Abstract
Background
Using allograft is an attractive alternative for flexor tendon reconstruction because of the lack of donor morbidity, and better matching to the intrasynovial environment. The purpose of this study was to use biolubricant molecules to modify the graft surface to decrease adhesions and improve digit function.
Methods
28 flexor digitorum profundus (FDP) tendons from the 2nd and 5th digits of 14 dogs were first lacerated and repaired to create a model with repair failure and scar digit for tendon reconstruction. Six weeks after the initial surgery, the tendons were reconstructed with FDP allograft tendons obtained from canine cadavers. One graft tendon in each dog was treated with saline as a control and the other was treated with gelatin, carbodiimide derivatized, hyaluronic acid and lubricin (cd-HA-Lubricin). Six weeks postoperatively, digit function, graft mechanics, and biology were analyzed.
Results
Allograft tendons treated with cd-HA-Lubricin had decreased adhesions at the proximal tendon/graft repair and within flexor sheath, improved digit function, and increased graft gliding ability. The treatment also reduced the strength at the distal tendon to bone repair, but the distal attachment rupture rate was similar for both graft types. Histology showed that viable cells migrated to the allograft, but these were limited to the tendon surface.
Conclusion
cd-HA-Lubricin treatment of tendon allograft improves digit functional outcomes after flexor tendon reconstruction. However, delayed bone-tendon healing should be a caution. Furthermore, the cell infiltration into the allograft tendons substance should be a target for future studies, to shorten the allograft self-regeneration period.
Keywords: Flexor Tendon, Allograft, Hyaluronic Acid, Lubricin
INTRODUCTION
While tendon grafts1–3 are no longer the primary treatment for flexor tendon lacerations in the fingers,4–6 they are still occasionally needed to treat complications following primary repair, including severe adhesion and rupture of the repaired tendon. Furthermore, tendon injures with large tendon defect in which direct tendon repair cannot be performed also require tendon grafting to restore hand function.7–9
The most common flexor tendon reconstruction uses autologous extrasynovial tendons, such as the palmaris longus, plantaris, or toe extensors. However, the flexor tendons in zone II are intrasynovial tendons. The surface structure of these two types of tendons is very different. Intrasynovial tendons are covered by a smooth membrane (epitenon) which contains a few layers of epitenon cells embedded in a matrix that is rich in lubricating macromolecules including hyaluronic acid, lubricin, and phospholipids. Furthermore, the lubricin on the intrasynovial tendon surface possesses a strong anti-adhesion effect, which reduces adhesion formation.10, 11 In contrast, the extrasynovial tendons are wrapped by loose connective tissues (paratenon).12 This surface structure is easily damaged with repetitive motion, as is the case when extrasynovial tendons are used to replace the finger flexor tendons.13, 14 Consequently, the use of extrasynovial tendon to reconstruct intrasynovial flexor tendons often results in poor functional outcomes in both clinical and experimental settings.15, 16 Unfortunately, the availability of autologous intrasynovial tendons is limited, providing clinicians with few options when faced with the need to reconstruct a finger flexor.
Although allograft FDP tendons are available for FDP tendon reconstruction, poor functional outcomes, possibly related to immunological reactions, have limited clinical use of this option.17–19,20 Decellularization and lyophilization can reduce immunogenicity, but these procedures also roughen the tendon surface. Thus, processed allograft intrasynovial tendons lose their superior functional properties.21 Interestingly, recent studies have shown that, in an animal model, graft surface modification with carbodiimide derivatized hyaluronic acid and gelatin (cd-HA) improve tendon surface gliding ability and durability in vitro and decrease adhesion in vivo.21, 22 Furthermore, in vitro experiments have revealed that further improvement of allograft gliding can been achieved by adding Lubricin to the cd-HA treatment.23 However, this chemically modified cd-HA plus lubricin (cd-HA-Lubricin) has not been tested in vivo. The purpose of the current study was to evaluate the results of allograft FDP tendon coated with cd-HA-Lubricin on digit function and adhesion formation using a clinically relevant canine in vivo model.
