Abstract
In vivo osteochondral defect models predominantly consist of small animals, such as rabbits. Although they have an advantage of low cost and manageability, their joints are smaller and more easily healed compared with larger animals or humans. We hypothesized that osteochondral cores from large animals can be implanted subcutaneously in rats to create an ectopic osteochondral defect model for routine and high-throughput screening of multiphasic scaffold designs and/or tissue-engineered constructs (TECs). Bovine osteochondral plugs with 4 mm diameter osteochondral defect were fitted with novel multiphasic osteochondral grafts composed of chondrocyte-seeded alginate gels and osteoblast-seeded polycaprolactone scaffolds, prior to being implanted in rats subcutaneously with bone morphogenic protein-7. After 12 weeks of in vivo implantation, histological and micro-computed tomography analyses demonstrated that TECs are susceptible to mineralization. Additionally, there was limited bone formation in the scaffold. These results suggest that the current model requires optimization to facilitate robust bone regeneration and vascular infiltration into the defect site. Taken together, this study provides a proof-of-concept for a high-throughput osteochondral defect model. With further optimization, the presented hybrid in vivo model may address the growing need for a cost-effective way to screen osteochondral repair strategies before moving to large animal preclinical trials.
Keywords: multiphasic scaffold, biomaterials, osteochondral repair, tissue engineering, in vivo model
1. Introduction
Articular cartilage is a unique tissue, which lacks vasculature, and has limited self-healing ability. When left untreated, cartilage damage caused by trauma or other degenerative conditions will not only cause pathological changes to the surrounding cartilage, but also to the subchondral bone [1]. Osteochondral lesions, which allow bone marrow infiltration into the damaged site, have better regenerative capabilities but they often result in fibrocartilage with inferior mechanical properties and diminished long-term durability. Current treatment strategies for osteochondral lesions, such as joint debridement and microfracture, have the disadvantage of fibrocartilage formation [2]. Osteochondral autografts or mosaicplasty are also commonly used to treat osteochondral defects and often have better outcomes, but these procedures have problems of donor tissue availability, topology mismatch and donor site morbidity [3]. Autologous chondrocyte implantation (ACI) and matrix-associated ACI (M-ACI) are some of the cell-based treatment methods that have shown positive outcomes [4,5] but they also fall short of fully recapitulating the characteristics of the native tissue [6].
In order to further improve current cell-based treatment methods, such as ACI and M-ACI, the effects of combining other factors such as scaffolds (cellular solids), matrices (hydrogels), growth factors and mechanical stimuli are actively being investigated [7]. When combined with growth factors such as TGF-β1, hydrogels such as alginate [8] and agarose [9] have been shown to help chondrocytes maintain their spherical morphology while supporting chondrogenic differentiation and cartilage-specific matrix deposition. Cartilage constructs can also be mechanically stimulated in vitro to enhance chondrocyte matrix synthesis and remodelling [10,11], and to recapitulate zonal characteristics in the construct [12,13]. For bone tissue engineering, polymeric and ceramic biomaterials are commonly used to design and fabricate scaffolds [14] with the addition of osteogenic growth factors, such as bone morphogenic protein (rh-BMP)-2 or rh-BMP-7 and bone-forming cells (e.g. osteoblasts) [15]. One of the most recent developments in osteochondral tissue engineering is the emergence of not only biphasic but multiphasic scaffolds [16]. By integrating cartilage- and bone-specific matrices and scaffolds, which are tailor-made to enhance chondrogenic or osteogenic development of the construct, multiphasic tissue-engineering approaches aim to enhance the integration and regenerative potential of the engineered grafts.
With an increasing number of concepts emerging in the cartilage and osteochondral tissue-engineering disciplines, there is a growing need for high-throughput and cost-effective in vivo animal models. Rabbits are commonly used for osteochondral defect studies, but their joint scales are much smaller than humans [17] (cartilage thickness: approx. 0.3 mm [18]), and osteochondral lesions in smaller animals, such as rabbits, are known to have the tendency to heal more readily compared with larger animals [19]. As a result, treatment strategies developed using small animals may not be as effective in larger animals, and there are obvious limitations in interpreting and extrapolating treatment outcomes from small-animal studies [20]. To this end, it is important to scale up and use larger animal models with comparable cartilage thickness and joint scale as humans for osteochondral tissue-engineering studies [21]. However, large animal models (e.g. porcine, dog or equine) are relatively expensive and facilities that can accommodate them may not be readily accessible, which make them less feasible in routine screening of newly developed tissue-engineering strategies. To bridge the gap of cost effectiveness by maintaining larger sample size, accessibility and anatomical tissue scales, we hypothesized that osteochondral cores from large animals, with osteochondral defects, implanted subcutaneously in rats would allow for screening of osteochondral repair potential of multiphasic constructs.
2. Material and methods
2.1. Chondrocyte and osteoblast isolation and culture
Chondrocytes were isolated from macroscopically normal parts of the cartilage from patients undergoing joint replacement surgeries with ethical approval from Queensland University of Technology and Prince Charles Hospital (n = 3). Superficial (S) and middle-deep (MD) zone chondrocytes were isolated, as described previously [22]. This technique has been optimized in our laboratory and involves manual dissection of the two layers using scalpel blades. Diced S and MD cartilage pieces were digested overnight in 0.15% collagenase type 2 (Worthington, Lakewood, NJ, USA) in serum-free basal medium (LG-DMEM, 2 mM GlutaMax, 110 mg l−1 sodium pyruvate, 50 U ml−1 penicillin, 50 µg ml−1 streptomycin). All reagents were obtained from Invitrogen (CA, USA) unless noted otherwise. Isolated chondrocytes were filtered through cell-strainers (100 µm), washed using phosphate-buffered saline (PBS) and seeded in T175 flasks at a density of 3000 cells cm−2 for expansion up to 1 passage (P1). Primary osteoblasts were isolated from the bone chips collected from the macroscopically normal bone off-cuts obtained from the joint replacement surgery patients. Bone chips were washed 10 times by vigorous shaking in PBS and incubated in 0.25% trypsin-EDTA for 30 min. After another wash in PBS, bone chips were cultured in T175 flasks. Chondrocytes and osteoblasts from each patient were cultured separately using the basal medium supplemented with 10% fetal bovine serum (FBS, Hyclone, UT, USA), and media (15–20 ml) were changed twice a week. Once confluent, cells were passaged using 0.1% trypsin-EDTA.
2.2. Chondrogenic differentiation of alginate discs seeded with surface or middle-deep chondrocytes and mechanical stimulation (cartilage compartment)
After expansion, S and MD chondrocytes (P1) were resuspended in 2% w/v alginate (Pronova UP LVG, FMC biopolymers, PA, USA) solution at 107 cells ml−1 and cross-linked using a custom-made mould immersed in 102 mM CaCl2 bath [22]. Each S and MD construct was 4 mm and 1.5 mm in diameter and thickness, respectively. Alginate discs were placed in 24-well plates and cultured in serum-free chondrogenic media (HG-DMEM (Invitrogen), 1.25 mg ml−1 bovine serum albumin, 1% ITS + 1, 10−7 M dexamethasone, 0.1 mM l-ascorbic acid (all Sigma, USA) and 10 ng ml−1 TGF-β3 (GroPrep Bioreagents, Adelaide, Australia)) for 12 weeks with media changed twice per week (0.6 ml/construct). Following the 12-week culture, S and MD alginate constructs were divided into either non-compressed (NC) or compressed (Comp) groups. Constructs selected for loading were subjected to a one-week intermittent dynamic compression in a commercial unconfined compression bioreactor (Cartigen C10–12c, Tissue Growth Technologies, Minnetonka, MN, USA) while those in the NC group were cultured under free-swelling conditions. The loading protocol was chosen based on a previous optimization study for chondrocytes in alginate gels [12] and consisted of 1 Hz sinusoidal compression for 3 h d−1 at 50% compressive strain.
