Summary
Background
We aimed to assess the sensitivity/specificity of serum and CSF antibody-testing in patients with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, and the correlation between titers, relapses, outcome, and epitope repertoire.
Methods
In this observational study, brain immunohistochemistry and cell-based assays (CBA) with fixed and live NMDAR-expressing cells were used to determine the sensitivity/specificity of antibody-testing in paired serum/CSF obtained at diagnosis of 250 patients with anti-NMDAR encephalitis and 100 control subjects. A patient was considered antibody-positive if either serum or CSF tested positive with both immunohistochemistry and CBA; titers were determined with serial sample dilution using brain immunohistochemistry. Samples from 45 patients (25 good-outcome: modified Rankin Scale [mRS] 0–2; 10 poor-outcome: mRS 3–6; 10 relapses) were examined at ≥3 disease time points. Epitope repertoire was determined with CBA expressing GluN1-NMDAR mutants
Findings
All 250 patients had NMDAR-antibodies in CSF but only 214/250 had antibodies in serum (sensitivity 100% [98.5–100%] versus 85.6% [80.7–89.4%], p<0.0001). Serum immunohistochemistry-testing was more often in agreement with CBA with fixed than live cells (77/108 versus 63/108, p=0.0056). In multivariable analysis, CSF and serum titers were higher in patients with poor-outcome than in those with good-outcome (CSF dilution 340 versus 129, difference 211, [95%-CI 1.1–421], p=0.049; serum 7370 versus 1243, difference 6127 [2369–9885], p=0.0025), and in patients with teratoma than in those without (CSF 395 versus 110, difference 285 [134–437], p=0.0079; serum 5515 versus 1644, difference 3870 [548–7193], p=0.024). Over time there was a decrease of antibody-titers regardless of outcome (from diagnosis to last follow-up: CSF 614 to 76, difference 538 [288–788]; serum 5460 to 1564, difference 3896 [2428–5362], both p<0.0001). Relapses correlated better with the titer-change in CSF than that in serum (14/19 versus 7/16, p=0.037). After recovery, 24/28 CSF and 17/23 serum from patients remained antibody-positive. Patients’ antibodies targeted a main epitope region at GluN1 aa369; the epitope repertoire did not differ between patients with different outcomes, and did not change during relapses.
Interpretation
NMDAR antibody-testing is more sensitive in CSF than serum. Antibody-titers in CSF and serum are higher in patients with poor-outcome or teratoma. The titer-change in CSF correlates better with relapses than that of serum.
Funding
The Dutch Cancer Society, the National Institute of Health, the McKnight Neuroscience of Brain Disorders award, the Fondo de Investigaciones Sanitarias, Erasmus MC fellowship and Fundació la Marató de TV3.
Keywords: anti-NMDA-receptor, encephalitis, serum, CSF, antibodies, titers
Introduction
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an immune-mediated disorder that associates with IgG antibodies against the GluN1 subunit of the NMDAR.1 The antibody reactivity depends on the conformation of GluN1 expressed alone or in combination with GluN2 (GluN1/N2) in HEK293 cells (cell-based assay)2 and it is always detectable with immunohistochemistry of rat brain and with cultures of dissociated rat hippocampal neurons.1 These findings serve to differentiate these antibodies from other antibodies against linear GluN1 or GluN2 epitopes that have been described in a variety of disorders and have little or no syndrome specificity.3,4,5 Patients with antibodies demonstrated with all three techniques, brain immunohistochemistry, neuronal cultures, and CBA with GluN1/2 develop a highly predictable syndrome that we termed anti-NMDAR encephalitis.1 Subsequent studies with 1500 consecutive patients with suspected autoimmune encephalitis, 100 of them with NMDAR antibodies,1 showed that all samples (either serum or CSF) that were positive with brain immunohistochemistry and CBA always showed reactivity with the cell surface of cultured neurons (data not published). This cell surface reactivity, although useful for basic research studies,6 was similar to that of any synaptic antibody and therefore we excluded it for routine testing. In contrast, brain immunohistochemistry which produces a highly characteristic pattern of reactivity7,8 and may reveal additional antibodies, combined with CBA with fixed cells expressing GluN1/2 which confirms the identity of the antigen,1 were kept as a set of techniques that since 2007 we have routinely used in serum or CSF, of all patients regardless of sex, age, or syndrome.
Over the years we have studied anecdotal patients who had a clinical picture characteristic of anti-NMDAR encephalitis but serum testing was negative in other laboratories. When these patients were examined by us, we confirmed that the serum was negative, but the CSF (not examined in other laboratories) was positive with both brain immunohistochemistry and CBA (unpublished observations). These findings suggested that in some patients with anti-NMDAR encephalitis the antibodies were only detectable in CSF, as demonstrated in several reports,9–12 and that examining both serum and CSF improved sensitivity.12 Despite these studies and the limited number of cases comparing paired serum and CSF reported by other investigators (e.g., 14 cases in one study,13 20 in another12), it has been suggested that serum testing using only CBA is sufficient for the specific identification of NMDAR antibodies and the diagnosis of anti-NMDAR encephalitis.14 While serum testing with CBA alone have identified NMDAR antibodies in patients with multiple neuropsychiatric disorders (schizophrenia, Creutzfeldt-Jakob, Parkinson),15,16,17,18 and healthy individuals,18 these findings have not been reproduced in studies using more comprehensive testing (CBA and brain immunohistochemistry) in serum and CSF1,19,20 These discrepancies are not trivial: serum CBA testing may miss the diagnosis of a disease that is severe but potentially treatable, such as anti-NMDAR encephalitis.9,10,12,21 On the other hand, serum CBA testing without confirmation with a second technique and/or demonstration of antibodies in CSF, may mislead the initial diagnosis of an unrelated disease, such as schizophrenia, Creutzfeldt-Jakob, or Parkinson disease, suggesting an immune mediated disorder. Therefore, clarification of appropriate antibody-testing is crucial but there are no studies directly comparing the sensitivity and specificity of different techniques in paired serum and CSF of patients with any of these disorders, not even anti-NMDAR encephalitis.
