Abstract
In this study, Artemia salina (crustacean filter feeders) larvae were used as a test model to investigate the toxicity of aluminum oxide nanoparticles (Al2O3 NPs) on marine microorganisms. The uptake, toxicity and elimination of α-Al2O3 (50 nm and 3.5 μm) and γ-Al2O3 (5 nm and 0.4 μm) NPs were studied. Twenty-four and ninety-six hour exposures of different concentrations of Al2O3 NPs to Artemia larvae were conducted in a seawater medium. When suspended in water, Al2O3 NPs aggregated substantially with the sizes ranging from 6.3 nm to > 0.3 μm for spherical NPs, and from 250 to 756 nm for rod-shaped NPs. The phase contrast microscope images revealed that NPs deposited inside the guts as aggregates. ICP-MS analysis showed that large particles (3.5 μm α-Al2O3) were not taken up by Artemia, while fine NPs (0.4 μm γ-Al2O3) and ultra-fine NPs (5 nm γ-Al2O3 and 50 nm α-Al2O3) accumulated substantially. Differences in toxicity were detected as changing with NP size and morphology. The malondialdehyde (MDA) levels indicated that smaller γ-Al2O3 (5 nm) NPs were more toxic than larger γ-Al2O3 (0.4 μm) particulates in 96 h. The highest mortality was measured as 34% in 96 h for γ-Al2O3 NPs (5 nm) at 100 mg/L (LC50 > 100 mg/L). γ-Al2O3 NPs were more toxic than α-Al2O3 NPs at in all conditions.
Keywords: Aluminum oxide, nanoparticles, accumulation, toxicity, oxidative stress, Artemia
INTRODUCTION
Nanoscience is becoming a prominent area of research in today's society. For years, nanoparticles (NPs), natural and manmade, have been entering the environment while their effects are unknown. The large increase in production, species and utilization of manufactured NPs has raised concerns that the release of these materials into the environment may pose a serious threat, leading to calls for an environmental risk assessment of NPs (Moore, 2006; Nowack and Bucheli, 2007; Handy et al., 2008; Klaine et al., 2008). As nanomaterials are being widely used in modern technology, there is an increasing need for information regarding their human health and environmental implications. To date, the human health impacts of NPs have received the greatest attention, which has been demonstrated through both in vivo and in vitro studies on mammalian test systems. The properties that make NPs so attractive for commercial applications (e.g. NPs’ size and increased surface area) also can be potentially responsible for undesirable health effects (Oberdörster et al., 2005; Meng et al., 2007; Papageorgiou et al., 2007; Singh et al., 2007; Poland et al., 2008).
Metal and metal oxide NPs are receiving increasing attention for a variety of applications. NPs have unique physicochemical properties, such as their chemistry, tiny size, large surface area, surface reactivity, charge, shape and media interactions. As a result, the properties of NPs differ substantially from their respective bulk materials of the same composition. However, certain novel properties of NPs could lead to adverse biological effects, with the potential for toxicity (Oberdörster et al., 2005; Nel et al., 2006). Aluminum oxide (Al2O3) NPs have been widely used as abrasives, as wear-resistant coatings on propeller shafts of ships, as a way to increase the specific impulse per weight of composite propellants used in solid rocket fuel and as drug delivery systems to increase solubility (Miziolek, 2002; Tyner et al., 2004; Wagner et al., 2007). However, the risks posed by the increased use of Al2O3 NPs are little known. Recently, several researchers studied the effects of Al2O3 NPs on organisms such as E. coli (Sadiq et al., 2009), bacterial cells (Jiang et al., 2009) and microalgae species like Scenedesmus sp. and Chlorealla sp. (Sadiq et al., 2011).
The use of aquatic models to address ecotoxicological issues has become prevalent in recent years (Rozita et al., 2010). Artemia salina, a zooplanktonic crustacean found in a variety of seawater systems from swamps to lakes – and the most popular live food for marine and aquarium fishes – was studied because the species filters a large amount of water per hour. This means that they have significant interaction with their aquatic environment, causing them to face a higher risk of exposure to pollutants than other aquatic species. Artemia larvae are sensitive to toxic substances (Milhem et al., 2008) and are commonly used for toxicity assays in pharmacology and ecotoxicity in a variety of nanoscale materials in marine ecosystems through laboratory experiments. An advantage of the Artemia test is the homogeneity in eggs and freshly-born individuals. Freshly-hatched individuals, called nauplii (larvae), were used for testing. Though the freshly-natal individuals are immediately applied in testing, difficult cultivation is not demanded.
Aluminium oxide exists in various metastable polymorphs (transition aluminas) including γ-phases of Al2O3 in addition to the thermodynamically stable α-Al2O3 form (corundum). The transition aluminas (especially the γ-form) have fine particle sizes and high surface areas with enhanced catalytic surface activity of their surfaces, and find industrial applications as adsorbents, catalysts or catalysts carriers, coatings and soft abrasives (Levin and Brandon 1998, Temujin et al., 2000). The aim of the current investigation was to study the difference (if any) in toxic response and characterization of uncoated α-Al2O3 and γ-Al2O3 NPs. The uptake, toxicity and depuration of these NPs also were compared to those of different sizes and crystal structures. To analyze the possible dose-dependent toxic effects of NPs in vivo, Artemia under acute exposure for 24 and 96 h were used.
MATERIALS AND METHODS
Reagent and Preparation of Nanoparticle Suspension
Dry α-Al2O3 (50 nm and 3.5 μm) and γ-Al2O3 (5 nm and 0.4 μm) NPs were obtained from Skyspring Nanomaterials Inc., in Houston, TX. The NPs were stored at room temperature in the laboratory until the implementation of the experimental studies. The NP stock solutions were prepared as described by Wang et al. (2009). The stock suspensions of α-Al2O3 and γ-Al2O3 were prepared separately by direct addition into MilliQ water (Barnstead E-pure system with resistivity of 18.0 MΩ cm−1) at a stock concentration of 20% w/v. The freshly-prepared stock solutions were vortexed (20 s, 2000 rpm) and ultrasonicated for maximum dispersion using a bath sonicator (10 min). Small magnetic bars were placed into the suspensions for stirring during dilution to avoid particle aggregation and deposition. Appropriate volumes of the stock suspension were immediately transferred into the exposure flask that already contained Artemia larvae in the seawater medium. All other chemicals were obtained from Sigma–Aldrich (St. Louis, MO).
Characterization of Nanoparticles
Powdered X-Ray Diffraction Analysis (XRD)
The powder samples were stored in a desiccator until they were needed. The crystal structures of the NPs were characterized by powdered X-ray powder diffraction (XRD) (D8 Advanced X-ray diffractometer, Bruker, Germany) and scanned with 2.2 kW Cu anode radiation at wavelength 1.54 Å produced by a Ceramic X-ray tube. About 250 mg of α-Al2O3 and γ-Al2O3 NPs were deposited separately on the sample holder for scanning in the range of 10° to 100°. The crystalline formation was determined from the diffraction pattern and the crystallite size was calculated with the Scherrer formula.