2.3. Biphasic construct fabrication, assembly and osteoblast seeding (bone compartment)
For the first layer, medical-grade polycaprolactone (PCL) was used to fabricate a composite scaffold via fused deposition modelling (FDM, Osteopore Inc., Singapore). The scaffolds measured 100 × 100 × 6 mm3 and had 100% interconnectivity, 70% porosity and a 0°/90° lay-down pattern. For the second layer, medical-grade PCL pellets (Lactel, USA) were loaded into a 2 ml syringe and were electrospun using an in-house melt electrospinning device (4 cm tip-to-collector distance, 80°C, 20 µl h−1, 7 kV) [23,24]. After 4 min of electrospinning, highly porous PCL melt-electrospun mesh with roughly 8 mm diameter was produced on top of the aluminium foil-covered glass slides placed over the collector. The biphasic scaffold was assembled as previously reported [25,26]. Briefly, 6 mm long FDM scaffold cylinders were punched out using a 4 mm biopsy punch and one side of the cylinder was placed 1 cm from a hot plate heated to 300°C for 4 s and then quickly press-fitted for 10 s onto the pre-cut PCL meshes (4 mm diameter, approx. 1 mm thick). This heat treatment partially melted the first layer of the FDM component enabling it to strongly bind to the electrospun scaffold upon cooling and solidification. Once the biphasic scaffolds were fused, they were soaked in 1 M NaOH solution overnight to increase hydrophilicity. Biphasic scaffolds were then washed in 70% ethanol and put under UV for 20 min to sterilize and were left to dry in a sterile laminar hood overnight. Once dry, biphasic scaffolds were placed in 24-well plates and each scaffold was seeded with 2.5 × 105 osteoblasts in 100 µl of basal media supplemented with 10% FBS. Cells were allowed to adhere to the scaffold for 4 h in the incubator before adding additional media (1.5 ml/construct). Scaffolds were cultured for 24 h prior to the final assembly into triphasic osteochondral constructs.
2.4. Preparation of bovine osteochondral defects and triphasic construct assembly
An adult bovine osteochondral joint was obtained from a local abattoir. The joint was securely clamped on top of a surgical bench and the joint capsule was opened using sterile scalpel blade and surgical forceps. Osteochondral plugs of 6 mm in diameter were cut from the patellofemoral groove using a sterile trephine and a hacksaw. The tissue was constantly irrigated with sterile PBS containing antibiotics to minimize heat-induced damage. To create 4 mm diameter full thickness defects, osteochondral plugs (n = 32) were clamped down and were drilled in the centre using a sterile trephine. The defect plugs were then cultured in basal media containing 10% FBS for 3 days until the triphasic construct insertion and rat subcutaneous implantation.
The triphasic construct was assembled 24 h prior to the in vivo implantation using a modified protocol from a previous study [22]. First, S and MD alginate discs (Comp or NC) were placed on sterile filter paper soaked with 200 mM EDTA for 3 s to partially de-cross-link one side on each disc. The de-cross-linked sides were then pressed together for 10 s before being transferred to a 102 mM CaCl2 bath for 10 min. To combine the alginate constructs with the PCL biphasic scaffolds, the MD side of the combined alginate was partially de-cross-linked using 200 mM EDTA-soaked filter paper and was press-fitted on top of the FDM side of the biphasic scaffold for 10 s to allow alginate to partially infiltrate the pores of the PCL-FDM scaffolds and were re-cross-linked in 102 mM CaCl2 bath. Combined triphasic constructs were cultured in basal media supplemented with 10% FBS for 24 h prior to combining with the osteochondral defect plugs and being implanted in rat subcutaneously. Cell-free alginates and biphasic PCL scaffolds were also prepared using the same method, as controls.
2.5. Subcutaneous implantation of multiphasic constructs in rat
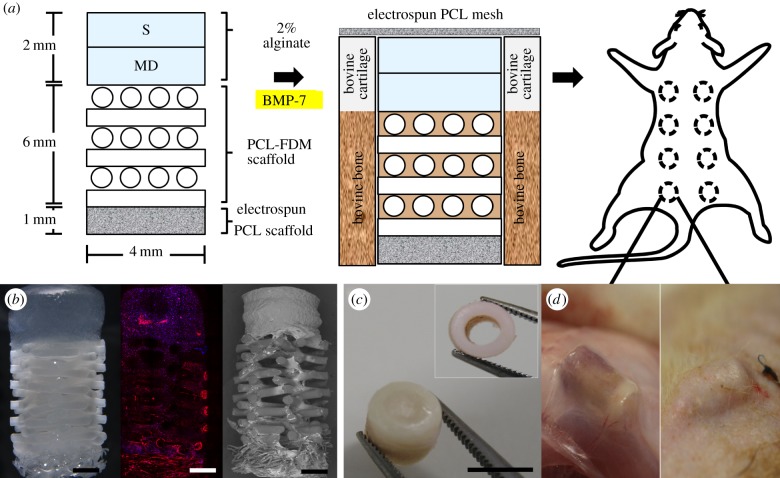
Figure 1a depicts the global strategy conducted in this study, which consisted of inserting a triphasic construct (figure 1b) in a bovine osteochondral defect plug (figure 1c) prior to subcutaneous implantation in a rodent model (figure 1d). Immediately before the surgical implantation, triphasic constructs were taken out of the media, gently dried using sterile gauze and inserted into the bovine osteochondral defect plugs. 15 µl of recombinant human bone morphogenic protein-7 (rhBMP-7, 1 mg ml−1, Olympus Biotech Corporation) mixed with 15 µl of fibrinogen and 15 µl of thrombin (TISSEL, Baxter, IL, USA) was injected into the bone compartment of the osteochondral defect. A thin PCL electrospun membrane (less than 0.5 mm thick, 8 mm diameter) was glued on top of the cartilage/alginate side using 5 µl of fibrinogen and 5 µl of thrombin to give an added protection against fibrous tissue infiltration into the cartilage compartment. Bovine defect plugs containing the triphasic constructs and rhBMP-7 were then implanted subcutaneously in athymic nude rats. This experimental design did not consider the implantation of osteoblast-seeded only (no rh-BMP-7) triphasic scaffolds as no ectopic bone formation was expected in this group, as we have recently demonstrated [27]. Animal ethics approval for the use of rats in this experiment was granted by the Animal Ethics Committee of Griffith University. Four 8-week-old male rats (Animal Resources Centre, Canning Vale, WA, Australia) were anaesthetized with isoflurane, and eight incisions were made longitudinally along the central line of the shaved dorsal area, approximately 1.5 cm apart, and subcutaneous pockets were made with a pair of surgical scissors. The assembled bovine defect plugs were inserted individually in each subcutaneous pocket. Rats were allowed to move freely within the cage and were closely monitored throughout the experiment. Following 12 weeks of implantation, animals of all groups were sacrificed, and the bovine defect plugs were fixed in 4% paraformaldehyde (PFA) for 2 h. Fixed explants were incubated in a solution of 50 mM BaCl2 and 100 mM sodium cacodylate trihydrate overnight to stabilize the remaining alginate gels and kept in 0.1% sodium azide in PBS solution at 4°C prior to further analysis.
Figure 1.
(a) Overview of the experimental design. Triphasic constructs were composed of chondrocyte-seeded bilayered (S, MD) 2% alginate gels and osteoblast-seeded PCL biphasic scaffolds. Alginate gels were cultured in chondrogenic media for 12 weeks and subjected to one week of compressive stimulation prior to in vivo implantation. Triphasic constructs seeded with chondrocytes and osteoblasts were imaged using stereo microscopy, confocal microscopy (rhodamine–phalloidin (red), DAPI (blue)) and scanning electron microscopy (b) (scale bar, 1 mm). Prior to the rat subcutaneous implantation, osteochondral cores prepared from bovine knees were filled with triphasic constructs and the cartilaginous compartment was covered with electrospun PCL mesh (c) (scale bar, 6 mm). Osteochondral constructs were implanted in rat subcutaneously in the dorsal pouches underneath the skin (d) and allowed to mature for 12 weeks.
2.6. Analysis of glycosaminoglycan, DNA and mechanical properties of superficial and middle-deep alginate constructs
After 12 weeks of chondrogenic culture and one week of loading, S and MD alginate constructs (NC and Comp) were analysed for glycosaminoglycan (GAG) and DNA content (n = 6), and also compressive modulus (n = 6). Prior to the biochemical assays, S and MD constructs were digested in 0.5 mg ml−1 proteinase K solution (Invitrogen) overnight at 60°C and thoroughly mixed using the vortex. A modified 1,9-dimethylmethylene blue (Sigma) assay at pH 1.5 [28] was used to determine GAG content. DNA content in the S and MD constructs was quantified using the Quant-iT PicoGreen dsDNA assay kit (Invitrogen) according to the manufacturer's protocol. The absorbance reading from the GAG plate and the fluorescence reading from the PicoGreen kit were measured on a POLARstar microplate reader (BMG Labtech, Mornington, Australia). The compressive moduli of the constructs were measured on an Instron 5848 microtester fitted with a 5 N load cell (Instron, Australia). S and MD constructs were kept in media until immediately before the compression test. The compression testing protocol comprised a slow downward ramp (10 µm s−1) from the contact point (0.02 N) until 50% compressive strain level was reached. The slope of the stress–strain curve in the 10–15% strain range was used to calculate the compressive modulus.
