Introduction
Recognizing the emerging field of therapeutic cell‐based treatments for a growing number of diseases, including the field of regenerative medicine, the National Heart, Lung, and Blood Institute (NHLBI) held a workshop in 2002, where experts in cellular product manufacturing and clinical trial design came together to discuss the state of the cellular therapy field and identify bottlenecks to its advancement.1 A consensus emerged that improved access to current Good Manufacturing Practice (cGMP) facilities, regulatory assistance, and training would foster the transition of cellular therapies into the clinic. The Production Assistance for Cellular Therapies (PACT) program was launched in 2003 with three cell processing facilities (Baylor College of Medicine, University of Minnesota, and University of Pittsburgh) and a coordinating center. The program was expanded in 2010 to five cell processing facilities. The goal of the PACT program is to facilitate the translation of promising cell therapies from the bench to bedside, provide leadership in the emerging field of cellular therapy, and provide education to researchers, clinicians and healthcare professionals in this rapidly expanding therapeutic area.2, 3 In this report we review the first 10 years of the NHLBI‐funded PACT program in cell and tissue therapies.
Participating institutions
Initially consisting of three cell processing facilities, PACT was expanded in 2010 to meet the increasing needs of the scientific community. Currently, 5 cell processing facilities are contracted to provide cell therapy production and translational services support for the PACT program. They are located at the Baylor College of Medicine, Center for Cell and Gene Therapy in Houston, TX, Boston Children's Hospital, Center for Human Cell Therapy in Boston, MA, City of Hope, Center for Applied Technology in Duarte, CA, the University of Minnesota, Molecular and Cellular Therapeutics Facility in St. Paul, MN, and the University of Wisconsin, Waisman Biomanufacturing in Madison, WI. The EMMES Corporation in Rockville, MD, serves as the Coordinating Center. A Steering Committee comprised of representatives from each cell processing facility, the Coordinating Center, NHLBI, and an independent NHLBI‐appointed Chair, provides overall governance for the program and oversees the conduct and maintenance of PACT projects.
PACT project applications
The PACT program is fueled by investigator‐initiated projects, where an investigator applies to PACT via a two‐stage web‐based process at www.pactgroup.net. Preliminary applications are evaluated based on their fit with the scope and mission of the NHLBI, significance to the cell therapy field, cell processing facility capabilities, and manufacturing feasibility. PACT applications are classified into one of two categories based on whether the applicant is requesting clinical product manufacturing support or translational development services and reviewed based on criteria specific for each of these categories (Figure 1 A and B). Following the preliminary review, the applicant may be invited to submit a more detailed application, which is reviewed by an independent external peer review panel comprised of experts in the clinical and translational cell therapy manufacturing field. The external review panel critiques are incorporated into the Steering Committee's overall assessment before a final decision of whether to support the project is made by the Steering Committee. For each approved PACT project, a team is assembled that includes administrative, scientific, manufacturing, quality systems, and regulatory affairs experts. Services are usually performed under a service agreement that is negotiated between the cell processing facility and the investigator and their associated organization or institution. Confidentiality and intellectual property considerations are negotiated prior to the start of any work and the cell processing facility staff work collaboratively with the investigator to develop the project timeline and milestones.
Figure 1.
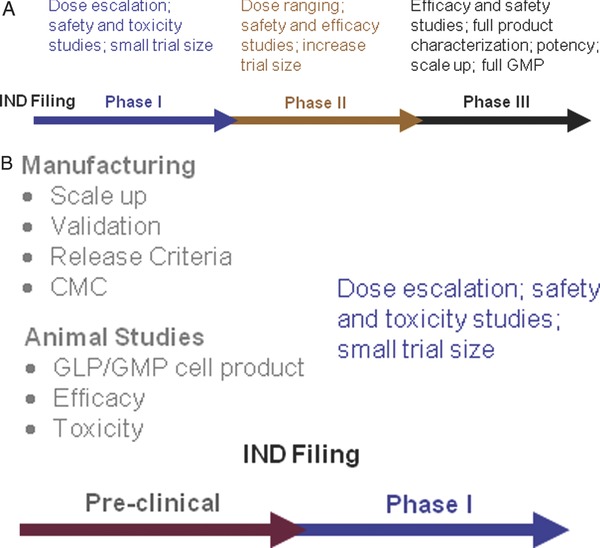
(A) Clinical product manufacturing support. (B) Translational development support.