Fourier Transform Infrared Spectroscopic Analysis (FT-IR)
All dry α-Al2O3 and γ-Al2O3 NP samples were prepared by an identical procedure for Fourier transform infrared spectroscopy (FT-IR). The FT-IR spectra were recorded using a spectrometer Nicolet Nexus 6700 FT-IR (Thermo Scientific Instruments Groups, Madison, WI) in the range of 4000 to 400 cm−1 and samples were prepared in potassium bromide pellets. Spectra were collected in single beam mode with 32 scans at 4 cm−1 resolution. The instrument detector was set on DTGS (deuterated triglycine sulfate – IR detector material), and the Beam Splitter was set on KBr. The gain parameter used to amplify the signal of the detector was set to Autogain, the velocity of the mirror was set at 0.6329 and the aperture was set at value 60. KBr (150 mg) and the sample (2 to 4 mg) were ground together in a mortar using a pestle, and pellets with good transparency were prepared. First, the background spectrum was recorded, then the sample spectrum was collected in the same conditions. Background was automatically subtracted from sample spectra, and final spectra were recorded in transmission mode. The management of spectra was realized with instrument software (Omnic User's Guide, 2006; Štengl et al., 2010; Palaniappan and Pramod, 2011).
Transmission Electron Microscopic Analysis (TEM)
The particle size and shape of α-Al2O3 and γ-Al2O3 particles were characterized by transmission electron microscopy (TEM) using a JOEL-1011 TEM instrument providing a resolution of JEM-1011 of 0.2 nm lattice with magnification of 50 to 1x106 under the accelerating voltage of 40 to 100 kV. For TEM measurements, a drop of the colloidal solution of NPs was placed onto 50 Å thick carbon-coated copper grids and allowed to dry to record the TEM images. The average particle diameter and size distribution were determined by using ImageJ software. The diameters of approximately 100 particles of α-Al2O3 and γ-Al2O3 were measured in random fields of view of three images to estimate their average size.
Hydrodynamic Light Scattering Analysis (DLS)
The actual size distribution of the α-Al2O3 and γ-Al2O3 NPs in the stock solution and exposure medium was determined by DLS measurements (Malvern Zetasizer Nano ZS Instrument). The actual size distributions of the NPs were examined after suspension in deionized water at the stock concentration. However, the concentration was too high; hence, it was further diluted and adjusted to a lower concentration, so that the device could acquire enough counts per second. A portion of each particular suspension was sonicated for a few minutes to break down visible clumps, then it was placed in a clean 1.5 ml square cuvette for DLS measurements. Five DLS measurements were taken successively for each solution, and an average size was obtained by the software from the intensity, volume and number distributions measured.
Test Organism
The acute toxicity of these NPs was also evaluated using the Artemia salina nauplii (larvae). The Artemia cysts were purchased from Artemia International LLC, Houston, TX and were kept at a temperature of 4°C in a moisture-free container in a refrigerator. The cysts were hatched in artificial seawater (3% m/v). The seawater was prepared by dissolving an appropriate amount of Instant Ocean® salt in deionized water, stirring it for 24 h under aeration and then filtering it through 30-μm Millipore cellulose filters. Artemia were hatched by using the procedure described by Persoone et al. (1989). Briefly, encysted Artemia were first hydrated in distilled water at 4 °C for 12 h and washed to separate the floating cysts from those that sink. The sinking cysts were collected on a Buchner funnel and washed with cold deionized water. Approximately 3 g of the pre-cleaned cysts were incubated in 1.5 L seawater in a conical plastic container with graduations at 30 ± 1 °C. A 1500 lux day-light was provided continuously by a fluorescent lamp. Aeration was maintained by a small line extending to the bottom of the hatching device from an aquarium air pump. Under these conditions, Artemia larvae hatched within 24 h.
Acute Toxicity Bioassays
A preliminary experiment was conducted to evaluate the Artemia larvae's response to different concentrations (5, 10, 50 and 100 mg/L) of the α-Al2O3 (50 nm and 3.5 μm) and γ-Al2O3 (5 nm and 0.4 μm) NPs separetely for 24 and 96 h exposure. Acute toxicity immobilization tests were performed on each of the NPs according to the Organization for Economic Cooperation and Development (OECD part 202) testing guidelines (OECD 2004). A control group also was setup without the target compound. Studies were carried out in triplicate measurements in conical plastic containers (1 L inner volume). Exposures were conducted in 500 mL of seawater for Artemia larvae. Sight aeration was provided by a line extending to the bottom of the conical flask to prevent the settling of NPs from suspension during the course of exposure. The number of Artemia larvae in each group visually counted under magnification lens after sequential dilutions before exposure. The number of larvae were found approximately in the range of 12.9 to 16.1 (x103). A salinity range of 2.9 to 3% m/v, a light regime ratio of 16:8 h light:dark, a water temperature of 24 ± 2 °C, and pH range of 7.6 to 8.8 were the conditions maintained during the experiment. There was no feeding during the tests.
Phase Contrast Microscopic Analysis
The process of quantifying different kinds of samples in terms of their phase contrast values was carried out in grayscale using a phase contrast microscope (Micromaster, Model 12-575-252, Fisher Scientific) equipped with a digital camera to elucidate the deposition of NPs. Artemia larvae from each group were examined at the end of the exposure period. Images were captured by Micron Imaging software from live Artemia in petri dishes.
Analytical Methods to Determine Aluminum Content
Trace metal grade nitric acid (HNO3, Fisher Scientific) was used for the dissolution of the Artemia samples collected after exposure to determine their total uptake and depuration levels. Stock Aluminum (Al) standard solution (100 μg mL−1) was purchased from SCP Science (Champlain, NY). Calibration standards for inductively coupled plasma mass spectrometry (ICP-MS) determinations were prepared within a range of 0 to 100 μg/L from the stock Al solution in 5% HNO3. At the end of the exposure and depuration experiments, Artemia were sampled and thoroughly washed with deionized water through a 40 μm plankton net. The cleaned samples were then filtered with 0.45 mm Whatman filter paper. For instrumental analysis, about 0.1 g of wet Artemia were weighed and digested in teflon vessels in 2 mL of concentrated HNO3 for 2 h using a digestion block (DigiPrep MS, SCP Science) at 160 °C, according to the protocols described previously (Arslan et al., 2011). Once completely mineralized, the contents were diluted to 50 mL with deionized water and visually inspected for complete dissolution of α-Al2O3 and γ-Al2O3 NPs (e.g., clear solution without any turbidity). The sample solutions were analyzed by ICP-MS using a Varian 820 MS ICP-MS instrument (Varian, Australia). The aluminum (Al) content of the solutions were measured to determine the accumulation pattern of the NPs across the dose of exposure. The total Al concentration detected was then translated into corresponding α-Al2O3 (50 nm and 3.5 μm) and γ-Al2O3 (5 nm and 0.4 μm) NP concentrations.
Malondialdehyde Assay
The malondialdehyde (MDA) assay is the most prominent assay being used as an index for lipid peroxidation in biological systems by the measurement of the thiobarbituric acid-reactive substances (TBARS) through the thiobarbituric acid (TBA) test (Gutteridge and Halliwell, 1990). TBARS were measured to determine the lipid peroxidation products as a measure of oxidative stress. The composition of lipid hydroperoxides leads to a variety of end products, one of them being MDA, which is now accepted as a reliable marker of lipid peroxidation (Ohkawa and Ohishi, 1979). The MDA concentration was measured as described by Van Ye et al. (1993). At the end of the exposure period, 0.1 g of Artemia were washed with cold water and assayed using the MDA assay kit obtained from Northwest Life Science Specialties, LLC, Vancouver, WA. Samples were homogenized in 2 mL of phosphate buffer solution (pH 7.2) by an ultrasonic homogenizer and centrifuged at 6,000 rpm for 10 min. The resulting supernatant was then immediately used for a biochemical assay. Briefly, 10 μL of butylated hydroxytoluene reagents (BHT), 0.25 mL of sample supernatant, 0.25 mL of phosphoric acid reagent and 0.25 mL of TBA reagent were added to a vial. A set of MDA assay standards was freshly prepared, also from tetramethoxypropane. All samples and standards were incubated at 90 °C for one hour and centrifuged after cooling at 13,000 rpm for 10 min to precipitate suspending tissue. The reaction mixture was then transferred to UV-vis cuvettes and the absorbance was measured at 532 nm. Measurements were performed in triplicate for all experimental groups. The values were expressed as total MDA concentration per gram of Artemia.
