With the advent of highly active antiretroviral therapy (HAART), human immunodeficiency virus Type 1 (HIV-1) infection has become a chronic disease with longer life expectancy.1 The HIV Outpatient Study showed that, with the addition of antiretroviral therapy (ART), mortality declined from 29.4 to 8.8 per 100 person-years.2 More recent data indicates that the proportion of patients expected to survive 5, 10, and 15 years after seroconversion in the HAART era are 99%, 93% and 89% respectively.3 With the increased life expectancy and decreased morbidity from opportunistic infections, the recognition and importance of chronic complications associated with HIV-1 infection is becoming more evident. Cardiac diseases are very common complications met in these patients. The spectrum of heart diseases varies significantly between developed and developing countries and in developed countries between pre-HAART and post- HAART eras.4 Among them, HIV-associated cardiomyopathy, broadly defined as a decreased left ventricular ejection fraction or dilated left ventricle by imaging studies, with or without symptoms of heart failure, is currently recognized as a major long-term complication of HIV-1 infection in developing countries; however, it is still prevalent in developed countries.4 Many questions regarding its pathogenesis and treatment remain unanswered.
Epidemiology
The epidemiology of HIV-associated cardiomyopathy has changed since the first report in 1986.5 The advent of HAART has significantly altered both the incidence and prevalence of this disease and the definition of HIV-associated cardiomyopathy has also evolved from one of primarily systolic dysfunction to now reflect the growing recognition of diastolic dysfunction in these patients.
Incidence
The incidence of HIV-associated cardiomyopathy is difficult to ascertain as very few studies actually evaluated this measure. In the pre-ART era, HIV-associated cardiomyopathy was defined as symptomatic, systolic dysfunction with a dilated left ventricle, and seen almost exclusively in patients with advanced HIV disease and acquired immunodeficiency syndrome (AIDS). These older studies, which are listed in Table 1,6–14 generally concluded that there was a high incidence of HIV-associated cardiomyopathy. However, this data is not all that useful in the current, post-ART era, since the phenotype of the disease has changed. The few studies in the post-ART era that have evaluated the incidence of HIV-associated cardiomyopathy have focused on the incidence of either asymptomatic systolic dysfunction and/or diastolic dysfunction (Table 1).15–22
Table 1.
Epidemiology of HIV-Associated Cardiomyopathy.
| Study | N | Incidence | Prevalence | Anti-retroviral therapy | Mean CD4 (cells/mm3) | Viral Load (copies/mL) | |
|---|---|---|---|---|---|---|---|
| Systolic Dysfunction (EF, if reported) | Diastolic Dysfunction (Stage, if reported) | ||||||
|
Pre-HAART
| |||||||
| Levy 1989 6 | 60 | NR | 16% | NR | None | NR (majority <100) | NR |
| DeCastro 1992 7 | 72 | NR | 16.6% (all with EF <50%; mean EF 35%) | NR | Zidovudine | NR | NR |
| Herskowitz 1993 8 | 69 | 18% per year | 14.5% (all with EF <45%; mean EF 34%) | NR | None | 30 | NR |
| DeCastro 1994 9 | 93 | 2% per 3 months | 7.5% (all with EF <45%) | NR | 69% on Zidovudine | 56 | NR |
| Akhras 1994 10 | 101 | NR | 20% (all EF <35%) | NR | Zidovudine | 67 | NR |
| Coudray 1995 11 | 51 | NR | NR | 4/6 diastolic function parameters worse in HIV vs. controls (no difference between symptomatic and asymptomatic HIV patients) | ~60% on Zidovudine | 172±198 in symptomatic- and 422 ±308 in asymptomatic patients | NR |
| Barbaro 1996 12 | 1236 | NR | Mean EF = 48% in HIV vs. 59% in controls | NR, but E/A, IVRT and LA parameters all significantly worse in HIV patients vs. controls | None | 670 | NR |
| Lipshultz 1998 13 | 196 | 4.7% per 2 years | 31% | NR | 63% on Zidovudine | 906 +/− 890 | NR |
| Pugilese 2000 14 | 1042 | NR | 8.1% in NRTI and 1.8% in ART treated patients | NR | 544 on NRTI alone 498 on ART | 42±15 in NRTI and 92±52 in HAART treated patients | NR |
|
| |||||||
|
Post-HAART
| |||||||
| Bijl 2001 15 | 105 | NR | 3% | NR | 91% on ART | Median 340 | Median 1.15 x104 (78% fully suppressed) |
| Kristofferson 2008 16 | 63 | <1% over 4.5 years | <1% | NR | 95% on ART | 710 +/− 350 | 16300±35800 (79% fully suppressed |
| Schuster 2008 17 | 30 | NR | 13% (lowest EF= 39%) | 64% (27% stage I, 37% stage II) | 100% on ART | 591 +/− 314 | 60% fully suppressed |
| Hsue 2010 18 | 196 | NR | 4% (EF range 33%–49%) | 49% (48% stage I, 1% stage II) | 82% on ART | Median 420 | 63% fully suppressed |
| Reinsch 2011 19 | 803 | NR | 34% (EF=32% 45–54%, 1.9% 30–44%, 0.4% 20–29%) | 48% (36% stage I, 9% stage II, 3% stage III) | 85% on ART | 509+/−301 | 66% fully suppressed |
| Mondy 2011 20 | 656 | NR | 18% (EF=17% 35–50%, 1% <35%) | 26% (15% stage I, 2% stage II, 9% stage III) | 73% on ART | Median 462 | 91% fully suppressed |
| Blaylock 2012 21 | 60 | 8.2 per 100 person years | NR | 47% (40% stage I, 7% stage II) | 78% on ART | Median 570 | 60 copies/mL |
| Cerrato 2013 (meta-analysis) 22 | 2242 | NR | 8.33% | 43.4% (31.85% stage I, 8.53% stage II, 3.02% stage III) | 98.5% on ART | Median 489 | 74% fully suppressed |
ART=Anti-Retroviral Therapy, EF=Ejection fraction, IVRT=Isovolumic Relaxation Time, LA=Left Atrium, NR = Not Reported, NRTI=Nucleoside Reverse Transcriptase Inhibitors
Prevalence
Far more studies have studied the prevalence of HIV-associated cardiomyopathy. Again, with the advent of ART, not only has the disease changed from a severe, dilated cardiomyopathy to one of often minimally symptomatic, mildly reduced LV systolic function or various degrees of impaired diastolic function. What is clear is that with the spread of ART, the prevalence of systolic dysfunction has decreased and the number of patients with severely impaired ejection fractions is quite low. On the contrary, the number of HIV-infected patients with abnormal diastolic parameters has increased significantly (Table 1). A meta-analysis of 11 studies in the HAART era assessed 2242 well-controlled, asymptomatic HIV-1 infected patients, who nevertheless had a prevalence of systolic dysfunction of 8.3% and diastolic dysfunction of 43.4%.22 Risk factors for systolic dysfunction included high sensitivity C-reactive protein >5 mg/L, tobacco use, and past myocardial infarction, whereas for diastolic dysfunction, risk factors were hypertension and older age.
