Abstract
Rhizobactin 1021 is a hydroxymate siderophore produced by the soil bacterium Sinorhizobium meliloti 2011. A regulon comprising rhtA, encoding the outer membrane receptor protein for the ferrisiderophore; the biosynthesis operon rhbABCDEF; and rhrA, the Ara-C-like regulator of the receptor and biosynthesis genes has been previously described. We report the discovery of a gene, located upstream of rhbA and named rhtX (for “rhizobactin transport”), which is required, in addition to rhtA, to confer the ability to utilize rhizobactin 1021 on a strain of S. meliloti that does not naturally utilize the siderophore. Rhizobactin 1021 is structurally similar to aerobactin, which is transported in Escherichia coli via the IutA outer membrane receptor and the FhuCDB inner membrane transport system. E. coli expressing iutA and fhuCDB was found to also transport rhizobactin 1021. We demonstrated that RhtX alone could substitute for FhuCDB to transport rhizobactin 1021 in E. coli. RhtX shows similarity to a number of uncharacterized proteins which are encoded proximal to genes that are either known to be or predicted to be involved in iron acquisition. Among these is PA4218 of Pseudomonas aeruginosa, which is located close to the gene cluster that functions in pyochelin biosynthesis and outer membrane transport. PA4218 was mutated by allelic replacement, and the mutant was found to have a pyochelin utilization-defective phenotype. It is proposed that PA4218 be named fptX (for “ferripyochelin transport”). RhtX and FptX appear to be members of a novel family of permeases that function as single-subunit transporters of siderophores.
Although iron is abundant, it is not readily available to microorganisms growing aerobically because it is chemically bound in complexes that have a low solubility at neutral pH. Iron is essential for growth, and bacteria have evolved a variety of strategies to overcome its limited availability (40). A common strategy is the production of ferric iron binding compounds, termed siderophores. There is considerable structural variation among siderophores (9), but they are all characterized by a high binding constant for ferric iron and the capacity to acquire iron from the local environment and facilitate its uptake by the bacterial cell. Siderophore receptor proteins that function in the transport of the ferrisiderophore complex into the cell are located in the outer membranes of gram-negative bacteria grown under conditions of iron depletion. The receptors display relatively tight specificity for their cognate siderophores. Translocation of the ferrisiderophore through the receptor and into the periplasm is dependent on the energy-transducing Ton system, comprising two cytoplasmic membrane proteins, ExbB and ExbC, in addition to the TonB protein that transverses the periplasm (27). The receptor proteins commonly possess a conserved sequence, the TonB box, and evidence suggests that the interaction between the siderophore and the receptor leads to a conformational change in the receptor and subsequent association between the TonB protein and the receptor, possibly at the TonB box. In this way TonB provides the energy necessary to transport the siderophore to the periplasm.
Ferrisiderophore transport across the periplasm and cytoplasmic membrane exhibits less specificity than that at the outer membrane. The ferric hydroxamate uptake (Fhu) system of Escherichia coli (23) facilitates the transport of different hydroxamate siderophores, including ferrichrome, coprogen, and aerobactin, each of which requires its cognate receptor at the outer membrane. The Fhu system is representative of an ABC transporter, composed of the cytoplasmic membrane integral protein FhuB, which resembles a heterodimer and has each half associated with the ATPase FhuC (16). In addition, the periplasmic protein FhuD binds the ferrisiderophore (24) and chaperones it across the periplasm to the cytoplasmic membrane FhuBC complex.
Infectious pathogens encounter strict iron limitation within an animal host due to the action of iron-withholding proteins such as transferrin and lactoferrin. The production of siderophores has been shown to correlate with bacterial pathogenicity in numerous studies. Pseudomonas aeruginosa, an important human opportunistic pathogen, produces two siderophores, pyoverdine and pyochelin (37). Both have been shown to have the capacity to remove iron from transferrin and lactoferrin and to contribute to the virulence of P. aeruginosa in animal infection models (47). Plant pathogens do not have to compete with iron binding proteins such as transferrin or lactoferrin. Nevertheless, they must compete with the plant host systems for iron transport and storage, which ensure that iron is accessible as a nutrient to the host while not being available to catalyze the formation of reactive free radicals (12). A role for the siderophore chrysobactin in the virulence of plant pathogenic Erwinia species has been established (11). In contrast, agrobactin produced by Agrobacterium tumefaciens is not necessary for crown gall formation (26). The interaction between root nodule bacteria, which are collectively called the rhizobia, and their leguminous plant hosts results in the formation of a nitrogen-fixing symbiotic interaction that is known to be demanding with regard to iron. However, rhizobactin 1021, produced by Sinorhizobium meliloti, and vicibactin, produced by Rhizobium leguminosarum, have been shown to be unnecessary for the symbiotic interactions with their respective plant hosts (6, 30). Rhizobia are found free living in soil, and it is likely that the siderophores that they produce function to provide them with an adequate supply of iron in the competitive soil environment.
