Abstract
Major life stressors, especially those involving interpersonal stress and social rejection, are among the strongest proximal risk factors for depression. In this review, we propose a biologically plausible, multilevel theory that describes neural, physiologic, molecular, and genomic mechanisms that link experiences of social-environmental stress with internal biological processes that drive depression pathogenesis. Central to this social signal transduction theory of depression is the hypothesis that experiences of social threat and adversity up-regulate components of the immune system involved in inflammation. The key mediators of this response, called proinflammatory cytokines, can in turn elicit profound changes in behavior, which include the initiation of depressive symptoms such as sad mood, anhedonia, fatigue, psychomotor retardation, and social-behavioral withdrawal. This highly conserved biological response to adversity is critical for survival during times of actual physical threat or injury. However, this response can also be activated by modern-day social, symbolic, or imagined threats, leading to an increasingly proinflammatory phenotype that may be a key phenomenon driving depression pathogenesis and recurrence, as well as the overlap of depression with several somatic conditions including asthma, rheumatoid arthritis, chronic pain, metabolic syndrome, cardiovascular disease, obesity, and neurodegeneration. Insights from this theory may thus shed light on several important questions including how depression develops, why it frequently recurs, why it is strongly predicted by early life stress, and why it often co-occurs with symptoms of anxiety and with certain physical disease conditions. This work may also suggest new opportunities for preventing and treating depression by targeting inflammation.
Keywords: early life stress, social threat, cytokines, mechanisms, disease
Depression is among the most common and costly of all psychiatric disorders. Nearly one in four women and one in six men experience depression during their lifetime (Kessler et al., 2010), and up to 65% of individuals have recurrent episodes of the disorder (Eaton et al., 2008; Monroe & Harkness, 2011; Yiend et al., 2009). Compounding the issue is the fact that many people with depression never receive diagnosis or treatment, and only about 30%–35% of adults achieve remission using current therapeutic approaches, leaving over two thirds of the disease burden intact (Alexopoulos, 2005; Andrews, Issakidis, Sanderson, Corry, & Lapsley, 2004; Chisholm, Sanderson, Ayuso-Mateos, & Saxena, 2004; Roose & Schatzberg, 2005). These features contribute to substantial social and economic burden (Greenberg et al., 2003; Vos et al., 2004). In fact, depression has been estimated to be the fourth leading cause of overall disease burden and the leading cause of nonfatal disease burden worldwide (Üstün, Ayuso-Mateos, Chatterji, Mathers, & Murray, 2004). Identifying biobehavioral factors that can be targeted for preventing and treating depression is thus of paramount public importance.
Central to most contemporary theories of depression is the notion that stress can initiate cognitive and possibly biological processes that increase risk for the disorder (Beck, 1967; Blatt, 2004; Brown & Harris, 1978). Consistent with these theories, major stressful life events are one of the best predictors of an impending onset of depression (Kendler, Karkowski, & Prescott, 1999; Kessler, 1997). Indeed, certain life events, such as those involving social rejection, confer a 21.6% increase in risk for onset of major depressive disorder (MDD; Kendler, Hettema, Butera, Gardner, & Prescott, 2003). Whereas an abundance of research has examined cognitive processes that may mediate the link between stress and depression (Gotlib & Joormann, 2010; Kircanski, Joormann, & Gotlib, 2012), though, relatively little is known about the biological processes that are influenced by stress and that, in concert with cognitive and affective processes, may lead to depression.
The development of tools for assessing neural activity, peripheral biology, genetic variation, and gene expression has been extremely impactful in this context because such methods have helped to identify mechanistic pathways that may link the external social environment with internal biological processes that have the ability to promote depression. One of the most recent and potentially important insights from this work is the discovery that components of the immune system that mediate inflammation may be intimately involved in depression. Inflammation is typically thought of as the body’s primary response to physical injury or infection. However, there is now substantial evidence that psychological stress can trigger significant increases in inflammatory activity (i.e., in the absence of physical injury; Glaser & Kiecolt-Glaser, 2005). Increases in inflammation can in turn elicit profound changes in behavior, which include the initiation of depressive symptoms such as sad mood, anhedonia, fatigue, psychomotor retardation, and social-behavioral withdrawal. These findings thus support the hypothesis that external social conditions, and even people’s mere perceptions of such conditions, may regulate molecular processes that initiate biological and behavioral changes that increase risk for depression. As we discuss later, these processes may also be relevant for the development of other disease conditions that have an inflammatory basis and tend to co-occur with depression, such as rheumatoid arthritis, chronic pain, obesity, diabetes, and cardiovascular disease.
The purpose of the present review is to provide an integrated account of how life stress affects inflammation and how stress–inflammation pathways may in turn be linked with depression. Although several excellent articles have reviewed the literature on stress and inflammation (e.g., Denson, Spanovic, & Miller, 2009; Irwin & Cole, 2011; Segerstrom & Miller, 2004; Steptoe, Hamer, & Chida, 2007) and reciprocal links between inflammation and depression (e.g., Bufalino, Hepgul, Aguglia, & Pariante, 2013; Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Dantzer, O’Connor, Lawson, & Kelley, 2011; Debnath et al., 2011; Leonard, 2010; Leonard & Maes, 2012; Maes, Leonard, Myint, Kubera, & Verkerk, 2011; Messay, Lim, & Marsland, 2012; A. H. Miller, Maletic, & Raison, 2009; Raedler, 2011; Raison, Capuron, & Miller, 2006; Raison & Miller, 2011), these literatures have historically represented separate and largely distinct lines of inquiry. As a result, no reviews to date have considered in detail how certain types of stress may up-regulate inflammatory processes in a way that promotes depression. This has occurred despite the possibility that integrating these literatures may help address some of the most important and pressing questions about depression, such as, how does stress up-regulate internal biological processes that evoke depressive symptoms? Why is depression often comorbid with certain somatic complaints and physical disease conditions? Why are people with a history of early life stress at elevated risk for depression and depression-related physical disease? Why is depression such a highly recurrent disorder for some individuals? And why do increases in anxiety frequently precede depression?
To integrate the literatures on stress, inflammation, and depression in a way that begins to address these questions, we first provide a general overview of the immune system and summarize components of this system that have been shown to be influenced by stress. Second, we review studies showing that experiences of stress, particularly interpersonal or social stress, up-regulate inflammatory activity, with an examination of neural and genetic factors that mediate and moderate this effect. Third, we synthesize the bodies of work demonstrating that biological mediators of the inflammatory response can communicate with the central nervous system to induce neural and behavioral alterations that may manifest into depressive symptoms. Fourth, based on this research, we propose an integrative, multilevel theory of depression that describes how stress-related increases in inflammation may lead to depression and, possibly, to other disorders that have an inflammatory component. Finally, we discuss the implications of this work for preventing and treating depression and suggest some possible avenues for future clarification and research.
The Immune System, Cytokines, and Inflammation
The immune system is critical for human health and well-being, as it helps coordinate the body’s response to physical injuries and infections that, if left unaddressed, could cause illness or death. The system is generally viewed as being composed of two interconnected branches. The first and evolutionally older of these branches, called innate immunity, is the body’s first line of defense against tissue damage and microbial infection (Medzhitov, 2007; Takeda, Kaisho, & Akira, 2003). Innate immunity is composed of immune cells such as monocytes/macrophages and dendritic cells that constantly circulate in the body and use invariant receptors to detect a wide variety of pathogens. These cells signal the occurrence of injury or infection and initiate a cascade of inflammatory processes that help contain an infection and promote healing and recovery (Medzhitov, 2007). When innate immune system defenses are insufficient, these cells activate the second branch of the immune system, called adaptive immunity (Barton, 2008). In contrast to innate immunity, which is nonspecific and does not confer long-lasting protection to the host, adaptive immunity involves the proliferation of microbial-specific white blood cells (i.e., lymphocytes) that attempt to neutralize or eliminate microbes based on an immunological memory of having responded to a specific pathogen in the past (Gruys, Toussaint, Niewold, & Koopmans, 2005; K. Murphy, 2011). Whereas the innate immune response is rapid, occurring over minutes or hours, the adaptive immune response takes days to fully develop (Barton, 2008).
Innate Immune System Dynamics
Initial activation of the innate immune system is called the acute-phase response, and it involves an increase in inflammatory activity that can occur both locally, at the site of tissue injury or infection, and systemically (Hennessy, Schiml-Webb, & Deak, 2009). This response is triggered when receptors of innate immune cells recognize hardwired and highly conserved features of microbes or pathogen-associated molecular patterns (PAMPs), which gives the system the ability to detect and respond to a wide range of microbial diversity (Barton, 2008; Medzhitov, 2007). This recognition strategy is termed pattern recognition, and innate immune receptors that use this strategy are called pattern recognition receptors (Barton, 2008). A prototypical PAMP (which we discuss later) is lipopolysaccharide (LPS), an endotoxin that is the major component of the outer membrane of Gram-negative bacteria (Raetz & Whitfield, 2002).
Pattern recognition receptors play a critical role in linking the recognition of microbial targets to inflammation. One of the best characterized families of these receptors are toll-like receptors (TLRs), and they are found on macrophages, neutrophils, and dendritic cells that drive inflammation (Akira, Takeda, & Kaisho, 2001; Medzhitov, 2001). TLRs recognize conserved components of microbes, including bacteria, viruses, and fungi, and in turn activate inflammatory and antimicrobial innate immune responses (Medzhitov, 2001). Examples of this family of TLRs include types that bind to, and become activated by, specific ligands such as LPS (TLR4), double-stranded RNA (TLR3), and single-stranded RNA (TLR7 and TLR8; Barton, 2008). When any of these TLRs are activated, a conserved signaling cascade is initiated that results in the activation of two principal intracellular transcription factors: nuclear factor-κB (NF-κB) and interferon (IFN) regulatory factors (Karin, 2006; Kawai & Akira, 2007). As we describe in more detail below, these transcription factors in turn drive the expression of proinflammatory immune response genes such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) that produce small protein molecules called cytokines, the main effectors of the inflammatory response (Karin, 2006; Raison et al., 2006).
Cytokines: The Key Mediators of Inflammation
Cytokines play a central role in immune system and inflammatory responding. In addition to coordinating cell-to-cell communication, they can alter neurochemical and neuroendocrine processes that have wide-ranging effects on physiology and behavior (Curfs, Meis, & Hoogkamp-Korstanje, 1997). Released from immune cells such as monocytes/macrophages, dendritic cells, and neutrophils, cytokines may be thought to function in a manner similar to neurotransmitters and hormones insofar as they mediate physiological responses, rely on receptor-ligand interactions, and have self (autocrine), local (paracrine), and distal (endocrine) effects (Jain & Mills, 2007). Among cytokines that coordinate cell functions related to inflammation, those that increase or up-regulate inflammation are referred to as proinflammatory, whereas those that down-regulate inflammation are called anti-inflammatory.
In this review, we focus primarily on the proinflammatory cytokines IL-1, interleukin-6 (IL-6), and TNF-α, which together coordinate a variety of cell functions that stimulate and enhance inflammation. For example, IL-1, IL-6, and TNF-α promote the differentiation of lymphocytes called cytotoxic T cells, which kill pathogens that are introduced into the body during physical wounding. Inflammatory cytokines also promote increased vascular permeability and cellular adhesion, which allows immune cells to leave the blood vessels (i.e., the “boulevards”) and migrate to tissues (i.e., the “battlefields”) where they can neutralize or eliminate pathogens (see Dhabhar, Malarkey, Neri, & McEwen, 2012). For example, IL-1 activates the expression of the endothelial adhesion molecule intercellular adhesion molecule-1, which, when bound to the properly conformed integrin (e.g., LFA-1) on the surface of immune cells, promotes firm adhesion to endothelial cells for eventual extravasation (i.e., migration of cells from the circulation to tissue; C. W. Smith, Marlin, Rothlein, Toman, & Anderson, 1989). TNF-α promotes a similar process for neutrophils by stimulating the production of the adhesion molecule E-selectin on the endothelium, which binds to adhesion molecules on neutrophils (Hubbard & Rothlein, 2000). This process of redistributing cells of the innate immune system to sites of injury or infection is aided by small polypeptides called chemokines. Chemokines are activated by the proinflammatory cytokines TNF-α, IL-6, and IL-1, and they continually survey the body to screen for pathogens in a process called immunosurveillance. Once a pathogen or infection has been identified, chemokines can act as chemoattractants that recruit other immune cells to the site of inflammatory activity (K. Murphy, 2011).
As summarized in Table 1, cytokines also have specific effects on the body that are commonly recognized as signs of inflammation. At sites of infection, for example, cytokines cause redness, heat, swelling, and pain. At a more systemic level, certain cytokines (i.e., IL-6) induce the production of the acute-phase protein (and key biomarker of inflammation) C-reactive protein (CRP), which, together with cytokines, can lead to increased body temperature, heart rate, respiratory rate, and fever (Poon, Ho, Chiu, & Chang, 2013; Ricciotti & FitzGerald, 2011). When combined, these effects help accelerate wound healing and limit the spread of infection in the host. They also promote social-behavioral withdrawal, which helps the organism recuperate and recover and reduces the likelihood that the infection will spread to conspecifics in the surrounding environment (K. Murphy, 2011).
Table 1.
Inflammatory Cytokines and Their Key Characteristics
| Cytokine | Family | Producer cells | Function |
|---|---|---|---|
| Proinflammatory cytokines | |||
| Interleukin-1β (IL-1β) | Unassigned | Macrophages | Key mediator of sickness behavior; promotes fever and pain hypersensitivity; involved in HPA axis activation, lymphocyte activation, macrophage and neutrophil activation, endothelial activation, prostanoid synthesis, and IL-6 synthesis |
| Interleukin-2 (IL-2) | Hematopoietins | T cells | Facilitates immunoglobulin production by B cells and differentiation and proliferation of NK cells |
| Interleukin-6 (IL-6)a | Hematopoietins | Macrophages, T cells | Key mediator of acute phase response; promotes fever and T and B cell differentiation and activation; can down-regulate inflammation by inhibiting TNF-α and IL-1 production |
| Interleukin-8 (IL-8) | Chemokines | Macrophages | Key mediator of inflammation; recruits neutrophils to the site of inflammation and induces chemotaxis in target cells |
| Tumor necrosis factor-α (TNF-α) | TNF family | Macrophages, NK cells | Key mediator of sickness behavior; promotes fever and suppresses appetite; stimulates HPA axis, endothelial activation, and neutrophil activation; induces apoptotic cell death |
| Anti-inflammatory cytokines | |||
| Interleukin-4 (IL-4) | Hematopoietins | T cells | Inhibits production of the proinflammatory cytokines TNF-α and IL-1; stimulates B and T cell proliferation |
| Interleukin-10 (IL-10) | Unassigned | Macrophages, T cells | Inhibits production of the proinflammatory cytokines IL-1, IL-6, and TNF-α; enhances B cell proliferation and antibody production |
Note. Adapted from Schiepers, Wichers, and Maes (2005), Dantzer, O’Connor, Freund, Johnson, and Kelley (2008), and DellaGioia and Hannestad (2010). HPA axis = hypothalamic–pituitary–adrenal axis; NK cells = natural killer cells.
Although IL-6 is listed as a proinflammatory cytokine, as described, it can also have anti-inflammatory effects.
Inflammation: Friend and Foe
From this brief overview, we can see that mounting a rapid innate immune system and inflammatory response to a specific trigger and then down-regulating the response once a pathogen has been cleared are critical for resolving infection, repairing tissue damage, and returning the body to a state of homeostasis (Kushner, 1982; Medzhitov, 2008). Recently, however, evidence has accumulated showing that when activation of the inflammatory response is altered or prolonged, it can actually cause more damage to a host than the pathogen itself (Barton, 2008). Indeed, it is now widely recognized that chronic inflammation plays a role in several major diseases including asthma, arthritis, diabetes, obesity, atherosclerosis, certain cancers, and Alzheimer’s disease (Couzin-Frankel, 2010). One factor that can alter adaptive innate immune system responding and prolong inflammation is stress (Segerstrom & Miller, 2004; Steptoe et al., 2007). In this review, therefore, we consider how stress influences the regulation of inflammation in a way that may be relevant for depression.
Regulation of Systemic Inflammation
Inflammation is a complex, tightly regulated process that is controlled at several levels by a number of different systems and processes. Many excellent articles have reviewed these regulatory dynamics in depth (e.g., Dantzer et al., 2008; Irwin & Cole, 2011; Maier & Watkins, 1998; Pavlov & Tracey, 2004; Radtke, Macdonald, & Tacchini-Cottier, 2013; Rivest, 2009; Sternberg, 2006). Here, therefore, we provide only a brief overview of proximal (i.e., genomic) and central processes that regulate inflammation, with a focus on the relevance of these dynamics for depression.
Genomic Regulation of Systemic Inflammation
The body’s complex, highly adaptive inflammatory response is regulated most proximally by intracellular processes that occur at the level of the genome. The specific pathway involved, though, depends on the type of threat to which a person is exposed (Amit et al., 2009). If the individual is exposed to an extracellular pathogen such as bacteria, then the intracellular transcription factors NF-κB and activator protein 1 (AP-1) are activated. These transcription factors induce the expression of proinflammatory immune response genes such as interleukin-1β (IL1B), IL6, interleukin-8 (IL8), and TNF, resulting in the increased production of the respective proinflammatory cytokines. In contrast, if the exposure involves an intracellular pathogen such as a virus, then transcription factors such as IFN regulatory factors are activated. These transcription factors induce antiviral immune response genes such as type I IFN genes, which through the translation of IFN can activate signal transducer and activator of transcription-1, leading to the production of proinflammatory cytokines. Regardless of the specific pathway that is activated, increases in inflammatory activity occur when extracellular signals cause transcription factors to translocate from the cytoplasm of an immune cell into the cell’s nucleus, where they activate immune response genes leading to the production of inflammatory cytokines that mediate systemic inflammation (Irwin & Cole, 2011; Medzhitov, 2008; Slavich & Cole, 2013).
Central Regulation of Systemic Inflammation
Inflammatory activity is also regulated more distally by processes occurring in the brain. This neuro-inflammatory link gives the innate immune system the ability to prepare the body for physical wounding or injury in advance of an assault that could lead to a pathogen-related infection. A key aspect of this preparatory pathogen host defense response is an anticipatory (i.e., pre-injury) redistribution and trafficking of innate immune cells to sites of possible injury or infection. When mobilized, this response is associated with enhanced postinjury wound healing and recovery, which can be critical for survival (Dhabhar, 2009; Rosenberger et al., 2009).
To mount a pathogen host defense response before an injury occurs, immune response genes “listen” for chemical signals indicating an increased risk for wound-related bacterial infection stemming from social-environmental danger. Threats of this nature historically involved the presence of predatory animals or hostile conspecifics. As summarized by Slavich and Cole (2013), however, recent research has demonstrated that this same innate immune system response can be activated in the contemporary social environment when an individual is exposed to adverse conditions involving social conflict, evaluation, rejection, isolation, or exclusion perhaps because of the implications these conditions historically had for physical danger.
The type of innate immune system response that is initiated by present-day social adversity depends in part of the temporal nature of the experience. For example, whereas acute stress has been found to enhance antiviral defenses (Edwards et al., 2006; Mays et al., 2010; Phillips, Carroll, Burns, & Drayson, 2009; Powell, Allen, Hufnagle, Sheridan, & Bailey, 2011), prolonged stress and depression have been associated with reduced antiviral immune responses (Irwin et al., 2013; Pedersen, Zachariae, & Bovbjerg, 2009). Assuming a more frequent or chronic activation of these pathways, we have proposed that the primary innate immune system response to contemporary social stressors involves up-regulation of proinflammatory immune response genes, which combat bacteria and other extracellular pathogens, and a reciprocal down-regulation of antiviral immune response genes, which target intracellular pathogens such as viruses (Irwin & Cole, 2011). As depicted in Figure 1 and described more fully by Slavich and Cole (2013), this increased proinflammatory/reduced antiviral skewing of our basal gene expression profile, called the basal transcriptome, appears to represent a conserved transcriptional response to adversity (CTRA) that is adaptive in countering injuries associated with actual physical threat (see Antoni et al., 2012; S. W. Cole et al., 2012; Fredrickson et al., 2013; Irwin & Cole, 2011; Powell et al., 2013). Activation of this ancestral host defense program by nonphysical social, symbolic, anticipated, or imagined threats, however, can increase an individual’s risk for both viral infection and inflammation-related disease.
Figure 1.
Conserved transcriptional response to adversity (CTRA). The innate immune system developed to counter physical threats from predatory animals and hostile conspecifics that dominated our ancestral environment. Exposure to these threats activates a CTRA that involves up-regulation of proinflammatory immune response genes, which combat extracellular pathogens and wound-related bacterial infections, and down-regulation of antiviral immune response genes, which target intracellular pathogens such as viruses. This redeployment of the leukocyte basal transcriptome is adaptive in the context of actual physical threat because it enhances wound healing and recovery from injury and infection. The CTRA can also be activated by modern-day social, symbolic, anticipated, and imagined threats, however, leading to increased risk for several inflammation-related conditions, including depression (see Antoni et al., 2012; S. W. Cole et al., 2012; Fredrickson et al., 2013; Irwin & Cole, 2011; Powell et al., 2013; Slavich & Cole, 2013). Saber-toothed cat image copyright 2013 by Dorling Kindersley; chimpanzee image copyright 2013 by Ronald van der Beek; pointing man image copyright 2013 by Craig Wactor; all other images copyright 2013 by Getty Images. All images reprinted with permission.
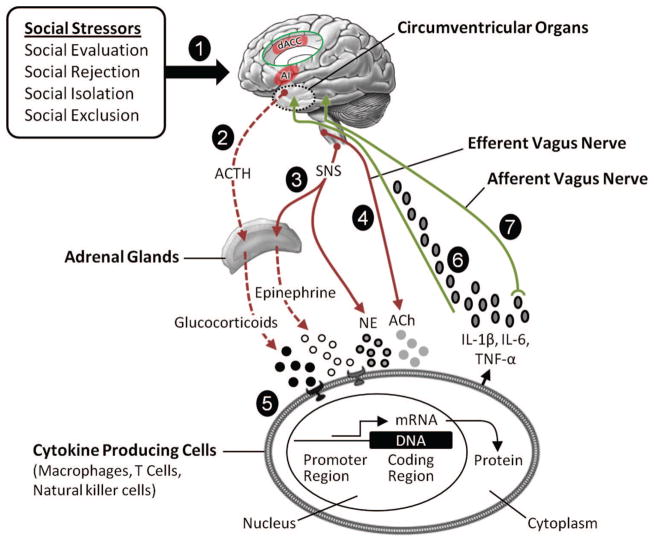
Two physiological pathways are responsible for converting social-environmental adversity into broad proinflammatory transcriptional programs such as the CTRA. The first pathway involves the sympathetic nervous system (SNS), and the second pathway involves the hypothalamic–pituitary–adrenal (HPA) axis (Irwin & Cole, 2011). Additional evidence suggests that the parasympathetic nervous system modulates immune responses at a regional level through both the efferent and afferent fibers of the vagus nerve, enabling it to prevent excessive inflammation (Borovikova et al., 2000; Sternberg, 2006; Tracey, 2009). Because the mechanisms underlying these pathways are described in detail elsewhere (Dantzer et al., 2008; Irwin & Cole, 2011; G. Miller, Chen, & Cole, 2009; Pavlov & Tracey, 2004; Slavich & Cole, 2013; Sternberg, 2006; Waldburger & Firestein, 2010), we only briefly summarize the pathways here, with an emphasis on how effector mechanisms in the SNS and HPA axis regulate inflammation.
