Abstract
Background
Cardiac dysfunction in failing hearts of human patients and animal models is associated with both microtubule densification and T-tubule remodeling. Our objective was to investigate whether microtubule densification contributes to T-tubule remodeling and excitation-contraction coupling dysfunction in heart disease.
Methods and Results
In a mouse model of pressure overload-induced cardiomyopathy by transaortic banding (TAB), colchicine, a microtubule depolymerizer, significantly ameliorated T-tubule remodeling and cardiac dysfunction. In cultured cardiomyocytes, microtubule depolymerization with nocodazole or colchicine profoundly attenuated T-tubule impairment, whereas microtubule polymerization/stabilization with taxol accelerated T-tubule remodeling. In situ immunofluorescence of heart tissue sections demonstrated significant disorganization of JP2, a protein that bridges the T-tubule and sarcoplasmic reticulum membranes, in TAB hearts as well as in human failing hearts, while colchicine injection significantly preserved the distribution of JP2 in TAB hearts. In isolated mouse cardiomyocytes, prolonged culture or treatment with taxol resulted in pronounced redistribution of JP2 from T-tubules to the peripheral plasma membrane, without changing total JP2 expression. Nocodazole treatment antagonized JP2 redistribution. Moreover, overexpression of a dominant-negative mutant of Kinesin 1, a microtubule motor protein responsible for anterograde trafficking of proteins, protected against JP2 redistribution and T-tubule remodeling in culture. Finally, nocodazole treatment improved Ca2+ handling in cultured myocytes by increasing the amplitude of Ca2+ transients and reducing the frequency of Ca2+ sparks.
Conclusions
Our data identify a mechanistic link between microtubule densification and T-tubule remodeling and reveal microtubule-mediated JP2 redistribution as a novel mechanism for T-tubule disruption, loss of E-C coupling, and heart failure.
Keywords: Cardiomyocytes, microtubules, T-tubules, Junctophilin-2, excitation-contraction coupling
INTRODUCTION
Microtubules, which are ubiquitous cytoskeletal fibers formed by polymerization of α- and β-tubulin dimers, regulate a wide range of cellular processes, including maintenance of cell shape, mitosis, and intracellular protein transport. In human hypertrophied and failing myocardium, tubulin expression levels were increased.1, 2 In patients and animal models with symptomatic aortic stenosis, an increase in microtubule density, namely, microtubule densification, was found to be inversely related with left ventricular (LV) fractional shortening.3, 4 Epidemiological studies demonstrate that the microtubule stabilizing agent taxol (also paclitaxel), an important antineoplastic chemotherapy agent, is associated with cardiotoxicity including myocardial infarction, ventricular tachycardia and congestive heart failure.5–7 Conversely, colchicine, a microtubule depolymerizing agent used to treat gout, decreases the incidence of myocardial infarction.8 Microtubule densification is a common observation in multiple animal models of cardiac disease.4, 9, 10 Studies performed in canine, feline, guinea pig, rat, and murine models consistently demonstrated that microtubule depolymerization attenuates cardiac dysfunction.11–15 For example, in a canine model of pressure overload-induced LV hypertrophy, treatment with colchicine normalizes both in vivo and in vitro cardiac contractile function.11 Thus, multiple lines of evidence suggest a clear role for microtubule destabilization in preservation of cardiac function, yet the mechanism by which microtubule densification results in loss of cardiac function remains incompletely understood.16, 17
Defective excitation-contraction (E-C) coupling is a hallmark of heart failure.18–20 Emerging evidence demonstrates that disruption of the transverse tubule (T-tubule) network is mechanistically involved in the development and progression of heart failure.21, 22 T-tubules are highly organized invaginations of surface membrane that form tightly physical couplings with the terminal cisternae of the sarcoplasmic reticulum (SR), termed cardiac dyads.23 Precise communication between the voltage-gated L-type Ca2+ channels (LTCCs) located mainly on the T-tubule membrane and Ca2+ release ryanodine receptor channels (RyRs) on the SR is essential for rapid electrical excitation, initiation and synchronous triggering of SR Ca2+ release, and therefore coordinated myocyte contraction.24, 25 Junctophilin-2(JP2), a member of the junctophilin family, spans the T-tubule and SR membranes and thereby plays an important role in the formation and maintenance of the cardiac dyad.26 Loss of JP2 expression results in T-tubule remodeling following pressure overload, as demonstrated by studies in cultured myocytes and a transgenic mouse model expressing JP2 shRNA.22, 27 Despite the substantial association in cardiac dysfunction between T-tubule remodeling and microtubule densification, it remains unclear if the latter two processes are mechanistically linked in the development of heart failure. In addition, progressive loss of T-tubule integrity is a long-standing and well-known phenotype in cultured adult cardiomyocytes.28–30 Related unresolved questions are how progressive T-tubule loss occurs in cultured myocytes and whether it shares common mechanisms with T-tubule remodeling in vivo in response to cardiac stress.
Using a transaortic banding (TAB) murine pressure overload cardiomyopathy model, we first established that treatment with the microtubule disrupting agent colchicine preserves cardiac function, and T-tubule integrity as demonstrated by in situ confocal imaging of Langendorff-perfused intact hearts. Our studies in cultured cardiomyocytes provided key mechanistic insights into how microtubules regulate T-tubule remodeling. Specifically, myocytes in culture undergo progressive loss of T-tubule organization with extended culture, accompanied by rearrangement and densification of microtubules. Microtubule polymerization/stabilization with taxol accelerates this T-tubule alteration, whereas microtubule depolymerization is protective. Genetic silencing of JP2 using an inducible cardiac-specific JP2 shRNA abrogates the protective effect of microtubule disruption. Moreover, pressure overload or microtubule stabilization induces marked redistribution of JP2 to the cell periphery, similar to that observed in failing human hearts. Lastly, Overexpression of a dominant-negative mutant of microtubule motor protein Kinesin 1 (also known as Kif5b) protected against JP2 redistribution and T-tubule remodeling. Analysis of Ca2+ handling properties demonstrate that microtubule depolymerization rescues E-C coupling, including an increase in the amplitude of Ca2+ transients and a reduction of Ca2+ sparks. These data collectively indicate that microtubule densification contributes to T-tubule remodeling in heart failure by altering JP2 distribution within the membrane system.
