Abstract
The Staphylococcus aureus lrg and cid loci are homologous operons that have been shown to regulate murein hydrolase activity and affect sensitivity to penicillin. Although the mode of action of these operons has not been demonstrated, a model based on the similarities of the lrgA and cidA gene products to the bacteriophage holin family of proteins has been proposed. In this study, the transcription organization and regulation of these operons were examined by Northern blot analyses. Unexpectedly, cidB and a gene located immediately downstream, designated cidC, were found to be cotranscribed on a 2.7-kb transcript. Maximal cidBC transcription occurred during early exponential growth, and high-level transcription of cidBC was dependent on the rsbU-mediated activation of the alternative sigma factor B (σB). In contrast, lrgAB transcription in stationary phase was negatively regulated by σB. Although cidABC transcription was not detected by Northern blot analysis, reverse transcriptase PCR revealed that these genes are also cotranscribed as a single RNA message in early exponential growth. Primer extension analysis revealed the presence of two cidBC transcription start sites, but no apparent σB-dependent promoter consensus sequence was identified in these regions. The rsbU gene was also shown to have a positive impact on murein hydrolase activity but a negligible effect on sensitivity to penicillin-induced killing. These results suggest that the lrgAB and cidBC genes may be part of the S. aureus σB-controlled stress regulon.
Staphylococcus aureus is a pernicious pathogen capable of infecting nearly every tissue and organ system in the human body. Strains resistant to multiple classes of antibiotics have emerged in the past several decades, and the first case of infection caused by vancomycin-resistant S. aureus was recently documented (6). Vancomycin has been long considered the last line of defense against infection by multidrug-resistant S. aureus. Therefore, we have entered an era in which it has become critical to discover novel targets to which therapeutic strategies can be developed. One avenue of research has been to determine the exact sequence of cellular events leading to bacterial lysis and killing by antibiotic agents such as penicillin. In this respect, our laboratory has focused on achieving a better understanding of cell wall physiology and the regulation of murein hydrolase activity in S. aureus. Although bacterial murein hydrolases are involved in many important processes during cell growth and development, the activity of these enzymes can also be detrimental to the cell in certain situations. For example, these enzymes have been shown to be responsible for the cellular autolysis that often occurs following exposure of bacteria to antibiotics such as penicillin (22, 29). This observation suggests that penicillin has evolved to disrupt the regulatory mechanism(s) that normally keeps murein hydrolase activity in check.
Work in our laboratory has recently identified and characterized the cid and lrg operons of S. aureus, whose gene products regulate murein hydrolase activity and tolerance to penicillin in a diametrically opposing manner (20, 42). An S. aureus lrgAB mutant displayed increased extracellular murein hydrolase activity and increased sensitivity to penicillin (20), whereas a cidA mutant displayed decreased murein hydrolase activity and decreased penicillin sensitivity (42). We have also shown that the cidA and lrgA gene products share structural similarities with the bacteriophage holin family of proteins, which control the timing and onset of host cell lysis (42, 48). Based on these findings, we have proposed that the members of the lrg and cid operons function in a manner similar to inhibitor and effector holins, respectively, to control murein hydrolase activity and antibiotic-induced killing (20, 41, 42). Although the precise mechanism by which the Lrg and Cid proteins regulate these two processes is currently unknown, it has been suggested that they may be components of a bacterial programmed cell death mechanism (1, 41) that functions to remove damaged cells from a bacterial population in response to a wide variety of bactericidal agents.
Recently, a number of studies have suggested that high-passage S. aureus laboratory strains display several phenotypic differences relative to low-passage clinical isolates. Specifically, S. aureus laboratory strains have been shown to display altered expression of the agr and sarA virulence gene regulators (4, 45), changes in virulence factor production (45), and decreased aconitase activity (45) relative to clinical isolates. Many of the traditionally studied S. aureus laboratory isolates, such as 8325-4 and RN6390, also contain a naturally occurring 11-bp deletion in rsbU, whose gene product regulates the activity of alternative sigma factor B (σB), and hence these strains contain a defect in the σB-mediated stress response (26). Therefore, we analyzed transcription of the cid and lrg operons in both the laboratory strain RN6390 as well as the clinical isolate UAMS-1. These experiments revealed that the cid operon is comprised of at least two overlapping transcripts, and the gene that lies immediately downstream of cidB (which we have designated cidC) comprises a third gene in this operon. Furthermore, transcription of cidBC was dramatically increased in UAMS-1 relative to that in RN6390, and this difference was shown to be likely due to the presence of an intact rsbU gene in UAMS-1. Finally, extracellular murein hydrolase activity was increased in the rsbU+ background of 8325-4, consistent with the effect that σB has on cid and lrg transcription.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. All S. aureus strains were grown in either tryptic soy broth (Difco Laboratories, Detroit, Mich.) or NZY broth (3% [wt/vol] N-Z Amine A [Sigma Chemical Co., St. Louis, Mo.], 1% [wt/vol] yeast extract [Fisher Scientific, Fair Lawn, N.J.]; pH 7.5), supplemented as necessary with 1.5% (wt/vol) granulated agar (Difco). All cultures were grown in Erlenmeyer flasks at 37°C with shaking at 250 rpm. All antibiotics were purchased from either Sigma Chemical Co. or Fisher Scientific and were used at the following concentrations: erythromycin (2 μg · ml−1), tetracycline (5 μg · ml−1).
TABLE 1.