After a patient is diagnosed with anti-NMDAR encephalitis, a constant question in clinical practice is the utility of following antibody titers during the disease. Some investigators assume that the degree of correlation between serum titers and the course of the disease is as good as that of CSF titers and therefore serum titers can be used to make clinical decisions.13,22 However, there is no convincing data supporting this concept. Case reports or small series are often affected by the bias of preferentially publishing cases with good outcome in which antibodies were only assessed in serum after treatments such as plasma exchange or intravenous immunoglobulin. These treatments may transiently decrease any type of serum antibodies but have little effect on CSF titers.23 In addition, there is limited information on the follow-up of serum titers in patients with poor outcome and of CSF titers in any clinical scenario.24 Recent studies suggest that after clinical recovery patients may still have detectable antibodies in serum and CSF.25,26 Therefore, at this time the degree of correlation between serum and CSF antibody titers, relapses, and outcome is unclear leaving physicians without direction on how to interpret antibody titers during the disease and whether serum and CSF titers provide equal clinical information.
To address these questions, we determined the sensitivity and specificity of NMDAR antibody testing in paired serum/CSF samples of 250 patients with anti-NMDAR encephalitis and 100 paired serum/CSF controls. We then focused on 45 patients selected from groups who had clinical relapses, good outcome, or poor outcome to investigate whether serum and/or CSF titers at three time points of the disease correlated with outcome. Finally, using 23 representative cases we determined if the pattern of epitope recognition could potentially help in the initial diagnosis or predict outcome.
Material and methods
Serum, CSF and clinical information
Patients were randomly selected from a cohort of 600 world-wide cases with anti-NMDAR encephalitis whose serum and CSF were studied in Dr. Dalmau’s laboratory at the Hospital of the University of Pennsylvania (until February 2011) or at Hospital Clínic, University of Barcelona (since February 2011). Anti-NMDAR encephalitis was defined as the new onset of neuropsychiatric symptoms associated with antibodies in either serum or CSF confirmed by at least two techniques: brain immunohistochemistry and CBA expressing GluN1/2 subunits of the NMDA-receptor.27 The highly characteristic syndrome of patients with anti-NMDAR encephalitis, including 577 of the indicated 600 cases, was recently reported.21 Clinical information was obtained by the authors or treating physicians at different time points during the course of the disease, as reported.21 Techniques used for antibody testing, and CBA with GluN1 deletion mutants are described in the appendix and have been reported.1,2
Clinical outcome was scored using the modified Rankin Scale (mRS).28 The mRS was assessed by the physicians taking care of the patients along with one of the investigators (MT) using questionnaires and phone calls placed regularly during the disease. The mRS was assessed before all serological studies used in the current manuscript. Good outcome was defined as mRS 0–2 at the last follow-up (median 26 months, IQR 16–38 months), and poor outcome as mRS >2.21 Clinical relapses were defined as the new onset or worsening of symptoms by ≥ 1 point on the modified Rankin scale (mRS).
For determination of the sensitivity of antibody testing in serum and CSF, paired samples from 250 patients with anti-NMDAR encephalitis obtained at the diagnosis of the disease were selected by use of an online random integer generator (http://www.random.org/integers/, a size calculation is provided in the Appendix). Only patients whose serum and CSF were obtained the same day were included in the random generator to avoid bias favoring one sample over the other. Serum and CSF of 100 consecutive patients prospectively studied for a variety of encephalopathies served as controls to determine the specificity of the tests.
The follow-up of antibody titers during the course of the disease was carried out with brain immunohistochemistry using serial dilutions of serum and CSF until the reactivity was no longer visible. The assessment of the samples and titers was done by two investigators blinded of information (NG and JD). A change of antibody titer in serum or CSF of any individual patient was considered substantial when between two time points of the disease there was a difference of titers of at least two serial dilutions (as an example, first time point CSF titer 1:320, second time point CSF titer either decreased to at least 1:80 or increased to at least 1:1280). For these studies serum and/or CSF samples of 45 patients including 10 with clinical relapses, 25 with good-outcome-, and 10 with poor-outcome were examined. No selection was performed other than availability of serum and/or CSF samples from 3 or more time points during the course of the disease.
Since amino acid G369 forms part of the main epitope of GluN1, we used 4 reported GluN1 mutants2 that modify this protein region to different degrees to determine whether the epitope repertoire predicts outcome or changes with relapses. For details on the GluN1 mutants see appendix (“cell-based studies with GluN1 deletion mutants”). Serum and/or CSF samples of 23 patients were included in these studies: 11 with good outcome, 8 with poor outcome, and 4 with relapses (see in appendix “information on patients’ serum and CSF samples used for epitope analysis). The cases were selected depending on the availability of samples to conduct the studies with all GluN1 mutants.
Written consent for studies was obtained from patients, families, or patients’ representatives. Studies were approved by the institutional review boards of the University of Pennsylvania and the University of Barcelona.
Statistical analysis
Sensitivity and specificity of serum and CSF, both for brain immunohistochemistry and CBA with fixed cells were calculated. A systematic difference in direction between the different tests (serum versus CSF, CBA with fixed cells versus CBA with live cells) was compared using McNemar’s paired test. Differences in sensitivity between patients with and without tumor were calculated with the Fisher Exact test. Diagnostic likelihood ratios (LR) were calculated with the use of sensitivity and specificity; if the specificity was 100%, the next control patient tested (without NMDAR-encephalitis) was considered to be a false positive to avoid infinite LRs. The titer change between different time points (provided as a number of dilutions), positive if the change was in the expected direction (decrease when clinically improving, increase when clinically deteriorating), was compared between CSF and serum by the Mann Whitney U test. Longitudinal mixed models for repeated measurements (MMRM)29 were used to analyze titers from patients with monophasic disease collected in 3 time points (first, intermediate and last), declaring the subject as random and the outcome (dichotomous, good outcome defined as mRS 0–2), tumor, and time point as fixed effects. Because of a skewed distribution, log-transformation was used for the titers. Negative samples were scored as if the next dilution step would be positive, i.e. undiluted CSF and serum diluted 1:100. The significance level alpha was set at 0.05 two-tailed. SAS, the mixed procedure, version 9.2 (SAS Institute Inc., Cary NC, USA) was used for the MMRM analysis.