2.7. Immunofluorescence staining of superficial and middle-deep constructs
In order to visualize the accumulation of collagen types I and II following the long-term chondrogenic culture and loading, S and MD alginate gels were immersed in a fixative solution containing 4% w/v PFA, 100 mM sodium cacodylate trihydrate (Sigma) and 10 mM CaCl2. To stabilize the alginate during the processing steps, constructs were incubated at 4°C overnight in 50 mM BaCl2 solution containing 100 mM sodium cacodylate trihydrate. Fixed constructs were paraffin-embedded and sectioned at 5 µm. Once de-paraffinized and rehydrated, sections were soaked in 10 mM sodium citrate/0.05% Tween 20 buffer at pH 6.0 and heated in a pressure cooker for 4 min at 94°C. Heat-treated sections were further incubated with 0.1% w/v pronase and 0.1% w/v hyaluronidase (all Sigma) solution at 37°C for 30 min. After the antigen retrieval process, sections were blocked with 2% FBS solution for 1 h and incubated overnight in primary antibody solution for collagen type I (Col I, dilution 1 : 500, I-8H5, MP Biomedicals, USA) and collagen type II (Col II, dilution 1 : 200, II-II6B3, Developmental Studies Hybridoma Bank (DSHB), USA). Sections were washed twice in PBS and incubated in fluorescence-labelled goat anti-mouse secondary antibody (Alexa Fluor 488, Invitrogen) for 1 h prior to mounting with Prolong Gold (Invitrogen). After allowing the mounting media to dry in the dark, sections were visualized using a fluorescence microscope (Axio Imager A1, Zeiss, Jena, Germany).
2.8. Morphological analysis of the multiphasic scaffold
Triphasic constructs seeded with zonal chondrocytes (alginate) and osteoblasts (biphasic PCL scaffold) were cultured in basal medium supplemented with 10% FBS for one week prior to imaging for morphological analysis (figure 1b). Triphasic constructs were fixed in 4% PFA for 30 min and immersed in PBS/5 mM CaCl2 solution to be imaged using the stereo microscope (DMS1000, Leica, Australia). PFA-fixed constructs stained with rhodamine–phalloidin and 4′,6-diamidino-2-phenylindole (DAPI) (all Invitrogen) in 5 mM CaCl2 in PBS solution were imaged using a Nikon A1R confocal laser scanning microscope system (Nikon Corp., Tokyo, Japan). Samples fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) were imaged without dehydration processes using Hitachi analytical table top microscope TM3000 (Hitachi, Tokyo, Japan) operating at 5 kV.
2.9. Micro-computed tomography
Osteochondral explants were fixed in 4% PFA and stored in 0.1% sodium azide in PBS at 4°C prior to imaging. In order to visualize the mineralized tissue, fixed osteochondral explants were scanned in a µCT40 (Scanco Medical, Brüttisellen, Switzerland) at a resolution of 12 µm, a voltage of 45 kVp and a current of 177 mA. The volume of newly formed bone within the bone compartment of the osteochondral construct was measured by the micro-computed tomography (µCT) software using a greyscale threshold of 220. For this experiment, the bovine bone was manually excluded for considering uniquely the newly formed bone matrix (cell-free constructs: n = 4; NC and Comp constructs: n = 14). Three-dimensional images of the scaffolds were reconstructed from the scans by the µCT system software package.
2.10. Equilibrium partitioning of an ionic contrast agent–micro-computed tomography
In order to visualize the GAG distribution in the cartilage and alginate components of the explants, equilibrium partitioning of an ionic contrast agent–µCT (EPIC-µCT) analysis was used, according to the protocol described by Benders et al. [29]. Explants were incubated in 1 ml of ionic contrast agent solution (40% ioxaglate (Hexabrix, Mallingckrodt, Hazelwood, MO, USA)/60% PBS) for 16 h at 4°C and scanned using µCT40 (Scanco Medical) at 45 kVp/177 µA. Scanned images were analysed using Scanco µCT software (Scanco Medical). Attenuation threshold levels were set between 1500 and 3500 Hounsfield units (HU) for all samples analysed. Gaussian filter was used with Sigma of 1.2 and a support of 2. Three cross-sectional images were taken at varying locations. As samples immersed in the contrast agent may not be suitable for other analyses, EPIC-µCT was first performed using limited sample numbers (n = 2 per group). Based on the result of the first analysis showing small differences between the groups, the rest of the samples were allocated for other analysis.
2.11. Histological and immunohistochemical analysis of osteochondral explants
Fixed osteochondral explants were decalcified in 5% formic acid at room temperature for 14 days under constant agitation. Formic acid was changed with fresh solution every other day. Following the decalcification process, explants were embedded in paraffin blocks and sectioned at 5 µm thickness. Haematoxylin and eosin (H/E) staining was carried out using a Leica ST5010 Autostainer (Leica Microsystems Pty Ltd, North Ryde, Australia). Sections were also stained with 0.1% w/v Safranin-O for 10 min and counter stained with 0.05% w/v fast green and Weigert's iron haematoxylin for 5 and 10 min, respectively. Stained sections were dehydrated in graded ethanol baths and xylene and mounted using Eukitt (all Sigma). Immunohistochemical analyses of the explants for collagen type I (Col I, dilution 1 : 500, I-8H5, MP Biomedicals), collagen type II (Col II, dilution 1 : 200, II-II6B3, DSHB), von Willebrand factor (vWF, dilution 1 : 3\0, Millipore AB7356) and alkaline phosphatase (ALP, dilution 1 : 500, B4–78, DSHB) were carried out using a commercially available kit (VectaStain ABC kit, Vector Labs Inc., Burlingame, CA, USA) using the instructions provided by the manufacturer. Prior to staining for Col I, Col II and ALP, sections were soaked in 10 mM sodium citrate/0.05% Tween 20 buffer at pH 6.0 and heated in a pressure cooker for 4 min at 94°C. For vWF, sections were incubated with few drops of proteinase K (Dako, Denmark). After rinsing in PBS, sections were treated with 3% H2O2 for 10 min and blocked with 2% normal horse serum supplied in the kit for 1 h. Sections were then incubated with primary antibody solution diluted in the blocking buffer for 1 h followed by two 5 min washes in PBS bath. Sections were further incubated in biotinylated secondary antibody solution and Vectastain ABC reagent provided in the kit for 30 min each. Bound peroxidase was visualized using ImmPACT DAB peroxidase substrate kit (Vector Laboratories, USA), and images were captured using a Leica SCN400 Slide Scanner (Leica Biosystems, Wetzlar, Germany). Decalcified human osteochondral sections were used as controls for histological and immunohistochemical staining.
2.12. Osteocyte etching
Osteochondral samples fixed in 4% PFA were dehydrated in graded series of ethanol and infiltrated using ethanol/resin solution with increasing resin concentration (from 30% v/v resin to 100% resin) for 4 days (Tecknovit 7200, Heraeus Kulzer, Germany) in an EXAKT 510 dehydration and infiltration system. Resin was then cured using an EXAKT 520 light polymerization system (EXAKT, Germany). Once cured, resin blocks were ground using an EXAKT 400 CS micro grinding system to expose the samples and subsequently cut using an EXAKT 300 cutting system equipped with a diamond saw. Resin blocks were polished using 400, 600 and 1200 grit sandpapers and a final polish achieved with 1.0 alpha alumina powder polishing using a soft cloth polishing wheel. Mirror-finished block faces were etched with 37% phosphoric acid for 2–5 s and washed with 12.5% sodium hypochlorite for 5 min followed by a brief rinse in deionized water [30]. Acid-etched blocks were sputter-coated with gold (Leica EM SCN005) and visualized using an FEI Quanta 200 environmental scanning electron microscope operating at 10 kV.
2.13. Statistics
SPSS (v. 19.0) was used to perform the statistical analysis. Data are presented as mean ± s.d. Two-factor ANOVA and Fisher's least significant difference post hoc tests were used to determine the effects of zone and pre-culture on GAG, DNA and compressive modulus in figure 2b (n = 6). In all tests, p < 0.05 was considered significant.
