Abstract
The antibiotic peptide nisin is the first known lantibiotic that uses a docking molecule within the bacterial cytoplasmic membrane for pore formation. Through specific interaction with the cell wall precursor lipid II, nisin forms defined pores which are stable for seconds and have pore diameters of 2 to 2.5 nm.
Nisin, an amphiphilic antibiotic peptide, is produced by a number of strains of Lactococcus lactis subsp. lactis. It has antibacterial activity against various gram-positive bacteria and is widely used as a food preservative (10, 15). Nisin belongs to the lantibiotics, which are ribosome synthesized, posttranslationally modified peptides characterized by intramolecular rings formed by the rare thioether amino acids lanthionine and 3-methyllanthionine (9, 12). Nisin is the most prominent member of the type A lantibiotics, which are elongated, amphiphilic, screw-shaped peptides with a net positive charge (11). The first report on the mode of action of nisin dates back to 1960, when Ramseier observed leakage of UV-absorbing intracellular compounds from treated cells, suggesting a detergent effect (16). Subsequent experiments showed that lantibiotics induce the rapid efflux of ions or cytoplasmic solutes such as amino acids and nucleotides. The concomitant depolarization of the cytoplasmic membrane resulted in a rapid termination of all biosynthetic processes (18, 20). These results led to the conclusion that the primary mode of action of nisin is the formation of channels in the cytoplasmic membrane. Although the pore formation process of nisin in artificial membranes was intensively studied and several models, such as the wedge model, were established (8), it was not possible to explain some special features of nisin activity in vivo. For example, nisin is active against some bacteria even in the nanomolar range, whereas in artificial membranes, pore formation required micromolar concentrations (4).
The first hints for the identification of a target molecule for nisin activity arose from observations by Linnett and Strominger (14); in an in vitro system with isolated membranes, nisin was shown to interfere with cell wall biosynthesis, which was later found to be based on interaction with the membrane-bound cell wall precursor lipid II [undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide)-GlcNAc] (17).
The cell wall precursor lipid II was purified, and it was demonstrated with intact cells and lipid II-doped multilamellar liposomes (7) that nisin uses lipid II as a docking molecule for pore formation. The specificity of the nisin-lipid II interaction resulting in high-level activity of nisin was further demonstrated in a comparative study with the pore-forming amphiphilic defense peptide magainin 2. In contrast to magainin, the activity of nisin was enhanced by a factor of 103 when lipid II was available for targeted pore formation (5).
To obtain information on the electrochemistry of the pore formation process in the presence of lipid II, we performed black lipid membrane bilayer experiments. The basic procedure for the formation of black lipid bilayer membranes was described previously (1). We painted a 1% solution of diphtanoyl-phosphatidylcholine (DiphPC; Avanti Polar Lipids) in n-decane across a circular hole connecting two aqueous compartments of a Teflon chamber. The membrane area was 1 mm2. The aqueous phase consisted of unbuffered 1 M KCl solutions, and the temperature was kept at 25°C. Membrane currents were measured with a pair of calomel electrodes switched in series with a voltage source and an electrometer (Keithley 602). The amplified signal was monitored with a storage oscilloscope (Tektronix 5115) and recorded by a strip chart recorder. Nisin was purchased from Koch & Light (Colmbrock, England). The orientation of the voltage was defined with respect to the addition of nisin (on the cis side); a trans-negative potential means that the negative side was opposite the side to which nisin was added.
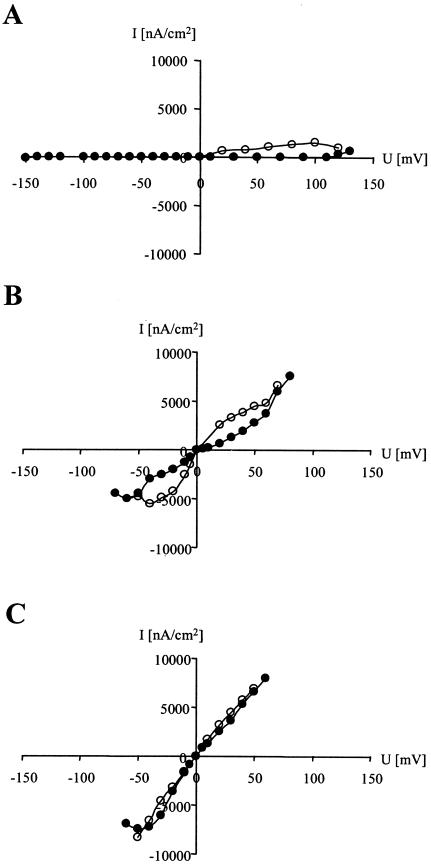
To obtain conductance within a reasonable time frame, a nisin concentration of 0.1 μM was routinely used. Nisin was added to the cis side, and the voltage was stepwise increased and decreased to zero (Fig. 1A); subsequently, the potential was increased with the opposite orientation and taken back to zero. The current was measured approximately 10 s after the application of the voltage. When the cis side was positive by about 110 mV, the membrane conductance started to increase, and when the voltage was taken back to zero, a strong hysteresis effect was observed. After the application of a trans-positive membrane potential (indicated by a minus sign), the membrane remained stable; however, membrane conductance was not observed, showing that the insertion of nisin into the membrane is an energy-dependent process that occurs only in the presence of a high trans-negative membrane potential (above 100 mV).
