Abstract
Reconsolidation is the process whereby consolidated memories are destabilized upon retrieval and restabilized to persist for later use. Although the neurobiology of reconsolidation of both appetitive and aversive memories has been intensively investigated, reconsolidation of memories of physiologically relevant social rewards has received little attention. Social play, the most characteristic social behaviour displayed by young mammals, is highly rewarding, illustrated by the fact that it can induce conditioned place preference (CPP). Here, we investigated the role of signaling mechanisms implicated in memory processes including reconsolidation, i.e. glucocorticoid, mineralocorticoid, NMDA glutamatergic and CB1 cannabinoid receptors, in the reconsolidation of social play-induced CPP in rats. Systemic treatment with the glucocorticoid receptor antagonist mifepristone before, but not immediately after retrieval, disrupted the reconsolidation of social play-induced CPP. Mifepristone did not affect social play-induced CPP in the absence of memory retrieval. Treatment with the NMDA receptor antagonist MK-801 modestly affected reconsolidation of social play-induced CPP. However, reconsolidation of social play-induced CPP was not affected by treatment with the mineralocorticoid and CB1 cannabinoid receptor antagonists spironolactone and rimonabant, respectively. We conclude that glucocorticoid neurotransmission mediates the reconsolidation of social reward-related memories in rats. These data indicate that the neural mechanisms of the reconsolidation of social reward-related memories only partially overlap with those underlying reconsolidation of other reward-related memories.
Keywords: Social play behaviour, Conditioned place preference, Reconsolidation, Reward, Glucocorticoid receptor, Mineralocorticoid receptor, NMDA receptor, CB1 receptor, rat
Introduction
Reconsolidation is the process whereby a retrieved memory enters a destabilized state and is subsequently restabilized (Nader et al., 2000). It has been suggested that this process provides an opportunity for updating or strengthening of existing memory traces (Lee, 2009; Inda et al., 2011). During the last decade, an extensive body of literature has emerged on the neural mechanisms underlying the reconsolidation of aversive memory traces, as well as appetitive food and drug memories. However, reconsolidation of memories of physiologically relevant natural rewards, such as social behaviour, has received little attention (Perrin et al., 2007).
To address this issue, we have recently demonstrated a long-term impairing effect of the beta-adrenoceptor antagonist propranolol on reconsolidation of social reward-related memory using social play behaviour-induced conditioned place preference (CPP) (Achterberg et al., 2012). Social play, the most characteristic social behaviour in juvenile and adolescent mammals, serves to facilitate social, physical and cognitive development (Panksepp et al., 1984; Vanderschuren et al., 1997; Špinka et al., 2001; Pellis and Pellis, 2009; Baarendse et al., 2013). Social play is highly rewarding (Vanderschuren et al., 1997; Trezza et al., 2010, -2011a), as is apparent from the observations that it can induce CPP (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Thiel et al., 2008; Trezza et al, 2009, -2011b). Because place conditioning relies on an associative mechanism, it can be used to study the dynamics of emotionally charged memories (Bernardi et al., 2006; Fricks-Gleason and Marshall, 2008).
Studies into the neural underpinnings of the reconsolidation process have identified a number of signaling mechanisms involved, including the beta noradrenergic, N-methyl-D-aspartate (NMDA), cannabinoid 1 (CB1) and glucocorticoid receptors in several paradigms and species (for reviews see Tronson and Taylor, 2007; Besnard et al., 2012). There is a large amount of literature showing that glucocorticoid hormones, such as corticosterone, strengthen memory of emotionally arousing experiences (De Quervain et al., 1998, -2009; Roozendaal et al., 2008). These hormones bind to glucocorticoid and mineralocorticoid receptors in brain areas involved in learning and memory, such as the hippocampus, amygdala and prefrontal cortex (De Kloet et al., 2005). Blocking glucocorticoid receptors has been found to impair reconsolidation of aversive events (Jin et al., 2007; Wang et al., 2008; Taubenfeld et al., 2009; Pitman et al., 2011; Nikzad et al., 2011), whereas blocking the mineralocorticoid receptor was found to interfere with the retrieval of fear memory in mice (Zhou et al., 2011). Interestingly, there is substantial evidence that the release of glucocorticoids is initiated not only in response to aversive stimuli but also in response to rewarding stimuli such as food, drugs of abuse, sex and social play (Piazza and Le Moal, 1997; Gordon et al., 2002; Koolhaas et al., 2011; Buwalda et al., 2012). Indeed, increased glucocorticoid levels have been shown to improve the acquisition and consolidation of appetitive memories (Micheau et al., 1981, 1985; Zorawski and Killcross, 2002; Wichmann et al. 2012).
Glutamatergic NMDA receptors have been widely implicated in the acquisition, (re)consolidation and extinction of both aversive and appetitive memory traces (Przybyslawski and Sara, 1997; Suzuki et al., 2004; Lee et al., 2006a; Lee and Everitt, 2008). In particular, blockade of NMDA receptors was found to interfere with reconsolidation of drug-induced CPP (Kelley et al., 2007; Sadler et al., 2007, Zhai et al., 2008; Wu et al., 2012). Cannabinoid CB1 receptors are expressed in brain regions involved in memory processing, including the hippocampus, amygdala and prefrontal cortex (Katona et al., 2001; Wilson and Nicoll, 2002; Li et al., 2008), and treatment with the CB1 receptor antagonist rimonabant has been shown to impair the reconsolidation process for both aversive and appetitive memories (Bucherelli et al. 2006; Yu et al. 2009, Fang et al. 2011). To the best of our knowledge, however, the effect of blocking glucocorticoid, mineralocorticoid, NMDA or CB1 receptors has not been investigated with respect to the reconsolidation of social reward-related memories.