METHODS
Human Heart Samples
Left ventricular samples from patients with ischemic or dilated cardiomyopathies were obtained from explanted hearts at the University of Iowa Heart Failure Transplant Program. Non-failing donor hearts without evidence of overt cardiac dysfunction were obtained through organ donor networks/organ procurement agencies. For immunostaining experiments, a total of 10 left ventricular samples were studied, including 3 rejected healthy donor hearts, 7 end-stage heart failure patients with either ischemic heart disease (4) or with dilated cardiomyopathy (3). All human heart tissue samples were obtained under organ research donation protocols approved by the Institutional Review Boards at University of Iowa and Mayo Clinic.
Animal studies and experimental methods
Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Iowa. All mice used in the study were in C57BL6 background. All experiments were performed in male mice 9 to 11 weeks of age. Numbers of mice and myocytes for each experimental group are provided in the figures or figure legends.
See the online-only Data Supplement for detailed experimental methods.
Statistics
Data are expressed as mean ± SE. One-way ANOVA with Bonferroni post-hoc test was applied to multiple group comparisons of in vivo animal experiments. Bonferroni procedure after a global test based on a linear mixed-effects model was performed for multiple group comparisons of in vitro cardiomyocyte experiments. A compound symmetry correlation structure was assumed for linear mixed-effects model tests. Student’s t-test was used for two group comparison. Statistical analyses were carried out using SPSS V.15.0 (SPSS). Values of p < 0.05 were considered statistically significant.
RESULTS
Microtubule depolymerization attenuates in vivo T-tubule remodeling following pressure overload-induced hypertrophy
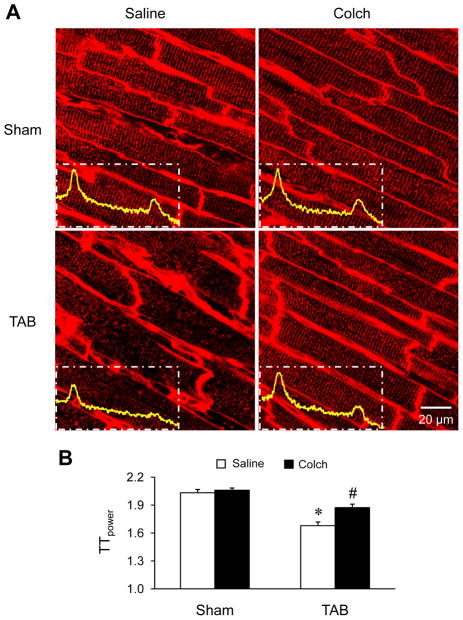
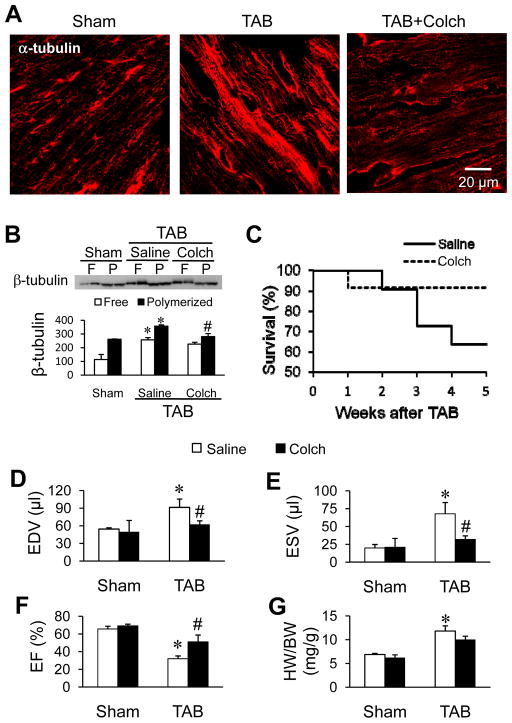
Several studies have demonstrated that microtubule stabilization and accumulation is associated with the loss of cardiac function following cardiac stress, yet the mechanisms remain incompletely understood. We hypothesized that microtubule stabilization is involved in T-tubule remodeling following pressure overload. In vehicle-treated mice, TAB produced severe T-tubule disorganization and subcellular T-tubule loss as demonstrated by in situ confocal imaging of the left ventricle (LV) (Figure 1A). Whereas colchicine injection had no effect on T-tubule organization in the LV of sham mice, it protected T-tubule structure against pressure overload-induced remodeling (Figure 1A). To quantify the integrity of T-tubules, we conducted a power spectrum analysis of the strength of T-tubule structural integrity (TTpower). TTpower analysis demonstrated a marked decrease in the T-tubule integrity in the LV of TAB mice compared to sham mice (Figure 1B). Colchicine treatment attenuated the decrease in TTpower. Consistent with previous reports,9 microtubule density (Figure 2A) and both free and polymerized β-tubulin (Figure 2B) were increased in pressure overloaded LV as compared to the sham group. Colchicine injection markedly attenuated the pressure overload-induced microtubule densification (Figure 2A and 2B, and Supplemental Figure S1). In addition, treatment with colchicine improved survival (Figure 2C), ameliorated TAB-induced cardiac dysfunction (Figure 2D–F), and had little effect on the heart weight to body weight ratio (Figure 2G). These data suggest that microtubule stabilization is involved in T-tubule remodeling and associated cardiac dysfunction induced by pressure overload.
Figure 1.
Microtubule depolymerization attenuates in vivo T-tubule remodeling in pressure overload mice. A. Representative T-tubule images stained with a lipophilic marker MM 4–64 in left ventricular (LV) from sham and TAB mice with saline or colchicine (Colch) intraperitoneal injection. Insets are power spectra of corresponding T-tubule images. B. Mean values of TTpower from different treatments. n = 5 mice per group. * p < 0.05 vs saline-treated sham group; # p < 0.05 vs saline-treated TAB group (One-way ANOVA).
Figure 2.
Depolymerization of microtubules in vivo improves survival and cardiac function following TAB. A. Representative confocal images of β-tubulin immunofluorescent staining of LV cryosections from age-matched sham and TAB mice injected with saline or colchicine (Colch). B. Representative immunoblot and summary data of free (F) and polymerized (P) β-tubulin in LV from sham and TAB mice injected with saline or colchicine (Colch). C. Kaplan-Meier survival curve for TAB mice treated with colchicine (Colch) or saline control (n = 22 and 24 mice for saline and colchicine injection, respectively). Mice died within the first two days post surgery were not included in this analysis. D–F. End diastolic volume (EDV, D), end systolic volume (ESV, E) and ejection fraction (EF, F) were assessed by echocardiography. G. HW/BW: the ratio of heart weight to body weight. n = 5 and 8 mice for sham and TAB, respectively.* p < 0.05 vs saline-treated sham group; # p < 0.05 vs saline-treated TAB group (One-way ANOVA).