Sympathetic nervous system
The first effector pathway that regulates systemic inflammation is the SNS. This pathway allows the central nervous system to “steer” innate immune responses between proinflammatory and antiviral phenotypes (S. W. Cole et al., 2010; Collado-Hidalgo, Sung, & Cole, 2006; Irwin & Cole, 2011). The SNS regulates proinflammatory cytokine production by releasing the neurotransmitter norepinephrine into peripheral tissues, primary and secondary lymphoid organs, and all other major organ systems including the vasculature and perivascular tissues. Once released, norepinephrine modulates immune response gene transcription via stimulation of β-adrenergic receptors (Irwin & Cole, 2011; Nance & Sanders, 2007), although α-adrenergic signaling has also been implicated (Grisanti et al., 2011; Huang et al., 2012). This adrenergic signaling cascade suppresses transcription of antiviral type I IFN genes (S. W. Cole, Korin, Fahey, & Zack, 1998; Lee et al., 2000) and up-regulates transcription of the proinflammatory immune response genes IL1, TNF, and IL6, leading to increases in systemic inflammatory activity (S. W. Cole et al., 2010; Grebe et al., 2010).
Hypothalamic–pituitary–adrenal axis
The second effector pathway involves the HPA axis. Under normal conditions, activation of the HPA axis suppresses (rather than promotes) transcription of both proinflammatory and antiviral immune response genes by stimulating the release of one of the body’s most potent anti-inflammatory substances, the glucocorticoid cortisol, from the adrenal cortex (Berkenbosch, van Oers, del Rey, Tilders, & Besedovsky, 1987; Besedovsky, del Rey, Sorkin, & Dinarello, 1986; Sapolsky, Rivier, Yamamoto, Plotsky, & Vale, 1987). At least three mechanisms mediate the inhibitory effects of glucocorticoid activation on immune response gene transcription. First, glucocorticoid receptor binding to gene promoter sequences can interrupt proinflammatory gene expression. Second, glucocorticoid receptor activation can lead to transcriptional induction of certain anti-inflammatory genes, which inhibit the function of the proinflammatory transcription factor NF-κB and block the inflammatory cascade initiated by this transcription factor. Finally, proinflammatory transcription factors such as NF-κB and AP-1 can antagonize gene transcription via protein–protein interactions (Irwin & Cole, 2011). These mechanisms are critical for ensuring that levels of inflammatory activity are appropriately elevated but do not exceed concentrations that would be dangerous for the organism. Indeed, glucocorticoid feedback inhibition of immune response gene transcription is now recognized as the most fundamental physiological mechanism for protection against diseases that involve excessive inflammation. It is therefore a prototype for some of the most effective anti-inflammatory drugs (Pace, Hu, & Miller, 2007; Rhen & Cidlowski, 2005).
Elevated HPA axis-related inflammation via glucocorticoid resistance
These dynamics characterize HPA axis functioning under normal conditions of typical, intermittent engagement. Under other circumstances, however, a different set of dynamics can emerge, leading to HPA axis-related increases (as opposed to decreases) in inflammation (Avitsur, Stark, & Sheridan, 2001; G. E. Miller, Cohen, & Ritchey, 2002). The process underlying this phenomenon is referred to as glucocorticoid resistance, or glucocorticoid insensitivity, and it occurs when immune cells become less sensitive to the anti-inflammatory effects of glucocorticoids in order to compensate for their persistent secretion (Schleimer, 1993).
Although the reasons for glucocorticoid resistance are unclear, one possibility is that the phenomenon exists because it allows cortisol and proinflammatory cytokines to increase in concert with one another, when such a dynamic would be adaptive. One instance is under conditions of frequent or chronic social-environmental threat, when exposure to actual or perceived danger is persistent (G. E. Miller et al., 2002). Another instance may be under conditions of acute stress that indicate increased social threat or the possibility of physical danger (Duman & Aghajanian, 2012). In such situations, cortisol provides the organism with the metabolic energy it needs to respond to threatening conspecifics; on the other hand, closely timed elevations in proinflammatory cytokines accelerate wound healing and limit infection if an injury occurs.
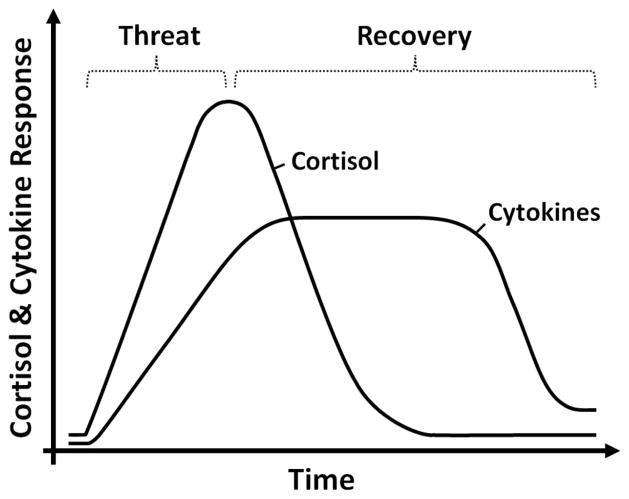
Evidence that even acute stress can alter glucocorticoid sensitivity is provided by research showing that exposure to social cues indicating an increased risk for possible physical danger induces glucocorticoid resistance. This has been demonstrated in animal models by exposing rodents to an aggressive intruder (Avitsur et al., 2001; Engler, Engler, Bailey, & Sheridan, 2005) and in two human studies in which participants were asked to give an impromptu speech in front of a socially rejecting panel of raters (Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009; Rohleder, Schommer, Hellhammer, Engel, & Kirschbaum, 2001). In both of these latter studies, however, the effects of acute stress on glucocorticoid resistance were confined to women, highlighting a need for additional research on this topic. A conceptual model of these cortisol–cytokine dynamics is depicted in Figure 2.
Figure 2.
Cortisol and proinflammatory cytokine responses to social cues indicating possible danger. The hypothalamic–pituitary–adrenal (HPA) axis and inflammatory system coordinate to keep an individual physically safe and biologically healthy. Increases in HPA axis activity and the associated release of cortisol prepare an individual for “fight-or-flight” when he or she is exposed to cues indicating the presence of socially threatening conspecifics. This initial cortisol response has a strong anti-inflammatory effect, which allows the organism to react to the impending threat without being hampered by the onset of sickness behaviors, such as fatigue and social-behavioral withdrawal. As cues of the social threat wane, the body up-regulates the inflammatory response to accelerate wound healing and limit infection caused by possible injury. Glucocorticoid resistance allows for elevations in systemic inflammation to occur together with, or closely after, increases in cortisol. Although these dynamics are adaptive during actual, intermittent physical threat, prolonged activation of the HPA axis and inflammatory response caused by persistent actual or perceived threat is biologically costly and can increase a person’s risk for several inflammation-related conditions including asthma, rheumatoid arthritis, cardiovascular disease, chronic pain, metabolic syndrome, and (possibly) certain cancers.
A complete review of the mechanisms underlying glucocorticoid resistance is beyond the scope of this article. However, it is important to note that because of glucocorticoid resistance, HPA axis processes that support adaptive “fight or flight” responses to social-environmental threat can become altered and promote excessive inflammation, especially if they are frequently or chronically engaged. These mechanisms thus have implications for mental and physical health (Marques, Silverman, & Sternberg, 2009; McEwen, 1998, 2008; McEwen & Seeman, 1999). In the context of depression, for example, individuals with MDD have been found to have flatter diurnal cortisol slopes (i.e., higher overall cortisol concentrations) than persons without MDD, and 46% of the variability in diurnal cortisol slope in depressed individuals may be explained by their levels of glucocorticoid sensitivity (Jarcho, Slavich, Tylova-Stein, Wolkowitz, & Burke, 2013; see also Anacker, Zunszain, Carvalho, & Pariante, 2011; Fries, Hesse, Hellhammer, & Hellhammer, 2005; Pace et al., 2007, 2011). Evidence of altered glucocorticoid sensitivity has also been found in several other disorders including anxiety, posttraumatic stress disorder, asthma, rheumatoid arthritis, cardiovascular disease, inflammatory bowel disease, autoimmune diseases, and some cancers (Chen & Miller, 2007; G. E. Miller & Chen, 2006; O’Donovan, Slavich, Epel, & Neylan, 2013; Pace & Heim, 2011; Pace & Miller, 2009). Although the role that altered glucocorticoid sensitivity plays in the etiology of these disease conditions remains unclear, there is little doubt that aberrant glucocorticoid signaling is a mechanism that can influence the maintenance or progression of these disorders (for reviews, see Barnes & Adcock, 2009; Biddie, Conway-Campbell, & Lightman, 2012; Marques et al., 2009).
Summary: The Immune System, Cytokines, and Inflammation
To summarize, the immune system plays a critical role in keeping the body biologically healthy, especially during times of physical injury, wounding, and infection. A key component of this system is the inflammatory response, which is mediated by pro-and anti-inflammatory cytokines that identify, neutralize, and eliminate foreign pathogens such as bacteria and viruses. Inflammation is regulated most proximally by the expression of immune response genes including IL1B, IL6, and TNF. When activated, these genes promote the secretion of proinflammatory cytokines that mediate systemic inflammation. Inflammation is also regulated more distally by processes occurring in the brain, which detects social-environmental cues indicating possible danger. This neuro-inflammatory link is highly adaptive insofar as it can activate the CTRA before a physical injury or bacterial infection takes place. A downside of central regulation of systemic inflammation, however, is that it gives social, symbolic, and anticipated threats—including those that have not yet happened or that may never actually occur—the ability to activate the CTRA in the absence of actual physical threat. Under normal conditions, the SNS up-regulates CTRA-related inflammatory activity via stimulation of β-adrenergic receptors, and the HPA axis down-regulates CTRA-related inflammatory activity via the production of cortisol. However, under conditions of prolonged actual or perceived threat, or possibly during acute stressors indicating social threat or physical danger, glucocorticoid resistance can develop, leading to excessive inflammation that increases a person’s risk for several disorders including depression, especially if activation of these pathways is prolonged.
Stress and Depression
The preceding section provides an overview of inflammatory biology and describes how systemic inflammation is regulated most proximally at the level of the genome and more distally by the SNS and the HPA axis. Because the SNS and the HPA axis originate in the brain and receive input from brain structures that monitor events in the external social world (Eisenberger & Cole, 2012), a biologically plausible pathway exists by which stressors occurring in the social environment may influence inflammatory activity (Irwin & Cole, 2011; G. Miller et al., 2009). A natural question based on the existence of these pathways is whether social stress is related to depression and, if so, whether inflammatory processes mediate this effect. To address this question, we first summarize the large literature demonstrating that stress is strongly associated with depression. Then, in the next section, we discuss studies showing that early life stress, adulthood life stress, and laboratory-based social stressors are associated with increased inflammatory activity.
Stress Is Associated With Major Depression
One of the most robust and well-documented effects in depression research involves the finding that stressful life events substantially increase a person’s risk for MDD (Kendler et al., 1995, 1999; Monroe, Slavich, Torres, & Gotlib, 2007a; for reviews, see Brown & Harris, 1978, 1989; Hammen, 2005; Kessler, 1997; Mazure, 1998; Monroe, Slavich, & Georgiades, 2009). One class of life events that has been found to be particularly strongly related to depression is major life events. These events cause substantial cognitive upheaval and disruption to a person’s goals, plans, and aspirations, and include stressors such as the termination of a close confidant or romantic relationship, a significant financial loss, major health-related events (e.g., cancer diagnosis, heart attack, death), and the ending of an important job (e.g., due to being laid off or fired; Brown & Harris, 1978).
As reviewed by Monroe et al. (2009), the most conservative estimate of the relation between stress and depression—which is based on a patient sample and restricted to fateful life events that are entirely independent of the person’s actions or behaviors—is that depressed persons have a 2.5-fold greater likelihood of experiencing a major life event prior to onset of depression compared to nondepressed persons during the same time period (Mazure, 1998; Shrout et al., 1989). A more liberal estimate, which includes any type of preonset major life event, raises the risk estimate several fold to an odds ratio of 9.38 for a first lifetime episode of MDD (Kendler, Thornton, & Gardner, 2000). Based on these estimates, exposure to recent major life stress is considered to be the strongest proximal risk factor for MDD in community samples, with up to 80% of major depressive episodes in the general population being precipitated by such stress (Mazure, 1998). This association persists across the entire life course and is especially strong for women in almost all age groups (Harkness et al., 2010). Somewhat remarkably, major life events emerge as the strongest risk factor for MDD even when examined in multifactorial models that simultaneously evaluate the effects of stress with other well-known risk factors for depression (Kendler, Gardner, & Prescott, 2002; Kendler, Kessler, Neale, Heath, & Eaves, 1993).
Interpersonal loss, social rejection, and depression
The vast majority of studies on stress and depression have conceptualized major life stress as a singular, unitary construct. Within this framework, life events of different types, with different social-psychological characteristics, have been regarded as functionally equivalent with respect to their impact on depression (Monroe & Slavich, 2007; Monroe et al., 2009). Against this backdrop, though, is a growing body of research that has taken a more refined approach to conceptualizing life stress. Emerging from this work is evidence that different types of life events may actually have different effects on depression depending on their core features and social-psychological characteristics (Cramer, Borsboom, Aggen, & Kendler, 2012; Keller, Neale, & Kendler, 2007; Muscatell, Slavich, Monroe, & Gotlib, 2009; Slavich & Epel, 2010).
The type of stress that has received the most attention in this context is interpersonal loss (Paykel, 2003). This is due in large part to the evolutionarily adaptive benefits that accompany the maintenance of close social bonds (Ainsworth, 1991; Baumeister & Leary, 1995; Bowlby, 1980; Gilbert, 1992; Sloman & Gilbert, 2000). As a result of these benefits, which include the provision of food, shelter, emotional comfort, and physical security, it has been hypothesized that interpersonal loss events that disrupt important social bonds may evoke intense distress, particularly if they involve social rejection, which signals a loss of social status, value, and regard (Dickerson, Gruenewald, & Kemeny, 2009; Kemeny, 2009). In sum, then, although many types of major life events can induce cognitive upheaval and threaten a person’s way of life, for the reasons outlined here, those that involve interpersonal loss and social rejection have been hypothesized to be particularly strongly associated with the type of emotional distress that is characteristic of depression (Slavich, O’Donovan, Epel, & Kemeny, 2010).
Consistent with this theorizing, a substantial body of evidence now exists showing that major life events involving interpersonal loss or social rejection are particularly strongly related to MDD (Slavich, O’Donovan, et al., 2010). An immediate question that comes to mind is whether such effects are simply driven by differences in the severity, or contextual threat, of interpersonal loss versus noninterpersonal loss events. Interestingly, however, a primacy for interpersonal loss and social rejection life events in predicting depression emerges even when interpersonal loss events are compared to other stressors of the same severity level (Slavich, O’Donovan, et al., 2010). As it turns out, major life events involving interpersonal loss are the most common precipitants of MDD, with up to 44% of depressive episodes being preceded by such stress (Brown, Harris, & Hepworth, 1995; Farmer & McGuffin, 2003). In addition, two large epidemiologic studies (one with adolescents and one with adults) have shown that risk for depression is greater following a major interpersonal loss event (hazard ratios = 1.70 and 1.76, respectively) than for any other type of major life stress (Kendler et al., 2003; Monroe, Rohde, Seeley, & Lewinsohn, 1999).
Interestingly, risk for depression appears to be greatest when interpersonal loss is coupled with social rejection. In one of the largest studies on this topic to date, more than 7,300 community-dwelling adults were administered a semistructured interview that assessed participants’ exposure to a range of life events that often precede depression. Reported events were subsequently rated in terms of their long-term contextual threat, in addition to the extent to which they involved loss, humiliation, danger, and entrapment. The most striking finding emerged in analyses that examined risk for MDD following different types of interpersonal loss. Specifically, these analyses revealed that major interpersonal loss events that were self-initiated (e.g., subject divorced his or her spouse) conferred a 10.2-fold increase in risk for depression. However, interpersonal loss events that were initiated by another person (e.g., subject was broken up with) conferred a 21.6-fold increase in risk for depression (Kendler et al., 2003). Thus, risk for depression more than doubled based on the presence of social rejection. Because all of the life events included in these analyses were rated as having high long-term contextual threat, this differential risk for MDD by event type is not likely due to differences in stressor severity.
In a more recent study, Slavich, Thornton, Torres, Monroe, and Gotlib (2009) assessed depressed participants’ exposure to major life events using the state-of-the-art Life Events and Difficulties Schedule (LEDS; Brown & Harris, 1978). This system employs a 2-hr semistructured interview that systematically inquires about many different types of stress and an independent panel of raters who judge the contextual threat and social-psychological characteristics of each stressor that is identified (Dohrenwend, 2006; Monroe, 2008). All life events that occurred prior to onset of MDD were also evaluated to determine whether they involved targeted rejection, defined as “social rejection that is directed at, and meant to affect, a single person, and that involves an active and intentional severing of relational ties with that person” (Slavich et al., 2009, p. 225). As predicted, individuals who experienced a recent major life event involving targeted rejection became depressed 3 times faster (i.e., in one third as many days) than their counterparts who experienced other forms of severe life stress (Slavich et al., 2009). Again, because the life events included in these analyses were all rated severe according to the LEDS system, the relatively quicker onset of MDD following targeted rejection versus nontargeted rejection life stress is likely due to the presence of targeted rejection, as opposed to differences in the severity of targeted rejection versus nontargeted rejection life events.
Summary: Stress and Depression
In sum, a large literature exists demonstrating that major life events, especially those involving interpersonal loss and social rejection, are a key proximal risk factor for MDD. As it turns out, these stressors have been implicated not just in the development of depression but in the onset, exacerbation, or progression of a variety of health problems. These conditions include several that, like depression, are thought (or known) to be mediated at least in part by inflammation, such as asthma, rheumatoid arthritis, cardiovascular disease, chronic pain, and certain cancers (Bower, Crosswell, & Slavich, 2014; Chrousos, 2009; Cohen, Janicki-Deverts, & Miller, 2007; Cutolo & Straub, 2006; Kivimäki et al., 2006; Loeser & Melzack, 1999; Lutgendorf et al., 2012, 2013; A. H. Miller, 2008; Reiche, Nunes, & Morimoto, 2004; Steptoe & Kivimäki, 2012; Walker, Littlejohn, McMurray, & Cutolo, 1999; R. J. Wright, 2011). As a result, we turn now to the question of whether stress is associated with inflammation.
Stress and Inflammation
A large number of naturalistic and laboratory-based studies have examined whether stress is associated with inflammation. As we review below, these studies strongly support such an association. Moreover, consistent with the CTRA model presented earlier (see Figure 1) and with the data just described linking social rejection and depression, associations between stress and inflammation appear to be particularly strong for stressors that indicate the presence of possible physical or social threat. To examine these effects, we first review studies showing that early life stress, adulthood life stress, and laboratory-based social stressors are associated with increased inflammatory activity. Then, we discuss neural, genetic, and genomic mechanisms that may be responsible for converting experiences of social stress into inflammation.
Early Life Stress Is Associated With Elevated Inflammation
The most well-developed body of research on early life stress and inflammation has examined the relation of childhood family environment and socioeconomic status to levels of inflammatory activity in healthy adolescents and adults. In this context, exposure to a childhood environment characterized by unpredictability and interpersonal stress has been related to greater in vitro LPS-stimulated IL-6 production in adolescents (G. E. Miller & Chen, 2010) and to elevated circulating levels of the inflammatory marker CRP in young adults (Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Taylor, Lehman, Kiefe, & Seeman, 2006). A socially tumultuous early environment has also been found to predict elevated levels of IL-6 in a population-based study of young adults (Cho, Bower, Kiefe, Seeman, & Irwin, 2012; Slopen et al., 2010), higher levels of IL-6 and TNF-α in a community sample of older adults (Kiecolt-Glaser, Gouin, et al., 2011; cf. Carpenter, Gawuga, Tyrka, & Price, 2012), and greater IL-6 responses to daily life stressors in a cross-sectional study of older adults (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012; see also Carroll et al., 2013). To examine whether early life stress prospectively predicts elevated inflammation, Slopen, Kubzansky, McLaughlin, and Koenen (2013) analyzed data from a population-based prospective longitudinal study of more than 4,600 children. Exposure to acute life events (e.g., physically or sexually abused, separated from mother or father, taken into foster care) was assessed at seven time points between 1.5 and 8 years of age, and levels of plasma IL-6 and CRP were measured at age 10. CRP was also measured again at age 15. As predicted, greater cumulative stress exposure before age 8 predicted higher levels of IL-6 and CRP at age 10 and higher levels of CRP at age 15 (Slopen et al., 2013).
Low socioeconomic status in childhood, in turn, has been related to higher levels of circulating IL-6 and CRP in adults (Appleton et al., 2012; Carroll, Cohen, & Marsland, 2011; Taylor et al., 2006). Low socioeconomic status in childhood has also been associated with a shifting of the leukocyte basal transcriptome toward a more proinflammatory phenotype in adolescence in a manner that is independent of a person’s socioeconomic status in adolescence (G. Miller & Chen, 2007). The transcriptional shift observed in these low early-life socioeconomic status individuals is characterized in part by increased expression of genes that code for TLR4, which is involved in activating the innate immune system response, and by decreased expression of genes that code for the glucocorticoid receptor, which (as we described earlier) is responsible for down-regulating inflammation in response to cortisol (see also S. W. Cole, 2008; G. Miller & Chen, 2007).
Early life stress and inflammation in depression
Early life stress has also been associated with inflammatory activity in the context of depression. In one of the largest prospective studies on this topic to date, depressed adults who experienced severe forms of early life stress (e.g., maternal rejection, harsh discipline, physical or sexual abuse) were 1.48 times more likely to have clinically high levels of CRP (>3 mg/L) than depressed adults who did not experience these severe forms of early life stress (Danese et al., 2008; see also Pace et al., 2012). Interestingly, although depression was also strongly associated with high CRP levels, this association was no longer significant when analyses adjusted for the effects of early life stress (Danese et al., 2008). In a more recent study that followed adolescent women at elevated risk for depression over 2.5 years, adolescents with a history of more common forms of early life stress, such as low socioeconomic status or parental separation, had greater increases in both IL-6 and CRP when becoming depressed than their counterparts without a history of these early life stressors. Moreover, whereas adolescents in this study who did not have a history of early life stress exhibited reductions in CRP as their depressive symptoms abated, those with a history of early life stress showed no such association, instead exhibiting elevations in CRP that persisted in the absence of depression (G. E. Miller & Cole, 2012).