In the present study, we therefore investigated whether retrieved social reward-related memories in a social play-induced CPP paradigm could be disrupted by administration of the glucocorticoid receptor antagonist mifepristone, the mineralocorticoid receptor antagonist spironolactone, the NMDA receptor antagonist MK-801 or the CB1 receptor antagonist rimonabant, in rats. We hypothesized that when social reward-related memories reconsolidate following memory retrieval, mifepristone, spironolactone, MK-801 and rimonabant would attenuate CPP on a subsequent test by persistently disrupting the memory trace. We predicted that this would also prevent reinstatement of CPP following extinction and retraining.
Methods
Ethics statement
All experiments were approved by the Animal Ethics Committee of Utrecht University and were in agreement with Dutch laws (Wet op Dierproeven 1996) and European regulations (Guideline 86/609/EEC).
Subjects
Male Wistar rats (Charles River, Sulzfeld, Germany) arrived in our animal facility at 21 days of age and were housed in groups of three or four in 40 × 26 × 20 cm (l × w × h) Macrolon cages under controlled conditions (i.e. temperature 20–24°C, 60–65% relative humidity and 12/12 h light cycle with lights on at 07.00 h). Upon arrival, the animals were allowed at least 5 days of acclimatization to the facility and were handled for 3 days before the start of the experiment. Food and water were freely available. All animals were experimentally naïve and were used only once.
Apparatus
Place conditioning was performed as previously described (Trezza et al., 2009; -2011b; Achterberg et al., 2012). The place conditioning setup (TSE System, Bad Homburg, Germany) comprised 8 boxes, each consisting of three compartments with removable Plexiglas lids: two equally sized large conditioning compartments (30 × 25 × 30 cm; l × w × h) separated by a smaller, neutral compartment (10 × 25 × 30 cm; l × w × h). The two conditioning compartments had different visual and tactile cues, which also differed from the cues in the middle compartment. The position of the animal in the apparatus was monitored by an array of photobeam sensors located 2.5 cm above the floor. A computer recorded the time (in ms) the animals spent in each compartment. All place conditioning experiments were performed in a sound attenuated and dimly lit room.
Experimental procedures
Effects of pre- or post-retrieval mifepristone on social play-induced CPP
The aim of this experiment was to investigate the effect of pre- or post-retrieval mifepristone treatment on reconsolidation and reinstatement of social play-induced CPP. At 26 days of age (day 1), each rat was placed in the middle compartment of the CPP apparatus and pre-conditioning side preference was determined by allowing the rats to move freely around the three compartments of the apparatus for 15 min (Pretest). On the basis of their Pretest scores, rats were assigned to a treatment group and to the compartment in which they would be allowed social interaction during conditioning. We used a counterbalanced place conditioning design (Tzschentke, 2007; Veeneman et al., 2011), meaning that the pre-conditioning preference in each experimental group for the to be social-paired or non-social paired compartment approximated 50%. As a result, based on their Pretest performance, half of the rats in each experimental group was conditioned in their preferred compartment and half was conditioned in their non-preferred compartment. This procedure rules out the possibility that preference shifts are the result of decreased avoidance of the non-preferred compartment. After the Pretest, rats were individually housed throughout the conditioning period to increase their motivation for social interaction and to facilitate the development of social play-induced CPP (Trezza et al., 2009).
Place conditioning began on day 2. Rats underwent eight consecutive days of conditioning, with two conditioning sessions per day. On days 2, 4, 6 and 8 of the experiment, rats were placed for 30 min in one compartment with an initially unfamiliar partner (social session) in the morning, and were placed alone in the other compartment (non-social session) in the afternoon. The composition of the pairs of rats during the social sessions was changed daily. As a result, the animals interacted with the same partner on every third conditioning session, in order to prevent the development of a dominance/subordination relationship within a test pair. All animals were used for analysis of CPP, i.e., no neutral `stimulus animals' were used. On days 3, 5, 7 and 9, the order of sessions was reversed, i.e. rats were placed alone in one side of the CPP apparatus during the morning session, and were placed in the other compartment with the social partner in the afternoon session. Social and non-social conditioning-sessions were separated by at least one hour. On day 10, rats were placed in the middle compartment, where they were allowed to explore the entire apparatus for 15 min (retrieval; RETR). The time spent in each compartment was recorded. The animals were treated with vehicle or mifepristone (30 mg/kg, s.c.) either 30 min before (pre-retrieval treatment) or immediately after the retrieval session (post-retrieval treatment). The next day, the animals were placed in the middle compartment again and were again allowed to move freely in the apparatus for 15 min to investigate the effect of mifepristone treatment (TEST); this test is also considered the first extinction session. This procedure was repeated once a day for the following days to extinguish place preference, i.e., until the mean difference between the time spent in the social-paired and the non-social-paired compartments was no longer statistically significant for four consecutive days in all the experimental groups. This took between 5 and 10 extinction sessions. Twenty-four hours after the last extinction session, the rats received a reconditioning session. Each rat was placed in the social compartment with a social partner for 30 min (social session) and at least 1 hour later, it was placed in the non-social compartment alone for 30 min (non-social session). The next day, the animals were exposed to the whole apparatus for 15 min and preference was determined again (reinstatement, REIN). As the pre-retrieval and the post-retrieval vehicle groups did not differ significantly in the time they spent in each compartment, the data of these groups were collapsed.
