Abstract
Haemochromatosis is due to excessive accumulation of iron in tissues and organs impairing their function. The most common haematologic disorders that are subject to an intensive transfusion regimen bringing excess iron in the body are: thalassemia and myelodysplastic syndrome. The value of serum ferritin in these patients (indicator of iron stores condition) reaches high values. Red cell substitution bringing additional iron intake must be accompanied by administration of chelation therapy in order to prevent haemochromatosis and related complications. We present the case of a patient with thalassemia intermedia, integumentary secondary haemochromatosis, cirrhosis with haemochromatosis, and secondary diabetes, who died at the age of 33 years because of upper gastrointestinal bleeding due to the rupture of oesophageal varices.
Keywords: thalassemia intermedia, haemochromatosis, cirrhosis, diabetes mellitus
Introduction
Haemochromatosis is the abnormal accumulation of iron in parenchymal organs, leading to organ toxicity. Secondary or acquired haemochromatosis can be caused by diseases such as thalassemia or myelodisplastic syndrome, especially if patients have received a large number of blood transfusions, rarely in hepatitis C, alcoholism, chronic liver disease, some types of anaemia, and other illnesses.
Patient, Methods and Results
Patient B.C. was diagnosed with thalassemia intermedia at the age of 3 years, by performing haemoglobin electrophoresis which showed a decreased A1 haemoglobin level, and an increase of A2 and foetal haemoglobin level of 45%. At the age of 8 years the patient was splenectomised due to giant splenomegaly, with hypersplenism and compression phenomena.
He was given a replacement and chelation iron therapy with intermittent Desferal at the Paediatric Clinic up to the age of 18, when he was guided towards the Clinic of Haematology.