Clinical product manufacturing support
PACT provides clinical product manufacturing support for Phase I and Phase II clinical trials (Figure 1 A) that evaluate the use of cellular products for indications such as cardiac repair, lung repair, immune reconstitution, treatment of complications after bone marrow transplantation such as graft‐versus‐host disease (GVHD) and infections, and hematologic diseases, except for primary treatment for hematological malignancy. Applications for clinical product manufacturing support may contain some level of translational work that would be needed prior to clinical product manufacturing.
Translational development services
PACT supports translational projects regardless of disease indication, unlike the clinical product manufacturing services that must support treatment of heart, lung, and blood diseases. Successful cell therapies require the translation of laboratory‐based techniques into Standard Operating Procedures (SOPs) and the development of production methods that can be conducted under cGMP. Successful applications for PACT translational services specify how the requested services fit into a product development plan with the eventual goal of a FDA‐approved Investigational New Drug (IND) application and clinical trial. PACT provides support for activities that will bring completed proof‐of‐concept discoveries to translational development. These activities include optimizing manufacturing processes, development of SOPs, cell manufacturing for relevant preclinical animal model studies, and development of the Chemistry, Manufacturing and Controls (CMC) section for an IND application (Figure 1 B).
The PACT program has seen an increasing number of applications since its inception in 2003 with a steadily increasing number of translational applications (Figures 2 A and B). As of September 16, 2013, a total of 110 full applications have been submitted to PACT of which 106 (53 clinical and 53 translational) were approved and initiated by the program (Figure 3).
Figure 2.
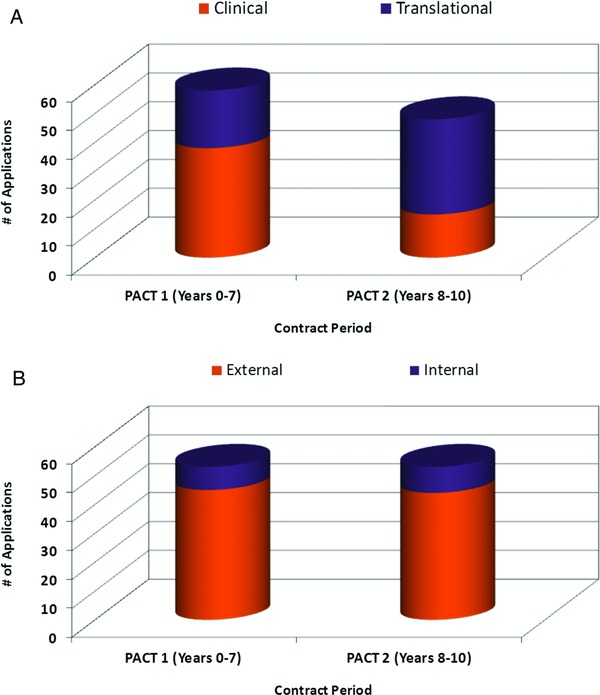
(A) Approved projects by application type between PACT 1 (Years 0–7) and PACT 2 (Years 8–10). (B) Projects approved in PACT 1 (Years 0–7) and PACT 2 (Years 8–10). PACT 1 initiated on average 7.5 projects per year and PACT 2 initiated an average of 10.0 projects per year. The investigators for over 80% of approved PACT projects are not affiliated with (external to) the PACT program.
Figure 3.

PACT project status Workflow product manufacturing, and product administrations associated with the current approved full applications to PACT during the period of September 2003 to September 2013.
PACT program activities and accomplishments
PACT clinical product manufacturing support
As of September 2013, PACT has manufactured over 650 products that have been administered under 32 clinical protocols. Twenty‐nine clinical projects have completed their manufacturing life cycle and all scheduled products were successfully delivered to the investigator for clinical use (Figure 3). Cell products manufactured by PACT and administered for a variety of treatment indications are shown in Table 1. This is best illustrated in the collaboration with the NHLBI Cardiovascular Cellular Therapy Research Network (CCTRN) to provide bone marrow‐derived mononuclear cells for three multicenter clinical trials. Myocardial infarction results from a blood clot forming in one of the coronary arteries. This blood clot blocks the flow of blood into the heart resulting in injury and death to some of the heart muscle. Although angioplasty removes blockage and restores blood flow, there can be permanent damage. In some cases, this injury may result in enlargement of the heart and congestive heart failure. The purpose of the Transplantation in Myocardial Infarction Evaluation (TIME) protocol was to determine if autologous bone marrow‐derived mononuclear cells can be transplanted into the injured heart and improve heart muscle function following a heart attack.4 This study had a companion study called LATE TIME which evaluated different time points postinfarction for transplantation.5 These studies required extensive coordination between all study cell processing facilities and the site clinical staff since standardized methods had to be developed and employed by all the centers for the extraction of bone marrow from the patients, processing of the cells at a cell processing facility, and timing of reinjection during surgery.
