Abstract
The role that atrial pacing therapy plays on the atrial fibrillation (AF) burden is still unclear. Aim of the study was to evaluate the effect of the atrial preference pacing algorithm on AF burden in patients affected by Myotonic Dystrophy type 1 (DM1) followed for a long follow up period. Sixty DM1 patients were -implanted with a dual chamber pacemaker (PM) for first degree or symptomatic type 1/type 2 second degree atrio-ventricular blocks- were followed for 2-years after implantation, by periodical examination. After 1 month of stabilization, they were randomized into two groups: 1) Patients implanted with conventional dual-chamber pacing mode (DDDR group) and 2) Patients implanted with DDDR plus Atrial Preference Pacing (APP) algorithm (APP ON group).
The results showed that atrial tachycardia (AT)/AF burden was significantly reduced at 1 year follow up in the APP ON group (2122 ± 428 minutes vs 4127 ± 388 minutes, P = 0.03), with a further reduction at the end of the 2 year follow up period (4652 ± 348 minutes vs 7564 ± 638 minutes, P = 0.005).
The data here reported show that the APP is an efficient algorithm to reduce AT/AF burden in DM1 patients implanted with dual chamber pacemaker.
Key words: Atrial overdrive algorithm, atrial preference pacing, supraventricular tachyarrhythmias, Myotonic Dystrophy type 1
Introduction
Myotonic dystrophy type 1 (DM1), or Steinert's disease, is a multisystem disorder with autosomal dominant inheritance. It is caused by an unstable expansion of the cytosine thymine-guanine (CTG) trinucleotide repeat located on the 3'UTR of chromosome 19q13.3. and'DMPK encoding a serine-threonine protein kinase (DMPK). The DM1 has an incidence of 1/8000 births and is characterized by highly variable clinical manifestation (1-3). Cardiac involvement is noticed in about 80% of cases and often precedes skeletal muscle manifestation. Heart block is the first and most clinically significant cardiac disease in this group of patients, likely related to fibrosis of the conduction system and fatty infiltration of the His bundle (4). To prevent cardiac sudden death, implantation of a pacemaker (PM) is required in 3-22% of cases (5-8). Modern PMs that include detailed diagnostic functions may facilitate the diagnosis and management of frequent paroxysmal atrial tachy-arrhythmias often undetected during conventional clinical follow-up (9). Paroxysmal atrial arrhythmias (atrial fibrillation, atrial flutter, atrial tachycardia) frequently occur in DM1 patients (10, 11). We have previously shown that the Atrial Preference Pacing (APP) is an efficient algorithm to prevent paroxysmal AF in DM1 patients implanted with dual chamber pacemaker (12, 13). However, the role that atrial pacing therapies play on the AF burden is still unclear. Aim of our study was to evaluate the effect of APP on atrial fibrillation burden in these patients during a long term follow up period.
Patients and methods
Patients selection
Among 278 DM1 patients, regularly followed at the Cardiomyology and Medical Genetics of Second Naples University, 60 patients with first or second degree atrioventricular block and indication for a permanent dual chamber cardiac pacing, were consecutively enrolled and addressed to our Unity to be implanted. The diagnosis of Steinert disease, firstly based on family history and clinical evaluation, had been subsequently confirmed by genetic test in all patients, to evaluate the CTG triplet expansion.
Six DM1 patients with patent foramen ovale, atrial septal aneurysm, severe mitral stenosis or regurgitation, left atrial enlargement, paroxysmal atrial fibrillation, sick sinus syndrome or inducible ventricular tachycardia were excluded from the study.
The study was conducted according to the declaration of Helsinki. A written informed consent was obtained from the patients before implantation, as approved by the Monaldi hospital ethical committee.
Study protocol
DM1 eligible patients were randomized one month following pacemaker implantation into two groups: 1). Patients implanted with conventional dual-chamber pacing mode (DDDR group) and 2): Patients implanted with DDDR plus APP algorithm (APP ON group). Patients were assessed every 3 months for the first year, and every 6 months thereafter up to 2 years. Atrial Tachycardia/Atrial Fibrillation (AT/AF) burden – defined as the quantity of AT/AF (minutes/day) retrieved from the device data logs – was determined at each follow-up visit. The baseline AT/ AF burden was measured just prior the randomization. Patients interrupted the follow-up, before completing the 2 years, in the case of severely symptomatic AT/AF requiring major changes in therapy.
