Abstract
Introduction
Suppression of dendritic cells (DCs) is a crucial mechanism by which tumor cells escape immune recognition and elimination. We have recently reported that MHC class I antigen processing machinery (APM) component expression in human DCs is down-regulated by tumor-derived gangliosides. However, the molecular mechanisms underlying this abnormality were not identified. Thus, the aim of this work was to analyze the role of interferon regulatory factor 8 (IRF-8) in APM protein expression and the antigen presenting capacity of DCs developed in the tumor microenvironment.
Results
We demonstrate that the expression of several MHC class I APM components, including delta, MB-1, LMP-10, ERp57, and tapasin, is significantly decreased in murine DCs generated in the presence of prostate cancer cells. APM component down-regulation was associated with decreased ability of DCs to present model antigen to antigen-specific T cells. Notable, impaired antigen-presenting activity of DCs co-cultured with tumor cells was accompanied by decreased levels of IRF-8. Transduction of DCs with the silencing RNA for the IRF-8 gene also led to reduced expression of APM components in DCs and decreased antigen presenting function.
Conclusion
Together, our data suggest that tumor-induced inhibition of antigen processing and presenting function of DCs is mediated by IRF-8, a member of the interferon regulatory factor family. These results provide a new molecular target for optimizing the generation of efficient DC vaccines for cancer therapy.
Keywords: Antigen-processing machinery, Dendritic cells, IRF-8, Prostate cancer
Introduction
DCs are the most potent antigen-presenting cells (APC); they are capable of recognizing, processing, and presenting tumor-associated antigens, and initiating tumor antigen-specific immune responses in both animals and humans [17, 19]. However, the development and progression of tumors indicates that tumor cells utilize a variety of mechanisms to escape immune recognition and elimination [20]. One of them is the inhibition and misbalancing of the DC system. Suppressed DC generation, differentiation, maturation, and function have been reported in tumor-bearing animals and in cancer patients [2, 12, 37, 38]. While it is well known that the antitumor activity of DCs depends on the processing and presentation of tumor-associated antigens, the mechanisms underlying down-regulation of MHC class I antigen-processing machinery (APM) components in DCs in cancer remain to be defined.
During the differentiation process, DCs develop the machinery required for the effective processing, transport, and assembly of antigenic peptides leading to the formation of the MHC class I-peptide complex [10]. In general, protein antigens are first marked for ubiquitination within the cytosol and subsequently degraded by the constitutive proteasome (consisting of delta (δ) (or β1), MB-1 (β5) and Z components) or interferon-gamma (IFN-γ) inducible proteasome (immunoproteasome) (consisting of LMP-2, LMP-7, and LMP-10 components). Peptides are then transported into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP1 and TAP2). MHC class I heavy chains are also transported into the ER, where they are associated with several chaperon proteins (BiP, calnexin, calreticulin, and ERp57) and β2 microglobulin (β2m). The MHC class I-β2m complex then binds with tapasin that brings the dimeric complex into association with TAP and ensures proper peptide loading onto the MHC class I-β2m complex. The trimeric MHC class I-β2m-peptide complex is transported to the plasma membrane [4]. Recently, we have demonstrated that tumor-derived factors downregulate MHC class I APM component expression in human peripheral blood monocyte (PBMC)-derived DCs, as well as their ability to present antigens to autologous antigen-specific T cells [37]. We have also reported earlier that the generation and function of DCs is significantly suppressed in the prostate cancer microenvironment in different murine models using RM1 and TRAMP-C2 cell lines in vitro and in vivo [38]. However, the effects of tumor-derived factors on MHC class I APM component expression by murine DCs and the molecular pathways leading to APM component down-regulation in DCs are not known.
It has been recently found that tumor-derived factors deregulate the IFN-γ signaling pathway through down-regulation of interferon regulatory factor 8 (IRF-8) in macrophages [22]. Interferons are multifunctional cytokines that are involved in many pathways in the cancer immunosurveillance process [27, 32]. IFN-γ has also been shown to stimulate expression of MHC class I and II molecules, costimulatory molecules, and APM proteins to enhance the antigen presenting capacity of DCs and macrophages. Promoters of IFN-γ regulated genes carry binding sites for various transcription factors [16]. The member of the interferon regulatory factor family of transcription factors (from IRF-1 to IRF-9), interferon consensus sequence-binding protein (ICSBP), also known as IRF-8, is important for IFN-γ-mediated signaling and plays an important role in myeloid cell development [24, 34]. IRF-8 affects both the differentiation and the functional maturation of DCs and has been suggested to regulate many aspects of DC-mediated T cell immunity [21]. However, the role of IRF-8 in the regulation of APM component expression and function in DCs in normal conditions and in the tumor microenvironment is not known.