FIG. 1.
Current-voltage relationship of nisin-induced membrane conductance. Nisin was added at a concentration of 0.1 μM to the cis-side of a membrane made of DiphPC in the absence (A) and in the presence (B) of 1 mol% lipid II. The voltage was increased stepwise (filled circles) and then taken back to zero (open circles). Once nisin was inserted into the membrane, the pores remained stable for hours and a linear current-voltage relationship without significant hysteresis was observed (C).
Purified lipid II was incorporated into a membrane of DiphPC at a concentration of 1 mol% with respect to total phospholipids. Lipid II was synthesized in vitro by using membrane preparations of Micrococcus luteus ATCC 4698. For extraction and purification, the protocol elaborated by Brötz for [14C]lipid II was applied (6). The concentration of lipid II stock solutions in chloroform-methanol (1:1, vol/vol) was determined by quantitative amino acid and amino sugar analysis by using the o-phthaldialdehyde derivatization technique (19).
When lipid II was present, membrane conductance was observed even at 5 to 10 mV (Fig. 1B). Remarkably, in contrast to the experiment without lipid II (Fig. 1A), nisin pore formation occurred after the application of both a trans-negative and a trans-positive membrane potential. Obviously, in the presence of the cell wall precursor, the energy needed to transfer the nisin molecules to a conducting state was strongly reduced. After nisin had been inserted into the membrane (Fig. 1B), the pores remained stable for hours. During this period, a linear current-voltage relationship was observed and the hysteresis was strongly reduced when the voltage was increased and decreased again (Fig. 1C). Thus, lipid II also seems to stabilize pores in such a way that complexes do not dissociate when the voltage is absent.
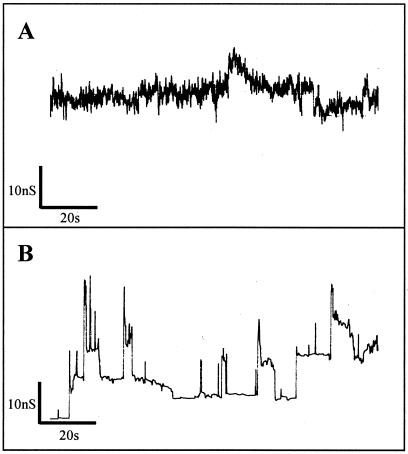
For further characterization of the nisin-lipid II pore complex, we performed single-channel experiments. In the absence of lipid II, a constant voltage of 100 mV had to be applied (Fig. 1A). The strong current noise observed indicates a high instability of the nisin-induced membrane conductance (Fig. 2A). In contrast, when the DiphPC membrane was doped with 1 mol% lipid II and nisin was added at a concentration of 0.1 μM, 5 mV was sufficient to produce regular patterns of membrane conductance (Fig. 2B). The most prominent conductance levels were used for the calculation (20) of an average pore diameter of 2 to 2.5 nm and an average pore lifetime of approximately 6 s (50 stable channels from two independent experiments were evaluated). In addition, particularly on pore openings, short-lived higher conductance levels that may represent unstable larger pores were observed.
FIG. 2.
(A) Single-channel conductance of a DiphPC membrane with 0.1 μM nisin added at the cis side of the membrane. The membrane potential was kept constant at 100 mV. The strong current noise indicates a high instability of the nisin-induced channels. (B) Single-channel conductance of a DiphPC membrane supplemented with 1 mol% lipid II in the presence of 0.1 μM nisin. The membrane potential applied was 5 mV. The average pore diameters calculated were 2 to 2.5 nm, and the pore lifetime was about 6 s.