Statistics
All experiments were repeated three times independently, and data were recorded as the mean with standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey's multiple comparisons was used to detect significant differences in mortality, uptake and elimination/depuration rates among the controls and treatments. In all data analyses, a p-value of < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Characterization of Nano α-Al2O3 and γ-Al2O3
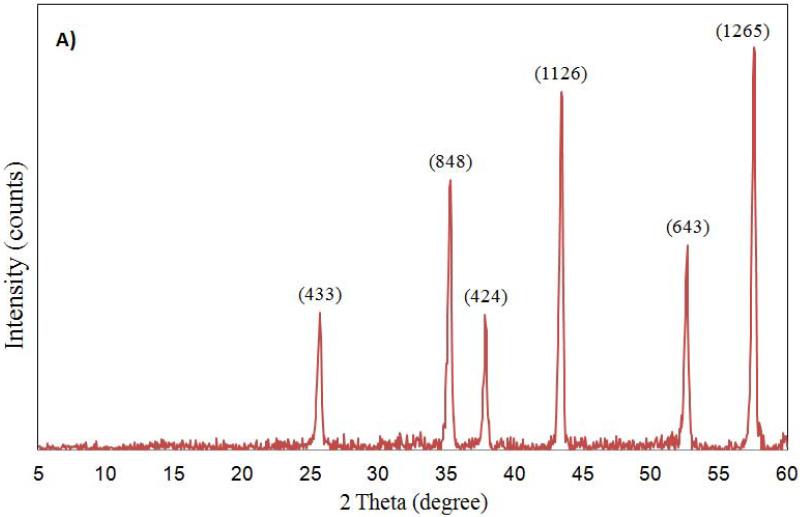
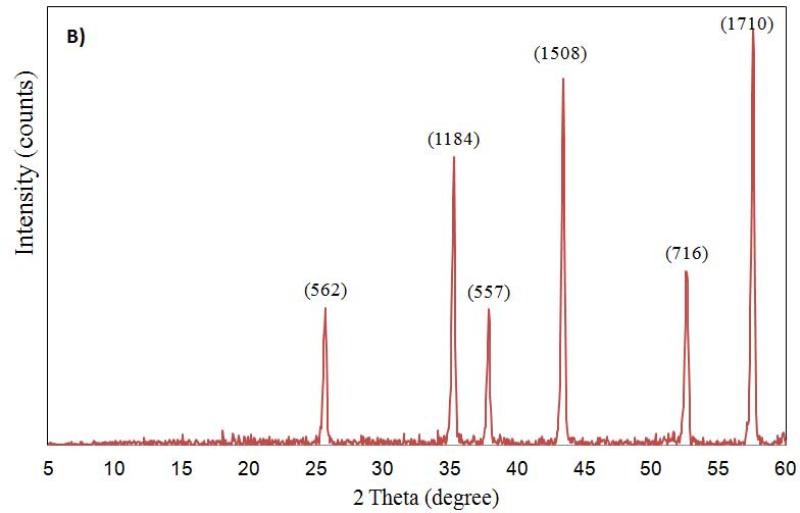
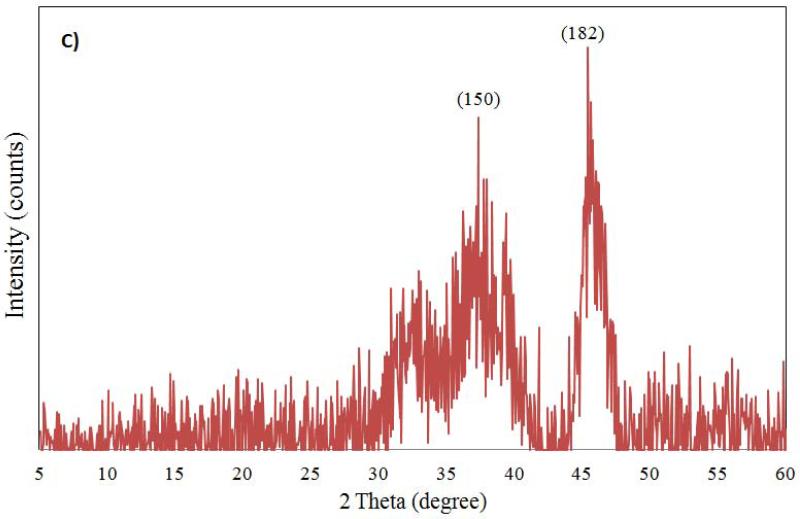
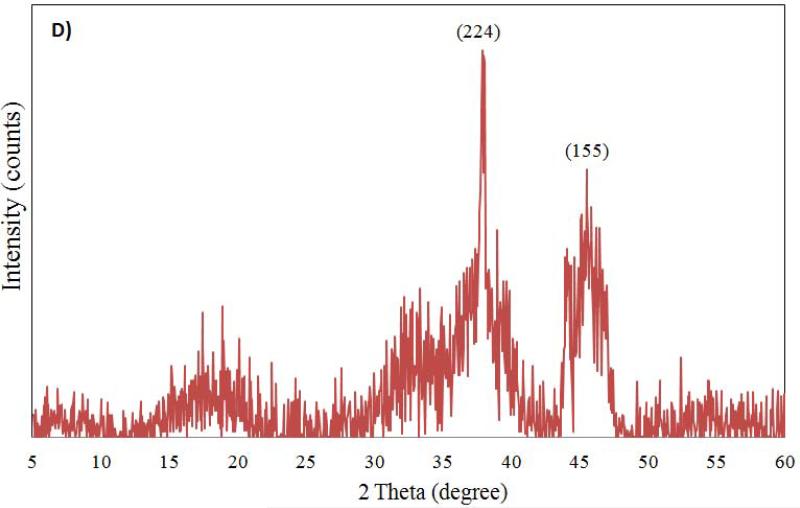
The characterization of aluminum oxide NPs was carried out by X-ray diffraction, FT-IR and TEM analysis before introducing them to test conditions. The X-ray diffractograms of nano aluminum oxide data are shown in Figure 1. For nano α-Al2O3 (50 nm and 3.5 μm) six diffraction peaks (at 57.0°, 53.8°, 44.5°, 37.5°, 35.5° and 25.0°) were observed (see Figure 1A, 1B). Sharp peaks of α-alumina proved its good crystallization (Sun et al., 2008; Shek et al., 1997). The data obtained were matched with the database of the Joint Committee on Powder Diffraction Standards (JCPDS) card file No. 46-1215, which confirmed the crystalline structure of alumina. The Debye-Scherrer equation was used to derive the crystallite size from the XRD data by determining the width of the 57.01° peak. The crystallite size was determined to be 17 ± 3 nm. Broad diffraction peaks were recorded for γ-Al2O3 5 nm and 0.4 μm (Figure 1C, 1D). They are similar to those previously reported and showed an amorphous structure with a small crystallization degree (Hosseini et al., 2012; Sun et al., 2008).