In the present study, we analyzed APM protein expression and the antigen presenting capacity of murine DCs developed in the presence of prostate cancer cells. Our findings reveal that tumor cells down-regulate antigen processing and presenting capacity in DCs and this effect is mediated by IRF-8, a member of the interferon regulatory factor family. These results demonstrate a new link between signal transduction pathways and function in DCs, which is targeted by tumor cells to inhibit an efficient tumor antigen-specific immune response.
Materials and methods
Animals
Male C57BL/6 mice, 6–8-week old, were obtained from Taconic. Animals were maintained at Central Animal Facility at the University of Pittsburgh according to the standard guidelines and IACUC approved protocols.
Cell lines and culture medium
Murine prostate cancer cell line RM1-wt was kindly supplied by Dr. T. C. Thompson (Baylor College of Medicine, Houston, TX). Specific OVA-recognizing T cell clone B3Z86/90.14 (B3Z) obtained from B3Z86/90.14 transgenic mice (H-2Kb, I-Ab) expressing the B3Z86/90.14 T cell receptor (TCR) that is specific for the OVA peptide fragment, 257–264, was kindly provided by Dr. B.A. Osborne (University of Massachusetts, Amherst, MA). All cells were cultured (at 37°C in a 5% CO2 atmosphere) in a complete medium (CM) [RPMI-1640 medium supplemented with 2 mM L-glutamine, 50 μg/ml gentamicin sulfate, 10 mM HEPES, 10% FBS, 10 mM non-essential amino-acids, and 1 mM sodium pyruvate (Gibco)].
APM component-specific antibodies
The MB-1-specific mAb SJJ-3, the delta- specific mAb SY-5, the low molecular weight protein (LMP-10)- specific mAb TO-7 and the tapasin-specific mAb TO-3 were developed and characterized as described elsewhere [5, 25]. Ab were purified from ascitic fluid by sequential precipitation with ammonium sulphate and caprylic acid [26]. The purity of the mAb preparation was assessed by SDS-PAGE. The activity of the mAb preparations was monitored by testing with the lymphoid cell lysate in Western blotting. The rabbit anti-ERp57, anti-calnexin, and anti-calreticulin polyclonal Ab were purchased from Stressgen Biotechnologies Corporation.
Dendritic cell cultures
Murine DCs were generated from the hematopoietic progenitors isolated from the bone marrow [38]. Bone marrow cells were collected from femurs, passed through a nylon cell strainer to remove pieces of bones and debris, depleted of red blood cells with a lysing buffer (Sigma) and incubated with anti-mouse B220, CD4 and CD8 antibodies for 1 h on ice followed by incubation with rabbit complement for 30 min at 37°C to deplete B and T lymphocytes. Cells (106 cells/ml) were cultured at 37°C in 6-well plates overnight. Then, non-adherent cells were collected, resuspended in a CM supplemented with murine GM-CSF (1,000 units/ml) and IL-4 (1,000 units/ml) (PeproTech) and incubated at 37°C for 5–7 days. Five-day-old DCs were used for transfection with siRNA. Six-day-old DCs were incubated with ovalbumin (OVA, Sigma) for antigen-presentation assay. Seven-day-old DCs were harvested, counted, and used for further analysis. DC precursors were incubated at 37°C in CM for 3 days with RM1 cells (106cells/well) separated by membrane inserts with 0.4 μm pore size (transwell system, Falcon). To control for tumor cell overgrowth and for nutrients in the medium, inserts were changed and fresh medium and tumor cells were added on day 2. After tumor cell removal, fresh medium supplemented with murine GM-CSF (1,000 units/ml) and IL-4 (1,000 units/ml) were added to DC cultures. Cells incubated in CM served as a control.
Antigen-presentation assay
To evaluate the capacity of DCs to process and present antigens, 6-day-old DCs (5 × 105 cells/ml/well) were incubated in a 24-well culture plate at 37°C overnight with different concentrations (0, 10, 100, and 1,000 μg/ml) of OVA. Following washing with CM, DCs (1 × 104/well) were cultured in 96-well flat-bottom plates at 37°C for 48 h with OVA-specific T cells (B3Z) (2 × 104/well). The ability of DCs to present OVA antigen to specific T cells was determined as IL-2 production by activated T cells [8, 9, 28]. IL-2 production was assessed in cell-free culture supernatants by ELISA (Endogen) according to the manufacturer’s instructions.
Flow cytometry analysis
Intracellular staining of DC was performed as described earlier [37]. Briefly, harvested cells were washed in PBS containing 1% BSA, fixed with 2% paraformaldehyde (PFA) (Sigma), washed again, resuspended in PBS containing 0.5% BSA, transferred to the glass flasks and treated in a microwave till the beginning of boiling. Cells were then chilled on ice, washed in PBS containing 1% BSA, permeabilized in PBS containing 1% BSA and 0.1% saponin (Sigma) at room temperature for 30 min and stained with primary Ab (mouse anti-human MB-1, Delta, LMP-10, and tapasin mAb or rabbit anti-mouse calnexin, calreticulin, and ERp57 Ab) at room temperature for 30 min. Then cells were washed in PBS containing 1% BSA and 0.1% saponin and incubated with secondary FITC-conjugated goat anti-mouse IgG or goat anti-rabbit IgG Ab (1:100) (Jackson) at room temperature for 30 min. Finally, cells were washed in PBS containing 1% BSA and 0.1% saponin, and fixed with 0.5% PFA. Fluorescence was measured using a FACScan flow cytometer. Data analysis was performed using the Cell Quest Software (Becton Dickinson). Results are expressed as percentage of positive cells and as mean fluorescence intensity (MFI).
