Abstract
Bullous pemphigoid (BP) and psoriasis vulgaris represent two clinically well characterized, inflammatory, chronic skin diseases. A 62 years old female patient, from rural areas, was admitted for the presence of erythematous plaques covered by large tense blisters with clear fluid, located symmetrically on the anterior part of the upper limbs, the trunk, the neck and the lower limbs. Also the lesions were intense itching. Lesions occurred three days before presentation at the clinic. Medical history revealed psoriasis diagnosed 28 years ago, breast cancer treated with surgery, radio and chemotherapy three years ago and Parkinson's disease diagnosed 3 weeks prior to presentation to the dermatology clinic. Histopathology examination revealed: atrophic epidermis with subepidermal presence of a blister containing numerous eosinophils and neutrophils. In the papillary dermis neutrophils and eosinophils predominantly vascular. Bullous pemphigoid has multiple etiology. Bullous pemphigoid is an autoimmune subepidermal bullous dermatosis which may be associated with psoriasis. Medical literature and cases reported in dermatology journals claim that bullous pemphigoid is often associated with psoriasis, though the immunogenetical and immunopathologycal mecanismes are still not known. Our patient has three different diseases but their etiology and pathogenesis can interfere.
Keywords: bullous pemphigoid, Parkinson’s disease, psoriasis
Introduction
Bullous pemphigoid is an immunobullous subepidermal dermatosis characterized by large, tense blisters on a erythematous skin. These usually occur on the flexural site of limbs and trunk. Blisters heal without scarring. Mucosal involvement is rare.
Among potential triggers, there are drugs (NSAIDs, ACE inhibitors, furosemide, antibiotics), UV radiation and X-rays. Usually occurs in patients older than 60 years, in children the condition can present after vaccination with distribution of lesions on the face, palms and plants. Can be found in children too (about 80 case reports), the youngest age being under 10 weeks.
The disease is characterized by the presence of Ig G autoantibodies against hemidesmosome’s molocules BPAG1 230kDa (intracellular) and BPAG2 180kDa (transmembrane).
Autoantibodies bind to antigens, leading to the activation of the complement, which together with inflammatory cells (mast cells, eosinophils, neutrophils) and proteolytic enzymes (neutrophilic elastase, gelatinases B/MMP-9) leads to cleavage in the lamina lucida and tense bullae formation.
Psoriasis is a chronic T cell mediated inflammatory disease affecting the skin, that can associated cardiovascular and other metabolic syndromes and also many cutaneous disorders [1-3 ].
Clinical case
We present the case of a 62 years old female patient, from rural areas, which was admitted for the appearance of erythematous plaques covered with large, tense blisters with clear fluid, located symmetrically on the anterior site of the upper limbs, the trunk, the cervical region and the lower limbs. Severe itching was present at the site of the lesions. The patient had an early menopause (at 30 years old) and from her past medical history we discovered psoriasis diagnosed in 1984, breast cancer treated with surgery, radio and chemotherapy in 2009, Parkinson's disease diagnosed in March 2012. The patient was following chronic treatment with 35 mg Preductal bd and Tanakan 40mg tds.
With 3 weeks before presentation at the dermatology clinic, was initiated the therapy with 6 mg Ropinirole od for Parkinson's disease.
The onset of the disease was three days before presentation to the clinic and it started with erythematous plaques, intensely pruritic, localized on the neck, then the plaques were covered by large, tense blisters with clear fluid and extended to the regions described above.
Clinical examination revealed average health condition and BMI=20.