It is important to point out that the data discussed above and listed in Table 1, represent studies looking at patients in the United States and Europe. However, more than two-thirds of HIV-infected people live in Sub-Sarahan Africa, where <20% of patients who need ART actually receive it. The Heart of Soweto Study was undertaken to investigate the impact of the HIV/AIDS epidemic on de novo manifestations of heart disease.23 In their analysis, 518 of 5328 (9.7%) of newly diagnosed heart disease were identified as HIV-positive. Of those, almost one-third presented with LV systolic dysfunction (N=148; 29%) and 196 (38%) had HIV-related cardiomyopathy (which encompassed both systolic and diastolic dysfunction in both symptomatic and asymptomatic patients). Furthermore, the incidence of coronary disease, which is rising in ART-treated HIV patients, was low, occurring in just 2.7% of patients. This study has important implications from a global health perspective, but it also reinforces the changing nature of HIV-associated heart disease in the era of widespread ART use.
With the high prevalence of diastolic abnormalities in HIV-infected patients, additional imaging modalities have been used to detect other impairments in cardiac function. In one study, 28 young HIV-1-infected patients age 7–29 years were compared to 28 controls and showed no abnormalities in gross systolic or diastolic parameters24, but the HIV-1 infected patients had impaired radial strain and longitudinal and circumferential strain and strain rate, compared to controls. Asymptomatic HIV-1 infection and the use of ARTs are associated with left ventricular hypertrophy and diastolic dysfunction independent of blood pressure.25 More recently, cardiac magnetic resonance imaging and spectroscopy found high rates of cardiac steatosis, altered myocardial function and a high rate of myocardial fibrosis in almost all 90 HIV-positive patients studied.26 The authors hypothesize that the cardiac steatosis, which is presumably secondary to ART, and the myocardial fibrosis—possibly representing subclinical myocarditis—may underlie the increased morbidity and mortality seen in HIV-infected patients with cardiac disease.
HIV-2 and Cardiomyopathy
There is a far greater experience with HIV-1 infection than HIV-2 with regard to cardiovascular disease. Some older evidence supports a different clinical expectation with regard to infection by HIV-2 compared to HIV-1. Left ventricular function was evaluated by echocardiography in a prospective study that included 98 consecutive HIV-infected patients and 40 HIV-seronegative controls. Only 8 (8%) of infected patients had symptomatic heart failure. In general cardiovascular function was better in earlier stages of the infection (fractional shortening in acquired immunodeficiency syndrome was 30%±6% and in asymptomatic HIV-seropositive patients was 34%±5%; p<0.005) and in HIV-2-infected patients, but more specific information about the smaller HIV-2 cohort was lacking.27
Prognosis
The severe systolic dysfunction that was a hallmark of pre-ART HIV-associated cardiomyopathy carried a grim prognosis (Table 2).28–34 Currie et al demonstrated a median survival among patients with AIDS with cardiomyopathy of 101 versus 472 days in those without,28 whereas another study showed an adjusted hazard for death of 5.86 compared to patients with idiopathic cardiomyopathy.29 However, with the widespread use of ARTs, not only has the epidemiology of the disease changed, but so has the prognosis. Cardiac diseases account for a quarter of deaths in the post-ART era compared to less than 10% in the pre-ART era. Furthermore, symptoms of heart failure or echocardiographic evidence of cardiomyopathy are associated with 6.5 and 4.0 times higher risk for death, respectively.32 Some studies have tried to further elucidate this increased risk. One such study looked at the risk of sudden cardiac death in HIV-1 infected patients.34 Sudden cardiac death in this population occurred at 4.5 times higher rate than expected. Furthermore, of those who died, 43% (N=13) had echocardiograms prior to death and half had known systolic and/or diastolic dysfunction. Another study of HIV-infected patients with systolic dysfunction (mean ejection fraction 28±11%) undergoing dobutamine stress echocardiography found 11 cardiac deaths (event rate 7.6%/year); all due to either worsening heart failure or arrhythmias.33 The presence of inotropic contractile reserve was associated with improved prognosis. Those without contractile reserve had a 7-times higher event rate (24%/year vs. 3.4%/year, p<0.0001). Furthermore, those with contractile reserve were more likely to improve ejection fraction over time (80% vs. 33%, P=0.003) from 30±11% to 44±11%, p<0.0001, vs. from 24±11% to 30±17%, despite no difference in use of anti-remodeling medication therapy between groups.33
Table 2.
Prognosis of HIV-Associated Cardiomyopathy.
| Study | Number of Patients | Length of Study | Definition of Cardiomyopathy | Hazard Ratio for Death | Median Survival | CD4 count (mean) | Mean Ejection Fraction | ART |
|---|---|---|---|---|---|---|---|---|
| Curie 1994 28 | 296 (13 had dilated cardiomyopathy) | 4 years | FS <28% with global LV hypokinesia | 11.68 for patients with dilated cardiomyopathy vs. controls with AIDS | 101 days for patients with dilated cardiomyopathy | 153 (7.4 in patients with dilated cardiomyopathy) | NR | No |
| Felker 2000 29 | 1230 (45 with HIV- cardiomyopathy) | 4.4 years | NR | 5.86 vs. idiopathic cardiomyopathy | NR | NR | NR | NR |
| Lipshultz 2000 30 | 193 (mean age 2.1 years old) | 60 months | LV contractility >2SD below the mean and LV end diastolic dimension >2 SD above mean* | FS: 1.31 RR for each SD decrease Wall thickness: 1.35 for each SD increase | 64% at 5 years (38% if FS <2 SD below normal) (45% if wall thickness >1 SD above normal) | 690 (normal value for 2 year old is 2298) | NR | NR |
| Sackoff 2006 31 | 68,669 | 5 years | NR (only evaluated “cardiovascular disease” as a cause of non-HIV related death | 29.2 age-adjusted mortality per 10,000 persons with AIDS for cardiovascular disease | NR | NR | NR | NR |
| Crum 2006 32 | 4241 (15 total deaths from HF or Cardiomyopathy in study cohort) | 13 years | Heart failure or cardiomyopathy diagnosed based on death certificate | 6.52 for Congestive Heart Failure 3.97 for Cardiomyopathy | NR | 123 pre-HAART era 202 early-HAART era 316 late-HAART era | NR | NR |
| Wever-Pinson 2011 33 | 60 | 2.4 +/− 2.1 years | Patients with EF<45%; DSE performed to assess for inotropic contractile reserve | 6.6 for the absence of contractile reserve 56% had improvement in EF (mean 48± 9%) | NR | 243 | 28 +/− 11% | 77% |
| Tseng 2012 34 | 2860 | Median 3.7 years | Causes of death were obtained from death certificates or National Death Index database | 4.46 for SCD (13% of deaths due to sudden cardiac death) | NR | Median 312 for patients who died of SCD | Patients with echo, 23% had moderate to severe low EF | NR |
ART= Anti-Retroviral Therapy, BSA= Body Surface Area, EF = Ejection fraction, FS= Fractional Shortening, LV= Left Ventricle, NR = Not Reported, NYHA= New York Heart Association, SCD= Sudden Cardiac Death, SD= Standard Deviation.