We also investigated whether memory retrieval is necessary for mifepristone to affect reconsolidation of social play-induced CPP. To that aim, the animals were conditioned as described above. On day 10, instead of a memory retrieval session, animals were treated with mifepristone or vehicle in their home cage. The next day, both groups were tested (TEST) as above.
Effects of pre- or post-retrieval spironolactone on social play-induced CPP
This experiment was designed to investigate the effect of administration of the mineralocorticoid receptor antagonist spironolactone (50 mg/kg, s.c.) on retrieval and reconsolidation of memory for social play-induced CPP. The animals were treated with vehicle or spironolactone either 30 min before (pre-retrieval treatment) or immediately after the retrieval session (post-retrieval treatment). Animals were trained and tested for retrieval (RETR), reconsolidation (TEST) and reinstatement (REIN) as in experiment 1.
Effects of pre- or post-retrieval MK-801 on social play-induced CPP
This experiment was designed to investigate the effect of treatment with the NMDA receptor antagonist MK-801 (0.1 or 0.2 mg/kg, i.p.) on retrieval and reconsolidation of memory for social play-induced CPP. The animals were treated with vehicle or MK-801 either 30 min before (pre-retrieval treatment) or immediately after the retrieval session (post-retrieval treatment). The 0.2 mg/kg dose was only used post-retrieval because of its disruptive effect on behaviour, which could interfere with memory processing and with the expression of CPP. Animals were trained and tested for retrieval (RETR), reconsolidation (TEST) and reinstatement (REIN) as in experiment 1.
Effects of pre- or post-retrieval rimonabant on social play-induced CPP
This experiment was designed to investigate the effect of treatment with the cannabinoid CB1 receptor antagonist rimonabant (1.0 mg/kg, i.p.) on retrieval and reconsolidation of memory for social play-induced CPP. The animals were treated with vehicle or rimonabant either 30 min before (pre-retrieval treatment) or immediately after the retrieval session (post-retrieval treatment). Animals were trained and tested for retrieval (RETR), reconsolidation (TEST) and reinstatement (REIN) as in experiment 1. Because rimonabant is known to have pruritic effects (Cook et al. 1998; Rubino et al. 2000; Tallett et al. 2007; Vickers et al. 2003), which may interfere with the expression of memory retrieval, scratching behaviour was scored for the animals that received rimonabant prior to retrieval.
Drugs
The glucocorticoid receptor antagonist mifepristone (RU38486, Tocris Bioscience, UK) and the mineralocorticoid receptor antagonist spironolactone (Tocris Bioscience, UK) were dissolved in propylene glycol (Sigma-Aldrich, Germany) and administered s.c. (mifepristone, 30 mg/kg; spironolactone, 50 mg/kg). The noncompetitive NMDA receptor antagonist (+)-5-methyl-10,11-dihydro-SH-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801, Tocris Bioscience, UK) was dissolved in saline and administered i.p. (0.1 or 0.2 mg/kg). The CB1 cannabinoid receptor antagonist rimonabant (SR141716A, National Institute of Mental Health's Chemical Synthesis and Drug Supply Program, National Institutes of Health, Bethesda, MD, USA) was dissolved in 5% Tween 80, 5% polyethylene glycol/saline and administered i.p. (1.0 mg/kg). In all the experiments, the injection volume was 2 ml/kg. Drug doses are based on literature about memory processing in rats (Pitman et al., 2011; Vafaei et al., 2011; Yu et al., 2009; Brown et al., 2008; Lee et al., 2006b).
Statistical analysis
Data were analyzed using SPSS software 15.0 for Windows. For each experiment, the time spent in the social paired and non-social paired compartments was expressed as mean ± SEM. Data were analyzed using ANOVA (mixed-model or two-way, depending on the experiment), using compartment (social or non-social) and treatment (mifepristone/spironolactone/MK-801/rimonabant or vehicle) as a between-subjects factor and test-day as a repeated-measures factor. The ANOVA was followed by Student's paired t-tests when appropriate, to investigate differences between the time spent in the social and non-social compartment. Differences in the time spent scratching were analyzed by a independent-samples t-test.
Results
Pre-retrieval treatment with the glucocorticoid receptor antagonist mifepristone disrupted reconsolidation but not retrieval of social reward-related memories
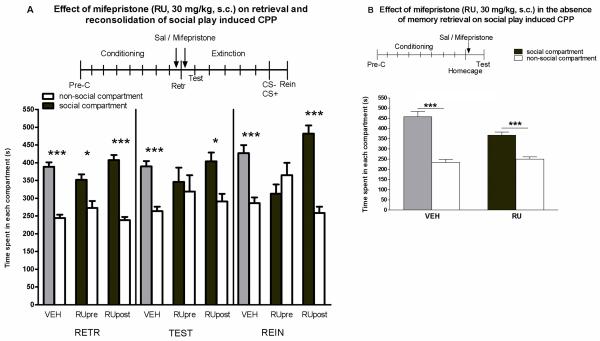
The mixed-model ANOVA revealed significant effects of test day (F2,248= 5.07, p=0.01) and compartment (F1,124= 78.38, p<0.001) and significant compartment × treatment (F2,124= 10.39, p<0.001) and test day × compartment × treatment (F4,248= 2.96, p<0.02) interaction. TThere was no significant main effect of treatment (F2,124= 0.88, n.s.) or other interaction effects (test day × compartment: F2,248= 1.57, n.s. and test day × treatment: F4,248= 0.23, n.s., figure 1a). Post hoc analysis revealed that on day 10 all the groups showed a significant social play-induced CPP (RETR: veh: t(31)= 7.41, p<0.001, n=32; pre: t(8)= 2.40, p<0.05, n=9; post: t(23)= 8.40, p<0.001, n=24), indicating that mifepristone treatment did not affect retrieval of social play-induced CPP. Twenty-four hours later (TEST), the vehicle- and the post-retrieval mifepristone-treated animals still showed a significant preference for the play-paired compartment (veh: t(31)= 4.81, p<0.001, post: t(24)= 2.55, p<0.001), whereas the pre-retrieval mifepristone-treated animals no longer showed a preference (pre: t(8)= 0.32, n.s.). Following the reconditioning session, both the vehicle-treated and the post-retrieval mifepristone-treated animals showed a significant social play-induced CPP (REIN: veh: t(31)= 3.88, p<0.001, post: t(24)= 5.65, p<0.001), whereas no significant reinstatement of CPP was found in the animals treated with mifepristone before retrieval (pre: t(8)= 0.88, n.s.). These findings indicate that the glucocorticoid receptor antagonist mifepristone disrupts reconsolidation of social reward-related memory when administered before, but not when administered immediately after a retrieval session.