The siderophore produced by S. meliloti 2011, termed rhizobactin 1021, is a citrate-based dihydroxamate that has a core structure identical to that of schizokinen from Bacillus megaterium (35). However, unlike shizokinen, rhizobactin 1021 has an unusual lipid moiety appended. The core structures of rhizobactin 1021 and schizokinen are not identical to, but do resemble, the core of aerobactin produced by some members of the Enterobacteriaciae and shown to be a virulence factor in E. coli. Genetic characterization of rhizobactin 1021 production and utilization by S. meliloti 2011 revealed an operon comprising the biosynthesis genes rhbABCDEF located upstream from rhtA, the outer membrane receptor gene for the siderophore (30). An Ara-C-like regulator encoded between the biosynthesis operon and the receptor gene was shown to positively regulate the production and transport of the siderophore. While some siderophore loci have clearly identifiable inner membrane transport systems encoded close to the biosynthesis and outer membrane receptor genes, there is no obvious transporter in the rhizobactin 1021 region of the sequenced genome of S. meliloti.
In S. meliloti over 400 transporter genes are predicted in the sequenced genome. Of the products of these genes, only the SitABCD metal-type permease, encoded close to the fur gene, has been investigated with regard to iron transport (36). It was determined that the SitABCD transporter is not involved in siderophore transport but does function in manganese acquisition. For P. aeruginosa there is remarkably little known about inner membrane transport of siderophores. We report the identification of novel genes that function in rhizobactin 1021 and pyochelin utilization. The products of these genes are members of a new family of proteins that appear to function at the inner membrane to facilitate the uptake of ferrisiderophore complexes in a variety of species, including S. meliloti and P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used are listed in Table 1. S. meliloti was cultured on TY medium (3). E. coli and P. aeruginosa were cultured on Luria-Bertani medium (43). SOC medium (20) was used in the transformation of E. coli. CAS (chrome azurol S) medium for the detection of siderophore production was prepared by the method of Schwyn and Neilands (44) with the modifications described by Reigh and O'Connell (41). Antibiotics were used at the following concentrations: for S. meliloti, kanamycin at 100 μg/ml, gentamicin at 30 μg/ml, tetracycline at 10 μg/ml, and streptomycin at 1 mg/ml; for P. aeruginosa, kanamycin at 500 μg/ml, gentamicin at 50 μg/ml, tetracycline at 50 μg/ml, and ampicillin at 100 μg/ml; and for E. coli, kanamycin at 30 μg/ml, gentamicin at 20 μg/ml, tetracycline at 20 μg/ml, and ampicillin at 100 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL-1Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIqZΔM15Tn10(Tetr)] | 43 |
| DH5α | endA1 gyrA96 thi-1 hsdR17 supE44 relA1 recA1 ΔlacU169(φ80ΔlacZΔM15) | 43 |
| A118 | Chromosomally located Tn5lac | 45 |
| H1443 | K-12 strain, aroB | 17 |
| RK4375 | fhuC::Tn10 fepA9 aroE24 (previously fhuB478::Tn10)b | E. coli Genetic Stock Center |
| S. meliloti | ||
| 2011 | Wild typec; Smr Nod+ Fix+ | |
| 2011rhtX1 | 2011 with Ω-Km cassette in rhtX (rhtX1) | This study |
| 2011rhtX43 | 2011 with Tn5lac insertion in rhtX (rhtX43) | This study |
| 2011Sma2335km | 2011 with Kmr cassette in Sma2335 | This study |
| 2011rhtA45 | Tn5lac insertion in rhtA | 30 |
| 2011rhrA26 | Tn5lac insertion in rhrA | 30 |
| 102F34 | Does not produce or utilize rhizobactin 1021; Nod+ Fix+ | 41 |
| P. aeruginosa | ||
| PA01 | Wild type; produces pyoverdine and pyochelin | |
| CDC5 | pvd-2; produces pyochelin, does not produce pyoverdine | 2 |
| DH143 | pvd-2 fptA | 19 |
| PA4218-km1 | CDC5 with a Kmr cassette in fptX (PA4218) | This study |
| B. megaterium ATCC 19213 (91-02) | Schizokinen producer | NCIMBd culture collection |
| Plasmids | ||
| pJQ200ks | Gmrmob sacB | 39 |
| pRK600 | Tra Cmr | 15 |
| pCR2.1 | Apr | Invitrogen |
| pMO 012405 | Cosmid carrying genomic region of PAO1 including fptX coding sequence | Pseudomonas Genetic Stock Center |
| pSUP104 | Tcrmob | 38 |
| pBBR1MCS-5 | Gmrmob | 25 |
| pPOC4 | pBBR1MCS-5::rhtX | This study |
| pPOC5 | pSUP104 with the region carrying rhrA and rhtA | This study |
| pEN7 | iutA | 18 |
| pRG13 | iucABCD | 18 |
| pHP45Ω-Km | Source of Ω-Km | 13 |
| pUC4K | Source of Kmr cassette | Amersham |
| pPC35 | pCR2.1 fhuCDB | This study |
| pPC29 | pCR2.1 fhuCD | This study |
| pPC20 | pCR2.1 fhuDB | This study |
Apr, Cmr, Gmr, Kmr, Smr, and Tcr, ampicillin, chloramphenicol, gentamicin, kanamycin, streptomycin, and tetracycline resistance, respectively; Tra, transfer.