Microtubule depolymerization protects T-tubule structure in cultured cardiomyocytes
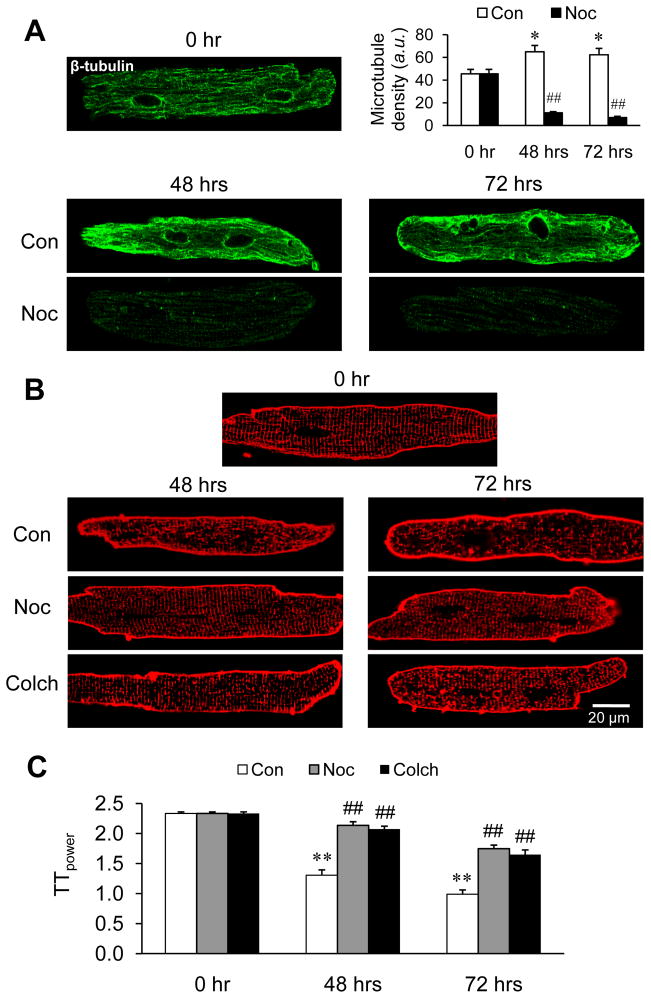
Studies were next extended to cultured cardiomyocytes to investigate the mechanisms underlying the T-tubule protection by microtubule depolymerization. We first established reorganization and assembly of the microtubule network adjacent to the sarcolemma after 48–72 hrs in culture (Figure 3A). Microtubule densification, as seen in culture for a longer time (48 hrs and 72 hrs), was abrogated by another microtubule depolymerizing agent nocodazole (Figure 3A). The increase in microtubule stability during culture was accompanied by progressive T-tubule loss and disorganization (Figure 3B, C). Similar to our in vivo data, treatment of cultured cardiomyocytes with colchicine or nocodazole pronouncedly attenuated the progressive loss of T-tubule organization (Figure 3B, C).
Figure 3.
Rearrangement and densification of microtubules is involved in T-tubule remodeling of cultured cardiomyocytes, and microtubule depolymerizing agents protect against T-tubule damage in cultured cardiomyocytes. Ventricular myocytes were isolated from adult mice and cultured for 48–72 hrs. A. Representative images and summary data of β-tubulin immunofluorescent staining in cells treated with or without 10 μM nocodazole. p<0.01 between Con and Noc groups at both 48 hrs and 72 hrs (Student’s t-test). B. Representative images of T-tubules stained with Di-8-ANNEPS in cells treated with or without 10 μM nocodazole or 1 μM colchicine. C. Average data of TTpower from different treatments. n = 25–30 cells per group. Con: DMSO control; Noc: nocodazole; Colch: cochicine.* p < 0.05; ** p < 0.01 vs 0 hr control; ## p < 0.01 vs corresponding control of drug treatment. p<0.01 among Con, Noc and Colch groups at 48 hrs and 72 hrs (by Linear mixed-effects model).
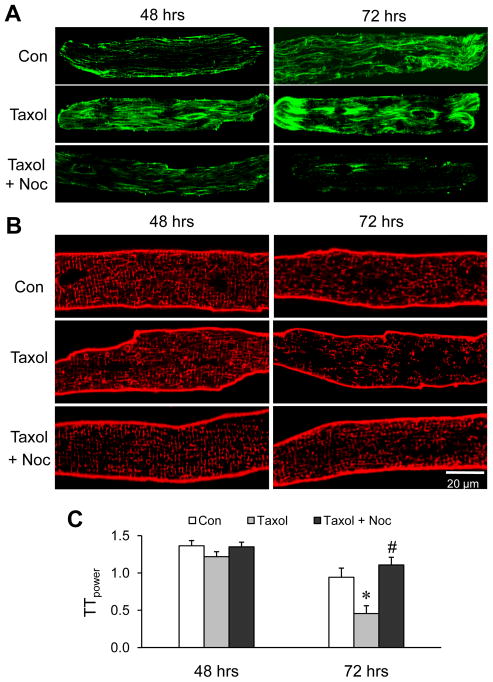
To confirm the role of microtubule stabilization in T-tubule remodeling, cardiomyocytes were treated with taxol, a microtubule polymerizing agent. Taxol treatment exaggerated the densification and aggregation of the microtubule network, especially at 72 hrs (Figure 4A). Disorganization of T-tubules was also exacerbated in cardiomyocytes treated with taxol for 72 hrs as compared to control cardiomyocytes, and this effect was antagonized by nocodazole treatment (Figure 4B, C). These data provide further evidence that microtubule stabilization is associated with loss of T-tubule integrity in vivo after TAB and in isolated, cultured myocytes.
Figure 4.
Stabilizing microtubules accelerates T-tubule damage in cultured cardiomyocytes. A, B. Representative confocal images of cultured cardiomyocytes stained with anti-β-tubulin antibody for microtubules (A) or Di-8-ANNEPS for T-tubules (B) after treatment with 20 μM taxol, or the combination of taxol and 10 μM nocodazole, C. Average data of TTpower from different treatments. Con: DMSO control; Noc: nocodazole. n = 25–30 cells per group. * p <0.05 vs corresponding control; # p < 0.05 vs Taxol treatment at 72 hrs. p<0.001 among the three groups of 72 hrs (tested by linear mixed-effects model).