Strains used in this study
| Strain | Descriptiona | Reference or source |
|---|---|---|
| S. aureus | ||
| RN6390 | Derivative of NCTC8325 that maintains its hemolytic pattern when grown on sheep blood agar, 11-bp deletion in rsbU | 33 |
| UAMS-1 | Clinical osteomyelitis isolate, rsbU+ | 17 |
| 8325-4 | NCTC8325, cured of prophages, 11-bp deletion in rsbU | 31 |
| GP269 | 8325-4 (rsbUVW sigB)+-tetL, Tcr | 16 |
| SH1000 | Functional rsbU derivative of 8325-4, rsbU+ | 25 |
| Newman | Clinical isolate (ATCC 25904), rsbU+ | 10 |
| IK184 | Newman ΔrsbUVW sigB, Emr | 26 |
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacqZΔM15 Tn10 (Tcr)] | Stratagene |
Abbreviations: Tcr, tetracycline resistant; Emr, erythromycin resistant.
Isolation of RNA.
For Northern blotting, overnight cultures of S. aureus strains were used to inoculate 200 ml of NZY in a 1-liter flask to an optical density at 600 nm (OD600) of 0.1 and grown for 12 h. Cells were harvested for RNA isolation at early exponential growth phase (2 h), late stationary phase (6 h), and stationary phase (12 h), and corresponding OD600 readings were taken to monitor growth. For reverse transcriptase PCR (RT-PCR) experiments, an overnight culture of S. aureus RN6390 was used to inoculate 400 ml of NZY broth in a 2-liter flask to an OD600 of 0.1. The culture was incubated for 12 h at 37°C with shaking at 250 rpm, and growth was monitored by measuring the OD600 at regular intervals. Total RNA was isolated using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, Calif.) and the FASTPREP FP120 instrument (BIO 101, Vista, Calif.) as previously described (9, 40).
Northern blot analysis.
RNA samples (10 μg) were separated by electrophoresis through a 1% (wt/vol) agarose gel containing 0.66 M formaldehyde and morpholinepropanesulfonic acid (MOPS) running buffer (20 mM MOPS, 10 mM sodium acetate, 2 mM EDTA; pH 7.0). The RNA samples were subsequently transferred to nylon membrane (Micron Separations Inc., Westboro, Mass.) by overnight capillary transfer in 20× SSC (0.3 M Na3-citrate, 3.0 M NaCl; pH 7.0) and fixed to the membrane by baking at 80°C for at least 2 h. Hybridization of the blots with digoxigenin (DIG)-labeled DNA probes and subsequent washing and detection steps were performed using buffers and reagents of the DIG system (Roche Applied Science, Indianapolis, Ind.), following the manufacturer's recommendations for Northern blot analysis. DIG-labeled DNA probes were synthesized by PCR using the reagents and cycling conditions supplied with the PCR-based DIG probe synthesis kit (Roche), using primer pairs cidB1-F-cidB1-R, cidC1-F-cidC1-R, and lrgA1-F-lrgA1-R (Table 2) to synthesize cidB-, cidC-, and lrgA-specific probes, respectively.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′)a | Location (8325 genome) or reference |
|---|---|---|
| cidB1-F | TGATTTTGTTGACTGTCGTT | 2625709-2625728 |
| cidB1-R | TCATGTGACACTTCGATACC | 2625335-2625354 |
| cidBp-F | cggatccTACAACTAGGAATCATCATTGTG | 2626088-2626110 |
| cidBp-R | cggctcgagGCTTGCACGTAATCATTCATAAGC | 2625737-2625760 |
| cidC1-F | CACCAAAATATAAAGACATCAAAAAAGCGG | 2624432-2624461 |
| cidC1-R | GCCGTtgtcgaCAATTGTGATAACCTTTCAATC | 2623252-2623278 |
| lrgA1-F | ccccatatgGTCGTGAAACAACAAAAGACGC | 254081-254103 |
| lrgA1-R | cccctcgagATCATGAGCTTGTGCCTCCTC | 254542-254562 |
| cidA1-F | AGACATATTTAGAAAGGGATCCCGCCATGCACAAAGTCC | 2626132-2626170 |
| cidB2-R | TTGTTGTAACCTTTTAGCG | 2625679-2625697 |
| gyrA-F | CGTGAAGGTGACGAAGTTGTAGG | 42 |
| gyrA-R | TAACTGGCGTACGTTTACCATAACC | 42 |
Lower case letters represent addition of a restriction site.
RT-PCR.
RT-PCRs were performed essentially as described by Rice et al. (42) with the following modifications. Briefly, cidABC cDNA was generated using RT and the reverse primer cidC1-R. The cidABC cDNA products were then detected by PCR using the primer pair cidA1-F and cidC1-R. The RT-PCR primers used for the detection of the gyrA transcripts were described previously (42). PCRs for both cidABC and gyrase were carried out in 50-μl aliquots and consisted of 2 μl of cDNA, a 0.2 μM concentration of each primer, 10× PCR buffer (Invitrogen Life Technologies), 3 mM MgCl2, 0.2 μM deoxynucleoside triphosphate, and 0.5 U of Taq polymerase (Invitrogen Life Technologies). Amplification was performed with an initial denaturation of 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 4 min, followed by a final extension step of 72°C for 4 min. The cidA1-F-cidC1-R primer pair generates a 2.9-kb amplicon, and the gyrA-specific primers amplify a 100-bp product (GenBank accession number D10489).
Primer extension analysis.
The +1 start site of cidBC transcription was mapped by primer extension, using previously described methods (42). Specifically, the reverse primer cidB2-R, complementary to the 5′ end of the cidB gene, was end labeled with [γ-32P]ATP and used in the primer extension reaction as previously described (42). One hundred micrograms of total RNA, isolated from an early exponential phase SH1000 culture, was used as template in the primer extension reaction. A DNA sequencing ladder of the cidBC promoter region was obtained with primer cidB2-R with the Sequenase kit (United States Biochemical Corporation, Cleveland, Ohio), according to the manufacturer's recommendations for using an end-labeled primer in the sequencing reaction. The plasmid pSJ9, containing 388 bp upstream of the ATG translational start site of cidB, was used as template in the sequencing reactions. Both the sequencing and primer extension products were resolved by electrophoresis through an 8% (wt/vol) denaturing polyacrylamide gel, and the bands were subsequently visualized by autoradiography.