LV contractility was defined as the relation between end-systolic LV wall stress and the rate-adjusted velocity of fiber shortening, which incorporates afterload and is independent of preload. Afterload was measured as meridional end-systolic LV wall stress. Peak systolic wall stress was measured as well.
It is important to recognize that no studies have evaluated the prognosis in HIV-infected patients with diastolic abnormalities. However, extrapolating evidence from studies in non-HIV-positive patient populations, diastolic dysfunction is a predictor of mortality, though not to the degree that systolic dysfunction is. Therefore, it seems reasonable to suggest that a screening echocardiogram should be performed on HIV-infected patients, particularly if they have any other cardiovascular risk factors, given the high prevalence of diastolic abnormalities as well as asymptomatic systolic dysfunction. The cost-effectiveness of such a strategy would need to be evaluated, especially given the lack of data supporting any treatments to reduce mortality in patients with diastolic dysfunction. At this time though, other imaging modalities such as MRI and even strain echocardiography need further research to determine what impact they may have on prognosis and how they should be used in daily practice. Table 2 presents the studies that have addressed prognosis.28–34
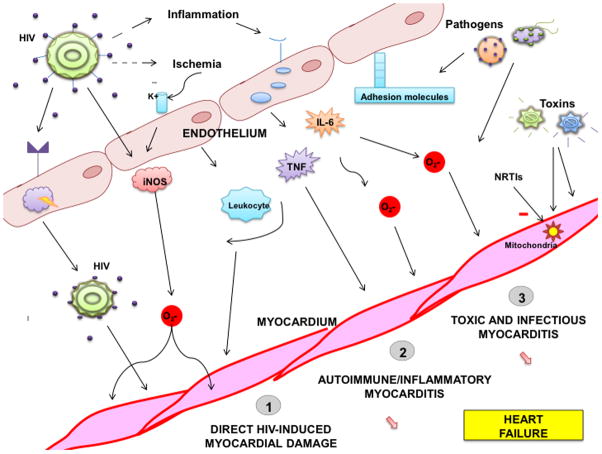
Pathophysiology
The pathophysiology of HIV-associated cardiomyopathy remains uncertain and is likely multifactorial (Figure 1). The proposed causes include direct infection of the myocardium by the HIV-1 virus with or without myocarditis, toxicity from the medications used to treat HIV-1 infection, opportunistic infections, as well as nutritional disorders, and others.35, 36 When HIV-associated cardiomyopathy was only thought of in terms of severe, dilated cardiomyopathy, the pathobiology was felt to be from opportunistic infections or as a result of myocarditis. However, as the disease has changed and grown to include more nuanced forms of myocardial involvement, the understanding of the mechanisms too has evolved.
Figure 1.
Pathophysiology of HIV Associated Heart Failure. HIV causes damages myocardium directly and also indirectly through inflammation and increased susceptibility to infections, toxins and eventually, ischemia. The endothelium serves as a reservoir of HIV and also acts to elaborate cytokines, such as tumor necrosis factor and interleukin-6, and free radicals in response to increased inflammation. Other causes of myocardial dysfunction among HIV infected individuals include mitochondrial damage resulting from HIV therapy such as NRTIs and other toxins. HIV=human immunodeficiency virus; TNF=tumor necrosis factor; IL-6=Interleukin-6; iNOS=inducible nitric oxide synthase; NRTIs=nucleoside reverse transcriptase inhibitors.
Direct HIV-induced Myocardial Damage
Infection of the heart with HIV-1 has been postulated as one of the key mechanisms for the development of impaired systolic function.35, 37–41 In situ hybridization of HIV-1 in myocardial samples from humans with AIDS41, 42 and in primates with Simian immunodeficiency virus43 revealed the cytologic identity of cardiac infection was the macrophage as compared to the myocyte. These facts are compatible with the notion that cardiomyocytes lack HIV-1 receptor proteins (gp 120 or gp 24). However, Wang et al. demonstrated that human cardiac fetal myocyte cell lines were capable of ingesting HIV-1 via specific Fc receptors despite the absence of CD4 receptors.44 Furthermore, HIV-1 infection within cardiac interstitial cells (dendritic cells or endothelial cells) rather than myocytes may play an important pathogenic role as these infected cells serve as viral reservoirs as well as antigen presenting cells mediating inflammation.45 Gene products of HIV-1 may also contribute and HIV-related proteins expressed in response to infection may lead to the development of cardiomyopathy.46, 47 As proof of principle it was shown experimentally that HIV-1 Tat expressed transgenically in the mouse causes systolic dysfunction, which could be relieved by antioxidants.48, 49
Autoimmune Mechanisms
There is evidence that common cardiotropic viruses may alter surface antigens leading to an autoimmune reaction to endogenous epitopes50 and cardiac specific autoantibodies are more common in HIV-1 infected people, especially those with some degree of myocardial disease, than in HIV-negative controls.51 This can result in increased myocardial expression of HLA class I antigens, which is seen more commonly in AIDS patients with symptomatic systolic dysfunction.52 Interestingly, there is experimental evidence that blocking some of these proteins may be cardioprotective,47, 51, 53, 54 and monthly intravenous immunoglobulin(s) in pediatric HIV-1 infected patients was shown to minimize left ventricular dysfunction and improve other markers of myocardial injury.13, 30
Inflammation
Pro-inflammatory cytokines, particularly interleukin-1β and tumor necrosis factor (TNF), have been shown to exert a negative inotropic effect and likely play a role in HIV-associated cardiomyopathy, specifically the form associated with depressed systolic function.55–57 Other investigators showed that TNF and inducible nitric oxide synthase expression was higher in patients with HIV-associated cardiomyopathy.45 Tumor necrosis factor in autopsies of HIV-associated cardiomyopathy patients suggest that it is a potent inducer of apoptosis.58 Some suggest that treatment to reduce oxidative stress may impact the development and outcome of impaired systolic function in these patients.59
Side Effects of HIV Medications
Some of the medications used to treat HIV infection may have a deleterious effect on myocardium. Mitochondrial toxicity is an acknowledged side effect of ART.60 Defects in mitochondrial DNA (mtDNA) replication and decreased energetics are caused by zidovudine (3′-azido-2′,3′-deoxythymidine, AZT)61, 62 as well as other nucleoside reverse transcriptase inhibitors (NRTIs), specifically fialuridine (FIAU; (1-[2-deoxy-2-fluoro-β-D-arabinofuranosyl]-5-iodouracil)63 clevudine (L-FMAU) and lodenosine, a purine NRTI (2′-fluoro-2′,3′-dideoxyadenosine), which had been used in the treatment of Hepatitis B.64