JP2 silencing abolishes the T-tubule protection by nocodazole
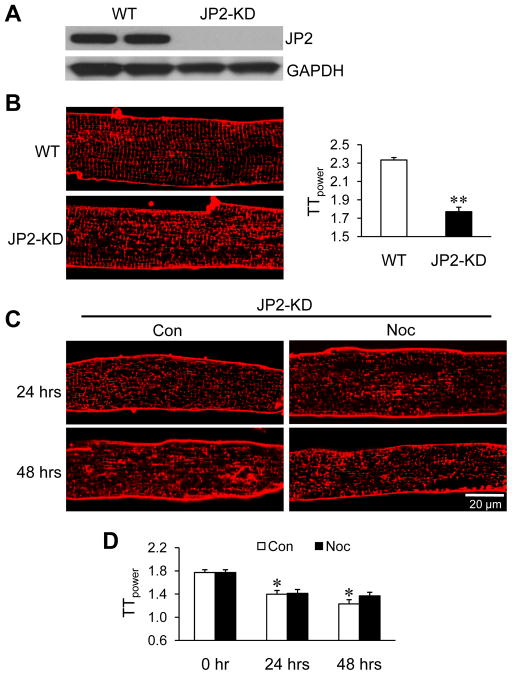
We recently reported that JP2, which tethers T-tubules to the SR and is a critical safeguard of the cardiac dyad, is required to maintain T-tubule integrity.22 To examine whether JP2 is involved in the microtubule densification-mediated T-tubule disorganization, we next performed studies in mice that express a cardiac-specific tamoxifen-inducible JP2 shRNA.27 Figure 5A demonstrates robust JP2 silencing in hearts from JP2 knockdown (JP2-KD) mice following 10-day tamoxifen injection (40 mg per kg per day; See Supplemental Figure S2 for an incomplete knockdown with lower dosage and/or shorter injection period). Cardiomyocytes freshly isolated from JP2 KD mice demonstrated significant disorganization of T-tubules (Figure 5B). In contrast to wild type hearts, treatment with nocodazole failed to protect JP2-KD myocytes from T-tubule remodeling associated with culture (Figure 5C, D). These data indicate that JP2 is required for mediating the protective effect of microtubule depolymerization on T-tubule architecture.
Figure 5.
JP2 knockdown abolishes the protective effect of microtubule depolymerization against T-tubule remodeling in culture. A. Representative immunoblots of JP2 expression in cardiomyocytes isolated from WT and JP2-KD mice, following 10-day tamoxifen injection. GAPDH: loading control. B. Representative images of T-tubules staining with Di-8-ANNEPS (left) and summarized average data of TTpower (right) in freshly isolated cardiomyocytes from WT or JP2-KD mice. n = 25–30 cells per group. ** p< 0.01 vs WT (Student’s t-test). C. Representative T-tubule images in cultured cardiomyocytes from JP2-KD mice in the absence or presence of 10 μM nocodazole during culture. D. Average data of TTpower from different treatments. Con: DMSO control; Noc: nocodazole. n= 25–30 cells per group. * p < 0.05 vs 0 hr control. No difference between Con and Noc groups of the same time point.
Microtubule stabilization induces JP2 redistribution from T-tubules to peripheral plasma membrane
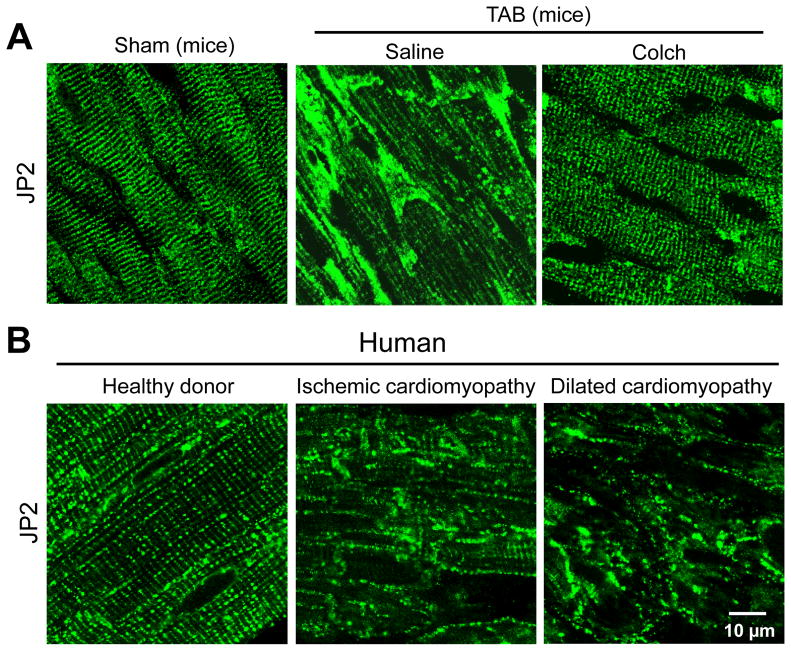
We next investigated how microtubule depolymerization affects JP2 expression and subcellular distribution. Nocodazole had no effect on JP2 mRNA or protein levels in cultured cardiomyocytes (Supplemental Figure S3A, B). Further western blotting assay with cytosolic and membrane fractions indicates that JP2 was primarily located on the membrane in cardiomyocytes. Nocodazole treatment for 48 hrs did not alter its membranous distribution (Supplemental Figure S3C). In intact hearts, in situ immunofluorescence staining showed that JP2 was organized in regular striations similar to T-tubules (Figure 6A, left). This staining pattern did not distinguish cell boundaries, which suggests that JP2 is primarily localized to the T-tubule regions in normal hearts. However, following TAB in mouse hearts, we detected a significant redistribution of JP2 protein, with punctate staining and aggregations (Figure 6A, middle). Colchicine injection pronouncedly attenuated the TAB-induced redistribution of JP2 (Figure 6A, right). More importantly, the same phenotype was observed in tissue sections of failing human hearts of both ischemic and dilated cardiomyopathies (Figure 6B). These data suggest that JP2 undergoes a profound reorganization following cardiac stress.
Figure 6.