Figure 1.
(A) Effects of pre- and post-retrieval mifepristone (RU486; RU) on social play-induced CPP. The experimental protocol is depicted above the graph (Pre-C: pre-conditioning test, CS+: conditioning session with a play-partner, CS−: conditioning session alone). Data represent the mean time (s + SEM) spent in the social compartment (grey and black bars) and the non-social compartment (white bars) during 15 min retrieval- (RETR), test- (TEST) and reinstatement- (REIN) sessions. Vehicle-treated animals (VEH: 2 ml/kg, s.c., n= 32), mifepristone-treated animals (30 mg/kg, s.c., treatment pre-retrieval: Rupre, n=:9; treatment post-retrieval: Rupost: n= 24). (B) Effects of mifepristone on social play-induced CPP in the absence of memory-retrieval. Vehicle-treated animals (VEH; 2ml/kg, i.p., n= 6), mifepristone-treated animals (RU, 30 mg/kg, i.p., n= 10). Post-hoc Student's paired t-tests for difference in time spent in the social- and non-social compartment *p<0.05, **p<0.01, ***p<0.001.
Treatment with mifepristone did not affect reconsolidation of social reward-related memories in the absence of memory retrieval (figure 1b). Twenty-four hours after administration of mifepristone in the home cage (i.e., without a retrieval session), both the vehicle and the mifepristone-treated rats showed a significant preference for the social compartment. The two-way ANOVA revealed significant effects of compartment (F1,28= 120.25, p<0.001) and treatment (F1,28= 8.45, p<0.01) and a significant compartment × treatment interaction (F1,28= 14.02, p=0.001). Post-hoc analysis showed that both the vehicle- and the mifepristone-treated animals showed a significant preference for the social-paired compartment (veh: t(5)= 6.98, p<0.001, n=6; mifepristone: t(9)= 5.06, p<0.001, n=10). These results indicate that mifepristone treatment without a retrieval session does not affect reconsolidation of social play-induced CPP.
The mineralocorticoid receptor antagonist spironolactone did not affect retrieval or reconsolidation of social reward-related memories
The mixed-model ANOVA showed significant effects of compartment (F1,52= 69.92, p<0.001) and test day (F2,104= 3.70, p<0.05). No other significant main or interaction effects were found (treatment: F2,52= 0.04; compartment × treatment: F2,52= 0.43; testday × compartment: F2,104= 0.89; test day × treatment: F4,104= 0.05; test day × treatment × compartment: F4,104= 1.01, all n.s.). All the treatment-groups showed a significant preference for the play-paired compartment at RETR and TEST and reinstatement of social play-induced CPP (figure 2, vehicle: n=12, pre-retrieval spironolactone: n=10, post-retrieval spironolactone: n=7). These results indicate that administering spironolactone either 30 min before or immediately after a retrieval session does not affect retrieval or reconsolidation of social play-induced CPP (figure 2).
Figure 2.
Effects of pre- and post-retrieval spironolactone on social play-induced CPP. The experimental protocol is depicted above the graph (Pre-C: pre-conditioning test, CS+: conditioning session with a play-partner, CS−: conditioning session alone). Data represent the mean time (s + SEM) spent in the social compartment (grey and black bars) and the non-social compartment (white bars) during 15 min retrieval- (RETR), test- (TEST) and reinstatement- (REIN) sessions. Vehicle-treated animals (VEH: 2 ml/kg, s.c., n= 12), spironolactone-treated animals (30 mg/kg, s.c., treatment pre-retrieval: Sprlpre: n= 10; treatment post-retrieval Sprlpost:, n= 7).