Two-plasmid experiments.
The plasmid pAC7-sigB, containing the S. aureus sigB gene under the control of the tightly regulated arabinose-inducible promoter (21), was constructed as previously described (24). Briefly, a 770-bp DNA fragment containing the sigB gene was PCR amplified from S. aureus COL chromosomal DNA, and the primers used in this reaction (24) introduced an NdeI site into the sigB translation initiation codon and a HindIII site downstream of the stop codon. This PCR fragment was then digested with NdeI and HindIII and ligated into pAC7 (38), cut with the same enzymes, and subsequently transformed into Escherichia coli XL1Blue, resulting in pAC7-sigB. The well-characterized S. aureus sigB-dependent asp23p promoter (15) was cloned in the promoter-probe plasmid pSB40N (38) as previously described (24). Briefly, an asp23p-containing 580-bp DNA fragment was PCR amplified from S. aureus COL chromosomal DNA, and the primers used in this reaction (24) incorporated a BamHI and XhoI site at the 5′ and 3′ ends of the amplicon, respectively. This PCR fragment was then digested with BamHI and XhoI, ligated into pSB40N cut with the same enzymes, and transformed into E. coli XL1Blue, resulting in pSB40N- asp23p1. For construction of pSB40N-cidBCp, a DNA fragment representing 376 bp of the cidBC promoter region of 8325-4 was generated by PCR using primer pair cidBp-F and cidBp-R. The PCR product was digested with BamHI and XhoI and cloned into promoter probe plasmid pSB40N (38) upstream of the lacZα reporter gene to obtain pSB40N-cidBCp. Sequence analysis confirmed the identity of the insert. Plasmid pSB40N-cidBCp was transformed into E. coli XL1Blue containing either compatible plasmids pAC7-sigB or empty pAC7 (24). Clones were selected on LBACX-ARA plates (Luria-Bertani [LB] medium with 5 g of lactose · liter−1, 100 μg of ampicillin · ml−1, 40 μg of chloramphenicol · ml−1, 20 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside · ml−1, and 2 μg of arabinose · ml−1) and analyzed for color production (39).
Penicillin sensitivity assays.
Penicillin-induced killing of S. aureus strains was assessed by dilution plating as described previously (20, 42), with the following modifications: the OD600 was determined for each overnight S. aureus culture, and the culture was then diluted in 10 ml of NZY broth to an OD600 of 1.06 × 10−3 (approximately a 1:10,000 dilution). Cultures were grown to exponential growth phase (2.5 h) prior to the addition of 20 times the MIC of penicillin G (0.04 μg · ml−1).
Murein hydrolase assays.
Overnight cultures of S. aureus strains were diluted to an OD600 of 0.1 in 10 ml of NZY broth in a 125-ml Erlenmeyer flask and grown for 16 h at 37°C and 250 rpm. The culture supernatant (containing extracellular murein hydrolases) was harvested by centrifugation for 20 min and 1,900 × g and concentrated approximately sixfold in a Centricon-3 concentrator (Millipore, Bedford, Mass.). Protein concentrations of the concentrated extracellular proteins were determined using the Bradford assay (Bio-Rad Laboratories, Hercules, Calif.), according to the manufacturer's recommended protocols. Quantitative cell wall hydrolysis assays were performed as previously described (20), except that 100 μg of concentrated extracellular protein was used to lyse a suspension of 1 mg of Micrococcus luteus cell wall (Sigma) · ml−1, and the turbidity of the samples was determined by measuring the absorbance at 580 nm (A580) with a spectrophotometer. Zymographic analysis was carried out as previously described (20).
RESULTS
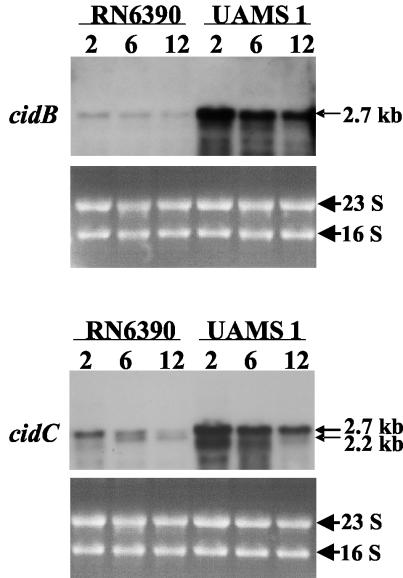
Analysis of cid operon transcription. The S. aureus cidA and cidB genes were recently identified and characterized because of their potential role as counterparts to the previously studied lrgAB operon. In contrast to the lrgAB operon, which inhibits murein hydrolase activity and penicillin-induced killing (20), the cid operon enhances these processes (42). This led to the hypothesis that these operons encode holin and antiholin proteins that function to regulate the murein hydrolase activity produced by the bacteria (20, 42). Previously, RT-PCR analysis demonstrated that the S. aureus cidA and cidB genes are cotranscribed and maximally expressed during early exponential growth (42). To examine the sizes of the transcripts produced by this operon, a Northern blot analysis was performed on RNA isolated from the laboratory strain, RN6390, as well as from the clinical isolate, UAMS-1, at early exponential (2 h), late exponential (6 h), and stationary phase (12 h) (Fig. 1). Unexpectedly, a DNA probe specific for the 5′ end of the cidB gene hybridized to a 2.7-kb transcript that was maximally expressed in the early exponential phase of growth in both RN6390 and UAMS-1 (Fig. 1). The size of this transcript was much larger than expected for cotranscription of the cidA and cidB genes, which is predicted to span 1,114 nucleotides.
FIG. 1.