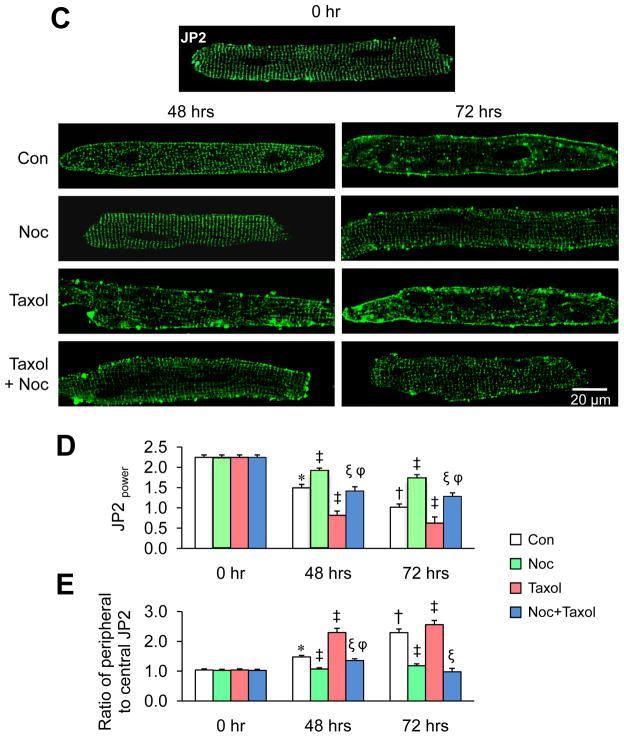
Microtubule polymerization accelerates JP2 redistribution in response to stress. A. Representative confocal images of in situ JP2 immunofluorescent staining in LV cryosections from age-matched sham and TAB mice injected with saline or colchicines (Colch). (n=3–4 hearts per group). B. Representative in situ JP2 immunofluorescence in LV cryosections from human “healthy” donor hearts (n=3), and hearts with ischemic (n=4) or dilated cardiomyopathy (n=3). 10–15 confocal images were acquired from each heart sample. C. Representative images of isolated cardiomyocytes stained with anti-JP2 antibody after treatment with 10 μM nocodazole, 20 μM taxol, or the combination of both in culture for 48 or 72 hrs. D. Average JP2power in cultured cardiomyocytes with different treatments. E. Semi-quantitative analysis of JP2 redistribution from T-tubule membrane to periphery sarcolemma. Con: DMSO control; Noc: nocodazole. n = 15–20 cells per group.* p<0.05 vs control at 0 hr; † p<0.05 vs control at 48 hrs; ‡ p<0.05 vs control at the same time point; ξ p<0.05 vs Taxol of the same time point; φ p<0.05 vs Noc at the same time point. p<0.001 among Con, Noc, Taxol, and Noc+Taxol groups at 48 hrs and 72 hrs (by Linear mixed-effects model).
To better understand the nature of JP2 disorganization following cardiac stress, we next performed studies in cultured cardiomyocytes. As compared to freshly isolated myocytes (0 hr), the organized JP2 distribution was altered after 48 hrs, with a more pronounced effect after prolonged culture for 72 hrs (Figure 6C). As a quantitative index of the regularity of JP2 staining, we calculated the peak JP2 power value at the dominant frequency (JP2power). JP2power was reduced after 48 hrs in culture and more dramatically at 72 hrs (Figure 6D). In addition, at 48 hrs in culture we observed a clear accumulation of JP2 around the edge of myocytes, suggesting a reorganization of JP2 protein during culture. More dramatically, at 72 hrs a significant amount of JP2 was redistributed from the T-tubule membrane to the periphery plasma membrane (Figure 6C, E). Microtubule depolymerization with nocodazole prevented the culture-associated disorganization of JP2 within the T-tubule network as well as the redistribution to the peripheral membrane (Figure 6C–E). Treatment with taxol accelerated the culture induced-redistribution of JP2, and this effect was antagonized by nocodazole (Figure 6C–E). These data collectively demonstrate that microtubule stabilization results in JP2 redistribution without changes in JP2 expression, suggesting that the T-tubule remodeling associated with microtubule stabilization is mediated by alteration of normal JP2 localization.
Kinesin 1 is involved in microtubule polymerization-mediated abnormal trafficking of JP2 protein
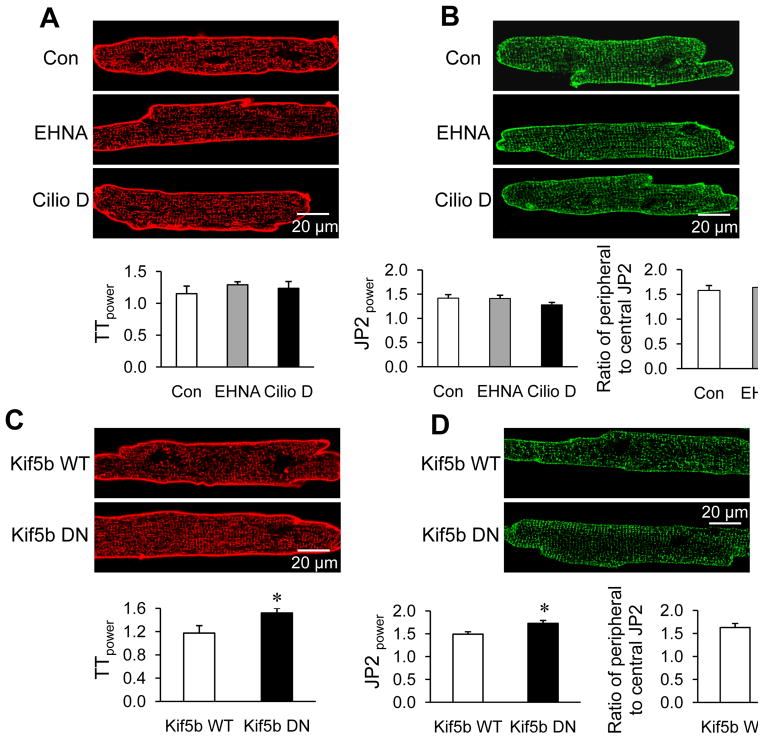
Microtubule motor proteins kinesin 1 and dynein are responsible for the forward and retrograde trafficking of cargos along microtubules, respectively. To test whether inhibition of retrograde trafficking affects T-tubule remodeling in cultured cardiomyocytes, we used dynein inhibitors erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA) and Ciliobrevin D (Cilio D). Neither 300 μM EHNA nor 50 μM Cilio D treatment for 48 hrs altered T-tubule structure and JP2 redistribution in cultured cardiomyocytes compared to that of control (Figure 7A–B). By contrast, overexpression of a dominant negative mutant of kinesin (Kif5b) protected against cultured-induced T-tubule remodeling as well as JP2 redistribution to the cell periphery (Figure 7C–D), compared to that of expressing wild type Kif5b. These data suggest a role for kinesin 1’s anterograde trafficking, but not dynein’s retrograde trafficking, of JP2 in microtubule densification-mediated T-tubule reorganization.