The strain carries the mutation previously described as fhuB478::Tn10 by Kadner et al. (22), which was determined to be fhuC::Tn10 in this work. See text for details.
S. meliloti 2011 is the parent strain of 1021 (31). 1021 was used to sequence the genome of the species and for the purification and chemical characterization of rhizobactin 1021 (35).
NCIMB, National Collections of Industrial, Food and Marine Bacteria.
Bacterial conjugation and transformation.
Bacterial conjugation was carried out as described by O'Connell et al. (33). Transformation was by the method of Inoue et al. (20). E. coli XL-1 Blue was used as a host for routine transformation and plasmid preparation.
DNA manipulations.
PCRs were undertaken with the temperature gradient block in a Thermo Hybaid PCR Express thermal cycler. DNA restriction and ligation reactions were carried out by standard procedures (43). Plasmid DNA was isolated by the alkaline lysis method (4). Cosmid DNA was prepared by the method of Little (29) but with the modification that the DNA was phenol extracted and was precipitated with 2.5 volumes of ethanol on ice for 30 min. Restriction fragments were cut from ethidium bromide-stained agarose gels as required for subcloning and were purified by using the Perfectprep gel cleanup kit as directed by the manufacturer (Eppendorf). Total genomic DNA was prepared from S. meliloti broth cultures by the method of Meade et al. (31) and from P. aeruginosa by the method of Chen and Kuo (7). Restricted genomic DNA was separated by agarose gel electrophoresis and transferred to nitrocellulose filters by standard methods (43). Probes for Southern hybridization were labeled and hybridized by using the digoxigenin DNA labeling and detection kit as directed by the manufacturer (Roche).
Strain and plasmid constructions.
The rhtX43 mutation in S. meliloti 2011rhtX43 was constructed by selecting for insertion of the transposon Tn5lac in rhtX (Sma2337 in the genome sequence) by using the fragment-targeted mutagenesis method described by Lynch et al. (30). E. coli A118 was the source of Tn5lac.
To construct the mutant S. meliloti 2011rhtX1, carrying an Ω kanamycin resistance cassette inserted in the rhtX gene, a 2-kb region, having the BamHI site within rhtX centrally located, was amplified by PCR (forward primer [Pf], 5′-AGATCTGTTGCCGAAGCCCTGCGGGTTC-3′; reverse primer [Pr], 5′-AGATCTGGAAGGCGGAGGCGGAGATGGC-3′). The fragment was ligated to the pCR2.1 vector by TA cloning and then subcloned into the BamHI site of pJQ200ks as a BglII fragment (BglII sites having been incorporated into the primers). This destroyed the BamHI recognition sequence in the vector and left the single BamHI site in the rhtX gene available for insertion of the Ω kanamycin resistance cassette, which was cut as a BamHI fragment from plasmid pHP45Ω-Km. The pJQ200ks derivative with rhtX::Ω-Km was mobilized into S. meliloti 2011 by a triparental mating with pRK600. Integration of the plasmid by a single crossover was selected on gentamicin and streptomycin. After purification, a second crossover and allelic replacement were selected on medium containing 5% sucrose with kanamycin. Gentamicin sensitivity was confirmed, and Southern hybridization was used to confirm the loss of the vector and the correct insertion of the cassette.
S. meliloti 2011 mutant Sma2335km was constructed as follows. A region encoding the Sma2335 gene was amplified on a 2-kb fragment from genomic DNA (Pf, 5′-GCGGCCGCCGATGGTCTGCTTCACCCCCTCG-3′; Pr, 5′-CCCGGGCATCCAGGCATTCGGCCGCCGG-3′). The primers introduced XmaI and NotI sites into the fragment, which was ligated to the pCR2.1 vector by TA cloning. The fragment was then subcloned into pJQ200ks that had been cut with XmaI and PspOM1, an enzyme that cuts pJQ200ks such that it can be ligated to the NotI-restricted fragment carrying Sma2335. This ligation destroyed the ApaI site from pJQ200ks, leaving a unique ApaI site in the ligated plasmid within the Sma2335 gene. The kanamycin resistance gene was amplified from plasmid pUC4K with ApaI sites introduced on the PCR primers (Pf, 5′-GGGCCCGACGTTGTAAAACGACGGCCAGTG-3′; Pr, 5′-GGGCCCGGAAACAGCTATGACCATGATTACG-3′). This fragment was initially ligated to the pCR2.1 plasmid by TA cloning and then subcloned into the ApaI site of the Sma2335 gene in pJQ200ks. Allelic replacement and confirmation of the insertion by Southern hybridization were carried out as described above.