Role of the funding source
The study sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
All 250 patients with anti-NMDAR encephalitis had CSF antibodies detectable with both brain immunohistochemistry and CBA using fixed cells expressing GluN1/N2 (sensitivity 100%, 95% CI 98.5%–100.0%). In contrast, only 214 (86%, 95% CI 80.7%–89.4%, p<0.0001) sera were positive with both techniques; 18 (7%) were negative with one of the techniques (15 [6%] CBA, and 3 [1%] immunohistochemistry, p=0.0095), and the other 18 (7%) were negative with both techniques (Table 1). Serum antibodies were more frequently detected in patients who had an underlying teratoma than in those who did not have a tumor (91/97, 94% vs 123/153, 80%, p=0.0029, Table 1). None of the 100 paired serum and CSF samples from controls showed NMDAR antibodies with either of the techniques (specificity 100%). The diagnostic LR for CSF and serum were 101 and 86, respectively.
Table 1.
Sensitivity and specificity of testing for NMDÁR antibodies
| Sensitivity | CSF | Serum | pMcNemar's test | Non-Tumor (serum) | Tumor (serum) | pFisher-Exact test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 250 | 250 | 153 | 97 | |||||||
| Both tests positive | 250 | 100.0% | 214 | 85.6% | < 0.0001 | 123 | 80.4% | 91 | 93.8% | 0.0029 | |
| 95% CI | (98.5 – 100.0% | (80.7 – 89.4%) | (73.4% – 85.9%) | 87.2% – 97.1%) | |||||||
| Inconsistent | |||||||||||
| IHC negative | 0 | 0.0% | 3 | 1.2% | 0.0095 * | 2 | 1.3% | 1 | 1.0% | ||
| CBA negative | 0 | 0.0% | 15 | 6.0% | 12 | 7.8% | 3 | 3.1% | |||
| Both tests negative | 0 | 0.0% | 18 | 7.2% | 16 | 10.5% | 2 | 2.1% | |||
| Specificity | CSF | Serum | pMcNemar's test | |||
|---|---|---|---|---|---|---|
| Number of controls** | 100 | 100 | ||||
| Both tests negative | 100 | 100.0% | 100 | 100.0% | 1.00 | |
| 95% CI | (96.3 – 100.0% | (96.3 – 100.0%) | ||||
CSF cerebrospinal fluid; CI confidence interval; IHC immunohistochemistry; CBA cell-based assay;
IHC serum versus CBA serum;
The 100 control patients with suspected encephalitis contained two patients with antibodies against LGI1 (Leucine-rich Glioma Inactivated 1), one against amphiphysin, one against GAD (glutamic acid decarboxylase) and one against DPPX (Dipeptidyl-Peptidase–Like Protein-6).
To determine whether live CBA expressing GluN1/2 would improve the serum findings, all patients whose serum was negative with fixed CBA and/or brain immunohistochemistry and 74 whose serum was positive with both techniques (information in appendix, “antibody testing”) were tested with live CBA. These experiments showed that live CBA was less specifically reactive than fixed CBA (63/108 positive vs 77/108, p = 0.0056; Table S1) and therefore, did not improve the results. Examples of the difficulties examining serum antibodies with CBA are shown in Figure S1.
Forty-five patients with anti-NMDAR encephalitis were included in titration studies to evaluate the evolution of serum and CSF titers during the course of the disease (see Tables S2 and S3 for detailed clinical information and time points when samples were obtained). Because previous experience and the above findings indicated that brain immunohistochemistry is more sensitive than CBA, the follow-up of titers after initial demonstration of antibodies with both techniques was performed with immunohistochemistry (Figure 1). Thirty-five of these 45 patients had a monophasic illness (25 good outcome, 10 poor outcome) and 10 had clinical relapses (Tables S2 and S3). Overall, paired serum/CSF samples were available from the three time points in 32/45 patients (15 good outcome, 10 poor outcome, and 7 relapses). From another 8 patients only CSF was available for each time point (6 good outcome, 2 relapses), and from 5 additional patients only serum was available for each time point (4 good outcome, 1 relapses). For all 45 patients the first samples were obtained at the time of diagnosis (median 0.9 month from symptom onset, interquartile range [IQR] 0.3–2 months; 80% had not started treatment). For the 35 monophasic cases (25 good outcome, 10 poor outcome) the second samples were obtained after a median follow-up of 3.6 months (IQR 2.6–6.4 months), and the third samples at the last follow-up (median 12.5 months, IQR 8.8–20.1 months). For 8 of 10 patients with relapses serum or CSF samples were obtained between relapses while patients were clinically improved or asymptomatic.