Figure 1.
Powdered XRD spectrum for Al2O3 nanoparticles (A: α-Al2O3 50 nm; B: α-Al2O3 3.5 μm; C: γ-Al2O3 5nm; D: γ-Al2O3 0.4 μm) sample.
FT-IR spectroscopy is widely used for the analysis of nano-size metal oxides (Prasad et al., 2006; Gondal et al., 2012; Guo et al., 2008; Nassar and Ahmed, 2011). Specific bands due to metal-oxygen bonds were recorded below 1000 cm−1 for these compounds (Alizadeh-Gheshlaghi et al., 2012). For α-Al2O3 50 nm, both a sharp band with a maximum of 454.2 cm−1 and a broad band with multiple peaks at 602.2, 644.3 and 729.1 cm−1 assigned to stretching and bending vibrations of Al-O were recorded. These were the most intense bands of the α-Al2O3 FT-IR spectrum, with transmittance values between 22.7 and 37.2% (Table 1). The peak of 1114.4 cm−1 may be assigned to Al-OH bending vibration (Manivasakan et al., 2011; Crisan et al., 2000). Also, a few small bands were observed: the first with maxima at 1436.8 and 1384.7 cm−1, the second with peaks at 1592.6 and 1630.6 cm−1 and the third with a large band at 3438.5 cm−1. The transmittance values of these were small, which ranged from 69.1 to 79.8%. The band centered at 3438.5 cm−1 was assigned to O-H stretching vibration of adsorbed water, and those from 1630.6 to 1592.6 cm−1 were assigned to bending vibrations of H-O-H (Gondal et al., 2012; Nassar and Ahmed, 2011). Bands with peaks at 1436.8 and 1384.7 cm−1 were assigned to carbon dioxide ν(O-C-O) symmetric vibration in the forms of carbonate and bicarbonate, respectively. Because alumina is an amphoteric oxide it contains both basic and acidic sites: by carbon dioxide adsorption both carbonate and bicarbonate species, respectively, may be formed (Galhotra, 2010; Crisan et al., 2000).
Table 1.
Specific wavelengths and their corresponding transmittance values for FT-IR spectra of nano-size α-Al2O3 and γ - Al2O3.
| α-50 nm | α-3.5 μm | γ- 5 nm | γ-0.4 μm | ||||
|---|---|---|---|---|---|---|---|
| cm−1 | % T | cm−1 | % T | cm−1 | % T | cm−1 | % T |
| 3438.5 | 75.7 | 3430.6 | 78.1 | 3440.3 | 39.4 | 3450.3 | 69.2 |
| 1630.6 | 77.1 | 1636.1 | 79.0 | 1631.2 | 65.1 | 1631 | 86.6 |
| 1592.6 | 79.8 | 1597 | - | 1588.5 | 71.5 | - | - |
| 1436.8 | 69.1 | 1436.8 | 71.8 | 1436.6 | 53.5 | 1437.5 | 80.4 |
| 1384.7 | 76.7 | 1380.7 | 82.4 | 1384.5 | 55.4 | 1383.8 | 89.8 |
| 1114.4 | 86.8 | 1111 | 86.5 | 1118.5 | 80.2 | 1116.4 | 94.3 |
| 729.1 | 27.1 | 731.2 | 27.4 | 807.9 | 23.8 | 822.5 | 29.6 |
| 644.3 | 25.2 | 641.1 | 25.2 | 750.7 | 22.1 | 752.9 | 28.9 |
| 602.2 | 22.7 | 594.1 | 16.5 | 701.6 | 22.7 | 692.8 | 38.9 |
| 452.2 | 37.2 | 449 | 28.1 | 624 | 20.5 | 630.4 | 31.3 |
| - | - | - | - | 572.6 | 20.3 | 570 | 30.8 |
The spectra of α-Al2O3 50 nm and 3.5 μm are similar, with minor differences between wavelengths of some absorption maxima: e.g., the peak recorded at 602.2 cm−1 for α-Al2O3 50nm was determined at 594.1 cm−1 for 3.5 μm (Table 1). The absorption maxima observed for α-Al2O3 were matched with those previously reported, and the presence of multiple peaks was associated with the crystalline form of α-alumina (Boumaza et al., 2010; Sun et al., 2008; Shek et al., 1997). For γ-Al2O3 5 nm, a broad and intense band with small peaks at 572.6, 624, 701.6, 750.7 and 807.9 cm−1 and transmittance values between 20.3 and 23.8 % were recorded. The band due carbon dioxide absorption had maxima at 1436.6 and 1384.5 cm−1 and those corresponding to water at 3440.3, 1631.2 and 1588.5 cm−1. The small peak at 1118.5 cm−1 was assigned to δAl-OH vibration. In the case of γ-Al2O3 0.4 μm, a similar spectra with a fine structure between 822.5 and 570 cm−1 was recorded. The broad bands of γ-Al2O3 with some fine structures are characteristic of amorphous powders with poor crystallization (Sun et al., 2008; Shek et al., 1997). Thus, it was determined that for the same polymorph phase, like α-Al2O3 (50 nm, 3.5 μm) and γ-Al2O3 (5 nm, 0.4 μm), the influence of particle size on FT-IR spectra was not significant. Both phases presented specific transmittance values ranging from 731.2 to 449 cm−1 and from 822.5 to 570 cm−1, respectively. The observations from FT-IR spectra analysis of α- Al2O3 and γ-Al2O3 are in agreement with XRD results.