To obtain the fptX (PA4218) gene of P. aeruginosa for mutagenesis, a 3.6-kb XhoI/XmaI fragment containing the coding region was subcloned from the cosmid pMO 012405 and inserted in pJQ200ks. Insertion in the multiple cloning site of the vector with these enzymes removed the SalI site from the vector and allowed the kanamycin resistance gene from pUC4K to be inserted into the SalI site within fptX. Allelic exchange to introduce the mutated gene into the P. aeruginosa genome, constructing strain PA4218-km1, was carried out. However, sucrose sensitivity as a result of the sacB gene on pJQ200ks was not as effective as in S. meliloti, and it was necessary to screen a large number of colonies by replica plating on medium containing kanamycin only and medium containing kanamycin and gentamicin to detect clones having the gentamicin-sensitive, kanamycin-resistant phenotype resulting from recombination events that excised the vector from the chromosome. Correct insertion of the cassette in the chromosome was confirmed by Southern hybridization.
Plasmid pPOC4, carrying rhtX expressed from a lac promoter, was constructed by amplifying the gene with its ribosome binding site by using primers that introduced XhoI and PstI restriction sites (Pf, 5′-CTCGAGGCCGGGCAGTGGCAGTTTTCGATGC-3′; Pr, 5′-CTGCAGTCATCGTGATCTTGAAGGACGCGCTTTC-3′) and inserting the gene in the pCR2.1 vector by TA cloning. The fragment was then subcloned into the mobilizable broad-host-range vector pBBR1MCS-5 that had been cut with XhoI and PstI, placing the gene in the correct orientation for expression from the lac promoter of the plasmid.
The plasmid pPOC5 carrying rhrA and rhtA was constructed by a two-step amplification as follows. First, the rhtA open reading frame was amplified (Pf, 5′-GAATTCCCTGTTGACGTTCGCATGC-3′; Pr, 5′-TCTAGATTAAAAAACCTTTCTCAGCGA-3′) and cloned by TA cloning in the pCR2.1 vector. Second, a fragment containing the rhrA gene and a section of rhtA including the XhoI site was amplified (Pf, 5′-GAATTCTCAAGCGGCGGCTGCCAGCC-3′; Pr, 5′-CTCGAGCGCGGAATCGCCCACG-3′) and cloned in the pCR2.1 vector. The first fragment was subsequently cloned in the broad-host-range vector pSUP104. The rhrA open reading frame was completed in this construct by inserting the region extending from the XhoI site to the end of the rhrA open reading frame, obtained from the second amplification described above. This construct then contained the rhrA and rhtA genes complete with their promoter regions cloned in pSUP104.
The plasmids pPC35, pPC29, and pPC20 were constructed by amplifying fhuCDB (Pf, 5′-GAATTCGTGCCCATTTCACAAGTTGGCTGTTATGC-3′; Pr, 5′-GGATCCTTAACGGCTCTGCTTTCTCAACAAATAGATAA-3′), fhuCD(Pf, as for fhuCDB; Pr, 5′-GGATCCTCACGCTTTACCTCCGATGGCGTTATCC-3′), and fhuDB (Pf, 5′-GAATTCCTGCACCTGTGAGTTTTGTTTATTGATGAGC-3′; Pr, as for fhuCDB), respectively, and inserting them in the pCR2.1 vector. The cloned genes were expressed with their own ribosome binding sites from the lac promoter of the vector.
Siderophore production and utilization assays.
Siderophore production was detected by the CAS assay. A 10-μl aliquot of culture adjusted to an optical density at 600 nm of 1.0 was delivered onto the surface of a CAS plate and the plate was incubated at 30°C for 3 days, after which the appearance of an orange halo indicated siderophore production.
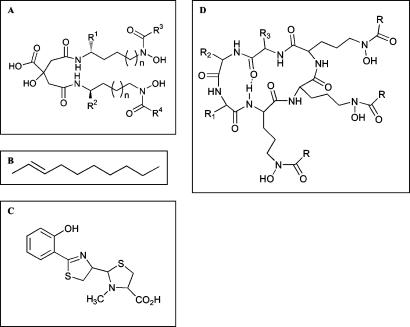
The siderophore utilization bioassay was carried out as described by Lynch et al. (30) with 2,2′-dipyridyl incorporated in the medium at concentrations of 300 μM for S. meliloti, 2 mM for P. aeruginosa, and 400 μM for E. coli. For the siderophore utilization bioassays, the supernatants containing the siderophores were prepared from strains as follows: rhizobactin 1021 from S. meliloti 2011, schizokinen from B. megaterium ATCC 19213 (91-02), pyochelin from P. aeruginosa CDC5, and aerobactin from E. coli H1443 carrying pRG13. The aroB mutation in E. coli H1443 abolishes the production of enterobactin, while aerobactin biosynthesis is encoded by iucABCD on pRG13. The strains were grown in medium containing 2,2′-dipyridyl at the concentrations described above and at 300 μM for B. megaterium. Ferrichrome was purchased from Sigma. Figure 1 shows the chemical structures of the siderophores used in this study. As negative controls, supernatants were prepared from strains grown under iron-replete conditions.