MATERIALS AND METHODS
Creation of Tendon Failure Model for Reconstruction
This study was approved by our Institutional Animal Care and Use Committee (IACUC). In order to mimic the clinical indication for flexor tendon reconstruction, a tendon repair failure model was created.22 In a second operation, the tendon graft was then inserted into the resulting scarred digit. Briefly, a total of 28 FDP tendons from the 2nd and 5th digits of 14 mixed-bred dogs with average weight of 20 kg were lacerated and repaired in zone II After tendon repair the dogs were allowed free cage activity with full weight bearing, resulting in rupture of all the repairs within one week.22
Allograft Preparation
28 FDP tendons were harvested from dogs that were euthanized for other IACUC approved studies. The tendons were immersed into liquid nitrogen for 1 minute and then thawed in 37°C saline for 5 minutes, and this procedure was repeat five times to create tenocyte necrosis. Previous studies have demonstrated that no immune responses from the host were observed with this procedure in vivo.22 Following tendon decellularization, the tendons were lyophilized (Millrock Technology Inc. Kingston NY). Then, each allograft was stored in a sealed a special plastic bag for gas sterilization. One day before reconstruction, the graft tendon was immersed in a 0.9% NaCl bath for rehydration in an incubator at 37°C.21 During flexor reconstruction surgery, two allograft FDP tendons were randomly assigned for either saline treatment as the control group or cd-HA-Lubricin surface coating with the following formula: 1% Sodium hyaluronate (Acros, 95%), 10% gelatin (from porcine skin, Sigma Chemical Co., St. Louis, MO, USA), 1% 1-ethy1-3 [(3-dimethylaminopropyl) carbodiimide hydrochloride] (EDC) (Sigma), and 1% N-hydroxysuccinimide (NHS) (Sigma) in 0.1 M NaCl pH 6.0 and 0.9% phosphate buffered saline (PBS). The tendons were immersed in this solution for 5 minutes, and then 260 μg/ml lubricin was added to the tendon surface.24
Flexor Tendon Reconstruction with Allograft
Six weeks after primary repair, the previously operated forelimb was prepared for the second surgical reconstruction. The distal attachment of the FDP tendon was approached through a lateral incision at the distal interphalangeal (DIP) joint to expose the normal FDP tendon/bone attachment and the distal end of the ruptured FDP tendon repair. The distal stump of the FDP host tendon was mobilized from adhesions and scar, and cut 5 mm from its insertion site. A bone tunnel for graft insertion was made at the base of the distal phalanx with a 3 mm diameter drill bit. Then, an incision was made at the mid metacarpal bone level to expose the proximal portion of the previously repaired tendon. A tunnel was created between these two incisions and the graft tendon was passed through this tunnel under the flexor digitorum superficialis tendon and within proximal pulley system. The distal end of the graft was fed into the bone tunnel at the distal phalanx and repaired with a suture-button technique.25 The proximal graft was repaired to the recipient FDP tendon using a two-weave interlacing technique.22 After the tendon reconstruction, a radial neurectomy was performed at the proximal humeral level to denervate the triceps muscle and prevent elbow and wrist extension and, thus, weight bearing.26, 27 The operated forelimb was protected with a custom-made canine jacket to hold the operated paw underneath the chest. At postoperative day 5, a synergistic wrist and digit rehabilitation protocol was initiated, and performed once daily, 7 days per week, until sacrifice.27, 28 Following sacrifice, the surgical and non-surgical contralateral normal digits were harvested for evaluation in different regions, as shown in Figure 1.
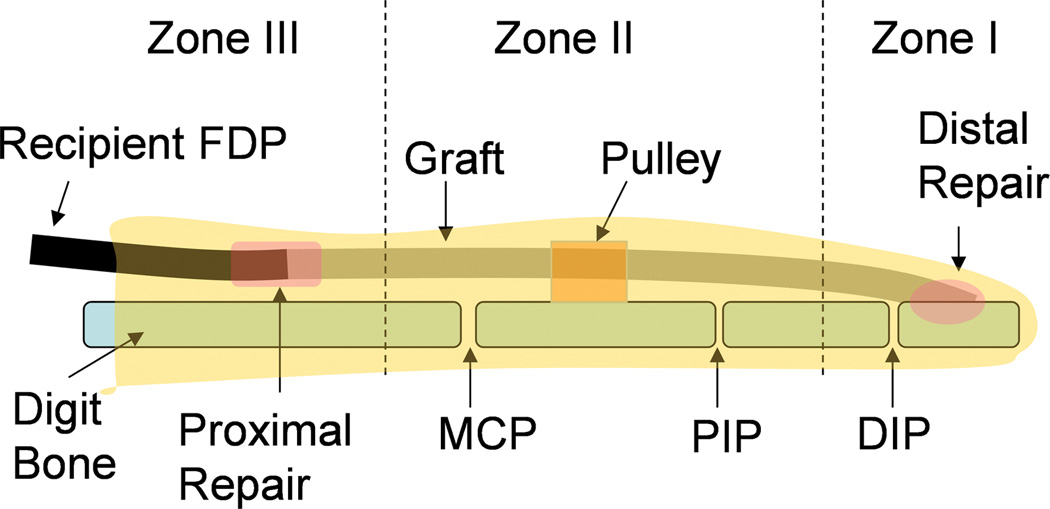
Figure 1.
Schematic diagram of the graft with digits zones I-III. The graft in zone I and zone II was assessed for work of flexion and adhesion formation. The graft in zone I was also tested for tendon to bone healing strength, and the graft in zone II was assessed for graft friction. The proximal host tendon to graft repair in zone III was used to evaluate the adhesion breaking strength. The graft segments in zone I, II, and III were also processed for histology. (DIP: distal interphalangeal joint; PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint)
Adhesion Evaluation of the Proximal Repair Site
6 weeks after reconstruction, the dogs were sacrificed and both fore paws were amputated at the wrist. The 2nd and 5th digits were dissected with zone I, II and III and the proximal and distal repair sites intact, including the entire flexor sheath. The specimen was mounted on a custom-made apparatus which included a clamp to hold the metacarpal bone and a clamp to secure the host FDP tendon. The tendon clamp was connected to a motor, actuator, and force transducer. The tendon was transected at the distal of proximal repair, and then the tendon was pulled proximally at a rate of 20 mm/minute until the proximal repair was fully pulled out. The force needed to break any adhesions between the proximal repair and the surrounding tissues (Figure 1, Zone III) was recorded at a sampling rate of 20 Hz.
Evaluation of Digit Work of Flexion and Adhesions in Zone II
The 2nd and 5th digits were further dissected and tested for digit work of flexion based on a previously published technique.25, 29 Breifly, the graft in zone II was clamped and pulled proximally by a motor connected to a force transducer, flexing the digit, including the PIP and DIP joints. The force, tendon motion, and digit joint anglar motion were recorded simultaneously using motion analysis system (Motion Analysis Corporation, Santa Rosa, CA). Work of flexion (WOF) data was calculated from the tendon displacement versus loading curve obtained during digit flexion. The WOF was then normalized by total PIP and DIP joint motion with a previously reported method, defined as normalized WOF (nWOF).25, 29
Following WOF testing, the digit was carefully exposed in the zone II area. The graft tendon within the flexor sheath and adhesions around the graft were assessed by two investigators who were blinded to treatment status (CZ and RLK), with an adhesion score ranging from 0 (no adhesions) to 8 (very severe adhesions) as described previously.30 Any disagreements in adhesion score were resolved by consensus.