Table 1.
PACT clinical cell products and their corresponding treatment indications
| Cell product | Treatment indication or objective |
|---|---|
| Cardiac stem/progenitor cells | • Cardiac regeneration in acute myocardial infarction |
| Mesenchymal stem cells (MSCs)* | • Repair cardiac damage following myocardial infarction• Sickle cell disease• Acute lung injury |
| Autologous bone marrow mononuclear cells* | • Cardiac repair left ventricular assist device (LVAD) placement• Stroke• Left ventricular function following acute myocardial infarction• Treatment of traumatic brain injury |
| T‐regulatory cells (umbilical cord blood and peripheral blood—derived) | • Prevent graft‐versus‐host disease (GVHD)• Enhance engraftment |
| Multivirus‐specific cytotoxic T lymphocytes (peripheral blood and bone marrow‐derived) | • Prevent and treat viral infections (CMV, EBV, adenovirus)• Treatment of refractory posttransplant lymphoproliferative disease |
| Dendritic cells pulsed with inactivated HIV‐1 infected apoptotic cells | • Therapeutic vaccine for HIV‐1 infected patients |
| Antisense oligonucleotide‐treated dendritic cells | • Preserve residual beta cell mass in type 1 diabetes |
| Allogeneic natural killer cells | • Gain durable remission• Hematopoietic stem cell transplant preparative regimen |
| CD133+ progenitor cells (peripheral blood and bone marrow‐derived) | • Critical limb ischemia• Chronic ischemic cardiomyopathy |
| Peripheral blood‐derived inducible T‐reg cells | • Prevention and treatment of GVHD |
| Gene modified CD34+ cells | • Treatment of SCID‐X1 |
*Multiple applications are grouped by cell product.
Often the clinical work has been supported by varying amounts of translational work to move the cellular therapy from bench to bedside as illustrated in the following examples. The first PACT project that involved the successful translation from bench to clinic is the manufacture of cytotoxic T cells (CTLs) specific for CMV, EBV, and adenoviruses for the prevention of posttransplant infections. It was assumed that three distinct cell lines would be required because the dominant antigens of each virus would compete for presentation to effector cells which would lack multivirus specificity. PACT services however led to the generation of a single process resulting in the generation of CTLs specific for CMV, EBV, and adenovirus.6 These cells can expand in response to viral challenge after administration and produce clinically relevant effects. Eleven stem cell recipients received these CTLs, all of which expanded in vivo, reduced the viral titer, and resolved disease symptoms in those with evidence of active CMV, EBV, and adenovirus infection.7 Initially this product required a long (>10 weeks) and complex manufacturing procedure as well as live virus and adenoviral vectors as a source of antigen. PACT‐supported services streamlined the manufacturing processes first using dendritic cells nucleofected with DNA plasmids to activate T cells in a 17‐day manufacturing process. Additional improvements were subsequently developed including the use of overlapping peptide sequences, adding BK and HHV6 virus coverage, and reducing the processing time to 10 days. Eleven subjects have now received pepmix‐activated T cells with results that are equally as promising as those seen in the first 11 subjects.8, 9, 10, 11
Natural killer (NK) cells are a major cell type in the innate immune system that are programmed to kill targets without prior antigen priming. This makes them an attractive cell population to exploit for antitumor therapy. NK cells are the first lymphocyte population to expand after hematopoietic stem cell transplantation (HSCT) and engraftment of these cells has many benefits including decreased rates of GVHD due to donor NK cells killing host dendritic cells,12 decreased rates of graft rejection as a result of NK lysis of host T cells,13 and antiviral protection, especially against CMV.14 PACT is supporting the manufacture of allogeneic NK cells for the first multicenter trial to assess infusion of adoptively transferred adult haploidentical interleukin‐2 activated NK cells prior to HSCT.15, 16 This is the first multisite Phase II study to examine the therapeutic benefit of NK cell‐based nonmyeloablative haploidentical transplantation for the treatment of high‐risk acute myeloid leukemia. The primary objective is to determine the rate of donor engraftment and complete disease response at Day 28 with secondary objectives of evaluating in vivo donor NK cell expansion in the patient's peripheral blood and the safety of the therapy by monitoring 6‐month disease‐free survival, treatment related mortality, and incidence of relapse. Two subjects have received products and other sites are preparing to enroll.