Children infected with HIV are exposed to ART for many years, including in utero exposure to HAART while the cardiovascular system is still developing. There may be an interaction between the effects of ART and HIV on the cardiovascular system of children; however, the direction and magnitude of such effects are unknown. Children and adolescents are unique populations to study the pathophysiologic mechanisms of HIV-associated cardiomyopathy, because they are less likely than adults to be exposed to other cardiovascular risk factors. HIV infection and ART exposure lead to subclinical abnormalities of cardiac structure and function in children that may eventually result in symptomatic cardiomyopathy in adulthood. Specifically, it has been reported that long-term HAART exposure may have cardioprotective properties early in life, but this cardioprotection decreases as HIV-infected children age into adolescence and early adulthood. Echocardiographic data from the NIH-funded Pediatric HIV/AIDS Cohort Study’s Adolescent Master Protocol showed that measures of LV structure and function were better in the long-term HAART-exposed group than in the relatively HAART-unexposed Vertically Transmitted HIV Infection cohort, but were not as normal as those in an HIV-exposed uninfected control group.65 Children exposed perinatally to either multi-drug ART or HAART had below-normal LV mass, LV dimension, and septal wall thickness. In a larger cohort of HIV-exposed uninfected perinatally HAART-exposed children showed that 16% of them had at least one abnormal echocardiographic measure.66 First trimester exposure to various ART agents has been associated with specific echocardiographic abnormalities. For instance, first trimester exposure to abacavir has been associated with decreased LV wall thickness. Also, in HIV-exposed uninfected children, serum cardiac biomarker measurements suggested that perinatal exposure to multiple ART agents might have subclinical myocardial inflammation. Specifically, abacavir exposure was potentially associated with deleterious cardiac effects.67
Nutritional effects
Selenium deficiency has been described in HIV-1 infected patients and is associated with a form of cardiomyopathy in China known as Keshan’s Disease.68, 69 However, the data on nutritional deficiencies resulting in cardiomyopathy are more closely related to socio-economic status rather than presence or absence of HIV-associated cardiomyopathy.50
Coronary Artery Disease
The growing burden of coronary artery disease in HIV-1 infected individuals may also significantly modify the risk for HIV-associated cardiomyopathy. It is well known that coronary disease can predispose patients to the development of cardiomyopathy and the mechanisms of coronary disease in this population are complex, though similar in many respects to non-HIV-1 infected patients.70 In a recent analysis, the prevalence of diabetes mellitus and hypertension—two of the most common and recognized risk factors for coronary artery disease—were found less frequently in HIV-infected patients who suffered an acute myocardial infarction (AMI) compared to AMI patients who were not infected with HIV.71 Furthermore, the authors showed similar 30-day and 1-year mortality and MACE in the HIV-positive patients with AMI compared to those without the disease. One of the more compelling findings of the study by Lorgis was that there was a 2-fold increased risk of hospitalization for heart failure in the year after the acute event in the HIV-positive group. The presence of diabetes conferred an almost 5-fold increased risk for the development of heart failure, as did the presence of HIV-infection itself. Prior studies have also shown that risk factors for the development of systolic impairment include smoking status, increased hs-CRP levels and prior myocardial infarction.20, 22 In contrast, the risk factors that seem to be associated with the development of diastolic abnormalities include higher body mass index and hypertension.20–22 The obvious next step would be to study whether aggressive control of these risk factors will delay or prevents the development of myocardial dysfunction.
Treatment
Medical Therapy
Little is known about the optimal therapy for HIV-cardiomyopathy and the response of known heart failure medications in HIV-1 infected patients. No randomized trials of heart failure medications have been performed in this patient population. With the privacy concerns and regulations, data on HIV status is not collected in clinical trials and registries and therefore no definitive data exists in this regard. Consequently, therapy is driven by consensus and data is derived from either retrospective analyses/case reports or from extrapolation from non-HIV-1 infected patients. General recommendations include standard, guideline-driven therapy, but no studies have assessed for the benefits of beta-blockers, angiotensin converting enzyme inhibitors or aldosterone antagonists in this specific subset of patients.
Devices
Little is known about the effect of device therapy in HIV-cardiomyopathy patients. Unfortunately neither the rate nor the effectiveness of implantable devices have been reported in the HIV-cardiomyopathy population. It has been suggested that HIV-1 infected patients may be less likely to receive an implantable defibrillator or cardiac resynchronization therapy due to either a belief that they have higher mortality and thus shorter lifespans or for fear of infectious complications.72 This concern is not without some merit, as a recent analysis showed a higher rate of bacteremia despite ART in HIV-1 infected patients as compared to the general population.73 However, in light of the findings by Tseng et al, the benefit of implantable defibrillator in this population to prevent the high incidence of sudden cardiac death should be studied.34
Immune Therapy
While sparse data exists, one retrospective review of intravenous immunoglobulin therapy in 49 children with HIV-1 infection found that it was associated with significant improvements in left ventricular wall thickness and decreases in peak wall stress.74 Favorable trends were also noted in fractional shortening and contractility. The therapeutic benefit of intravenous immunoglobulin may result from its ability to inhibit TNF and interleukin production. Etanercept, another immune modulating agent has been used in a small study of patients with heart failure with moderate success.75 In an animal study, monkeys infected with simian immunodeficiency virus as well as killed Mycobacterium avium complex bacteria developed severe left ventricular dysfunction.57 However, monkeys treated with etanercept did not develop cardiomyopathy, suggesting not only that TNF may play a causative role in the development of HIV cardiomyopathy (as discussed above), but that therapy directed at TNF may treat the cardiomyopathy as well. However, this therapy has not been tested in human HIV-1 infected patients.