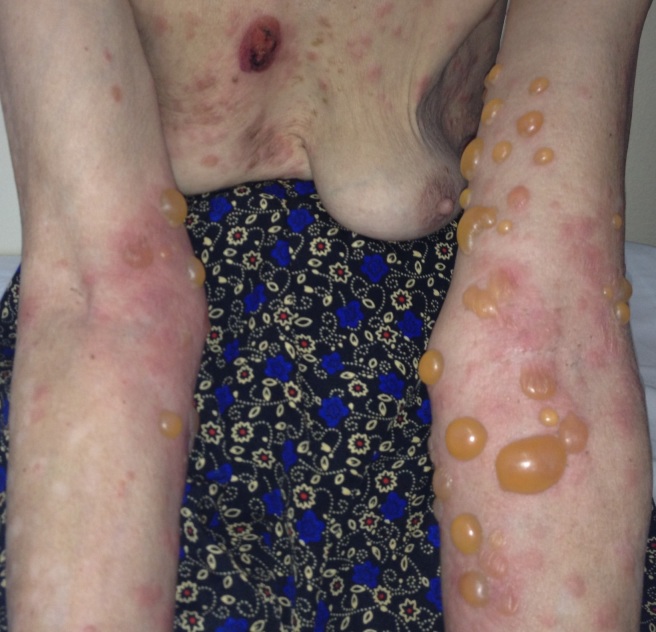
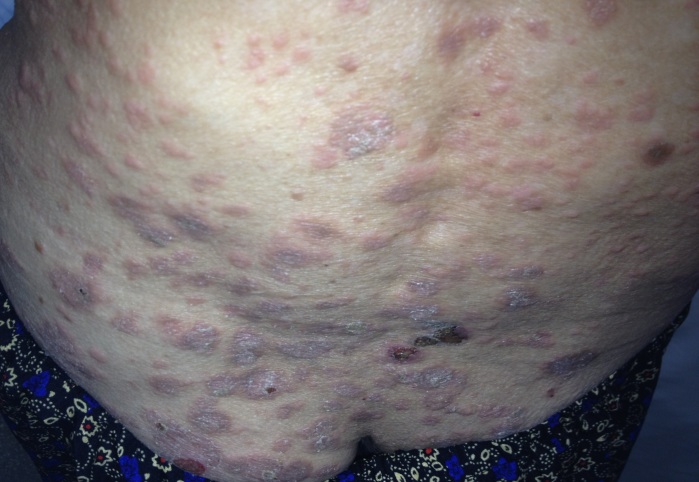
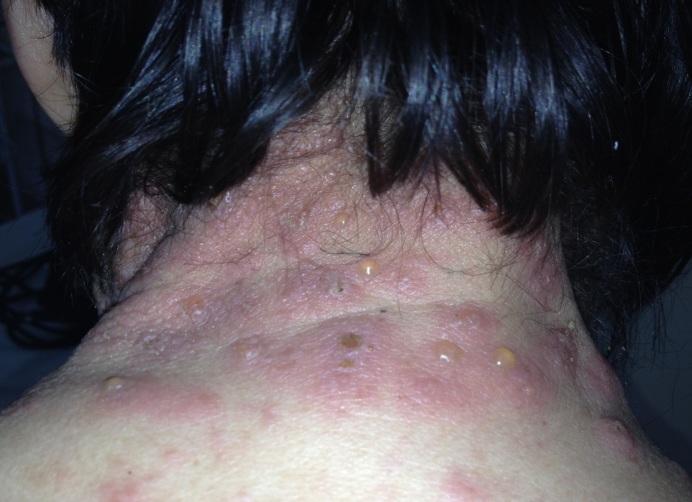
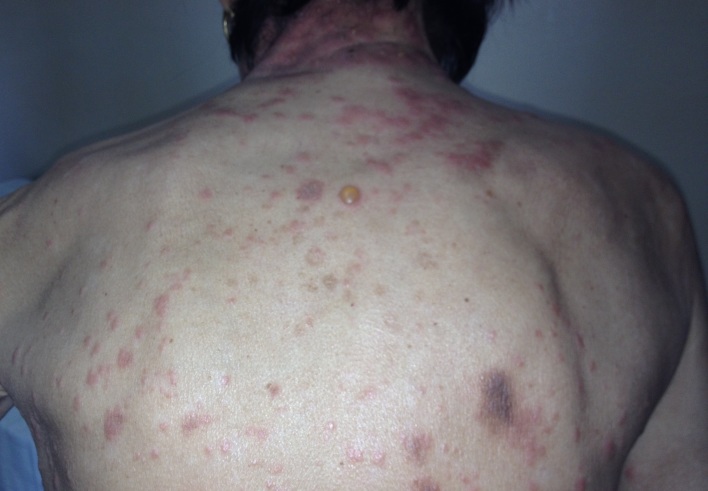
Dermatological examination revealed erythematous plaques covered with large, tense blisters with clear fluid, located symmetrically on the flexural site of the upper limbs, the trunk, the cervical region and the lower limbs, an erosion of 2-3cm diameter on the anterior thorax covered by hematic crust, erythematous plaques with clearly defined edges, covered with pearly white scales, located in the sacral region; hypochromic plates located on the posterior sites of the upper limbs and the lower limbs, atrophic skin covered by fine scales on the lower limbs (Fig.1-4).
Fig.1.
Multiple tense bullae-clinical aspect of bullous pemphigoid
Fig.4.
Erythematous scaly plaques-clinical aspect of psoriasis vulgaris
Fig.2.
Erythematous plaques covered with tense blisters with clear fluid-clinical aspect of bullous pemphigoid
Fig.3.
