recipient. Pediatr. Res. 58, 685–688. ( 10.1203/01.PDR.0000180558.03164.E8) [DOI] [PubMed] [Google Scholar]
- 84.Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. 2000. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am. J. Obstet. Gynecol. 182, 417–426. ( 10.1016/S0002-9378(00)70233-X) [DOI] [PubMed] [Google Scholar]
- 85.Lewi L, et al. 2007. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am. J. Obstet. Gynecol. 197, 587.e1–587.e8. ( 10.1016/j.ajog.2007.05.009) [DOI] [PubMed] [Google Scholar]
- 86.Saunders NJ, Snijders RJ, Nicolaides KH. 1991. Twin–twin transfusion syndrome during the 2nd trimester is associated with small intertwin hemoglobin differences. Fetal Diagn. Ther. 6, 34–36. ( 10.1159/000263622) [DOI] [PubMed] [Google Scholar]
- 87.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. 1999. Staging of twin–twin transfusion syndrome. J Perinatol. 19, 550–555. ( 10.1038/sj.jp.7200292) [DOI] [PubMed] [Google Scholar]
- 88.Haverkamp F, Lex C, Hanisch C, Fahnenstich H, Zerres K. 2001. Neurodevelopmental risks in twin-to-twin transfusion syndrome: preliminary findings. Eur. J. Paediatr. Neurol. 5, 21–27. ( 10.1053/ejpn.2001.0400) [DOI] [PubMed] [Google Scholar]
- 89.Senat M-V, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. 2004. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl. J. Med. 351, 136–144. ( 10.1056/NEJMoa032597) [DOI] [PubMed] [Google Scholar]
- 90.Baschat AA, Barber J, Pedersen N, Turan OM, Harman CR. 2013. Outcome after fetoscopic selective laser ablation of placental anastomoses vs equatorial laser dichorionization for the treatment of twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 209, 234.e1–234.e8. ( 10.1016/j.ajog.2013.05.034) [DOI] [PubMed] [Google Scholar]
- 91.Rossi AC, D'Addario V. 2009. The efficacy of Quintero staging system to assess severity of twin–twin transfusion syndrome treated with laser therapy: a systematic review with meta-analysis. Am. J. Perinatol. 26, 537–544. ( 10.1055/s-0029-1215430) [DOI] [PubMed] [Google Scholar]
- 92.Rossi AC, Vanderbilt D, Chmait RH. 2011. Neurodevelopmental outcomes after laser therapy for twin–twin transfusion syndrome: a systematic review and meta-analysis. Obstet. Gynecol. 118, 1145–1150. ( 10.1097/AOG.0b013e318231827f) [DOI] [PubMed] [Google Scholar]
- 93.Walsh CA, McAuliffe FM. 2012. Recurrent twin–twin transfusion syndrome after selective fetoscopic laser photocoagulation: a systematic review of the literature. Ultrasound Obstet. Gynecol. 40, 506–512. ( 10.1002/uog.11105) [DOI] [PubMed] [Google Scholar]
- 94.Chmait RH, Assaf SA, Benirschke K. 2010. Residual vascular communications in twin–twin transfusion syndrome treated with sequential laser surgery: frequency and clinical implications. Placenta 31, 611–614. ( 10.1016/j.placenta.2010.04.006) [DOI] [PubMed] [Google Scholar]
- 95.Lopriore E, Slaghekke F, Middeldorp JM, Klumper FJ, Oepkes D, Vandenbussche FP. 2009. Residual anastomoses in twin-to-twin transfusion syndrome treated with selective fetoscopic laser surgery: localization, size, and consequences. Am. J. Obstet. Gynecol. 201, 66.e1–66.e4. [DOI] [PubMed] [Google Scholar]
- 96.Lewi L, et al. 2006. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am. J. Obstet. Gynecol. 194, 790–795. ( 10.1016/j.ajog.2005.08.062) [DOI] [PubMed] [Google Scholar]
- 97.Robyr R, Lewi L, Salomon LJ, Yamamoto M, Bernard JP, Deprest J, Ville Y. 2006. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 194, 796–803. ( 10.1016/j.ajog.2005.08.069) [DOI] [PubMed] [Google Scholar]