Acute lung injury (ALI) is a major cause of mortality and morbidity that affects 200,000 patients annually in the US alone. It is a diffuse and heterogeneous lung injury characterized by hypoxemia, noncardiogenic pulmonary edema, low lung compliance, and widespread capillary leakage. It is caused by inflammation and is typically associated with sepsis. There is no specific therapy that reduces lung injury and increases survival.17 Recent preclinical studies demonstrate a major potential for ALI therapy with bone marrow‐derived mesenchymal stromal cells (MSCs).18 MSCs are multipotent stromal cells that can differentiate into many types of cells including osteoblasts, chrondocytes, and adipocytes19 and the mechanisms for the potential therapeutic effects of MSCs are not completely understood. Animal studies have demonstrated that MSCs can repair alveolar epithelium, although it is not known whether this is through a direct effect or a paracrine effect.19 A clinical trial has recently begun that will test both cell‐contact‐dependent and cell‐contact‐independent mechanisms for the therapeutic benefit of MSCs in repairing the injured lung. PACT is manufacturing the MSCs for this Phase I/II safety and dosing trial. PACT was also involved in the translational phase of this project. PACT facilitated discussions between investigators and the FDA regarding the establishment of appropriate animal models and produced GMP‐grade MSCs for an IND‐enabling ovine study.20 This project exemplifies the role that PACT can play in assisting investigators through the translational phase and into the early clinical phases of cellular therapy.
PACT translational development services
PACT provides services for qualified projects that bring proof‐of‐concept studies into the translational developmental stage. The community's need for translational services in cell therapy product development has grown during the course of the PACT program (Figure 2 A). Translational work has continued to increase as described below with 24 translational projects completed (Table 2). In addition to the acute lung injury work described above, PACT has developed the production of bone marrow and lung‐derived MSCs for stroke, bronchiolitis obliterans, and emphysema.
Table 2.
PACT‐supported translational work
| Translational work | Treatment indication or objective |
|---|---|
| Human embryonic stem cell—derived cardiomyocytes | • Acute and chronic myocardial ischemia |
| Corneal and oral epithelial stem cells | • Corneal transplantation in patients with corneal limbal stem cell deficiency |
| Lung‐derived mesenchymal stem cells (MSCs) | • End‐stage emphysema |
| Bone marrow‐derived MSCs* | • Myocardial infarction• Pulmonary arterial hypertension• Acute lung injury• Lung rejection• 3‐D human lung for transplant• Bronchiolitis obliterans• Stroke• Graft‐versus‐host disease (GVHD)• Vocal fold scarring |
| Peripheral blood—derived iTregs (expansion optimization studies) | • Treatment/prevention of GVHD following hematopoietic stem cell transplantation (HSCT) |
| Gene modified CD34+ cells | • Treatment of Wiskott‐Aldrich syndrome |
| Peripheral blood—derived HIV‐1—specific cytotoxic T lymphocytes (CTLs) | • HIV‐1—specific CTL therapy |
| Fibroblasts | • Wound healing/therapies |
In addition to providing clinical product for multicenter clinical trials, PACT also has a history of working with other NHLBI programs to share translational development resulting in a clinical trial. Wiskott‐Aldrich syndrome (WAS) is a rare disease that is X‐linked and recessive resulting in eczema, thrombocytopenia, and immune deficiency. Mutations in the WAS gene leading to reduced or absence of WAS protein (WASP) are responsible for the clinical manifestations. The WAS protein is a master regulator of actin cytoskeleton; thus, its absence compromises multiple hematopoietic cellular functions including phagocytosis, migration, immune synapse formation and proliferation.21 Investigators at Boston Children's Hospital are conducting a single center, pilot and feasibility study using an infusion of autologous CD34+ cells transduced with the lentiviral vector containing the human WASP gene (w1.6_WASP_WPRE(VSVg)).22 The primary objectives of the trial are to safely administer the vector‐modified hematopoietic progenitor cells and achieve engraftment of WASP‐expressing transduced T cells. PACT optimized and standardized the transduction procedures and developed standard operating procedures for the manufacture of the transduced cells. PACT collaborated with the NHLBI Gene Therapy Resource Program (www.gtrp.org), which provides support for the trial and products manufacturing. Of note, the same lentiviral vector is used in clinical trials in France, Great Britain and Italy.