Erythematous plaques covered with tense blisters with clear fluid-clinical aspect of bullous pemphigoid
We did the biopsy of an early blister and conducted Tzanck’s cytodiagnosis and histopathological examination.
Tzanck’s cytodiagnosis showed numerous polymorphonuclear leukocytes, both neutrophils and eosinophils (up to 60%), rare lymphocytes and plasma cells, rare red blood cells and cellular debris. The presence of inflammatory infiltrate.
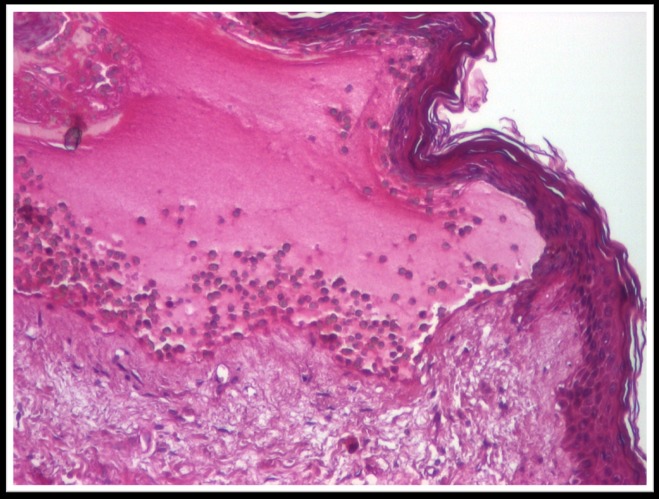
Histopathological examination revealed an atrophic epidermis with sub-epidermal blister containing numerous eosinophils and neutrophils. The papillary dermis infiltrated with neutrophils and eosinophils predominantly perivascular (Fig.5-7).
Fig.5.
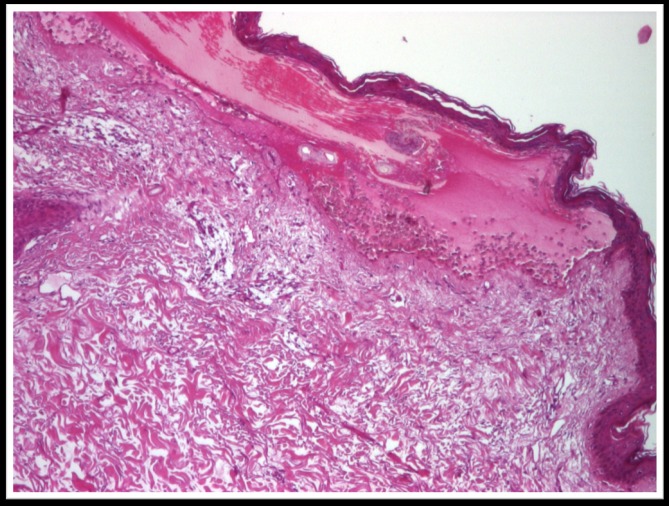
Atrophic epidermis with sub-epidermal blister containing numerous eosinophils and neutrophils, col. HE x 40
Fig.7.
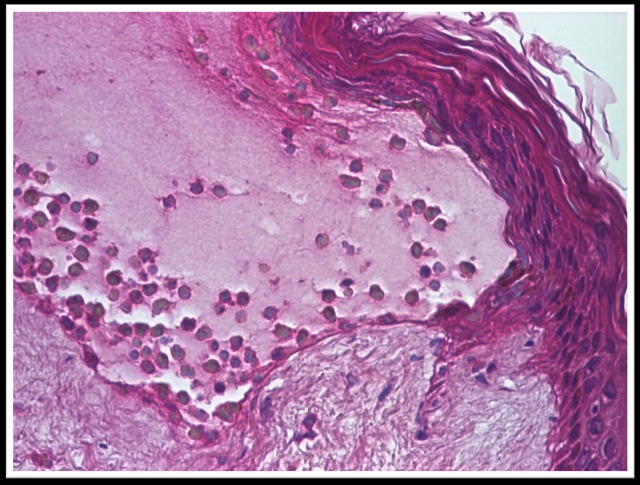
Atrophic epidermis with sub-epidermal blister containing numerous eosinophils and neutrophils, col. HE x 200
Fig.6.
Atrophic epidermis with sub-epidermal blister containing numerous eosinophils and neutrophils, col. HE x 100
Histopathological examination suggests the diagnosis of bullous pemphigoid.
Biological explorations were in normal range.
Treatment was initiated with iv corticosteroids then orally, antihistamines and PPI’s. The blisters were deflated with a sterile needle and topical steroids were applied.
The evolution was favorable, with no complications of the therapy or the disease.