Northern blot analysis of cid operon transcription in S. aureus RN6390 and UAMS-1. Ten-microgram samples of total RNA were collected at 2, 6, and 12 h, separated in a 1.0% formaldehyde-agarose gel, and hybridized to cidB- and cidC-specific DIG-labeled probes. The sizes of the 2.7- and 2.2-kb transcripts were determined by comparison to an RNA ladder (Invitrogen) run on the same gel. The corresponding ethidium bromide-stained gels are shown underneath each blot.
Subsequent inspection of the sequence downstream from the cidB gene revealed the presence of an open reading frame (ORF) spanning 1,739 nucleotides (nucleotides 2623281 to 2625020 of the S. aureus 8325 genome [http://www.genome.ou.edu/staph.html]) and separated by only 46 bp from the cidB stop codon. The relatively short intergenic sequence separating cidB and the downstream ORF (46 bp), along with the size of the transcript detected by the cidB probe, suggested that this downstream ORF may be part of the cid operon. As shown in Fig. 1, a probe specific for the downstream ORF also hybridized to a 2.7-kb transcript that was maximally expressed in the early exponential growth phase in both strains. Additionally, this probe hybridized to a 2.2-kb transcript in the RN6390 6- and 12-h RNA (Fig. 1), but this transcript was not detected in the corresponding UAMS-1 samples. These data indicate that the downstream ORF, subsequently designated cidC, is cotranscribed with the cidB gene. The cidC gene is predicted to encode a relatively large, 579-amino-acid protein with a molecular mass of 63.7 kDa and a pI of 6.9. Comparison of the cidC gene product to the proteins in the GenBank database revealed that it exhibits 33% identity with known pyruvate oxidases from E. coli (18) and Lactobacillus plantarum (43). It also exhibits a high degree of sequence similarity with putative pyruvate oxidase enzymes from a variety of gram-positive bacteria, including Staphylococcus epidermidis, Bacillus subtilis, and Listeria monocytogenes. However, with the exception of S. epidermidis, the putative pyruvate oxidase genes from the above-mentioned organisms do not appear to be associated with cidAB homologues on their respective genomes.
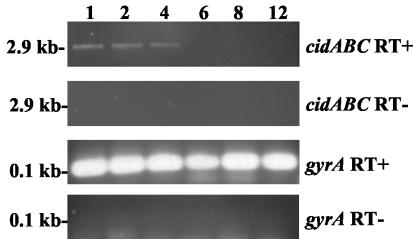
Interestingly, neither probe detected a transcript corresponding in size to cotranscription of all three cid genes, which would be predicted to be at least 2.9 kb. Additionally, a cidA-specific probe did not detect any transcripts by Northern blot analysis (data not shown), suggesting that this transcript is expressed at low levels compared to the cidBC transcript. Therefore, confirmation that all three genes are cotranscribed was accomplished by RT-PCR analysis of RNA samples collected from RN6390, using a cidA-specific forward primer and a reverse primer specific for cidC (Fig. 2). A 2.9-kb PCR product was detected during exponential growth (at 1, 2, and 4 h), a pattern of growth-phase-dependent expression that is consistent with the previously observed pattern using cidAB-specific primers (42). As expected, expression of the gyrA gene was detectable at all time points examined. Collectively, these experiments demonstrate that the cid operon is comprised of two overlapping transcripts: a full-length cidABC transcript that is expressed under these conditions at low levels during exponential growth and a cidBC transcript that is also expressed maximally in exponential growth phase but at higher levels relative to the cidABC transcript.
FIG. 2.
RT-PCR analysis of cidABC expression. RNA was isolated from S. aureus over a 12-h time course experiment and subjected to RT-PCR analysis using primers that detect cidABC and gyrA transcription (labeled cidABC RT+ and gyrA RT+, respectively). The time points (in hours) when RNA was collected are indicated above each lane, and the sizes of the RT-PCR products are shown to the left of the gels. Control reactions (labeled cidABC RT- and gyrA RT-), lacking RT enzyme, failed to generate RT-PCR products, indicating the absence of contaminating genomic DNA.
Transcription of cidBC and lrgAB is altered in strains with an intact sigB locus.
In addition to the transcription organization of the cid operon, it was also observed that cidBC transcription was greatly increased at all time points in the clinical isolate UAMS-1, relative to that in the laboratory strain RN6390 (Fig. 1). Laboratory isolates such as RN6390 and 8325-4 contain a naturally occurring 11-bp deletion in rsbU, a gene that is involved in positively activating σB, encoded downstream of rsbU (26). Therefore, we hypothesized that the altered cidBC transcription observed in UAMS-1 may be due the presence of an intact rsbU gene in this strain. To test whether rsbU is involved in cidBC transcription, we assessed the levels of cidBC transcript accumulation in several rsbU mutant strains and compared them to the levels produced by their otherwise isogenic parents (Fig. 3A). As predicted, the rsbU+ strains SH1000 and GP269 displayed dramatically increased cidBC expression relative to their parental rsbU-negative strain 8325-4. Likewise, transcription of cidBC was abolished in strain IK184, a derivative of the rsbU+ strain Newman in which the entire rsbUVWsigB locus has been deleted (Fig. 3A). Therefore, high-level cidBC transcription is dependent on an intact rsbU gene. Furthermore, transcription of lrgAB also appeared to be affected by the presence of an intact rsbU gene. Specifically, lrgAB transcription was decreased at all time points in the rsbU+ derivatives of 8325-4, but this decrease was most apparent in stationary phase (6- and 12-h growth) (Fig. 3B). By comparison, lrgAB transcription in strain Newman and its ΔrsbUVWsigB derivative IK184 was similar in exponential growth phase (2-h growth) but was decreased in Newman relative to IK184 in stationary phase (6- and 12-h growth). Collectively, these results suggest that transcription of both lrgAB and cidBC is affected by σB.
FIG. 3.