Furthermore, SOPs developed during the translational phase of PACT projects have been utilized to manufacture gene‐modified CD34+ cells for Phase I clinical trials for the X‐linked form of severe combined immunodeficiency (SCID‐X1) and MSCs for lung and stroke indications.20, 23
There is increasing interest in the cell therapy community to harness the differentiation potential of pluripotent stem cells to regenerate damaged cardiac tissues since myocardial infarction and heart failure are leading causes of death in the United States and worldwide. Under proper culturing conditions, these cells can be differentiated into cardiomyocytes which could theoretically be infused into damaged heart tissue to repair and regenerate as healthy cells. The production of pluripotent stem cells has been accomplished on a scale appropriate for limited research, but they have not yet been produced in a GMP‐compliant manner with accompanying scale‐up methodology to be used in future human clinical studies. One of the PACT translational projects involves the development of a human embryonic stem cell (hESC) master cell bank and a GMP‐compliant cardiomyocyte differentiation process. A scalable suspension culture system for hESC has been established that is suitable to seed the clinical production of cardiomyocytes (Table 2).
Over 35 peer‐reviewed publications have resulted from PACT‐supported clinical manufacturing and translational development services. A list of publications that have put forward key proof‐of‐principle studies as well as reports on clinical applications of human cellular therapy can be found on the PACT Website at www.pactgroup.net (Table 3).
Table 3.
Representative NHLBI PACT cell therapy products: from key scientific rationale in the literature to clinical application in human cellular therapy
| Cell product | Clinical rationale | Scientific basis citations | Clinical application citations |
|---|---|---|---|
| LMP1–and LMP2‐specific cytotoxic T‐lymphocytes | Epstein‐Barr virus—associated lymphoma | 31 | 31 |
| Tri‐virus—specific cytotoxic T‐lymphocytes (nucleofected with plasmids) | Prevention and treatment of cytomegalovirus (CMV), EBV, and adenovirus post hematopoietic stem cell transplantation (HSCT) | 32, 33 | 33–39 |
| Bone marrow—derived mononuclear cells | Traumatic brain injury | 40 | 41 |
| Bone marrow—derived mononuclear cells | Acute myocardial infarction | – | 42, 43 |
| Expanded T‐regulatory cells | Prevention of GVHD | 44–48 | 48 |
| Autologous mature apoptotic dendritic cells with HIV‐1 | Improving host immune control of residual host immune HIV infection during highly active antiretroviral therapy (HAART) | 49 | 50 |
| Allogeneic haploidentical natural killer cells | Gain durable remission/HSCT preparative regimen | 51 | 52 |
PACT development projects
In the past 10 years, PACT has played a leadership role in the identification and development of best‐in‐class technologies for cell therapy. The PACT program identifies and evaluates important new technologies and assesses current methods to advance the field of cell therapy. Through its unique consortium of five academic centers, PACT is able to leverage the program to address issues facing the cell manufacturing community. PACT is currently involved in several development projects such as cell characterization, potency assay development, validation of storage conditions and device evaluations. Early PACT development projects have generated peer‐reviewed publications on shipping validation, comparison of endotoxin testing methods, and cell selection for small volume cell samples24, 25, 26 and helped to define best practices in the field of cell therapy.
PACT education and outreach
An important part of PACT's mission is to provide continuing education to physicians, scientists, and cell therapy professionals. In order to accomplish this goal, PACT provides interactive webinars and onsite workshops that offer avenues for learning and networking. PACT also serves as a resource center for cell processing facility operations and disseminates information on best practices through publications and contributions to textbooks.
Webinar topics have covered a wide range of subjects in cellular therapy. Over 25 topics have been covered from ethical and policy issues regarding unproven stem cell therapies, facility management, equipment and process validation, cell product cryopreservation, deviation management of type 351 and 361 products, and data management systems to informational topics on cGMP facility management and operations. The webinars are available for on demand learning by visiting the PACT Website (www.pactgroup.net). Furthermore, through a partnership with AABB, PACT provides continuing education credits.