Figure 7.
Effects of microtubule motor proteins on JP2 distribution and T-tubule integrity. A. Representative images of T-tubules stained with Di-8-ANNEPS (upper) and summarized average data (lower) of TTpower in cells treated with or without 300 μM EHNA or 50 μM Ciliobrevin D (Cilio D) for 48 hrs. n = 25–30 cells per group. p>0.05 among the three groups (Con, EHNA and Cilio D), linear mixed-effects model. B. Isolated cardiomyocytes stained with anti-JP2 antibody after treatment with or without EHNA or Cilio D for 48 hrs. Upper: Representative images; lower-left: average JP2power in cultured cardiomyocytes with different treatments; lower-right: semi-quantitative analysis of JP2 distribution between T-tubule and periphery sarcolemma. n = 15–20 cells per group. p>0.05 among the three groups (Con, EHNA and Cilio D), linear mixed-effects model). C. Representative images of T-tubules stained with Di-8-ANNEPS (upper) and summarized average data (lower) of TTpower in cells after infection with WT or DN Kif5b for 48 hrs. n = 25–30 cells per group. * p<0.05 Kif5b DN vs WT (Student t-test) D. Cardiomyocytes stained with anti-JP2 antibody after infection with adenoviral constructs containing WT or dominant negative (DN) Kif5b for 48 hrs. Upper: Representative images; lower-left: average JP2power; lower-right: semi-quantitative analysis of JP2 redistribution from T-tubule membrane to periphery sarcolemma. n = 15–20 cells per group. * p<0.05 vs Kif5b WT (Student t-test).
Protected T-tubule structure by microtubule depolymerization preserves Ca2+ handling
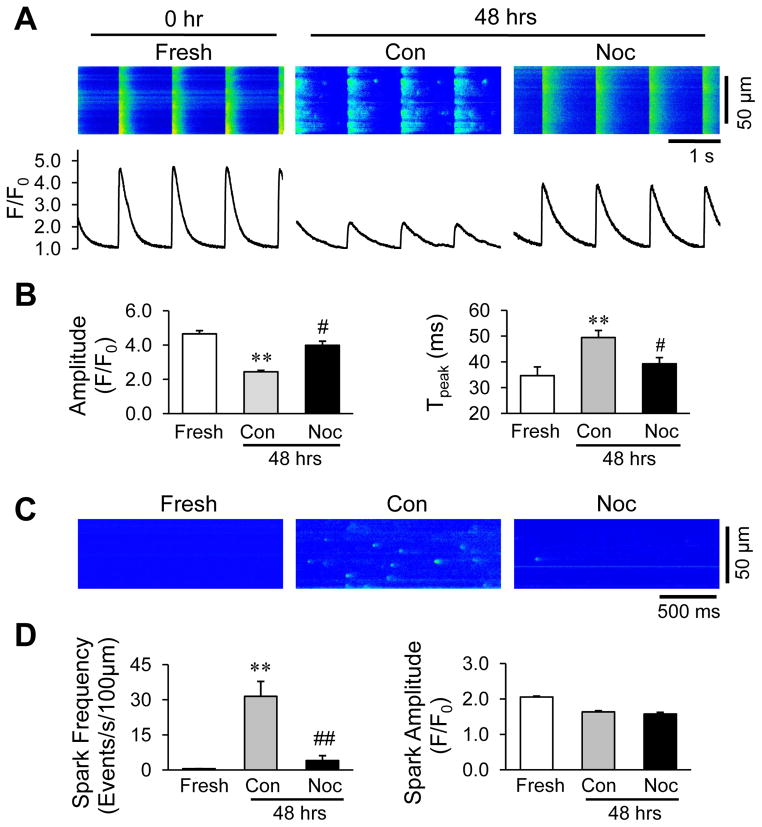
A fundamental impact of T-tubule remodeling in cardiomyocytes is defective E-C coupling between T-tubule and SR. Accordingly, cultured cardiomyocytes paced at 1Hz displayed depressed amplitude and prolonged time to peak of Ca2+ transients compared to freshly isolated myocytes (Figure 8A, B). Nocodazole treatment normalized these alterations. Under resting conditions, the activity of spontaneous Ca2+ sparks reflects the gating of ryanodine receptors on the SR. We detected a dramatic increase in spontaneous Ca2+ sparks in control cells after culture for 48 hrs as compared to freshly isolated cardiomyocytes (Figure 8C, D). Nocodazole treatment markedly reduced the frequency of Ca2+ sparks without affecting other parameters (Figure 8C, D). These data suggest that microtubule depolymerization plays an important role in preserving myocyte Ca2+ handling function by maintaining the normal architecture of E-C coupling machinery.
Figure 8.
Microtubule depolymerization increases the amplitude of Ca2+ transients and reduces spontaneous Ca2+ release. A. Representative cytosolic Ca2+ images (upper) and traces (lower) in freshly isolated cardiomyocytes (within 2 hrs of isolation) or cells cultured for 48 hrs in the absence or presence of 10 μM nocodazole. B. Average data of the amplitude and time to peak of Ca2+ transients. p<0.001 for both measurements among the fresh, Con and Noc groups of 48 hrs by linear mixed-effects model. C. Representative Ca2+ spark images in cardiomyocytes freshly isolated or cultured for 48 hrs. D. Mean values of the frequency and amplitude of Ca2+ sparks. Con: DMSO control; Noc: nocodazole. n = 15–25 cells per group. ** p < 0.01 vs fresh; # p < 0.05, ## p < 0.01, vs cultured control. p<0.001 for spark frequency among the fresh, Con and Noc groups of 48 hrs by linear mixed-effects model.