Northern blot analysis of cidBC (A) and lrgAB (B) transcription in S. aureus strains with or without an intact rsbU gene at 2, 6, and 12 h of growth. Ten micrograms of total RNA was subjected to Northern blot analysis using either a cidB-specific (A) or lrgA-specific (B) DIG-labeled DNA probe. The corresponding ethidium bromide-stained gels are shown underneath each blot.
Mapping of the cidBC promoter region.
To determine the 5′ end of the cidBC transcript and to localize the promoter region involved in cidBC transcription, a primer extension analysis was employed. This study revealed two primer extension products, indicating that cidBC transcription is initiated 242 and 109 bp upstream of the cidB start codon (Fig. 4A and B). However, no apparent −10 and −35 elements that match the B. subtilis σB-dependent promoter consensus sequence GTTTAAn13-15GGG(A/T)A(A/T) (34, 35) were identified in the sequence preceding the cidB gene (Fig. 4B). Instead, possible −10 elements that resemble the consensus for σA-dependent promoters were identified upstream of the two possible transcription start sites (Fig. 4B). In order to analyze whether the cidBC promoter is recognized directly by σB, irrespective of the lack of an obvious σB consensus sequence upstream of the cidBC transcriptional start point, the cidBC promoter region was cloned into reporter plasmid pSB40N, and the resulting plasmid was used in the heterologous two-plasmid system that was recently shown to be suitable for the detection of σB-dependent S. aureus promoters (24). Plasmid pSB40N-asp23p1, harboring the σB-dependent asp23 p1 promoter, which was used as a positive control, and pSB40N-cidBCp were each transformed into E. coli XL1-Blue cells containing either pAC7 or pAC7-sigB, respectively, and the clones obtained were selected on LBACX-ARA plates (39). Both negative (containing pAC7) and positive (containing pAC7-sigB) clones grew with comparable growth rates. Transformants containing pAC7 produced uncolored colonies, indicating that neither the S. aureus asp23 p1 promoter nor the cidBC promoter was recognized by any form of E. coli RNA polymerase holoenzyme. However, transformants containing pAC7-sigB and pSB40N-asp23p1 were blue on selective LBACX-ARA plates, demonstrating that the activity of the asp23 p1 promoter was dependent upon arabinose-induced heterologous expression of the S. aureus sigB in E. coli and indicating that the S. aureus σB-E. coli RNA polymerase holoenzyme hybrid was capable of recognizing the heterologous S. aureus asp23 promoter. In contrast, transformants containing pAC7-sigB and pSB40N-cidBCp remained uncolored on selective LBACX-ARA plates, demonstrating that the cidBC promoter region was not recognized directly by σB-containing RNA polymerase holoenzyme. Collectively, these observations suggest that high-level expression of cidBC is mediated indirectly by an as-yet-unidentified σB-regulated factor. The possibility that the rsbU gene product itself is affecting the activity of other regulatory proteins controlling cidBC and lrgAB expression can be ruled out, since overexpression of sigB in an rsbU-defective background increases cidBC expression and decreases lrgAB expression, while the presence of an intact rsbU gene in a sigB background has no effect on either cidBC or lrgAB expression when compared with an ΔrsbUVWsigB mutant (data not shown).
FIG. 4.
Primer extension analysis (A) and promoter sequence (B) of the cidBC transcript. (A) Primer extension of total cellular RNA (100 μg) (lane 1) from SH1000 yielded two cDNA products of 320 and 189 bp, mapping the cidBC transcription start sites to adenine residues located 242 and 109 bp upstream of the cidB ATG start codon. Both primer extension products depicted in this panel were obtained from the same reaction and represent two portions of the same sequencing gel. The sizes of the extension products were determined by comparison with the DNA sequencing ladder (shown to the left of lane 1) of the cidBC promoter region. Primer extension and sequencing reactions were performed with the same primer. (B) Nucleotide sequence of the cidBC promoter region. The cidBC transcription start sites determined in panel A are indicated by asterisks, whereas the start codons of cidA and cidB and the stop codon of cidA are each indicated by a single bar. The putative −10 regions are each indicated by a double bar.
Effect of an intact sigB locus on penicillin-induced killing and extracellular murein hydrolase activity.
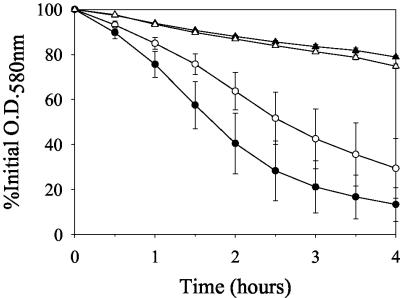
Previously, it was shown that the cid operon increased sensitivity to penicillin, whereas the lrg operon decreased penicillin sensitivity (20, 42). Based on these observations, we speculated that decreased lrgAB expression and increased cidBC expression in the rsbU+ backgrounds of 8325-4 and Newman may also be associated with increased sensitivity to penicillin. To test this, the viability of exponential-phase 8325-4 (rsbU-negative), SH1000 (rsbU+), GP269 (rsbU+), Newman (rsbUVWsigB+), and IK184 (ΔrsbUVWsigB) cultures were compared after exposure to 20 times the MIC of penicillin G. As shown in Fig. 5, neither GP269 nor SH1000 displayed a difference in sensitivity to penicillin relative to 8325-4. Furthermore, Newman displayed only a very minor increase in penicillin sensitivity compared to IK184. Based on these results, it appears that the sigB locus does not play a role in penicillin-induced killing in the 8325-4 and Newman genetic backgrounds.
FIG. 5.
Comparison of penicillin sensitivity of S. aureus strains with or without an intact rsbU gene. (Top) Penicillin (20 times the MIC) was added to exponential-phase cultures of the rsbU-negative strain, 8325-4 (open circles), and its rsbU+ derivatives, SH1000 (closed circles) and GP269 (closed squares). (Bottom) Penicillin (20 times the MIC) was added to exponential-phase cultures of the rsbUVWsigB+ strain, Newman (closed triangles), and its ΔrsbUVWsigB derivative, IK184 (open triangles). Viable cell counts of each culture were determined by dilution plating on TSA. These data are the average of three independent experiments, and error bars correspond to the standard errors of the means.