Pacemaker programming
All DM1 patients were implanted with a dual-chamber PM system (Medtronic Adapta ADDR01, Medtronic Inc., Minneapolis, MN, USA). The right ventricular lead (Medtronic 4074 CapSure Sense) was positioned in the apex, under fluoroscopic guidance; the bipolar atrial screw-in lead (Medtronic 5076 CapSureFix) was positioned in the right atrial appendage or on the right side of the inter-atrial septum in the region of Bachmann's bundle, according to optimal site, defined as the location with lowest pacing and highest sensing thresholds. To minimize confounding variables with different electrode materials and inter-electrode spacing, an identical model lead was used in all patients. Similarly, PMs with identical behaviour and telemetric capabilities were used to assure accuracy in comparing measurements among patients. To minimize atrial lead oversensing, the sensitivity configuration was bipolar. All devices were programmed in DDDR mode with a lower rate of 60 bpm and an upper rate of 130 bpm. Mode switches were programmed for atrial rates > 200 bpm, persisting for more than 12 ventricular beats. Managed Ventricular Pacing algorithm (MVP, Medtronic Inc., Minneapolis, MN, USA) was enable in order to promote the intrinsic conduction and reduce the possible influence of high percentage ventricular pacing on atrial fibrillation incidence. Atrial Preference Pacing (APP, Medtronic Inc., Minneapolis, MN, USA) was enable according to the prospective programming compliance criteria. The devices used in this study were programmed to detect the episodes of atrial tachycardia, and to record summary and detailed data, atrial and ventricular electrograms (EGMs) included.
Study endpoints and data analysis
The primary efficacy endpoint was identified as the effect of pacing therapies on AT/AF burden over time. Permanent AT/AF was defined as an AT/AF burden of 24 h/day for at least 6 months.
Statistical analysis was performed using Student's ttest for paired data. Continuous variables are presented as mean ± SD. P values < 0.05 were considered to be statistically significant. Analyses were performed using the statistical package SPSS 11.0 software for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patients population
The study group included sixty DM1 patients (mean age 53,2 ± 8,5; 43 M;17 F) who underwent dual chamber pacemaker implantation for the following indications: 1. first degree atrioventricular block with a pathological infra- Hissian conduction (25 patients); symptomatic type 1 (19 patients) and type 2 (16 patients) second degree block. The study population was randomized and treated according to the study protocol. The mean period of FU was 24 ± 6 months. Six DM1 patients were censored at 11 ± 2 months before the study completion due to far-field ventricular sensing despite refractory periods reprogramming (2 cases), atrial undersensing (2 cases) or persistent AF during follow-up (2 cases).
Table 1 shows that the baseline characteristics of the 2 groups of study population were not significantly different. Out of 4 DM1 patients who needed a major change in therapy, 3 were in the DDD/R mode group, and 1 in the APP ON mode group. Amiodarone was initiated in one patient and electrical cardioversion performed in 3 patients.
Table 1.
Clinical and echocardiographic parameters of the study groups.
| DDDR Group | APP ON Group | P | |
|---|---|---|---|
| Patients (n) | 27 | 27 | n.s. |
| Age (years) | 51 ± 8 | 53 ± 6 | n.s. |
| Sex (M:F) | 19:8 | 20:7 | n.s. |
| First degree AV block | 12 | 13 | n.s. |
| Type 1 second degree AV block | 9 | 10 | n.s. |
| Type 2 second degree AV block | 6 | 4 | n.s. |
| QRS duration (ms) | 95 ± 15 | 93 ± 16 | n.s. |
| LVEDD (mm) | 43,2 ± 3,2 | 44,3 ± 3,6 | n.s. |
| LVESD (mm) | 26,2 ± 2,8 | 25,5 ± 3,1 | n.s. |
| IVSDD (mm) | 6,3 ± 1,3 | 6,9 ± 1,1 | n.s. |
| LVWDD (mm) | 6,9 ± 1,7 | 7,2 ± 0,9 | n.s. |
| LAD (cm) | 3,5 ± 0,4 | 3,3 ± 0,5 | n.s. |
| Ejection fraction (%) | 55 ± 4 | 54 ± 3 | n.s. |
Atrial pacing and atrial fibrillation burden
Table 2 shows the device reported data during the follow-up period. At baseline, before randomization, the AF/AT burden of the entire population was 7875 ± 532 minutes. At 1 year follow-up, the AF/AT burden was significantly decreased in the APP ON group (2122 ± 428 minutes vs 4127 ± 388 minutes, p = 0,03) with a further decrease at the end of the 2 year follow-up (4652 ± 348 minutes vs 7564 ± 638 minutes, p = 0,005) (Fig. 1) At the end of the follow-up, the number of AF episodes in APP ON group was lower than those registered during no treatment (126 ± 14 vs 293 ± 46,p = 0,03). Furthermore, the atrial premature beats count was significantly greater in DDDR group than in APP ON group (46689 ± 13534 vs 14717 ± 8806 beats, p = 0,004). However no statistically significant difference was observed in the mean duration of AF episodes between the two groups (84 ± 21 vs 78 ± 19 minutes, p = 0,4) (Fig. 2). In the DDDR group the atrial and ventricular pacing percentages were 28% and 17% respectively; in APP On group 98% and 14%. No difference in the percentage of ventricular pacing percentage between the two groups was also observed (14 vs 17%, p = 0,2).