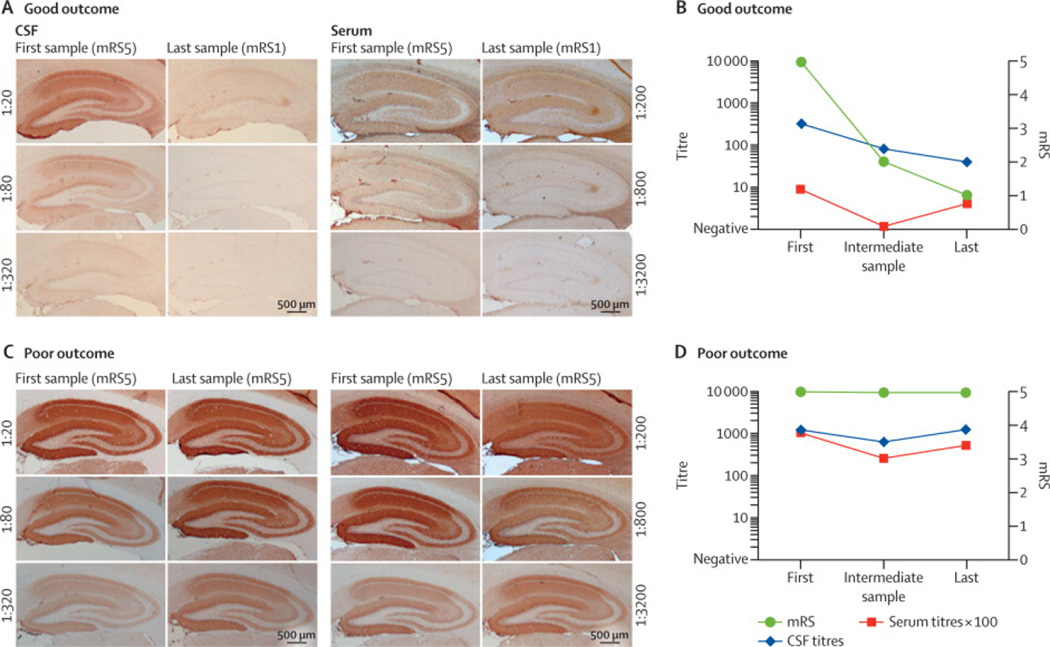
Figure 1. Determination of antibody titers using rat brain immunohistochemistry.
Brain reactivity of serial dilutions of serum and CSF of a patient with good outcome (A) and a patient with poor outcome (C). Samples were obtained at the time of diagnosis (first sample) and at the last follow-up (last sample); the intermediate samples are not shown. During the interval between first and last samples, the modified Ranking Scale (mRS) of the patient with good outcome improved from 5 to 1, and the CSF titers decreased substantially; however, the serum titers were only mildly changed, showing a poor correlation between serum titers and clinical improvement. During a similar interval, the mRS of the patient with poor outcome did not improve, and the CSF and serum titers remained unchanged. Scale bars: 500µm.
Panels B and D show a graphic representation of the change of titers in CSF (blue line) and serum (red), and the change of mRS (green) during the three time points studied. CSF titer and serum titer (× 100) are represented in the left Y axis and mRS is represented in the right Y axis.
Analysis of the follow-up of serum and CSF titers in patients with monophasic illness (good outcome or poor outcome) as a group is shown in Figure 2. In multivariable analysis, both CSF and serum titers were significantly higher in patients with poor-outcome than in those with good-outcome (CSF titer dilution 340 versus 129, difference 211, [95%-CI 1.1–421], p=0.049; serum 7370 versus 1243, difference 6127 [2369–9885], p=0.0025, Table 2), and in patients with a tumor (all teratomas) than in those without (CSF 395 versus 110, difference 285 [134–437], p=0.0079; serum 5515 versus 1644, difference 3870 [548–7193], p=0.024). Regardless of outcome, CSF and serum antibody titers decreased with prolonged follow-up (median follow-up 12.7 months; in CSF titers decreased from 614 at diagnosis to 76 at last follow-up, difference 538 [288–788]; and in serum from 5460 to 1564, difference 3896 [2428–5362], p<0.0001 p<0.0001 for both).
Figure 2. Change of antibody titers in patients with good and poor outcome.
A difference of magnitude of at least two dilutions up is colored in red, while a decrease of at least two dilutions down is colored in blue; minor changes are colored in grey. Each patient has a unique symbol which facilitates the comparison of patient’s serum and CSF. Patients with a tumor are depicted by circles or hexagons. Clinical details are provided in Table S2. The median decrease of antibody titers in CSF and serum between the indicated time points (originating from the initial median titer at time point 1) are shown in panels E and F; in these two panels patients with poor outcome are represented with dark colored lines, and patients with good outcome with light colored lines. Note that the median titer decrease was greater and occurred faster in CSF of patients with good outcome than in CSF of patients with poor outcome, or in serum of patients regardless of outcome.
Table 2.
Multivariate analysis of the change in titers in CSF (A) and serum (B)
| A. CSF | p | Estimate | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Good | 0.049 | 129 | 75 | 221 | ||||
| Poor | 340 | 158 | 735 | ||||||
| Tumor | No | 0.0079 | 110 | 57 | 213 | ||||
| Yes | 395 | 211 | 750 | ||||||
| time point | First | < 0.0001 | 614 | 347 | 1086 | ||||
| Intermediate | 198 | 118 | 330 | ||||||
| Last | 76 | 46 | 125 | ||||||
| interactions | |||||||||
| tumor * time point | 0.058 | tumor | no tumor | ||||||
| First | 1054 | 469 | 2368 | 358 | 161 | 796 | |||
| Intermediate | 305 | 147 | 633 | 129 | 62 | 268 | |||
| Last | 194 | 96 | 395 | 29 | 14 | 60 | |||
| outcome * tumor | 0.60 | ||||||||
| outcome * time point | 0.55 | ||||||||
| outcome * tumor * time point | 0.45 | ||||||||
| B. serum | p | Estimate | 95% CI | |||
|---|---|---|---|---|---|---|
| Outcome | Good | 0.0025 | 1243 | 655 | 2334 | |
| Poor | 7370 | 3119 | 17243 | |||
| Tumor | No | 0.024 | 1644 | 785 | 3447 | |
| Yes | 5515 | 2684 | 11443 | |||
| time point | First | < 0.0001 | 5460 | 2908 | 10149 | |
| Intermediate | 3214 | 1091 | 5515 | |||
| Last | 1564 | 921 | 2684 | |||
| interactions | ||||||
| tumor * time point | 0.18 | |||||
| outcome * tumor | 0.21 | |||||
| outcome * time point | 0.42 | |||||
| outcome * tumor * time point | 0.13 | |||||
The ln (titer) was used to correct for skewing. After creation of the best fitting model, recalculation of the estimated titer (and confidence interval) was performed; therefore the confidence interval is skewed. CSF cerebrospinal fluid; CI confidence interval
Analysis of the follow-up of serum and CSF titers not at a group level, but in individual patients with monophasic disease, showed a substantial decrease of titers between the first and second time points in the CSF of 12/21 (57%) cases with good outcome (Figure 2A) while this only occurred in 3/10 (30%) cases with poor outcome (Figure 2C). Limitations in power prevented a statistical difference (p=0.25). Similar assessments with serum titers between first and second time points (Figure 2B and D), or with serum and CSF titers between second and third time points (later stage of the disease) did not suggest any correlation.