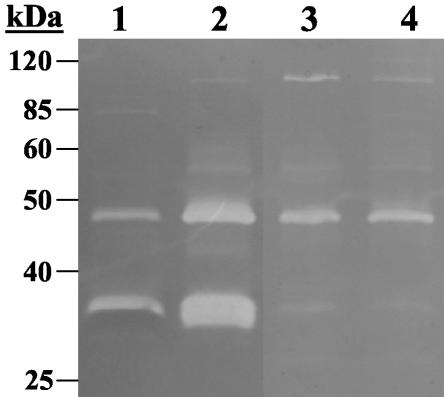
Furthermore, the cid operon has been previously shown to have a positive effect on extracellular murein hydrolase activity (42), whereas the lrgAB operon negatively regulates murein hydrolase activity (20). Therefore, we predicted that the presence of an intact rsbU gene would have a positive impact on murein hydrolase activity. The extracellular murein hydrolase activity of isogenic strains with or without an intact rsbU gene was measured by both quantitative murein hydrolase assays and by zymography (Fig. 6 and 7). As indicated in Fig. 6, the extracellular murein hydrolase activity of SH1000 (rsbU+) was reproducibly increased relative to its rsbU counterpart, 8325-4. By comparison, the murein hydrolase activities of strain Newman and its isogenic rsbUVWsigB mutant IK184 were virtually identical to each other, although there was greatly decreased murein hydrolase activity in these strains compared to 8325-4 and SH1000 (Fig. 6). These quantitative results were confirmed by zymography (Fig. 7). Two major protein bands of 35 and 48 kDa with murein hydrolase activity were observed in the culture supernatants of both 8435-4 and SH1000 (Fig. 7, lanes 1 and 2, respectively), and both of these bands displayed increased murein hydrolase activity in SH1000 (rsbU+) compared to 8325-4. As well, both IK184 (Newman ΔrsbUVWsigB) and Newman displayed identical secreted murein hydrolase profiles (Fig. 7, lanes 3 and 4, respectively), with greatly decreased activity of the 35-kDa band compared to that in 8325-4 and SH1000. These results demonstrate that extracellular murein hydrolase activity is influenced by σB in certain S. aureus strains.
FIG. 6.
Quantitative extracellular murein hydrolase assays of S. aureus strains with or without an intact rsbU gene. Aliquots of 100 μg of extracellular proteins isolated from 16-h cultures of 8325-4 (rsbU-negative; open circles), SH1000 (rsbU+; closed circles), IK184 (ΔrsbUVWsigB; open triangles), and Newman (rsbUVWsigB+; closed triangles) were each added to a 1.0-mg/ml suspension of M. luteus cells, and the murein hydrolase activity of each sample was measured as a decrease in turbidity over a 4-h time course experiment. These data are the average of three independent experiments. The error bars on the graph correspond to the standard errors of the means.
FIG. 7.
Zymographic analysis of S. aureus strains with or without an intact rsbU gene. Extracellular proteins were isolated from stationary-phase culture supernatants, and 15 μg of each sample was separated in an sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel containing 1.0 mg of M. luteus cells/ml, followed by overnight incubation at 37°C in a buffer containing Triton X-100 and staining with methylene blue. This zymogram is representative of results obtained from three independent experiments. Molecular mass markers are indicated in kilodaltons to the left of the gel. Lane numbers correspond to 8325-4 (rsbU-negative; lane 1), SH1000 (rsbU+; lane 2), IK184 (ΔrsbUVWsigB; lane 3), and Newman (rsbUVWsigB+; lane 4).
DISCUSSION
Previous work from our laboratory described the S. aureus cidAB operon, which was shown to positively regulate extracellular murein hydrolase activity and increase sensitivity to penicillin (42). In the study presented here it was found that this operon also encodes a third gene, cidC, which lies immediately downstream of cidB. Furthermore, the cid operon is comprised of two distinct and overlapping transcripts: a cidABC message that is expressed during exponential growth and is only detectable under these conditions using RT-PCR, and a smaller cidBC message that is also expressed maximally during exponential growth and is upregulated in S. aureus strains that contain a functional rsbU gene. The presence of these overlapping transcripts is similar to the lrgAB operon, which was also previously shown by Northern blotting to contain two overlapping transcripts, lrgAB and lrgB (5). Other parallels can be drawn between the organization of the lrg and cid operons, such as the fact that both the lrgAB and cidAB ORFs appear to be translationally coupled, as the 5′ ends of the lrgB and cidB ORFs overlap with the 3′ ends of lrgA and cidA, respectively (20, 42). Furthermore, this study has demonstrated that expression of both the cidBC and lrgAB transcripts is affected by the rsbU gene, albeit in an opposing manner: lrgAB transcription is downregulated in strains containing a functional rsbU gene (and, hence, an intact sigB response) especially in stationary phase, whereas cidBC transcription appears to be upregulated in these strains at all phases of growth. Similar transcription profiles of cidBC and lrgAB were also recently observed by microarray analysis of isogenic S. aureus strains, showing that cidBC expression was at least 18-fold higher in strains harboring an intact sigB operon, while lrgAB expression was more than 6-fold lower in sigB+ strains compared to their isogenic ΔrsbUVWsigB mutants (M. Bischoff et al., submitted for publication). The effect of rsbU on expression of these two gene loci also reflects the diametrically opposing regulation that the lrg and cid operons confer on extracellular murein hydrolase activity and penicillin-induced killing (42).