Small interfering RNA and DC transfection
Small interfering RNA (siRNA) for IRF-8, control siRNA, fluorescein conjugated control siRNA and siRNA transfection reagent were purchased from Santa Cruz Biotechnology. Transfection efficiency was determined using fluorescein conjugated control siRNA by fluorescence microscopy and varied between 50 and 60%. Transfection was conducted according to the manufacturer’s protocol with minor modifications to optimize the procedure for murine DCs. Briefly, 5-day-old DCs were collected, sequentially washed in PBS and in transfection medium and seeded in 48-well plates at the concentration of 2 × 106 cells/0.8 ml transfection medium/well. IRF-8 and control siRNA were diluted in transfection medium (1 μg/100 μl/well), and mixed by pipetting the solution with transfection reagent, which was also diluted in transfection medium (8 μl/100 μl/well). Following a 30 min incubation at room temperature the mixture was added to DCs and the incubation was continued for an additional 24 h at 37°C. CM supplemented with murine GM-CSF (1,000 units/ml) and IL-4 (1,000 units/ml) was then added to the cells which were cultured at 37°C for an additional 24 h.
Western blot
The protein expression of the IRF-8 in DCs was assessed by Western blot. Nuclear extracts were prepared from DCs using the Nuclear Extraction Kit (Marligen) according to the manufacturer’s instructions. The protein concentration was determined in the supernatants by the Bradford method using the BioRad protein kit. Total proteins (40 μg) were dissolved in electrophoresis sample buffer, separated by 4–12% polyacrylamide gel electrophoresis (NuPAGE) and transferred to a nitrocellulose membrane (NOVEX). The membrane was blocked with 0.5% nonfat milk, 0.1% Tween-20 (Fisher) in 20 mM Tris–HCl buffer (pH 7.2). Protein was detected using an optimal amount of IRF-8/ICSBP-specific goat antibodies (Santa Cruz Biotechnology), and rabbit anti-goat secondary antibody (1:100,000 dilution; Pierce). Expression of β-actin was evaluated as a housekeeper control protein using an optimal amount of mouse anti-actin mAb (1:100,000 dilution; Sigma) and secondary antibody (Pierce). The membrane was processed and treated with chemiluminescent reagents (Pierce). The bands were visualized on a Kodak film (Eastman Kodak) prior to densitometric evaluation of protein levels using UN-SCAN-II software (Silk Sci Corp.).
Statistical analysis
Statistical significance of differences was determined using the Student t test and the nonparametric Mann-Whitney test. For all statistical analyses, the level of significance was set at a probability of 0.05 to be considered significant. Data are presented as the mean ± SEM. All experiments were repeated at least three times.
Results
Tumor cells downregulate APM component expression in murine DC
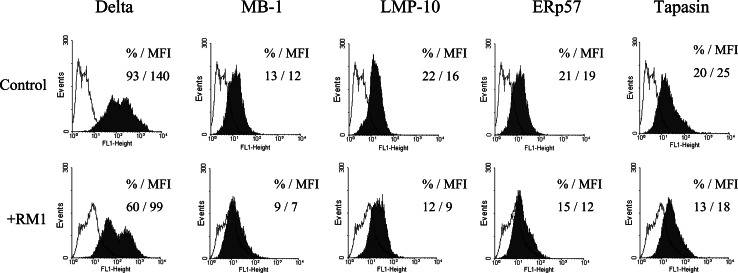
First, because it has been previously reported that expression of several MHC class I APM components, including MB-1 (β5), LMP-7, LMP-10, and ERp57 is significantly down-regulated in human DCs generated in the presence of tumor cells [37], we evaluated expression of several MHC class I APM components in murine DCs in the tumor microenvironment. The levels of delta, MB-1, LMP-10, ERp57, and tapasin proteins were significantly down-regulated in murine DCs generated from bone marrow precursors co-incubated with RM1 cells for 72 h at 37°C (Fig. 1). The extent of down-regulation ranged from 30% for ERp57 to 50% for LMP-10. Similar results were obtained by analyzing the corresponding MFI values. In contrast, expression of calnexin and calreticulin was not significantly altered in DCs incubated with RM1 tumor cells. These results suggest that antigen degradation and association with molecules of the MHC class I complex in DCs might be deregulated by tumors, which may be responsible for low levels of antigen presentation by DCs in cancer.