Graft Friction Measurement in Zone II
The graft tendon was dissected in the zone II area with removal of any adhesions between the graft and the surrounding tissue. The frictional force was then measured between the graft and the proximal pulley during tendon gliding, using previously described methods.13
Distal Graft Tendon to Bone Healing Strength Test in Zone I
The distal phalanx with distal 15 mm graft stump was isolated for the evaluation of the distal tendon to bone repair healing. The tendon stump and distal phalanx were secured with a custom-made clamp system, which was mounted on a servohydraulic test machine (MTS, Minneapolis MN). The graft tendon was distracted at 20 mm/min starting until failure. Force and actuator displacement data were recorded at a sample rate of 50 Hz. The peak force and stiffness were determined. Stiffness was determined by calculating the slope of the linear region of the load-displacement curve.
An additional 12 FDP tendons, prepared as for the allograft procedure, were used to test the mechanical strength of the distal tendon to bone repair at time 0 in order to compare to the strength 6 weeks after reconstruction. The repair procedure in this in vitro group followed the same procedure as described above for the in vivo model.
Histology
Two graft tendons in each group were harvested immediately after sacrifice. One centimeter segment in zone II were dissected and assessed by calcein-AM (cal AM) and ethidium homodimer (EthD-1) for cell viability.31 A second 1-cm segment in zone II from the same dogs was evaluated with hematoxylin and eosin (H&E) staining of paraffin embedded sections. Two samples of the distal tendon to bone insertion were also evaluated with H&E staining following specimen decalcification.
Statistical Analysis
One-way ANOVA was used to analyze the differences on proximal repair adhesion breaking strength, adhesion score, nWOf , graft friction, and distal tendon to bone healing strength using JMP software (SAS institute Inc, Cary, NC). If a significant difference was detected by one way ANOVA, then the Tukey Studentized range (honestly significant difference, HSD) post hoc test was used to compare between groups. A p < 0.05 was considered significant.
RESULTS
All wounds healed by primary intention without severe infection after reconstruction. In five grafts in three dogs the distal tendon to bone attachment had ruptured. Three of these grafts had been treated with cd-HA-Lubricin. No ruptures were observed at the proximal tendon to tendon repair site.
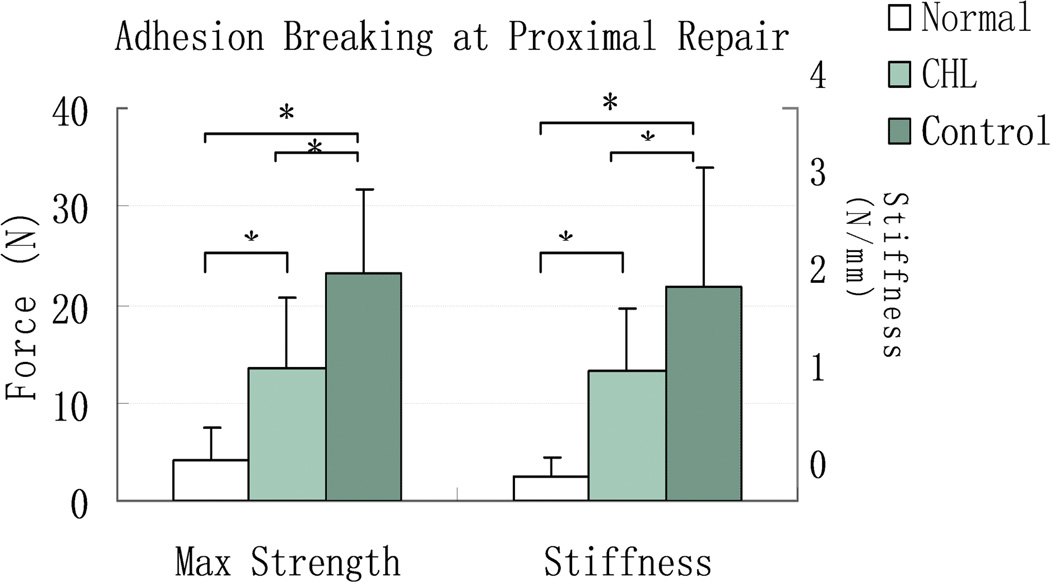
The adhesion breaking strength and stiffness of the proximal repair in both graft digits were significantly higher than in the normal contralateral FDP tendon (p < 0.05). However, the proximal repair adhesion breaking strength and stiffness in the cd-HA-Lubricin graft digits were significantly lower than in the saline treated graft digits (p < 0.05) (Figure 2).
Figure 2.
The mean and standard deviation of adhesion breaking strength and stiffness at the proximal tendon/graft repair site for normal FDP tendon (Normal), cd-HA-Lubricin (CHL) treated, and saline treated control graft tendons. Asterisk denotes a significant difference (p < 0.05).