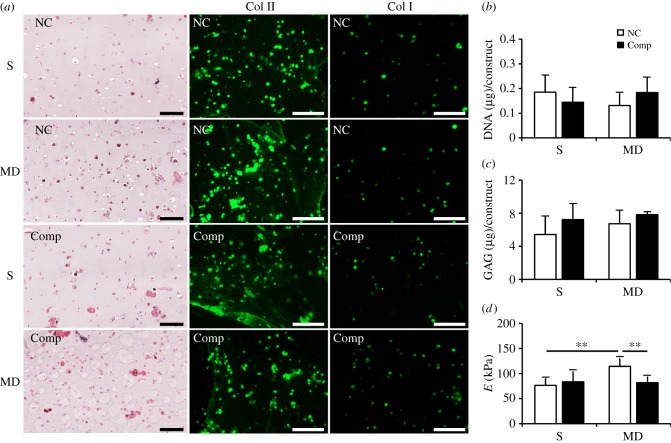
Figure 2.
Zonal construct analysis after 12 weeks of pre-culture and one week of compressive loading (Comp) or free-swelling (NC) culture. (a) S and MD constructs expressed both collagen types I and II during the long-term culture. (b) DNA and (c) GAG content in constructs did not vary significantly regardless of zones or loading conditions. MD constructs were significantly stiffer compared with the S constructs under free-swelling conditions, and one-week compression decreased the stiffness in MD zone constructs (d). **p < 0.01. Scale bars, 100 µm.
3. Results
3.1. Analysis of zonal (surface and middle-deep) constructs following long-term culture and compressive stimulation
Following 12 weeks of in vitro culture under chondrogenic conditions, S and MD constructs stained intensely for collagen type II (Col II) around chondrocytes but limited staining was observed in spaces further away from the cells (figure 2a). Collagen type I (Col I) was also expressed in some zonal chondrocytes and was less intense compared with Col II. Both Col II and I stained similarly between groups that were compressed for one week (Comp) and those that were NC. DNA and GAG levels did not vary significantly between groups regardless of zones or loading conditions (figure 2b,c). Unconfined compression tests revealed that MD constructs were significantly more stiff compared with the S constructs under free-swelling conditions (figure 2d; p = 0.002). Compressive stimulation decreased stiffness of MD constructs but not in S constructs (p = 0.009).
3.2. Triphasic scaffold assembly
Alginate discs seeded with surface and middle-deep chondrocytes were well integrated, with visibly smooth transition line, as shown under the stereo microscope (figure 1b). The PCL-FDM scaffold and the melt-electrospun PCL mesh were also well integrated. Scanning electron microscopy images show that top portions of the thin mesh filaments are fused with the larger FDM scaffold fibres, which had been partially melted by the heat treatment (figure 1b). The interface between the zonal alginate construct and the PCL scaffolds was relatively more fragile than the other interfaces, yet was able to withstand manual handling using forceps. Partial de-cross-linking of the alginate and press-fitting it on top of the porous FDM scaffold resulted in alginate infiltration through the pores of the PCL scaffold and the topmost layer of the PCL scaffold was partially encapsulated in alginate gel (figure 1b). Fibrin glue used for the rh-BMP-7 delivery also served to hold the construct in place within the osteochondral defect (figure 1c), and construct loosening did not occur during the implantation procedures (figure 1d).
3.3. Analysis of osteochondral constructs following in vivo implantation: equilibrium partitioning of an ionic contrast agent–micro-computed tomography and micro-computed tomography
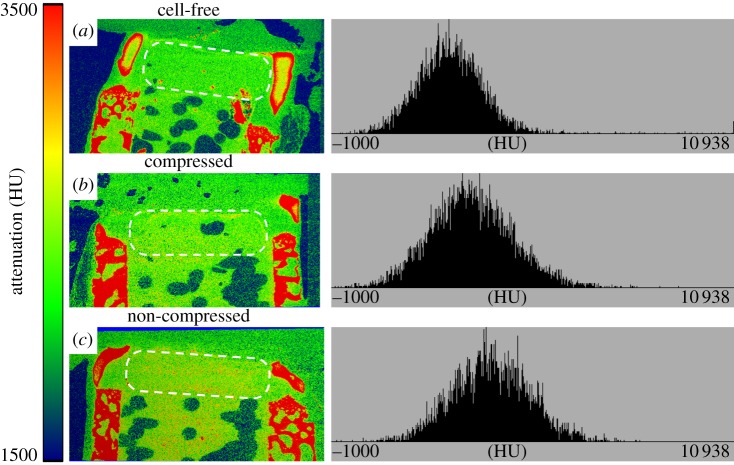
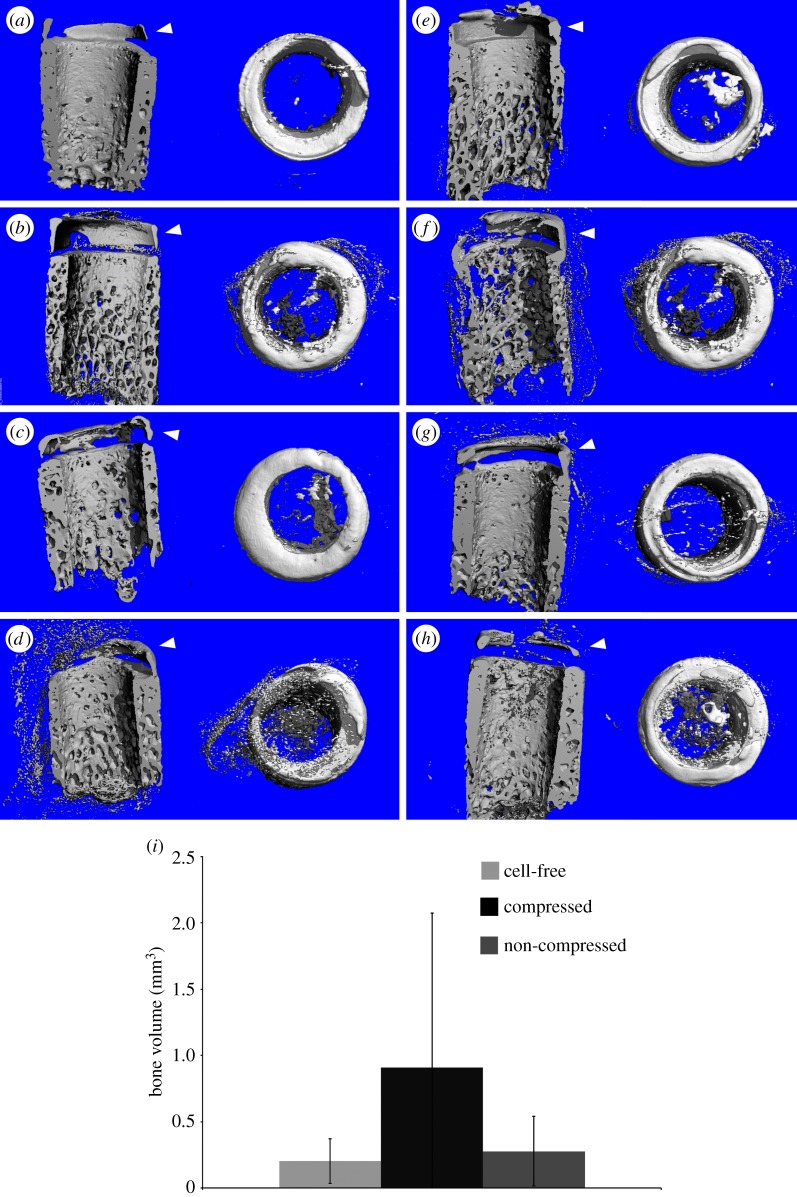
High degree of attenuation in the EPIC-µCT images has been reported to show low GAG concentration in cartilage [29]. The upper portion of the osseous compartment and the inner parts of the cartilage compartment of the construct had lower attenuation levels (figure 3). Zonal differences in attenuation levels between the S and MD alginate layers were not apparent. Attenuation level distributions were also similar in cell-free (figure 3a) and cell-containing alginate layers cultured with (figure 3b) or without compression (figure 3c). The outer edge of the bovine cartilage, along with the bovine bone had high attenuation in the EPIC-µCT images, which is most likely owing to calcification of the cartilage in these regions. This is apparent from the three-dimensional µCT images of explants not stained with Hexabrix, which show that the outer rims of the bovine cartilage and the upper portion of the bovine bone had substantial mineralization in most explants (figure 4). Surprisingly, there was limited mineralization in the PCL compartment with or without the pre-seeded osteoblasts (figure 4e) and no significance differences were found between the groups (figure 4i).
Figure 3.