Fig. 1.
MHC class I APM component down-regulation in DCs co-incubated with RM1 prostate cancer cell line. DCs from bone marrow precursors (106 cells/well) were generated with GM-CSF and IL-4 as described in “Materials and methods”. RM1 cells (106 cells/well) were added in a transwell system and incubated for the first 3 days in cultures. Tumor-treated and control DCs were intracytoplasmatically stained with delta-, MB-1-, LMP-10-, ERp57-, and tapasin-specific Ab, and analyzed by flow cytometry. Data represent the percentage of positive cells and MFI. The results of one representative out of three independent experiments are shown
Murine prostate cancer cells inhibit antigen-presenting activity of DC
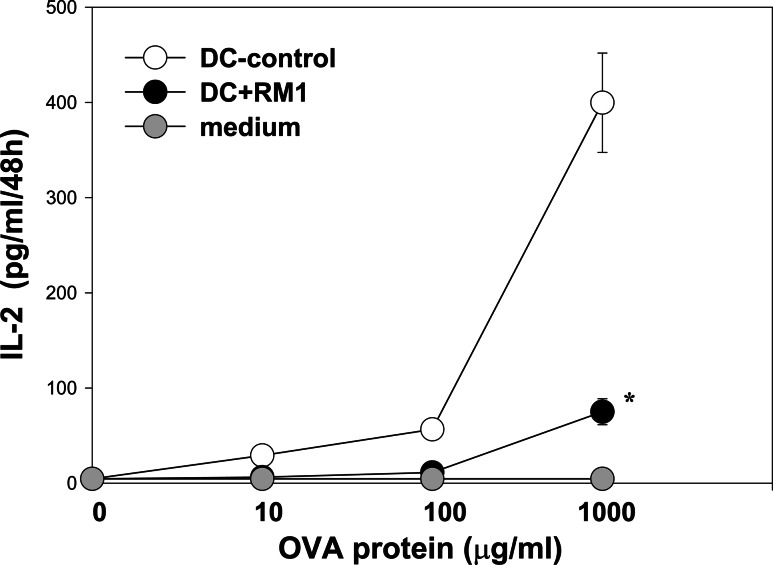
To test whether APM component down-regulation in DCs generated in the presence of tumor cells has functional consequences on antigen presentation, we evaluated the ability of DCs to present OVA antigen to antigen-specific T cells. Six-day-old RM1 cell-treated and control DCs were pulsed with different concentrations of OVA (0, 10, 100, and 1,000 μg/ml) and co-incubated with OVA-antigen-specific T cell clone B3Z. The ability of DCs to present OVA antigen to specific T cells, determined as IL-2 production by activated T cells, was significantly decreased when DCs had been co-incubated with RM1 cells, in comparison to control DCs (Fig. 2). For instance, at the concentration of OVA 1,000 μg/ml, IL-2 production was reduced by 80% ± 12% (P < 0.05). Thus, prostate cancer cells suppress the ability of murine DCs to present antigens to antigen-specific T cells.
Fig. 2.
RM1 cells inhibited antigen-presenting capacity of DCs. DCs from murine bone marrow precursors were generated as described in the Fig. 1 legend. RM1 cells were added in a transwell system and incubated for the first 3 days in cultures. Tumor-treated and control 6-day-old DCs were loaded with OVA overnight, washed and cultured in 96-well flat-bottom plates in complete medium with OVA257–264-specific T cells (B3Z) as described in “Materials and methods”. The ability of DCs to present OVA antigen to specific T cells was determined as IL-2 production by activated T cells and assessed by ELISA. Data represent the mean ± SEM of triplicate measurements from three independent experiments. * P < 0.05
IRF-8 protein expression is decreased in DCs generated with RM1 cells
It has been observed that tumor cells might disrupt the IFN-γ signaling pathway through down-regulation of IRF-8 in macrophages [22]. We speculated that a similar mechanism might be involved in tumor-induced dysregulation of APM function in DCs. To test the hypothesis that tumor-mediated inhibition of APM components in DCs may be mediated by IRF-8, the expression of IRF-8 protein in RM1-treated and control DCs was first assessed by Western blot. The bands corresponding to IRF-8 were quantified by densitometry as relative intensity units (RIU); the results are presented as the ratio of RIU of IRF-8 to the RIU of β-actin. The ratio of IRF-8 to β-actin was reduced from 0.73 ± 0.09 in non-treated DCs to 0.34 ± 0.05 in RM1 cells-treated DCs (P < 0.05) (Fig. 3a). These results show that prostate cancer cells also decrease IRF-8 expression in murine DCs.
Fig. 3.