PACT workshops have brought together experts in cell therapy trials and manufacturing for 1–2‐day events to promote information exchange, research, and to contribute to the advancement of the field. PACT cell processing facilities have hosted regional workshops focusing on the core requirements for cGMP, translational development and scale‐up of cell therapy products, and manufacturing and regulatory considerations in getting to an IND. PACT also holds national workshops. In 2009, a national workshop focused on key aspects of the latest scientific, clinical, and technologic developments in cell therapy which resulted in a publication in Cytotherapy.27 A 2011 national workshop outlined the challenges in pediatric cell therapies and identified potential solutions.28
PACT serves as a significant educational resource to the cell therapy community though other activities as well. For example, PACT has fulfilled, through its Website, over 400 requests for PACT facility SOPs for such activities as validation processes, quality management, and personnel training to researchers across 11 countries. PACT investigators have also added to the field of cellular therapy through the publication of a multiauthor contemporary textbook along with a new chapter in the 6th edition of Hematology.29, 30
Discussion/conclusion
Since its inception, PACT has successfully improved access to cGMP facilities and manufacturing expertise, provided regulatory assistance, and led multiple training initiatives to foster the advancement of cellular therapy. PACT is an active and successful early stage cell therapy manufacturing resource in the US, addressing translational development and training, while forging relationships among academia, industry, and participating institutions and is representative of a coordinated approach to bringing cell‐based therapies to the clinic. The PACT program serves as a unique model advancing the cell therapy field and delivering over 650 clinical products that otherwise would not have been possible. Clinical efficacy usually requires iterative Phase I clinical trials in which small steps are taken to improve an initially promising therapy. This may mean the addition of a novel gene that alters the biological behavior of the cells, but more often addressing practical issues, involving optimization of manufacturing procedures. These changes are essential to pave the way for pivotal late phase clinical trials that could be supported by industry. PACT is ideally suited to shepherd this critical work across the “valley of death” and has allowed for the clinical evaluation of cell therapy products that might not otherwise have been tested. Leadership evidenced through peer‐reviewed journals, books, webinars, and workshops has further advanced the field of cellular therapy.
As cell therapy has evolved, PACT has been a critical resource to advance knowledge in these areas and continues to lead the field with the development of new technologies and innovations. As regenerative medicine expands and develops, delivery systems, scaffolds, combination products, and tracking of administered cells will require further investigation and development. PACT is a unique infrastructure of centers and is in an ideal position to further develop these areas.
Financial Support
This project was supported by NHLBI contract #s. HHSN268201000006C, HHSN268201000007C, HHSN268201000008C, HHSN268201000009C, HHSN268201000010C, and HHSN268201000011C.
Acknowledgments
The PACT program is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services under contract numbers HHSN268201000006C, HHSN268201000007C, HHSN268201000008C, HHSN268201000009C, HHSN268201000010C, and HHSN268201000011C. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. All authors have each signed a conflict of interest form and hereby attest that they have no conflicts of interest to disclose regarding the publication of this manuscript. The authors express their appreciation to David Styers of EMMES for providing data tables and figures and his assistance and dedication in bringing this manuscript to fruition.
References
- 1. Stroncek D, Harvath L, Barrett J. National Heart, Lung, and Blood Institute of the National Institutes of Health forum on immune reconstitution and cellular therapy following hematopoietic stem‐cell transplantation. Cytotherapy. 2002; 4: 415–418. [DOI] [PubMed] [Google Scholar]
- 2. Mondoro TH, Thomas JW, Henslee‐Downey PJ, Peterson CM. NHLBI plans for the promise of cell‐based therapies. Cytotherapy. 2005; 7: 317–327. [DOI] [PubMed] [Google Scholar]