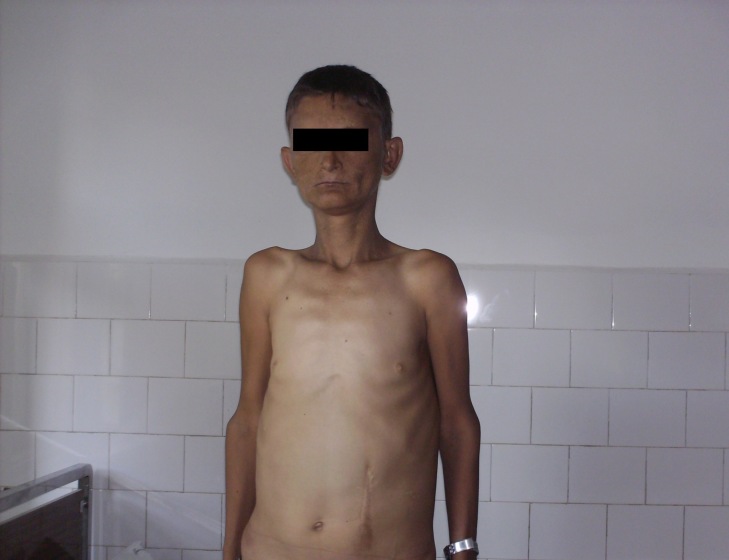
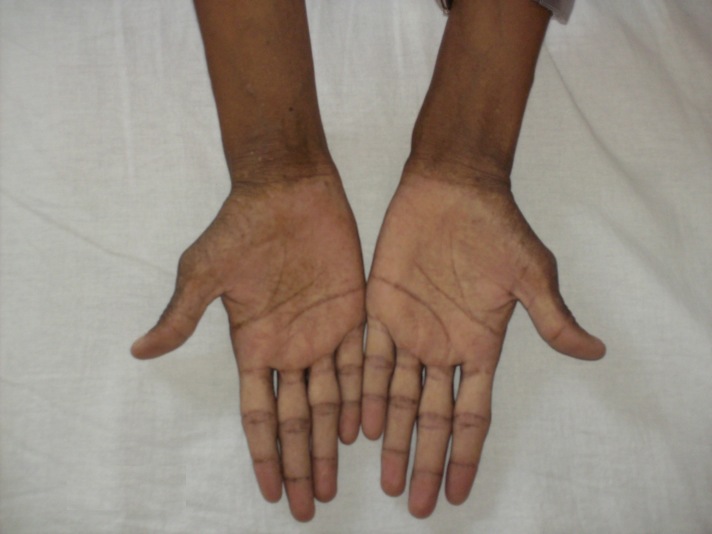
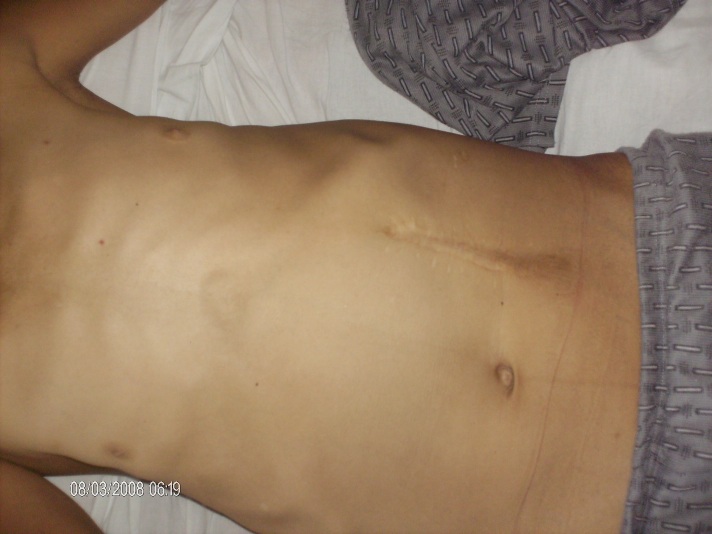
Objective examination: longilin asthenic constitution type (Fig. 1), skin hyper-pigmentation, ogival palate, dental implant defects, simian hand (Fig. 2), weakness of scapular girdle muscles, irregular heart sounds, tachycardia (a.v. 100 beats/min), unorganized extrasystoles, enlargement of abdomen with postoperative scar, caused by giant hepatomegaly occupying the entire abdomen, increased onsistency(Fig. 4).
Fig.1.
Longilin asthenic constitution type, weakness of scapular girdle muscles
Fig.2.
Simian hand appearance, with hyperpigmentation of the palmar creases
Fig.3.
Abdomen enlargement caused by giant hepatomegaly occupying the entire abdomen. Postoperative scar (splenectomy)
Paraclinical testing: ESR 86mm/1h, Hb 5.2g/dl, L 18 200/mm3, T 168 000/mm3, Mtc 1%, NS 2%, S 43%, Ly 21%, Eo 2%, Mn 6% erythroblasts 25% marked poikilocytosis, erythrocytes that have the appearance of a ‘shooting target’, Jolly bodies.
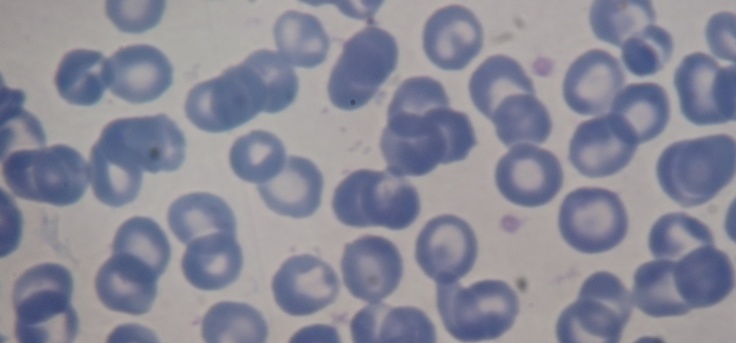
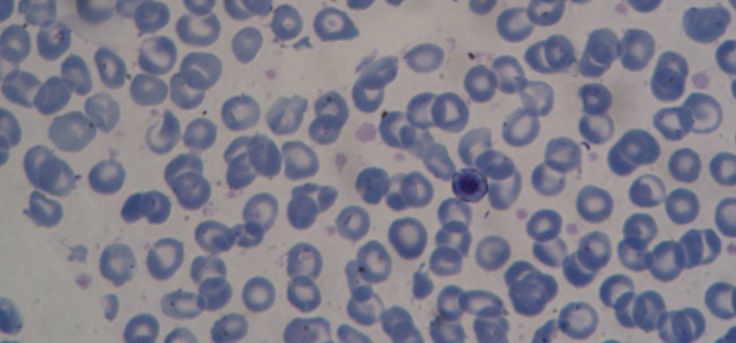
Peripheral blood smear (Fig.4,5)-microcytes, hypochromia, great erythrocyte disorder, marked poikilocytosis with erythrocytes that have the appearance of a ‘shooting target’, presence of erythroblasts on smear (a sign of the presence of extramedullary haematopoiesis outbreaks) GPT 145 UI/l, GOT 121 UI/L, FA 345 UI/L, serum protein electrophoresis PT 6.8g/dl, albumins 59%, alpha 1 4%, alpha 2 5.4%, beta 12%, gamma globulins19.6%, BT 3.2mg/dl, BI 2.1mg/dl, creatinine 0.67mg/dl, blood glucose 376mg/dl, serum iron 279mg/dl, serum ferritin 6 800mg/ml, blood glucose 338mg/dl-245mg/dl-112mg/dl treated with rapid-acting insulin, requiring higher doses to regulate blood glucose values.