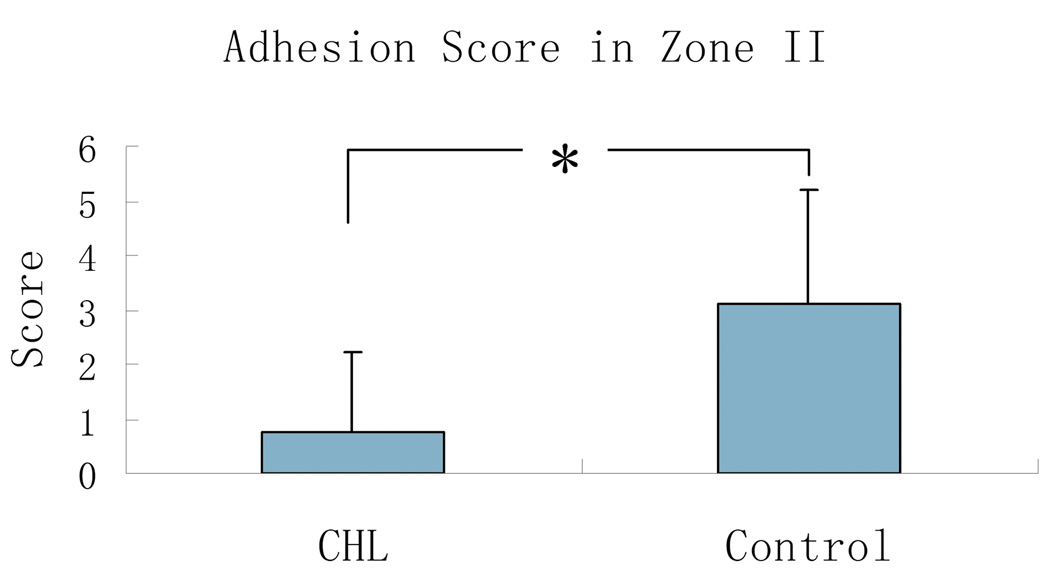
The adhesion score in zone II in grafts treated with cd-HA-Lubricin was 0.75 ± 1.5 , which was significantly lower than the grafts that were treated with saline (3.1 ± 2.1) (p < 0.05) (Figure 3).
Figure 3.
The mean and standard deviation of adhesion score between cd-HA-Lubricin (CHL) and saline control group. Asterisk denotes a significant difference (p < 0.05).
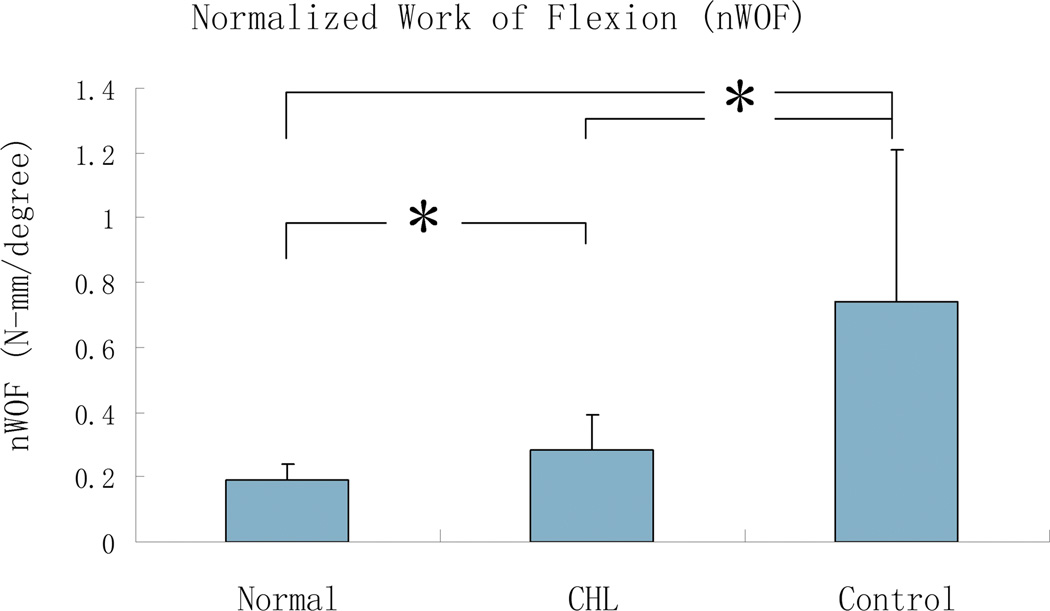
The nWOF and graft frictional force in the cd-HA-Lubricin group were all significantly lower than that in the saline group, but still higher than in the normal contralateral digit group (p < 0.05) (Figure 4).
Figure 4.
The mean and standard deviation of normalized work of flexion (nWOF) for normal digit (Normal), cd-HA-Lubricin (CHL), and saline treated control groups. Asterisk denotes a significant difference (p < 0.05).
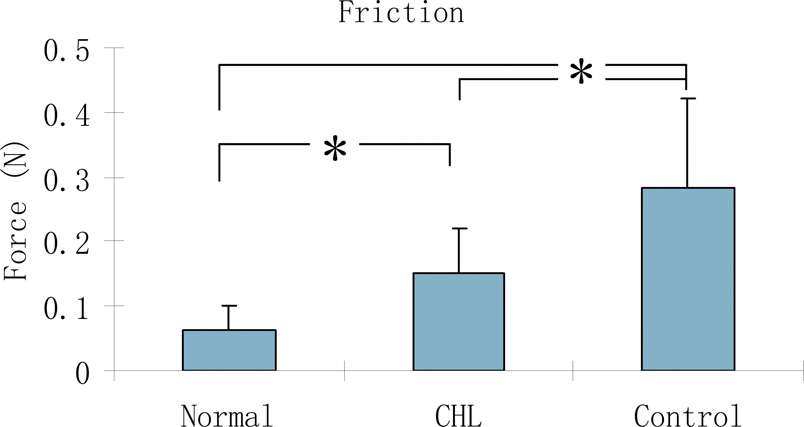
The tendon gliding resistance in zone II in the cd-HA-Lubricin graft digits was significantly lower than in the saline graft digits, although both grafts were significantly higher than the normal FDP contralateral tendons in gliding resistance (p < 0.05) (Figure 5).
Figure 5.
The mean and standard deviation of tendon gliding resistance for normal FDP tendon (Normal), cd-HA-Lubricin (CHL) treated, and control graft tendons. Asterisk denotes a significant difference (p < 0.05).