Table 2.
Device reported data during the follow-up period.
| APP ON Group | DDDR Group | P | |
|---|---|---|---|
| Atrial Pacing (%) | 98 | 28 | 0,003 |
| Ventricular Pacing (%) | 14 | 17 | 0,2 |
| Total AT/AF burden at 1 year follow-up (min) | 2122 ± 428 | 4127 ± 388 | 0,03 |
| Total AT/AF burden at 2 years follow-up (min) | 4652 ± 348 | 7564 ± 638 | 0,005 |
| AF episodes (n) | 126 ± 14 | 293 ± 46 | 0,03 |
| AF mean duration (min) | 84 ± 21 | 78 ± 19 | 0,4 |
| Atrial premature beats (n) | 14717 ± 8806 | 46689 ± 13534 | 0,004 |
Figure 1.
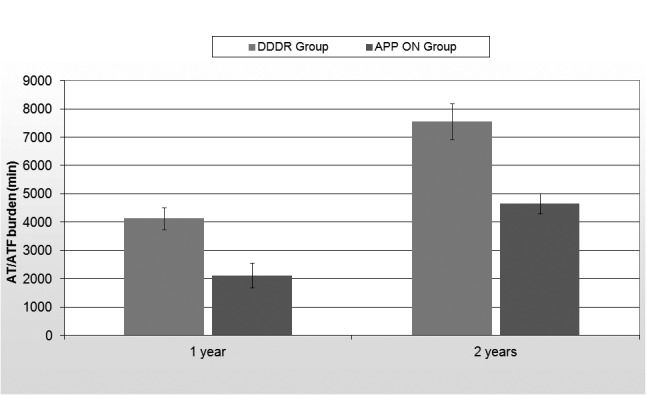
Total AT/AF burden during the follow-up period.
Figure 2.
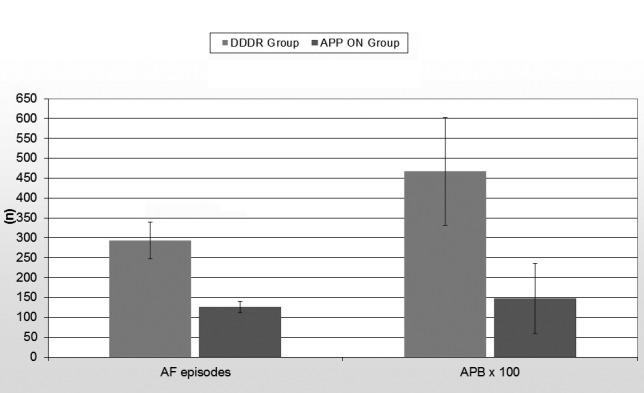
Total atrial fibrillation episodes and atrial premature beats at the end of the follow-up period.
Discussion
The most frequent clinical event in DM1 patients is the development of a supraventricular arrhythmia, commonly found on 12 lead ECG or 24 hour Holter monitoring and often asymptomatic (14, 15). The most common arrhythmias are atrial fibrillation, atrial flutter (AFL) and atrial tachycardia, observed in up to 30% of patients both as un-sustained and sustained forms. Compared to other conditions (16-24) or cardiomyopathies (25-34), little is still known about electrocardiographic predictors of ventricular and supraventricular tachyarrhythmias in DM1 patients. AF/AFL are easily inducible by electrophysiological study even in the absence of previously documented spontaneous episodes, however the clinical implications of these findings remain uncertain.