Fig.4,5.
Marked poikiloctosis, with presence of erythrocytes that have the appearance of a ‘shooting target’, erythroblasts in peripheral blood (a sign of the presence of extramedullary haematopoiesis outbreaks), and appearance of great erythrocyte disorder
Fig.4.
Fig.5.
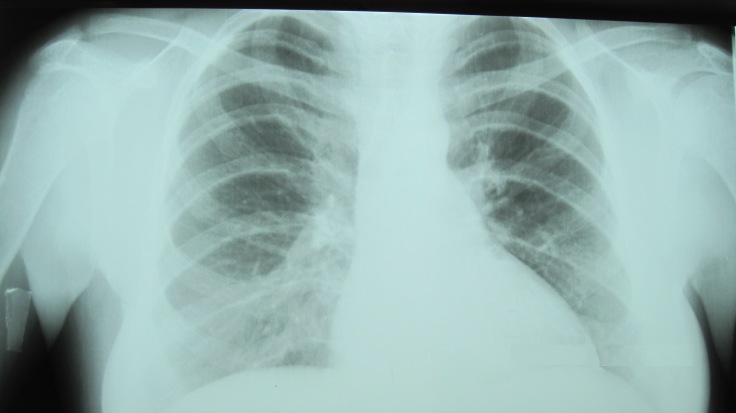
Rx heart lung PA (Fig.6) - slightly enlarged heart, there can be seen disseminated haemosiderin deposits on both lung areas.
Fig.6.
An increased interstitial drawing with the presence of nodules with a diameter of 2-3 mm, due to haemosiderin deposits
Echocardiogram - left ventricular ejection fraction (LVEF) 72%, without obvious heart disease. No NMR examination was performed.
Discussion
In some patients, noticeably those with thalassemia major, sickle cell disease, myelodysplastic syndrome, aplastic anaemia, hemolytic anaemia, and refractory sideroblastic anaemia, who may become transfusion-dependent and receive excess iron with each transfusion (that the body has no means to excrete), iron gradually accumulates in various tissues, causing morbidity and mortality. Each unit of transfused blood has approximately 250mg of iron. Haemosiderin is an abnormal, insoluble form of iron storage. The human body has no active mechanism for the excretion of iron [1]. Iron homeostasis thus relies on the amount that is absorbed from the small intestine [2]. The ferritin does not circulate in blood but is deposited in tissues and is unavailable when cells need iron. Major organs affected by this surplus iron include the heart, lung, liver, and endocrine glands.
When the plasma iron-binding protein transferrin is oversaturated, as in transfusion-induced iron overload, the excess iron circulates as relatively free non-transferrin-bound iron (NTBI). This NTBI is rapidly taken up by liver and other tissues. Transferrin-bound iron (TBI) is also taken up by these cells through the hepcidin mechanism, which is increased in such states [3]. It is this excessive iron that damages tissues.
The most common cause of morbidity is cardiomyopathy (30%) that is induced by iron overload [4]. Cardiac involvement in haemochromatosis typically results in congestive cardiomyopathy; a restrictive cardiomyopathy due to haemochromatosis is distinctly rare. The average time for the development of heart failure in transfused, unchelated patients is 10 years [5]. Iron chelation can reverse cardiac changes and improve performance [6]. In the presented patient there were not revealed any echocardiogram changes, LVEF value was preserved without rhythm or management disturbances.