- 3. Reed W, Noga SJ, Gee AP, Rooney CM, Wagner JE, McCullough J, McKenna DH, Whiteside TL, Donnenberg AD, Baker AK, et al. Production Assistance for Cellular Therapies (PACT): Five‐year experience from the United States National Heart Lung and Blood Institute (NHLBI) Contract Research Program in Cell and Tissue Therapies. Transfusion. 2009; 49(4): 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao D, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA. 2012; 308(22): 2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson J, Zhao D, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function. JAMA. 2011; 306(19): 2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy‐Nasser AA, Leung KS, Gee AP, Krance RA, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein‐Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009; 114(19): 4283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, et al. Monoculture‐derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006; 12: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 8. Gerdemann U, Vera JF, Rooney CM, Leen AM. Generation of multivirus‐specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J Vis Exp. 2011; (51): e2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H, Dilloo D, Heslop HE, Brenner MK, Rooney CM, et al. Nucleofection of DCs to generate Multivirus‐specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009; 17(9): 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, Perna SK, Ennamuri S, Gottschalk S, Brenner MK, et al. Rapidly generated multi‐virus specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012; 20(8): 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, Martinez CA, Kennedy‐Nasser A, Leung KS, Gottschalk SM, et al. Safety and clinical efficacy of rapidly‐generated trivirus‐directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;. 21: 2113—2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen‐presenting cells. Science. 1999; 285(5426): 412–415. [DOI] [PubMed] [Google Scholar]
- 13. Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49‐mismatched NK cells from MHC‐matched donors. Blood. 2007; 109(8): 3603–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaia JA, Sun JY, Gallez‐Hawkins GM, Thao L, Oki A, Lacey SF, Dagis A, Palmer J, Diamond DJ, Forman SJ, et al. The effect of single and combined activating killer immunoglobulin‐like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009; 15(3): 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller JS, Soignier Y, Panoskaltsis‐Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005; 105: 3051–3057. [DOI] [PubMed] [Google Scholar]
- 16. McKenna DH Jr., Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six‐year single‐institution experience. Transfusion. 2007; 47(3): 520–528. [DOI] [PubMed] [Google Scholar]
- 17. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005; 353(16): 1685–1693. [DOI] [PubMed] [Google Scholar]
- 18. Gupta, N , Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow‐derived mesenchymal stem cells improves survival and attenuates endotoxin‐induced acute lung injury in mice. J Immuno. 2007 Aug 1; 179(3): 1855–1863. [DOI] [PubMed] [Google Scholar]
- 19. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell. 2001; 105(3): 369–377. [DOI] [PubMed] [Google Scholar]
- 20. Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013; 187(7): 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massaad MJ, Ramesh N, Geha RS. Wiskott‐Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci. 2013; 1285(1): 26–43. [DOI] [PubMed] [Google Scholar]
- 22. Galy A, Thrasher AJ. Gene therapy for the Wiskott‐Aldrich syndrome. Curr Opin Allergy Clin Immunol. 2011; 11(6): 545–550. [DOI] [PubMed] [Google Scholar]
- 23. Hanley PJ, Mei Z, da Graca Cabreira‐Hansen M, Klis M, Li W, Zhao Y, Durett AG, Zheng X, Wang Y, Gee AP, et al. Manufacturing mesenchymal stromal cells for phase I clinical trials. Cytotherapy. 2013 Apr; 15(4): 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gee AP, Sumstad D, Stanson J, Watson P, Proctor J, Kadidlo D, Koch E, Sprague J, Wood D, Styers D, et al. A multicenter comparison study between the Endosafe® PTS™ rapid‐release testing system and traditional test methods for detecting endotoxin in cell therapy products. Cytotherapy. 2008; 10(4): 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKenna D Jr, Adams S, Sumstad D, Sumstad T, Kadidlo D, Gee AP, Durett A, Griffin D, Donnenberg A, Amrani D, et al. CD34+ cell selection using small volume marrow aspirates: a platform for novel cell therapies and regenerative medicine. Cytotherapy. 2010; 12: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whiteside T, Griffin D, Stanson J, Gooding W, McKenna D Jr., Sumstad D, Kadidlo D, Gee AP, Durett A, Lindblad R, et al. Shipping of therapeutic somatic cell products. Cytotherapy. 2011; 13: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stroncek D, Berlyne D, Fox B, Adrian Gee AP, Heimfeld S, Lindblad R, Loper K, McKenna D, Rooney C, Sabatino M, et al. Developments in clinical cell therapy. Cytotherapy 2010; 12: 425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez R, Silberstein L, Lindblad R, Welniak L, Mondoro T, Wagner J. Strategies for more rapid translation of cellular therapies for children: A U.S. perspective. Pediatrics. 2013; 132(2): 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adrian Gee. (editor in chief) et al. Cell Therapy: cGMP Facilities and Manufacturing. Springer Publishing, 2009. [Google Scholar]
- 30. Lindblad R, Heath Mondoro T, Wood D. Preclinical Process of Cell‐Based Therapies Silberstein L. editor: Hoffman: Hematology 6th edition: Basic Principles and Practice e‐book: Chapter 97. Elsevier Inc, 2012. [Google Scholar]