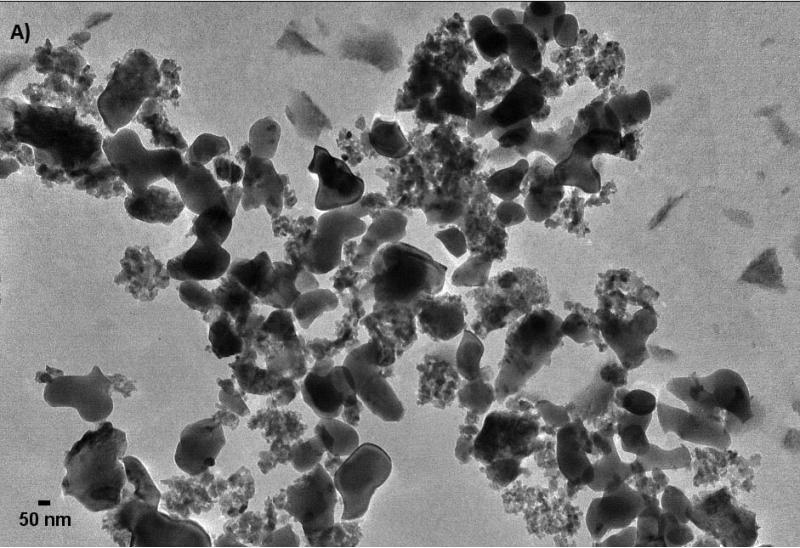
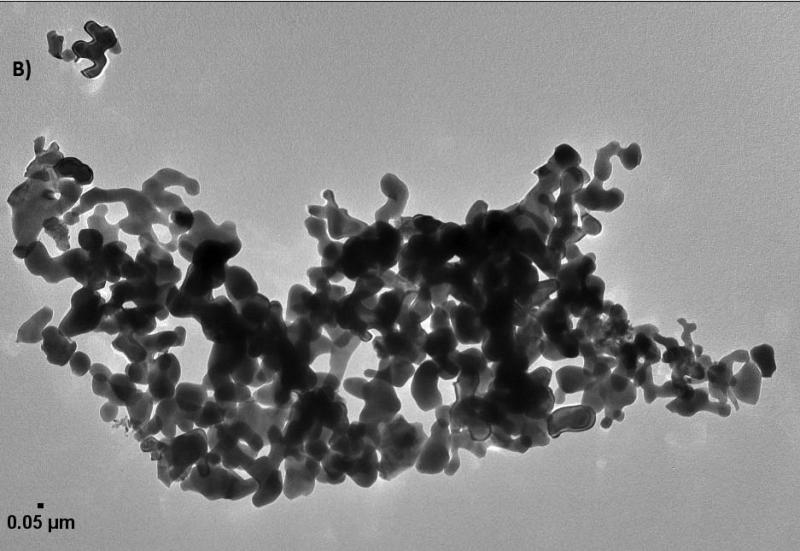
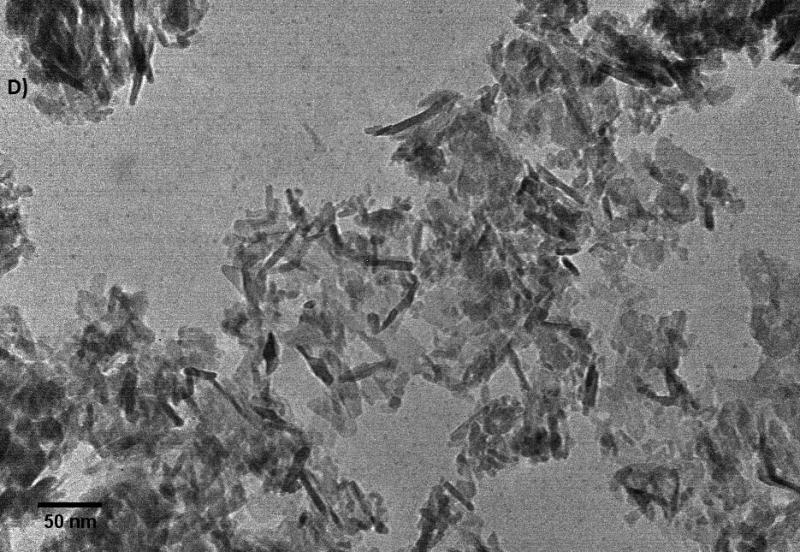
Further characterization of the size and shape of α-Al2O3 and γ-Al2O3 NPs was measured in different media and carried out by TEM images (Figure 2). The sizes measurements in dried suspension of small and large spherical NPs ranged from 45 to 100 nm, from 6 to 45 nm and > 0.3 μm for α-Al2O3 (50 nm), γ-Al2O3 (5 nm) and γ-Al2O3 (0.4 μm) NPs, excluding α-Al2O3 (3.5 μm), respectively. Nanorod lengths of α-Al2O3 and γ-Al2O3 were also measured from 240 to 750 nm with diameters of 10 nm. DLS results showed a wide distribution of particle size for all types of NPs, excluding α-Al2O3 (3.5 μm), which typically showed two distinctive populations in both the several hundred nanometers region and the several microns domain, a result that is consistent with previous reports (Franklin et al., 2007; Peng et al., 2011; Ates et al., 2013). DLS provided the size distribution for the hydrated forms the NPs as 412.1, 561.9 and 1096 nm for α-Al2O3 (50 nm), γ-Al2O3 (5 nm), γ-Al2O3 (0.4 μm) NPs, respectively. Particle size increased by a factor of above 40 for spherical NPs, indicative of the considerable degree of aggregation within NPs as a consequence of the high surface-area-to-volume ratio of α-Al2O3 and γ-Al2O3 NPs. Zeta potential were measured negatively charged for ultrafine α-Al2O3 (50 nm) and γ-Al2O3 (5 nm) NPs, and positively charged for larger particles. In fact, it is not uncommon for particles to appear as agglomerates in solution, since there was no surfactant capping on their surface (Adams et al., 2006; Miller et al., 2010). The colloidal suspensions were found to be enhanced with smaller α-Al2O3 and γ-Al2O3 NPs compared to larger particles, even though the NPs were present in solution as aggregates with hydrodynamic diameters ranging from 1 to 5 μm. It is clear from these results that the aggregation of α-Al2O3 and γ-Al2O3 NPs in water is inevitable, unless a suitable surface modification is made to render the NPs water-soluble.
Figure 2.
The images of α-Al2O3 and γ-Al2O3 nanoparticles (A: α-Al2O3 50 nm; B: α-Al2O3 3.5 μm; C: γ-Al2O3 5nm; D: γ-Al2O3 0.4 μm).
Uptake and Depuration of Nano α-Al2O3 and γ-Al2O3 in Artemia
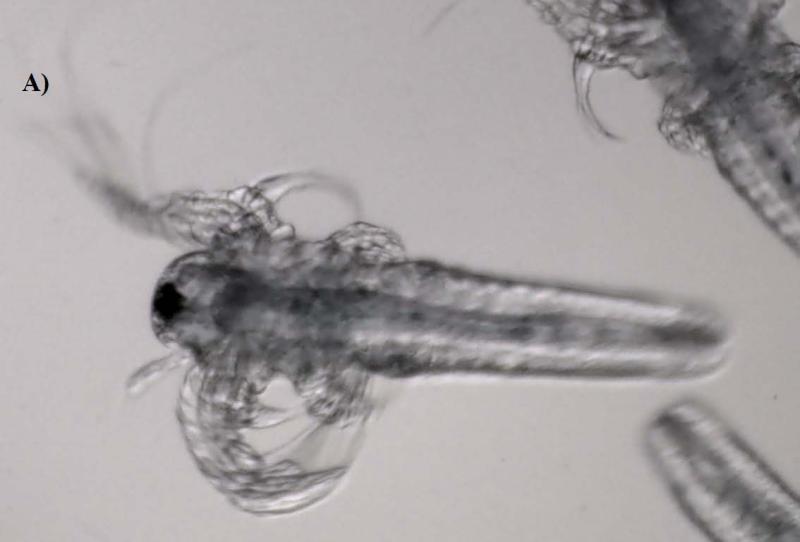
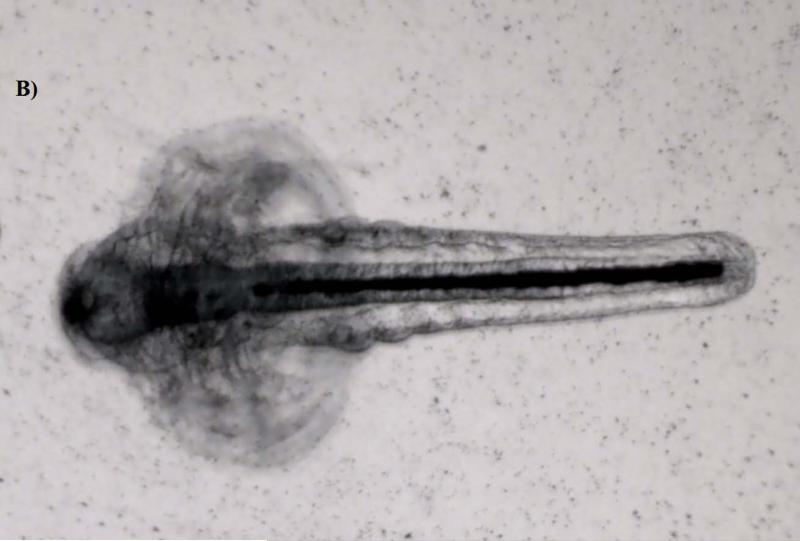
Artemia, especially large taxa like Daphnia, are considered non-selective filter feeders; thus they can ingest readily fine particles smaller than 50 μm (Hund-Rinke and Simon, 2006). Accumulation was clearly visible under the microscope, manifested by a dark line inside the guts of the larvae (Figure 3). The images demonstrated that the guts for the control were empty (transparent), whereas they were entirely filled with NPs in the treatment, especially for those in Groups C and D which were exposed to 50 and 100 mg/L levels, respectively. The results indicate that Artemia larvae did not show any discrimination to particle size variation among the NP suspensions, and that they readily accumulated the aggregates of α-Al2O3 and γ-Al2O3 NPs to elevated levels (e.g., mg/g) in their guts (see Figure 3). But the largest particulates of α-Al2O3 (3.5 μm) were not taken up by Artemia in either exposure period and its image was similar to that of the control (Figure was not shown).