FIG. 1.
Chemical structures of the siderophores used in this study. (A) Aerobactin (R1 = R2 = COOH; R3 = R4 = H; n = 2), schizokinen (R1 = R2 = H; R3 = R4 = H; n = 0), rhizobactin 1021 (R1 = R2 = R3 = H; R4 = the structure in panel B; n = 0). (C) Pyochelin. (D) Ferrichrome (R = CH3; R1 = R2 = R3 = H).
Plant nodulation and nitrogen fixation assays.
Alfalfa (Medicago sativa) seeds were surface sterilized in ethanol and domestic bleach and examined for the absence of contaminants by germination on TY medium. Germinated seedlings were transferred to Jensen medium, as described by Ogawa et al. (34), in test tubes and inoculated with approximately 105 S. meliloti cells per tube. Nitrogen fixation by whole plants was determined after 30 days by the acetylene reduction assay (49).
DNA sequencing and sequence analysis.
DNA was sequenced by MWG Biotech, Milton Keynes, United Kingdom. Database searches were undertaken with BLASTX and BLASTP (1). Multiple-sequence alignments were performed with Multalin (8) and Genedoc. The Tmpred program was used to predict protein structure and folding. Access to genome sequence data was obtained at http://sequence.toulouse.inra.fr/meliloti.html for S. meliloti and at http://www.pseudomonas.com for P. aeruginosa.
RESULTS
Identification and mutagenesis of rhtX.
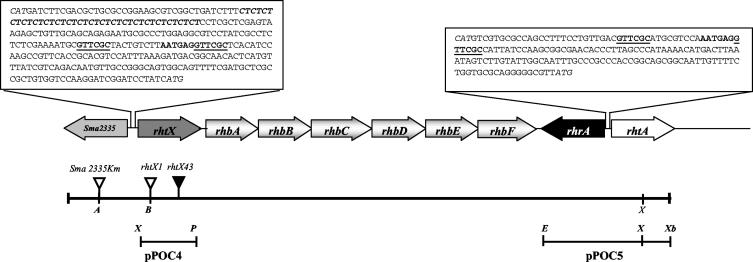
To fully delineate the rhizobactin regulon, the region upstream from the biosynthesis operon rhbABCDEF (Fig. 2) was mutagenized. A Tn5lac insertion in the open reading frame upstream of rhbA was isolated by the fragment-targeted transposon mutagenesis method described by Lynch et al. (30). The strain carrying the insertion, S. meliloti 2011rhtX43, was tested for its ability to produce rhizobactin 1021 and to utilize the siderophore. The plate bioassay revealed that the mutant was totally defective in rhizobactin 1021 utilization. In the CAS assay the mutant produced a diminished halo, which initially suggested that the rhbABCDEF operon located downstream from the transposon insertion site may be transcribed from two promoters, one located between the transposon insertion site and the operon and the second upstream from the transposon. However, it came to our attention that the Tn5lac transposon can have outward promoter activity which could be responsible for the low-level expression of the rhbABCDEF operon in the mutant. We therefore constructed a mutation in the same gene with the Ω kanamycin resistance cassette, to ensure complete termination of transcription by the cassette insertion. Mutant S. meliloti 2011rhtX1 carrying the cassette was found to be defective in rhizobactin 1021 utilization. However, unlike S. meliloti 2011rhtX43, it was totally defective in the production of the siderophore as tested by the CAS assay, indicating that there was a polar effect on siderophore production in S. meliloti 2011rhtX1. It can be concluded, therefore, that the diminished halo which had been observed in S. meliloti 2011rhtX43 was due to the polar effect of the Tn5lac insertion, which was partially relieved by the outward promoter activity from the Tn5lac transposon transcribing the rhbABCDEF genes at a low level. The gene carrying the mutations, called Sma2337 in the published genome sequence, was named rhtX (for “rhizobactin transport”) in recognition of the observed phenotype and the homology to YbtX from Yersinia pestis (discussed below). The evidence implies that rhtX is the first gene in an operon that extends through rhbABCDEF.
FIG. 2.
Genetic organization of the region encoding rhizobactin biosynthesis (rhb), regulation (rhr), and transport (rht) genes in S. meliloti. The DNA sequences upstream of rhtX and between rhrA and rhtA are shown, having the six-base repeat (underlined) interspersed by 15 bases. Identical sequences in the two regions are shown in boldface. The locations of the kanamycin cassette insertions in the mutants 2011Sma2335km and 2011rhtX1 are shown (▿). The location of the Tn5lac insertion in mutant 2011rhtX43 is also shown (▾). Start codons are in italic. The CT repeats are in boldface italic. Restriction sites: A, ApaI; B, BamHI; E, EcoRI; P, PstI; X, XhoI; Xb, XbaI.