HAART Therapy
The role of HAART in HIV-cardiomyopathy is complicated. On one hand, most studies suggest that systolic dysfunction is more pronounced and prevalent with poorly controlled HIV-1 infection. On the other hand, therapy with ART has been associated with higher incidence of coronary disease, which is a risk factor for myocardial impairment. Some case reports have been published that showed regression and normalization of cardiomyopathy in adults76 and children77 that were treated with HAART. In the largest study to date of pediatric patients, over 3,000 children with HIV infection were longitudinally followed for incident cardiomyopathy and to assess the effect of HAART.78 Over a median of 5.5 years of follow-up, 99 cases of cardiomyopathy were observed, yielding an incidence of 5.6 cases per 1,000 person years. The authors noted that the incidence decreased dramatically in the post-HAART era from 25.6 cases per 1,000 person-years to 3.9 cases per 1,000 person-years. While this study did not specifically address the effect of HAART on “curing” HIV-cardiomyopathy (specifically LV systolic dysfunction), it did demonstrate the “protective” effect of HAART in reducing its incidence. Despite this, the incidence of cardiomyopathy in pediatric HIV-1 infected patients is still 40 times higher than the reported annual incidence of 1.13 per 100,000 children from the US Pediatric Cardiomyopathy Registry.79 Thus, the question of whether HAART can actually reverse HIV-cardiomyopathy is not answered and warrants further investigation,
Transplant and Mechanical Circulatory Assist Devices
HIV-1 infection has generally been considered a contraindication for cardiac transplant due to historically poor survival and concerns over progression to AIDS with immunosuppression,80 despite recent evidence that suggests that immunosuppressant medications can actually increase the efficacy of HAART in treating HIV infection.81 A survey of cardiac transplant programs revealed that the 70% considered infection with HIV-1 an absolute contraindication to transplant. Indeed, early reports of cardiac transplant in patients subsequently found to have HIV-1 infected after transplantation showed poor outcomes.82 However, since 2003 when the first cardiac transplant was performed in a known HIV-positive patient83, outcomes have generally been favorable.84 No increase in rejection or worsening of HIV status with immunosuppression have been reported. Larger case-series in the US85 and Europe86 have shown similar results. Hence calls for re-evaluation of HIV-1 infection as an absolute contraindication have been made.80
In 2009, 2 reports of destination therapy with HeartMate XVE implanted in HIV-1 infected patients were published.87, 88 Both patients did well and did not suffer complications much different from those by non-HIV-1 infected left ventricular assist device recipients. A subsequent case demonstrated no major infectious related complications89 and no significant increased risk of allosensitization.90 Thus, while the data on mechanical assist devices in HIV-1 infected patients are limited, case series indicate reasonable outcomes and no significant adverse events attributed to HIV-1 infection. These findings warrant further investigation.
Conclusion
Since the first report in 1986, our understanding of the HIV-associated cardiomyopathy has evolved, but nevertheless remains inadequate. What was once thought of as strictly systolic dysfunction and associated with poorly controlled HIV-1 infection, the widespread use of ART in the Western world has changed the disease from a severe, dilated cardiomyopathy to one of less severe LV systolic function and one with various degrees of impaired diastolic function, often independent of traditional cardiac risk factors. The prevalence of systolic dysfunction has decreased in developed countries and unfortunately in the parts of the world where HIV is most prevalent, ART is not widespread and so the disease remains one of severe systolic impairment with high rates of morbidity and mortality. While the exact incidence, prevalence and pathophysiology remain to be elucidated, it is clear that these patients have poor prognosis if they develop systolic dysfunction. The exact significance of diastolic abnormalities among these patients is not known, necessitating further research to determine their prognosis and how best to prevent its development. Related to the fact that its pathophysiology is poorly understood, the therapeutic approach to these patients remains unknown as well. Whether or not drug- and device-based therapies that have been shown to be of benefit in heart failure patients without HIV-1 infection will benefit those who are infected, and to the same degree, is unknown. Considering the epidemiologic significance of cardiac functional abnormalities among HIV-1 infected individuals and the lack of definitive data on how to treat these patients, further research is urgently needed in this group particularly in sub-Saharan Africa
Supplementary Material
Acknowledgments
Funding Source: Dr. Ofotokun’s work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS, R01AR059364), National Institute of Aging (NIA, R01AG040013), National Institute of Allergy and Infectious Diseases (NIAID, U01AI103408), the Emory Center For AIDS Research (CFAR, P30AI050409), and the Atlanta Clinical and Translational Science Institute (UL1TR000454)
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio Monforte A, Knysz B, Dietrich M, Phillips AN, Lundgren JD. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Ewings FM, Bhaskaran K, McLean K, Hawkins D, Fisher M, Fidler S, Gilson R, Nock D, Brettle R, Johnson M, Phillips A, Porter K. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS. 2008;22:89–95. doi: 10.1097/QAD.0b013e3282f3915e. [DOI] [PubMed] [Google Scholar]
- 4.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nat Clin Pract Cardiovasc Med. 2009;6:120–127. doi: 10.1038/ncpcardio1437. [DOI] [PubMed] [Google Scholar]
- 5.Cohen IS, Anderson DW, Virmani R, Reen BM, Macher AM, Sennesh J, DiLorenzo P, Redfield RR. Congestive Cardiomyopathy in Association with the Acquired Immunodeficiency Syndrome. N Engl J Med. 1986;315:628–630. doi: 10.1056/NEJM198609043151007. [DOI] [PubMed] [Google Scholar]
- 6.Levy WS, Simon GL, Rios JC, Ross AM. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Am J Cardiol. 1989;63:86–89. doi: 10.1016/0002-9149(89)91081-3. [DOI] [PubMed] [Google Scholar]