Figure 3.
Phase contrast microscope images of the nanoparticles inside Artemia larvae. (A: The guts are empty in controls. B: Nanoparticles are visible as a dark line inside the guts of treatments).
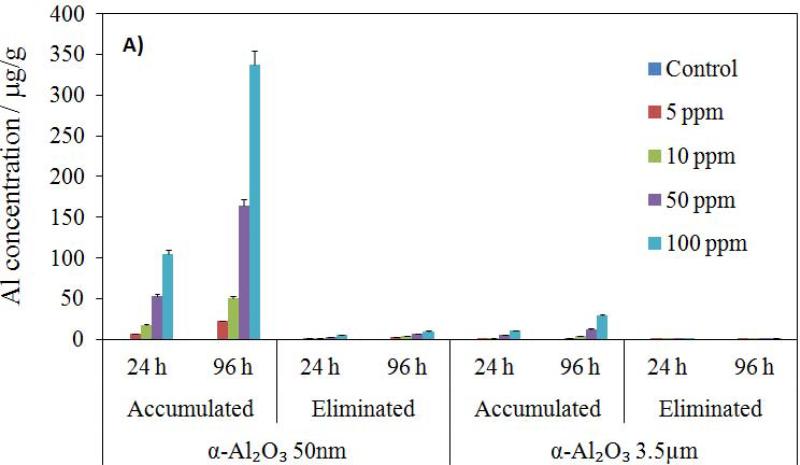
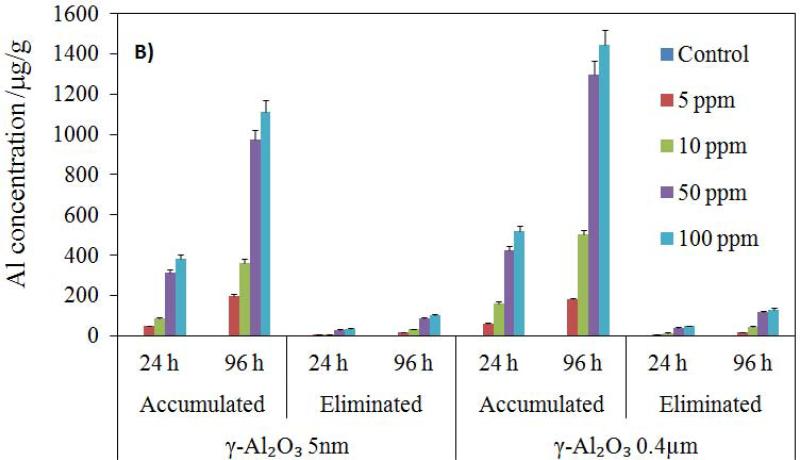
The total α-Al2O3 and γ-Al2O3 NP contents of Artemia samples were determined by ICPMS. The concentration values were based on the wet weight of Artemia, reflecting the total body burden across the concentration gradient from 10 to 100 mg NP L−1. ICP-MS analysis showed that accumulation was dependent on NP size, and that no aluminum uptake was detected in the controls and groups exposed to the largest particulate of α-Al2O3 (3.5 μm) in either exposure period. The accumulation profiles of all experimental groups were illustrated in Figure 4. Total NP uptake by Artemia increased with increasing initial NP concentration in the medium and with exposure time. The accumulations of both NPs in Artemia were significantly higher in 96 h than in 24 h (p < 0.05). Although larger particles (α-Al2O3 3.5 μm) were not taken up by Artemia, fine NPs (0.4 μm γ-Al2O3) and ultra-fine NPs (5 nm γ-Al2O3 and 50 nm α-Al2O3) accumulated substantially. Average Al concentration resulting from exposure to the suspension of 50 nm α-Al2O3 NPs ranged from 6.33 to 105 ppb and from 22.15 to 337.18 ppb for 24 and 96 h, respectively, with increasing initial NP concentration in the medium. For 5 nm γ-Al2O3 NPs, the uptake ranged from 48.46 to 384.11 ppb and from 198.68 to 1113.6 ppb for 24 and 96 h, respectively. For 0.4 μm γ-Al2O3 particulate, the uptake ranged from 61.95 to 520.5 ppb and from 179.65 to 1445.12 ppb for 24 and 96 h, respectively. Average Al uptake was higher for larger γ-Al2O3 particulates than it was for all smaller NP groups within the same exposure regimes.
Figure 4.
Accumulation and elimination (in 24 h) profiles of α-Al2O3 (a) and γ-Al2O3 (b) nanoparticles by Artemia larvae for 24 h and 96 h exposure (μg/g).
The ability of Artemia to depurate the ingested α-Al2O3 and γ-Al2O3 NPs was also investigated. After exposure, Artemia were transferred to a freshly-prepared seawater culture medium. The entire transfer process for each test was completed within 24 h without rinsing with clean seawater to minimize the damage to Artemia larvae. After Artemia were being collected in a mesh, they were filtered, washed and digested in acid to determine the loss in Al2O3 NP content. The elimination profiles for the accumulated Al are illustrated in Figure 4. No mortality was observed during the depuration process. These results demonstrated that the excretion of the ingested NPs appears to be very slow, and that Artemia were unable to eliminate the NPs as rapidly as they accumulated, most likely due to the formation of larger aggregates inside the guts. As expected, the highest loss occurred for the groups exposed to 100 mg L−1 levels for which the average concentration of NPs eliminated in 24 h was about 5.0 and 34.65 ppb for α-Al2O3 and γ-Al2O3 NPs, respectively. These values increased to about 9.8 and 103.2 ppb, respectively, in 96 h. The elimination of the 0.4 μm γ-Al2O3 in 24 and 96 h increased from 48.1 to 130.5 ppb. The observed effects may not reflect those of NPs but aggregates of NPs, which points to the fact that the physicochemical properties (size, shape and reactivity) of ingested particles/aggregates are important factors that should be considered when evaluating the toxicity of α-Al2O3 and γ-Al2O3 NPs and other similar metal oxide NPs.