Liver involvement is common in those who undergo long-term transfusions. Early cirrhotic changes can be observed as early as age 7 years in some people with thalassemia [7]. Iron overload has important clinical5 consequences in patients with thalasemia intermedia. Iron accumulation primarily occurs in hepatocytes can predispose to liver fibrosis and cirrhosis, and potentially hepato-cellular carcinoma [8,9,10]. The presented patient developed cirrhosis with haemochromatosis after 22 years of evolution of the disease, vascularly modified after another six years of evolution, with the presence of oesophageal varices at endoscopic examination, the hepatic portal vein echography 16mm. Patient death occurred in upper gastrointestinal bleeding due to the rupture of oesophageal varices, followed by irreversible hemorrhagic shock and cardiopulmonary arrest. If NMR is available, cardiac iron levels and cardiac function should also be measured by NMR yearly and every 6 months in patients who have intensive chelation therapy [11]. How iron overload contributes to vascular morbidity in thalassemia imtermedia is unclear. More frequent vascular disorders are atherosclerosis, microangiopathic haemolytic anemia, vasculitis [12]. T2-NMR value is inversely proportional to the degree of haemochromatosis. T2<20ms indicates a high iron load and is associated with low ejection fraction. By performing NMR there can be identified patients with high risk of deterioration of cardiac function before the onset of clinical picture and the intensifying of chelation therapy [13,14].
Pulmonary hypertension is another complication that occurs with a relatively high frequency in patients with thalassemia intermedia, and several factors were involved in its pathogenesis: chronic anemia and hypoxia, iron overload, splenectomy, hypercoagulability, microthrombotic disease [15,16,17]. More than one third of transfusion-dependent patients with β-thalassemia major exhibit a restrictive lung function defect, which may improve with chelation therapy [18].
Although heart-lung radiography revealed the presence of haemosiderin deposits, ventilatory function was not impaired, ventilatory tests being within normal limits.
There was also reported an association between elevated liver iron concentration and the occurrence of endocrinopathy, bone disease and vascular morbidity [18]. Up to 14% may develop insulin-dependent diabetes mellitus (IDDM) [19]. Our patient developed secondary diabetes at the age of 28 years, requiring therapy with high doses of insulin in order to correct blood glucose values.
Failure of puberty was the major clinical endocrine problem, and it was present in 51% of boys and 47% of girls, all older than 15 years. Secondary amenorrhea was recorded in 23% of the patients with β-thalassemia major [20].
All patients who are transfusion dependent require careful monitoring of their iron stores. It is advisable to measure ferritin levels at least every 3 months and iron studies every year. Liver iron levels should be measured annually (either by biopsy or noninvasively) and every 3-6 months in patients who undergo intensive chelation for heart failure.
Conclusions
The case presented in this paper is that of a patient with thalassemia intermedia, integumentary secondary haemochromatosis, cirrhosis with haemochromatosis, and secondary diabetes, who died at the age of 33 years because of upper gastrointestinal bleeding due to the rupture of oesophageal varices. Complications caused by secondary haemochromatosis appeared after the age of 20 years. Chelation therapy with Deferoxamine was intermittently used because it was not consistently available.