IRF-8 protein expression was decreased in DCs generated with RM1 cells to the same extent as in DCs transfected with siRNA IRF-8. DCs from murine bone marrow precursors were generated as described in the Fig. 1 legend. a RM1 cells were added in a transwell system and incubated for the first 3 days in cultures. Nuclear extracts were prepared from tumor-treated (DC-RM1) and control (DC-cnt) 7-day-old DCs using the Nuclear Extraction Kit as described in “Materials and methods”. Western blot was performed as described in “Materials and methods”. The results of one representative out of three independent experiments are shown. b 5-day-old DCs were transfected with siRNA for IRF-8 as described in “Materials and methods”. The level of DC transfection using protocol developed by Santa Cruz Biotechnology was between 50 and 60% based on fluorescein conjugated control siRNA expression. Western blot for DCs transfected with IRF-8 siRNA (DC-siRNA) and control siRNA (DC-cnt) was performed as described in “Materials and methods”. The results of one representative out of three independent experiments are shown
Transfection of DCs with siRNA for IRF-8 inhibits APM component expression and antigen presenting capacity of DCs
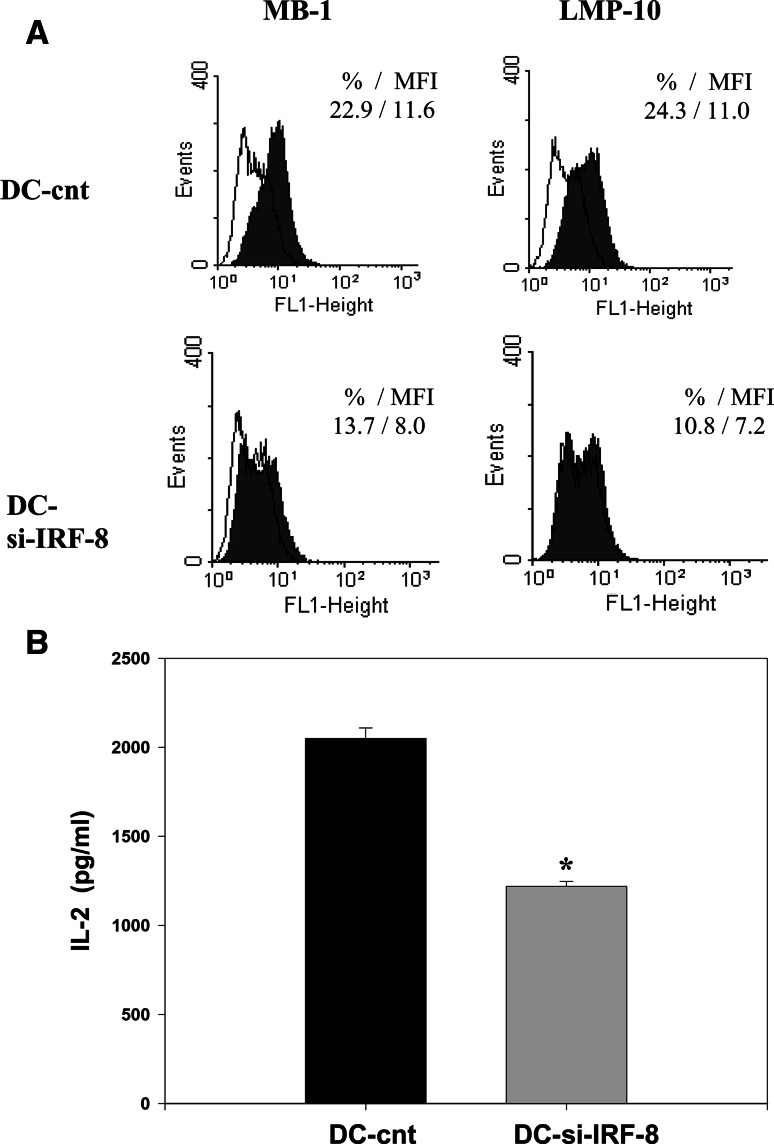
To determine whether IRF-8 deficiency affects APC function of DCs, we evaluated expression of different components of the MHC class I APM in DCs transfected with IRF-8 siRNA and their ability to present OVA. Transfection efficiency (50–60%) was determined using fluorescein conjugated control siRNA by fluorescence microscopy and confirmed by evaluation of the IRF-8 protein expression in DCs using Western blotting (Fig. 3b). Transfection of DCs with si-IRF-8 decreased the ratio of IRF-8 to β-actin from 0.58 ± 0.07 in control-siRNA-transfected DCs to 0.26 ± 0.04 in si-IRF-8-transfected DCs (P < 0.05). The silencing of IRF-8 expression significantly reduced the expression of the MHC class I APM components in DCs (Fig. 4a). The IRF-8 silencing-induced inhibition of APM component expression in DCs was about 40–45% for MB-1 and LMP-10, and about 25–30% for delta, ERp57 and tapasin (P < 0.05). Similar results were obtained by analyzing the corresponding MFI data. The IRF-8 deficiency also significantly decreased the capacity of DCs to present OVA antigen to specific T cells (Fig. 4b). For instance, at the concentration of OVA 1,000 μg/ml, IL-2 production was reduced by 40.5% ± 5.6% (P < 0.01). Notable, IL-2 secretion by B3Z cells activated by OVA-pulsed tumor-treated DCs was reduced by 80%, while IL-2 production induced by siRNA-treated DCs was decreased by only 40%. However, this difference is due to the transfection efficiency that was about 50–60%. These results indicate that the loss of IRF-8 expression via RNA silencing significantly impairs DC antigen processing and presenting activity to the same extent as in DCs generated in the presence of prostate cancer cells.