EPIC-µCT images and histograms of attenuation in the cartilaginous compartments (dotted area) following in vivo implantation. Lower attenuation levels indicate higher GAG content and high attenuation levels indicate bone or tissues with low GAG content. (a) Cell-free alginate gel, (b) alginate gel subjected to one week of compression and (c) non-loaded gel implanted within the cartilaginous compartment had similar attenuation levels between the different groups, and depth-dependent zonal variations were not observed. Bone-like attenuation patterns were visible in the outer rims of the bovine cartilage in all explants.
Figure 4.
µCT images showing mineralized tissues in the scaffold. Explants of the triphasic scaffold inserted in bovine core, which had cell-free (osteoblast) (a,e) and osteoblast-seeded bone compartments (patient 1: b,f; patient 2: c,g; patient 3: d,h), had little bone formation in the osseous compartment. Explants that contained alginate gels subjected to a one-week compressive loading prior implantation are shown in (e–h), and those that had non-loaded alginates are shown in (a–d). Mineralization was visible on the outer rims of the cartilage in all groups including the cell-free controls. (i) Bone volume within the bovine osteochondral defect as measured by µCT. There was no statistical difference among the three groups (cell-free n = 4, Comp and NC n = 14). (Online version in colour.)
3.4. Analysis of cartilage compartment: histology and immunohistochemistry
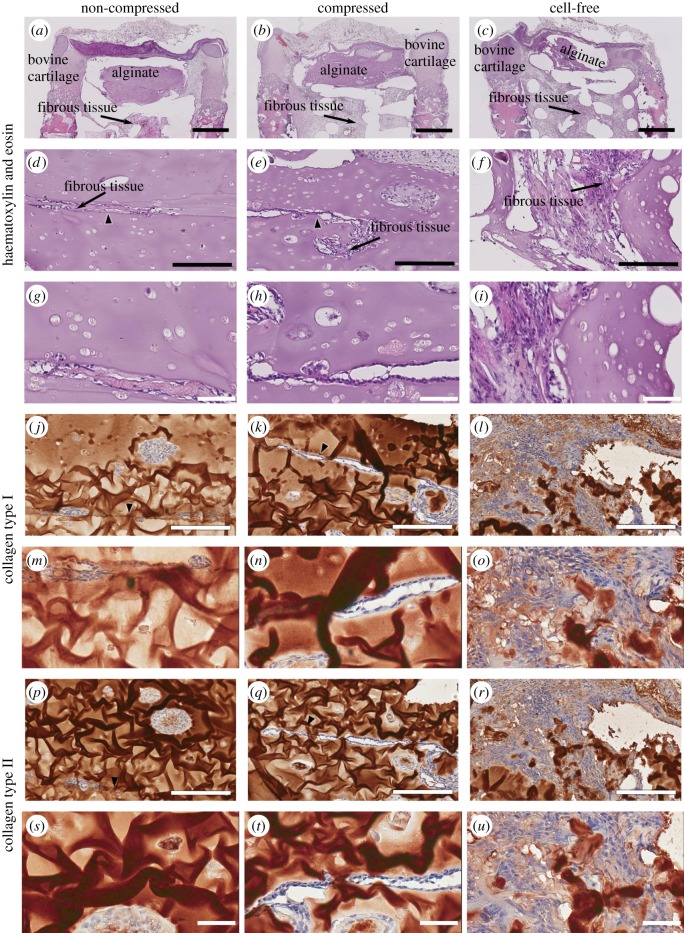
The intensity of Safranin-O within the bovine tissue was the strongest in the hypertrophic zone of the cartilage near the bone and the newly mineralized regions on the outer edge of the cartilage (figure 5a,b). These areas stained faintly of collagen type II and had distinct boundaries (figure 5c,d). Layered alginate constructs were largely intact in constructs pre-seeded with cells compared with those that were cell-free at the time of implantation (figure 6a–i). A thin line of cells was found in some areas in between the S and MD layers. Cells in the alginate appeared either as isolated cells or were in large aggregates. Cell aggregates stained faintly of collagen type II in the centre. Cell-free alginate gels were fully infiltrated with fibrous tissues and many multinucleated cells were found in between the alginate debris (figure 6j–u).
Figure 5.
(a,b) Safranin-O and (c,d) collagen type II-stained images of bovine cartilage tissue following in vivo implantation. Areas of the cartilage corresponding to the mineralized regions are indicated by the open arrow. (b,d) Scale bars, 200 µm. (Online version in colour.)
Figure 6.
Histological and immunohistochemical images of explant cartilaginous compartment. While explants implanted with both NC (a,d,g,j,m,p,s) and Comp (b,e,h,k,n,q,t) alginate gels had retained much of the alginate constructs during the 12-week in vivo culture, those in the cell-free group had little alginate left (c,f,i,l,o,r,u). While S and MD alginate gels remained intact in the cell-seeded groups, fibrous tissue infiltration was visible in the interface between the two gels (arrows). Cells found within the alginate compartment in all groups formed dense aggregates but did not stain strongly for either collagen type I (j–o) or II (p–u). Scale bars: (a–c), 1 mm; (d–f, j–l, p–r), 200 µm; (g–j, m–o, s–u), 50 µm. (Online version in colour.)
3.5. Analysis of bone compartment: histology, immunohistochemistry and osteocyte etching
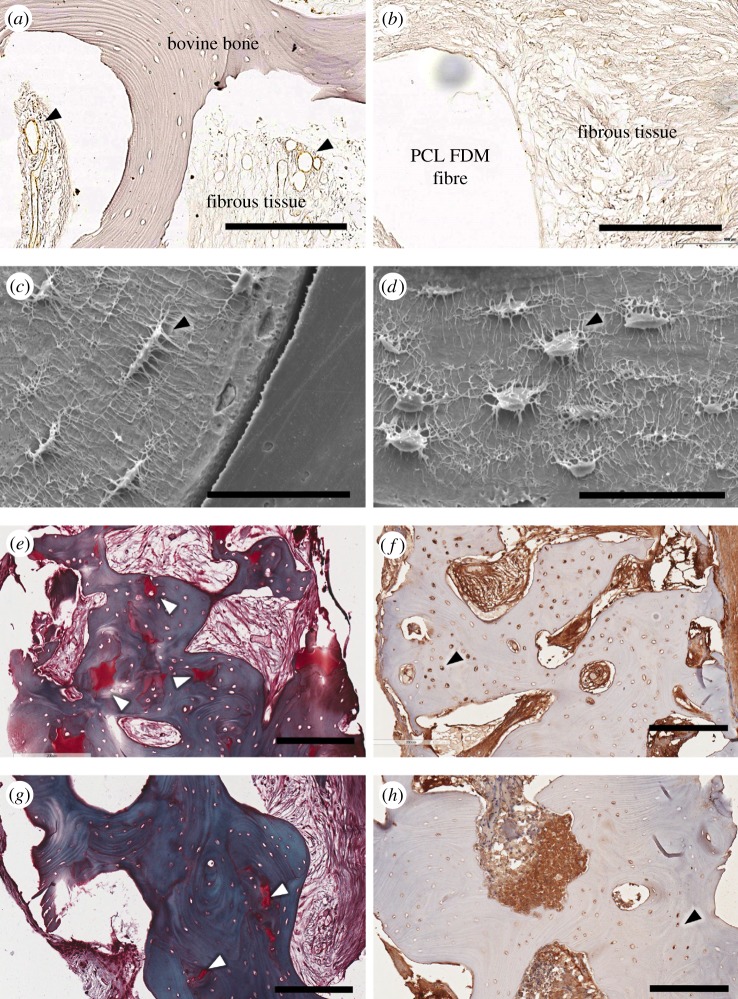
Sections stained for vWF indicated a limited blood vessel network within the osseous construct while there were many blood vessels found within the bovine bone (figure 7a,b, black arrowheads). Scanning electron micrographs of acid-etched explants also show that osteocytes within the bovine bone had well-preserved processes, which were extensively interconnected with each other (figure 7c,d, black arrowheads) although those within approximately 100 µm of inner and outer edges of the bone had relatively diminished interconnectivity and some empty lacunae, possibly from heat or mechanically induced cell injury during the osteochondral plug preparation. The upper regions of the bovine bone, which were also densely mineralized according to the µCT analysis, had numerous patches, which stained strongly with Safranin-O (figure 7e, white arrowheads). These regions of bone also contained many cells, which stained positive for ALP, as indicated by the black arrowhead in figure 7f. Lower parts of the bone also exhibited Safranin-O (figure 7g, white arrowheads)-stained regions and ALP-positive cells (figure 7h, black arrowhead), but to a lesser degree. Although the fibrous interstitial tissue stained intensively for ALP in both the top and bottom portion of the bovine osteochondral plug (figure 7f,h), there was a clear difference within the bovine bone with the top portion having a large number of ALP-positive stained cells as opposed to the bottom portion where only a very few cells were positively stained.