- 7.De Castro S, Migliau G, Silvestri A, D’Amati G, Giannantoni P, Cartoni D, Kol A, Vullo V, Cirelli A. Heart involvement in AIDS: a prospective study during various stages of the disease. Eur Heart J. 1992;13:1452–1459. doi: 10.1093/oxfordjournals.eurheartj.a060085. [DOI] [PubMed] [Google Scholar]
- 8.Herskowitz A, Vlahov D, Willoughby S, Chaisson RE, Schulman SP, Neumann DA, Baughman KL. Prevalence and incidence of left ventricular dysfunction in patients with human immunodeficiency virus infection. Am J Cardiol. 1993;71:955–958. doi: 10.1016/0002-9149(93)90913-w. [DOI] [PubMed] [Google Scholar]
- 9.De Castro S, D’Amati G, Gallo P, Cartoni D, Santopadre P, Vullo V, Cirelli A, Migliau G. Frequency of development of acute global left ventricular dysfunction in human immunodeficiency virus infection. J Am Coll Cardiol. 1994;24:1018–1024. doi: 10.1016/0735-1097(94)90864-8. [DOI] [PubMed] [Google Scholar]
- 10.Akhras F, Dubrey S, Gazzard B, Noble MI. Emerging patterns of heart disease in HIV infected homosexual subjects with and without opportunistic infections; a prospective colour flow Doppler echocardiographic study. Eur Heart J. 1994;15:68–75. doi: 10.1093/oxfordjournals.eurheartj.a060382. [DOI] [PubMed] [Google Scholar]
- 11.Coudray N, de Zuttere D, Force G, Champetier de Ribes D, Pourny JC, Antony I, Lecarpentier Y, Chemla D. Left ventricular diastolic function in asymptomatic and symptomatic human immunodeficiency virus carriers: an echocardiographic study. Eur Heart J. 1995;16:61–67. doi: 10.1093/eurheartj/16.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Barbaro G, Barbarini G, Di Lorenzo G. Early impairment of systolic and diastolic function in asymptomatic HIV-positive patients: a multicenter echocardiographic and echo-Doppler study. The Gruppo Italiano Per lo Studio Cardiologico dei Pazienti Affetti da AIDS. AIDS Res Hum Retroviruses. 1996;12:1559–1563. doi: 10.1089/aid.1996.12.1559. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, McIntosh K, Schluchter MD, Colan SD. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 1998;97:1246–1256. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugliese A, Isnardi D, Saini A, Scarabelli T, Raddino R, Torre D. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect. 2000;40:282–284. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 15.Bijl M, Dieleman JP, Simoons M, van der Ende ME. Low prevalence of cardiac abnormalities in an HIV-seropositive population on antiretroviral combination therapy. J Acquir Immune Defic Syndr. 2001;27:318–320. doi: 10.1097/00126334-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kristoffersen US, Lebech AM, Gerstoft J, Hesse B, Petersen CL, Gutte H, Kjaer A. Right and left cardiac function in HIV-infected patients investigated using radionuclide ventriculography and brain natriuretic peptide: a 5-year follow-up study. HIV Med. 2008;9:180–186. doi: 10.1111/j.1468-1293.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 17.Schuster I, Thoni GJ, Ederhy S, Walther G, Nottin S, Vinet A, Boccara F, Khireddine M, Girard PM, Mauboussin JM, Rouanet I, Dauzat M, Cohen A, Messner-Pellenc P, Obert P. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol. 2008;101:1213–1217. doi: 10.1016/j.amjcard.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 18.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, Martin JN, Deeks SG, Bolger AF. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinsch N, Kahlert P, Esser S, Sundermeyer A, Neuhaus K, Brockmeyer N, Potthoff A, Erbel R, Buck T, Neumann T. Echocardiographic findings and abnormalities in HIV-infected patients: results from a large, prospective, multicenter HIV-heart study. Am J Cardiovasc Dis. 2011;1:176–184. [PMC free article] [PubMed] [Google Scholar]
- 20.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, Hammer J, Carpenter CC, Kojic E, Patel P, Brooks JT. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 21.Blaylock JM, Byers DK, Gibbs BT, Nayak G, Ferguson M, Tribble DR, Porter C, Decker CF. Longitudinal assessment of cardiac diastolic function in HIV-infected patients. Int J STD AIDS. 2012;23:105–110. doi: 10.1258/ijsa.2011.011099. [DOI] [PubMed] [Google Scholar]
- 22.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, Castagno D, Omede P, Quadri G, Sciuto F, Presutti D, Frati G, Bonora S, Moretti C, Gaita F. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–1436. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 23.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–874. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims A, Frank L, Cross R, Clauss S, Dimock D, Purdy J, Mikhail I, Hazra R, Hadigan C, Sable C. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J Am Soc Echocardiogr. 2012;25:741–748. doi: 10.1016/j.echo.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandi AM, Nicolini E, Giola M, Gianni M, Maresca AM, Marchesi C, Guasti L, Balsamo ML, Venco A, Grossi PA. Left ventricular remodelling in asymptomatic HIV infection on chronic HAART: comparison between hypertensive and normotensive subjects with and without HIV infection. J Hum Hypertens. 2012;26:570–576. doi: 10.1038/jhh.2011.81. [DOI] [PubMed] [Google Scholar]
- 26.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive Cardiac Magnetic Resonance Imaging and Spectroscopy Reveal a High Burden of Myocardial Disease in HIV Patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso JS, Moura B, Martins L, Mota-Miranda A, Rocha Goncalves F, Lecour H. Left ventricular dysfunction in human immunodeficiency virus (HIV)-infected patients. Int J Cardiol. 1998;63:37–45. doi: 10.1016/s0167-5273(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 28.Currie PF, Jacob AJ, Foreman AR, Elton RA, Brettle RP, Boon NA. Heart muscle disease related to HIV infection: prognostic implications. BMJ. 1994;309:1605–1607. doi: 10.1136/bmj.309.6969.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying Causes and Long-Term Survival in Patients with Initially Unexplained Cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 30.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, Sopko G, Colan SD. Cardiac dysfunction and mortality in HIV-infected children: The Prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 2000;102:1542–1548. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 32.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 33.Wever-Pinzon O, Bangalore S, Romero J, Silva Enciso J, Chaudhry FA. Inotropic contractile reserve can risk-stratify patients with HIV cardiomyopathy: a dobutamine stress echocardiography study. JACC Cardiovasc Imaging. 2011;4:1231–1238. doi: 10.1016/j.jcmg.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis W. Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis. 2000;43:151–170. doi: 10.1053/pcad.2000.9031. [DOI] [PubMed] [Google Scholar]