Discussion
In 1953 Walter Lever classified bullous pemphigoid as a disease with different clinical and histological features than pemphigus vulgaris. A decade later Jordon and Beutner proved that patients with bullous pemphigoid have autoantibodies against structures in the dermo-epidermal junction.
Bullous pemphigoid is a disease that usually affects elderly patients over 60 years. The annual incidence is estimated to be 6-7 new cases/million of inhabitants. So far there is no evidence for a geographical predisposition to disease.
From the pathogenesis point of view is characterized by the presence of IgG autoantibodies against hemidesmosomes. BPAG1 antigens (BP 230) of 230kDa (intracellular) and BPAG2 (BP 180) of 180kDa (transmembrane) are located at the hemidesmosomes level.
Autoantibodies bind the antigen, leading to activation of complement. Complement, inflammatory cells (mast cells, eosinophils, neutrophils) and proteolytic enzymes (neutrophilic elastase, gelatinases B/MMP-9) lead to cleavage at lamina lucida causing the formation of the blisters.
The association of bullous pemphigoid- psoriasis was found by Bloom in 1929 [4]. Blisters were found on psoriasis plaques. It has been speculated that the chronic inflammatory response in the dermo-epidermal junction lead to exposure of antigens to autoreactive T lymphocytes, resulting in a secondary immune response, but immunogenetic and immunopathological mechanisms are incompletely understood [5,6]. Medical literature and cases reported in dermatology journals claim that bullous pemphigoid is often associated with psoriasis, though the immunogenetical and immunopathologycal mecanismes are still not known. Studies showed that the therapy for psoriasis (UV-B, UV-A) can be trigger for bullous pemphigoid [7,8,9].
The triggering factor in this association remains unknown. It is possible to participate the pathological events at the basement membrane zone in psoriasis itself or common immunological or immunogenetic mechanisms [10,11]. Psoriasis preceded in most cases the development of bullous disease, with an average time interval of 20 years [9]
Regarding bullous pemphigoid and related diseases, there is increasing evidence showing its association with malignancy (gastric cancer, lung cancer) probably due to age of onset of the disease [12,13].
Another association is that of bullous pemphigoid with autoimmune diseases (rheumatoid arthritis, Hashimoto thyroiditis, dermatomyositis, autoimmune thrombocyto-penia) reflecting genetic susceptibility to autoimmune diseases.
There have been cases in which the disease was induced by trauma, burns, radiotherapy and ultraviolet radiation, vaccines [14]. Also, bullous pemphigoid was associated with the consumption of drugs. The most commonly involved were NSAIDs, diuretics, neuroleptics, antibiotics, ACE inhibitors, cyclosporine, azathioprine [11,15,16].
Another trigger involved is the neurological diseases. Case reports of bullous pemphigoid at patients with Parkinson's disease, epilepsy, multiple sclerosis were described.
From the clinical aspect point of view, the disease is characterized by the appearance of blisters on a erythematous, pruritic, or normal skin. Blisters are in tension, usually with clear fluid, sometimes with hemorrhagic fluid, with evolution to erosion and crusting. Typically they are found on the flexion site of the limbs, trunk. Involvement of mucous membranes is rare.
The diagnosis is supported by the clinical appearance, Tzanck’s cyotdiagnosis (no acantholysis, the presence of eosinophils), histopathology (subepidermal blisters, eosinophils in the papillary dermis, no acantholysis). Direct immunofluorescence, in almost all patients, will show linear deposits of IgG and/or C3 at the basement membrane. IgG1 and IgG4 are the predominant IgG classes. Indirect immunofluorescence shows auto-antibodies against BPAG1 and BPAG2. Electron microscopy shows that IgG antibodies are predominantly located on the upper site of lamina lucida beneath the hemidesmosomes. Immunoprecipitation and immunoblotting show that the antigens are BPAG1 and BPAG2.
Laboratory exams will show the presence of inflammatory syndrome, peripheral blood eosinophilia and sometimes disorders of electrolytes.
As a therapeutic attitude, the patient is admitted for diagnosis and treatment. Systemic corticosteroid is the basic therapy for generalized forms. Doses are between 40-80mg/day.
Good results were obtained with low-dose prednisone, from 0.6mg/kg/zi in mild forms of the disease, to 1mg/kg/zi for severe forms. For localized forms, potent topical steroids.
Potent topical steroid-clobetasol propionate-for extended forms of disease, will lower the doses for the systemic steroids. Azathioprine 100-150 mg/day and oral cyclophosphamide 2-2.5mg/kg/day were also used.