- 98.DeLia JE, Cukierski MA, Lundergan DK, Kochenour NK. 1989. Neodymium: yttrium–aluminum–garnet laser occlusion of rhesus placental vasculature via fetoscopy. Am. J. Obstet. Gynecol. 160, 485–489. ( 10.1016/0002-9378(89)90477-8) [DOI] [PubMed] [Google Scholar]
- 99.Taylor MJ, Farquharson D, Cox PM, Fisk NM. 2000. Identification of arterio-venous anastomoses in vivo in monochorionic twin pregnancies: preliminary report. Ultrasound Obstet. Gynecol. 16, 218–222. ( 10.1046/j.1469-0705.2000.00227.x) [DOI] [PubMed] [Google Scholar]
- 100.Khalek N, Johnson MP. 2013. Management of prenatally diagnosed lung lesions. Semin. Pediatr. Surg. 22, 24–29. ( 10.1053/j.sempedsurg.2012.10.005) [DOI] [PubMed] [Google Scholar]
- 101.Cavoretto P, Molina F, Poggi S, Davenport M, Nicolaides KH. 2008. Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet. Gynecol. 32, 769–783. ( 10.1002/uog.6218) [DOI] [PubMed] [Google Scholar]
- 102.Adzick NS. 2010. Open fetal surgery for life-threatening fetal anomalies. Semin. Fetal Neonatal Med. 15, 1–8. ( 10.1016/j.siny.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 103.Ruano R, da Silva MM, Salustiano EM, Kilby MD, Tannuri U, Zugaib M. 2012. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat. Diagn. 32, 1127–1132. ( 10.1002/pd.3969) [DOI] [PubMed] [Google Scholar]
- 104.Nicolini U, Cerri V, Groli C, Poblete A, Mauro F. 2000. A new approach to prenatal treatment of extralobar pulmonary sequestration. Prenat. Diagn. 20, 758–760. () [DOI] [PubMed] [Google Scholar]
- 105.Bermudez C, Perez-Wulff J, Bufalino G, Sosa C, Gomez L, Quintero RA. 2007. Percutaneous ultrasound-guided sclerotherapy for complicated fetal intralobar bronchopulmonary sequestration. Ultrasound Obstet. Gynecol. 29, 586–589. ( 10.1002/uog.3944) [DOI] [PubMed] [Google Scholar]
- 106.Witlox RS, Lopriore E, Oepkes D. 2011. Prenatal interventions for fetal lung lesions. Prenat. Diagn. 31, 628–636. ( 10.1002/pd.2778) [DOI] [PubMed] [Google Scholar]
- 107.Merchant AM, Peranteau W, Wilson RD, Johnson MP, Bebbington MW, Hedrick HL, Flake AW, Adzick NS. 2007. Postnatal chest wall deformities after fetal thoracoamniotic shunting for congenital cystic adenomatoid malformation. Fetal Diagn. Ther. 22, 435–439. ( 10.1159/000106350) [DOI] [PubMed] [Google Scholar]
- 108.Swamy R, Embleton N, Hale J. 2008. Sacrococcygeal teratoma over two decades: birth prevalence, prenatal diagnosis and clinical outcomes. Prenat. Diagn. 28, 1048–1051. ( 10.1002/pd.2122) [DOI] [PubMed] [Google Scholar]
- 109.Donnellan WL, Swenson O. 1969. Benign and malignant sacrococcygeal teratomas. CA Cancer J. Clin. 19, 344–351. ( 10.3322/canjclin.19.6.344) [DOI] [PubMed] [Google Scholar]
- 110.Okada T, Sasaki F, Cho K, Honda S, Naito S, Hirokata G, Todo S. 2008. Management and outcome in prenatally diagnosed sacrococcygeal teratomas. Pediatr. Int. 50, 576–580. ( 10.1111/j.1442-200X.2008.02703.x) [DOI] [PubMed] [Google Scholar]
- 111.Bond SJ, et al. 1990. Death due to high-output cardiac failure in fetal sacrococcygeal teratoma. J. Pediatr. Surg. 25, 1287–1291. ( 10.1016/0022-3468(90)90535-H) [DOI] [PubMed] [Google Scholar]