To determine whether the gene further upstream of rhtX (Fig. 2) also functioned in the production or utilization of the siderophore, a mutation was constructed in the neighboring open reading frame. By allelic replacement a kanamycin resistance cassette was inserted, as described in Materials and Methods, in the small open reading frame annotated in the S. meliloti genome as Sma2335, to give strain S. meliloti 2011Sma2335km. The phenotype of the strain was determined by using the CAS assay to detect siderophore production and the plate bioassay to examine siderophore utilization. A phenotype identical to that of the wild type was observed for the strain, indicating that Sma2335 does not function in production or utilization of rhizobactin 1021. Further upstream from rhtX and next to Sma2335 there is an operon showing very good homology to the kdp operon present in many bacterial species and determined to function in potassium transport. This operon is unlikely to function in transport of the siderophore.
Complementation of the mutations in rhtX was undertaken with plasmid pPOC4, carrying the rhtX gene in the broad-host-range plasmid pBBR1MCS-5, under the control of the lac promoter of the plasmid. The presence of plasmid pPOC4 in S. meliloti 2011rhtX1 restored the wild-type phenotype for siderophore transport in the bioassay but did not restore the wild-type phenotype for siderophore production on CAS plates. This further confirmed the conclusion that rhtX does not have a role in siderophore biosynthesis and that the reason for the low-level siderophore production in the S. meliloti 2011rhtX43 mutant was the effect of the outward gene expression from the Tn5lac transposon on the downstream biosynthesis genes.
Analysis of the sequence upstream of rhtX revealed direct repeats of the sequence GTTCGC separated by 15 bases (Fig. 2). Moreover, 20 repeats of the CT dinucleotide are found consecutively in the published genome, upstream as shown in Fig. 2. Interestingly, we sequenced this region independently and found 19 repeats of the dinucleotide at the same location.
Mutagenesis of rhtX did not affect the ability of S. meliloti to nodulate alfalfa and fix nitrogen. Nitrogenase activity, as measured by the acetylene reduction assay, of plants nodulated by S. meliloti 2011rhtX43 was comparable to that of the wild type (data not shown).
Introduction of rhtX and rhtA confers the ability to utilize rhizobactin 1021 in S. meliloti 102F34, a strain that otherwise cannot utilize the siderophore.
It has been shown that rhtA encodes the outer membrane receptor for rhizobactin 1021 (30, 41). The RhrA activator positively regulates transcription of the gene. Plasmid pPOC5, carrying rhtA and rhrA in the broad-host-range vector pSUP104, was shown to complement mutations in rhtA and rhrA when introduced into S. meliloti 2011rhtA45 and S. meliloti 2011rhrA26, respectively. S. meliloti 102F34 is a strain that does not produce or utilize rhizobactin 1021. When pPOC5 was introduced into S. meliloti 102F34, it did not confer the ability to utilize the siderophore. However, when pPOC4, carrying rhtX, was introduced into S. meliloti 102F34 along with pPOC5, the strain was able to utilize rhizobactin 1021 in the plate bioassay (Table 2; Fig. 3). The presence of both plasmids also conferred the ability to utilize shizokinen but not aerobactin. The introduction of pPOC4 alone did not confer the ability to utilize rhizobactin 1021 on S. meliloti 102F34.
TABLE 2.
Siderophore utilization by S. meliloti
| Strain | Relevant genotype | Utilization of:
|
|||
|---|---|---|---|---|---|
| Rhizobactin 1021 | Schizokinen | Aerobactin | Ferrichrome | ||
| 2011 | rhrA+rhtA+rhtX+ | + | + | − | + |
| 2011rhtX43 | rhrA+rhtA+rhtX43 | − | − | − | + |
| 2011rhtA45 | rhrA+rhtA45 rhtX+ | − | − | − | + |
| 102F34 | Lacks rhrA, rhtA, and rhtX | − | − | − | + |
| 102F34(pPOC4)a | rhtX+ | − | − | − | + |
| 102F34(pPOC5)b | rhrA+rhtA+ | − | − | − | + |
| 102F34(pPOC4, pPOC5) | rhrA+rhtA+rhtX+ | + | + | − | + |
rhtX is expressed constitutively.
rhtA expression is activated by RhrA.
FIG. 3.
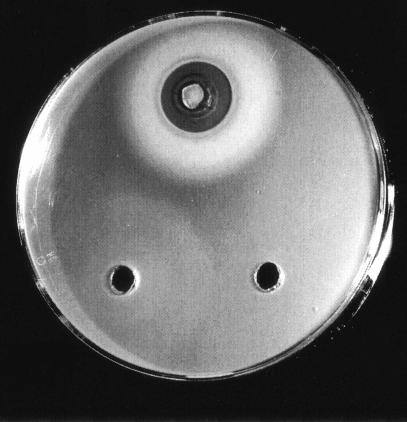
Iron nutrition bioassay. The medium was seeded with S. meliloti 102F34, carrying plasmids pPOC4 and pPOC5. Ferric chloride (100 mM) was added to the top well, a test solution containing rhizobactin 1021 was added to the well on the left, and a test solution lacking rhizobactin 1021 was added to the well on the right.