Figure 7.
Representative imaging of the bovine bone across the different groups. vWF staining shows well-developed blood vessels in and around the bovine bone (black arrowhead) (a), while little to no defined vWF staining is visible within the osseous scaffold within the defect (b) from a NC sample. Osteocyte etching reveals well-preserved cells in the bone that are in close proximity to the infiltrated fibrous tissues (black arrowhead) (c) and those that are well within the bone (d) from a NC sample. Representative histological images from a NC sample, which indicate areas within the bone staining intensely with Safranin-O (top (e), bottom (g), white arrowhead). Sections of a compressed sample stained for ALP show depth-dependent staining patterns with more cells staining for ALP near the top portion of the bone (f) compared with those in the bottom (h) (black arrowheads). Scale bars: (a,b,e–h), 200 µm; (c,d), 50 µm. (Online version in colour.)
3.6. Fibrous tissue infiltration
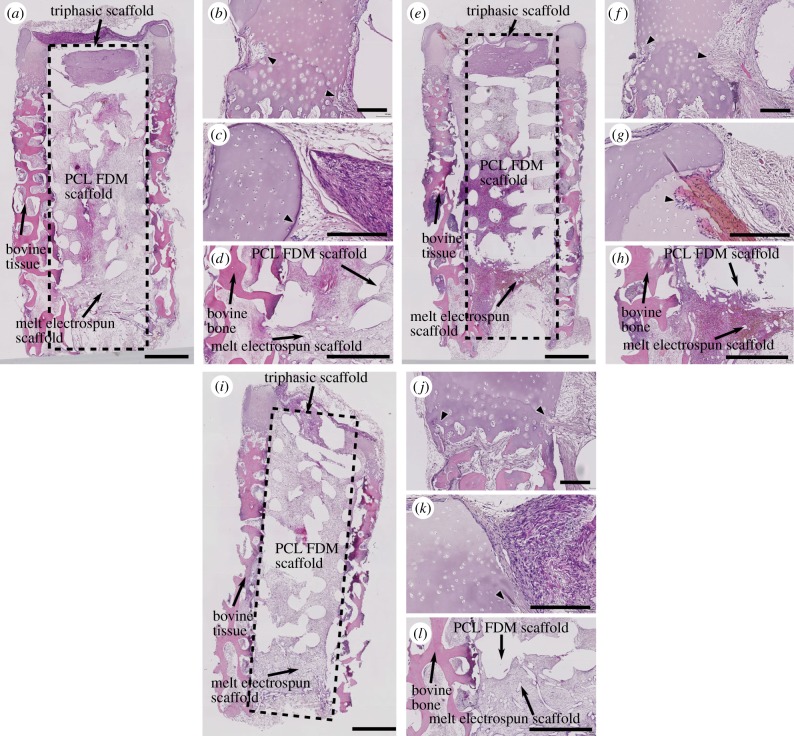
Fibrous tissue infiltration occurred throughout the osteochondral explants in all samples. In the bovine cartilage, fibrous tissues were found to penetrate towards the periphery of the mineralizing areas (figure 8a,b,e,f,i,j) from the both outer and inner edge of the bovine cartilage. Fibrous tissue infiltration also occurred from the top portion of the cartilage and immediately below the mineralized regions (figure 8c,g,k). Rat fibroblasts also saturated the electrospun mesh and formed a cellular bridge between the mesh and cartilage (figure 8c,g,k). Fibrous tissues fully surrounded the PCL-FDM/melt-electrospun scaffold in the osseous compartment, and they were also present throughout the bovine bone (figure 8d,h,l). Macrophage-like multinucleated cells were visible in some areas of the fibrous tissue, which may indicate inflammatory responses.
Figure 8.
Haematoxylin-/eosin-stained sections of osteochondral explants. (a–d) NC, (e–h) Comp and (i–l) cell-free. Fibrous tissue infiltration was seen in both the inner and outer edges near the lower portion of the bovine cartilage in all explants (a,e,i, and arrowheads in b,f,j). Fibrous tissue infiltration was also seen in the upper part of the cartilage and they were seen primarily from the inner edges of the cartilage (arrowheads in c,g,k). Fibrous tissues also fully infiltrated the bovine bone, PCL-FDM scaffolds and the PCL mesh (a,e,i,d,h,l). Scale bars: (a,e,i), 1 mm; (b–d,f–h,j–l), 200 µm. (Online version in colour.)
4. Discussion
Preclinical animal testing is an important step towards the translation of newly developed tissue-engineering concepts from bench to bedside. Immunodeficient rodents are widely used for xenograft-based studies and have the benefit of low cost and manageability. Subcutaneous implantation in severe combined immunodeficiency mouse or rat is a well-established method of studying human tissue development and has been used to study the development of tumours [31], bone [32] and cartilage [33]. Bovine osteochondral cores have been used in the past to study cartilage defect repair in vitro [34], as they have comparable cartilage thickness to humans. Combining the use of bovine osteochondral defect plugs and subcutaneous implantation strategies is a new concept that has not been well characterized. In this study, we developed a hybrid in vivo animal model to implant multiphasic osteochondral constructs and evaluated the outcomes after 12 weeks. Our results indicate that our hybrid in vivo defect model requires further optimization to support robust bone and cartilage formation.
Multiphasic scaffold design is a growing trend in osteochondral tissue engineering, with a recent increase in the number of studies exploring the biphasic concepts. There are advantages in using multiphasic designs over monophasic constructs for osteochondral repair. For instance, scaffold types and growth factor additives can be optimized for the development of cartilage and bone separately, and the option of post-assembly allows for chondrogenic and osteogenic pre-culture prior to the in vivo implantation [35]. However, unlike monophasic constructs, designing the cartilage–bone interface in multiphasic tissue graft is a major challenge. Although the presence of bovine defect plug offers a degree of protection from external mechanical stresses, histological analysis of the implanted constructs shows that the alginate constructs had been separated from the PCL scaffolds in some explants possibly owing to gradual weakening of the interface region (figure 6a–i).
Recapitulation of zonal architecture and mechanical properties similar to native articular cartilage remain important goals in functional regeneration of cartilage. As a result, recent developments in cartilage tissue engineering show a growing number of investigators attempting to incorporate zonal architecture [36] and mechanical pre-conditioning [37] to further improve the functionality of the cartilage construct in vitro. In our study, one week of mechanical loading did not significantly affect collagen types I, II, total DNA and GAG levels and using S and MD zone chondrocytes to fabricate layered constructs did not result in zonal differences (figure 2). Not surprisingly, EPIC-µCT images of the explants also show that we were not able to achieve zone-specific differences in the GAG distribution in constructs with or without the mechanical loading (figure 3). Compared to the 12 weeks of culture, one week is a relatively short period of time, and it is possible that mechanical loading should be applied earlier and for a longer duration for optimal results. Also, as indicated by others, expanded chondrocyte subpopulations gradually lose zonal phenotypes in vitro [38] and may require zone-specific differentiation strategies. In future studies, improvements in zonal cartilage formation may be achieved by applying different types of mechanical load (e.g. shear stress, hydrostatic pressure) to better represent zone-specific physiological load.
One of the benefits of implanting osteochondral constructs within the osteochondral defect is that resident chondrocytes and osteoblasts in the surrounding osteochondral tissues may influence the integration and the differentiation processes of the immature construct. Our analysis of the µCT images shows that some parts of the bovine cartilage had gone through hypertrophy, especially at the outer edges of the bovine cartilage (figure 4). There was a clear boundary around the mineralized areas of the cartilage, which stained intensely with Safranin-O, but had little to no stain for collagen types I and II (figure 5). This observation is comparable to the calcification process of the growth plate cartilage where concentration of aggregating proteoglycans and collagen type II in the growth plate cartilage is at its highest immediately before hypertrophy, followed by a dramatic decrease, as the tissue gets mineralized [39]. Sharp transitions of proteoglycans and collagen content seen near the edges of the mineralized cartilage may be indicative of an ongoing endochondral ossification-like process. Factors that may have contributed to cartilage calcification are chondrocyte hypertrophy and apoptosis, and the presence of inflammatory cytokines and rh-BMP-7 [40].