Fig. 4.
IRF-8 deficiency impaired antigen processing and presenting capacity of DCs. DCs from murine bone marrow precursors were generated and transfected with siRNA for IRF-8 as described in the Fig. 4 legend. a DCs transfected with IRF-8 siRNA and control siRNA DCs were intracytoplasmatically stained with delta-, MB-1-, LMP-10-, ERp57-, and tapasin-specific Ab and analyzed by flow cytometry as described in the Fig. 1 legend. Expression of LMP-10 and MB-1 is shown. Data represent the percentage of positive cells and MFI. The results of one representative out of three independent experiments are shown. b DCs transfected with IRF-8 siRNA and control siRNA DCs were loaded with OVA overnight, then washed and cultured in 96-well flat-bottom plates in complete medium with OVA-specific T cells (B3Z) as described in the Fig. 2 legend. The ability of DCs to present OVA antigen to specific T cells was determined as IL-2 production by activated T cells and assessed by ELISA. Data represent the mean ± SEM of triplicate measurements from three independent experiments. * P < 0.01
Discussion
In this study we investigated the suppression of antigen processing and presentation of bone marrow-derived DCs by prostate cancer cells, and the role of IRF-8, a member of the interferon regulatory factor family of transcription factors, in this suppression. Prostate cancer remains the most common malignancy, and the second leading cause of cancer death among American men. Prostate cancer-induced suppression of DC function is an important mechanism of tumor escape from immune recognition. The number of DCs is lower in the human prostate cancer tissue in comparison with normal prostatic and benign prostate hyperplasia (BPH) tissues [39]. Clinical observations suggest that the number of DCs in prostate carcinoma lesions inversely correlates with the histopathological grade of the tumor [6]. We found that the generation of DCs is significantly suppressed in the prostate cancer microenvironment in different murine models both in vitro and in vivo [38]. We have also shown that following co-incubation with prostate cancer cell lines in vitro human DCs express lower levels of costimulatory molecules CD80 and CD86 and are less potent inducers of T cell proliferation in MLR [1, 31].
Here, we focused on the molecular mechanisms of DC suppression in prostate cancer and analyzed IRF-8-mediated tumor-induced suppression of APM component expression in DCs and the ability of DCs to present antigens to antigen-specific T cells. We found for the first time that reduced expression of APM components (LMP-10, MB-1, ERp57, tapasin, delta), and impaired antigen presenting activity of DCs co-cultured with RM1 tumor cells was associated with decreased level of IRF-8 protein expression (Figs. 1, 2, 3). IRF-8 is known to be essential for DC differentiation and maturation [33, 35]. IRF-8 is also known to play an important role in the regulation of DC/T cell interaction. IRF-8−/− mice display an impairment in monocyte, macrophage, and DC functions [14]. Murine bone marrow-derived DCs from IRF-8−/− mice were characterized as immature DCs with altered chemotactic behavior and defective responses to inflammatory stimuli. In addition, DCs from IRF-8−/− mice appeared to be much less able to stimulate antigen-specific naive CD4+ T cells than DCs from wild-type mice indicating that IRF-8 controls MHC class II presentation by DCs [21, 29, 35, 40]. Using siRNA to silence IRF-8 expression in DCs, we have revealed that IRF-8 deficiency leads to reduced expression of the MHC class I APM components LMP-10, MB-1, ERp57, tapasin, and delta, and suppresses the ability of DCs to present OVA antigen to specific T cells (Figs. 3, 4).
IRF-8 was originally identified as a transcription factor binding to the interferon-stimulated response element (ISRE) motif in the promoter region of the MHC class I gene [11]. IRF-8 regulates its transcriptional activity through multiple target DNA elements, such as ISRE, composite ets/IRF-cis elements (also referred to as EICE sequences), and IFN-γ activation site (GAS). IRF-8 acts either as an activator or a repressor, depending on interacting factors and target DNA elements [18, 36]. For instance, interactions with IRF-1 or IRF-2 on ISRE lead to the formation of a heterocomplexes with repression activity. IRF-8 also cooperates with PU.1 and IRF-1 or PU.1 and IRF-2 resulting in the formation of transcriptional activation heterocomplexes that regulate transcription of genes in the immune system [15, 16, 18]. The involvement of IRF-8 in the regulation of APM protein expression has not been reported before.