This study also revealed the presence of a third gene, cidC, in the cid operon. The cidC gene encodes a protein that shares 33% identity with the well-characterized pyruvate oxidases of E. coli (18, 19) and L. plantarum (43) and shares a high degree of similarity with pyruvate oxidases from a number of other organisms, such as S. epidermidis, B. subtilis, and L. monocytogenes. Interestingly, our analysis of the genomes of these organisms has revealed that the association of a putative pyruvate oxidase gene with cidAB homologues appears to be unique to S. epidermidis and S. aureus, but the biological implications of this genetic organization are as yet unknown. The pyruvate oxidase family of enzymes catalyzes the oxidative decarboxylation of pyruvate to specific end products. For example, the E. coli pyruvate oxidase PoxB catalyzes the oxidative decarboxylation of pyruvate to acetate and carbon dioxide (7, 14), whereas the LpPOX pyruvate oxidase of L. plantarum catalyzes the oxidative decarboxylation of pyruvate to acetyl phosphate, carbon dioxide, and hydrogen peroxide (47). Whether the cidC gene product actually belongs to this family of enzymes and the biological implications of its genetic association with cidAB are currently under investigation in our laboratory. However, it is interesting that, similar to the apparent control of cidBC expression by σB in S. aureus, PoxB expression is under control of RpoS (8), a sigma factor that controls the E. coli general stress response (23).
Since both the lrg and cid operons have been previously shown to regulate extracellular murein hydrolase activity (20, 42), we predicted that extracellular murein hydrolase activity would be altered in strains containing an intact rsbU gene. Interestingly, both quantitative analysis and zymography revealed that murein hydrolase activity was increased in the rsbU+ background of 8325-4, and this increased activity may be partly a consequence of increased cidBC expression and/or decreased lrgAB expression (Fig. 6 and 7). However, extracellular murein hydrolase activity in strain Newman was substantially reduced compared to 8325-4 and was not affected by the presence of an intact sigB locus, illustrating that other factors are likely involved in the regulation of murein hydrolase activity in this strain. These could include the level of transcription of various murein hydrolases, such as atl (32), lytM (37), and lytN (46), the amount of protease activity produced by these strains (11), and/or control of murein hydrolase activity by other regulators, such as agr and sarA (12).
Although this study has shown that both the lrgAB and cidBC transcripts are affected by the rsbU mutation, the specific mode of this regulation remains to be elucidated. Putative σA-dependent −10 elements were identified upstream to the two possible transcription start sites mapped by primer extension (Fig. 4A and B), but a clear −35 element was not identified in either case. Furthermore, a σB promoter consensus sequence was not present (Fig. 4B), and results from the two-plasmid experiment indicated that (i) σB does not interact directly with the cidBC promoter, and (ii) that the cidBC promoter is not recognized by any form of E. coli RNA polymerase. Therefore, it is likely that the high-level transcription of cidBC observed in rsbU+ S. aureus strains is dependent on an as-yet-unknown σB-dependent transcription factor or pathway of regulators, such as SarA, which has been previously shown to positively regulate lrgAB expression (13). In B. subtilis, the σB-mediated stress response protects the cells against a wide variety of metabolic and environmental stresses, such as acid, heat, oxidative stress, and starvation for glucose or phosphate (reviewed in reference 36). In S. aureus, σB activity is maximal in late exponential growth phase (3) and is required for tolerance of the cells to exposure to environmental stresses such as heat shock (15, 27) and exposure to hydrogen peroxide (25, 26). In this study, we have shown that both cidBC and lrgAB expression are affected by rsbU in S. aureus, implying that these genes are also members of the σB stress regulon. However, the precise role of these genes in the general stress response remains to be determined. Both the lrg and cid operons have been previously shown to be involved in penicillin-induced killing (20, 42), and σB has been shown to affect the resistance of S. aureus to various antibiotics, including teicoplanin (2), vancomycin (44), and methicillin (oxacillin) (30, 44, 49). Therefore, one possibility is that the σB stress regulon is involved in antibiotic resistance, and likewise the lrg and cid operons may also play a role in this response. In fact, it has been recently proposed that the elimination of bacterial cells damaged by antibiotics is actually achieved by programmed cell death (PCD), an altruistic response that would ultimately benefit the bacterial population as a whole (28). Based on the similarities in structure and function between the LrgA and CidA proteins to bacteriophage-encoded holins, along with their roles in tolerance to penicillin (20, 41, 42), these proteins have also been recently suggested to control bacterial PCD (1, 41), perhaps in a manner analogous to the eukaryotic Bcl-2 family of proteins (1).
In contrast to our finding that the S. aureus laboratory strains 8325-4 and Newman did not display an altered tolerance to penicillin compared to their isogenic rsbU+ and ΔrsbUVWsigB counterparts (Fig. 5), previous studies in other laboratories found that methicillin-resistant S. aureus and glycopeptide-intermediate S. aureus isolates displayed increased resistance to methicillin (oxacillin) and vancomycin compared to their isogenic sigB-negative counterparts, respectively (30, 44, 49). These results suggest that the molecular components involved in resistance to cell wall antibiotics may differ from those that confer tolerance. As well, previous work in our laboratory has shown that the cidA gene enhances penicillin-induced killing in RN6390 (42). Thus, it is possible that σB did not affect penicillin tolerance because of its lack of effect on cidA expression. Furthermore, other unidentified strain-dependent factors may be responsible for the apparent lack of effect that σB had on penicillin sensitivity in 8325-4 and Newman. In this respect, other studies have shown that high-passage S. aureus laboratory strains display several phenotypic differences relative to low-passage clinical isolates (4, 45), and S. aureus laboratory isolates such as 8325-4 and RN6390 contain a naturally occurring deletion in rsbU, whose gene product is required for σB activity (26). Collectively, these observations support the recently proposed idea that highly propagated laboratory isolates have been selected for defects in their PCD mechanism and therefore do not respond to stress or undergo PCD in the same way as they would in low-passage strains (41). Therefore, it is important to perform future studies of the lrg and cid operons in a clinical isolate in order to determine what role their gene products play in the σB-mediated stress response and/or PCD.