Toxicity and Lipid Peroxidation Detection by Melondialdehyde Levels
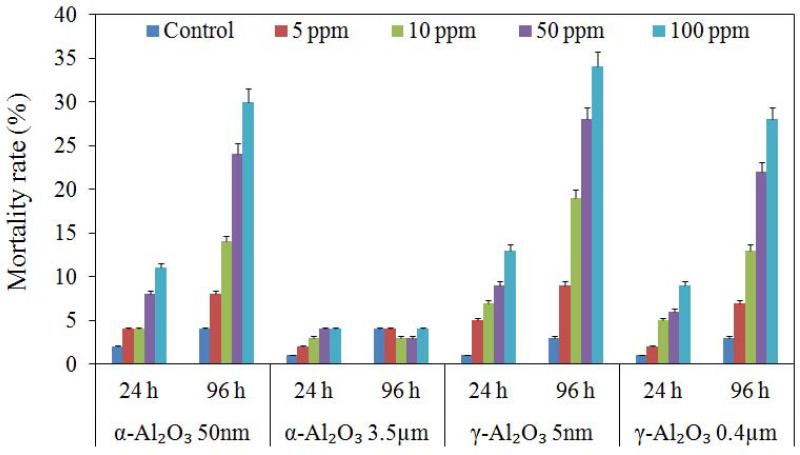
The toxic effects of α-Al2O3 and γ-Al2O3 NPs were different on Artemia for 24 and 96 h exposure. The controls and 3.5 μm α-Al2O3 showed about 1 to 4% mortality in 24 and 96 h, which were not statistically significant (p > 0.05). As aforementioned, the exposures were conducted in the absence of food. The experimental mortalities for the controls clearly demonstrated that deprivation from food did not induce any lethal effect on Artemia larvae, even up to 96 h. The mortality ranges were about 4 to 11%, 5 to 13%, 2 to 9% in 24 h, but they were 8 to 30%, 9 to 34% and 7 to 28% in 96 h for α-Al2O3 (50 nm), γ-Al2O3 (5 nm) NPs, and γ-Al2O3 (0.4 μm) particulates, respectively (Figure 5). In the treatments, the mortalities significantly increased with increasing NP concentration and time (p < 0.05). These results demonstrated that Al2O3 NPs were relatively benign to Artemia larvae during 24 h exposure, even at the highest test concentration. The lethal effects recorded for 96 h exposure were more prominent. Results also indicated that γ-Al2O3 NPs were more toxic than α-Al2O3 NPs at all conditions, and the highest mortality was measured from γ-Al2O3 NPs (5 nm) at 100 mg/L (LC50 > 100 mg/L). Moreover, smaller NPs exhibited a more toxic effect than larger particles.
Figure 5.
Percent mortality rates for Artemia salina larvae from exposure to α-Al2O3 and γ-Al2O3 particles for 24 h and 96 h.
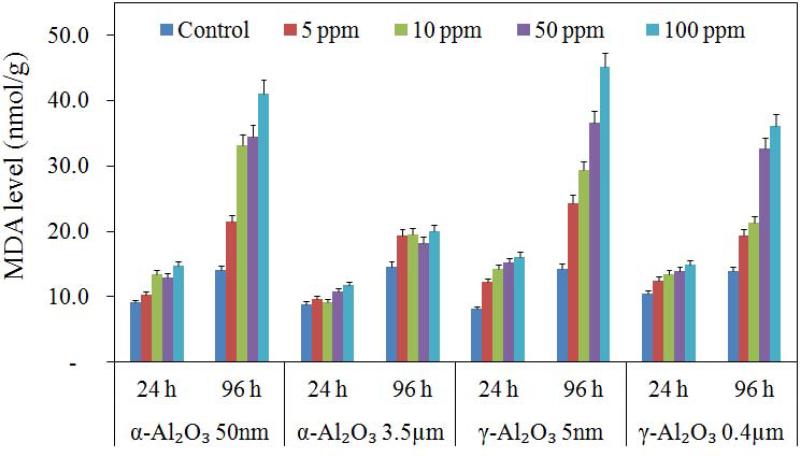
Oxidative deterioration of cell membrane lipids has been used extensively as a marker of oxidative stress in lipid peroxidation detection measured as total MDA concentration. The MDA results of this study are displayed in Figure 6. The data were consistent with the mortality rates for 24 and 96 h exposure in different concentrations of all Al2O3 particles. MDA values detected in treatments were not statistically different than the control after exposure of both NPs for 24 h (p > 0.05).; however, a significant increase in MDA values was found after exposure of NPs for 96 h (p < 0.05). This indicated that Artemia were undergoing oxidative stress, which was consistent with our results of a higher concentration of α-Al2O3 and γ-Al2O3 NPs exhibiting more potent effects on disturbance to the antioxidant defense systems of Artemia in 96 h (Figure 6). Increasing NP concentration from 5 to 100 mg/L also caused a significant increase in 96 h MDA levels (p < 0.05). MDA levels indicated that γ-Al2O3 (5 nm) NPs (MDA = 45.1 nmol/g) were more toxic than α-Al2O3 (50 nm) NPs (MDA = 41.1 nmol/g) in 96 h. It was found that larvae were more vulnerable and always experienced relatively higher mortalities in 96 h, and that the toxic effects of α-Al2O3 and γ-Al2O3 NPs were mediated by oxidative stress. The 96 h mortality percentage showed a high correlation with 96 h MDA values (r2 = 0.951), suggesting that lethal effects were due to the elevated oxidative stress induced by α-Al2O3 and γ-Al2O3 NPs.
Figure 6.
Malondialdehyde levels measured in Artemia larvae exposed to α-Al2O3 and γ-Al2O3 nanoparticles for 24 h and 96 h exposure (nmol/g)
CONCLUSIONS
In this study, the uptake, toxicity and elimination of α-Al2O3 (50 nm and 3.5 μm) and γ-Al2O3 (5 nm and 0.4 μm) NPs were studied. The responses observed from Artemia exposed to NPs are consistent with those of marine organisms. Thus, Artemia could be used as a robust test model for studying the ecological risks of nanomaterials on marine ecosystems. Both α-Al2O3 and γ-Al2O3 NPs aggregate in saltwater to form micrometer-size particles. However, this phenomenon does not have any effect on the uptake of particles from water. Larvae could readily accumulate α-Al2O3 and γ-Al2O3 NPs to elevated levels, excluding larger ones like α-Al2O3 (3.5 μm). While no acute toxicity was detected in 24 h, mortality and MDA levels increased in 96 h. The results demonstrate that α-Al2O3 and γ-Al2O3 NPs are not totally benign to Artemia, but that they exhibit marginal acute toxicity. The toxic effects are mediated by oxidative stress, which induces lethal effects after prolonged exposure. An important conclusion from this study is that the sensitivity of each assay is dependent on the physico-chemical characteristics of the particle, emphasizing the importance of a multi-trophic approach. The principal concern related to the release of NPs and their smaller particles may change the materials’ transport and potential toxicity to aquatic organisms compared to larger particles.
ACKNOWLEDGMENTS
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS); (Contract #W912HZ-10-2-0045). The views expressed herein are those of authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors thank Jackson State University, Biostatistical Support Unit for assistance in statistical analysis.