3Effect of the NMDA receptor antagonist MK-801 on retrieval and reconsolidation of social reward-related memories
In the experiment where the effect of 0.1 mg/kg MK-801 was tested, the mixed-model ANOVA revealed significant effects of compartment (F1,172= 146.53, p<0.001) and test day (F2,344= 4.42, p<0.02), and significant compartment × treatment (F2,178= 10.33, p<0.001), test day × compartment (F2,344= 6.83, p<0.002) and test day × compartment × treatment (F4,344= 3.36, p<0.02) interactions. There was no significant main effect of treatment (F2,172= 1.16, n.s.) or test day × treatment interaction (F4,344= 0.56, n.s., figure 3a). Post hoc analysis revealed that at RETR and TEST, all groups showed a significant preference for the play-paired compartment (RETR: veh: t(39)= 9.12, p<0.001, n=40; pre: t(28)= 2.48, p<0.02, n=29; post: t(20)= 7.21, p<0.001, n=19; TEST: veh: t(39)= 6.83, p<0.001, pre: t(28)= 2.19, p<0.05, post: t(19)= 2.31, p<0.05). The vehicle and post-retrieval MK-801 treated animals showed significant reinstatement of social play-induced CPP (REIN: veh: t(39)= 2.27, p<0.05, post: t(20)= 3.21, p<0.01), whereas the pre-retrieval MK-801 treated animals did not (REIN: pre: t(28)= 0.79, n.s.).
Figure 3.
Effects of MK-801 treatment on social play-induced CPP. The experimental protocol is depicted above the graph (Pre-C: pre-conditioning test, CS+: conditioning session with a play-partner, CS-: conditioning session alone). Data represent the mean time (s + SEM) spent in the social compartment (grey and black bars) and the non-social compartment (white bars) during 15 min retrieval- (RETR), test- (TEST) and reinstatement- (REIN) sessions. (A) Effects of pre- and post-retrieval MK-801 (0.1 mg/kg). Vehicle-treated animals (VEH: 2 ml/kg, s.c., n= 40), MK-801-treated animals (0.1 mg/kg, i.p., treatment pre-retrieval: Mkpre:, n= 29; treatment post-retrieval: Mkpost: n= 19). Post-hoc Student's paired t-tests for difference in time spent in the social- and non-social compartment *p<0.05, **p<0.01, ***p<0.001. (B) Effects of post-retrieval MK-801 (0.2 mg/kg). Vehicle-treated animals (VEH: 2 ml/kg, i.p., n= 8), MK-801-treated animals (0.2 mg/kg, i.p., MKpost: n= 8).
In the experiment where the effect of 0.2 mg/kg MK-801 was tested, the mixed-model ANOVA revealed a significant effect of compartment (F1,28= 53.00, p<0.001). No other significant main or interaction effects were found (test day: F2,56= 1.20; treatment: F1,28= 0.08; test day × compartment: F2,56= 1.02; test day × treatment: F2,56= 0.21; test day × compartment × treatment: F2,56= 0.02, all n.s., figure 3b). All groups showed a significant preference for the social-paired compartment at RETR, TEST and REIN (Figure 3b, vehicle: n=8, post-retrieval MK-801: n=8). These results indicate that treatment with 0.2 mg/kg MK-801 immediately after a retrieval session does not affect reconsolidation of social play-induced CPP.
The cannabinoid receptor antagonist rimonabant did not affect retrieval or reconsolidation of social reward-related memories
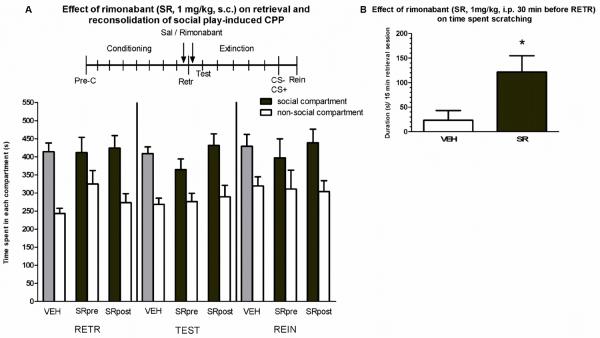
The mixed-model ANOVA revealed a significant effect of compartment (F1,68= 55.59, p<0.001) but no other significant main or interaction effects (test day: F2,136= 1.16; treatment: F2,68= 0.23; treatment × compartment: F2,68= 1.19; test day × compartment: F2,136= 0.27; test day × treatment: F4,136= 0.85; test day × compartment × treatment: F4,136= 0.17, n.s.). All groups showed a significant preference for the social-paired compartment at RETR, TEST and REIN (Figure 4a, vehicle: n= 19, pre-retrieval rimonabant: n=10, post-retrieval rimonabant: n=8). These results show that treatment with rimonabant (1.0 mg/kg) either 30 min before or immediately after a retrieval session does not affect retrieval, reconsolidation or reinstatement of social play-induced CPP. We also found that rimonabant-pretreated animals spent significantly more time scratching during the 15 min test compared to vehicle-treated animals (t(12.87)= −2.52, p<0.05, figure 4b).
Figure 4.
(A) Effects of pre- and post-retrieval rimonabant (SR141716; SR) on social play-induced CPP. The experimental protocol is depicted above the graph (Pre-C: pre-conditioning test, CS+: conditioning session with a play-partner, CS-: conditioning session alone). Data represent the mean time (s + SEM) spent in the social compartment (grey and black bars) and the non-social compartment (white bars) during 15 min retrieval- (RETR), test- (TEST) and reinstatement- (REIN) sessions. Vehicle-treated animals (VEH: 2 ml/kg, i.p., n= 19), rimonabant-treated animals (1.0 mg/kg, i.p., treatment pre-retrieval: SRpre: n= 10; treatment post-retrieval: SRpost: n= 8). (B) Time spent scratching during the 15 min test in pre-retrieval rimonabant-treated animals. Independent samples t-test, *p<0.05.