Finally, there is evidence that associations between stress, inflammation, and depression may be detectable at a relatively young age. For example, a recent longitudinal study found that children who were exposed to physical maltreatment before age 10 and depressed at age 12 exhibited significantly higher levels of CRP relative to children with depression only, maltreatment only, and neither depression nor maltreatment (Danese et al., 2011; for a review, see Taylor, Way, & Seeman, 2011). Considered together, then, at least three studies on early life stress, inflammation, and depression suggest that exposure to early life stress may contribute uniquely to elevations in inflammation that are evident in depression.
Adulthood Life Stress Is Associated With Elevated Inflammation
There is also a large body of research demonstrating that naturally occurring social stressors in adolescence and adulthood are associated with elevated levels of inflammatory activity. These effects have been detected for both acute and chronic forms of life stress and are particularly strong for social stressors involving conflict, threat, isolation, and rejection (for reviews, see Herbert & Cohen, 1993b; Kiecolt-Glaser, Gouin, & Hantsoo, 2010; Segerstrom & Miller, 2004). Paralleling the early life stress findings, for example, lower socioeconomic status in adulthood has been found to be related to increased levels of CRP and IL-6 (Gruenewald, Cohen, Matthews, Tracy, & Seeman, 2009; see also Petersen et al., 2008; Pollitt et al., 2007). Moreover, at least two studies have shown prospective associations between lower socioeconomic status and elevated levels of CRP (Deverts, Cohen, Kalrab, & Matthews, 2012; Gimeno et al., 2007). In addition, several studies have demonstrated that negative social interactions involving friends, peers, teachers, or family members in daily life are associated with elevations in several markers of inflammatory activity, including CRP, IL-6, and a soluble receptor for TNF-α (Chiang, Eisenberger, Seeman, & Taylor, 2012; Fuligni et al., 2009; Marin, Chen, Munch, & Miller, 2009).
Although most studies have examined the effects of social stressors occurring over several days or years, there is also some evidence that experiencing one major life event may be sufficient for up-regulating inflammatory activity if it involves interpersonal loss or social rejection. For example, Schultze-Florey and colleagues (2012) found that older adults who experienced the recent death of their spouse had higher IL-1 and IL-6 activity than nonbereaved older adults. This effect was moderated by a variant in the IL-6 gene (IL6–174), which modified individuals’ likelihood of exhibiting high levels of inflammation following bereavement. Specifically, SNS activation caused by bereavement-related distress led to GATA1 transcription factor activation that mediates up-regulation of IL-6 production but only for individuals with two GATA1-sensitive-174G alleles (Schultze-Florey et al., 2012). A different but conceptually similar study suggested that these effects may be due in part to the fact that at least some recently bereaved individuals have flatter diurnal cortisol slopes across the day (O’Connor, Wellisch, Stanton, Olmstead, & Irwin, 2012).
Associations between recent major life events and increased inflammatory activity have also been found for social rejection. For example, M. L. M. Murphy, Slavich, Rohleder, and Miller (2013) assessed 147 adolescent girls at elevated risk for depression every 6 months for 2.5 years. Exposure to recent life stress was measured using the UCLA Life Stress Interview (Adrian & Hammen, 1993), and levels of inflammatory gene expression were indexed by quantifying leukocyte mRNA for the proinflammatory transcription factor NF-κB and inhibitor of κB (I-κB), which regulates the effects of NF-κB. Multilevel modeling analyses designed to test within-person associations between targeted rejection and inflammatory gene expression revealed that participants had significantly higher levels of mRNA for both NF-κB and I-κB at visits when they had experienced a recent targeted rejection life event compared to visits when no such event had occurred. Consistent with the notion that these effects may also be due in part to altered glucocorticoid dynamics, a different study reported that experiencing even one recent major life event (as rated by the LEDS) predicts greater glucocorticoid resistance (Cohen et al., 2012). As suggested previously, therefore, social stress-related reductions in glucocorticoid sensitivity may be one mechanism linking social threat and rejection with elevated inflammation and risk for depression (Slavich, O’Donovan, et al., 2010).
One of the largest bodies of research on adulthood social stress and inflammation has focused on the strength and quality of a person’s social connection to other people. This work has been fueled by the provocative finding that having stronger social bonds—as characterized by better social integration and/or social support—decreases risk for mortality by up to 50% (Holt-Lunstad, Smith, & Layton, 2010). This effect is striking because it equals the health benefits conferred by quitting smoking and exceeds the benefits associated with the absence of other major risk factors for disease, including excessive alcohol consumption, obesity, and physical inactivity (Holt-Lunstad et al., 2010).
Consistent with the possibility that these effects of social connection and isolation on health are mediated at least in part by inflammatory processes, a community-based case-cohort study and a nationally representative cohort study recently revealed that socially isolated individuals are approximately 2.0–2.5 times more likely to have clinically high levels of CRP than socially integrated individuals (Ford, Loucks, & Berkman, 2006; Heffner, Waring, Roberts, Eaton, & Gramling, 2011). Social isolation has also been related to the up-regulated expression of proinflammatory immune response genes and a reciprocal down-regulation of genes involved in antibody production (S. W. Cole, Hawkley, Arevalo, & Cacioppo, 2011). Finally, one study found that levels of CRP and IL-6 are 2.0 and 3.8 times higher, respectively, for socially isolated depressed men than for socially integrated nondepressed men, suggesting that the combination of social isolation and depression has a robust effect on inflammation (Häfner et al., 2011; see also Goldman-Mellor, Brydon, & Steptoe, 2010).
Laboratory-Based Social Stressors Trigger Inflammatory Activity
The most carefully controlled research on stress and inflammation has occurred in the laboratory setting, where stressors can be standardized and changes in inflammatory activity can be closely monitored. This research has revealed that social stressors are potent triggers of systemic inflammation (Steptoe et al., 2007). Paralleling findings from the life events and depression literature (described above), stressors that evoke the strongest inflammatory responses in the laboratory are those that involve social conflict, rejection, or exclusion (Denson et al., 2009; Dickerson, Gruenewald, & Kemeny, 2009; Kemeny, 2009). Examples of such stressors include writing about traumatic experiences involving self-blame, which has been found to trigger increases in a soluble receptor for TNF-α (i.e., sTNF-RII), as well as higher levels of self-reported shame (Dickerson, Kemeny, Aziz, Kim, & Fahey, 2004). Shame, in turn, is a cardinal feature of many depressive experiences (Kim, Thibodeau, & Jorgensen, 2011). In a more recent study, married couples were brought into a laboratory and asked to engage in a social support interaction during a first study visit and a hostile marital interaction during a second study visit. Although couples who were judged to be low in hostility were relatively unaffected by these tasks, couples who were high in hostility exhibited significantly greater increases in plasma IL-6 and TNF-α following the hostile marital interaction than following the social support interaction (Kiecolt-Glaser et al., 2005).
Most laboratory studies on stress and inflammatory biology have utilized a social stress task called the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993). Participants in this paradigm are asked to prepare and give an impromptu speech and to perform difficult mental arithmetic in front of a nonresponsive, socially rejecting panel of raters. In a recent study that examined the effects of the TSST on proinflammatory cytokine regulation, individuals who completed the TSST in the presence of socially rejecting raters exhibited greater in vitro LPS-stimulated production of TNF-α and greater glucocorticoid resistance than individuals who performed the TSST in the absence of these raters (Dickerson, Gable, et al., 2009). Interestingly, participants judged these two conditions to be equally challenging, controllable, and difficult. However, individuals who felt more evaluated in both conditions of the TSST exhibited greater increases in TNF-α production, even after controlling for participants’ perceptions of TSST-related challenge, controllability, and difficulty. A related TSST study found that exposure to socially rejecting raters triggered increases in cortisol, but only when the raters were being evaluative (Dickerson, Mycek, & Zaldivar, 2008; cf. Taylor et al., 2010). Consistent with the hypothesis that rejection-related stressors are the strongest regulators of HPA axis processes that influence inflammation, a meta-analytic review of 208 laboratory studies revealed that stressors characterized by low controllability and high social-evaluative threat trigger the greatest cortisol responses and the slowest recovery of cortisol to baseline levels (Dickerson & Kemeny, 2004).
Individual differences in laboratory-based inflammatory responding
Generally speaking, these studies have investigated the question of whether laboratory-based social stressors trigger increases in inflammation on average. Because the brain plays a critical role in appraising stressors, as well as in modulating immune system reactivity to physical and social threat (McEwen & Gianaros, 2010; Pavlov & Tracey, 2004; Sternberg, 2006), it might be expected that individual differences exist in the extent to which people mount an inflammatory response to social stress (Slavich, O’Donovan, et al., 2010). The few studies that examined this issue assessed participants’ psychological perceptions of, or emotional reactions to, a laboratory-based social stressor and then used this information to predict their inflammatory responses to the task. These studies provide preliminary evidence that, as expected, stress-related appraisals and reactions appear to modulate inflammatory responding to acute social stress.
In this context, for example, individuals who report experiencing more fear to the TSST have been found to exhibit greater increases in the proinflammatory marker sTNF-RII (Moons, Eisenberger, & Taylor, 2010). In a second study, greater levels of TSST-induced perceived stress predicted greater increases in circulating IL-1β (Yamakawa et al., 2009; see also Prather et al., 2009). A third study reported that a public speaking task similar to the TSST induced feelings of anxiety, depression, and anger; greater increases in anxiety and anger were, in turn, independently related to greater increases in circulating IL-6 (Carroll, Low, et al., 2011). Finally, a fourth study found that greater difficulty in maintaining a positive cognitive–affective state during the TSST was associated with greater increases in circulating IL-1β and that increases in circulating IL-1β in turn predicted increases in depressive symptoms over the following year. In fact, IL-1β reactivity to the TSST significantly mediated the relation between cognitive–affective responses to the TSST and future increases in depressive symptoms over the 1-year follow-up period (Aschbacher et al., 2012; for a review, see Campbell & Ehlert, 2012).
Factors that moderate laboratory-based inflammatory responding
Finally, a small number of studies have examined factors that moderate inflammatory responses to acute social stress in the laboratory. One such study exposed psychiatrically healthy adults with differing histories of early life stress to the TSST (Carpenter et al., 2010). Experiencing more early life stress, as assessed by the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994), was unrelated to baseline levels of IL-6 but strongly associated with greater increases in IL-6 in response to the TSST. Subsequent analyses compared individuals with little or no early life stress to persons with moderate to severe early life stress. Again, although these groups had similar baseline levels of IL-6, participants who experienced moderate to severe early life stress exhibited significantly greater IL-6 responses to the TSST than their counterparts with little or no early life stress (Carpenter et al., 2010). In another report of two separate studies, participants who had more feelings of trait loneliness exhibited greater LPS-stimulated production of TNF-α, IL-6, and IL-1β in response to the TSST compared to those who were less lonely (Jaremka et al., 2013; see also Hackett, Hamer, Endrighi, Brydon, & Steptoe, 2012).
In addition, at least three studies have examined whether depression status moderates the effects of acute social stress on inflammatory responding in the laboratory. Weinstein and colleagues (2010) exposed depressed and nondepressed adults to a variant of the TSST in which participants were asked to give a speech about a recent incident in which they experienced anger or frustration; next, they completed a mental arithmetic task. Compared to nondepressed participants, depressed participants exhibited greater TSST-induced increases in several markers of SNS and HPA axis activity, including epinephrine, norepinephrine, adrenocorticotropic hormone, and cortisol. Depressed participants also exhibited greater increases in plasma levels of the proinflammatory cytokines TNF-α and IL-6 and the inflammatory marker CRP (Weinstein et al., 2010).
G. E. Miller, Rohleder, Stetler, and Kirschbaum (2005) also exposed depressed and nondepressed adults (all women) to a variant of the TSST that involved preparing and then giving a 17-min mock job interview, followed by a difficult puzzle-solving task. This experience evoked increases in anxiety and shame and greater LPS-stimulated production of TNF-α and IL-6 for both depressed and nondepressed women. Compared to nondepressed women, however, depressed women exhibited elevations in CRP that persisted following the TSST (G. E. Miller et al., 2005). This study did not identify specific mechanisms that accounted for these differences in temporal CRP response, but several interrelated factors that are independent risk factors for depression have been found to be associated with persistently high levels of CRP and may contribute to these effects, including having a sedentary lifestyle, high body mass index, and being female (Ishii et al., 2012).
Finally, Pace and colleagues (2006) examined inflammatory responses to the TSST for depressed individuals (all male) with elevated early life stress compared to nondepressed persons. Compared to nondepressed individuals, depressed individuals with early life stress exhibited greater increases in IL-6 and NF-κB to the TSST, as well as higher levels of IL-6 during the post-TSST recovery period. When examined together in a multivariate regression model, greater depression severity, as assessed by the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), was an independent predictor of TSST-induced increases in both IL-6 and NF-κB while adjusting for the effects of early life stress on inflammatory activity. In contrast, early life stress, as assessed by the CTQ (Bernstein et al., 1994), was unrelated to TSST-induced changes in IL-6 and NF-κB while adjusting for the effects of depression on inflammation (Pace et al., 2006).
Neural Mechanisms Underlying Stress-Induced Inflammatory Responding
Although there is growing interest in understanding neural mechanisms that underlie inflammatory responding to social stress, to our knowledge, only one study has examined this issue to date (Slavich, Way, Eisenberger, & Taylor, 2010). In this study, 124 healthy young adults completed the TSST while levels of IL-6 and sTNF-RII were quantified from oral fluids. In a separate experimental session, a subset of these participants (n = 31) had their neural activity assessed using fMRI while they played a computerized ball-tossing game called Cyberball, in which they were ultimately excluded by two other supposed players. As predicted, the social rejection experience evoked while playing Cy-berball engaged brain regions that have been previously implicated in processing negative affect and social rejection-related distress—namely, the bilateral anterior insula and dorsal anterior cingulate cortex (dACC). Greater activity in these brain regions was in turn related to greater sTNF-RII responses to the TSST, indicating that individuals who are more neurally sensitive to social rejection exhibit greater inflammatory responses to acute social stress (Slavich, Way, et al., 2010). Although the manner by which these brain regions regulate inflammation is complex (Pavlov & Tracey, 2004), the anterior insula and dACC are key nodes in a network of brain regions known to be engaged during experiences of physical pain (Eisenberger, 2012a, 2012b). This may thus explain, at least in part, why activity in these brain regions is associated with inflammatory responding to social stress.
Using a different strategy for examining this issue, Eisenberger, Inagaki, Rameson, Mashal, and Irwin (2009) randomly assigned 39 healthy adults to receive either an inflammatory challenge (i.e., bacterial endotoxin) or placebo (i.e., saline) via intravenous injection. Participants then had their neural activity assessed using fMRI while they played Cyberball. As expected, administration of bacterial endotoxin triggered increases in cortisol, IL-6, physical sickness symptoms, and depressive mood. Increases in IL-6 were in turn associated with greater neural activity in the anterior insula and dACC, which are the same brain areas that emerged as being relevant for inflammation in the study described above. Further confirming the relevance of these regions for inflammation, activity in these brain regions mediated the association between endotoxin-induced increases in IL-6 and depressive mood in female participants. Endotoxin-induced increases in IL-6 were also related to greater activity in several neural regions that have been implicated in mentalizing, or the process of thinking about the content of other people’s minds, including the medial and dorso-medial prefrontal cortex, posterior cingulate cortex, and precuneus (Eisenberger et al., 2009).
When considered together with the results of the previous study, these data suggest a possible bidirectional link between systemic inflammation and pain-related neural systems in the brain. Namely, whereas the former study showed that activity in pain-related neural circuitry is associated with inflammatory responses to acute social stress, the latter study showed that greater endotoxin-induced increases in inflammation are associated with greater activity in pain-related neural systems. This bidirectional link between systemic inflammation and brain regions that process experiences of pain may be adaptive in dangerous environments insofar as it prepares the body to deal with possible physical threat or injury. As we illustrate in Figure 3, however, the fact that neural responses to contemporary threats like social evaluation and rejection may up-regulate systemic inflammation, and that increases in inflammation may in turn promote neural sensitivity to social rejection, suggests that activity in pain-related neural systems and systemic inflammation may be mutually promoting and, over time, become engaged in a recursive loop that increases levels of inflammation and risk for depression (see Slavich & Cole, 2013).
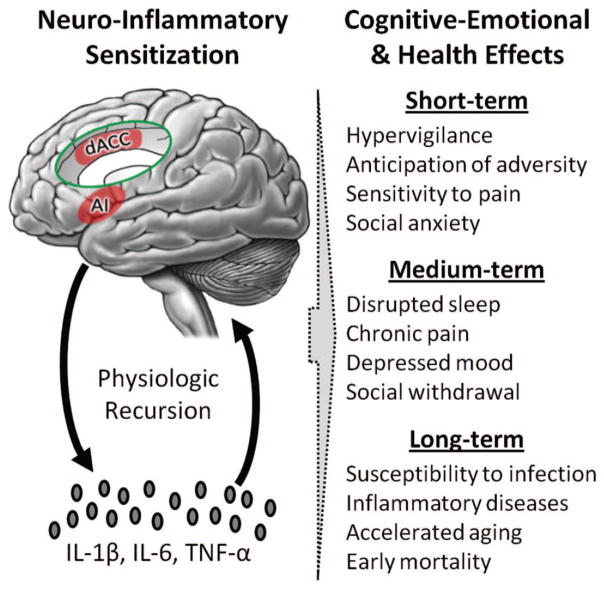
Figure 3.
Neuro-inflammatory sensitization to adversity. Bidirectional links between the brain and periphery allow the brain to regulate inflammatory activity, and inflammatory activity to in turn influence neural processes in the brain. This dynamic is initiated by experiences of early life stress or chronic adversity, which promote a proinflammatory skewing of the leukocyte basal transcriptome (i.e., the conserved transcriptional response to adversity [CTRA]) that feeds back on pain-related neural systems to perpetuate subjective perceptions of threat. Brain regions involved in this process include the anterior insula (AI) and dorsal anterior cingulate cortex (dACC, shown in the insert). As a result of this physiologic recursion, experiences of social-environmental adversity can become biologically embedded and sustain perceptions of threat for months or years after the original social-environmental impetus has passed. The consequences of these dynamics are multifold and start with increased hypervigilance, chronic anticipation of adversity, sensitivity to pain, and symptoms of social anxiety. As activation of the CTRA persists, somatic and affective symptoms of depression may develop. Finally, after years of sustained engagement, these dynamics may confer increased risk for inflammation-related disorders, infection, accelerated biological aging, and early mortality (see Eisenberger & Cole, 2012; G. E. Miller, Chen, & Parker, 2011; G. E. Miller & Cole, 2012; Slavich & Cole, 2013). IL-1β = interleukin-1β; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α.
Genetic and Genomic Mechanisms Underlying Stress-Induced Inflammatory Responding
Recent studies have also examined genetic factors that moderate inflammatory responding to social stress and genome-wide transcriptional changes that may give experiences of social threat the ability to affect health. One line of research on this topic has involved identifying genetic variants, or single-nucleotide polymorphisms (SNPs), that predict differences in inflammatory responses to social stress. A locus of interest in this context is a functionally active regulatory SNP in the human IL6 promoter (rs1800795; Fishman et al., 1998). A recent study that examined the effects of this SNP on inflammation found that high levels of social-environmental stress were associated with increased mortality risk for rs1800795 G homozygotes; C allele carriers, in contrast, exhibited no increases in mortality risk under high levels of stress (S. W. Cole et al., 2010). Far from negligible, this effect amounted to a 2.8-year shorter average life span for persons who were homozygous for the G allele. Consistent with the prediction that this association is mediated by inflammation, the effect of this SNP on mortality was completely attenuated when the survival analyses adjusted for levels of CRP. In addition, the effect of this genotype on mortality risk was entirely specific to inflammation-related causes of death (e.g., cardiovascular and neurodegenerative diseases).
These findings demonstrate that genetic factors interact with social-environmental conditions to predict systemic inflammation in ways that are relevant for health and mortality. The effect of a particular polymorphism on health, however, is realized only when a gene is expressed—that is, when DNA is transcribed into RNA and translated into protein (Slavich & Cole, 2013). As a result, a handful of studies have searched for differences in the expression of immune response genes that are involved in inflammation.
As we have reviewed here, some studies have investigated this issue by testing whether levels of mRNA for the intracellular transcription factor NF-κB differ for individuals experiencing high versus low levels of adversity. As we have also discussed, however, several studies have demonstrated that literally hundreds of genes are differentially expressed as a function of several types of social adversity, including being socially isolated or low in social status, or experiencing high levels of chronic interpersonal stress (e.g., Chen et al., 2009; S. W. Cole et al., 2007, 2011; G. E. Miller et al., 2008; G. E. Miller, Rohleder, & Cole, 2009; see also Tung et al., 2012). This work is provocative insofar as it indicates that whole gene programs or gene profiles, such as the CTRA, can be activated by adverse social conditions (S. W. Cole, 2010; Slavich & Cole, 2013). Consistent with the fact that these gene programs are relevant for health, a small number of studies have found genome-wide transcriptional shifts in persons with asthma, ovarian cancer, breast cancer, and posttraumatic stress disorder (Bower, Ganz, Irwin, Arevalo, & Cole, 2011; Chen et al., 2009; Lutgendorf et al., 2009; O’Donovan et al., 2011). To our knowledge, however, no studies to date have tested for differences in the leukocyte basal transcriptome of depressed versus nondepressed individuals (for related work, see S. W. Cole, 2009, 2010; Fredrickson et al., 2013; A. H. Miller et al., 2009; Pace & Miller, 2009).
Summary: Stress and Inflammation
To summarize, data from several lines of research converge to demonstrate that stress is associated with elevated inflammatory activity. These effects are apparent for both early life stress and adulthood life stress, and they have been demonstrated at the protein level (i.e., proinflammatory cytokines), intracellular signaling level (i.e., transcription factors), and genome-wide expression level (i.e., gene programs, transcriptional skewing). Although little attention is typically paid to the social-psychological characteristics of stress that are most relevant for depression, studies that have taken this information into account have suggested that the types of stressors most strongly associated with inflammation are those that involve cues indicating possible increased risk for physical or social threat. Such stressors include social conflict, evaluation, rejection, isolation, and exclusion. Social-environmental conditions related to these experiences are also implicated, including low socioeconomic status, which can be accompanied by a more risky or dangerous physical environment, and low social status, which can indicate elevated risk for social isolation or exclusion. The fact that these stressors are most consistently related to increased inflammatory activity is interesting insofar as it directly parallels the literature on life stress and depression, which has shown that life events involving interpersonal loss and social rejection are the types of stressors that are most strongly associated with onset of depression.
Research has just begun to examine neural, genetic, and genomic mechanisms that underlie inflammatory responding to social stress. This work is intriguing insofar as it has revealed that neural systems involved in processing physical pain (e.g., resulting from intense cold or heat) also play a role in processing social pain (e.g., resulting from social evaluation or rejection). This discovery may highlight answers to several questions, including how social stressors up-regulate inflammatory activity (Slavich, Way, et al., 2010) and why people often describe negative interpersonal events such as breakups in physical terms (e.g., “he broke my heart,” “she hurt my feelings”; Eisenberger, 2012b).