E. coli can utilize rhizobactin 1021 via either the Fhu transport system or RhtX.
We investigated the utilization of rhizobactin 1021 and the structurally related siderophores schizokinen and aerobactin in E. coli. The outer membrane receptor IutA and the FhuCDB transport system are required for utilization of aerobactin by E. coli (23). We found that rhizobactin 1021 was utilized in the presence of iutA and the fhu operon (Table 3). The strain also utilized schizokinen, a hydroxamate siderophore produced by B. megaterium.
TABLE 3.
Siderophore utilization by E. coli
| Strain | Relevant genotype | Utilization of:
|
|||
|---|---|---|---|---|---|
| Rhizobactin 1021 | Schizokinen | Aerobactin | Ferrichrome | ||
| H1443 | fhuC+fhuD+fhuB+ | − | − | − | + |
| H1443(pEN7) | fhuC+fhuD+fhuB+iutA+ | + | + | + | + |
| RK4375a | fhuC::Tn10 | − | − | − | − |
| RK4375(pEN7) | fhuC::Tn10 iutA+ | − | − | − | − |
| RK4375(pPOC4) | fhuC::Tn10 rhtX+ | − | − | − | − |
| RK4375(pPOC4, pEN7) | fhuC::Tn10 iutA+rhtX+ | + | + | − | − |
RK4375 carries a Tn10 insertion in fhuC that is polar on fhuD and fhuB (see text).
To determine whether the utilization of rhizobactin 1021 in E. coli was dependent on the Fhu system, we sought mutants with mutations in the fhuCDB genes. E. coli RK4375 carries the mutation described as fhuB478::Tn10 by Kadner et al. (22). This mutation was constructed prior to the discovery of the fhuC and fhuD genes within the same operon, and therefore it was necessary to determine the precise gene within the fhu operon into which Tn10 had inserted. Plasmids pPC35, pPC29, and pPC20 were constructed to express the fhuCDB, fhuCD, and fhuDB genes, respectively, and complementation of the fhuB478::Tn10 mutation was undertaken with each of the plasmids. Plasmid pPC35 complemented the mutation, resulting in restoration of the ability to transport ferrichrome as determined by bioassay. Neither pPC29 nor pPC20 restored the phenotype. On this basis it was concluded that the Tn10 insertion in E. coli RK4375 is in the fhuC gene, from which it exerts a polar effect on the downstream fhuDB genes.
Plasmid pEN7 was introduced into E. coli RK4375 to express iutA, and the strain was tested for the ability to utilize rhizobactin 1021, schizokinen, aerobactin, and also ferrichrome (the FhuA receptor is intact in this strain). The negative result that was observed (Table 3) indicated that the Fhu system is necessary for the transport of both rhizobactin 1021 and schizokinen in E. coli. As expected, aerobactin utilization and ferrichrome utilization were also abolished by the mutation in fhuC. However, when pPOC4, expressing rhtX, was introduced into this mutant, in the presence of pEN7, it restored the ability to utilize rhizobactin 1021 and schizokinen, although aerobactin and ferrichrome were still not utilized (Table 3). The results indicate that RhtX can act as an alternative to the inner membrane FhuCDB functions to facilitate the utilization of rhizobactin 1021 and schizokinen in E. coli.
A homologue of RhtX functions in the utilization of pyochelin by P. aeruginosa.
Homologues of RhtX identified by a BLAST search included PA4218, the product of a gene located close to the regulon for pyochelin production in the genome of P. aeruginosa. A mutation in the PA4218 gene was constructed by inserting a kanamycin resistance cassette in the genomic copy of the gene in P. aeruginosa PA4218-km1. This mutant is a derivative of P. aeruginosa CDC5, a pyochelin-producing strain that does not produce pyoverdine. The mutant was tested for pyochelin utilization in the plate bioassay and was found to have lost the ability to utilize the siderophore. The elimination of pyochelin utilization was similar to that observed for the fptA mutant P. aeruginosa DH143. We propose that the gene annotated as PA4218 in the P. aeruginosa genome be named fptX (for “ferripyochelin transport”) in recognition of its role in pyochelin utilization and the sequence homology to RhtX that the encoded protein displays.