Discussion
In this study, we investigated the involvement of glucocorticoid, mineralocorticoid, NMDA and cannabinoid CB1 receptors in retrieval and reconsolidation of social reward-related memories in rats. Our hypothesis was that blocking these receptors would disrupt the reconsolidation of social play-induced CPP. We show that: (1) the glucocorticoid receptor antagonist mifepristone disrupts reconsolidation of social play-induced CPP when administered before a retrieval session; (2) neither the mineralocorticoid receptor antagonist spironolactone, nor the CB1 cannabinoid receptor antagonist rimonabant affected retrieval or reconsolidation of social play-induced CPP, whereas pre-retrieval treatment with the NMDA receptor antagonist MK-801 modestly affected social play-induced CPP. Together, our data show that glucocorticoid neurotransmission mediates the reconsolidation of social play-induced CPP without affecting the retrieval process whereas mineralocorticoid, NMDA and CB1 cannabinoid receptors are not primarily involved in the dynamics of social reward-related memories.
In the first experiment, vehicle- and post-retrieval mifepristone treated animals showed a preference for the social-paired compartment 24h after retrieval, whereas pre-retrieval mifepristone-treated animals did not. This effect of mifepristone was not the result of a non-specific memory impairment, since mifepristone-treatment in the absence of retrieval did not alter social play-induced CPP (Tronel and Alberini, 2007; Jin et al, 2007; Taubenfeld et al., 2009; Nikzad et al., 2011; Pitman et al., 2011). Furthermore, following extinction of CPP, vehicle- and post-retrieval mifepristone-treated animals showed reinstatement of CPP 24h after a reconditioning session, whereas pre-retrieval mifepristone-treated animals did not. The inability to reinstate social play-induced CPP in the pre-retrieval mifepristone-treated group suggests that acute pre-retrieval mifepristone persistently disrupted the social play-CPP memory trace, rather than inducing a retrieval deficit or facilitating extinction learning (for discussion see Achterberg et al., 2012). Our findings are consistent with previous reports showing that mifepristone treatment (either systemic or intra-amygdala/hippocampus) blocks reconsolidation of fear memories, while sparing retrieval (Tronel and Alberini, 2007; Jin et al, 2007; Taubenfeld et al., 2009; Nikzad et al., 2011; Pitman et al., 2011), although it should be noted that most of these previous studies employed post-retrieval mifepristone treatment, which was ineffective in our study. One likely explanation for this apparent discrepancy is that we used a relatively long retrieval session, because in our experience, the expression of CPP is difficult to detect using shorter retrieval sessions. In this scenario, post-retrieval mifepristone is less effective in interfering with reconsolidation since the glucocorticoid receptor-dependent processes involved in the reconsolidation process may take less than 15 min. Interestingly, all the above studies that showed glucocorticoid receptor involvement in reconsolidation were conducted in fear-learning paradigms. Therefore, the present study extends the involvement of glucocorticoid receptors to reconsolidation of appetitive memories. Pleasurable stimuli such as food, drugs of abuse or sex are known to cause a rise in corticosterone levels (Piazza and Le Moal, 1997; Koolhaas et al., 2011; Buwalda et al., 2012). Indeed, an episode of social play also evokes an increase in corticosterone levels in rats (Gordon et al., 2002). Moreover, increasing glucocorticoid levels improves acquisition and/or consolidation of appetitive memory (Micheau et al., 1981, 1985; Zorawski and Killcross, 2002; Wichmann et al. 2012) suggesting a role for glucocorticoid receptors in the initial stages of appetitive memory formation. Our data add to this by demonstrating that reconsolidation of reward-related memory can be disrupted by antagonizing glucocorticoid receptors. Whether other reward-related memories, such as drug-reward memory, are affected by antagonizing glucocorticoid receptors remains to be elucidated. The mineralocorticoid receptor antagonist spironolactone did not interfere with retrieval or reconsolidation of social reward-related memories. Consistent with our findings, Vafaei et al. (2011) found no effect of spironolactone (either systemically and intra-hippocampus) on reconsolidation of inhibitory avoidance memory. On the other hand, in a fear conditioning paradigm, blocking the mineralocorticoid receptors with spironolactone before a brief context retrieval-session, but not a cue-tone retrieval session, disrupted subsequent expression of fear, although post-retrieval treatment with spironolactone was ineffective (Zhou et al., 2011). Thus, mineralocorticoid receptors may be involved in the reconsolidation of certain aversive rather than appetitive memories. However, the contribution of other factors to the discrepancies between the studies (i.e. reliance on cues vs. contextual information, and species and age differences of the animals tested) can at this point not be ruled out, since literature on the role of the mineralocorticoid receptor in reconsolidation is very limited.