Research has also begun to identify genetic factors, such as a regulatory SNP in the human IL6 promoter, that moderate inflammatory responses to social stress (S. W. Cole et al., 2010) and genomic processes, including stress-associated transcriptional shifts, that reveal that social stressors can impact the molecular activation of inflammation (for a review, see Slavich & Cole, 2013). Whether these processes are relevant for depression is an important question, so we turn to this issue next. First, we describe how inflammatory cytokines communicate with the brain to influence mood, cognition, and behavior. Then, we review basic studies showing that cytokines can induce depressive-like behaviors in animal model systems. Finally, we synthesize the bodies of research showing that (a) several inflammation-related disorders frequently co-occur with depression, (b) levels of inflammatory activity are elevated in depression, (c) inflammatory challenges (e.g., bacterial endotoxin administration) evoke depressive symptoms and alter the functioning of depression-relevant neural systems, and (d) anti-inflammatory agents can help alleviate depressive symptoms.
Communication Pathways Between Inflammatory Cytokines and the Brain
We have already alluded to the fact that proinflammatory cytokines communicate with the brain and can alter neural activity (see Figure 3). This can occur via at least three pathways, involving cellular, molecular, and neural mechanisms. First, because cytokines are relatively large proteins, they do not efficiently cross the blood–brain barrier via passive transport (Quan & Banks, 2007). However, circulating cytokines can enter the central nervous system in areas where the blood–brain barrier is either incomplete (e.g., circumventricular sites) or permeable (e.g., organum vasculosum of the lamina terminalis). Second, peripheral cytokines can communicate with the brain by binding to the cerebral vascular endothelium, which facilitates the release of second messengers and the induction of local cytokine activity in the brain. Finally, carrier-mediated mechanisms can actively transport cytokines across the blood–brain barrier (Schiepers, Wichers, & Maes, 2005; Watkins, Maier, & Goehler, 1995). Together, these communication pathways involving (a) macrophage-like cells residing in circumventricular organs, (b) second-messenger activation, and (c) active transport provide three overlapping cellular and molecular mechanisms by which the brain actively senses circulating cytokines in the body and monitors changes in the composition of the internal inflammatory milieu (Quan & Banks, 2007).
Neural pathways also play a critical role in linking immune system processes with the brain. Throughout the body, there are innervating afferent nerves that can be stimulated by inflammatory cytokines. In fact, afferent nerve activation in response to inflammatory signaling in the periphery induces the release of proinflammatory cytokines in the brain (Watkins & Maier, 2000). This is evident with the afferent vagal fibers, for example, that, when activated, send neural signals from the periphery to sites in the brain, thereby promoting local production and release of proinflammatory cytokines (Watkins & Maier, 1999, 2000). Considered together, these interactions are what enable bidirectional communication between peripheral cytokine activity and the brain. Specifically, the brain can modulate inflammatory activity throughout the body via the processes described earlier—namely, SNS-related β-adrenergic signaling and HPA axis-related cortisol production—and inflammatory cytokines can in turn signal the brain via the cellular, molecular, and neural mechanisms described here (Dantzer et al., 2008; Harrison, Brydon, Walker, Gray, Steptoe, Dolan, & Critchley, 2009; Irwin & Cole, 2011; Lane et al., 2009; Pavlov & Tracey, 2004; Sternberg, 2006).
Inflammatory Cytokines and Effects on Behavior: Evidence From Basic Studies
Empirical evidence of bidirectional communication between peripheral cytokine activity and the brain is provided by basic studies of inflammation and behavior. One method for investigating these links entails exposing animals (typically rodents) to immunological challenges that up-regulate inflammatory activity. Then, inflammation-induced changes in a variety of behaviors can be observed. Such experiments involve administration of innate immune cytokines (e.g., IL-1β, TNF-α) or bacterial endotoxin (e.g., LPS), both of which trigger increased inflammatory activity in the periphery, as well as in the brain. As we discuss below, central activation of inflammatory cytokine activity in animals may be especially relevant for understanding the pathogenesis of depression, since these processes evoke a social-behavioral state that closely resembles depression in humans.
Cytokine-to-brain communication is initiated when, in response to inflammatory signaling in the periphery, certain classes of cells in the brain—specifically, microglial cells and astrocytes—begin secreting proinflammatory cytokines that bind to cytokine receptors throughout the brain (Camacho-Arroyo, López-Griego, & Morales-Montor, 2009). In turn, these cytokines promote the release of the neurotransmitters norepinephrine, dopamine, and serotonin (Anisman & Merali, 2002; Camacho-Arroyo et al., 2009), implicating central inflammatory cytokines in the initiation or modulation of neurochemical cascades that directly affect behavior. Via these interactions, for example, central cytokine activation leads to disturbances in sleep–wake activity, as characterized by alterations in measures of sleep continuity and architecture. These interactions also evoke decreases in daytime activity, as well as decreased interest in feeding, grooming, socializing, and mating and hedonic behaviors. These behaviors have been collectively called sickness behaviors, and they are thought to facilitate an organism’s recuperation and recovery from injury or infection (Hart, 1988). The fact that inflammatory cytokines can induce sickness behaviors is highly relevant for depression since these behaviors are strikingly similar to somatic and behavioral symptoms of depression (Capuron, Ravaud, & Dantzer, 2001; Yirmiya et al., 1999). These effects thus argue for the possibility that cytokines may be able to induce major depression in humans by altering the activity of neurotransmitters and neural systems that regulate cognition, mood, and behavior.
Hundreds of basic studies have been conducted demonstrating that inflammatory processes affect behavior. A complete discussion of this body of work is beyond the scope of this review. In short, though, this research has shown that inflammatory challenges that involve administration of the proinflammatory cytokines IL-β or TNF-α trigger a constellation of several sickness behaviors in rodents that includes hypersomnia, fatigue, anorexia, impaired cognitive abilities, psychomotor retardation, and reduced social and exploratory behavior (Anisman & Matheson, 2005; De La Garza, 2005; Pecchi, Dallaporta, Jean, Thirion, & Troadec, 2009). Similarly, injection of bacterial endotoxin (i.e., LPS) inhibits sexual activity and reduces the consumption of sweetened solutions that are strongly preferred by rats (Yirmiya, 1996). Finally, basic studies have found that depressive-like behaviors that are evoked by bacterial endotoxin administration can be alleviated by antidepressant medication administration or eliminated almost entirely by pretreatment with cytokine synthesis blockers or cytokine antagonists (Castanon, Bluthé, & Dantzer, 2001; Yirmiya, 1996; Yirmiya et al., 1999). Taken together, these findings provide evidence that inflammation may be implicated in at least some depressive symptoms (e.g., somatic, vegetative symptoms), insofar as they show that immunological challenges that up-regulate inflammation induce a constellation of behaviors that closely resembles MDD in humans.
Inflammation and Depression
Given that stress up-regulates proinflammatory cytokine activity and that cytokines can in turn signal the brain to induce a depressive-like state in animal model systems, it is reasonable to consider the hypothesis that stress-induced increases in inflammation may play a role in MDD. As it turns out, the observation that depressive disorders may be mediated at least in part by inflammation is not new. Probably without realizing the psychoneuroimmunological implications of his insight, for example, Sigmund Freud wrote in 1917 that “the complex of melancholia behaves like an open wound” (Freud, 1917/1957, p. 253). Years later, N. E. Miller (1964) proposed that feeling sick during times of infection helps organisms to conserve energy and prioritize behaviors that are critical for survival.
These early observations paved the way for more sophisticated thinking on the topic. In the late 1980s, scientists articulated that sickness behaviors represent an organized, highly adaptive response to infection (Hart, 1988) and that this response is mediated by cytokines (Dantzer & Kelley, 1989, 2007). Soon after, the connection between sickness behaviors, cytokines, inflammation, and depression was made. In his pioneering work on this issue, R. S. Smith (1991) developed a macrophage theory of depression, which described for the first time the notion that cytokines can act on the brain to cause depression. This perspective was subsequently elaborated by Michael Maes, who observed that depression is characterized by an activated innate immune system (Maes, 1993; Maes, Smith, & Scharpe, 1995). Raz Yirmiya, in turn, was among the first to show that cytokines can directly elicit depressive symptoms and that symptoms elicited in this way can be alleviated with antidepressant medications (Yirmiya et al., 1999, 2000; see also Musselman et al., 2001, and Capuron et al., 2002).
With these observations as the backdrop, a tremendous number of studies were conducted in the 2000s to better understand how inflammation is related to depression. Generally speaking, these studies support the formulation that inflammatory processes promote depressive symptoms and, in addition, that they likely cause at least some forms of the disorder. These lines of research are reviewed next, focusing first on correlational evidence and then on experimental data.
Several Inflammation-Related Disorders Frequently Co-Occur With Depression
Among the first evidence to suggest that inflammation may contribute to depression came in the form of naturalistic clinical observations that patients who have certain somatic and physical disorders with an underlying inflammatory component also have a high likelihood of being or becoming depressed. These conditions include asthma, rheumatoid arthritis, inflammatory bowel disease, metabolic syndrome, coronary heart disease, and chronic pain (Barton, 2008; Calder, 2006). In a prospective study of more than 1,200 young adults over a 21-year period, for example, asthma in adolescence and young adulthood was associated with a significantly increased likelihood of being diagnosed with MDD (odds ratio = 1.7, 95% confidence interval [CI] [1.3, 2.3]; Goodwin, Fergusson, & Horwood, 2004).
Likewise, several studies have shown that individuals with rheumatoid arthritis and inflammatory bowel disease are 2 to 3 times more likely to have major depression than the general population (Graff, Walker, & Bernstein, 2009; Katz & Yelin, 1993; Regier et al., 1988). In the context of metabolic syndrome, a recent meta-analytic review of all existing epidemiological studies revealed that across nine prospective cohort samples, the pooled adjusted odds of metabolic syndrome predicting future risk for depression were 1.49 (95% CI [1.19, 1.89]; Pan et al., 2012). Similarly, depression has been found to be approximately twice as likely in individuals with coronary heart disease and 3 times as likely in persons with congestive heart failure compared to prevalence rates in the general population (Whooley, 2006; see also Barth, Schumacher, & Herrmann-Lingen, 2004; Wulsin & Singal, 2003).
Increasing evidence suggests that chronic pain is also associated with elevated levels of inflammation and that such inflammation drives hyperalgesia, or increased sensitivity to pain (Sandkühler, 2009; Sommer & Kress, 2004; Watkins & Maier, 2000). In this context, it is notable that chronic pain has also been found to be highly comorbid with depression (Backonja, Coe, Muller, & Schell, 2008; Bair, Robinson, Katon, & Kroenke, 2003; Marchand, Perretti, & McMahon, 2005; Ren & Dubner, 2010; Samad et al., 2001). Even though mood disorders can often go unrecognized in persons with chronic pain (Agüera, Failde, Cervilla, Díaz-Fernández, & Mico, 2010), chronic pain is a strong, independent predictor of both onset and duration of depressive symptoms in the general population (Ohayon & Schatzberg, 2003). When the presence of depression in chronic pain patients is assessed using gold-standard methods such as the Structured Clinical Interview for DSM–IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995), up to 72% of patients with chronic pain meet threshold diagnostic criteria for concurrent MDD. When self-report measures of depression are used, the prevalence of depression in individuals experiencing chronic pain is as high as 86% (Poole, White, Blake, Murphy, & Bramwell, 2009; see also Bair et al., 2004).
Levels of Inflammatory Activity Are Elevated in Depression
If the high comorbidity between these disorders and depression can be explained (at least in part) by a common underlying biological process involving inflammation, then one would expect proinflammatory cytokine levels to be elevated in depressed individuals. Dozens of studies have been conducted on this topic, and these studies have been interrogated in at least six meta-analytic reviews. To summarize, compared to nondepressed individuals, depressed individuals (who are otherwise healthy) exhibit higher circulating levels of several proinflammatory cytokines including IL-1, IL-6, and TNF-α, as well as higher levels of the systemic inflammatory biomarker CRP (Dowlati et al., 2010; Hiles, Baker, de Malmanche, & Attia, 2012; Howren, Lamkin, & Suls, 2009; Kuo et al., 2005; Zorrilla et al., 2001; see also Herbert & Cohen, 1993a; cf. O’Donovan et al., 2010). Although information on anti-inflammatory cytokines in depression is more limited, at least one study found that concentrations of the anti-inflammatory cytokine IL-10 are lower for depressed than for nondepressed individuals (Dhabhar et al., 2009; cf. Hiles et al., 2012).
These data do not provide evidence that inflammatory cytokines are directly involved in the pathogenesis of depression, but several observations favor this interpretation. First, consistent with the formulation that cytokines cause at least some depressive symptoms, increases in IL-6 and CRP have been found to prospectively predict the development of depressive symptoms (Gimeno et al., 2009; van den Biggelaar et al., 2007; see also Wium-Andersen, Ørsted, Nielsen, & Nordestgaard, 2013). Depressive symptoms have also been found to predict increases in inflammation (Stewart, Rand, Muldoon, & Kamarck, 2009), but in at least one study, these effects were not robust when controlling for possible confounding factors, such as body mass index, physical activity, and smoking (Matthews et al., 2010). Nevertheless, the possibility that inflammation–depression links are bidirectional should be considered. Second, antidepressant medication treatments have been found to be associated with decreases in levels of the proinflammatory cytokines IL-1β, IL-2, and IL-6 in a manner that is correlated with the expected reductions in depressive symptoms (Frommberger et al., 1997; Hernández et al., 2008; Służewska et al., 1995; see Schleifer, Keller, & Bartlett, 1999), although the evidence for this effect is mixed (see Supplement 1 in A. H. Miller et al., 2009).
Finally, cytokines have been found to reduce levels of serotonin and possibly other related neurotransmitters by decreasing the availability of its serotonin precursor, an essential amino acid called tryptophan (Konsman, Parnet, & Dantzer, 2002; Mattson, Maudsley, & Martin, 2004; Raison et al., 2010; Schwarcz, Bruno, Muchowski, & Wu, 2012; van Donkelaar et al., 2011). Additional evidence for this tryptophan depletion hypothesis of depression is provided by animal model studies, which have shown that depressive-like behaviors in rodents are mediated by an enzyme that degrades tryptophan, called indoleamine 2,3-dioxygenase (IDO; O’Connor et al., 2009; see also Maes et al., 2011). Because serotonin is intimately involved in regulating mood, motivation, and behavior, cytokine-related tryptophan depletion may well represent a key event in the pathogenesis of depression (Capuron & Miller, 2004, 2011; Capuron et al., 2011; Wichers & Maes, 2002).
As reviewed elsewhere, elevated proinflammatory cytokine levels are not the only indication that inflammation may be a key biological mediator of depression (Irwin & Miller, 2007; Maes, 2011; Zorrilla et al., 2001). In fact, depression is accompanied not just by increases in circulating markers of inflammation but also by cell-mediated immune activation, which involves macrophages, monocytes, and T-lymphocytes. For example, individuals with depression show increased numbers of T cells bearing T cell activation markers, including CD4+ (T helper cells) and CD8+ (T suppressor/cytotoxic cells; Maes et al., 1992, 1996); increased production of IFN-γ (Gabbay et al., 2009; Maes et al., 1994); and elevated levels of the soluble receptors for IL-2 and TNF-α (Himmerich et al., 2008). These findings are intriguing because it has been proposed that cell-mediated immune activation causes tryptophan-related reductions in serotonin, which in turn lead to somatic and neurovegetative symptoms of depression (Maes, 2011). Consistent with this hypothesis, there is some evidence that alterations in these markers of immune system functioning are most pronounced for individuals with melancholic forms of depression, suggesting that inflammation-related processes may be particularly responsible for somatic and physical symptoms of depression (Cover & Irwin, 1994; Maes, 1995, 1999; see also Andréasson, Arborelius, Erlanson-Albertsson, & Lekander, 2007; Anisman, Ravindran, Griffiths, & Merali, 1999; cf. Marques-Deak et al., 2007).
Depression has also been associated with immune suppression, including with decreases in antiviral immunity (Irwin et al., 2011, 2013). This may sound counterintuitive, but increases in inflammation and decreases in other aspects of cellular immunity (e.g., natural killer cell activity) have been found to co-occur in depression (Pike & Irwin, 2006). Moreover, as we suggested earlier, perhaps because of activation of the SNS pathway and increased β-adrenergic signaling, the SNS outflow that occurs in depression (see Irwin et al., 1991) may suppress transcription of antiviral immune response genes (S. W. Cole et al., 1998; Lee et al., 2000), leading to increased susceptibility to viruses. Additionally, SNS activation enhances transcription of proinflammatory immune response genes (S. W. Cole et al., 2010; Grebe et al., 2010), which increases systemic inflammatory activity that aids in combating bacteria and other extracellular pathogens. This simultaneous increase in inflammation and reduction in antiviral immunity is central to the model presented in Figure 1 and discussed by Irwin and Cole (2011) and Slavich and Cole (2013).
Inflammatory Challenges Elicit Depressive Symptoms and Major Depression in Humans
The associations described thus far are largely correlational; as such, they do not indicate whether elevated cytokine levels cause depression or whether they are somehow secondary to the disorder (e.g., the result of sleep disturbance or physical disease). This issue of causality has been examined, however, and several lines of research support the formulation that cytokines can cause depressive symptoms and even clinically diagnosable forms of depression. We have already reviewed basic studies on this topic, which demonstrate that immunological challenges that up-regulate inflammatory activity cause increases in depressive-like sickness behaviors in rodents. Similar research has been conducted with humans by examining alterations in cognitive and behavioral functioning that result from vaccination or immunotherapy. Three models have been used for this purpose: an IFN-α model, a typhoid vaccination model, and an endotoxin model.
Early studies investigating inflammation-related changes in mood and behavior examined patients with hepatitis C and cancer who were receiving IFN-α to boost their immune system and facilitate recovery. In these quasi-experimental studies, up to 50% of patients receiving IFN-α developed clinically significant levels of depression (Capuron & Miller, 2004; Raison et al., 2006). Specific symptoms induced by IFN-α administration include anxiety, pain, depressed mood, anhedonia, fatigue, cognitive impairment, sleep disturbance, anger, hostility, loss of appetite, and suicidal ideation (Capuron et al., 2002; Janssen, Brouwer, van der Mast, & Schalm, 1994; Lotrich, Rabinovitz, Gironda, & Pollock, 2007). Among individuals treated with IFN-α, onset of depression has been found to be especially likely for patients who develop vegetative-depressive symptoms early on during the course of IFN-α (Robaeys et al., 2007), as well as for those who exhibit potentiated adrenocorticotropic hormone, cortisol, and p38 mitogen-activated protein kinase responses within 12 hr of a first infusion or injection (Capuron et al., 2003; Felger et al., 2011). Several factors may be involved in the development of depression during chronic IFN-α treatment. As reviewed by Raison and Miller (2011), these include increased TNF-α and IL-6 signaling, reduced HPA axis diurnal variation, increased glucocorticoid insensitivity, reduced plasma concentrations of brain-derived neurotrophic factor, reduced plasma concentrations of tryptophan, increased plasma and cerebrospinal fluid concentrations of kynurenine and quinolinic acid, and reduced slow-wave sleep and sleep continuity.
A few studies have also examined factors that moderate risk for depression in response to IFN-α treatment. These studies have found that individuals with a history of depression are more likely to develop cognitive and affective symptoms of depression (e.g., depressed mood, anxiety, irritability, memory and attentional problems) following IFN-α treatment for cancer or hepatitis C than persons without a history of depression. However, nearly all patients who receive IFN-α treatment for these conditions appear to experience a sudden onset of neurovegetative symptoms of depression, including anorexia, fatigue, psychomotor retardation, and disrupted sleep (Capuron & Miller, 2011).
In addition, at least two longitudinal studies of hepatitis C patients have examined whether a functional SNP in the promoter region of the serotonin transporter gene (i.e., 5-HTTLPR) is related to IFN-α treatment-related depression. Consistent with the large body of research showing that individuals with one or two copies of the short allele at 5-HTTLPR are at greater risk for MDD than their long-allele homozygous counterparts (Caspi et al., 2003; Karg, Burmeister, Shedden, & Sen, 2011; Uher & McGuffin, 2010), Bull and colleagues (2009) found that short-allele carriers developed more depressive symptoms during the course of IFN-α treatment than did participants with the long/long 5-HTTLPR genotype. Similar effects were found by Lotrich and colleagues, although in this study, changes in depression over time were assessed with the SCID (in addition to self-report measures of depression; Lotrich, Ferrell, Rabinovitz, & Pollock, 2009; for a review, see Lotrich, El-Gabalawy, Guenther, & Ware, 2011). Although a complete review of this literature is beyond the scope of this discussion, it should be noted that functional SNPs in the promoter regions of the genes encoding both IDO (rs9657182) and IL-6 (rs1800795) have also been found to moderate the effects of IFN-α treatment on depression (Bull et al., 2009; A. K. Smith et al., 2012).
Acute administration of typhoid vaccination or bacterial endotoxin has been shown to produce effects that are similar to those seen in long-term IFN-α treatment. For example, typhoid vaccination, which is a relatively mild inflammatory challenge, elicits significant increases in negative mood, confusion, and fatigue, and these effects are mediated by changes in IL-6 (Harrison, Brydon, Walker, Gray, Steptoe, & Critchley, 2009; Strike, Wardle, & Steptoe, 2004; C. E. Wright, Strike, Brydon, & Steptoe, 2005). Similarly, administration of bacterial endotoxin, which is a relatively strong inflammatory challenge, elicits heightened anxiety, as well as several symptoms of depression including sad mood, anhedonia, cognitive impairment, fatigue, reduced food intake, altered sleep (e.g., disrupted sleep continuity, increased REM latency, and REM suppression), and social-behavioral withdrawal (Eisenberger, Inagaki, Mashal, & Irwin, 2010; Eisenberger et al., 2009; Reichenberg et al., 2001; for a review, see DellaGioia & Hannestad, 2010).
Consistent with the hypothesis that inflammatory processes are a key mediator linking these immunological challenges with depression, increases in depressive symptoms following endotoxin administration have been associated with changes in levels of the proinflammatory cytokines IL-6 and TNF-α in peripheral blood (Hannestad, DellaGioia, Ortiz, Pittman, & Bhagwagar, 2011). Furthermore, several studies have found that the effects of endotoxin-induced increases in depressive symptoms can be blunted or abated with antidepressant medications (see Hannestad et al., 2011; Musselman et al., 2001). Although data on the association between inflammation and social-cognitive aspects of depression are limited, there is some evidence that endotoxin administration induces feelings of social disconnection and a desire to be alone, which are hallmarks of some depressive episodes (DellaGioia & Hannestad, 2010; Eisenberger, Inagaki, et al., 2010).
Inflammatory Challenges Alter Activity in Depression-Relevant Neural Systems
If systemic inflammatory activity can signal the brain to induce depressive symptoms, then peripherally administered immunological challenges should be associated with altered neural activity in depression-relevant brain systems. Although this research is difficult to conduct given that it involves combining immunological and neuroimaging methods in the same experimental context, a handful of studies have employed these techniques together and have revealed that immunological challenges that up-regulate inflammation also alter neural activity in several brain regions that have been implicated in depression. These regions include the basal ganglia, cerebellum, anterior cingulate cortex (ACC), and ventral striatum.