According to Brembilla-Perrot et al. (35), atrial fibrillation is associated with a significant risk of death in association with the age of DM1 patients. The anatomo-pathological substrate of myotonic dystrophy, characterized by progressive selective fibrosis and scar replacement of myocardial tissue, not only limited to the specialized conduction system, may facilitate the onset and the perpetuation of atrial fibrillation in these patients. We have previously shown that a) AF episodes increase in DM1 patients with a high percentage of right ventricular pacing and a lower percentage of atrial stimulation (36); b) right atrial septal stimulation in the Bachmann's bundle region is a safe and feasible procedure (37), with less atrial pacing and sensing defects than the right atrial appendage stimulation (38), though it does not seem to provide significant benefits for prevention of paroxysmal atrial fibrillation (39). Atrial pacing may prevent the onset of atrial fibrillation through the following mechanisms: a) prevention of the relative bradycardia that triggers paroxysmal AF; b) prevention of the bradycardia-induced dispersion of refractoriness; c) suppression or reduction of premature atrial contractions that initiate the re-entry and predispose to AF; and d) preservation of atrio-ventricular synchrony, which may prevent switch-induced changes in atrial repolarization, predisposing to AF (40, 41). APP algorithm adapts the atrial pacing rate to a value slightly higher than the intrinsic sinus rate; this can result in suppression and/or prevention of atrial ectopy favorably modifying the arrhythmogenic substrate.
Previous studies, though based on short term follow- up data, have shown that APP is an efficacy algorithm for preventing paroxysmal AF in DM1 patients implanted with dual-chamber PM for atrioventricular conduction disorders (12, 13). Our study further shows that APP may significantly reduce the atrial fibrillation burden in DM1 patients, regardless of the site of atrial stimulation (Backmann's bundle or right atrial appendage). This effect can be explained by the high percentage of atrial pacing, warranted by atrial overdrive algorithm, that may prevent the relative bradycardia and reduce the number of premature atrial contractions, causing the reentry and predisposing to AF. However a more extensive study, including a greater number of patients will confirm these preliminary data.
Conclusions
Heart blocks and supraventricular arrhythmias are an integral part of the clinical picture of myotonic dystrophies. Implantation of a pacemaker (PM) is the only possibility to prevent cardiac sudden death, while atrial pacing seems to prevent the onset of atrial fibrillation.
Therefore we recommend that all patients with Myotonic Dystrophy type 1, once molecularly diagnosed, are carefully monitored for the development of conduction defects and arrhythmias, even in the absence of cardiac signs/symptoms and in the early stages of the disease.
Furthermore we suggest to implant APP as it significantly reduces the AT/AF burden over a long-term follow-up in DM1 patients implanted with dual chamber pacemaker, compared with those implanted with conventional DDD/R pacing alone.
Acknowledgements
The work was in part supported by Telethon grants (GUP07013A and GTB12001H) to LP. DNA samples from patients with DM1 derive from the NHMGB bank, that is partner of Eurobiobank and Telethon Network of Genetic Biobanks.
References
- 1.Reardon W, Harper PS. Advances in myotonic dystrophy: a clinical and genetic perspective. Curr Opin Neurol Neurosurg. 1992;5:605–609. [PubMed] [Google Scholar]
- 2.Phillips MF, Harper PS. Cardiac disease in myotonic dystrophy. Cardiovasc Res. 1997;33:13–22. doi: 10.1016/s0008-6363(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 3.Hawley RJ, Gottdiener JS, Gay JA, et al. Families with myotonic dystrophy with and without cardiac involvement. Arch Intern Med. 1983;143:2134–2136. [PubMed] [Google Scholar]
- 4.Nguyen HH, Wolfe JT, III, Holmes DR, Jr, et al. Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J Am Coll Cardiol. 1988;11:662–671. doi: 10.1016/0735-1097(88)91547-1. [DOI] [PubMed] [Google Scholar]