DISCUSSION
The relationship between microtubule densification and loss of myocyte contractility in decompensated hypertrophied hearts was established over 20 years ago.1, 31–33 Our study using a murine model of chronic pressure overload-induced cardiomyopathy provides new insights by mechanistically linking microtubule accumulation and T-tubule remodeling, which leads to impaired E-C coupling and heart failure. The major findings of our study are as follows: (1) depolymerizing microtubules in vivo mitigated TAB-induced T-tubule remodeling and heart failure; (2) densification of microtubules was accompanied by T-tubule impairment, and attenuation of microtubule densification using microtubule depolymerizers mitigated T-tubule alterations; (3) The normal distribution of JP2 was altered in pressure overloaded mouse hearts as well as in human diseased hearts; (4) microtubule densification resulted in JP2 redistribution from T-tubules to the cell periphery, which was preventable by microtubule depolymerization; (5) the protective effect of microtubule depolymerization on T-tubules was abolished in cardiomyocytes from JP2-KD mice; (6) overexpressing a dominant negative mutant of Kif5b prevented the culture-induced JP2 redistribution and T-tubule damage; (7) microtubule depolymerization preserved cytosolic Ca2+ transients and markedly reduced spontaneous Ca2+ sparks in cultured cardiomyocytes. Taken together, these data implicate the critical role of microtubule densification in the mechanism of T-tubule remodeling and loss of E-C coupling in pressure overload-induced cardiomyopathy.
Cardiac E-C coupling relies on precise positioning of ion channels, transporters and related regulatory/structural proteins. Microtubules form an intricate and dynamic network capable of shuttling protein containing vesicles to proper destinations.34–37 It appears clear that microtubule is in close approximation with E-C coupling machinery such as T-tubular-SR junctions,38 whereby microtubules may modulate adjacent RyR2 activities.39, 40 It is also suggested that microtubules are critical in maintaining the structural integrity of the SR network in ventricular myocytes.41
Microtubule accumulation and densification due to increased expression of tubulin and formation of stable microtubules have been observed in multiple models of cardiac hypertrophy, both at the compensated and decompensated stages.1, 10, 14 It was soundly established that the densification of microtubule network contributes to the cardiac dysfunction in decompensated hypertrophy by two reported mechanisms. First, accumulation of microtubules increases the stiffness of the cell and mechanically impedes sarcomere motion.14, 31 Second, microtubule densification alters E-C coupling, including Ca2+ influx and SR Ca2+ release, but this remains controversial.16, 17 Elevation of stable microtubules occurred during both compensated and decompensated stages.1, 10, 14 It began at the onset of cardiac hypertrophy and was involved in early hypertrophic response of myocardium induced by pressure overload.10 These studies led us to hypothesize that microtubule densification might also be involved in chronic cardiac remodeling such as T-tubule remodeling.22, 42 Like microtubule densification, loss of T-tubule structural integrity is evident in compensated hypertrophy and progressively deteriorates with the development of heart failure.22, 43 Loss of T-tubule integrity alters the spatial distribution of L-type Ca2+ channel and increases ‘orphaned’ RyRs,44 which leads to reduced amplitude and slower rise of Ca2+ release.29, 44 We found that, in both intact pressure oveloaded hearts and cultured cardiomyocytes, treatment with microtubule depolymerizing agents attenuated T-tubule impairment. Moreover, microtubule depolymerization preserved the amplitude of Ca2+ transients and reduced the frequency of Ca2+ sparks. Thus, our data provide a clear mechanistic link between microtubule densification, T-tubule remodeling, and E-C coupling dysregulation. Our study also resolves a long-lasting question in the field: what mediates the progressive loss of T-tubules associated with prolonged culture of isolated primary cardiomyocytes?28–30, 45 In the present study, our data not only suggest a link between microtubule densification and T-tubule remodeling, but also provides mechanistic insights by implicating JP2 redistribution in this process in cultured myocytes. Moreover, our data also suggest that this is a common mechanism in cultured cardiomyocytes and in vivo following cardiac stress.
We and others have reported a critical role for JP2 in T-tubule integrity such that deficiency of JP2 impairs T-tubule maturation during cardiac development,46 and accelerates T-tubule remodeling and loss of E-C coupling under pathological conditions.22, 27 JP2 expression is markedly reduced in diseased hearts.22, 42, 47, 48 One mechanism is through miRNA-mediated gene silencing.49 JP2 is also regulated at the post-translational level via degradation by the Ca2+-dependent proteolysis.50 Our data herein complement these studies by providing new evidence that JP2 is required for the protective effect of microtubule depolymerization on T-tubule structure following cardiac stress. As an additional mechanism, we identified altered JP2 localization from the T-tubule membrane to the surface plasma membrane in response to stresses that induce microtubule densification. The mechanism likely involves enhanced anterograde trafficking of JP2 to the cell periphery. Our data indicate that the densification and likely reorganized microtubule network influence the redistribution of JP2 within the membrane system, thereby inducing T-tubule remodeling and E-C coupling dysfunction and contributing to the development of heart failure.
We have recently reported that β-adrenergic blockage improves T-tubule remodeling and cardiac function after myocardial infarction through restoration of JP2 protein expression.42 In the present study our data suggest that microtubule densification-mediated JP2 redistribution represents another mechanism associated with heart failure. Thus, in addition to maintaining expression of JP2, proper localization of JP2 to the T-tubule system is also critical for normal myocyte function.
In summary, we identify densification of microtubules as a cause of T-tubule remodeling in heart disease and in cultured adult cardiomyocytes. Our data implicate redistribution of JP2, a critical safeguard of the cardiac dyad, as a mechanism by which microtubule densification leads to T-tubule disruption, loss of E-C coupling, and heart failure. Our study also identify a novel mechanism for JP2 dysregulation, that is, microtubule densification-mediated JP2 mis-trafficking, complementary to other mechanisms of JP2 downregulation such as by miRNA-mediated gene silencing 49 and post-translational degradation.50 Taken together, these findings provide novel insights into the roles of microtubules and JP2 in the pathogenesis of heart failure, which may have important implications for future development of new therapeutic strategies to ameliorate cardiac remodeling and the progression of heart failure.
Supplementary Material
Acknowledgments
We thank Dr. Guangmao Cheng (Medical University of South Carolina) for help on isolation of free and polymerized tubulin.