IRF-1 has been shown to be essential for the mediation of constitutive expression and cytokine induction of the immunoproteasomes, TAP1, and tapasin, indicating its important role in the MHC class I antigen processing [3, 7, 13, 23]. It has recently been shown that overexpression of a constitutively active form of IRF-7 positively regulates the promoter of IRF-1 and LMP-2 [30]. Our findings demonstrate a new role for IRF-8 in APM function of DC suggesting that IRF-8 might play in concert with IRF-1 (and/or other IRF family members) to form transcriptional heterocomplexes. These molecules may have not yet determined role in regulating antigen processing and presentation in health and cancer. It was reported that tumor-derived immunosuppressive cytokines disrupted the IFN-γ signaling pathway through down-regulation of IRF-8 in macrophages [22]. We have found here that tumor-derived factors inhibit antigen processing and presenting activity of DCs through the down-regulation of IRF-8 in DCs.
Collectively, prostate cancer suppresses DC generation, differentiation, maturation, and function, including antigen uptake, processing, and presentation. This raises the possibility that defects in the DC system in patients with prostate cancer may be an important contributor to immune escape and immune tolerance pathways. Characterization of the molecular mechanisms underlying the dysfunction of the DC system in cancer will lead to a variety of therapeutic strategies, such as stimulation of DC proliferation, promotion of antigen uptake and processing, activation of DC-induced T cell proliferation, and protection of DCs from tumor-induced apoptosis. Since the antitumor activity of DCs depends on their main function, i.e., antigen presentation, knowledge of the molecular mechanisms regulating processing and presentation of tumor antigens by DCs in the prostate cancer microenvironment is essential for further improvement of therapeutic efficacy of different immunotherapeutic modalities including DC-based vaccines, which are currently under evaluation in several clinical trials.
Acknowledgments
This work was supported by National Institutes of Health grants 2RO1 CA 084270 (MRS), RO1CA110249 (SF), RO1CA113861 (SF) and PO1CA109688 (SF) and DOD grants 06-1-0151 (MRS) and 7-01-0096 (SF).
References
- 1.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46:68–75. doi: 10.1002/1097-0045(200101)46:1<68::AID-PROS1010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol. 2003;170:2026–2030. doi: 10.1097/01.ju.0000091264.46134.b7. [DOI] [PubMed] [Google Scholar]
- 3.Abarca-Heidemann K, Friederichs S, Klamp T, Boehm U, Guethlein LA, Ortmann B. Regulation of the expression of mouse TAP-associated glycoprotein (tapasin) by cytokines. Immunol Lett. 2002;83:197–207. doi: 10.1016/S0165-2478(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–4188. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 5.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66:185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 6.Bigotti G, Coli A, Castagnola D. Distribution of Langerhans cells and HLA class II molecules in prostatic carcinomas of different histopathological grade. Prostate. 1991;19:73–87. doi: 10.1002/pros.2990190108. [DOI] [PubMed] [Google Scholar]
- 7.Brucet M, Marques L, Sebastian C, Lloberas J, Celada A. Regulation of murine Tap1 and Lmp2 genes in macrophages by interferon gamma is mediated by STAT1 and IRF-1. Genes Immun. 2004;5:26–35. doi: 10.1038/sj.gene.6364035. [DOI] [PubMed] [Google Scholar]
- 8.Canaday DH, Chakravarti S, Srivastava T, Tisch DJ, Cheruvu VK, Smialek J, Harding CV, Ramachandra L. Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol. 2006;243:96–106. doi: 10.1016/j.cellimm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Lu J, Wang L, Gan YH. Mycobacterial heat shock protein 65 enhances antigen cross-presentation in dendritic cells independent of Toll-like receptor 4 signaling. J Leukoc Biol. 2004;75:260–266. doi: 10.1189/jlb.0703341. [DOI] [PubMed] [Google Scholar]
- 10.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driggers PH, Ennist DL, Gleason SL, Mak WH, Marks MS, Levi BZ, Flanagan JR, Appella E, Ozato K. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esche C, Lokshin A, Shurin GV, Gastman BR, Rabinowich H, Watkins SC, Lotze MT, Shurin MR. Tumor’s other immune targets: dendritic cells. J Leukoc Biol. 1999;66:336–344. doi: 10.1002/jlb.66.2.336. [DOI] [PubMed] [Google Scholar]
- 13.Foss GS, Prydz H. Interferon regulatory factor 1 mediates the interferon-gamma induction of the human immunoproteasome subunit multicatalytic endopeptidase complex-like 1. J Biol Chem. 1999;274:35196–35202. doi: 10.1074/jbc.274.49.35196. [DOI] [PubMed] [Google Scholar]