- 5.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices. Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 6.Russo V, Rago A, Antonio Papa A, et al. Cardiac resynchronization improves heart failure in a patient with myotonic dystrophy type 1. A case report. Acta Myol. 2012;31:154–155. [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro G, Papa AA, Politano L. The heart and cardiac pacing in Steinert disease. Acta Myol. 2012;31:110–116. [PMC free article] [PubMed] [Google Scholar]
- 8.Nigro G, Politano L, Passamano L, et al. Cardiac treatment in neuro- muscular diseases. Acta Myol. 2006;25:119–123. [PubMed] [Google Scholar]
- 9.Lazarus A, Varin J, Babuty D, et al. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: a multicenter diagnostic pacemaker study. JACC. 2002;40:1645–1652. doi: 10.1016/s0735-1097(02)02339-2. [DOI] [PubMed] [Google Scholar]
- 10.Russo AD, Mangiola F, Della Bella P, et al. Risk of arrhythmias in myotonic dystrophy: trial design of the RAMYD study. J Cardiovasc Med (Hagerstown) 2009;10:51–58. doi: 10.2459/jcm.0b013e328319bd2c. [DOI] [PubMed] [Google Scholar]
- 11.Cudia P, Bernasconi P, Chiodelli R, et al. Risk of arrhythmia in type I myotonic dystrophy: the role of clinical and genetic variables. J Neurol Neurosurg Psychiatry. 2009;80:790–793. doi: 10.1136/jnnp.2008.162594. [DOI] [PubMed] [Google Scholar]
- 12.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a prospective, randomized, single-bind cross over study. Europace. 2012;14:486–489. doi: 10.1093/europace/eur373. [DOI] [PubMed] [Google Scholar]
- 13.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a long term follow-up study. Acta Myol. 2012;31:139–143. [PMC free article] [PubMed] [Google Scholar]
- 14.Politano L, Palladino A, Nigro G, et al. Usefulness of heart rate variability as a predictor of sudden cardiac death in muscular dystrophies. Acta Myol. 2008;27:114–122. [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro G, Comi LI, Nigro G, et al. Cardiomyopathies: diagnosis of types and stages. Acta Myol. 2004;23:97–102. [PubMed] [Google Scholar]
- 16.Santangelo L, Ammendola E, Russo V, et al. Influence of biventricular pacing on myocardial dispersion of repolarization in dilated cardiomyopathy patients. Europace. 2006;8:502–505. doi: 10.1093/europace/eul054. [DOI] [PubMed] [Google Scholar]
- 17.Santangelo L, Ammendola E, Russo V, et al. Relationship between transmural dispersion of repolarization, Tpeak-Tend interval and ventricular arrhythmias: Reply. Europace. 2007;9:61–61. [Google Scholar]
- 18.Russo V, Ammendola E, Crescenzo I, et al. Effect of weight loss following bariatric surgery on myocardial dispersion of repolarization in morbidly obese patients. Obes Surg. 2007;17:857–865. doi: 10.1007/s11695-007-9160-9. [DOI] [PubMed] [Google Scholar]
- 19.Russo V, Ammendola E, Crescenzo I, et al. Severe obesity and P-wave dispersion: the effect of surgically induced weight loss. Obes Surg. 2008;18:90–96. doi: 10.1007/s11695-007-9340-7. [DOI] [PubMed] [Google Scholar]
- 20.Nigro G, Russo V, Chiara A, et al. Autonomic nervous system modulation before the onset of sustained atrio-ventricular nodal re-entry tachycardia. Ann Noninvasive Electrocardiol. 2010;15:49–55. doi: 10.1111/j.1542-474X.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigro G, Russo V, Salvo G, et al. Increased heterogeneity of ventricular repolarisation in obese non hypertensive children. PACE. 2010;33:1533–1539. doi: 10.1111/j.1540-8159.2010.02889.x. [DOI] [PubMed] [Google Scholar]
- 22.Russo V, Rago A, Pannone B, et al. Dispersion of repolarisation and beta thalassemia major: the prognostic role of QT and JT dispersion for identifying the high risk patients for sudden death. Eur J Haematol. 2011;86:324–331. doi: 10.1111/j.1600-0609.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 23.Russo V, Rago A, Pannone B, et al. Early electrocardiographic evaluation of atrial fibrillation risk in beta thalassemia major patients. Int J Hematol. 2011;93:446–451. doi: 10.1007/s12185-011-0801-3. [DOI] [PubMed] [Google Scholar]
- 24.Nigro G, Russo V, Rago A, et al. Heterogeneity of ventricular repolarisation in newborns with severe aortic coarctation. Pediatr Cardiol. 2012;33:302–306. doi: 10.1007/s00246-011-0132-4. [DOI] [PubMed] [Google Scholar]