The median decrease of titers between the different time points was greater and occurred earlier (between the first and second time points) in the CSF of patients with good outcome than in the CSF of patients with poor outcome (decrease by 2 serial dilutions per time interval versus 1 serial dilution), or than in the serum of patients with good or bad outcome (decrease by 1 serial dilution per time interval) (Figure 2E–F). At the last follow-up, most patients with good outcome (20/21 CSF samples and 14/19 serum samples) and patients with relapses and good outcome (4/7 CSF samples and 3/4 serum samples) still had detectable antibodies. In patients with clinical relapses, 14/19 episodes of clinical improvement or deterioration (Figure 3A) associated with a substantial decrease or increase of CSF antibody titer, respectively (median 2 dilutions). In contrast, less than half of the episodes (7/16) had a similar correlation when serum titers were considered (median 1 dilution, 14/19 versus 7/16, p=0.037, Fisher Exact test; Figure 3B).
Figure 3. Change of antibody titers in patients with clinical relapses.
A difference of magnitude of at least two dilutions in the expected direction (decrease at remission, and increase at relapse) is colored in blue, smaller change of titers are shown in grey. Each patient has a unique symbol which facilitates the comparison of patient’s serum and CSF. Patients with an underlying tumor are depicted by circles. Clinical details are provided in Table S3.
The experiments of epitope region analysis were conducted on samples of 23 patients, demonstrating that changes in amino acid G369I (glycine for isoleucine) abrogated the reactivity of 27 of 36 serum or CSF samples regardless of clinical outcome (Table 3, Figure 4), and substantially decreased the reactivity of the remaining 9 samples (Table S4, and Figure S2). In contrast, mutants of GluN1 at sites different from G369 had limited and inconsistent effects on the reactivity of patients’ samples: only 4/36 samples were affected by the change at the top lobe of GluN1, and only one with the construct ATD-TM4. Moreover, the pattern of reactivity in the initial episode of encephalitis did not change during relapses. These findings indicate that the main epitope region targeted by antibodies from patients with good outcome is similar to that of patients with poor outcome, and that there is no epitope spreading or change in the main epitope region during relapses.
Table 3.
Distribution of reactivity of patients’ serum and CSF with GluN1 mutants
| Outcome | Sample | WT | G369I | G369S | Top lobe | ATD-TM4 |
|---|---|---|---|---|---|---|
| Good (11 patients) | Serum | 8 | 1 | 6 | 5 | 7 |
| CSF | 10 | 4 | 10 | 10 | 10 | |
| Poor (8 patients) | Serum | 6 | 0 | 6 | 5 | 6 |
| CSF | 8 | 4 | 8 | 8 | 8 | |
| Relapse (4 patients) 1st episode/relapse | CSF | 4/4 | 0/0 | 4/4 | 4/4 | 4/4 |
The table shows the number of serum and CSF samples reacting with HEK cells expressing the indicated GluN1 mutants. Samples include 8 sera and 10 CSF of 11 patients with good outcome, and 6 sera and 8 CSF samples of 8 patients with poor outcome. For patients with relapses, a CSF sample obtained during the first episode and a sample obtained at relapse were examined. Note that mutation G369I abolished the reactivity of 27/36 samples, and substantially decreased the reactivity of the other 9 (the decrease of reactivity is shown in Table S4 and Figure S2).
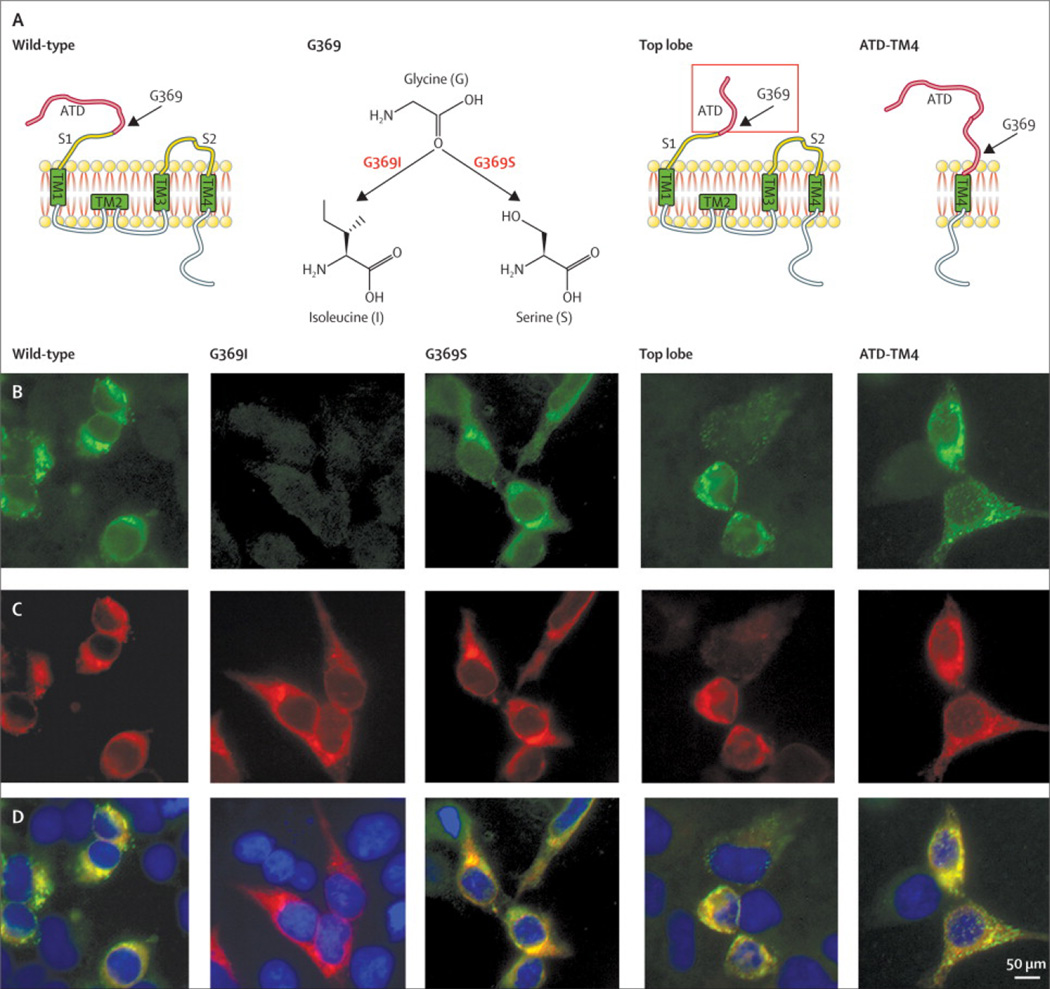
Figure 4. Typical pattern of reactivity of patients’ antibodies with GluN1 deletion constructs.