The prognosis in bullous pemphigoid is significantly affected by age and Karnofsky score<40 (range 0-100). Associated disorders (myocardial infarction, diabetes mellitus) antibodies against BPAG2 and peripheral blood eosinophilia influence morbidity and mortality at patients with bullous pemphigoid.
For our case the diagnosis was made based on dermatological examination, Tzanck’s cytodiagnosis and histopathological examination. The evolution under therapy with oral corticosteroids was positive and there were no complications.
Conclusions
Our patient has three different diseases but their etiology and pathogenesis can interfere. Common immunological or immunogenetic mechanisms may play an important role in this association.
References
- 1.De Felice C, Chimenti S. Overview of Psoriasis. In: Chimenti S, editor. Psoriasis. Florence: Ed.SEE- Firenze; 2005. pp. 19–21. [Google Scholar]
- 2.Christophers E, Mrowietz U. Psoriasis. In: Burgdorf WH, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco's Dermatology. 3. Heidelberg: Springer; 2009. pp. 506–518. [Google Scholar]
- 3.Saraceno R, Chimenti S. Associated conditions of psoriasis. In: Chimenti S, editor. Psoriasis. Florence: Ed.SEE- Firenze; 2005. pp. 105–111. [Google Scholar]
- 4.Bloom D. Psoriasis with superimposed bullous eruption. Med J Rec. 1929;130:246–undefined. [Google Scholar]
- 5.Burnett PE. Bullous pemphigoid and psoriasis vulgaris. Dermatology Online Journal. 2003 Oct;9(4):19–undefined. [PubMed] [Google Scholar]
- 6.Al-Otaibi A, Al-Abdulrazzaq A, Najem N. Coexistence of generalized pustular psoriasis and bullous pemphigoid: Pathogenic relationship or coincidence? The Gulf Journal of Dermatology and Venereology. 2010;17(2):47–49. [Google Scholar]
- 7.Barnadas MA, Gilaberte M, Pujol R, Agustí M, Gelpí C, Alomar A. Bullous pemphigoid in a patient with psoriasis during the course of PUVA therapy: Study by ELISA test. Int J Dermatol. 2006;45:1089–1092. doi: 10.1111/j.1365-4632.2004.02517.x. [DOI] [PubMed] [Google Scholar]
- 8.Jankowski M, Czajkowski R, Scibior K, Schwartz RA. Coexistence of psoriasis vulgaris and vitiligo with bullous pemphigoid: a case report. Nov 21;2013 Int J Dermatol. doi: 10.1111/ijd.12074. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Wilczek A, Sticherling M. Concomitant psoriasis and bullous pemphigoid: coincidence or pathogenic relationship? Int J Dermatol. 2006 Nov;45(11):1353–1357. doi: 10.1111/j.1365-4632.2006.02861.x. [DOI] [PubMed] [Google Scholar]
- 10.Rao R, Gupta A, Yunis F, Handettu S, Chandrashekar B. Coexistence of psoriasis with bullous pemphigoid. Indian Dermatol Online J. 2012 May;3(2):119–121. doi: 10.4103/2229-5178.96707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirtschig G, Chow ET, Venning VA, Wojnarowska FT. Acquired subepidermal bullous diseases associated with psoriasis: A clinical, immunopathological and immunogenetic study. Br J Dermatol. 1996;135:738–745. [PubMed] [Google Scholar]
- 12.Ogawa H, Sakuma M, Marioka S, Kitamura K, Sasai Y, Imamura S, Inaba Y. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Derm Science. 1995 May;9(2):136–141. doi: 10.1016/0923-1811(94)00371-k. [DOI] [PubMed] [Google Scholar]
- 13.Lindelof B, Islam N, Eklund G, Arfors L. Pemphigoid and cancer. Arch Dermatol. 1990 Jan;126(1):66–68. doi: 10.1001/archderm.1990.01670250072011. [DOI] [PubMed] [Google Scholar]
- 14.Mul VE, van Geest AJ, Pijls-Johannesma MC, Theys J, Verschueren TA, Jager JJ, Lambin P, Baumert BG. Radiation-induced bullous pemphigoid: A systematic review of an unusual radiation side effect. Radiotherapy & Oncology. 2007;82(1):5–9. doi: 10.1016/j.radonc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, Salagnac V, Lok C, Roujeau JC. Drugs Associated With Bullous Pemphigoid: A Case-Control Study. Arch Dermatol. 1996 Jan;132(3):272–276. [PubMed] [Google Scholar]
- 16.Perry A, Sparling JD, Pennington M. Bullous pemphigoid following therapy with an oral beta-blocker. Journal of Drugs in Dermatology. 2005 Nov-Dec;4(6):746–748. [PubMed] [Google Scholar]