- 112.Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, Wilson RD, Adzick NS. 2004. Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J. Pediatr. Surg. 39, 430–438 (discussion-8) ( 10.1016/j.jpedsurg.2003.11.005) [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi Y, Tsukimori K, Hojo S, Nakanami N, Nozaki M, Masumoto K, Taguchi T, Wake N. 2006. Spontaneous rupture of sacrococcygeal teratoma associated with acute fetal anemia. Ultrasound Obstet. Gynecol. 28, 720–722. ( 10.1002/uog.3821) [DOI] [PubMed] [Google Scholar]
- 114.Adzick NS, Crombleholme TM, Morgan MA, Quinn TM. 1997. A rapidly growing fetal teratoma. Lancet 349, 538 ( 10.1016/S0140-6736(97)80088-8) [DOI] [PubMed] [Google Scholar]
- 115.Rychik J, et al. 2004. Acute cardiovascular effects of fetal surgery in the human. Circulation 110, 1549–1556. ( 10.1161/01.CIR.0000142294.95388.C4) [DOI] [PubMed] [Google Scholar]
- 116.Makin EC, Hyett J, Ade-Ajayi N, Patel S, Nicolaides K, Davenport M. 2006. Outcome of antenatally diagnosed sacrococcygeal teratomas: single-center experience (1993–2004). J. Pediatr. Surg. 41, 388–393. ( 10.1016/j.jpedsurg.2005.11.017) [DOI] [PubMed] [Google Scholar]
- 117.Ruano R, Duarte S, Zugaib M. 2009. Percutaneous laser ablation of sacrococcygeal teratoma in a hydropic fetus with severe heart failure—too late for a surgical procedure? Fetal Diagn. Ther. 25, 26–30. ( 10.1159/000188663) [DOI] [PubMed] [Google Scholar]
- 118.Ding J, Chen Q, Stone P. 2010. Percutaneous laser photocoagulation of tumour vessels for the treatment of a rapidly growing sacrococcygeal teratoma in an extremely premature fetus. J. Matern. Fetal Neonatal Med. 23, 1516–1518. ( 10.3109/14767051003678085) [DOI] [PubMed] [Google Scholar]
- 119.Paek BW, Jennings RW, Harrison MR, Filly RA, Tacy TA, Farmer DL, Albanese CT. 2001. Radiofrequency ablation of human fetal sacrococcygeal teratoma. Am. J. Obstet. Gynecol. 184, 503–507. ( 10.1067/mob.2001.110446) [DOI] [PubMed] [Google Scholar]
- 120.Ibrahim D, Ho E, Scherl SA, Sullivan CM. 2003. Newborn with an open posterior hip dislocation and sciatic nerve injury after intrauterine radiofrequency ablation of a sacrococcygeal teratoma. J. Pediatr. Surg. 38, 248–250. ( 10.1053/jpsu.2003.50055) [DOI] [PubMed] [Google Scholar]
- 121.Voogt MJ, van Stralen M, Ikink ME, Deckers R, Vincken KL, Bartels LW, Mali WPTM, Bosch MAAJ. 2012. Targeted vessel ablation for more efficient magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Cardiovasc. Intervent Radiol. 35, 1205–1210. ( 10.1007/s00270-011-0313-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu F, Chen W-Z, Bai J, Zou J-Z, Wang Z-L, Zhu H, Wang ZB. 2002. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med. Biol. 28, 535–542. ( 10.1016/S0301-5629(01)00515-4) [DOI] [PubMed] [Google Scholar]
- 123.Aoki H, Ichizuka K, Ichihara M, Matsuoka R, Hasegawa J, Okai T, Umemura S. 2013. Application of high-intensity focused ultrasound for fetal therapy: experimental study using an animal model of lower urinary tract obstruction. J. Med. Ultrasonics 40, 107–10. ( 10.1007/s10396-012-0398-z) [DOI] [PubMed] [Google Scholar]
- 124.Sapareto SA, Dewey WC. 1984. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 10, 787–800. ( 10.1016/0360-3016(84)90379-1) [DOI] [PubMed] [Google Scholar]
- 125.Evans N, Kluckow M, Simmons M, Osborn D. 2002. Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 87, F181–F184. ( 10.1136/fn.87.3.F181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill CR, Rivens I, Vaughan MG, ter Haar GR. 1994. Lesion development in focused ultrasound surgery: a general model. Ultrasound Med. Biol. 20, 259–269. ( 10.1016/0301-5629(94)90066-3) [DOI] [PubMed] [Google Scholar]