Schematic representation of GluN1 and GluN1 mutants (A). For all studies in this manuscript the wild type (WT) form of GluN1was used; the other GluN1 constructs were used to determine changes in the pattern of epitope recognition. The typical pattern of reactivity of a patient’s CSF is shown in row B, which demonstrates recognition of all constructs except G369I. The reactivity of the indicated GluN1 rabbit polyclonal antibody with the same mutants is shown in row C, and the merged reactivities in row D. Mutation G369I abolished the reactivity of 27/36 samples (Table 3) and substantially decreased the reactivity of the other 9 (Table S4 and Figure S2). Scale bar: 10µm.
Discussion
This study provides several novel findings that are relevant for the diagnosis and interpretation of antibody titers during the course of anti-NMDAR encephalitis: 1) it shows that by the time of diagnosis of the disease, NMDAR antibodies are always present in CSF but 13.2% (95% CI 9.6% – 18.0%) of the patients do not have serum antibodies detectable with CBA; 2) identifies an association between high antibody titers and poor outcome and/or the presence of a teratoma; 3) demonstrates that the change of titers in CSF correlates better with clinical relapses than that of serum; 4) suggests that an early decrease of CSF antibody titers during the first months of the disease associates with good outcome, and 5) shows that all patients’ antibodies target a main epitope region at GluN1 (aa 369) regardless of outcome.
The diagnosis of anti-NMDAR encephalitis would have been missed in 13% of the patients if only serum and CBA with fixed cells had been used. These results did not improve with CBA with live cells. In fact, the latter was found significantly worse than the CBA with fixed cells. Overall, 7% of patients did not have detectable serum antibodies with any of the techniques used, either brain immunohistochemistry or CBA with fixed or live cells. In contrast, all patients had NMDAR antibodies in CSF that were detectable with all techniques.
Recent studies using serum-testing on CBA with live cells expressing GluN1/2 or GluN1 subunits of the NMDAR receptor have shown IgG antibodies in some patients with Creutzfeldt-Jakob disease (CJD),16 schizophrenia,15 or degenerative diseases.17 Five healthy controls (0.4%) tested positive for IgG GluN1/2 antibodies30, and another study indicates a 3% false-positivity rate of serum testing using serum and a fixed CBA.31 These findings, however, have not been reproduced in other series using serum testing with CBA with fixed cells,32 or serum or CSF testing with combined techniques (brain immunohistochemistry and CBA with fixed cells), suggesting that this set of techniques has higher specificity for anti-NMDAR encephalitis.19,20 For example, none of 459 patients with acute schizophrenia, major depression, or borderline personality disorder had GluN1-specific antibodies except two patients who were reclassified as having misdiagnosed anti-NMDAR encephalitis based on the characteristic syndrome with multiple neurological and psychiatric symptoms and presence of serum antibodies against the GluN1 subunit of the NMDAR (CSF was not examined).32 Another study showed that 23 of 571 (4%) patients with anti-NMDAR encephalitis developed episodes of pure psychosis,33 but none of the 571 patients has developed schizophrenia or CJD to date. Moreover, comprehensive CSF testing of 49 patients with pathologically confirmed CJD did not reveal NMDAR antibodies.20 Overall, these studies show variable syndrome specificity among laboratories when antibodies are tested using only serum and CBA. In contrast, a remarkable syndrome specificity comparable among laboratories (100% of patients with symptoms of anti-NMDAR encephalitis) occurs when NMDAR antibodies are tested with CSF using CBA and brain immunohistochemistry (regardless of serum results).31,34
Patients with poor outcome or with a systemic teratoma had significantly higher titers of serum and CSF NMDAR antibodies than those with good outcome or without teratoma, confirming in part the findings of previous studies.1,13 In addition, patients with teratoma were more likely to have antibodies detectable in serum than those without tumor, suggesting that the tumor contributes in triggering the immune response. However, a systemic trigger is not always the cause of anti-NMDAR encephalitis. Recent studies show that in some patients the novel synthesis of NMDAR antibodies is triggered by herpes simplex encephalitis.35,36,37,38
In the current study, the importance of CSF antibody titers is demonstrated in patients with clinical relapses in whom the fluctuation of CSF titers correlated better with symptom recurrence and improvement than that of serum titers; despite this, we did not find a substantial change of CSF titers (> 2 serial dilutions) in 5 of 19 (26%) episodes of relapses (in these patients a potential correlation could have been obtained comparing CSF titers at shorter intervals). In patients with monophasic illness, a similar trend in the change of antibody titers was suggested between the first and second time points of the disease, although the limited number of cases did not provide statistical power. Moreover, when compared with patients with poor outcome, those with good outcome usually had a more rapid and robust decrease of CSF antibody titers. Of note, the actual difference in the decrease of CSF titers between groups of good and poor outcome is probably larger than that shown in Figure 2E for two reasons; first, the titers of patients with tumors (over-represented in the poor outcome group) tended to decrease faster than those of patients without tumors (shown in Table 2, p=0.058), and second, due to the lack of improvement, patients with poor outcome had received more intensive immunotherapy by the second time point of the disease (further discussed below).