In an early study that employed a wait-list control design, patients with hepatitis C virus infection who were assigned to receive chronic, low-dose IFN-α treatment exhibited greater activity in the dACC during a visuospatial attention task compared to control participants who were not yet receiving the treatment. Greater activity in the dACC was in turn associated with more errors during the visuospatial task for IFN-α-treated patients but not for control participants (Capuron et al., 2005). As noted previously, the dACC has been implicated in processing experiences of physical pain (Eisenberger, 2012a), and activity in this region has been found to be associated with inflammatory responses to social stress (Slavich, Way, et al., 2010). In another study using this same model, IFN-α administration was found to be associated with increased glucose metabolism in the basal ganglia and cerebellum, which are involved in motor activity and motivation (Capuron et al., 2007). Finally, at least one study has shown that long-term administration of IFN-α is associated with reduced neural responses to a hedonic reward task in the bilateral ventral striatum, a brain region that is involved in reward-related responding. Reduced activation of the ventral striatum, in turn, was significantly correlated with greater symptoms of anhedonia, depression, and fatigue (Capuron et al., 2012).
Along similar lines, typhoid vaccination has been associated with altered neural activity in the substantia nigra, a part of the basal ganglia that is involved in motor planning and reward seeking (Brydon, Harrison, Walker, Steptoe, & Critchley, 2008), as well as with greater activity in the subgenual ACC, which has been repeatedly implicated in depression (Harrison, Brydon, Walker, Gray, Steptoe, & Critchley, 2009; see also Eisenberger et al., 2009). Finally, at least one study has shown that bacterial endotoxin administration also reduces neural activity in the ventral striatum (Eisenberger, Berkman, et al., 2010). Consistent with the results obtained by Capuron et al. (2012), this study also found that neural activity in the ventral striatum mediated the effects of endotoxin administration on increases in depressed mood, as rated by an independent observer (Eisenberger, Berkman, et al., 2010).
Anti-Inflammatory Agents Alleviate Depression
The last line of research indicating that inflammation is a key mediator of depression involves the discovery that anti-inflammatory agents alleviate depressive symptoms. We have already reviewed studies showing that antidepressant medications lead to reductions in proinflammatory cytokine activity, which decline in concert with the abatement of depression. The fact that anti-inflammatory agents also reduce both cytokine activity and depression severity provides converging experimental evidence that inflammatory processes mediate depressive symptomatology.
In this context, a recent double-blind, placebo-controlled study examined the role of anti-inflammatory agents on depression. Patients with MDD were randomly assigned to receive either the antidepressant medication reboxetine plus placebo or reboxetine plus the anti-inflammatory medication celecoxib (Müller et al., 2006). Celecoxib is a cyclooxygenase (COX)-2 inhibitor used for treating excessive inflammation and pain. Patients were followed for 6 weeks and had their depressive symptoms assessed using the clinician-administered HRSD (Hamilton, 1960). Patients in the reboxetine plus celecoxib group exhibited reductions in depression severity that were nearly twice as great (55% reduction) as those experienced by patients in the control group (33% reduction). Moreover, 75% of patients receiving reboxetine plus celecoxib were categorized as responders, compared to only 45% of patients receiving reboxetine plus placebo (Müller et al., 2006; see also Akhondzadeh et al., 2009). Consistent with these results implicating COX-2 as a mediator in depression, a recent study found that expression of the gene that codes for COX-2 was greater for depressed than nondepressed individuals (Gałecki et al., 2012).
A related double-blind, placebo-controlled study examined the antidepressant effects of the TNF-α antagonist etanercept (Tyring et al., 2006). Etanercept is an anti-inflammatory agent that works by mimicking the inhibitory effects of naturally occurring soluble TNF receptors; it has thus been used to treat rheumatoid arthritis and other inflammatory conditions. In this study, patients with psoriasis were randomly assigned to receive either etanercept or placebo and were followed for 12 weeks. Depressive symptoms were assessed using the HRSD and Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996), and fatigue symptoms were assessed using the Functional Assessment of Chronic Illness Therapy–Fatigue scale (Webster, Cella, & Yost, 2003). At Week 12, 43% and 55% of patients in the etanercept treatment group were categorized as responders as assessed by the HRSD and BDI, respectively, compared to only 32% and 39% of patients in the placebo group. In addition, whereas only 43% of patients in the placebo group experienced clinically significant improvements in fatigue at Week 12, 58% of those in the etanercept group experienced such improvements (Tyring et al., 2006; see also Persoons et al., 2005).
Finally, a recent double-blind, placebo-controlled study examined whether inhibiting TNF-α reduced depressive symptoms in 60 medically stable outpatients with treatment-resistant depression. Participants were randomly assigned to receive either three infusions of the TNF-α antagonist infliximab or placebo at baseline, Week 2, and Week 6 of a 12-week clinical trial (Raison et al., 2013). Depressive symptoms were measured by the HRSD. Although no overall differences in depression severity were found between the two groups over the trial, patients with baseline CRP levels of greater than 5 mg/L exhibited a treatment response (i.e., ≥50% reduction in HRSD score at any point) of 62% for infliximab-treated patients compared to only 33% for placebo-treated patients. For participants in the high-CRP group, infliximab improved anxiety symptoms and a wide range of depressive symptoms including depressed mood, psychomotor retardation, suicidal ideation, and performance of work and other activities (i.e., anhedonia/fatigue). Moreover, infliximab-treated responders exhibited significantly greater decreases in levels of CRP from baseline to Week 12 compared to placebo-treated responders (Raison et al., 2013).
In sum, research on the antidepressant effects of anti-inflammatory agents is currently limited. Nonetheless, the results from the three studies just described converge with a large literature showing that anti-inflammatory agents block inflammation-related behavioral changes in animal models of depression (Dantzer et al., 2008). Results from these studies raise important questions, such as whether anti-inflammatory agents are efficacious for all depressed individuals or for only a subgroup of patients (e.g., those with elevated inflammation; see Raison et al., 2013). Despite these lingering questions, however, these findings have already prompted an enormous amount of interest in the possibility of using anti-inflammatory agents to treat, and perhaps even prevent, depression (Capuron & Miller, 2011; Haroon, Raison, & Miller, 2012; Hayley, 2011; Li, Soczynska, & Kennedy, 2011; Rook, Raison, & Lowry, 2012).
Summary: A Role for Inflammation in Depression
To summarize, five lines of research point to the fact that inflammation plays a prominent role in depression, particularly when somatic or neurovegetative symptoms are present. First, several somatic disorders and physical disease conditions that have an inflammatory basis frequently co-occur with depression, including rheumatoid arthritis, inflammatory bowel disease, metabolic syndrome, coronary heart disease, and chronic pain. Second, multiple markers of inflammatory activity are elevated in depressed compared to nondepressed individuals, including plasma concentrations of the proinflammatory cytokines IL-1, IL-6, and TNF-α, which mediate systemic inflammation; levels of CRP, a key biomarker of systemic inflammatory activity; and several markers of cell-mediated immune activation, which involves macrophages, monocytes, and T-lymphocytes. Elevations in these biomarkers appear to precede the development of depression (although bidirectional effects have also been reported), and at least some depressive symptoms that are associated with inflammation can be alleviated with antidepressant medications. Third, immunological challenges that up-regulate inflammatory activity trigger depressive-like behaviors in animal model systems of depression and diagnosable forms of MDD in humans. The alterations evoked by these immunological challenges are hallmark symptoms of depression and include both neurovegetative symptoms (e.g., psychomotor retardation, fatigue, sleep disturbance, social-behavioral withdrawal) and cognitive and affective symptoms (e.g., depressed mood, anhedonia, difficulty concentrating, irritability). In addition, depressive symptoms that are elicited by these immunological challenges can be blocked with cytokine synthesis blockers or cytokine antagonists in animals and potentially blunted or alleviated with antidepressants in humans. Fourth, at least three different inflammatory challenges (i.e., IFN-α administration, typhoid vaccination, and endotoxin administration) have been shown to alter metabolic or neural activity in brain regions that have been implicated in depression, including the basal ganglia, cerebellum, ACC, and ventral striatum. Because these regions are involved in modulating mood, motivation, motor control, and responsiveness to reward, these neuroimaging results provide an important neurobiological account of how inflammation might alter central nervous system functioning to cause depression. Finally, three anti-inflammatory agents (i.e., the COX-2 inhibitor, celecoxib, and the TNF-α antagonists, etanercept and infliximab) have been found to alleviate depressive symptoms in double-blind, randomized, placebo-controlled studies. Considered together, these data provide compelling correlational and experimental evidence that inflammation is intimately involved in the pathogenesis of depression.
Social Signal Transduction Theory of Depression
The central question of this review is whether stress influences inflammatory processes in a way that is relevant for depression. The literatures on stress, inflammation, and depression support this hypothesis by demonstrating that (a) social stressors up-regulate proinflammatory cytokine activity, particularly if they involve social threat or rejection, and (b) proinflammatory cytokines can signal the central nervous system to induce neurobiological and behavioral alterations that include several hallmark symptoms of depression. Additional evidence that stress-induced increases in inflammation play a key role in depression is provided by basic and human research on these topics. This work has begun to elucidate neural systems that are involved in processing social threat, how activity in these brain regions gets converted into physiological processes that regulate proinflammatory cytokine activity, and how central and peripheral cytokines in turn induce depressive symptoms.
Together, these data begin to provide the empirical basis for biologically plausible theories that describe how perceptions of the external social environment get converted, or transduced, into cellular inflammation and depression. We propose one such theory in Figure 4, called the social signal transduction theory of depression. Based on earlier thinking by S. W. Cole (2009) and Slavich and Cole (2013), this theory specifies that situations involving social threat are represented neurally by brain regions such as the anterior insula and dACC, which process experiences of negative affect and rejection-related distress (Eisenberger, 2012b; Slavich, O’Donovan, et al., 2010; Slavich, Way, et al., 2010). Although these regions do not directly regulate inflammatory activity, they have anatomical connections to lower level brain regions, including the hypothalamus and brainstem autonomic control nuclei, which influence systemic inflammation by modulating the activity of the HPA axis and SNS (Irwin & Cole, 2011; Pavlov & Tracey, 2004; Sternberg, 2006). Whereas HPA axis-related production of cortisol suppresses inflammatory activity, SNS-related production of epinephrine and norepinephrine promotes inflammation by interacting with receptors on immune cells to initiate a complex set of intracellular interactions that result in the activation of the transcription factors NF-κB and AP-1. These interactions cause NF-κB and AP-1 to translocate into the nucleus of the cell where they bind to cis-regulatory DNA sequences and up-regulate the expression of proinflammatory immune response genes including IL1B, IL6, IL8, and TNF (S. W. Cole, 2012; Slavich & Cole, 2013). Once activated, these genes produce amino acid sequences that form the basis for different proteins, such as the proinflammatory cytokines IL-1, IL-6, IL-8, and TNF-α. The central nervous system can also influence peripheral inflammation via efferent vagus nerve activity, which down-regulates inflammation by strongly suppressing TNF gene transcription (Chiu, von Hehn, & Woolf, 2012; Libert, 2003; Pavlov, Wang, Czura, Friedman, & Tracey, 2003).
Figure 4.
Social signal transduction theory of depression. Social signal transduction theory of depression describes mechanisms that convert, or transduce, experiences of the external social environment into the internal biological environment of depression pathogenesis. (1) Social-environmental experiences indicating possible social threat or adversity (e.g., social evaluation, rejection, isolation, or exclusion) are represented neurally, especially in brain systems that process experiences of social and physical pain. Key nodes in this neural network include the anterior insula (AI) and dorsal anterior cingulate cortex (dACC, shown in the insert). These regions project to lower level brain areas (e.g., hypothalamus, brainstem autonomic control nuclei) that have the ability to initiate and modulate inflammatory activity via three pathways that involve (2) the hypothalamic–pituitary–adrenal axis, (3) sympathetic nervous system (SNS), and (4) efferent vagus nerve. (5) Activation of these pathways leads to the production of glucocorticoids, epinephrine, norepinephrine (NE), and acetylcholine (ACh), which interact with receptors on cytokine-producing cells. Whereas glucocorticoids and acetylcholine have anti-inflammatory effects, epinephrine and norepinephrine activate intracellular transcription factors (e.g., nuclear factor-κB and activator protein 1) that bind to cis-regulatory DNA sequences to up-regulate inflammatory gene expression. When this occurs and immune response genes are expressed, DNA is transcribed into RNA and then translated into protein. The resulting change in cell function leads to the production of proinflammatory cytokines (e.g., interleukin-1β [IL-1β], interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α]) that signal the brain to induce cognitive, emotional, and behavioral alterations that include several hallmark symptoms of depression (e.g., sad mood, anhedonia, fatigue, psychomotor retardation, altered appetite and sleep, and social-behavioral withdrawal). Cytokines can exert these effects on the central nervous system by (6) passing through leaky or incomplete regions of the blood–brain barrier (e.g., circumventricular organs, organum vasculosum of the lamina terminalis) and by (7) stimulating primary afferent nerve fibers in the vagus nerve, which relays information to brain systems that regulate mood, motor activity, motivation, sensitivity to social threat, and arousal. Although these neurocognitive and behavioral responses are adaptive during times of actual threat, as depicted in Figure 1, these social signal transduction pathways can also be initiated by purely symbolic, anticipated, or imagined threats—that is, situations that have not yet happened or that may never actually occur. Moreover, activation of these pathways can become self-promoting over time due to neuro-inflammatory sensitization and, as a result, remain engaged long after an actual threat has passed (see Figure 3). In such instances, these dynamics can increase risk for depression in the short-term and possibly promote physical disease, accelerate biological aging, and hasten mortality over the long run. ACTH = adrenocorticotropic hormone; mRNA = messenger ribonucleic acid.
Increases in inflammatory activity in turn induce somatic, vegetative, and some cognitive and affective symptoms of depression, an effect that is mediated by several nonmutually exclusive pathways. As revealed by a combination of animal model and human studies, for example, cytokines can act on the brain to promote depressive behaviors in several ways including via macrophage-like cells residing in circumventricular organs, second-messenger activation, and active transport, as well as by inflammatory signaling-related afferent nerve activation in the periphery that induces the release of proinflammatory cytokines from microglial cells and astrocytes in the brain. These processes promote the release of the neurotransmitters norepinephrine, dopamine, and serotonin (Anisman & Merali, 2002; Camacho-Arroyo et al., 2009) and lead to widespread neurocognitive and behavioral alterations that include aberrations in mood, cognition, motivation, eating patterns, sleep–wake rhythms, pain sensitivity, psychomotor activity, and social and exploratory behavior (Dantzer et al., 2008; Hart, 1988).
These biobehavioral responses are critical for protecting the human body when they occur intermittently and in response to actual threat. As illustrated in Figure 3, however, these social signal transduction pathways can also be activated by purely symbolic, anticipated, or imagined threats, which include things that have not yet happened or that may never actually occur (Slavich & Cole, 2013). Moreover, engagement of these pathways may become self-promoting as a result of neuro-inflammatory sensitization and thus drive heightened levels of inflammation and exaggerated perceptions of social threat for months or years after the original social-environmental impetus has passed (see Figure 3; G. E. Miller, Chen, & Parker, 2011; G. E. Miller & Cole, 2012; Slavich & Cole, 2013). Ultimately, it is this biological embedding of exaggerated neuro-inflammatory responses to threat that is hypothesized to increase a person’s risk for depression and, possibly, other physical and aging-related disorders that have an inflammatory component (Barton, 2008; Calder, 2006; O’Donovan, Slavich, et al., 2013; O’Donovan et al., 2012; Wolkowitz, Epel, Reus, & Mellon, 2010).
Inflammation: Necessary or Sufficient?
New, multilevel theories of disease risk are inevitably complex, giving rise to more questions than answers. That is certainly true here. One of the most important questions that can be asked of this social signal transduction theory of depression is whether inflammation is necessary and/or sufficient for depression. Given the state of the literature, there are currently no definitive answers to these questions. But it is possible to speculate on these issues based on what we do know.
Is inflammation sufficient for depression?
Some of the best evidence that elevated inflammatory activity may not be sufficient for depression to develop in all individuals comes from research showing that only up to 50% of patients who receive chronic IFN-α treatment meet full criteria for MDD (Capuron & Miller, 2004). Thus, a full 50% of people (or more) do not develop MDD despite receiving an immunological challenge that substantially up-regulates systemic inflammation. Although the reasons for these differential effects remain unclear, one possibility is that these latter individuals do not demonstrate a cognitive vulnerability for depression, which could be required for a full episode of MDD to develop. Consistent with this possibility, almost all patients who receive IFN-α treatment develop neurovegetative symptoms of depression (e.g., psychomotor retardation, fatigue, and disrupted appetite or sleep), but only a subset develops cognitive and affective symptoms of the disorder (e.g., depressed mood, irritability, difficulty concentrating, and excessive worthlessness or guilt). Moreover, those who do develop cognitive and affective symptoms of depression are more likely to have had a prior depressive episode, which indicates the presence of cognitive vulnerability for depression (Capuron & Miller, 2011). It is possible, therefore, that while stress-related increases in inflammation may elevate a person’s risk for depression, these biological drivers of depression may need to act in concert with certain cognitive vulnerability factors (e.g., negative automatic thoughts, core beliefs) for certain individuals to meet full diagnostic criteria for MDD.
Another body of research that addresses whether elevated inflammatory activity is sufficient for the development of MDD comes from the early life stress literature. Early life stress is one of the strongest predictors of lifetime risk for depression (Gilman, Kawachi, Fitzmaurice, & Buka, 2003). In one study of more than 9,500 adults, for example, exposure to multiple forms of early life stress was associated with a 4.6-fold increase in risk of being depressed over the past year (Felitti et al., 1998). In another study, it was estimated that early life stress explains 20%–25% of all mood disorders in adulthood (Green et al., 2010).
In addition to these findings, research has shown that at least some links between inflammation and depression are influenced by exposure to early life stress. As discussed previously, for example, Danese et al. (2008) found that although depression was associated with elevated levels of CRP, this association was not robust when adjusting for early life stress. In addition, G. E. Miller and Cole (2012) reported that while adolescents with no early life stress showed reductions in CRP levels as their depressive symptoms abated, their counterparts with early life stress exhibited elevations in CRP that persisted even when their depressive symptoms abated. Finally, in a laboratory-based study, Carpenter et al. (2010) found that early life stress was unrelated to baseline levels of IL-6 but was strongly associated with greater TSST-induced increases in IL-6. Although none of these studies show that early life stress is a critical factor linking inflammation and onset of depression, they are consistent with a small but growing body of work suggesting that inflammation may be more strongly associated with depression when other preexisting vulnerability factors are present. Given the paucity of studies on this topic, much more research is needed to identify factors such as past depressive episodes and early life stress that increase risk for MDD and that may thus make elevated inflammation more likely to be followed by depression (Monroe, Slavich, & Gotlib, 2013; Slavich, Monroe, & Gotlib, 2011; Slavich, O’Donovan, et al., 2010). Only after acquiring these additional data will we know whether elevated inflammatory activity is sufficient for evoking depression.
Is inflammation necessary for depression?
The question of whether inflammation is necessary for depression is different, but equally important. One indication that high levels of inflammation may not be necessary for depression to develop in all people comes from the fact that each of the meta-analytic reviews on inflammation and depression referenced here revealed substantial variability across studies. Although many studies have found clear evidence for elevated levels of inflammation in depressed versus nonde-pressed individuals, these effects are not equally strong across all studies. This variability is likely due in part to differences in both sample characteristics (e.g., participants’ age, health status, history of depression, early life stress exposure, etc.) and sampling method (e.g., inpatient vs. outpatient vs. community studies). However, it could also be due to the fact that depression is an etiologically and symptomatically heterogeneous disorder (Lux & Kendler, 2010), which may indicate the existence of different biological subtypes of depression (Fanous & Kendler, 2005; Holtzheimer & Mayberg, 2011; Savitz & Drevets, 2009).
Consistent with this thinking, some researchers have proposed that an inflammatory subtype of depression may exist, characterized primarily by somatic and vegetative symptoms (Baune et al., 2012; see Raison & Miller, 2011). Although tenable, this hypothesis has been undoubtedly influenced by the fact that out of the many studies that have been conducted on inflammation and depression to date, only a small number have examined the role inflammation plays in social, cognitive, and affective aspects of MDD. What makes these latter studies important is that they provide experimental and naturalistic evidence demonstrating that inflammation is associated not just with somatic and vegetative symptoms of depression but also with cognitive and affective symptoms such as feelings of sadness, hopelessness, guilt, indecisiveness, suicidal ideation, and desire to be alone (e.g., Eisenberger, Inagaki, et al., 2010; Kupper, Widdershoven, & Pedersen, 2012; O’Donovan, Rush, et al., 2013). In sum, then, although it is too early to know for sure whether inflammation is more intimately involved in some forms of depression than others, the possibility that inflammation might promote all symptoms of depression must be seriously considered.
Regardless of whether inflammation itself is necessary or sufficient for MDD, we believe that the neural, physiologic, molecular, and genomic mechanisms outlined in this social signal transduction theory of depression are relevant for all persons. In our mind, the main question is not whether these social signal transduction pathways exist for certain people and not others but rather whether activation of these pathways perhaps results in the development of depression for some people and not others. Addressing this question is an important task for future research, but one thing is already clear: Inflammation has wide-ranging effects on cognition, emotion, motivation, and behavior, making this biological process relevant for understanding many aspects of depression.
Implications and Future Directions
Some of the best theorizing in psychology over the past century has focused on depression (e.g., Allen & Badcock, 2003; Beck, 1967; Blatt, 2004; Bowlby, 1980; Brown & Harris, 1978; Freud, 1917/1957; Gilbert, 1992; Post, 1992). Despite this impressive body of work, though, the field has lacked a comprehensive theory that can account for the complete social, emotional, biological, and clinical phenomenon that is depression. Social signal transduction theory of depression may help address this issue by shedding light on several outstanding issues regarding depression, including how depression develops, why depression is comorbid with certain somatic complaints and physical disease conditions, why early life stress is a strong predictor of elevated lifetime risk for depression, why depression is highly recurrent, and why anxiety disorders often precede depression. The theory may also reveal new ways for treating, and perhaps even preventing, depression. We briefly consider these topics below, with a focus on identifying possible avenues for future research.
Development of Depression
Social signal transduction theory of depression is perhaps most relevant for elucidating how adverse life events and social-environmental conditions may lead to internal biological changes that evoke MDD. As we alluded to at the outset of this article, literally thousands of books and articles have considered the issue of how stress promotes depression (e.g., Allen & Badcock, 2003; Beck, 1967, 2008; Brown & Harris, 1978; Coyne & Downey, 1991; Dohrenwend, 2006; Hammen, 2005; Kendler et al., 1999; Kessler, 1997; Mazure, 1998; Monroe & Simons, 1991). Although this literature has shown that stress undoubtedly increases risk for depression, it has not convincingly identified biological processes that could be plausible mediators of this effect. Social signal transduction theory of depression begins to address this issue, given that inflammatory activity is both up-regulated by social adversity and a mediator of neurobiological and behavioral alterations that are key features of depression. These alterations include depressed mood, blunted responsiveness to reward, psychomotor retardation, fatigue, irritability, disrupted appetite and sleep, difficulty concentrating, and social-behavioral withdrawal. Moreover, as we have discussed, several studies have now shown that stress is prospectively associated with increased inflammatory activity (e.g., Danese et al., 2008; Deverts et al., 2012; M. L. M. Murphy et al., 2013) and that increases in inflammation are in turn related to the development of depression (e.g., Gimeno et al., 2009; van den Biggelaar et al., 2007).