References
- 1.Anderson GJ. Mechanisms of iron loading and toxicity. Am J Hematol. 2007 Dec;82(12 suppl):1128–1131. doi: 10.1002/ajh.21075. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Vulpe CD. Regulation of intestinal iron transport. In: Templeton DM, editor. Molecular and Cellular Iron Transport. New York: Marcel Dekker Inc; 2002. pp. 559–596. [Google Scholar]
- 3.Kohgo Y, Ohtake T, Ikuta K, et al. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005 Nov;29(11 suppl):189S–193S. doi: 10.1097/01.alc.0000189274.00479.62. [DOI] [PubMed] [Google Scholar]
- 4.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007 Apr;82(4):255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe L, Olivieri N, Sallan D, et al. Prevention of cardiac disease by subcutaneous deferoxamine in patients with thalassemia major. N Engl J Med. 1985 Jun 20;312(25):1600–1603. doi: 10.1056/NEJM198506203122503. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Chang JS, Wu KH, Peng CT. Regression of myocardial dysfunction after switching from desferrioxamine to deferiprone therapy in beta-thalassemia major patients. Hemoglobin. 2006;30(2):229–238. doi: 10.1080/03630260600642559. [DOI] [PubMed] [Google Scholar]
- 7.Jean G, Terzoli S, Mauri R, Borghetti L, Di Palma A, Piga A, et al. Cirrhosis associated with multiple transfusions in thalassaemia. Arch Dis Child. 1984 Jan;59(1):67–70. doi: 10.1136/adc.59.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restivo Pantalone G, Renda D, Valenza F, et al. Hepatocelullar carcinoma in patients with thalassemia syndromes: clinical characteristics and outcome in a long term single centre experience. Br J Haematol. 2010;150:245–247. doi: 10.1111/j.1365-2141.2010.08180.x. [DOI] [PubMed] [Google Scholar]
- 9.Musallam KM, Cappellini MD, Wood JC, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with beta thalassemia intermedia. Hematologica. 2011;96:1605–1612. doi: 10.3324/haematol.2011.047852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancuso A. Hepatocelullar carcinoma in thalassemia: a critical review. World J Hepatol. 2010;2:171–174. doi: 10.4254/wjh.v2.i5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JC. Diagnosis and management of transfusion iron overload: the role of imaging. Am J Hematol. 2007 Dec;82(12 suppl):1132–1135. doi: 10.1002/ajh.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malyutin Z, Shai E, Dana M, et al. Shorter carotid artery occlusion in a thalassemic mouse model: a potential role foroxidative stress affecting both RBCs and platelets. 17th Congress of the European Hematology Association; June 14–17, 2012 ; Amsterdam, The Netherlands. [Google Scholar]
- 13.Aypar E, Alehan D, Hazirolan T, et al. The efficacy of tissue Doppler imaging in predicting myocardial iron load in patients with beta-thalassemia major: correlation with T2 cardiovascular magnetic resonance. Int.J.Cardiovasc Imaging. 2010;26(4):413–421. doi: 10.1007/s10554-010-9591-6. [DOI] [PubMed] [Google Scholar]
- 14.Deborah Chirnomas S., Geukes-Foppen M, Barry K, et al. Practical implication of liver and heart iron load assessment by T2-MRI in children and adults with transfusion-dependent anemias. Am J Hematol. 2008;83(10):781–783. doi: 10.1002/ajh.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokhtar GM, Adly AA, El Alfy MS, et al. N-terminal natriuretic peptide and ventilation-perfusion lung scan in sickle cell disease and thalassemia patients with pulmonary hypertension. Hemoglobin. 2010;34:78–94. doi: 10.3109/03630260903554621. [DOI] [PubMed] [Google Scholar]
- 16.Amoozgar H, FarhaniN , Khodadadi N, et al. Comparative study of pulmonary circulation and myocardial function in patients with beta thalassemia intermedia with and without hydroxyureea, a case control study. Eur J Haematol. 2011;87:61–67. doi: 10.1111/j.1600-0609.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 17.Karimi M, Musallam KM, Cappellini MD, et al. Risk factors for pulmonary hypertension in patients with beta thalassemia intermedia. Eur J Intern Med. 2011;22:607–610. doi: 10.1016/j.ejim.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Piatti G, Allegra L, Fasano V, et al. Lung function in beta-thalassemia patients: a longitudinal study. Acta Haematol. 2006;116(1):25–29. doi: 10.1159/000092344. [DOI] [PubMed] [Google Scholar]
- 19.Gamberini MR, Fortini M, De Sanctis V, Gilli G, Testa MR. Diabetes mellitus and impaired glucose tolerance in thalassaemia major: incidence, prevalence, risk factors and survival in patients followed in the Ferrara Center. Pediatr Endocrinol Rev. 2004 Dec;2(suppl 2):285–291. [PubMed] [Google Scholar]
- 20.Italian Working Group on Endocrine Complications in Non-endocrine Diseases. Multicentre study on prevalence of endocrine complications in thalassaemia major. Clin Endocrinol (Oxf. 1995 Jun;42(6):581–586. doi: 10.1111/j.1365-2265.1995.tb02683.x. [DOI] [PubMed] [Google Scholar]