A surprising finding was that by the last follow-up most patients had a decrease of serum and CSF titers regardless of outcome. One possibility could be a slow spontaneous fading of the immune response, providing an explanation for some cases of spontaneous clinical improvement and disappearance of antibodies many months after symptom onset.39 Another plausible explanation could be the more intense immunotherapy that patients with poor outcome received eventually leading to a decrease of titers regardless of the clinical deficits (burnt out stage).
In a third set of experiments we found that the epitope repertoire was similar in patients with good outcome and poor outcome, and did not change during relapses. Given that all patients’ antibody reactivity is highly dependent on the integrity of the G369 epitope region, the mutant that abrogates (or greatly diminishes) the antibody reactivity can be potentially used to confirm the presence of disease-relevant antibodies (e.g., antibodies that define anti-NMDAR encephalitis).
This study has limitations that are unavoidable without prospective assessment of serum and CSF antibody titers. Although for most patients we had paired samples obtained at different time points of the disease, in a few cases only serum or CSF was available for all time points. Moreover, while the second time point was in most cases ~3–4 months after symptom presentation, the last time point (last follow-up) was widely variable among patients. We specifically chose the second time point at ~3–4 months from onset because experience suggests that this is the time when decisions on continuation or change of treatment are frequently made.21 Finally, the restrictions that we put in the study design (e.g., inclusion of cases with paired serum and CSF samples, and/or availability of samples from at least 3 time points of the disease) resulted in a small number of subjects, diminishing statistical power in some assessments. Nevertheless, the better correlation of CSF over serum antibody titers with symptoms was demonstrated during relapses and was suggested at early stages of patients with monophasic disease.
Findings from this study have important practical implications: First, CSF antibody determination should be included in the initial diagnostic testing; examining only serum is not sufficient. Second, atypical clinical settings such as the occurrence of a characteristic syndrome with negative serum antibodies, or a mismatch between serum and CSF antibodies (e.g., positive serum and negative CSF) should be viewed with suspicion of being false results and should lead to comprehensive testing (serum and CSF with two different techniques). Third, patients with high antibody titers and limited or no decrease of CSF antibodies during the first 4 months of the disease are less likely to have good outcome than those with low titers and/or clear decrease of CSF titers, although the prognostic value needs prospective confirmation. Fourth, after recovery, most patients still have antibodies in serum and CSF; therefore, determination of baseline serum and CSF titers after recovery is potentially useful for characterization of new onset symptoms as possible relapses. Fifth, clinical relapses correlate better with the increase of CSF titers than that of serum titers. Finally, in practice, if regular follow-up of CSF titers cannot be obtained, clinical decisions should be based more on clinical assessment than on serum titers alone or on sporadic symptom-driven determination of titers in serum or CSF. This is supported by the outcome in a series of 577 patients where treatment decisions were primarily based on clinical status and 81% of the patients had substantial recovery at 2-year follow-up.21
Future studies should determine whether the levels of antibodies that persist after clinical recovery predict relapses and need of chronic immunotherapy. These studies should include an early and prospective assessment of CSF antibody titers as a possible prognostic biomarker.
Research in context
Systematic review
We searched in Medline and Embase databases up until June 01, 2013 for articles published in any language with the search terms “Anti-N-Methyl-D-Aspartate Receptor Encephalitis” [MeSH Terms], “Receptors, N-Methyl-D-Aspartate” [MeSH Terms], and “Encephalitis” [MeSH Terms]. We restricted searches to human studies.
Interpretation
Asystematic assessment of paired serum and CSF for NMDAR antibodies using more than one technique or confirmatory test at the time of diagnosis of anti-NMDAR encephalitis has not been previously conducted. There are only two series from other investigators (including 14 and 20 patients) in which paired serum and CSF studies were investigated. Both series used CBA (other confirmatory tests not described) leading to conflicting recommendations; while the investigators of one series suggested that serum and CSF analysis increased sensitivity for antibody testing, the investigators of the other (based on 14 patients) suggested that serum with CBA alone was sufficient. The current study provides the sensitivity and specificity of several techniques used for antibody determination in serum and CSF and demonstrates that CSF analysis should be included in the determination of IgG antibodies to GluN1 subunit of the NMDAR. The data shows that patients with high titers of antibodies in serum and CSF are more likely to have an underlying tumor or have worse outcome compared with those with low titers. A suggested correlation between serum antibody titers and outcome is not demonstrated in this study. CSF titers correlated better than serum titers with clinical relapses. Moreover, the data suggest that patients with good outcome have a faster and greater decrease of CSF antibodies than patients with poor outcome. On the other hand, a main epitope region at aa369 of GluN1 is identified by all patients’ antibodies, a property that can potentially be used as a distinctive marker of this disorder. Overall, these findings have important implications for the diagnosis and management of patients with anti-NMDAR encephalitis by emphasizing the importance of including CSF in antibody studies, and indicating that antibody titers should be viewed as complementary information to the clinical assessment.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JD and DL receive royalties from licensing fees to Euroimmun for a patent for the use of NMDAR as autoantibody test. The rest of the authors have nothing to disclose. None of the funding sources had any influence on the collection, analysis or interpretation of the data, or in the writing process. All authors had full access to the data of the study. The role and specific contribution of the authors is as follows: NG and MJT:literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript, funding. MT: statistical analysis. AT: data interpretation, statistical analysis. EA: data collection and interpretation; LM: data collection and interpretation; FL: data collection and critical approval of final manuscript; AG: plasmid construction and critical approval final manuscript; RB-G: data interpretation, and critical approval final manuscript, funding; MRR: data collection, writing, and critical approval final manuscript; DL: plasmid construction, critical approval final manuscript, funding; FG: data collection, data analysis, data interpretation, critical approval final manuscript, funding; JD: figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript, funding.