These findings provide evidence that stress-related increases in inflammation play a key role in the pathogenesis of depression. However, several issues remain unaddressed. First, it is important to remember that, to date, researchers have not generally assessed life stress, inflammation, and depression together in the context of the same study. Additional research is thus needed to confirm the expected temporal ordering of these associations and to directly test the hypothesis that increases in inflammatory activity mediate the relation between stress and depression. Second, as we have alluded to already, MDD is a symptomatically heterogeneous disorder, and it is less clear what role inflammation plays in cognitive and affective aspects of depression versus somatic and vegetative symptoms of the disorder. Third and relatedly, it is unclear whether inflammation plays an equally important role in all forms of depression. Finally, it is unclear whether the fact that some stressors (e.g., social isolation, rejection) up-regulate inflammatory activity to a greater extent than others (e.g., financial problems, deaths) might explain why different forms of social adversity are associated with distinct patterns of cognitive, affective, and somatic symptoms of depression (Cramer et al., 2012; Keller et al., 2007; Muscatell et al., 2009). To examine these issues, the next iteration of studies will need to follow individuals longitudinally and examine how different stressors relate to increases in inflammation and, in turn, to different symptoms of depression. Experimental studies that examine social and affective consequences of inflammatory challenges in the laboratory will also be important for addressing these questions.
Comorbidity of Depression With Somatic and Physical Disease Conditions
Social signal transduction theory of depression may also have implications for understanding comorbidity in depression. Depression is frequently associated with several somatic conditions, including asthma, rheumatoid arthritis, chronic pain, metabolic syndrome, cardiovascular disease, and neurodegeneration. These comorbidities foster significant impairment and contribute to MDD’s status as a leading cause of disability in the United States (Üstün et al., 2004). It has historically been unclear why depression is associated with increased risk for such a wide range of health problems. However, over the past 10 years or so, research has identified a key role for inflammation in the pathophysiology of each of the aforementioned conditions (Chen & Miller, 2007; Choy & Panayi, 2001; Glass, Saijo, Winner, Marchetto, & Gage, 2010; Mantovani, Allavena, Sica, & Balkwill, 2008; Marx, 2004; Perry, Cunningham, & Holmes, 2007; Ridker, Cushman, Stampfer, Tracy, & Hennekens, 1997; Schrepf et al., 2013; Yaffe et al., 2004). This raises the interesting possibility that at least some comorbidity in depression may be explained by the fact that these physical conditions and depression share a common biological basis that involves the activation of inflammation.
Because we are just now beginning to understand the role that inflammation plays in these disorders, additional research is needed to evaluate this comorbidity hypothesis and to examine several important issues, including why depression co-occurs with different physical health disorders for different people. Research is also needed to examine the effects of inflammation on physical health in depression vis-à-vis other factors that are also known to impact health, such as physical environment, chemical exposure, genetic liability, exercise, smoking, adiposity, sleep quality, and diet (e.g., Duivis et al., 2011; Hamer, Molloy, de Oliveira, & Demakakos, 2009; Shelton & Miller, 2010). Inflammation may be one factor linking depression with physical disease, but it is certainly not the only factor and, in certain cases, may not even be the most central factor.
Early Life Stress and Lifetime Risk for Depression and Related Diseases
Another possible implication of social signal transduction theory of depression is that it may provide clues to how early life stress increases a person’s risk for depression across the life span. Events occurring in utero and early childhood have been consistently related to higher lifetime rates of depression, as well as to diabetes, cardiovascular disease, osteoporosis, and metabolic syndrome (Gluckman, Hanson, Cooper, & Thornburg, 2008; Heim, Owens, Plotsky, & Nemeroff, 1997). Moreover, as reviewed earlier, several forms of early life stress (e.g., low socioeconomic status, abuse, neglect) predict elevated levels of inflammation in later life (Cho et al., 2012; Kiecolt-Glaser, Gouin, et al., 2011; Slopen et al., 2010, 2013), with some data suggesting that such effects persist despite subsequent improvements in the surrounding environment (G. Miller & Chen, 2007). One possibility, therefore, is that early life stress increases lifetime risk for depression and depression-related disease conditions in part by heightening an individual’s sensitivity to stress, which in turn drives the emergence of an increasingly proinflammatory phenotype (see G. E. Miller et al., 2011).
This phenomenon of exaggerated inflammatory responses to stress as a function of exposure to early life stress may make sense, insofar as an adaptive leukocyte basal transcriptome is one that is shaped by a developing organism’s likely exposure to social-environmental threat. In fact, several theorists have proposed ideas that are consistent with this hypothesis by suggesting that childhood adversity leads to exaggerated cognitive, emotional, and biological responses to stress (Boyce & Ellis, 2005; Cicchetti & Toth, 2005; Gibson, 2008; Raison & Miller, 2013; Way & Taylor, 2010; Zhang et al., 2006). This response pattern is thought to confer short-term advantages in adverse environments, insofar as it calibrates neurobiological responses to threat based on the surrounding environment and perceived likelihood of danger. However, these advantages come with biological costs that can outweigh the benefits, especially if the current surrounding environment is not hostile, but favorable.
To date, research on early life stress and lifelong disease risk has given rise to more questions than answers. One question concerns how, exactly, early stress exposure leads to biological changes, such as persistent elevations in inflammation, that eventually damage health. A number of interrelated mechanisms may be implicated (McCrory, De Brito, & Viding, 2010). These include neurobiological factors, such as structural and functional remodeling of limbic and cortical brain systems (Eiland & Romeo, 2013; Fernald & Maruska, 2012; McEwen, 2007, 2010; Pascual-Leone, Amedi, Fregni, & Merabet, 2005); physiologic processes, such as exaggerated or prolonged SNS or HPA axis responsivity (Boyce & Ellis, 2005; Boyce, Sokolowski, & Robinson, 2012; Gunnar & Quevedo, 2007; Hertzman, 1999; Taylor, 2010); immune system dynamics, such as increased proinflammatory cytokine responses to challenge and decreased immune cell sensitivity to anti-inflammatory signals (G. E. Miller, Chen, et al., 2009; G. E. Miller et al., 2011); and genetic and epigenetic processes, such as transcriptional skewing of the leukocyte basal transcriptome (S. W. Cole, 2010; Slavich & Cole, 2013) and DNA methylation and histone modification (Danese & McEwen, 2012; Gluckman et al., 2008; G. E. Miller et al., 2011; Robinson, Grozinger, & Whitfield, 2005; Vialou, Feng, Robison, & Nestler, 2013). Several social and cognitive factors may also be implicated. For example, early life stress can lead to interpersonal stress generation, poor psychosocial coping, negative thinking styles, maladaptive core beliefs, and negative social expectations that persist into adulthood and combine to increase risk for depression (Beck, 1967; Evraire & Dozois, 2011; Fagundes, Bennett, Derry, & Kiecolt-Glaser, 2011; Gotlib & Joormann, 2010; Hankin & Abramson, 2001; Hazel, Hammen, Brennan, & Najman, 2008; Joiner, Metalsky, Katz, & Beach, 1999; Taylor & Stanton, 2007).
Finally, there are several lifestyle and environmental factors that may link early life stress with increased lifelong risk for depression and poor health. Although some of these pathways may be mediated in part by inflammatory processes, others may not. As reviewed by Marsland (2013), for example, early life stress is associated with sedentary behavior, poor diet, aberrant sleep continuity and architecture, and less preventative health care. Environmental stressors have also been implicated, such as noisy surroundings, overcrowding, high unemployment, crime, chemical exposure, and pollution (see also Evans & Kim, 2010; Repetti, Taylor, & Seeman, 2002). Many of these factors have been hypothesized to influence the programming of physiological and neuroendocrine systems that both regulate inflammation and are highly sensitive to remodeling by social-environmental input, especially during childhood and early adolescence (G. E. Miller et al., 2011). Given the paucity of longitudinal studies incorporating measures of stress, inflammation, and health, however, the empirical evidence for these models is currently limited. Consequently, it also remains unclear whether early life stress is a primary driver of the link between inflammation and depression. This is possible, but only a handful of studies to date have actually tested this hypothesis.
Recurrence in Depression
In addition, social signal transduction theory of depression may have implications for understanding recurrence in depression. Based on current estimates, approximately 60% of individuals who have a first lifetime episode of depression experience a second episode, 70% of individuals with two episodes experience a third, and 90% of those with three episodes experience a fourth (American Psychiatric Association, 2000; Solomon et al., 2000). One framework for understanding this phenomenon, generally referred to as stress sensitization, posits that individuals become increasingly vulnerable for depression as a result of neurobiological kindling and behavioral sensitization that develops in response to experiences with stress and depression (Monroe & Harkness, 2005, 2011). Consistent with this framework, individuals with a history of major early life stress and persons with more lifetime episodes of depression have been found to develop MDD following lower levels of recent life stress than their less vulnerable counterparts (i.e., persons with no early life stress and fewer lifetime episodes of MDD; Hammen, Henry, & Daley, 2000; Harkness, Bruce, & Lumley, 2006; Rudolph & Flynn, 2007; Slavich et al., 2011).
The concept of neuro-inflammatory sensitization depicted in Figure 3 provides one way of understanding the role that inflammation may play in stress sensitization and depression recurrence. The basic idea is that exposure to early adversity and successive experiences with depression potentiate SNS and HPA axis responding to stress and, in doing so, galvanize the regulatory pipeline between the brain and the inflammatory system (see G. E. Miller et al., 2011; G. E. Miller & Cole, 2012; Slavich & Cole, 2013). As a result, we speculate that the level of stress required to elicit inflammation and evoke depression may decrease over time. Presumably, it is possible that these bidirectional links between the brain and inflammatory system may become so strong over time that neural and physiological pathways that regulate inflammation could remain active in the absence of actual adversity, thereby leading to recurrent or chronic depression (see Figure 3).
To test these hypotheses about neuro-inflammatory sensitization and depression, future studies might examine whether individuals with a history of early life stress or prior depression have elevated levels of inflammation despite relatively low levels of recent life stress. Studies could also examine whether individuals with a history of early life stress or prior depression are more neurally sensitive to social threat than their less vulnerable counterparts. Given some evidence that recurrent episodes of depression are due in part to a confluence of social and biological factors including stress generation (Hammen, 1991), excessive reassurance seeking (Evraire, & Dozois, 2011; Joiner & Metalsky, 2001), and neurobiological kindling (Post, 1992, 2007), identifying how different factors interact to contribute to depression recurrence will be an important goal of future research.
Temporal Ordering of Anxiety and Depression
The idea of neuro-inflammatory sensitization embedded in this social signal transduction theory of depression may also have implications for understanding why anxiety disorders temporally precede depression. Converging evidence for this phenomenon has accumulated from several sources, including retrospective studies (de Graaf, Bijl, Spijker, Beekman, & Vollebergh, 2003; Essau, 2003, 2008; Warner, Weissman, Mufson, & Wickramaratne, 1999), longitudinal studies (Burke, Loeber, Lahey, & Rathouz, 2005; D. A. Cole, Peeke, Martin, Truglio, & Seroczynski, 1998; Giaconia et al., 1994; Lewinsohn, Zinbarg, Seeley, Lewinsohn, & Sack, 1997; Orvaschel, Lewinsohn, & Seeley, 1995; Wittchen, Kessler, Pfister, & Lieb, 2000), and even daily diary studies of anxiety and depression (Starr & Davila, 2012a, 2012b; for a review, see Wittchen, Beesdo, Bittner, & Goodwin, 2003). In a longitudinal community study of more than 2,500 adolescents and young adults, for example, having generalized anxiety disorder at baseline conferred a 4.5-fold increase in subsequent risk for developing a first lifetime episode of MDD, even after adjusting for other psychiatric disorders and factors that could have contributed to participants’ increased risk for depression (Bittner et al., 2004). Similar effects were found in a recent community-based sample of midlife women, in which having an anxiety disorder at baseline conferred an increase in risk for a first lifetime onset of MDD that was comparable to experiencing a recent stressful life event (Bromberger et al., 2009).
Although this research demonstrates that anxiety disorders often precede depression, the reasons for this temporal ordering have been unclear. Our view is that this ordering makes sense from the perspective of the social signal transduction theory of depression. The model is depicted in a static state in Figure 4 but can also be viewed from a developmental perspective, as seen in Figure 5. From this view, genetic factors (e.g., regulatory SNPs in the serotonin transporter or IL-6 gene), personality traits (e.g., neuroticism), and social-environmental conditions during childhood and adolescence (e.g., social stress, uncertainty, abuse, and neglect) all play a role in shaping a person’s neuro-inflammatory responding to stress. If an individual possesses genetic or personality characteristics that heighten his or her sensitivity to threat or if a person’s environment signals an increased likelihood of possible threat, then mild, preclinical levels of inflammation may develop during childhood and adolescence. This low-level inflammatory activity prepares the developing body for possible threat, but it might also feed back on the brain to alter sleep; promote hypervigilance, anxiety, and exaggerated perceptions of threat; and eventually lead to feelings of anhedonia and social disconnection (Eisenberger, Inagaki, et al., 2010; Eisenberger et al., 2009). These neuro-inflammatory interactions may not have immediate effects on health. As the neuro-inflammatory pipeline becomes more galvanized over time, though, it may skew the leukocyte basal transcriptome toward an increasingly proinflammatory state, eventually leading to increases in prodromal symptoms of depression and, possibly, clinical depression. Because perceptions of social threat are a critical mechanism driving these dynamics over time, we refer to this formulation as the social threat hypothesis of anxiety, inflammation, and depression (see Figure 5).
Figure 5.
Social threat hypothesis of anxiety, inflammation, and depression. Individuals’ perceptions of social threat and adversity are shaped by several influences including genetic factors (e.g., regulatory single nucleotide polymorphisms in the serotonin transporter or IL6 gene), personality traits (e.g., neuroticism, interpersonal and rejection sensitivity), and social-environmental conditions during childhood and adolescence (e.g., experiences of social stress, uncertainty, abuse, and neglect). Elevated levels of actual or perceived threat that result from these factors are hypothesized to increase feelings of anxiety and activate social signal transduction pathways that up-regulate inflammatory activity. Relatively mild, preclinical levels of inflammation may have limited effects on health in childhood and adolescence, especially for persons who are otherwise healthy. However, sustained engagement of these systems resulting from prolonged experiences of social threat or adversity may drive neuro-inflammatory sensitization, which could lead to both elevated levels of inflammation and exaggerated perceptions of social threat. Transcriptional changes that occur during this process may in turn foster an increasingly proinflammatory milieu that elevates risk for depression as an individual grows older. From this perspective, genetic, personality, and social-environmental factors early in life promote elevated perceptions of social threat, symptoms of anxiety, and preclinical levels of inflammation; then, at least for some individuals, anxiety symptoms become eclipsed by symptoms of depression that include sad mood, anhedonia, fatigue, altered appetite and sleep, and social-behavioral withdrawal.
At least three lines of research are consistent with this developmental model. First, several epidemiological studies have shown that the average age of onset for a first lifetime anxiety disorder is well before adolescence, when individuals typically experience a first lifetime depressive episode. In a recent analysis of data from the National Comorbidity Survey, for example, the median age of first onset for generalized anxiety disorder was 9.8 years old and 10.8 years old for males and females, respectively, and the median age of first onset for social phobia was 8.6 years old and 8.1 years old (again, for males and females, respectively; Burstein et al., 2012). In contrast, the average age of first onset for MDD is between 13 and 15 years old (Lewinsohn, Clarke, Seeley, & Rohde, 1994; Weissman & Olfson, 1995). Therefore, when considered together with the prospective studies of anxiety and depression, there appears to be substantial evidence that anxiety disorders frequently precede onset of depression, both across people and within the same person over time.
Second, there is evidence that perceptions of social threat develop early in life and increase a person’s subsequent risk for depression. In a recent neuroimaging study of young adolescents, for example, participants were exposed to a brief episode of social rejection (i.e., Cyberball) and then followed prospectively for 1 year. As expected, greater neural responses to the acute episode of social rejection predicted greater increases in depressive symptoms over the year (Masten et al., 2011). Although these data are limited, it is interesting to speculate that exaggerated sensitivity to social threat might serve as a neurocognitive precursor of depression and, perhaps, an endophenotype for MDD.
Finally, there is emerging evidence that individuals who have experienced early life stress, and who are thus at high risk for developing depression during their lifetime, exhibit significant increases in systemic inflammatory activity from early childhood (1.5 years old) through late childhood (10 years old) and into midadolescence (15 years old; Slopen et al., 2013). Although the development of anxiety, perceptions of social threat, and inflammation and depression have not been studied in concert with one another, the fact that all three precede depression—and that heightened inflammation and perceptions of social threat appear to be mechanistically involved in the pathogenesis of depression—raises the interesting possibility that anxiety and depression may be sequentially timed consequences of a slowly evolving inflammatory milieu that is driven by exaggerated sensitivity to social threat.
Although the idea that inflammation may play a role in linking anxiety and depression is new, the notion that anxiety and depression may be phenotypic expressions of a common underlying risk factor has been long debated. In this context, some theorists have proposed that anxiety and depression are largely inseparable, perhaps arising from a similar personality trait or biological process (Brady & Kendall, 1992; Clark & Watson, 1991; Griffith et al., 2010; Krueger & Markon, 2006; Watson, 2005). Our formulation is consistent with this perspective but extends this work by focusing on exaggerated sensitivity to social threat and related increases in inflammation as key common mediators that drive aspects of both anxiety and depression.
Clearly, much more research is needed to evaluate this social threat hypothesis of anxiety, inflammation, and depression. To begin with, longitudinal studies are needed to map the developmental trajectories of exaggerated sensitivity to social threat, anxiety, inflammation, and depression. Second, although anxiety and inflammation are known to increase in parallel with one another, additional research is needed to examine whether inflammatory processes are mechanistically involved in anxiety or simply an epiphenomenon of the disorder—for example, the result of other processes like increased β-adrenergic signaling, which is implicated in both anxiety and inflammation (see Hanke, Powell, Stiner, Bailey, & Sheridan, 2012). Third, a new generation of studies is needed that incorporates neuroimaging into longitudinal study designs. These studies will be important insofar as they will enable investigators to elucidate the full set of neurocognitive processes that predict increased risk for anxiety, heightened inflammation, and depression. Here, we have suggested that neural systems involved in processing social threat may play an important role in anxiety, inflammation, and depression. However, several other neurocognitive processes are also likely involved, including those implicated in safety processing (e.g., “Am I safe?” or “Am I secure?”) and social cognition (e.g., “What does that person think of me?” or “Does he like me?”; see Muscatell & Eisenberger, 2012).
Finally, additional research is needed to identify genetic, personality, and social-environmental factors that might moderate associations between anxiety, inflammation, and depression, perhaps making these phenomena more tightly linked in some people versus others. Considered together, these lines of research may help reveal why anxiety disorders often precede, and are so frequently comorbid with, depression, as well as why negative thoughts about the self and others are typically a cardinal feature of MDD. Perhaps more important, though, this research may provide clues for how to prevent depression by targeting predepression anxiety disorders and exaggerated perceptions of social-environmental threat (Garber & Weersing, 2010; O’Donovan, Slavich, et al., 2013).
Prevention and Treatment of Depression
Finally, social signal transduction theory of depression may generate new ideas for how to treat and prevent depression. Episodes of depression can be extremely debilitating, especially when they involve sickness behaviors such as anhedonia, fatigue, psychomotor retardation, and social-behavioral withdrawal. These symptoms disrupt interpersonal relationships and can severely impair occupational functioning. Insofar as inflammatory processes mediate these neurovegetative symptoms, presumably new pharmacological and psychosocial interventions can be developed that target cytokine activity in order to alleviate, and perhaps even prevent, depression (Capuron & Miller, 2011; Haroon et al., 2012; Hayley, 2011; Li et al., 2011; A. H. Miller et al., 2009; Rook et al., 2012).
Pharmacologic interventions that target inflammation
Although this research is still in its infancy, some evidence exists demonstrating that cytokine-related mechanisms can be targeted to help alleviate depression. As alluded to previously, for example, TNF-α antagonists such as etanercept or infliximab and the COX-2 inhibitor celecoxib have all been found to alleviate depressive symptoms in double-blind, randomized, placebo-controlled studies (Müller et al., 2006; Raison et al., 2013; Tyring et al., 2006; see also Monk et al., 2006). These targets are just the tip of the iceberg, though. As reviewed by Rook et al. (2012), additional possibilities include inhibiting NF-κB (e.g., via curcumin, Vitamin E, or resveratrol) or p38 mitogen-activated protein kinase (e.g., via minocycline); antagonizing IDO (e.g., using 1-MT) or chemokines receptors (e.g., using maraviroc, plerixafor); enhancing glucocorticoid sensitivity (e.g., using salbutamol or salmeterol); and activating the anti-inflammatory cholinergic reflex (e.g., using α7nAChR agonists or vagus nerve stimulation devices).
More commonly used substances that impact inflammation may also alleviate depression. In an open-label study of treatment-resistant depression, for example, 52.4% of depressed individuals who were treated with acetylsalicylic acid (i.e., aspirin) in addition to receiving a selective reuptake inhibitor (SSRI) were considered responders after having not responded to a first SSRI treatment. In fact, patients who were considered responders to the combined SSRI–acetylsalicylic acid treatment saw their HRSD scores decrease from 29.3 at Day 0 to 8.4 at Day 28 of the study, which was a reduction of 20.9 HRSD points (Mendlewicz et al., 2006). Consistent with these effects, a recent study that combined daily diary and neuroimaging techniques showed that healthy young adults who were randomly assigned to take acetaminophen twice a day for 3 weeks exhibited significantly fewer hurt feelings on a daily basis and less neural sensitivity to a laboratory-based episode of social rejection (i.e., Cyberball) compared to their counterparts in the placebo condition (DeWall et al., 2010). Although acetaminophen is not known to have anti-inflammatory effects, it does have the ability to attenuate pain perception in the brain, which may potentially reduce experiences of both physical and social pain in the context of depression. Finally, in a recent double-blind, randomized, placebo-controlled trial, medical students who received omega-3 supplementation exhibited a 14% reduction in LPS-stimulated IL-6 production and a 20% reduction in anxiety symptoms compared to those in the control group (Kiecolt-Glaser, Belury, Andridge, Malarkey, & Glaser, 2011). Although omega-3 supplementation was unrelated to changes in depression in this study, a recent meta-analytic review of 35 randomized controlled trials found that omega-3 fatty acid supplementation is associated with a 0.41-0.57-SD difference in depression severity for individuals with MDD (see Appleton, Rogers, & Ness, 2010).