REFERENCES
- Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale ZnO and Zn, SiO2, and ZnO water suspensions. Water. Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Alizadeh-Gheshlaghi E, Shaabani B, Khodayari A, Azizian-Kalandaragh Y, Rahimi R. Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012;217:330–339. [Google Scholar]
- Arslan Z, Ates M, McDuffy W, Agachan MS, Farah IO, Yu WW, Bednar AJ. Probing metabolic stability of CdSe nanoparticles: alkaline extraction of free cadmium from liver and kidney samples of rats exposed to CdSe nanoparticles. J. Haz. Mat. 2011;192:192–199. doi: 10.1016/j.jhazmat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah OI. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ. Monit. Asses. 2013;185:3339–3348. doi: 10.1007/s10661-012-2794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumaza A, Djelloul A, Guerrab F. Specific signatures of α-alumina powders prepared by calcinations of boehmite or gibbsite. Powder Technol. 2010;201:177–180. [Google Scholar]
- Crisan M, Jitianu A, Crisan D, Balasoiu M, Dragan N, Zaharescu M. Sol-gel monocomponent nano-sized oxide powders. J. Optoelectron Adv. M. 2000;2:339–344. [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007;41(24):8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- Pragati Galhotra. Carbon dioxide adsorption on nanomaterials. University of Iowa: 2010. dissertation. http://ir.uiowa.edu/etd/670. [Google Scholar]
- Gondal MA, Saleh TA, Drmosh QA. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl. Surf. Sci. 2012;258:6982–6986. [Google Scholar]
- Guo Z, Lei K, Li Y, Ng HW, Prikhodko S, Hahn HT. Fabrication and characterization of iron oxide nanoparticles reinforced vinyl-ester resin nanocomposites. Compos. Sci. Technol. 2008;68:1513–1520. [Google Scholar]
- Gutteridge MJ, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends. Biochem. Sci. 1990;15:129–134. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Handy R, von der Kammer F, Lead J, Hassellöv M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- Hosseini SY, Nikou RKM. Effects of precipitation/aging temperature on catalytic activity of γ-Al2O3 nanocatalysts for dehydration of methanol to dimethyl ether. J. Am. Sci. 2012;8:628–632. [Google Scholar]
- Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles (ZnO and Zn) on algae and daphnids. Environ. Sci. Pollut. Res. 2006;13:225–232. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano- and micro-scale oxide particles. Environ. Pollut. 2009;157:1619–1625. doi: 10.1016/j.envpol.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Levin I, Brandon D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998;81:1995–2012. [Google Scholar]
- Manivasakan P, Rajendran V, Rauta PR, Sahu BB, Panda BK. Effect of mineral acids on the production of alumina nanopowder from raw bauxite. Powder. Technol. 2011;211:77–84. [Google Scholar]
- Meng H, Chen Z, Xing G, Yuan H, Chen V, Zhao F, Zhang C, Zhao Y. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Milhem MM, Al-Hiyasat AS, Darmani H. Toxicity testing of restorative dental materials using brine shrimp larvae (Artemia salina). J. Appl. Oral. Sci. 2008;16(4):297–301. doi: 10.1590/S1678-77572008000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Lenihan HS, Muller EB, Tseng N, Hanna SK, Keller AA. Impacts of metal oxide nanoparticles on marine phytoplankton. Environ. Sci. Technol. 2010;44:7329–7334. doi: 10.1021/es100247x. [DOI] [PubMed] [Google Scholar]
- Miziolek A. Nanoenergetics: an emerging technology area of national importance. AMPTIAC Q. 2002;6:43–48. [Google Scholar]
- Moore MN. Do nanoparticles present toxicological risks for the health of the aquatic environment? Environ. Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nassar MY, Ahmed IS. Hydrothermal synthesis of cobalt carbonates using different counter ions: An efficient precursor to nano-sized cobalt oxide (Co3O4). Polyhedron. 2011;30:2431–2437. [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;3576(311):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nowack B, Bucheli TD. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particle. Environ. Health. Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Organisation for Economic Co-operation and Development (OECD). Guideline for the Testing of Chemicals (Part 202) 2004 [Google Scholar]
- Ohkawa H, Ohishi N. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omnic User`s Guide, Version 7.3. Copyright© 2006 by Thermo Electron Corporation; 5225 Verona Road, Madison: WI. 53711-4495, U.S.A. [Google Scholar]
- Palaniappan PlRM Pramod KS. The effect of titanium dioxide on the biochemical constituents of the brain of Zebrafish (Danio rerio): An FT-IR Study. Spectrochim. Acta. A. 2011;79:206–212. doi: 10.1016/j.saa.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28:2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Peng TY, Lv HJ, Zeng P, Zhang XH. Preparation of ZnO Nanoparticles and Photocatalytic H2 Production Activity from Different Sacrificial Reagent Solutions. Chin. J. Chem. Phys. 2011;24:464. [Google Scholar]
- Persoone G, Van de Vell A, Van Steertegem M, Nayer B. Predictive value for laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquat. Toxicol. 1989;14:149–166. [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Prasad V, D'Souza C, Yadav D, Shaikh AJ, Vigneshwaran N. Spectroscopic characterization of zinc oxide nanorods synthesized by solid-state reaction. Spectrochim. Acta. A. 2006;65:173–178. doi: 10.1016/j.saa.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Rozita Y, Brydson R, Scott J. An investigation of commercial gamma-Al2O3 nanoparticles. Journal of Physics: Conference Series. 2010;241:12096. [Google Scholar]
- Sadiq MI, Chowdhury B, Chandrasekaran N, Mukherjee A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2009;5:282–286. doi: 10.1016/j.nano.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Sadiq MI, Pakrashi S, Chandrasekaran N, Mukherjee A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011;13:3287–3299. [Google Scholar]
- Shek CH, Lai JKL, Gu TS, Lin GM. Transformation evolution and infrared absorption spectra of amorphous and crystalline nano Al2O3 powders. Nanostruct. Mater. 1997;8:605–610. [Google Scholar]
- Singh S, Shi T, Duffin R, Albrecht C, van Berlo D, Hohr D, Fubini B, Martra G, Fenoglio I, Borm PJ, Schins RP. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007;222:141–151. doi: 10.1016/j.taap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Štengl V, Houšková V, Bakardjieva S, Marafa N, Maříková M, Opluštil F, Nĕmec T. Zirconium doped nano-dispersed oxides of Fe, Al and Zn for destruction of warfare agents. Mater. Charact. 2010;61:1090–1088. [Google Scholar]
- Sun ZX, Zheng TT, Bo QB, Du M, Forsling W. Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. J. Colloid. Interf. Sci. 2008;319:247–251. doi: 10.1016/j.jcis.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Temuujin J, Jadambaa TS, Mackenzie KJD, Angerer P, Porte F, Riley F. Thermal formation of corundum from aluminium hydroxides prepared from various aluminium salts. Bull. Mater. Sci. 2000;23:301–304. [Google Scholar]
- Tyner KM, Schiffman SR, Giannelis EP. Nanobiohybrids as delivery vehicles for camptothecin. J. Control. Release. 2004;95:501–514. doi: 10.1016/j.jconrel.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Van Ye TM, Roza AM, Pieper GM. Inhibition of intestine lipid peroxidation does not minimize morphologic damage. J. Surg. Res. 1993;55:553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- Wagner AJ, Bleckmann CA, Murdock RC, Schrand AM, Schlager JJ, Hussain SM. Cellular interaction of different forms of aluminium nanoparticles in rat alveolar macrophages. J. Phys. Chem. Biol. 2007;111:7353–7359. doi: 10.1021/jp068938n. [DOI] [PubMed] [Google Scholar]
- Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009;157:1171–1177. doi: 10.1016/j.envpol.2008.11.004. [DOI] [PubMed] [Google Scholar]