- 14.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC, 3rd, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/S0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Horvath E, Eklund EA. PU.1, interferon regulatory factor (IRF) 2, and the interferon consensus sequence-binding protein (ICSBP/IRF8) cooperate to activate NF1 transcription in differentiating myeloid cells. J Biol Chem. 2007;282:6629–6643. doi: 10.1074/jbc.M607760200. [DOI] [PubMed] [Google Scholar]
- 16.Kanno Y, Levi BZ, Tamura T, Ozato K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25:770–779. doi: 10.1089/jir.2005.25.770. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/S0092-8674(01)00455-X. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Ma X. Interferon regulatory factor 8 regulates RANTES gene transcription in cooperation with interferon regulatory factor-1, NF-kappaB, and PU.1. J Biol Chem. 2006;281:19188–19195. doi: 10.1074/jbc.M602059200. [DOI] [PubMed] [Google Scholar]
- 19.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/S0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 20.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 21.Mattei F, Schiavoni G, Borghi P, Venditti M, Canini I, Sestili P, Pietraforte I, Morse HC, 3rd, Ramoni C, Belardelli F, Gabriele L. ICSBP/IRF-8 differentially regulates antigen uptake during dendritic-cell development and affects antigen presentation to CD4+ T cells. Blood. 2006;108:609–617. doi: 10.1182/blood-2005-11-4490. [DOI] [PubMed] [Google Scholar]
- 22.Mullins DW, Martins RS, Elgert KD. Tumor-derived cytokines dysregulate macrophage interferon-gamma responsiveness and interferon regulatory factor-8 expression. Exp Biol Med (Maywood) 2003;228:270–277. doi: 10.1177/153537020322800305. [DOI] [PubMed] [Google Scholar]
- 23.Namiki S, Nakamura T, Oshima S, Yamazaki M, Sekine Y, Tsuchiya K, Okamoto R, Kanai T, Watanabe M. IRF-1 mediates upregulation of LMP7 by IFN-gamma and concerted expression of immunosubunits of the proteasome. FEBS Lett. 2005;579:2781–2787. doi: 10.1016/j.febslet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/S1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 25.Ogino T, Wang X, Ferrone S. Modified flow cytometry and cell-ELISA methodology to detect HLA class I antigen processing machinery components in cytoplasm and endoplasmic reticulum. J Immunol Methods. 2003;278:33–44. doi: 10.1016/S0022-1759(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 26.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 27.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 28.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 29.Schiavoni G, Mattei F, Borghi P, Sestili P, Venditti M, Morse HC, 3rd, Belardelli F, Gabriele L. ICSBP is critically involved in the normal development and trafficking of Langerhans cells and dermal dendritic cells. Blood. 2004;103:2221–2228. doi: 10.1182/blood-2003-09-3007. [DOI] [PubMed] [Google Scholar]
- 30.Sgarbanti M, Marsili G, Remoli AL, Orsatti R, Battistini A. IRF-7: new role in the regulation of genes involved in adaptive immunity. Ann N Y Acad Sci. 2007;1095:325–333. doi: 10.1196/annals.1397.036. [DOI] [PubMed] [Google Scholar]
- 31.Shurin GV, Aalamian M, Pirtskhalaishvili G, Bykovskaia S, Huland E, Huland H, Shurin MR. Human prostate cancer blocks the generation of dendritic cells from CD34+ hematopoietic progenitors. Eur Urol. 2001;39(Suppl 4):37–40. doi: 10.1159/000052584. [DOI] [PubMed] [Google Scholar]
- 32.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 33.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/S1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 34.Tamura T, Ozato K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interferon Cytokine Res. 2002;22:145–152. doi: 10.1089/107999002753452755. [DOI] [PubMed] [Google Scholar]
- 35.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 36.Tamura T, Thotakura P, Tanaka TS, Ko MS, Ozato K. Identification of target genes and a unique cis element regulated by IRF-8 in developing macrophages. Blood. 2005;106:1938–1947. doi: 10.1182/blood-2005-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tourkova IL, Shurin GV, Chatta GS, Perez L, Finke J, Whiteside TL, Ferrone S, Shurin MR. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J Immunol. 2005;175:3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 38.Tourkova IL, Yamabe K, Foster B, Chatta G, Perez L, Shurin GV, Shurin MR. Murine prostate cancer inhibits both in vivo and in vitro generation of dendritic cells from bone marrow precursors. Prostate. 2004;59:203–213. doi: 10.1002/pros.10369. [DOI] [PubMed] [Google Scholar]
- 39.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol. 1998;160:214–219. doi: 10.1016/S0022-5347(01)63093-3. [DOI] [PubMed] [Google Scholar]
- 40.Tsujimura H, Tamura T, Kong HJ, Nishiyama A, Ishii KJ, Klinman DM, Ozato K. Toll-like receptor 9 signaling activates NF-kappaB through IFN regulatory factor-8/IFN consensus sequence binding protein in dendritic cells. J Immunol. 2004;172:6820–6827. doi: 10.4049/jimmunol.172.11.6820. [DOI] [PubMed] [Google Scholar]