These data suggest that inflammation can be targeted to reduce depressive symptoms. However, some research has also suggested that the link between inflammatory cytokines and the antidepressant response is complex and likely more nuanced than previously thought. In a recent study, for example, Raison and colleagues (2013) found that although infliximab had antidepressant effects in treatment-resistant depressed patients with high baseline levels of inflammation, placebo unexpectedly outperformed this TNF blockade in patients with low baseline levels of inflammation. Therefore, it is possible that alleviating depressive symptoms with either an antidepressant or anti-inflammatory compound requires some optimal level of cytokine activity. Consistent with this possibility, low levels of cytokine activity are essential for neuroplasticity and neurogenesis, which generally lessen risk for depression (Yirmiya & Goshen, 2011). In addition, a recent study found that the effects of serotonergic antidepressants on behavior are mediated by TNF-related processes (Warner-Schmidt, Vanover, Chen, Marshall, & Greengard, 2011). Finally, anti-inflammatory drugs have been found to interfere with the antidepressant effects of SSRIs by disrupting TNF-related increases in p11, a small acidic protein that can have strong antidepressant properties (Warner-Schmidt et al., 2011). As such, although targeting inflammation to alleviate depression sounds intuitively promising, additional research is needed to understand when exactly these pharmacologic interventions are beneficial.
Psychosocial interventions that target inflammation
In addition, there is now evidence that cognitive- and meditation-based interventions that are known to alleviate depression may also lead to reductions in inflammation. These interventions include mindfulness-based cognitive therapy (Britton, Shahar, Szepsenwol, & Jacobs, 2012; Ma & Teasdale, 2004; see Hofmann, Sawyer, Witt, & Oh, 2010), tai chi (Lavretsky et al., 2011), and yoga (Pilkington, Kirkwood, Rampes, & Richardson, 2005; Sengupta, 2012). In one of the first studies to examine this effect, healthy adults were exposed to a laboratory-based social stressor (i.e., TSST) after being randomized to either 6 weeks of compassion meditation training or a health discussion control group. Although there were no between-group effects, within the meditation group greater meditation practice was associated with fewer social stress-induced increases in IL-6 and emotional distress (Pace et al., 2009). In a second study, individuals completed the TSST after being randomly assigned to either participate in tai chi classes for 3 months or be in a wait-list control group. Compared to participants in the control group, those in the tai chi group perceived the TSST as less stressful and exhibited fewer TSST-induced increases in heart rate, cortisol, and α-amylase (Nedeljkovic, Ausfeld-Hafter, Streitberger, Seiler, & Wirtz, 2012). This last finding is interesting since α-amylase serves a proxy for adrenergic activation, and adrenergic signaling is known to up-regulate proinflammatory cytokine production (Desser, Rehberger, & Paukovits, 1994). In addition, tai chi administration has been reported to reduce SNS activity (Motivala, Sollers, Thayer, & Irwin, 2006), which may lead to lower inflammation (Irwin & Olmstead, 2012; Lavretsky et al., 2011). Finally, in a third study, levels of inflammatory activity were assessed in novice and expert yoga practitioners before, during, and after three separate study visits that included a restorative hatha yoga session, a movement control session, and a passive-video control session. Although the yoga intervention did not impact inflammatory activity, novices’ IL-6 levels were 41% higher than those of experts across the sessions (Kiecolt-Glaser, Christian, et al., 2010).
In addition to this research, at least three studies have demonstrated that cognitive- and meditation-based interventions that have been employed for treating depression can reverse stress-induced genome-wide transcriptional responses like the CTRA. In the first study, healthy older adults were randomized to either an 8-week mindfulness-based stress reduction (MBSR) program or a wait-list control condition. The MBSR program reduced self-reported feelings of loneliness and resulted in 143 genes being differentially expressed in MBSR versus wait-list control participants. Compared to participants in the wait-list control condition, those in the MBSR group exhibited reduced activity of NF-κB target genes (Creswell et al., 2012). In the second study, individuals who were caregiving for a family member with dementia were randomly assigned to either participate in Kirtan Kriya meditation or listen to relaxing music for 12 min daily over 8 weeks. A total of 68 genes were differentially expressed between the two groups, with down-regulated genes again primarily being NF-κB-associated proinflammatory genes that are normally up-regulated under conditions of high chronic stress (Black et al., 2013). Finally, in the third study, women undergoing primary treatment of stage 0-III breast cancer were randomized to either a 10-week cognitive behavioral stress management intervention designed to target anxiety-related affective and behavioral processes or an active control condition. Consistent with the other two studies just described, this intervention counteracted the effects of stress-related transcriptional skewing by reducing expression of proinflammatory and metastasis-related genes and by increasing expression of IFN-α genes (Antoni et al., 2012).
Considered together, these studies suggest that psychotherapeutic and meditative interventions that are already in our clinical toolbox may be helpful for reducing inflammation. None of the studies just reviewed, though, sampled depressed individuals. As such, additional research is needed to examine whether these cognitive, behavioral, and meditative interventions alleviate depressive symptoms in depressed individuals and, if so, whether they exert these effects by reducing inflammation.
Concluding Comments
In conclusion, we know a lot about the adverse social-environmental conditions that typically precipitate depression and about cognitive and emotional processes that mediate these effects. With the advent of new neuroimaging, immunological, and genome-wide profiling techniques, we are now poised to go one step deeper and elucidate the full set of biological mechanisms that link stress with depression. Inflammation is undoubtedly a key player in this link. As we have discussed, two general phenomena are consistent with the hypothesis that stress-related increases in inflammation are involved in depression. First, a large number of naturalistic and laboratory-based experimental studies have shown that stress is a potent activator of inflammation, and second, it is now well known that vaccinations and immunological challenges that up-regulate inflammatory activity evoke depressive-like behaviors in rodents and clinically significant episodes of depression in at least some people. In addition, these challenges have been shown to up-regulate peripheral and central cytokine production and to alter metabolic and neural activity in brain regions that have been implicated in depression. Many questions remain unanswered regarding these effects, including whether inflammation is necessary or sufficient for all cases of MDD. Nonetheless, based on existing data, we conclude that stress likely increases risk for depression in a substantial number of people by up-regulating inflammatory activity and by altering social, cognitive, and affective processes that are known to promote this disorder.
These insights are important because they can help update contemporary theories of depression with information about biological mechanisms that are involved in the pathogenesis of MDD. For the potential of these insights to be fully realized, they will need to be translated into new strategies for modifying processes that promote depression (Sanislow et al., 2010). At a very general level, such processes include neurocognitive mechanisms like negative cognitive appraisals and neural sensitivity to social threat, which have been associated with inflammation and depression (Masten et al., 2011; Monroe, Slavich, Torres, & Gotlib, 2007b; Rueggeberg, Wrosch, Miller, & McDade, 2012); immunological processes such as preclinical levels of inflammation, which could presage the development of chronic inflammation and disease (G. E. Miller et al., 2011); and psychosocial factors such as parental behaviors, which have been found to influence the effects of social-environmental adversity on proinflammatory signaling (Chen, Miller, Kobor, & Cole, 2011; see also Carroll et al., 2013). The hope is that by targeting these and other dynamics, we may one day be able to reduce the prevalence of depression and the substantial financial burden and personal suffering associated with this common and costly disorder.
Acknowledgments
Preparation of this review was supported by National Institutes of Health (NIH) Grant R01 CA140933 and by a Society in Science—Branco Weiss Fellowship to George M. Slavich; by NIH Grants R01 AG034588, R01 AG026364, R01 CA160245, R01 CA119159, R01 HL095799, R01 DA032922, and P30 AG028748 to Michael R. Irwin; and by seed grants from the UCLA Clinical and Translational Science Institute (UL1 TR000124) and the Cousins Center for Psychoneuroimmunology. We thank Keely Muscatell and Aoife O’Donovan for their very helpful comments on a previous version of the manuscript.
References
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology. 1993;61:354–359. doi: 10.1037/0022-006X.61.2.354. [DOI] [PubMed] [Google Scholar]
- Agüera L, Failde I, Cervilla JA, Díaz-Fernández P, Mico JA. Medically unexplained pain complaints are associated with underlying unrecognized mood disorders in primary care. BMC Family Practice. 2010;11:Article 17. doi: 10.1186/1471-2296-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MDS. Attachments and other affectional bonds across the life cycle. In: Parkes CM, Stevenson-Hinde J, Marris P, editors. Attachment across the life cycle. London, England: Routledge; 1991. pp. 33–51. [Google Scholar]
- Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: A double blind and placebo controlled trial. Depression and Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nature Immunology. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Allen NB, Badcock PB. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129:887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, Regev A. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009 Oct 9;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415– 425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain, Behavior, and Immunity. 2007;21:147–152. doi: 10.1016/j.bbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Andrews G, Issakidis C, Sanderson K, Corry J, Lapsley H. Utilising survey data to inform public policy: Comparison of the cost-effectiveness of treatment of ten mental disorders. British Journal of Psychiatry. 2004;184:526–533. doi: 10.1192/bjp.184.6.526. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neuroscience and Biobehavioral Reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain, Behavior, and Immunity. 2002;16:513–524. doi: 10.1016/S0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Cole SW. Cognitive behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biological Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Kubzansky LD. The association between childhood emotional functioning and adulthood inflammation is modified by early-life socioeconomic status. Health Psychology. 2012;31:413– 422. doi: 10.1037/a0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n–3 long-chain polyunsatu-rated fatty acids on depressed mood. American Journal of Clinical Nutrition. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabhar FS. Maintenance of a positive outlook during acute stress protects against proinflammatory reactivity and future depressive symptoms. Brain, Behavior, and Immunity. 2012;26:346–352. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and Behavior. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. Journal of Neuroimmunology. 2008;195:157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Eckert GJ, Stang PE, Croghan TW, Kroenke K. Impact of pain on depression treatment response in primary care. Psychosomatic Medicine. 2004;66:17–22. doi: 10.1097/01.PSY.0000106883.94059.C5. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: A meta-analysis. Psychosomatic Medicine. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- Barton GM. A calculated response: Control of inflammation by the innate immune system. Journal of Clinical Investigation. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, Berger K. The relationship between subtypes of depression and cardiovascular disease: A systematic review of biological models. Translational Psychiatry. 2012;2:Article e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York, NY: Harper & Row; 1967. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Biddie SC, Conway-Campbell BL, Lightman SL. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology. 2012;51:403–412. doi: 10.1093/rheumatology/ker215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner A, Goodwin RD, Wittchen HU, Beesdo K, Höfler M, Lieb R. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? Journal of Clinical Psychiatry. 2004;65:618–626. doi: 10.4088/JCP.v65n0505. [DOI] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Lavretsky H. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38:348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt SJ. Experiences of depression: Theoretical, clinical, and research perspectives. Washington, DC: American Psychological Association; 2004. [DOI] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 Jan 12;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress: Risk factors for cancer-related fatigue. Clinical Psychological Science. 2014;2:108–115. doi: 10.1177/2167702613496243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: Increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain, Behavior, and Immunity. 2011;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 3. Loss: Sadness and depression. New York, NY: Basic Books; 1980. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Sokolowski MB, Robinson GE. Toward a new biology of social adversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17143–17148. doi: 10.1073/pnas.1121264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychological Bulletin. 1992;111:244–255. doi: 10.1037/0033-2909.111.2.244. [DOI] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: Results from a randomized controlled trial. Behavior Therapy. 2012;43:365–380. doi: 10.1016/j.beth.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychological Medicine. 2009;39:55– 64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. New York, NY: Free Press; 1978. [Google Scholar]
- Brown GW, Harris TO. Life events and illness. New York, NY: Guilford Press; 1989. [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: A patient and non-patient comparison. Psychological Medicine. 1995;25:7–21. doi: 10.1017/S003329170002804X. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain, Behavior, and Immunity. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-α and ribavirin treatment. Molecular Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB, Rathouz PJ. Developmental transitions among affective and behavioral disorders in adolescent boys. Journal of Child Psychology and Psychiatry. 2005;46:1200–1210. doi: 10.1111/j.1469-7610.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- Burstein M, Georgiades K, Lamers F, Swanson SA, Cui L, He JP, Merikangas KR. Empirically derived subtypes of lifetime anxiety disorders: Developmental and clinical correlates in U.S. adolescents. Journal of Consulting and Clinical Psychology. 2012;80:102–115. doi: 10.1037/a0026069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. n–3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition. 2006;83(Suppl):S1505–S1519. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, López-Griego L, Morales-Montor J. The role of cytokines in the regulation of neurotransmission. Neuroimmunomodulation. 2009;16:1–12. doi: 10.1159/000179661. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-α in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-α. Biological Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacology and Therapeutics. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-α therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. American Journal of Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosomatic Medicine. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biological Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Price LH. C-reactive protein, early life stress, and wellbeing in healthy adults. Acta Psychiatrica Scandinavica. 2012;126:402– 410. doi: 10.1111/j.1600-0447.2012.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain, Behavior, and Immunity. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul 18;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castanon N, Bluthé RM, Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1β in the rat. Psychopharmacology. 2001;154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain, Behavior, and Immunity. 2007;21:993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on proinflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1878–1882. doi: 10.1073/pnas.1120972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D, Sanderson K, Ayuso-Mateos JL, Saxena S. Reducing the global burden of depression: Population-level analysis of intervention cost-effectiveness in 14 world regions. British Journal of Psychiatry. 2004;184:393–403. doi: 10.1192/bjp.184.5.393. [DOI] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature Neuroscience. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain, Behavior, and Immunity. 2012;26:859–865. doi: 10.1016/j.bbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EHS, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cole DA, Peeke LG, Martin JM, Truglio R, Seroczynski AD. A longitudinal look at the relation between depression and anxiety in children and adolescents. Journal of Consulting and Clinical Psychology. 1998;66:451–460. doi: 10.1037/0022-006X.66.3.451. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain, Behavior, and Immunity. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression. Current Directions in Psychological Science. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Elevating the perspective on human stress genomics. Psychoneuroendocrinology. 2010;35:955–962. doi: 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of gene expression in the immune system. In: Segerstrom SC, editor. The Oxford handbook of psychoneuroimmunology. New York, NY: Oxford University Press; 2012. pp. 254–273. [DOI] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:Article R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. Journal of Immunology. 1998;161:610–616. [PubMed] [Google Scholar]
- Collado-Hidalgo A, Sung C, Cole S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain, Behavior, and Immunity. 2006;20:552–563. doi: 10.1016/j.bbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. Inflammation bares a dark side. Science. 2010 Dec 17;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- Cover H, Irwin M. Immunity and depression: Insomnia, retardation, and reduction of natural killer cell activity. Journal of Behavioral Medicine. 1994;17:217–223. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Downey G. Social factors and psychopathology: Stress, social support, and coping processes. Annual Review of Psychology. 1991;42:401–425. doi: 10.1146/annurev.ps.42.020191.002153. [DOI] [PubMed] [Google Scholar]
- Cramer AO, Borsboom D, Aggen SH, Kendler KS. The pathoplasticity of dysphoric episodes: Differential impact of stressful life events on the pattern of depressive symptom inter-correlations. Psychological Medicine. 2012;42:957–965. doi: 10.1017/S003329171100211X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Cole SW. Mindfulness-based stress reduction training reduces loneliness and proinflammatory gene expression in older adults: A small randomized controlled trial. Brain, Behavior, and Immunity. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: Sources, receptors, effects, and inducers. Clinical Microbiology Reviews. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Straub RH. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation. 2006;13:277–282. doi: 10.1159/000104855. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: An integrated view of relationships between the brain and the immune system. Life Sciences. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath M, Doyle KM, Langan C, McDonald C, Leonard B, Cannon DM. Recent advances in psychoneuroimmunology: Inflammation in psychiatric disorders. Translational Neuroscience. 2011;2:121–137. doi: 10.2478/s13380-011-0019-0. [DOI] [Google Scholar]
- de Graaf R, Bijl RV, Spijker J, Beekman AT, Vollebergh WA. Temporal sequencing of lifetime mood disorders in relation to comorbid anxiety and substance use disorders: Findings from the Netherlands Mental Health Survey and Incidence Study. Social Psychiatry and Psychiatric Epidemiology. 2003;38:1–11. doi: 10.1007/s00127-003-0597-4. [DOI] [PubMed] [Google Scholar]
- De La Garza R., II Endotoxin- or proinflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neuroscience and Biobehavioral Reviews. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neuroscience and Biobehavioral Reviews. 2010;34:130–143. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychological Bulletin. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Desser L, Rehberger A, Paukovits W. Proteolytic enzymes and amylase induce cytokine production in human peripheral blood mononuclear cells in vitro. Cancer Biotherapy. 1994;9:253–263. doi: 10.1089/cbr.1994.9.253. [DOI] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, Kalrab P, Matthews KA. The prospective association of socioeconomic status with C-reactive protein levels in the CARDIA study. Brain, Behavior, and Immunity. 2012;26:1128–1135. doi: 10.1016/j.bbi.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, MacDonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Eisenberger NI. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychological Science. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. Journal of Psychiatric Research. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells—From barracks to boulevards to battlefields: A tale of three hormones—Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37:1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: An experimental laboratory investigation. Psychological Science. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Psychobiological responses to social self threat: Functional or detrimental? Self and Identity. 2009;8:270–285. doi: 10.1080/15298860802505186. [DOI] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004;66:124–131. doi: 10.1097/01.PSY.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology. 2008;27:116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: Prospective findings from the Heart and Soul Study. American Journal of Psychiatry. 2011;168:913–920. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012 Oct 5;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee HB, Lee BH, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry. 2008;65:513–520. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain, Behavior, and Immunity. 2006;20:159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The neural bases of social pain: Evidence for shared representations with physical pain. Psychosomatic Medicine. 2012a;74:126–135. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012b;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. Journal of Neuroimmunology. 2005;163:110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Essau CA. Comorbidity of anxiety disorders in adolescents. Depression and Anxiety. 2003;18:1–6. doi: 10.1002/da.10107. [DOI] [PubMed] [Google Scholar]
- Essau CA. Comorbidity of depressive disorders among adolescents in community and clinical settings. Psychiatry Research. 2008;158:35–42. doi: 10.1016/j.psychres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status–health gradient. Annals of the New York Academy of Sciences. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Evraire LE, Dozois DJ. An integrative model of excessive reassurance seeking and negative feedback seeking in the development and maintenance of depression. Clinical Psychology Review. 2011;31:1291–1303. doi: 10.1016/j.cpr.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and inflammation across the lifespan: Social developmental pathways to disease. Social and Personality Psychology Compass. 2011;5:891–903. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: Searching for a framework. Molecular Psychiatry. 2005;10:6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- Farmer AE, McGuffin P. Humiliation, loss and other types of life events and difficulties: A comparison of depressed subjects, healthy controls and their siblings. Psychological Medicine. 2003;33:1169–1175. doi: 10.1017/S0033291703008419. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Pace TW, Woolwine BJ, Hu F, Raison CL, Miller AH. Early activation of p38 mitogen activated protein kinase is associated with interferon-alpha-induced depression and fatigue. Brain, Behavior, and Immunity. 2011;25:1094–1098. doi: 10.1016/j.bbi.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Maruska KP. Social information changes the brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17194–17199. doi: 10.1073/pnas.1202552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. Journal of Clinical Investigation. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among US adults. Annals of Epidemiology. 2006;16:78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Coffey KA, Algoe SB, Firestine AM, Arevalo JMG, Cole SW. A functional genomic perspective on human well-being. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. Mourning and melancholia. In: Strachey J, translator. The standard edition of the complete psychological works of Sigmund Freud. Vol. 14. London, England: Hogarth Press; 1957. pp. 243–258. Original work published 1917. [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. European Archives of Psychiatry and Clinical Neuroscience. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki P, Gałecka E, Maes M, Chamielec M, Orzechowska A, Bobińska K, Szemraj J. The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. Journal of Affective Disorders. 2012;138:360–366. doi: 10.1016/j.jad.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Garber J, Weersing VR. Comorbidity of anxiety and depression in youth: Implications for treatment and prevention. Clinical Psychology: Science and Practice. 2010;17:293–306. doi: 10.1111/j.1468-2850.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaconia RM, Reinherz HZ, Silverman AB, Pakiz B, Frost AK, Cohen E. Ages of onset of psychiatric disorders in a community population of older adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:706–717. doi: 10.1097/00004583-199406000-00012. [DOI] [PubMed] [Google Scholar]
- Gibson G. The environmental contribution to gene expression profiles. Nature Reviews Genetics. 2008;9:575–581. doi: 10.1038/nrg2383. [DOI] [PubMed] [Google Scholar]
- Gilbert P. Depression: The evolution of powerlessness. New York, NY: Guilford Press; 1992. [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Family disruption in childhood and risk of adult depression. American Journal of Psychiatry. 2003;160:939–946. doi: 10.1176/appi.ajp.160.5.939. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Brunner EJ, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. European Journal of Epidemiology. 2007;22:675–683. doi: 10.1007/s10654-007-9171-9. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Mellor S, Brydon L, Steptoe A. Psychological distress and circulating inflammatory markers in healthy young adults. Psychological Medicine. 2010;40:2079–2087. doi: 10.1017/S0033291710000267. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Fergusson DM, Horwood LJ. Asthma and depressive and anxiety disorders among young persons in the community. Psychological Medicine. 2004;34:1465–1474. doi: 10.1017/S0033291704002739. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine. 2012;44:287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflammatory Bowel Diseases. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- Grebe KM, Takeda K, Hickman HD, Bailey AL, Embry AC, Bennink JR, Yewdell JW. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. Journal of Immunology. 2010;184:540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM–IV disorders. Archives of General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: How did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/JCP.v64n1211. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Zinbarg RE, Craske MG, Mineka S, Rose RD, Waters AM, Sutton JM. Neuroticism as a common dimension in the internalizing disorders. Psychological Medicine. 2010;40:1125–1136. doi: 10.1017/S0033291709991449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. α1-adrenergic receptors positively regulate toll-like receptor cytokine production from human monocytes and macrophages. Journal of Pharmacology and Experimental Therapeutics. 2011;338:648–657. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in Black and White men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Social Science & Medicine. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. Journal of Zhejiang University Sciences B. 2005;6:1045–1056. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. 2012;37:1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Häfner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W KORA Study Investigators. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: Results from the MONICA/KORA study. Brain, Behavior, and Immunity. 2011;25:1701–1707. doi: 10.1016/j.bbi.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Persistent depressive symptomatology and inflammation: To what extent do health behaviours and weight control mediate this relationship? Brain, Behavior, and Immunity. 2009;23:413–418. doi: 10.1016/j.bbi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. The generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037/0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. Journal of Consulting and Clinical Psychology. 2000;68:782–787. doi: 10.1037/0022-006X.68.5.782. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain, Behavior, and Immunity. 2012;26:1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability–transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain, Behavior, and Immunity. 2011;25:256–259. doi: 10.1016/j.bbi.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Bruce AE, Lumley MN. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. Journal of Abnormal Psychology. 2006;115:730–741. doi: 10.1037/0021-843X.115.4.730. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Tabari N, Monroe SM, Slavich GM, Gotlib IH, Bagby RM. Sex differences in life events prior to onset of major depressive disorder: The moderating effect of age. Journal of Abnormal Psychology. 2010;119:791–803. doi: 10.1037/a0020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biological Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayley S. Toward an anti-inflammatory strategy for depression. Frontiers in Behavioral Neuroscience. 2011;5:Article 19. doi: 10.3389/fnbeh.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel NA, Hammen C, Brennan PA, Najman J. Early childhood adversity and adolescent depression: The mediating role of continued stress. Psychological Medicine. 2008;38:581–589. doi: 10.1017/S0033291708002857. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Waring ME, Roberts MB, Eaton CB, Gramling R. Social isolation, C-reactive protein, and coronary heart disease mortality among community-dwelling adults. Social Science & Medicine. 2011;72:1482–1488. doi: 10.1016/j.socscimed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: Focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml-Webb PA, Deak T. Separation, sickness, and depression: A new perspective on an old animal model. Current Directions in Psychological Science. 2009;18:227–231. doi: 10.1111/j.1467-8721.2009.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychological Bulletin. 1993a;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: A meta-analytic review. Psychosomatic Medicine. 1993b;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Hernández ME, Mendieta D, Martínez-Fong D, Loría F, Moreno J, Estrada I, Pavón L. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. European Neuropsychopharmacology. 2008;18:917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: Exploring the causes of heterogeneity. Brain, Behavior, and Immunity. 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Pollmächer T. Depression, comorbidities and the TNF-α system. European Psychiatry. 2008;23:421– 429. doi: 10.1016/j.eurpsy.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7:Article e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Stuck in a rut: Rethinking depression and its treatment. Trends in Neurosciences. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang JL, Zhang YL, Wang CC, Zhou JR, Ma Q, Wang X, Jiang CL. Enhanced phosphorylation of MAPKs by NE promotes TNF-α production by macrophage through α adrenergic receptor. Inflammation. 2012;35:527–534. doi: 10.1007/s10753-011-9342-4. [DOI] [PubMed] [Google Scholar]
- Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radical Biology and Medicine. 2000;28:1379–1386. doi: 10.1016/S0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Irwin M, Brown M, Patterson T, Hauger R, Mascovich A, Grant I. Neuropeptide Y and natural killer cell activity: Findings in depression and Alzheimer caregiver stress. FASEB Journal. 1991;5:3100–3107. doi: 10.1096/fasebj.5.15.1743441. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, Oxman MN. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain, Behavior, and Immunity. 2011;25:759–766. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, Oxman MN. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clinical Infectious Diseases. 2013;56:1085–1093. doi: 10.1093/cid/cis1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: A randomized controlled trial of tai chi chih. American Journal of Geriatric Psychiatry. 2012;20:764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DR, Jr, Cho HJ, Seeman TE. Gender, obesity and repeated elevation of C-reactive protein: Data from the CARDIA cohort. PLoS One. 2012;7:Article e36062. doi: 10.1371/journal.pone.0036062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Mills PJ. Cytokines, chronic stress, and fatigue. In: Fink G, editor. Encyclopedia of stress. 2. Oxford, England: Academic Press; 2007. pp. 698–704. [DOI] [Google Scholar]
- Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. Journal of Hepatology. 1994;21:241–243. doi: 10.1016/S0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biological Psychology. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Loneliness promotes inflammation during acute stress. Psychological Science. 2013;24:1089–1097. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Jr, Metalsky GI. Excessive reassurance seeking: Delineating a risk factor involved in the development of depressive symptoms. Psychological Science. 2001;12:371–378. doi: 10.1111/1467-9280.00369. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Metalsky GI, Katz J, Beach SRH. Depression and excessive reassurance-seeking. Psychological Inquiry. 1999;10:269–278. doi: 10.1207/S15327965PLI1004_1. [DOI] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006 May 25;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Katz PP, Yelin EH. Prevalence and correlates of depressive symptoms among persons with rheumatoid arthritis. Journal Rheumatology. 1993;20:790–796. [PubMed] [Google Scholar]
- Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. Journal of Biochemistry. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. American Journal of Psychiatry. 2007;164:1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. Psychobiological responses to social threat: Evolution of a psychological model in psychoneuroimmunology. Brain, Behavior, and Immunity. 2009;23:1–9. doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–848. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: Toward an integrated etiologic model. American Journal of Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. American Journal of Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. American Journal of Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: Results from the National Comorbidity Survey Replication (NCS-R) Psychological Medicine. 2010;40:225–237. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior, and Immunity. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, Glaser R. Stress, inflammation, and yoga practice. Psychosomatic Medicine. 2010;72:113–121. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kim S, Thibodeau R, Jorgensen RS. Shame, guilt, and depressive symptoms: A meta-analytic review. Psychological Bulletin. 2011;137:68–96. doi: 10.1037/a0021466. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Joormann J, Gotlib IH. Cognitive aspects of depression. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3:301–313. doi: 10.1002/wcs.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Virtanen M, Elovainio M, Kouvonen A, Väänänen A, Vahtera J. Work stress in the etiology of coronary heart disease: A meta-analysis. Scandinavian Journal of Work, Environment, and Health. 2006;32:431–442. doi: 10.5271/sjweh.1049. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends in Neurosciences. 2002;25:154–159. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: Systematic review and meta-analysis. Lancet Neurology. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Kupper N, Widdershoven JW, Pedersen SS. Cognitive/affective and somatic/affective symptom dimensions of depression are associated with current and future inflammation in heart failure patients. Journal of Affective Disorders. 2012;136:567–576. doi: 10.1016/j.jad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Annals of the New York Academy of Sciences. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, Part I: Historical context, methods, and relevant basic science. Psychosomatic Medicine. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, Irwin MR. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: A randomized controlled trial. American Journal of Geriatric Psychiatry. 2011;19:839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O’Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. Journal of Experimental Medicine. 2000;192:105–116. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. The concept of depression as a dysfunction of the immune system. Current Immunology Reviews. 2010;6:205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience and Biobehavioral Reviews. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Zinbarg R, Seeley JR, Lewinsohn M, Sack WH. Lifetime comorbidity among anxiety disorders and between anxiety disorders and other mental disorders in adolescents. Journal of Anxiety Disorders. 1997;11:377–394. doi: 10.1016/S0887-6185(97)00017-0. [DOI] [PubMed] [Google Scholar]
- Li M, Soczynska JK, Kennedy SH. Inflammatory biomarkers in depression: An opportunity for novel therapeutic interventions. Current Psychiatry Reports. 2011;13:316–320. doi: 10.1007/s11920-011-0210-6. [DOI] [PubMed] [Google Scholar]
- Libert C. Inflammation: A nervous connection. Nature. 2003 Jan 23;421:328–329. doi: 10.1038/421328a. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Melzack R. Pain: An overview. Lancet. 1999;353:1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: Different treatments and their effects. Journal of Rheumatology. 2011;88:48–54. doi: 10.3899/jrheum.110903. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biological Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Rabinovitz M, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: Characteristics and vulnerability. Journal of Psychosomatic Research. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Sood AK. Social influences on clinical outcome of ovarian cancer patients. Journal of Clinical Oncology. 2012;30:2885–2890. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, Lucci J, III, Cole SW. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain, Behavior, and Immunity. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Slavich GM, DeGeest K, Goodheart M, Bender D, Thaker PH, Sood AK. Non-cancer life stressors contribute to impaired quality of life in ovarian cancer patients. Gynecologic Oncology. 2013;131:667–673. doi: 10.1016/j.ygyno.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux V, Kendler KS. Deconstructing major depression: A validation study of the DSM–IV symptomatic criteria. Psychological Medicine. 2010;40:1679–1690. doi: 10.1017/S0033291709992157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Maes M. A review on the acute phase response in major depression. Reviews in the Neurosciences. 1993;4:407– 416. doi: 10.1515/REVNEURO.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: A review and hypothesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-M. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Advances in Experimental Medicine and Biology. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, Raus J. Evidence for a systemic immune activation during depression: Results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychological Medicine. 1992;22:45–53. doi: 10.1017/S0033291700032712. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new “5-HT” hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpé S, Meltzer HY, Okayli G, Bosmans E, D’Hondt P, Cosyns P. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: Further evidence for an immune response. Psychiatry Research. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-J. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Ranjan R, Bosmans E, Van Gastel A, Bergmans R, Desnyder R. Increased serum soluble CD8 or suppressor/cytotoxic antigen concentrations in depression: Suppressive effects of glucocorticoids. Biological Psychiatry. 1996;40:1273–1281. doi: 10.1016/0006-3223(95)00627-3. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295X.105.1.83. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008 Jul 24;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature Reviews Neuroscience. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosomatic Medicine. 2009;71:378–384. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, Silverman MN, Sternberg EM. Glucocorticoid dysregulations and their clinical correlates: From receptors to therapeutics. Annals of the New York Academy of Sciences. 2009;1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Deak AH, Neto FL, Dominguez WV, Solis AC, Kurcgant D, Sato F, Prado EB. Cytokine profiles in women with different subtypes of major depressive disorder. Journal of Psychiatric Research. 2007;41:152–159. doi: 10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Marsland AL. Adversity and inflammation among adolescents: A possible pathway to long-term health risk. Psychosomatic Medicine. 2013;75:438–441. doi: 10.1097/PSY.0b013e3182983ea6. [DOI] [PubMed] [Google Scholar]
- Marx J. Inflammation and cancer: The link grows stronger. Science. 2004 Nov 5;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain, Behavior, and Immunity. 2010;24:96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in Neurosciences. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. Journal of Immunology. 2010;184:2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clinical Psychology: Science and Practice. 1998;5:291–313. doi: 10.1111/j.1468-2850.1998.tb00151.x. [DOI] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204:38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nature Reviews Immunology. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007 Oct 18;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008 Jul 24;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: A pilot open-label study. International Clinical Psychopharmacology. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biology of Mood & Anxiety Disorders. 2012;2:Article 4. doi: 10.1186/PREACCEPT-1461493759628561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Elucidating the consequences of chronic stress on immune regulation and behavior in rheumatoid arthritis. Brain, Behavior, and Immunity. 2008;22:22–23. doi: 10.1016/j.bbi.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69:402– 409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and β2-adrenergic receptor in children with asthma. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker HA, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving towards a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Cole SW. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biological Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of proinflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Miller NE. Some psychophysiological studies of the motivation and of the behavioral effects of illness. Bulletin of the British Psychological Society. 1964;17:1–20. [Google Scholar]
- Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose intensive chemotherapy in cancer patients. Journal of Clinical Oncology. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Recurrence in major depression: A conceptual analysis. Psychological Review. 2011;118:655– 674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. Journal of Abnormal Psychology. 1999;108:606–614. doi: 10.1037/0021-843X.108.4.606. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM. Psychological stressors, overview. In: Fink G, editor. Encyclopedia of stress. 2. Vol. 3. Oxford, England: Academic Press; 2007. pp. 278–284. [DOI] [Google Scholar]
- Monroe SM, Slavich GM, Georgiades K. The social environment and life stress in depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York, NY: Guilford Press; 2009. pp. 340–360. [Google Scholar]
- Monroe SM, Slavich GM, Gotlib IH. Life stress and family history for depression: The moderating role of past depressive episodes. Journal of Psychiatric Research. 2013 doi: 10.1016/j.jpsychires.2013.11.005. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, Gotlib IH. Major life events and major chronic difficulties are differentially associated with history of major depressive episodes. Journal of Abnormal Psychology. 2007a;116:116–124. doi: 10.1037/0021-843X.116.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, Gotlib IH. Severe life events predict specific patterns of change in cognitive biases in major depression. Psychological Medicine. 2007b;37:863– 871. doi: 10.1017/S0033291707000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai chi chih acutely decreases sympathetic nervous system activity in older adults. Journals of Gerontology: Series A Biological Sciences and Medical Sciences. 2006;61:1177–1180. doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway’s immunobiology. New York, NY: Garland Science; 2011. [Google Scholar]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clinical Psychological Science. 2013;1:30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Social and Personality Psychology Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. Journal of Nervous and Mental Disease. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. New England Journal of Medicine. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeljkovic M, Ausfeld-Hafter B, Streitberger K, Seiler R, Wirtz PH. Taiji practice attenuates psychobiological stress reactivity: A randomized controlled trial in healthy subjects. Psychoneuroendocrinology. 2012;37:1171–1180. doi: 10.1016/j.psyneuen.2011.12.007. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Olmstead R, Irwin MR. Diurnal cortisol in complicated and non-complicated grief: Slope differences across the day. Psychoneuroendocrinology. 2012;37:725–728. doi: 10.1016/j.psyneuen.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM. Clinical anxiety, cortisol, and interleukin-6: Evidence for specificity in emotion–biology relationships. Brain, Behavior, and Immunity. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depression and Anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neuroscience and Biobehavioral Reviews. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Disease Markers. 2011;30:123–132. doi: 10.1155/2011/560572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Archives of General Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Lewinsohn PM, Seeley JR. Continuity of psychopathology in a community sample of adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:1525–1535. doi: 10.1097/00004583-199511000-00020. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain, Behavior, and Immunity. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Hu F, Miller AH. Activation of cAMP–protein kinase A abrogates STAT5-mediated inhibition of glucocorticoid receptor signaling by interferon-alpha. Brain, Behavior, and Immunity. 2011;25:1716–1724. doi: 10.1016/j.bbi.2011.07.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Miller AH. Cytokines and glucocorticoid receptor signaling: Relevance to major depression. Annals of the New York Academy of Sciences. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163:1630–1633. doi: 10.1176/appi.ajp.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain, Behavior, and Immunity. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cellular and Molecular Life Sciences. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Molecular Medicine. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- Paykel ES. Life events and affective disorders. Acta Psychiatrica Scandinavica. 2003;108:61–66. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: Old story, new insights. Physiology & Behavior. 2009;97:279–292. doi: 10.1016/j.physbeh.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: A meta-analysis. Brain, Behavior, and Immunity. 2009;23:427–433. doi: 10.1016/j.bbi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Persoons P, Vermeire S, Demyttenaere K, Fischler B, Vandenberghe J, Van Oudenhove L, Rutgeerts P. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Alimentary Pharmacology, and Therapeutics. 2005;22:101–110. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosomatic Medicine. 2008;70:646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Carroll D, Burns VE, Drayson M. Cardiovascular activity and the antibody response to vaccination. Journal of Psychosomatic Research. 2009;67:37–43. doi: 10.1016/j.jpsychores.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain, Behavior, and Immunity. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Pilkington K, Kirkwood G, Rampes H, Richardson J. Yoga for depression: The research evidence. Journal of Affective Disorders. 2005;89:13–24. doi: 10.1016/j.jad.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: Prevalence and measurement. Pain Practice. 2009;9:173–180. doi: 10.1111/j.1533-2500.2009.00274.x. [DOI] [PubMed] [Google Scholar]
- Poon DC, Ho YS, Chiu K, Chang RC. Cytokines: How important are they in mediating sickness? Neuroscience and Biobehavioral Reviews. 2013;37:1–10. doi: 10.1016/j.neubiorev.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neuroscience and Biobehavioral Reviews. 2007;31:858–873. doi: 10.1016/j.neubiorev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Powell ND, Allen RG, Hufnagle AR, Sheridan JF, Bailey MT. Stressor-induced alterations of adaptive immunity to vaccination and viral pathogens. Immunology and Allergy Clinics of North America. 2011;31:69–79. doi: 10.1016/j.iac.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain, Behavior, and Immunity. 2009;23:622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain, Behavior, and Immunity. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Radtke F, Macdonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nature Reviews Immunology. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Current Opinion in Psychiatry. 2011;24:519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Molecular Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Current Psychiatry Report. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The evolutionary significance of depression in pathogen host defense (PATHOS-D) Molecular Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Boyd JH, Burke JD, Jr, Rae DS, Myers JK, Kramer M, Locke BZ. One-month prevalence of mental disorders in the United States: Based on five Epidemiologic Catchment Area sites. Archives of General Psychiatry. 1988;45:977–986. doi: 10.1001/archpsyc.1988.01800350011002. [DOI] [PubMed] [Google Scholar]
- Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncology. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nature Medicine. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: New mechanisms for old drugs. New England Journal of Medicine. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. New England Journal of Medicine. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nature Reviews Immunology. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Robaeys G, De Bie J, Wichers MC, Bruckers L, Nevens F, Michielsen P, Buntinx F. Early prediction of major depression in chronic hepatitis C patients during peg interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World Journal of Gastroenterology. 2007;13:5736–5740. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: Social life in molecular terms. Nature Reviews Genetics. 2005;6:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA. Can we vaccinate against depression? Drug Discovery Today. 2012;17:451–458. doi: 10.1016/j.drudis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Roose SP, Schatzberg AF. The efficacy of antidepressants in the treatment of late-life depression. Journal of Clinical Psychopharmacology. 2005;25:S1–S7. doi: 10.1097/01.jcp.0000162807.84570.6b. [DOI] [PubMed] [Google Scholar]
- Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Dhabhar FS. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery: A prospective study. Journal of Bone & Joint Surgery. 2009;91:2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueggeberg R, Wrosch C, Miller GE, McDade TW. Associations between health-related self-protection, diurnal cortisol, and C-reactive protein in lonely older adults. Psychosomatic Medicine. 2012;74:937–944. doi: 10.1097/PSY.0b013e3182732dc6. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Woolf CJ. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001 Mar 22;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiological Reviews. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: Genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Bartlett JA. Depression and immunity: Clinical factors and therapeutic course. Psychiatry Research. 1999;85:63–69. doi: 10.1016/S0165-1781(98)00133-4. [DOI] [PubMed] [Google Scholar]
- Schleimer RP. An overview of glucocorticoid anti-inflammatory actions. European Journal of Clinical Pharmacology. 1993;45:S3–S7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Lutgendorf SK. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain, Behavior, and Immunity. 2013;30:S126–S134. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Florey CR, Martínez-Maza O, Magpantay L, Crabb Breen E, Irwin MR, Gündel H, O’Connor MF. When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity. 2012;26:1066–1071. doi: 10.1016/j.bbi.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: When physiology meets pathology. Nature Reviews Neuroscience. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. Health impacts of yoga and pranayama: A state-of-the-art review. International Journal of Preventive Medicine. 2012;3:444–458. [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Progress in Neurobiology. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Link BG, Dohrenwend BP, Skodol AE, Stueve A, Mirotznik J. Characterizing life events as risk factors for depression: The role of fateful loss events. Journal of Abnormal Psychology. 1989;98:460–467. doi: 10.1037/0021-843X.98.4.460. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clinical Psychological Science. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Epel ES. The Stress and Adversity Inventory (STRAIN): An automated system for assessing cumulative stress exposure. Los Angeles: University of California, Los Angeles; 2010. [Google Scholar]
- Slavich GM, Monroe SM, Gotlib IH. Early parental loss and depression history: Associations with recent life stress in major depressive disorder. Journal of Psychiatric Research. 2011;45:1146–1152. doi: 10.1016/j.jpsychires.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman L, Gilbert P. Subordination and defeat: An evolutionary approach to mood disorders and their therapy. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and Whites in the Midlife in the United States survey. Psychosomatic Medicine. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Służewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Annals of the New York Academy of Sciences. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Smith AK, Simon JS, Gustafson EL, Noviello S, Cubells JF, Epstein MP, Miller AH. Association of a polymorphism in the indoleamine-2,3-dioxygenase gene and interferon-α-induced depression in patients with chronic hepatitis C. Molecular Psychiatry. 2012;17:781–789. doi: 10.1038/mp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. Journal of Clinical Investigation. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Medical Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-Z. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, Endicott J. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience Letters. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Starr LR, Davila J. Responding to anxiety with rumination and hopelessness: Mechanism of anxiety-depression symptom co-occurrence? Cognitive Therapy and Research. 2012a;36:321–337. doi: 10.1007/s10608-011-9363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr LR, Davila J. Temporal patterns of anxious and depressed mood in generalized anxiety disorder: A daily diary study. Behaviour Research and Therapy. 2012b;50:131–141. doi: 10.1016/j.brat.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nature Reviews Immunology. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression–inflammation relationship. Brain, Behavior, and Immunity. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. Journal of Psychosomatic Research. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8507– 8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98:47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Annual Review of Clinical Psychology. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Development and Psychopathology. 2011;23:939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised Phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. British Journal of Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frölich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Experimental Gerontology. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, Blokland A, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J. Mechanism of acute tryptophan depletion: Is it only serotonin? Molecular Psychiatry. 2011;16:695–713. doi: 10.1038/mp.2011.9. [DOI] [PubMed] [Google Scholar]
- Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annual Review of Pharmacology and Toxicology. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Haby MM, Barendregt JJ, Kruijshaar M, Corry J, Andrews G. The burden of major depression avoidable by longer-term treatment strategies. Archives of General Psychiatry. 2004;61:1097–1103. doi: 10.1001/archpsyc.61.11.1097. [DOI] [PubMed] [Google Scholar]
- Waldburger JM, Firestein GS. Regulation of peripheral inflammation by the central nervous system. Current Rheumatology Reports. 2010;12:370–378. doi: 10.1007/s11926-010-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JG, Littlejohn GO, McMurray NE, Cutolo M. Stress system response and rheumatoid arthritis: A multilevel approach. Rheumatology. 1999;38:1050–1057. doi: 10.1093/rheumatology/38.11.1050. [DOI] [PubMed] [Google Scholar]
- Warner V, Weissman MM, Mufson L, Wickramaratne PJ. Grandparents, parents, and grandchildren at high risk for depression: A three-generation study. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:289–296. doi: 10.1097/00004583-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin re-uptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. The pain of being sick: Implications of immune-to-brain communication for understanding pain. Annual Review of Psychology. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sciences. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-M. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM–V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. Social influences on health: Is serotonin a critical mediator? Psychosomatic Medicine. 2010;72:107–112. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health and Quality of Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biological Psychology. 2010;84:228–234. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Olfson M. Depression in women: Implications for health care research. Science. 1995 Aug 11;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- Whooley MA. Depression and cardiovascular disease: Healing the broken-hearted. JAMA. 2006;295:2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmunopathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Beesdo K, Bittner A, Goodwin RD. Depressive episodes: Evidence for a causal role of primary anxiety disorders? European Psychiatry. 2003;18:384–393. doi: 10.1016/j.eurpsy.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Kessler RC, Pfister H, Lieb M. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatrica Scandinavica. 2000;102(Suppl s406):14–23. doi: 10.1111/j.0065-1591.2000.acp29-03.x. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry. 2013;70:176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: Do stress and depression accelerate cell aging? Depression and Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain, Behavior, and Immunity. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Epidemiology of stress and asthma: From constricting communities and fragile families to epigenetics. Immunology and Allergy Clinics of North America. 2011;31:19–39. doi: 10.1016/j.iac.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine. 2003;65:201–210. doi: 10.1097/01.PSY.0000058371.50240.E3. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M, Ohira H. Transient responses of inflammatory cytokines in acute stress. Biological Psychology. 2009;82:25–32. doi: 10.1016/j.biopsycho.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Yiend J, Paykel E, Merritt R, Lester K, Doll H, Burns T. Long term outcome of primary care depression. Journal of Affective Disorders. 2009;118:79–86. doi: 10.1016/j.jad.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Research. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Pollmächer T. Illness, cytokines, and depression. Annals of the New York Academy of Sciences. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Advances in Experimental Medicine and Biology. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, Schmidt K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain, Behavior, and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]