Funding Sources: This work was supported by NIH R01 HL090905 (L.S.S.), American Heart Association Scientific Development Grant 0635056N (L.S.S.); American Heart Association Midwest Postdoctoral Fellowship 13POST14630077 (A.G.); NIH R00 HL092235 (J.D.M.); NIH R01 HL079031, HL62494, HL70250 and HL113001 (M.E.A.) and HL085686 (L.F.S.).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 2.Aquila-Pastir LA, DiPaola NR, Matteo RG, Smedira NG, McCarthy PM, Moravec CS. Quantitation and distribution of beta-tubulin in human cardiac myocytes. J Mol Cell Cardiol. 2002;34:1513–1523. doi: 10.1006/jmcc.2002.2105. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper G. Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J Am Coll Cardiol. 2001;37:1080–1084. doi: 10.1016/s0735-1097(00)01207-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Li F, Campbell SE, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: II. Cytoskeletal remodeling. J Mol Cell Cardiol. 1999;31:319–331. doi: 10.1006/jmcc.1998.0885. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MC, Donehower RC. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9:1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- 6.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, Spreafico C, Laffranchi A, Caraceni A, Martini C, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol. 1995;13:2688–2699. doi: 10.1200/JCO.1995.13.11.2688. [DOI] [PubMed] [Google Scholar]
- 8.Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, Cronstein BN, Sedlis SP, Pillinger MH. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39:1458–1464. doi: 10.3899/jrheum.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagawa H, Koide M, Sato H, Zile MR, Carabello BA, Cooper G. Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res. 1998;82:751–761. doi: 10.1161/01.res.82.7.751. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Tsutsui H, Tagawa H, Igarashi-Saito K, Imanaka-Yoshida K, Takeshita A. Microtubules are involved in early hypertrophic responses of myocardium during pressure overload. Am J Physiol. 1998;275:H341–H348. doi: 10.1152/ajpheart.1998.275.2.H341. [DOI] [PubMed] [Google Scholar]
- 11.Koide M, Hamawaki M, Narishige T, Sato H, Nemoto S, DeFreyte G, Zile MR, Cooper GI, Carabello BA. Microtubule depolymerization normalizes in vivo myocardial contractile function in dogs with pressure-overload left ventricular hypertrophy. Circulation. 2000;102:1045–1052. doi: 10.1161/01.cir.102.9.1045. [DOI] [PubMed] [Google Scholar]
- 12.Cheng G, Zile MR, Takahashi M, Baicu CF, Bonnema DD, Cabral F, Menick DR, Cooper Gt. A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am J Physiol Heart Circ Physiol. 2008;294:H2231–2241. doi: 10.1152/ajpheart.91515.2007. [DOI] [PubMed] [Google Scholar]
- 13.Tagawa H, Koide M, Sato H, Cooper Gt. Cytoskeletal role in the contractile dysfunction of cardiocytes from hypertrophied and failing right ventricular myocardium. Proc Assoc Am Physicians. 1996;108:218–229. [PubMed] [Google Scholar]
- 14.Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res. 1997;80:281–289. doi: 10.1161/01.res.80.2.281. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi Y, Tsutsui H, Yamamoto S, Takahashi M, Imanaka-Yoshida K, Yoshida T, Urabe Y, Sugimachi M, Takeshita A. Role of microtubules in myocyte contractile dysfunction during cardiac hypertrophy in the rat. Am J Physiol. 1996;271:H1978–1987. doi: 10.1152/ajpheart.1996.271.5.H1978. [DOI] [PubMed] [Google Scholar]
- 16.Gomez AM, Kerfant BG, Vassort G. Microtubule disruption modulates Ca2+ signaling in rat cardiac myocytes. Circ Res. 2000;86:30–36. doi: 10.1161/01.res.86.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Calaghan SC, Le Guennec JY, White E. Modulation of Ca2+ signaling by microtubule disruption in rat ventricular myocytes and its dependence on the ruptured patch-clamp configuration. Circ Res. 2001;88:E32–37. doi: 10.1161/01.res.88.4.e32. [DOI] [PubMed] [Google Scholar]
- 18.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: Altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 19.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 20.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 21.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of t-tubule remodelling in heart failure. Cardiovasc Res. 2013;98:204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969;42:1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 25.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 26.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: A novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 27.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Arch. 1996;431:814–827. doi: 10.1007/s004240050073. [DOI] [PubMed] [Google Scholar]
- 29.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Pavlovic D, McLatchie LM, Shattock MJ. The rate of loss of T-tubules in cultured adult ventricular myocytes is species dependent. Exp Physiol. 2010;95:518–527. doi: 10.1113/expphysiol.2009.052126. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, Cooper G. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation. 1994;90:533–555. doi: 10.1161/01.cir.90.1.533. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993;260:682–687. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- 33.Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 34.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. Bin1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zadeh AD, Cheng Y, Xu H, Wong N, Wang Z, Goonasekara C, Steele DF, Fedida D. Kif5b is an essential forward trafficking motor for the kv1.5 cardiac potassium channel. J Physiol. 2009;587:4565–4574. doi: 10.1113/jphysiol.2009.178442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 38.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerfant BG, Vassort G, Gomez AM. Microtubule disruption by colchicine reversibly enhances calcium signaling in intact rat cardiac myocytes. Circ Res. 2001;88:E59–65. doi: 10.1161/hh0701.090462. [DOI] [PubMed] [Google Scholar]
- 40.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 41.Vega AL, Yuan C, Votaw VS, Santana LF. Dynamic changes in sarcoplasmic reticulum structure in ventricular myocytes. J Biomed Biotechnol. 2011;2011:382586. doi: 10.1155/2011/382586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, Zimmerman K, Weiss RM, Miller FJ, Anderson ME, Song LS. β-adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J. 2012;26:2531–2537. doi: 10.1096/fj.11-199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, Mathias RT, Cohen IS, Song LS, Entcheva E, Lin RZ. PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PLoS One. 2011;6:e24404. doi: 10.1371/journal.pone.0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XH, Song LS. Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res. 2013;100:54–62. doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minamisawa S, Oshikawa J, Takeshima H, Hoshijima M, Wang Y, Chien KR, Ishikawa Y, Matsuoka R. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem Biophys Res Commun. 2004;325:852–856. doi: 10.1016/j.bbrc.2004.10.107. [DOI] [PubMed] [Google Scholar]
- 48.Zhang HB, Li RC, Xu M, Xu SM, Lai YS, Wu HD, Xie XJ, Gao W, Ye H, Zhang YY, Meng X, Wang SQ. Ultrastructural uncoupling between t-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res. 2013;98:269–276. doi: 10.1093/cvr/cvt030. [DOI] [PubMed] [Google Scholar]
- 49.Xu M, Wu HD, Li RC, Zhang HB, Wang M, Tao J, Feng XH, Guo YB, Li SF, Lai ST, Zhou P, Li LL, Yang HQ, Luo GZ, Bai Y, Xi JJ, Gao W, Han QD, Zhang YY, Wang XJ, Meng X, Wang SQ. Mir-24 regulates junctophilin-2 expression in cardiomyocytes. Circ Res. 2012;111:837–841. doi: 10.1161/CIRCRESAHA.112.277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD. Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol. 2013;591:719–729. doi: 10.1113/jphysiol.2012.243279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.