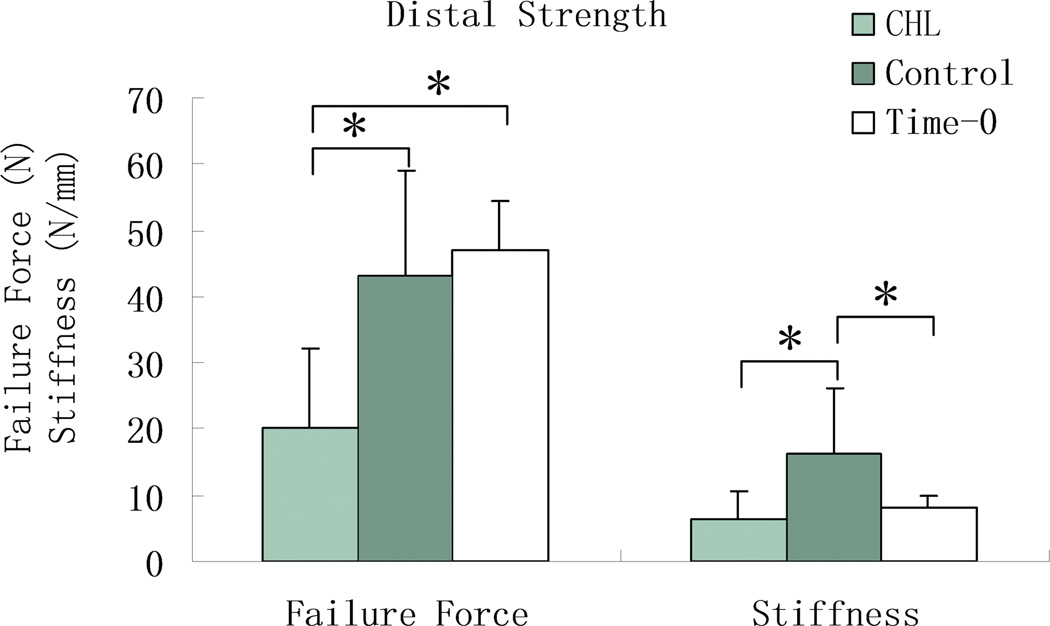
The force to failure of the distal tendon to bone attachment in the cd-HA-Lubricin group was significantly lower than that in saline control group and in the time-0 repair group (p < 0.05) (Figure 6). There was no significant difference in maximal failure force between the six week grafts treated with saline and the grafts repaired to the distal phalanx at time 0 (Figure 6). However, the stiffness of the saline grafts was significantly higher than both the grafts treated with cd-HA-Lubricin and the grafts in the time-0 group (p < 0.05).
Figure 6.
The mean and standard deviation of failure strength and stiffness at the distal graft to bone attachment for time-0, cd-HA-Lubricin (CHL) treated, and saline treated control graft tendons. Asterisk denotes a significant difference (p < 0.05).
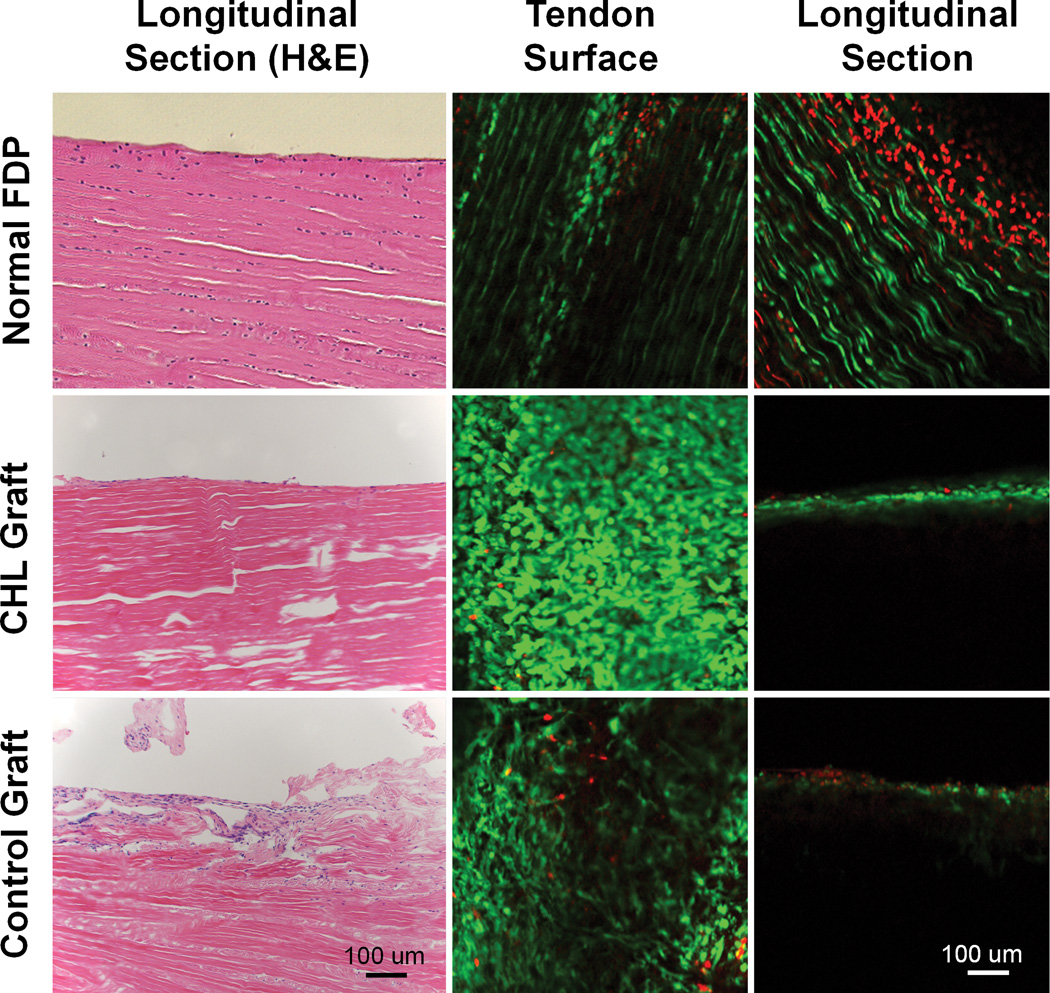
H&E staining (Figure 7 left column) showed that cells were distributed throughout the tendon section in the normal FDP tendons. In contrast, cells were present only on the graft surface in the grafts treated with either cd-HA-Lubricin or saline. Cell viability staining demonstrated a large of amount of viable cells randomly distributed on the graft surface (Figure 7 middle column), with no viable cells observed within the graft substance in longitudinal sections 6 weeks after transplantation (Figure 7 right column). The distal tendon to bone insertion in the normal FDP tendons displayed a fibrocartilage transitional zone. However, this normal tendon to bone interface was not found in the graft tendons, regardless of treatment method (Figure 8).