- 31. Bollard CM, Gottschalk S, Huls MH, Leen AM, Gee AP Rooney C. Good manufacturing practice‐grade cytotoxic T lymphocytes specific for latent membrane proteins (LMP)‐1 and LMP2 for patients with Epstein‐Barr virus‐associated lymphoma. Cytotherapy. 2011; 13: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK, Rooney CM. Fiber‐modified adenoviruses generate subgroup cross‐reactive, adenovirus‐specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004; 103: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 33. Sili U, Huls MH, Davis AR, Gottschalk S, Brenner MK, Heslop HE, Rooney CM. Large‐scale expansion of dendritic cell‐primed polyclonal human cytotoxic T‐lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003; 26: 241–256. [DOI] [PubMed] [Google Scholar]
- 34. Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, et al. Complete responses of relapsed lymphoma following genetic modification of tumor‐antigen presenting cells and T‐lymphocyte transfer. Blood. 2007; 110: 2838–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kennedy‐Nasser AA, Bollard CM. T cell therapies following hematopoietic stem cell transplantation: surely there must be a better way than DLI? Bone Marrow Transplant. 2007; 40: 93–104. [DOI] [PubMed] [Google Scholar]
- 36. Leen AM, Myers GD, Bollard CM, Huls MH, Sili U, Gee AP, Heslop HE, Rooney CM. T‐cell immunotherapy for adenoviral infections of stem‐cell transplant recipients. Ann N Y Acad Sci. 2005; 1062: 104–115. [DOI] [PubMed] [Google Scholar]
- 37. Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, Demmler‐Harrison G, Heslop HE, Rooney CM, Gottschalk S, et al. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein‐Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011; 13: 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sili U, Leen AM, Vera JF, Gee AP, Huls H, Heslop HE, Bollard CM, Rooney CM. Production of good manufacturing practice‐grade cytotoxic T lymphocytes specific for Epstein‐Barr virus, cytomegalovirus and adenovirus to prevent or treat viral infections post‐allogeneic hematopoietic stem cell transplant. Cytotherapy. 2012; 14: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gerdemann U, Katari U, Christin AS, Cruz CR, Tripic T, Rousseau A, Gottschalk SM, Savoldo B, Vera JF, Heslop HE, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor‐associated antigens to treat EBV negative lymphoma. Mol Ther. 2011; 19(12): 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harting MT, Baumgartner JE, Worth LL, Ewing‐Cobbs L, Gee AP, Day MC, Cox CS Jr. Cell therapies for traumatic brain injuries. Neurosurgical Focus. 2008; 24(3–4): E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cox CS Jr., Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, Ewing‐Cobbs L, Hasan KM, Day MC, Lee D, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011; 68: 588–600. [DOI] [PubMed] [Google Scholar]
- 42. Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Piller LB, Penn MS, Byrne BJ, et al. Rationale and design for TIME: A phase II, randomized, double‐blind, placebo‐controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009; 158: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Simpson LM, Penn MS, Byrne BJ, et al. Late TIME: a phase‐II, randomized, double‐blinded, placebo‐controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010; 37: 412–420. [PMC free article] [PubMed] [Google Scholar]
- 44. Blazar BR, Taylor PA. Regulatory T cells. Biol Blood Marrow Transplant. 2005; 11(2 Suppl 2): 46–49. [DOI] [PubMed] [Google Scholar]
- 45. Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro‐expanded human CD4(+)CD25(+) T‐regulatory cells can markedly inhibit allogeneic dendritic cell‐stimulated MLR cultures. Blood. 2004; 104: 453–461. [DOI] [PubMed] [Google Scholar]
- 46. Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)‐derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005; 105: 750–758. [DOI] [PubMed] [Google Scholar]
- 47. Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, et al. Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Sci Transl Med. 2011; 3(83): 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen K, McKenna DH, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011 Jan 20; 117(3): 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease‐a review and rationale for new therapeutic approaches. AIDS Rev. 2005; 7: 168–180. [PubMed] [Google Scholar]
- 50. Whiteside TL, Piazza P, Reiter A, Stanson J, Connolly N, Rinaldo CR Jr., Riddler SA. Production of DC‐based vaccine containing inactivated autologous virus for therapy of patients with chronic HIV‐1 infection. Clin Vaccine Immunol. 2009; 16(2): 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koepsell SA, Kadidlo DM, Fautsch S, McCullough J, Klingemann H, Wagner JE, Miller JS, McKenna DH Jr. Successful “in‐flight” activation of natural killer cells during long‐distance shipping. Transfusion. 2013; 53(2): 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo DM, Klein AK, Sprague KA, Miller KB, Comenzo RL, Kewalramani T, et al. Autologous stem cell transplant recipients tolerate haploidentical related‐donor natural killer cell‐enriched infusions. Transfusion. 2013; 53(2): 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]