- 25.Russo V, Rago A, Politano L, et al. Increased dispersion of ventricular repolarisation in Emery-Dreifuss muscular dystrophy patients. Med Sci Monit. 2012;18:CR643–CR647. doi: 10.12659/MSM.883541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo V, Rago A, D'Andrea A, et al. Early onset "electrical" heart failure in Myotonic Dystrophy type 1 patients: the role of ICD biventricular pacing. Anadolu Kardiyol Derg. 2012;12:517–519. doi: 10.5152/akd.2012.161. [DOI] [PubMed] [Google Scholar]
- 27.Ammendola E, Russo V, Politano L, et al. Is heart rate variability (HRV) a valid parameter to predict sudden death in Becker muscular dystrophy patients? Heart. 2006;92:1686–1687. doi: 10.1136/hrt.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigro G, Russo V, Rago A, et al. Regional and transmural dispersion of repolarisation in patients with Emery-Dreifuss muscular dystrophy. Kardiol Pol. 2012;70:1154–1159. [PubMed] [Google Scholar]
- 29.Nigro G, Russo V, Ventriglia VM, et al. Early onset of cardiomyopathy and primary prevention of sudden death in X-linked Emery Dreifuss muscular dystrophy. Neuromusc Disord. 2010;20:174–177. doi: 10.1016/j.nmd.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Russo V, Rago A, Palladino A, et al. P wave duration and dispersion in Emery-Dreifuss Muscular Dystrophy. J Invest Med. 2011;59:1151–1154. doi: 10.2310/JIM.0b013e31822cf97a. [DOI] [PubMed] [Google Scholar]
- 31.Lancioni A, Rotundo IL, Kobayashi YM, et al. Combined deficiency of alpha and epsilon sarcoglycan disrupts the cardiac dystrophin complex. Hum Mol Genet. 2011;20:4644–4654. doi: 10.1093/hmg/ddr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotundo IL, Faraso S, Leonibus E, et al. Worsening of cardiomyopathy using deflazacort in an animal model rescued by gene therapy. PLoS One. 2011;6:e24729–e24729. doi: 10.1371/journal.pone.0024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belfiore MP, Berritto D, Iacobellis F, et al. A longitudinal study on BIO14.6 hamsters with dilated cardiomyopathy: micro-echocardiographic evaluation. Cardiovasc Ultrasound. 2011;9:39–39. doi: 10.1186/1476-7120-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitiello C, Faraso S, Sorrentino NC, et al. Disease rescue and increased lifespan in a model of cardiomyopathy and muscular dystrophy by combined AAV treatments. PLoS One. 2009;4:e5051–e5051. doi: 10.1371/journal.pone.0005051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brembilla-Perrot B, Schwartz J, Huttin O, et al. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in Myotonic Dystrophy. Pacing Clin Electrophysiol. 2014;37:329–335. doi: 10.1111/pace.12260. [DOI] [PubMed] [Google Scholar]
- 36.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in Myotonic Dystrophy type 1 patients? Kardiol Pol. 2013;71:1147–1153. doi: 10.5603/KP.2013.0295. [DOI] [PubMed] [Google Scholar]
- 37.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in myotonic dystrophy patients. The role of Bachmann's Bundle stimulation. PACE. 2008;31:1463–1466. doi: 10.1111/j.1540-8159.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 38.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann’s bundle stimulation: a two year comparative study of electrical parameters in Myotonic Dystrophy type 1 patients. PACE. 2009;32:1192–1197. doi: 10.1111/j.1540-8159.2009.02464.x. [DOI] [PubMed] [Google Scholar]
- 39.Nigro G, Russo V, Politano L, et al. Does Bachmann's bundle pacing prevent atrial fibrillation in myotonic dystrophy type 1 patients? A 12 months follow-up study. Europace. 2010;12:1219–1223. doi: 10.1093/europace/euq170. [DOI] [PubMed] [Google Scholar]
- 40.Mehra R, Hill MRS. Saksena S, Luderlitz B. Interventional Electrophysiology: a Textbook. 2nd Edition. Armonk, NY: Futura Publishing; 1996. Prevention of atrial fibrillation/flutter by pacing techniques; pp. 521–540. [Google Scholar]
- 41.Carlson M, Ip J, Messenger J, et al. A new pacemaker algorithm for the treatment of atrial fibrillation. Results of the Atrial Dynamic Overdrive Pacing Trial (ADOPT) JACC. 2003;42:627–633. doi: 10.1016/s0735-1097(03)00780-0. [DOI] [PubMed] [Google Scholar]