Treatment with MK-801 modestly affected reconsolidation of social play-induced CPP. Thus, post-retrieval treatment with MK-801 did not alter the expression of social play-induced CPP during the tests for reconsolidation and reinstatement. After pre-retrieval treatment with 0.1 mg/kg MK-801, there was significant CPP during retrieval and the test for reconsolidation, albeit of a lesser magnitude than seen in the vehicle-treated rats. Interestingly, after reconditioning, there was no reinstatement of CPP in the animals treated with 0.1 mg/kg MK-801 pre-retrieval. This suggests that pre-retrieval NMDA receptor blockade impaired the integrity of the memory trace to some extent. Previously, systemic blockade of NMDA receptors has been found to block reconsolidation of aversive (Suzuki et al., 2004; Lee et al., 2006b) as well as drug- and food reward memory (Kelley et al, 2007; Sadler et al, 2007; Brown et al, 2008; Itzak 2008; Lee and Everitt, 2008; Milton et al, 2008). There are several explanations for our findings that MK-801 treatment did not profoundly disrupt reconsolidation of social play-induced CPP in the present study. Thus, Ben Mamou et al. (2006) and Milton et al. (2013) have shown a role for different subtypes of NMDA receptors in the destabilization and reconsolidation of memory. Blocking NR2B-containing NMDA receptors in the basolateral amygdala prevents the reactivation of a conditioned fear memory, whereas that NR2A-containing NMDA receptors are specifically implicated in reconsolidation of fear memory. It is therefore possible that pre-retrieval MK-801 administration inhibited the reactivation of the social play-CPP memory trace. As a result, reconsolidation could not be completely blocked because the memory trace was not in a fully active state. This retrieval-inhibition explanation is consistent with the reduced magnitude of CPP after pre-retrieval MK-801 treatment. Furthermore, treatment with NMDA receptor antagonists disrupts extinction learning (Suzuki et al, 2004; Lee et al, 2006b; Chan and McNally, 2009). According to Suzuki et al. (2004) there is a brief time window for reconsolidation after retrieval (approximately 3 min), whereas extinction only occurs after prolonged exposure (30 min). As explained above, we used a 15 min reactivation session, which may result in competing reconsolidation and extinction processes, whereby MK-801 administration could affect both, so that the social play CPP memory trace would remain relatively intact.
Neither retrieval nor reconsolidation of social play-induced CPP was disrupted by administration of the CB1 receptor antagonist rimonabant. There is no consensus in the literature on the effect of CB1 antagonists on aversive memory, as disruption (Bucherelli et al. 2006), facilitation (De Oliviera Alvares et al. 2008) and lack of an effect (Suzuki et al. 2008) on reconsolidation have been found. Interestingly, systemic treatment with rimonabant has been shown to disrupt reconsolidation of nicotine-induced and methamphetamine-induced CPP (Fang et al., 2011; Yu et al., 2009). However, these studies used a higher dose of rimonabant (3.0 mg/kg), which leaves the possibility open that this reconsolidation blockade occurred through a non-CB1 receptor-dependent mechanism of action of rimonabant. Moreover, rimonabant is known to be pruritogenic (Cook et al. 1998; Rubino et al. 2000; Tallett et al. 2007; Vickers et al. 2003). Indeed, we found a significant increase in scratching in rimonabant-treated animals. We therefore did not test the 3.0 mg/kg dose of rimonabant, since scratching severely disrupts behaviour, which may interfere with memory processing in the CPP box. Treatment with CB1 receptor antagonists has been shown to disrupt extinction learning in aversive paradigms (Marsicano et al. 2002; Suzuki et al. 2004; Niyuhire et al. 2007) but their role in extinction of appetitive memories is not clear (Hernandez and Cheer, 2011, Manwell et al. 2009). This makes it unlikely that the lack of effect of rimonabant on social play-induced CPP is the result of interference with reconsolidation and extinction at the same time. However, CB1 receptors are thought to be required for memory destabilization (Suzuki et al. 2004; -2008). In conclusion, our data do not support a role for CB1 receptors in the reconsolidation of social reward memories, but the contribution of a destabilization blockade in our findings can as yet not be excluded.
In conclusion, the present study extends our knowledge about reconsolidation of social reward-related memories in rats, showing that this type of reward memory is subject to the impairing effects of glucocorticoid receptor antagonism. However, our data do not support a primary role for mineralocorticoid, NMDA or CB1 receptors in reconsolidation of social reward-related memories in rats.
Acknowledgements
We thank Dr. Henk Karst for valuable advice on the study.
Source of funding Supported by the National Institute on Drug Abuse Grant R01 DA022628 (L.J.M.J.V.), Netherlands Organization for Scientific Research (NWO) Veni grant 91611052 (V.T.), and Marie Curie Career Reintegration Grant PCIG09-GA-2011-293589 (V.T.).
Footnotes
Conflict of interest The authors declared that no conflict of interest.