- 36.Lewis W. AIDS cardiomyopathy: physiological, molecular, and biochemical studies in the transgenic mouse. Ann N Y Acad Sci. 2001;946:46–56. [PubMed] [Google Scholar]
- 37.Welch K, Finkbeiner W, Alpers CE, Blumenfeld W, Davis RL, Smuckler EA, Beckstead JH. Autopsy findings in the acquired immune deficiency syndrome. JAMA. 1984;252:1152–1159. [PubMed] [Google Scholar]
- 38.Cammarosano C, Lewis W. Cardiac lesions in acquired immune deficiency syndrome (AIDS) J Am Coll Cardiol. 1985;5:703–706. doi: 10.1016/s0735-1097(85)80397-1. [DOI] [PubMed] [Google Scholar]
- 39.Reilly JM, Cunnion RE, Anderson DW, O’Leary TJ, Simmons JT, Lane HC, Fauci AS, Roberts WC, Virmani R, Parrillo JE. Frequency of myocarditis, left ventricular dysfunction and ventricular tachycardia in the acquired immune deficiency syndrome. Am J Cardiol. 1988;62:789–793. doi: 10.1016/0002-9149(88)91223-4. [DOI] [PubMed] [Google Scholar]
- 40.Lewis W. AIDS: cardiac findings from 115 autopsies. Prog Cardiovasc Dis. 1989;32:207–215. doi: 10.1016/0033-0620(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 41.Grody WW, Cheng L, Lewis W. Infection of the heart by the human immunodeficiency virus. Am J Cardiol. 1990;66:203–206. doi: 10.1016/0002-9149(90)90589-s. [DOI] [PubMed] [Google Scholar]
- 42.Lipshultz SE, Fox CH, Perez-Atayde AR, Sanders SP, Colan SD, McIntosh K, Winter HS. Identification of human immunodeficiency virus-1 RNA and DNA in the heart of a child with cardiovascular abnormalities and congenital acquired immune deficiency syndrome. Am J Cardiol. 1990;66:246–250. doi: 10.1016/0002-9149(90)90603-x. [DOI] [PubMed] [Google Scholar]
- 43.Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, Lackner AA. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation. 2000;101:185–193. doi: 10.1161/01.cir.101.2.185. [DOI] [PubMed] [Google Scholar]
- 44.Wang YC, Neckelmann N, Mayne A, Herskowitz A, Srinivasan A, Sell KW, Ahmed-Ansari A. Establishment of a human fetal cardiac myocyte cell line. In Vitro Cell Dev Biol. 1991;27:63–74. doi: 10.1007/BF02630896. [DOI] [PubMed] [Google Scholar]
- 45.Barbaro G, Di Lorenzo G, Soldini M, Giancaspro G, Grisorio B, Pellicelli A, Barbarini G. Intensity of myocardial expression of inducible nitric oxide synthase influences the clinical course of human immunodeficiency virus-associated cardiomyopathy. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS (GISCA) Circulation. 1999;100:933–939. doi: 10.1161/01.cir.100.9.933. [DOI] [PubMed] [Google Scholar]
- 46.Kan H, Xie Z, Finkel MS. HIV gp120 enhances NO production by cardiac myocytes through p38 MAP kinase-mediated NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2000;279:H3138–3143. doi: 10.1152/ajpheart.2000.279.6.H3138. [DOI] [PubMed] [Google Scholar]
- 47.Kan H, Xie Z, Finkel MS. p38 MAP kinase-mediated negative inotropic effect of HIV gp120 on cardiac myocytes. Am J Physiol Cell Physiol. 2004;286:C1–7. doi: 10.1152/ajpcell.00059.2003. [DOI] [PubMed] [Google Scholar]
- 48.Raidel SM, Haase C, Jansen NR, Russ RB, Sutliff RL, Velsor LW, Day BJ, Hoit BD, Samarel AM, Lewis W. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am J Physiol Heart Circ Physiol. 2002;282:H1672–1678. doi: 10.1152/ajpheart.00955.2001. [DOI] [PubMed] [Google Scholar]
- 49.Fang Q, Kan H, Lewis W, Chen F, Sharma P, Finkel MS. Dilated cardiomyopathy in transgenic mice expressing HIV Tat. Cardiovasc Toxicol. 2009;9:39–45. doi: 10.1007/s12012-009-9035-5. [DOI] [PubMed] [Google Scholar]
- 50.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. AIDS. 2003;17 (Suppl 1):S21–28. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 51.Currie PF, Goldman JH, Caforio AL, Jacob AJ, Baig MK, Brettle RP, Haven AJ, Boon NA, McKenna WJ. Cardiac autoimmunity in HIV related heart muscle disease. Heart. 1998;79:599–604. doi: 10.1136/hrt.79.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herskowitz A, Wu TC, Willoughby SB, Vlahov D, Ansari AA, Beschorner WE, Baughman KL. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol. 1994;24:1025–1032. doi: 10.1016/0735-1097(94)90865-6. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Y, Kan H, Fang Q, Chen F, Finkel MS. CXCR4 receptor antagonist blocks cardiac myocyte p38 MAP kinase phosphorylation by HIV gp120. Cardiovasc Toxicol. 2008;8:173–180. doi: 10.1007/s12012-008-9026-y. [DOI] [PubMed] [Google Scholar]
- 54.Berzingi C, Chen F, Finkel MS. p38 MAP kinase inhibitor prevents diastolic dysfunction in rats following HIV gp120 injection in vivo. Cardiovasc Toxicol. 2009;9:142–150. doi: 10.1007/s12012-009-9047-1. [DOI] [PubMed] [Google Scholar]
- 55.Hofmann U, Heuer S, Meder K, Boehler J, Lange V, Quaschning T, Ertl G, Bonz A. The proinflammatory cytokines TNF-alpha and IL-1 beta impair economy of contraction in human myocardium. Cytokine. 2007;39:157–162. doi: 10.1016/j.cyto.2007.07.185. [DOI] [PubMed] [Google Scholar]
- 56.Monsuez JJ, Escaut L, Teicher E, Charniot JC, Vittecoq D. Cytokines in HIV-associated cardiomyopathy. Int J Cardiol. 2007;120:150–157. doi: 10.1016/j.ijcard.2006.11.143. [DOI] [PubMed] [Google Scholar]
- 57.Yearley JH, Mansfield KG, Carville AA, Sokos GG, Xia D, Pearson CB, Shannon RP. Antigenic stimulation in the simian model of HIV infection yields dilated cardiomyopathy through effects of TNFalpha. AIDS. 2008;22:585–594. doi: 10.1097/QAD.0b013e3282f57f61. [DOI] [PubMed] [Google Scholar]
- 58.Pozzan G, Pagliari C, Tuon FF, Takakura CF, Kauffman MR, Duarte MI. Diffuse-regressive alterations and apoptosis of myocytes: possible causes of myocardial dysfunction in HIV-related cardiomyopathy. Int J Cardiol. 2009;132:90–95. doi: 10.1016/j.ijcard.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 59.Chen F, Lewis W, Hollander JM, Baseler W, Finkel MS. N-acetylcysteine reverses cardiac myocyte dysfunction in HIV-Tat proteinopathy. J Appl Physiol. 2012;113:105–113. doi: 10.1152/japplphysiol.00068.2012. [DOI] [PubMed] [Google Scholar]
- 60.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Lewis W, Copeland WC, Day B. Mitochondrial DNA Depletion, Oxidative Stress and Mutation: Mechanisms of Nucleoside Reverse Transcriptase Inhibitor Toxicity. Lab Invest. 2001;81:777–790. doi: 10.1038/labinvest.3780288. [DOI] [PubMed] [Google Scholar]
- 62.Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM, Bruggeman L, Klotman P. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest. 2000;80:187–197. doi: 10.1038/labinvest.3780022. [DOI] [PubMed] [Google Scholar]