When the results of the present study were compared to previously published details on nisin pores in the absence of lipid II (i.e., a threshold potential of −100 mV [as confirmed here], a pore size of 1 nm, and pore lifetime in the millisecond range [2, 13]), it was obvious that lipid II both facilitated pore formation and influenced pore architecture. Nisin is presumed to form a stoichiometric 1:1 complex with lipid II (21), and it has been suggested that lipid II actually could be an integral part of the pore (3). A pore diameter of 2 nm, as observed here, requires that several such complexes be associated to form a functional pore. The number of nisin-lipid II complexes participating in the stable pore appeared to be constant; intermediate conductance levels or regular conductance fluctuations, which could indicate variations in pore size, could not be resolved in the single-channel recording. The short-lived conductance spikes might represent unstable pore aggregates that rapidly dissociate into more stable pores of the dimensions calculated above. Further experimentation will be necessary to understand the dynamics and architecture of this unique pore.
Acknowledgments
We gratefully thank the Deutsche Forschungsgemeinschaft (grant no. Sa 292/9-1) and the BONFOR program for financial support.
We thank Elke Maier for technical assistance.
REFERENCES
- 1.Benz, R., K. Janko, W. Boos, and P. Lauger. 1978. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 511:305-319. [DOI] [PubMed] [Google Scholar]
- 2.Benz, R., G. Jung, and H.-G. Sahl. 1991. Mechanism of channel-formation by lantibiotics in black lipid membranes, p. 359-372. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. Escom, Leiden, The Netherlands.
- 3.Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. [DOI] [PubMed] [Google Scholar]
- 4.Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. [DOI] [PubMed] [Google Scholar]
- 5.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H.-G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 6.Brötz, H., G. Bierbaum, K. Leopold, P. E. Reynolds, and H.-G. Sahl. 1998. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brötz, H., M. Josten, I. Wiedemann, U. Schneider, F. Götz, G. Bierbaum, and H.-G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 8.Driessen, A. J. M., H. W. van den Hooven, W. Kuiper, M. van de Kamp, H.-G. Sahl, R. N. H. Konings, and W. N. Konings. 1995. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry 34:1606-1614. [DOI] [PubMed] [Google Scholar]
- 9.Gross, E., and J. L. Morell. 1971. The structure of nisin. J. Am. Chem. Soc. 93:4634-4635. [DOI] [PubMed] [Google Scholar]
- 10.Hurst, A. 1981. Nisin. Adv. Appl. Microbiol. 27:85-123. [Google Scholar]
- 11.Jung, G. 1991. Lantibiotics—ribosomally synthesized biologically active polypeptides containing sulfide bridges and α,β-didehydroamino acids. Angew. Chem. Int. Ed. Engl. 30:1051-1068. [Google Scholar]
- 12.Jung, G., and H.-G. Sahl. 1991. Lantibiotics: a survey, p. 1-34. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. Escom, Leiden, The Netherlands.
- 13.Kordel, M., and H.-G. Sahl. 1986. Susceptibility of bacterial, eukaryotic and artificial membranes to the disruptive action of the cationic peptides Pep5 and nisin. FEMS Microbiol. Lett. 34:139-144. [Google Scholar]
- 14.Linnett, P. E., and J. L. Strominger. 1973. Additional antibiotic inhibitors of peptidoglycan synthesis. Antimicrob. Agents Chemother. 4:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattick, A. T., and A. Hirsch. 1944. A powerful inhibitory substance produced by group N streptococci. Nature 154:551. [Google Scholar]
- 16.Ramseier, H. R. 1960. Die Wirkung von Nisin auf Clostridium butyricum. Arch. Mikrobiol. 37:57-94. [DOI] [PubMed] [Google Scholar]
- 17.Reisinger, P., H. Seidel, H. Tschesche, and P. Hammes. 1980. The effect of nisin on murein synthesis. Arch. Microbiol. 127:187-193. [DOI] [PubMed] [Google Scholar]
- 18.Ruhr, E., and H.-G. Sahl. 1985. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob. Agents Chemother. 27:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahl, H.-G., M. Grossgarten, W. R. Widger, W. A. Cramer, and H. Brandis. 1985. Structural similarities of the staphylococcin-like peptide Pep5 to the peptide antibiotic nisin. Antimicrob. Agents Chemother. 27:836-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahl, H.-G., M. Kordel, and R. Benz. 1987. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch. Microbiol. 149:120-124. [DOI] [PubMed] [Google Scholar]
- 21.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. de Kruijff, and H. G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]