Figure 7.
Left column displays H&E staining of normal FDP tendon (top, left) Note that tenocytes are aligned along tendon fascicles, with a smooth surface. The graft treated with CHL also showed a smooth surface with a cell layer attached (Center, left). No cells are present within the CHL grafts. The saline treated graft show a rough surface with adhesion formation (bottom, left).
Middle column (tendon surface) and right column (tendon longitudinal section) show viable cells (green) identified by calcein-AM and ethidium homodimer probe under confocal microscopy. All three groups showed a large amount of live cells on the tendon surface. Cells are well aligned longitudinally in the normal FDP tendon (top, middle). However, the cells on both grafts are disorganized (center, middle and bottom, middle). In the tendon longitudinal sections, there are cell layers within the normal FDP tendon (top, right). Many dead cells (in red) were also observed on the normal tendon longitudinal section, which might be related to sample preparation (sectioning or manipulating) before staining that caused the cell death. No viable cells or dead cells were observed within either graft tendons (center, right and bottom, right).
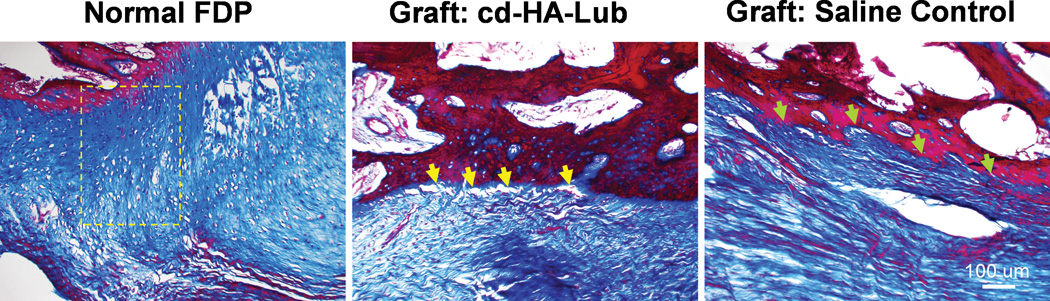
Figure 8.
Masson-Trichrome staining image of normal FDP tendon distal insertion (A) displays a gradient of chondrocytes within the transitional zone between tendon and bone (yellow frame). However, there was no such fibrocartilage transitional zone at the tendon to bone interface in either graft group. Note gaps at the tendon/bone interface in the cd-HA-lubricin treated graft (yellow arrows) compared to a solid connection at the tendon/bone interface in the saline control group (green arrows).
DISCUSSION
Allograft tendon has been considered an attractive alternative to an autograft for tendon or ligament reconstruction because of its ready availability, absence of donor site morbidity, good match of tissue type and size, and decreased operating time.32–37 In 1976, Peacock et al. reported 11 cases of composite allograft use to restore finger flexion using the entire flexor mechanism, including the FDP and FDS tendons, flexor sheath, and volar plate. Although 7 of his patients regained some active finger motion, the functional outcomes were not reported in detail.17 The long-term immune response was also not described. Also in 1976, Iselin and Peze described a chemically preserved tendon allograft using Cialit for flexor tendon reconstruction.19 They found that 40% of 30 FDP reconstruction resulted in poor function due to adhesion and rupture. In a 3-year prospective study over a decade later, Cookson BD et al. reported that Cialit preservation for allograft tissue preservation had a high risk of contamination which led to transplant infection.38 More recently, Asencio et al. reported two other cases of tendon allograft surgery, with encouraging results.39 However, the surgical procedure was very complicated with an extensive exposure that resulted in significant scarring. Thus the authors suggested this procedure should only be considered as an alternative to amputation, after failure of more conventional tendon reconstructions.39 Liu et al. used allograft tendons to treat flexor tendon injuries with a two-staged reconstruction in which the silicone rod was implanted at the first stage to create a flexor canal, and then allograft tendons were transplanted at the second stage, following removal of the silicone rod. However, the results after 2 years follow-up functional performance was reported as only 8% good, 71% fair and 21% poor.18 Thus, although the use of allograft tendons for knee anterior cruciate ligament reconstruction has yielded results comparable autografts,40, 41 the use of allografts for flexor tendon reconstruction has not achieved similar levels of success. Improvement in the results of flexor tendon allografts outcomes would therefore be clinically important, and could improve the functional recovery following complex flexor tendon injures.