Bone morphogenic protein-7 is well known for its ability to stimulate bone formation in vivo [41], but robust bone formation and long-term survival are also dependent on successful angiogenesis. In this study, the use of rh-BMP-7 in the implanted osseous constructs did not sufficiently augment bone development even though there was prominent mineralization in the bovine cartilage. Studies suggest that vascular invasion and mineralization can occur in tissue-engineered cartilage following ectopic implantation in mouse, especially when they are developed using pre-differentiated human mesenchymal stem cells [42] or expanded chondrocytes [43]. These studies also show that constructs developed from fully differentiated (i.e. freshly isolated) chondrocytes tend to resist vascular invasion and mineralization. However, what was apparent in our study was that most pronounced mineralization occurred mainly in the bovine cartilage, rather than in the engineered cartilage containing the expanded chondrocytes. A possible reason for this, other than rh-BMP-7, may be the close proximity of the mineralized parts of the bovine cartilage to the host vasculature, increasing its vulnerability to hypertrophy.
On the other hand, the lack of bone formation in the osseous compartment may be owing to insufficient vascular ingrowth. Sections stained for vWF indicate higher concentration of blood vessels within the bovine bone compared with the osseous compartment of the construct (figure 7a,b). Highly vascularized areas of the bovine bone also had well-preserved osteocytes (figure 7c,d), and in these areas histological images show a number of small defined areas which stained intensely with Safranin-O, an indicator of cartilage-like tissue formation. Cells that stained for ALP were found in larger numbers near the top portion of the bovine bone, coinciding with the higher number of Safranin-O-stained areas near the top portion of the bone (figure 7e,f). These observations show that the role of angiogenesis in bone formation is prominent and that it often dictates the success of bone regeneration.
It is possible that dense bovine bone may have acted as a barrier for vascular infiltration. Although the bovine bone itself was vascularized, spaces within the bone were occupied by fibrous tissues, which together with the dense mineralized matrix can be a physical barrier to vascular ingrowth. Delayed or compromised vascular infiltration may affect the construct development in two ways. Firstly, prolonged deprivation of oxygen and nutrients would have decreased the viability of the pre-seeded osteoblasts, rendering them ineffective in initiating bone regeneration. While longer in vitro osteogenic differentiation of osteoblasts on the PCL scaffolds may help to kick start mineralization, the vascularization issue will likely remain an issue for post-implantation bone regeneration. Secondly, the delivery method of rh-BMP-7 to the osseous compartment was through fibrin glue [44], with the resorption time frame of two weeks during an in vivo implantation [45]. If rh-BMP-7 leached out of the defect site prior to sufficient cellular and vascular infiltration, it may have induced bone formation elsewhere, with limited rh-BMP-7-induced bone formation within the osseous compartment. Synthetic oxygen carrier-enriched hydrogels as rh-BMP-7 delivery vehicles may be considered as an alternative to fibrin glue, as they have been shown to help bone regeneration [46]. The use of hydrogel-coated microspheres to deliver VEGF (gelatin hydrogel) prior to rh-BMP-7 (PLGA) has been reported to improve ectopic bone formation in rat through improved vascular network [47]. Increasing the duration of the release profile of rh-BMP-7 and combining other growth factors, such as VEGF, may help better bone regeneration in the multiphasic constructs in future studies.
While the osteochondral plugs provided structural support for the implanted constructs, they did not shield them from fibrous tissue infiltration. Not only was fibrous tissue infiltration observed in the bovine bone and the osseous compartment of the triphasic scaffold, fibrous tissue penetration was visible within the non-mineralized areas of the bovine cartilage and alginate constructs (figure 8a–l). A fibrous tissue layer had bridged the alginate and bovine cartilage together and had infiltrated into the alginate compartment (figure 6d–o). They also filled the interface region between the two alginate layers (figure 6d–i) and aggregated into small spheroids in some areas within the alginate. Interestingly, the morphology of the alginate constructs and the degree of rat fibroblast infiltration varied between the chondrocyte-seeded and the cell-free groups. Chondrocyte-seeded alginate constructs retained their overall shape while cell-free alginate constructs did not. Large multinucleated cells were also visible around the remaining alginate in the cell-free constructs (figure 6d–o). While it is not clear as to why the chondrocyte-seeded alginate gels fared better against fibrous tissue infiltration, other groups have also noted that constructs seeded with cells survive better when implanted in osteochondral defects compared with the acelluar constructs [48].
A limiting factor in the presented model is the lack of mechanical stress, which routinely occurs in the knee joint. It is evident that mechanical stimulation through compressive, tensile and hydrostatic loading improves matrix production in both chondrocytes and osteoblasts [49–51]. Mechanical stimulation also appears to influence osteoblast and osteoclast activity and encourages mineralization [52,53]. While it will be difficult to apply physiological loads in this hybrid in vivo model, it does allow for optimization of several other factors important in osteochondral repair, such as cell source and biomaterial type. The repair tissue in the hybrid in vivo defect model may have arisen from a mixture of cells from three different species (human, bovine and rat). The use of tissues from different species may actually be advantageous, by identifying the source of the cells and tissues using species-specific antibodies. Nonetheless, the use of three different species is not a requirement, and using osteochondral cores from normal or osteoarthritic human donors may offer the potential to study the effects of the osteoarthritic environment on cartilage repair, but they are difficult to obtain frequently or in large quantities. Finally, the model described here employed multiphasic constructs with hydrogels and composite scaffolds of different scales and fabrication techniques but could be used with virtually any biomaterial and scaffold repair approach. Taken together, this study provides a proof-of-concept for a novel in vivo osteochondral defect model. With further optimization, the presented hybrid in vivo model may help address the growing need for a cost-effective way to screen osteochondral repair strategies before moving to large-animal preclinical trials.
Acknowledgement
Antibodies II-II6B3 (developed by Thomas F. Linsenmayer) and B4–78 (developed by Jerry A. Katzmann) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Funding statement
The research leading to these results has received financial support from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 309962 (project HydroZONES) and National Health and Medical Research Council Australian–European Union Health Research Collaboration APP1067108. Additionally, T.K. and D.W.H. were funded through ARC Future Fellowships. The authors have no conflict of interest with regard to the work.