Acknowledgments
This work was funded by NIH grant no. R01-AI038901, NIH-NRRI grant no. P20RR15587, and DOD grant no. DAAD 19-03-1-0191.
We thank Simon Foster (University of Sheffield, Sheffield, England) for kindly providing S. aureus strain SH1000 and Jan Kormanec (Slovak Academy of Sciences, Slovak Republic) for kindly providing plasmids pAC7 and pSB40N.
REFERENCES
- 1.Bayles, K. W. 2003. Are the molecular strategies that control apoptosis conserved in bacteria? Trends Microbiol. 11:306-311. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, M., and B. Berger-Bachi. 2001. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunskill, E. W., and K. W. Bayles. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178:5810-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 7.Chang, Y. Y., and J. E. Cronan, Jr. 1983. Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J. Bacteriol. 154:756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. Y., A. Y. Wang, and J. E. Cronan, Jr. 1994. Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS(katF) gene. Mol. Microbiol. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, A. L., K. J. Eberhardt, and V. A. Fischetti. 1994. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal. Biochem. 222:511-514. [DOI] [PubMed] [Google Scholar]
- 10.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto, D. F., and K. W. Bayles. 1998. Opposing roles of the Staphylococcus aureus virulence regulators, Agr and Sar, in Triton X-100- and penicillin-induced autolysis. J. Bacteriol. 180:3724-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto, D. F., E. W. Brunskill, and K. W. Bayles. 2000. Analysis of genetic elements controlling Staphylococcus aureus lrgAB expression: potential role of DNA topology in SarA regulation. J. Bacteriol. 182:4822-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennis, R. B., and L. P. Hager. 1976. Pyruvate oxidase, p. 493-504. In A. Martonosi (ed.), The enzymes of biological membranes, vol. 2. Plenum, New York, N.Y. [Google Scholar]
- 15.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 16.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabau, C., and J. E. Cronan, Jr. 1984. Molecular cloning of the gene (poxB) encoding the pyruvate oxidase of Escherichia coli, a lipid-activated enzyme. J. Bacteriol. 160:1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabau, C., and J. E. Cronan, Jr. 1986. Nucleotide sequence and deduced amino acid sequence of Escherichia coli pyruvate oxidase, a lipid-activated flavoprotein. Nucleic Acids Res. 14:5449-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groicher, K. H., B. A. Firek, D. F. Fujimoto, and K. W. Bayles. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidrich, C., M. F. Templin, A. Ursinus, M. Merdanovic, J. Berger, H. Schwarz, M. A. de Pedro, and J.-V. Holtje. 2001. Involvement of N-acetylmuramyl-l-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 41:167-178. [DOI] [PubMed] [Google Scholar]
- 23.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli, p. 161-178. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 24.Homerova, D., M. Bischoff, A. Dumoulin, and J. Kormanec. 2004. Optimization of a two-plasmid system for the identification of promoters recognized by RNA polymerase containing Staphylococcus aureus alternative sigma factor σB. FEMS Microbiol. Lett. 232:173-179. [DOI] [PubMed] [Google Scholar]
- 25.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kullik, I. I., and P. Giachino. 1997. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151-159. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreillon, P., Z. Markiewicz, S. Nachman, and A. Tomasz. 1990. Two bactericidal targets for penicillin in pneumococci: autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob. Agents Chemother. 34:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morikawa, K., A. Maruyama, Y. Inose, M. Higashide, H. Hayashi, and T. Ohta. 2001. Overexpression of sigma factor, varσB, urges Staphylococcus aureus to thicken the cell wall and to resist beta-lactams. Biochem. Biophys. Res. Commun. 288:385-389. [DOI] [PubMed] [Google Scholar]
- 31.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 32.Oshida, T., M. Sugai, H. Komatsuzawa, Y. M. Hong, H. Suginaka, and A. Tomasz. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 92:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Volker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Volker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price, C. W. 2000. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria, p. 179-197. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 37.Ramadurai, L., and R. K. Jayaswal. 1997. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J. Bacteriol. 179:3625-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezuchova, B., and J. Kormanec. 2001. A two-plasmid system for identification of promoters recognized by RNA polymerase containing extracytoplasmic stress response sigma(E) in Escherichia coli. J. Microbiol. Methods 45:103-111. [DOI] [PubMed] [Google Scholar]
- 39.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli sigmaE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 40.Rice, K., R. Peralta, D. Bast, J. de Azavedo, and M. J. McGavin. 2001. Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect. Immun. 69:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice, K. C., and K. W. Bayles. 2003. Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 50:729-738. [DOI] [PubMed] [Google Scholar]
- 42.Rice, K. C., B. A. Firek, J. B. Nelson, S.-J. Yang, T. G. Patton, and K. W. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in the regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sedewitz, B., K. H. Schleifer, and F. Gotz. 1984. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 160:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, V. K., J. L. Schmidt, R. K. Jayaswal, and B. J. Wilkinson. 2003. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int. J. Antimicrob. Agents 21:256-261. [DOI] [PubMed] [Google Scholar]
- 45.Somerville, G. A., S. B. Beres, J. R. Fitzgerald, F. R. DeLeo, R. L. Cole, J. S. Hoff, and J. M. Musser. 2002. In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 184:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugai, M., T. Fujiwara, H. Komatsuzawa, and H. Suginaka. 1998. Identification and molecular characterization of a gene homologous to epr (endopeptidase resistance gene) in Staphylococcus aureus. Gene 224:67-75. [DOI] [PubMed] [Google Scholar]
- 47.Tittmann, K., R. Golbik, S. Ghisla, and G. Hubner. 2000. Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum. Biochemistry 39:10747-10754. [DOI] [PubMed] [Google Scholar]
- 48.Wang, I. N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]
- 49.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]