In the current study, we demonstrated that surface modification with native lubricating molecules has greatly reduced adhesion formation and improved digit function in our in vivo model. Adhesions at the proximal repair site were also decreased compared to the saline control grafts. On the other hand, this surface modification also interfered with graft –host healing, as indicated by decreased repair strength and stiffness. However, the decreased strength in the cd-HA-Lub group seemed to have no effect on the graft failure rate, with two failures in the control group and three in the cd-HA-Lub treated group. All five failures were due to early weight bearing, in which the load far exceeded the repair strength.
Histology showed that the native transitional fibrocartilage zone of the tendon to bone insertion site was not reestablished in either graft group after six weeks. Since the failure strength at the distal attachment included both repair suture holding strength and healing strength, it is difficult to accurately assess the distal healing in the two treatment groups. However, the stiffness of the saline control group was significantly increased compared to the time 0 repair, which could imply that in this group at least there might be some degree of healing taking place at the tendon to bone interface.
Both allograft groups appeared to undergo a surface recellularization after six weeks in vivo. Thus, the lubricating coating did not appear to interfere with the recellularization process. However, the cells were only found on the graft surface. Due to the dense tendon extracellular matrix, cell migration into the tendon substance is a challenge. Although chemical detergents have been used to increase tendon porosity for cell penetration, it has been difficult to optimize the tendon porosity to satisfy both cell infiltration and tendon strength requirements.42, 43
There were several limitations to the study. First, we only investigated the outcomes at one time point, six weeks after allograft reconstruction. This period of time is relatively short for allograft regeneration. However, a six-week follow-up was appropriate to study the adhesion status. Second, although the cell viability was observed under confocal microscopy, a quantitative measure such cell counting was not employed. Finally, we focused on biomechanical and functional evaluation, and graft biochemistry assessments, such as extracellular matrix synthesis, were not performed.
In summary, the current study presented a novel surface coating technique to improve the functional outcomes after flexor tendon reconstruction using a decellularized native flexor tendon as a donor allograft in canine in vivo model. The decellularized allograft and the chemical modification did not induce any obvious immunogenetic response. The results, as expected, showed decreased adhesion formation and improved digit function for the allografts treated with cd-HA-Lubricin. However, some side effects were also noted. The distal tendon to bone interface was weakened by this surface treatment. And, while a large amount of viable cells was observed on the allografts the cells were limited to the tendon surface. Future studies should assess acceleration of graft regeneration and incorporation by increasing cell penetration.
ACKNOWLEDGMENT
This study was funded by a grant from NIH/NIAMS (AR 57745).
Footnotes
Financial Disclosures: None
AUTHOR'S ROLE/PARTICIPATION
| Chunfeng Zhao: | Contribute to the study conception and design, experiments, data analysis and interpretation, and manuscript drafting, editing and finalization. |
| Zhuang Wei: | Contribute to study design, experiment, data collection and analysis, and manuscript revision. |
| Ramona L. Kirk: | Contribute to experiment, data collection and analysis, manuscript revision. |
| Andrew R. Thoreson: | Contribute to experiment, data collection and analysis, manuscript editing and revision. |
| Gregory D. Jay: | Contribute to study design and conception, data interpretation, and manuscript editing and revision. |
| Steven L. Moran: | Contribute to study design and conception, data analysis and interpretation, and manuscript editing and revision. |
| Kai-Nan An: | Contribute to study design and conception, data analysis and interpretation, and manuscript editing and revision. |
| Peter C. Amadio: | Contribute to study design and conception, data analysis and interpretation, and manuscript editing and revision. |
REFERENCES
- 1.Pulvertaft RG. Tendon grafts for flexor tendon injuries in the fingers and thumb; a study of technique and results. J Bone Joint Surg Br. 1956;38-B:175–194. doi: 10.1302/0301-620X.38B1.175. [DOI] [PubMed] [Google Scholar]
- 2.White WL. Tendon grafts: A conssideration of their source, procurement and suitability. Surg Clin North Am. 1960;40:403–413. doi: 10.1016/s0039-6109(16)36047-9. [DOI] [PubMed] [Google Scholar]
- 3.Williams SB. New dynamic concepts in the grafting of flexor tendons. Plast Reconstr Surg. 1965;36:377–419. doi: 10.1097/00006534-196510000-00001. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie AR. An experimental multiple barbed suture for the long flexor tendons of the palm and fingers. Preliminary report. J Bone Joint Surg Br. 1967;49:440–447. [PubMed] [Google Scholar]
- 5.Green WL, Niebauer JJ. Results of primary and secondary flexor-tendon repairs in no man's land. J Bone Joint Surg Am. 1974;56:1216–1222. [PubMed] [Google Scholar]
- 6.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–876. [PubMed] [Google Scholar]
- 7.Soucacos PN, Beris AE, Malizos KN, Xenakis T, Touliatos A, Soucacos PK. Two-stage treatment of flexor tendon ruptures. Silicon rod complications analyzed in 109 digits. Acta Orthop Scand Suppl. 1997;275:48–51. [PubMed] [Google Scholar]
- 8.Bertelli JA, Santos MA, Kechele PR, Rost JR, Tacca CP. Flexor tendon grafting using a plantaris tendon with a fragment of attached bone for fixation to the distal phalanx: A preliminary cohort study. J Hand Surg Am. 2007;32:1543–1548. doi: 10.1016/j.jhsa.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Tang L, Chen H, Cui T, Cheng G, Fang G, Ding X. [long-term effectiveness of tendon allograft for repairing tendon defect] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:341–343. [PubMed] [Google Scholar]