References
- 1.Goldring MB, Goldring SR. 2010. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. NY Acad. Sci. 1192, 230–237. ( 10.1111/j.1749-6632.2009.05240.x) [DOI] [PubMed] [Google Scholar]
- 2.Saris DB, et al. 2008. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 36, 235–246. ( 10.1177/0363546507311095) [DOI] [PubMed] [Google Scholar]
- 3.Grayson WL, Chao P-HG, Marolt D, Kaplan DL, Vunjak-Novakovic G. 2008. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 26, 181–189. ( 10.1016/j.tibtech.2007.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. 1994. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl. J. Med. 331, 889–895. ( 10.1056/NEJM199410063311401) [DOI] [PubMed] [Google Scholar]
- 5.Tuan RS. 2007. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res. Ther. 9, 109 ( 10.1186/ar2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. 2011. Failures, re-operations, and complications after autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage 19, 779–791. ( 10.1016/j.joca.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 7.Williams DF. 1999. The Williams dictionary of biomaterials. Liverpool, UK: Liverpool University Press. [Google Scholar]
- 8.Klein TJ, Rizzi SC, Schrobback K, Reichert JC, Jeon JE, Crawford RW, Hutmacher DW. 2010. Long-term effects of hydrogel properties on human chondrocyte behavior. Soft Matter 6, 5175–5183. ( 10.1039/c0sm00229a) [DOI] [Google Scholar]
- 9.Mauck RL, Yuan X, Tuan RS. 2006. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179–189. ( 10.1016/j.joca.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 10.Mauck RL, Soltz MA, Wang CCB, Wong DD, Valhmu WB, Hung CT, Ateshian GA, Chao PG. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122, 252–260. ( 10.1115/1.429656) [DOI] [PubMed] [Google Scholar]
- 11.Nagel T, Kelly DJ. 2012. Mechanically induced structural changes during dynamic compression of engineered cartilaginous constructs can potentially explain increases in bulk mechanical properties. J. R. Soc. Interface 9, 777–789. ( 10.1098/rsif.2011.0449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon JE, Schrobback K, Hutmacher DW, Klein TJ. 2012. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis Cartilage 20, 906–915. ( 10.1016/j.joca.2012.04.019) [DOI] [PubMed] [Google Scholar]
- 13.Ng KW, Mauck RL, Statman LY, Lin EY, Ateshian GA, Hung CT. 2006. Dynamic deformational loading results in selective application of mechanical stimulation in a layered, tissue-engineered cartilage construct. Biorheology 43, 497–507. [PubMed] [Google Scholar]
- 14.Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. 2007. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 1, 245–260. ( 10.1002/term.24) [DOI] [PubMed] [Google Scholar]
- 15.Bessa PC, Casal M, Reis RL. 2008. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2, 1–13. ( 10.1002/term.63) [DOI] [PubMed] [Google Scholar]
- 16.Vaquette C, Cooper-White J. 2013. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomater. 9, 4599–4608. ( 10.1016/j.actbio.2012.08.015) [DOI] [PubMed] [Google Scholar]
- 17.Simon WH. 1970. Scale effects in animal joints. I. Articular cartilage thickness and compressive stress. Arthritis Rheum. 13, 244–256. ( 10.1002/art.1780130305) [DOI] [PubMed] [Google Scholar]
- 18.Frisbie DD, Cross MW, McIlwraith CW. 2006. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet. Comp. Orthop. Traumatol. 19, 142–146. [PubMed] [Google Scholar]
- 19.Sah RL, Ratcliffe A. 2010. Translational models for musculoskeletal tissue engineering and regenerative medicine. Tissue Eng. B 16, 1–3. ( 10.1089/ten.teb.2009.0726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahern BJ, Parvizi J, Boston R, Schaer TP. 2009. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17, 705–713. ( 10.1016/j.joca.2008.11.008) [DOI] [PubMed] [Google Scholar]
- 21.Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO. 2010. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. B 16, 123–145. ( 10.1089/ten.teb.2009.0658) [DOI] [PubMed] [Google Scholar]
- 22.Jeon J, Malda J, Schrobback K, Irawan D, Masuda K, Sah RL, Hutmacher DW, Klein TJ. 2010. Engineering cartilage tissue with zonal properties. In Methods in bioengineering: 3D tissue engineering (eds Berthiaume F, Morgan J.), pp. 205–224. Norwood, MA: Artech House. [Google Scholar]
- 23.Brown TD, Dalton PD, Hutmacher DW. 2011. Direct writing by way of melt electrospinning. Adv. Mater. 23, 5651–5657. ( 10.1002/adma.201103482) [DOI] [PubMed] [Google Scholar]
- 24.Dalton PD, Vaquette C, Farrugia BL, Dargaville TR, Brown TD, Hutmacher DW. 2013. Electrospinning and additive manufacturing: converging technologies. Biomater. Sci. 1, 171–185. ( 10.1039/c2bm00039c) [DOI] [PubMed] [Google Scholar]
- 25.Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. 2012. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 33, 5560–5573. ( 10.1016/j.biomaterials.2012.04.038) [DOI] [PubMed] [Google Scholar]
- 26.Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW. 2014. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 41, 283–294. ( 10.1111/jcpe.12214) [DOI] [PubMed] [Google Scholar]
- 27.Vaquette C, Ivanovski S, Hamlet SM, Hutmacher DW. 2013. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 34, 5538–5551. ( 10.1016/j.biomaterials.2013.03.088) [DOI] [PubMed] [Google Scholar]
- 28.Enobakhare BO, Bader DL, Lee DA. 1996. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal. Biochem. 243, 189–191. ( 10.1006/abio.1996.0502) [DOI] [PubMed] [Google Scholar]
- 29.Benders KE, Malda J, Saris DBF, Dhert WJA, Steck R, Hutmacher DW, Klein TJ. 2010. Formalin fixation affects equilibrium partitioning of an ionic contrast agent-microcomputed tomography (EPIC-muCT) imaging of osteochondral samples. Osteoarthritis Cartilage 18, 1586–1591. ( 10.1016/j.joca.2010.10.005) [DOI] [PubMed] [Google Scholar]
- 30.Feng JQ, et al. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315. ( 10.1038/ng1905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Prestwich GD. 2010. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer 116, 1739–1750. ( 10.1002/cncr.24907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim B-S. 2006. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 27, 1399–1409. ( 10.1016/j.biomaterials.2005.08.016) [DOI] [PubMed] [Google Scholar]
- 33.Malda J, Woodfield TBF, van der Vloodt F, Wilson C, Martens DE, Tramper J, van Blitterswijk CA, Riesle J. 2005. The effect of PEGT/PBT scaffold architecture on the composition of tissue engineered cartilage. Biomaterials 26, 63–72. ( 10.1016/j.biomaterials.2004.02.046) [DOI] [PubMed] [Google Scholar]
- 34.Tam HK, Srivastava A, Colwell CW, D'Lima DD. 2007. In vitro model of full-thickness cartilage defect healing. J. Orthop. Res. 25, 1136–1144. ( 10.1002/jor.20428) [DOI] [PubMed] [Google Scholar]
- 35.Augst A, et al. 2008. Effects of chondrogenic and osteogenic regulatory factors on composite constructs grown using human mesenchymal stem cells, silk scaffolds and bioreactors. J. R. Soc. Interface 5, 929–939. ( 10.1098/rsif.2007.1302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein TJ, Malda J, Sah RL, Hutmacher DW. 2009. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. B 15, 143–157. ( 10.1089/ten.teb.2008.0563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung CT, Mauck RL, Wang CCB, Lima EG, Ateshian GA. 2004. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann. Biomed. Eng. 32, 35–49. ( 10.1023/B:ABME.0000007789.99565.42) [DOI] [PubMed] [Google Scholar]
- 38.Darling EM, Athanasiou KA. 2005. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432. ( 10.1016/j.orthres.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 39.Alini M, Matsui Y, Dodge GR, Poole AR. 1992. The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif. Tissue int. 50, 327–335. ( 10.1007/BF00301630) [DOI] [PubMed] [Google Scholar]
- 40.Ea HK, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, Daudon M, Lioté F. 2011. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 63, 10–18. ( 10.1002/art.27761) [DOI] [PubMed] [Google Scholar]
- 41.Geesink RG, Hoefnagels NH, Bulstra SK. 1999. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J. Bone Joint Surg. Br. 81, 710–718. ( 10.1302/0301-620X.81B4.9311) [DOI] [PubMed] [Google Scholar]
- 42.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. 2006. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 54, 3254–3266. ( 10.1002/art.22136) [DOI] [PubMed] [Google Scholar]
- 43.Dell'Accio F, De Bari C, Luyten FP. 2001. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 44, 1608–1619. () [DOI] [PubMed] [Google Scholar]
- 44.Patel VV, Zhao L, Wong P, Pradhan BB, Bae HW, Kanim L, Delamarter RB. 2006. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J. 6, 397–403. ( 10.1016/j.spinee.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 45.Brennan M. 1991. Fibrin glue. Blood Rev. 5, 240–244. ( 10.1016/0268-960X(91)90015-5) [DOI] [PubMed] [Google Scholar]
- 46.Kimelman-Bleich N, et al. 2009. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials 30, 4639–4648. ( 10.1016/j.biomaterials.2009.05.027) [DOI] [PubMed] [Google Scholar]
- 47.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. 2009. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30, 2816–2825. ( 10.1016/j.biomaterials.2009.01.031) [DOI] [PubMed] [Google Scholar]
- 48.Cao Z, Hou S, Sun D, Wang X, Tang J. 2012. Osteochondral regeneration by a bilayered construct in a cell-free or cell-based approach. Biotechnol. Lett. 34, 1151–1157. ( 10.1007/s10529-012-0884-9) [DOI] [PubMed] [Google Scholar]
- 49.Sah RL, Kim Y-J, Doong J-YH, Grodzinsky AJ, Plass AHK, Sandy JD. 1989. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 7, 619–636. ( 10.1002/jor.1100070502) [DOI] [PubMed] [Google Scholar]
- 50.Vanderploeg EJ, Wilson CG, Levenston ME. 2008. Articular chondrocytes derived from distinct tissue zones differentially respond to in vitro oscillatory tensile loading. Osteoarthritis Cartilage 16, 1228–1236. ( 10.1016/j.joca.2008.02.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. 1993. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch. Biochem. Biophys. 300, 458–465. ( 10.1006/abbi.1993.1062) [DOI] [PubMed] [Google Scholar]
- 52.Kadow-Romacker A, Hoffmann JE, Duda G, Wildemann B, Schmidmaier G. 2009. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs 190, 61–68. ( 10.1159/000178022) [DOI] [PubMed] [Google Scholar]
- 53.Palmoski M, Perricone E, Brandt KD. 1979. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 22, 508–517. ( 10.1002/art.1780220511) [DOI] [PubMed] [Google Scholar]