References
- Achterberg EJM, Trezza V, Vanderschuren LJMJ. β-Adrenoreceptor stimulation mediates reconsolidation of social reward-related memories. PLoS ONE. 2012;7:e39639. doi: 10.1371/journal.pone.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–9. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–7. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Besnard A, Caboche J, Laroche S. Reconsolidation of memory: A decade of debate. Prog Neurobiol. 2012;99:61–80. doi: 10.1016/j.pneurobio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–65. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn Mem. 2006;13:426–30. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Scholte J, de Boer SF, Coppens CM, Koolhaas JM. The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm Behav. 2012;61:218–26. doi: 10.1016/j.yhbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspects of social interaction in juvenile rats. Physiol Behav. 1992;51:667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Chan WYM, McNally GP. Conditioned stimulus familiarity determines effects of MK-801 on fear extinction. Behav Neurosci. 2009;123:303–14. doi: 10.1037/a0014988. [DOI] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–6. [PubMed] [Google Scholar]
- Crowder WF, Hutto CW. Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–24. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- Fang Q, Li F-Q, Li -Q, Xue Y-X, He Y-Y, Liu J-F, et al. Cannabinoid CB 1 receptor antagonist rimonabant disrupts nicotine reward-associated memory in rats. Pharmacol Biochem Behav. 2011;99:738–42. doi: 10.1016/j.pbb.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval β-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–8. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull. 2002;57:651–9. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF. Extinction learning of rewards in the rat: is there a role for CB1 receptors? Psychopharmacology. 2011;217:189–97. doi: 10.1007/s00213-011-2275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: From reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–43. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann NY Acad Sci. 2008;1139:350–7. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- Jin X-C, Lu Y-F, Yang X-F, Ma L, Li B-M. Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci. 2007;25:3702–12. doi: 10.1111/j.1460-9568.2007.05621.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: Disruption and reinstatement. Neuroreport. 2007;18:777–80. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: A critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–21. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem. 2008;90:147–54. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006a;26:5881–7. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci. 2006b;26:10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Fang Q, Liu Y, Zhao M, Li D, Wang J, Lu L. Cannabinoid CB(1) receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. Eur J Pharmacol. 2008;589:122–6. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav. 2009;94:154–62. doi: 10.1016/j.pbb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascioll MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Micheau J, Destrade C, Soumireu-Mourat B. Intraventricular corticosterone injection facilitates memory of an appetitive discriminative task in mice. Behav Neural Biol. 1981;31:100–4. doi: 10.1016/s0163-1047(81)91162-6. [DOI] [PubMed] [Google Scholar]
- Micheau J, Destrade C, Soumireu-Mourat B. Time-dependent effects of posttraining intrahippocampal injections of corticosterone on retention of appetitive learning tasks in mice. Eur J Pharmacol. 1985;106:39–46. doi: 10.1016/0014-2999(84)90675-7. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JLC, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–7. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B-and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. J Neurosci. 2013;33:1109–15. doi: 10.1523/JNEUROSCI.3273-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nikzad S, Vafaei AA, Rashidy-Pour A, Haghighi S. Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress. 2011;14:459–64. doi: 10.3109/10253890.2010.548171. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology. 2007;191:223–31. doi: 10.1007/s00213-006-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience. 2008;154:1648–55. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–92. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The playful brain: venturing to the limits of neuroscience. Oneworld Publications; Oxford: 2009. [Google Scholar]
- Perrin G, Ferreira G, Meurisse M, Mouly A, Levy F. Social recognition memory requires protein synthesis after retrieval. Behav Neurosci. 2007;121:148–55. doi: 10.1037/0735-7044.121.1.148. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Res Rev. 1997;25:359–72. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Milad MR, Igoe SA, Vangel MG, Orr SP, Tsareva A, et al. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; Propranolol prevents this effect. Behav Neurosci. 2011;125:632–8. doi: 10.1037/a0024364. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–6. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–70. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Zagato E, Sala M, Parolaro D. In vivo characterization of the specific cannabinoid receptor antagonist,SR141716A: behavioral and cellular responses after acute and chronic treatments. Synapse. 2000;35:8–14. doi: 10.1002/(SICI)1098-2396(200001)35:1<8::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sadler R, Herzig V, Schmidt WJ. Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol. 2007;18:699–703. doi: 10.1097/FBP.0b013e3282effb81. [DOI] [PubMed] [Google Scholar]
- Špinka M, Newberry RC, Bekoff M. Mammalian play: Training for the unexpected. Q Rev Biol. 2001;76:141–68. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–95. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Mukawa T, Tsukagoshi A, Frankland PW, Kida S. Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Mem. 2008;15:426–33. doi: 10.1101/lm.888808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology. 2007;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biol Psychiatry. 2009;65:249–57. doi: 10.1016/j.biopsych.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–69. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–9. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011a;1:444–57. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011b;31:6362–70. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Alberini CM. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry. 2007;62:33–9. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–75. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vafaei AA, Pakdel R, Nikzad S, Rashidy-Pour A. Effects of mineralocorticoid receptors blockade on fear memory reconsolidation in rats. Basic Clin Neurosci. 2011;2:58–66. [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Veeneman MMJ, Boleij H, Broekhoven MH, Snoeren EMS, Guitart Masip M, Cousijn J, et al. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology. 2011;214:863–76. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist,SR 141716, on food intake and body weight gain of obese (fa/fa)compared to lean Zucker rats. Psychopharmacology. 2003;167:103–11. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Wang X-Y, Zhao M, Ghitza UE, Li Y-Q, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–10. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann R, Fornari RV, Roozendaal B. Glucocorticoids interact with the noradrenergic arousal system in the nucleus accumbens shell to enhance memory consolidation of both appetitive and aversive taste learning. Neurobiol Learn Mem. 2012;98:197–205. doi: 10.1016/j.nlm.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li Y, Gao J, Sui N. Differential effect of NMDA receptor antagonist in the nucleus accumbens on reconsolidation of morphine -related positive and aversive memory in rats. Eur J Pharmacol. 2012;674:321–6. doi: 10.1016/j.ejphar.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Yu L-L, Wang X-Y, Zhao M, Liu Y, Li Y-Q, Li F-Q, et al. Effects of cannabinoid CB1 receptor antagonist rimonabant in consolidation and reconsolidation of methamphetamine reward memory in mice. Psychopharmacology. 2009;204:203–11. doi: 10.1007/s00213-008-1450-y. [DOI] [PubMed] [Google Scholar]
- Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L. Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol. 2008;19:211–6. doi: 10.1097/FBP.0b013e3282fe88a0. [DOI] [PubMed] [Google Scholar]
- Zhou M, Bakker EH, Velzing EH, Berger S, Oitzl M, Joëls M, Krugers HJ. Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiol Learn Mem. 2010;94:530–7. doi: 10.1016/j.nlm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kindt M, Joëls M, Krugers HJ. Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. PLoS ONE. 2011;6:e26220. doi: 10.1371/journal.pone.0026220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem. 2002;78:458–64. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]