- 10.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funakoshi T, Martin SD, Schmid TM, Spector M. Distribution of lubricin in the ruptured human rotator cuff and biceps tendon: A pilot study. Clin Orthop Relat Res. 2010;468:1588–1599. doi: 10.1007/s11999-009-1108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momose T, Amadio PC, Zobitz ME, Zhao C, An K-N. Effect of paratenon and repetitive motion on the gliding resistance of tendon of extrasynovial origin. Clin Anat. 2002;15:199–205. doi: 10.1002/ca.10009. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the a2 pulley. J Bone Joint Surg Am. 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. The marshall r. Urist young investigator award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997:239–247. [PubMed] [Google Scholar]
- 16.Gelberman RH, Seiler JG, 3rd, Rosenberg AE, Heyman P, Amiel D. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg. 1992;26:257–264. doi: 10.3109/02844319209015268. [DOI] [PubMed] [Google Scholar]
- 17.Peacock EE, Jr, Madden JW. Human composite flexor tendon allografts. Ann Surg. 1967;166:624–629. doi: 10.1097/00000658-196710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu TK. Clinical use of refrigerated flexor tendon allografts to replace a silicone rubber rod. J Hand Surg Am. 1983;8:881–887. doi: 10.1016/s0363-5023(83)80087-2. [DOI] [PubMed] [Google Scholar]
- 19.Iselin F, Peze W. Use of chemically preserved tendon allografts in hand surgery. Hand. 1976;8:167–172. doi: 10.1016/0072-968x(76)90042-5. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Zhang Y, Li X, Deng J. analysis of reasons of tendon adhesion post tendon allograft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:346–348. [PubMed] [Google Scholar]
- 21.Ikeda J, Zhao C, Sun Y-L, An K-N, Amadio PC. Carbodiimide-derivatized hyaluronic acid surface modification of lyophilized flexor tendon: A biomechanical study in a canine in vitro model. J Bone Joint Surg Am. 2010;92:388–395. doi: 10.2106/JBJS.H.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Sun Y-L, Ikeda J, Kirk RL, Thoreson AR, Moran SL, et al. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: Study of a primary repair failure model. J Bone Joint Surg Am. 2010;92:2817–2828. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taguchi M, Zhao C, Sun YL, Jay GD, An KN, Amadio PC. The effect of surface treatment using hyaluronic acid and lubricin on the gliding resistance of human extrasynovial tendons in vitro. J Hand Surg Am. 2009;34:1276–1281. doi: 10.1016/j.jhsa.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Sun Y-L, Amadio PC, Tanaka T, Ettema AM, An K-N. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg. Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop AT, Cooney WP, 3rd, Wood MB. Treatment of partial flexor tendon lacerations: The effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301–312. doi: 10.1097/00005373-198604000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84-A:78–84. [PubMed] [Google Scholar]
- 28.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Sun Y-L, Kirk RL, Thoreson AR, Jay GD, Moran SL, et al. Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2010;92:1453–1461. doi: 10.2106/JBJS.I.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karabekmez FE, Zhao C. Surface treatment of flexor tendon autograft and allograft decreases adhesion without an effect of graft cellularity: A pilot study. Clin Orthop Relat Res. 2012;470:2522–2527. doi: 10.1007/s11999-012-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto T, Sun Y, An K, Amadio P, Zhao C. The effect of tendon surface treatment on cell attachment for potential enhancement of tendon graft healing: An ex vivo model. Med Eng Phys. 2012 doi: 10.1016/j.medengphy.2012.01.001. Published online Feb. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole DW, Ginn TA, Chen GJ, Smith BP, Curl WW, Martin DF, et al. Cost comparison of anterior cruciate ligament reconstruction: Autograft versus allograft. Arthroscopy. 2005;21:786–790. doi: 10.1016/j.arthro.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 33.Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for acl reconstruction. Clin Orthop Relat Res. 2008;466:2238–2246. doi: 10.1007/s11999-008-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kokkalis ZT, Zanaros G, Sotereanos DG. Ligament reconstruction with tendon interposition using an acellular dermal allograft for thumb carpometacarpal arthritis. Tech Hand Up Extrem Surg. 2009;13:41–46. doi: 10.1097/BTH.0b013e31818be857. [DOI] [PubMed] [Google Scholar]
- 35.Furlow LTJ. Homologous flexor mechanism replacement in four fingers of one hand. Case report. Plast Reconstr Surg. 1969 May;:531–535. doi: 10.1097/00006534-196905000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Peacock EE., Jr A study of the circulation in normal tendons and healing grafts. Ann Surg. 1959;149:415–428. doi: 10.1097/00000658-195903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peacock EE., Jr Homologous composite tissue grafts of the digital flexor mechanism in human beings. Transplant Bull. 1960;7:418–421. doi: 10.1097/00006534-196004000-00043. [DOI] [PubMed] [Google Scholar]
- 38.Cookson BD, Hoffman PN, Price T, Webster W, Fenton O. 'Cialit' as a tissue preservative: A microbiological assessment. J Hosp Infact. 1988;11:263–270. doi: 10.1016/0195-6701(88)90104-1. [DOI] [PubMed] [Google Scholar]
- 39.Asencio G, Abihaidar G, Leonardi C. Human composite flexor tendon allografts. A report of two cases. J Hand Surg Br. 1996;21:84–88. doi: 10.1016/s0266-7681(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 40.Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:661–681. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:292–298. doi: 10.1016/j.arthro.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Tischer T, Aryee S, Wexel G, Steinhauser E, Adamczyk C, Eichhorn S, et al. Tissue engineering of the anterior cruciate ligament-sodium dodecyl sulfate-acellularized and revitalized tendons are inferior to native tendons. Tissue Eng Part A. 2010;16:1031–1040. doi: 10.1089/ten.TEA.2009.0043. [DOI] [PubMed] [Google Scholar]
- 43.Vavken P, Joshi S, Murray MM. Triton-x is most effective among three decellularization agents for acl tissue engineering. J Orthop Res. 2009;27:1612–1618. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]