- 63.McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B.[see comment] N Engl J Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 64.Comereski CR, Kelly WA, Davidson TJ, Warner WA, Hopper LD, Oleson FB. Acute cardiotoxicity of nucleoside analogs FddA and FddI in rats. Fundam Appl Toxicol. 1993;20:360–364. doi: 10.1006/faat.1993.1046. [DOI] [PubMed] [Google Scholar]
- 65.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, Sopko G, Schluchter MD, Colan SD, Pediatric P Cardiovascular Complications of Vertically Transmitted HIVISG. Cardiovascular status of infants and children of women infected with HIV-1 (P(2)C(2) HIV): a cohort study. Lancet. 2002;360:368–373. doi: 10.1016/S0140-6736(02)09607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipshultz SE, Miller TL, Wilkinson JD, Scott GB, Somarriba G, Cochran TR, Fisher SD. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597. doi: 10.7448/IAS.16.1.18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkinson JD, Williams PL, Leister E, Zeldow B, Shearer WT, Colan SD, Siberry GK, Dooley LB, Scott GB, Rich KC, Lipshultz SE, Pediatric HCS. Cardiac biomarkers in HIV-exposed uninfected children. AIDS. 2013;27:1099–1108. doi: 10.1097/QAD.0b013e32835cf21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keshan Disease Research Group of the Chinese Academy of Medical Sciences. Observations on effect of sodium selenite in prevention of Keshan disease. Chin Med J (Engl) 1979;92:471–476. [PubMed] [Google Scholar]
- 69.Chariot P, Perchet H, Monnet I. Dilated Cardiomyopathy in HIV-Infected Patients [letter] N Engl J Med. 1999;340:732–735. doi: 10.1056/NEJM199903043400911. [DOI] [PubMed] [Google Scholar]
- 70.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, Capeau J, Cohen A. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 71.Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, Touzery C, Hamblin J, Gudjoncik A, Cottin Y, Quantin C. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127:1767–1774. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 72.Escaffre N, Morin M, Bouhnik AD, Fuzibet JG, Gastaut JA, Obadia Y, Moatti JP. Injecting drug users’ adherence to HIV antiretroviral treatments: physicians’ beliefs. AIDS Care. 2000;12:723–730. doi: 10.1080/09540120020014264. [DOI] [PubMed] [Google Scholar]
- 73.Yehia BR, Fleishman JA, Wilson L, Hicks PL, Gborkorquellie TT, Gebo KA. Incidence of and risk factors for bacteraemia in HIV-infected adults in the era of highly active antiretroviral therapy. HIV Med. 2011;12:535–543. doi: 10.1111/j.1468-1293.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 74.Lipshultz SE, Orav EJ, Sanders SP, Colan SD. Immunoglobulins and left ventricular structure and function in pediatric HIV infection. Circulation. 1995;92:2220–2225. doi: 10.1161/01.cir.92.8.2220. [DOI] [PubMed] [Google Scholar]
- 75.Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–1047. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 76.Rangasetty UC, Rahman AM, Hussain N. Reversible right ventricular dysfunction in patients with HIV infection. South Med J. 2006;99:274–278. doi: 10.1097/01.smj.0000202698.25909.97. [DOI] [PubMed] [Google Scholar]
- 77.Diogenes MS, Carvalho AC, Succi RC. Reversible cardiomyopathy subsequent to perinatal infection with the human immunodeficiency virus. Cardiol Young. 2003;13:373–376. [PubMed] [Google Scholar]
- 78.Patel K, van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR., 3rd The Impact of HAART on Cardiomyopathy among Children and Adolescents Perinatally Infected with HIV-1. AIDS. 2012;26:2027–2037. doi: 10.1097/QAD.0b013e3283578bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 80.Roland ME, Havlir DV. Responding to Organ Failure in HIV-Infected Patients. New Engl J Med. 2003;348:2279–2281. doi: 10.1056/NEJMp030074. [DOI] [PubMed] [Google Scholar]
- 81.Ciuffreda D, Pantaleo G, Pascual M. Effects of immunosuppressive drugs on HIV infection: implications for solid-organ transplantation. Transpl Int. 2007;20:649–658. doi: 10.1111/j.1432-2277.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 82.Anthuber M, Kemkes BM, Heiss MM, Schuetz A, Kugler C. HIV infection after heart transplantation: a case report. J Heart Lung Transplant. 1991;10:611–613. [PubMed] [Google Scholar]
- 83.Calabrese LH, Albrecht M, Young J, McCarthy P, Haug M, Jarcho J, Zackin R. Successful cardiac transplantation in an HIV-1-infected patient with advanced disease. N Engl J Med. 2003;348:2323–2328. doi: 10.1056/NEJMoa022935. [DOI] [PubMed] [Google Scholar]
- 84.Gupta S, Markham DW, Mammen PP, Kaiser P, Patel P, Ring WS, Drazner MH. Long-term follow-up of a heart transplant recipient with documented seroconversion to HIV-positive status 1 year after transplant. Am J Transplant. 2008;8:893–896. doi: 10.1111/j.1600-6143.2008.02154.x. [DOI] [PubMed] [Google Scholar]
- 85.Uriel N, Jorde UP, Cotarlan V, Colombo PC, Farr M, Restaino SW, Lietz K, Naka Y, Deng MC, Mancini D. Heart transplantation in human immunodeficiency virus-positive patients. J Heart Lung Transplant. 2009;28:667–669. doi: 10.1016/j.healun.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Castel MA, Perez-Villa F, Miro JM. Heart transplantation in HIV-infected patients: more cases in Europe. J Heart Lung Transplant. 2011;30:1418. doi: 10.1016/j.healun.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 87.Fieno DS, Czer LS, Schwarz ER, Simsir S. Left ventricular assist device placement in a patient with end-stage heart failure and human immunodeficiency virus. Interact Cardiovasc Thorac Surg. 2009;9:919–920. doi: 10.1510/icvts.2009.215244. [DOI] [PubMed] [Google Scholar]
- 88.Mehmood S, Blais D, Martin S, Sai-Sudhakar C. Heartmate XVE destination therapy for end-stage heart failure in a patient with human immunodeficiency virus. Interact Cardiovasc Thorac Surg. 2009;9:909–910. doi: 10.1510/icvts.2009.212076. [DOI] [PubMed] [Google Scholar]
- 89.Sims DB, Uriel N, Gonzalez-Costello J, Deng MC, Restaino SW, Farr MA, Takayama H, Mancini DM, Naka Y, Jorde UP. Human immunodeficiency virus infection and left ventricular assist devices: a case series. J Heart Lung Transplant. 2011;30:1060–1064. doi: 10.1016/j.healun.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Joyce DL, Southard RE, Torre-Amione G, Noon GP, Land GA, Loebe M. Impact of Left Venticular Assist Device (LVAD)-mediated Humoral Sensitization on Post-transplant Outcomes. J Heart Lung Transplant. 2005;24:2054–2059. doi: 10.1016/j.healun.2005.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.