Reference List
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA Receptor Encephalitis Antibody Binding Is Dependent on Amino Acid Identity of a Small Region within the GluN1 Amino Terminal Domain. J Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumakura A, Miyajima T, Fujii T, Takahashi Y, Ito M. A patient with epilepsia partial is continua with anti-glutamate receptor epsilon 2 antibodies. Pediatr Neurol. 2003;29:160–163. doi: 10.1016/s0887-8994(03)00151-6. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRepsilon2 in patients with Rasmussen's encephalitis and chronic progressive epilepsia partialis continua. Epilepsia. 2005;46(Suppl 5):152–158. doi: 10.1111/j.1528-1167.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 5.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 6.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansing LH, Tuzun E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pruss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 10.Greiner H, Leach JL, Lee KH, Krueger DA. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure. 2011;20:266–270. doi: 10.1016/j.seizure.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Punja M, Pomerleau AC, Devlin JJ, Morgan BW, Schier JG, Schwartz MD. Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis: an etiology worth considering in the differential diagnosis of delirium. Clin Toxicol (Phila) 2013;51:794–797. doi: 10.3109/15563650.2013.829235. [DOI] [PubMed] [Google Scholar]
- 12.Suh-Lailam BB, Haven TR, Copple SS, Knapp D, Jaskowski TD, Tebo AE. Anti-NMDA-receptor antibody encephalitis: performance evaluation and laboratory experience with the anti-NMDA-receptor IgG assay. Clin Chim Acta. 2013;421:1–6. doi: 10.1016/j.cca.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–772. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 15.Zandi MS, Irani SR, Lang B, et al. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. 2011;258:686–688. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol. 2012;259:1979–1981. doi: 10.1007/s00415-012-6489-3. [DOI] [PubMed] [Google Scholar]
- 17.Deakin J, Lennox BR, Zandi MS. Antibodies to the N-Methyl-D-Aspartate Receptor and Other Synaptic Proteins in Psychosis. Biol Psychiatry. 2013 Aug 26; doi: 10.1016/j.biopsych.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Hammer MS, Larsen MB, Stack CV. Outcome of children with opsoclonus-myoclonus regardless of etiology. Pediatr Neurol. 1995;13:21–24. doi: 10.1016/0887-8994(95)00083-r. [DOI] [PubMed] [Google Scholar]
- 19.Masdeu JC, Gonzalez-Pinto A, Matute C, et al. Serum IgG antibodies against the NR1 subunit of the NMDA receptor not detected in schizophrenia. Am J Psychiatry. 2012;169:1120–1121. doi: 10.1176/appi.ajp.2012.12050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grau-Rivera O, Sánchez-Valle R, Saiz A, et al. Determination of neuronal antibodies in suspected and definite Creutzfeldt-Jakob disease. JAMA Neurol. doi: 10.1001/jamaneurol.2013.4857. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent A, Bien CG. Anti-NMDA-receptor encephalitis: a cause of psychiatric, seizure, and movement disorders in young adults. Lancet Neurol. 2008;7:1074–1075. doi: 10.1016/S1474-4422(08)70225-4. [DOI] [PubMed] [Google Scholar]
- 23.Furneaux HF, Reich L, Posner JB. Autoantibody synthesis in the central nervous system of patients with paraneoplastic syndromes. Neurology. 1990;40:1085–1091. doi: 10.1212/wnl.40.7.1085. [DOI] [PubMed] [Google Scholar]
- 24.Thomas A, Rauschkolb P, Gresa-Arribas N, Schned A, Dalmau J, Fadul CE. Anti-N-methyl-D-aspartate receptor encephalitis: a patient with refractory illness after 25 Months of intensive immunotherapy. JAMA Neurol. 2013 Oct 28; doi: 10.1001/jamaneurol.2013.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexopoulos H, Kosmidis ML, Dalmau J, Dalakas MC. Paraneoplastic anti-NMDAR encephalitis: long term follow-up reveals persistent serum antibodies. J Neurol. 2011;258:1568–1570. doi: 10.1007/s00415-011-5982-4. [DOI] [PubMed] [Google Scholar]
- 26.Hansen HC, Klingbeil C, Dalmau J, Li W, Weissbrich B, Wandinger KP. Persistent Intrathecal Antibody Synthesis 15 Years After Recovering From Anti- N-methyl-D-aspartate Receptor Encephalitis. JAMA Neurol. 2013;70:117–119. doi: 10.1001/jamaneurol.2013.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 29.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2010. [Google Scholar]
- 30.Hammer C, Stepniak B, Schneider A, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2013 Sep 3; doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- 31.Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. doi: 10.1212/WNL.0000000000000126. in press. [DOI] [PubMed] [Google Scholar]
- 32.Steiner J, Walter M, Glanz W, et al. Increased prevalence of diverse N -methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N -methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 33.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013;70:1133–1139. doi: 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titulaer MJ, Dalmau J. Seizures as first symptom of anti-NMDA receptor encephalitis are more common in men. Neurology. doi: 10.1212/WNL.0000000000000131. in press. [DOI] [PubMed] [Google Scholar]
- 35.Armangue T, Titulaer MJ, Malaga I, Bataller L, Gabilondo I, Graus F, Dalmau J. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162:850–856. doi: 10.1016/j.jpeds.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leypoldt F, Titulaer FJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: Case Report. Neurology. 2013;81:1637–1639. doi: 10.1212/WNL.0b013e3182a9f531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post herpes simplex virus encephalitis neurological relapse. Mov Disord. 2013 Sep 6; doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- 38.Mohammad SS, Sinclair K, Pillai S, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2-receptor. Mov Disord. 2013 Oct 1; doi: 10.1002/mds.25623. [DOI] [PubMed] [Google Scholar]
- 39.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.