DISCUSSION
The transport of siderophores across the periplasm and cytoplasmic membrane of gram-negative bacteria has been found to be facilitated by periplasmic binding protein-dependent transport (PBT) systems of the ABC superfamily. Here we report that rhizobactin 1021 can be utilized by E. coli expressing the aerobactin outer membrane receptor IutA and a functional Fhu PBT system. The recognition of rhizobactin 1021 by IutA is not surprising given the structural similarity between aerobactin and rhizobactin 1021 and the high identity (40%) and similarity (61%) between IutA and RhtA, the receptor for rhizobactin 1021 in S. meliloti. It is also not surprising that utilization is dependent on the Fhu system, as it is known that a number of citrate hydroxamate siderophores such as aerobactin are transported by the Fhu PBT system in E. coli. Many gram-negative bacteria possess transport systems that show homology to the Fhu system of E. coli. Among the rhizobia, R. leguminosarum biovar viciae has been shown to have a Fhu system that is required for the uptake of the trihydroxamate siderophore vicibactin (46). However, analysis of the sequenced genome of S. meliloti reveals no gene products with significant homology to the components of the Fhu transport system, apart from two homologues of FhuA. No homologue of FhuD has been identified, while any homology to FhuB and FhuC is of negligible significance (our unpublished observations). The absence of recognizable Fhu genes in S. meliloti raises a question regarding the transport system that facilitates the utilization of rhizobactin 1021 and ferrichrome. The identification of rhtX in the region encoding rhizobactin 1021 biosynthesis and the observation that mutants with mutations in this gene are defective in utilization of the siderophore suggested that RhtX may be a novel permease for rhizobactin 1021. This was supported by the observation that the introduction of rhtX and rhtA into S. meliloti 102F34, a strain that does not utilize rhizobactin 1021, was sufficient to confer the ability to utilize the siderophore. Further evidence for the role of RhtX as a transporter is provided by the restoration of rhizobactin 1021 utilization in an E. coli fhuC polar mutant by the introduction of rhtX, in the presence of the outer membrane receptor IutA. This result supports a role for RhtX as a transporter of either intact ferric rhizobactin 1021 or a derivative of the siderophore. RhtX does not function in the transport of ferrichrome in S. meliloti (Table 2) or E. coli (Table 3), and it is not clear how ferrichrome is transported in S. meliloti, given the absence of a recognizable FhuBCD transport system. The absence of FhuBCD in S. meliloti and the inability of RhtX to facilitate ferrichrome transport imply that another novel transport system for a hydroxamate siderophore must exist in the organism.
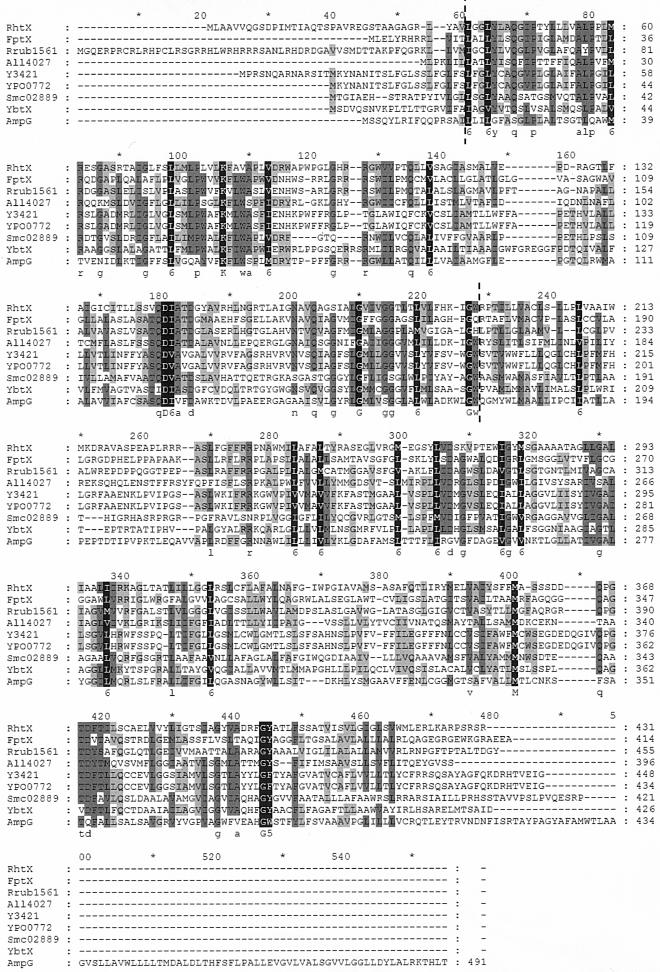
The Tmpred program predicts that RhtX has 12 transmembrane domains. Comparison of the protein sequence by BLASTP (1) analysis identified homology to a number of proteins, two of which have been investigated in detail, namely, AmpG from E. coli and YbtX from Y. pestis (which is 99% similar to Irp8 from Yersinia enterocolitica). AmpG is a permease for muropeptides that functions in cell wall recycling and generates signal molecules for the induction of β-lactamase (10, 21, 28). While the similarity to AmpG was low over the entire length of RhtX, the 155 amino acids extending from position 38 to 193 in RhtX show 51% similarity to the aligned region of AmpG. The genes encoding YbtX and Irp8 are located proximal to the genes that encode biosynthesis and uptake of yersiniabactin in their respective hosts. They have 52% similarity in the region extending from position 38 to 193 in RhtX. Both ybtX and irp8 have been mutagenized in the analysis of yersiniabactin transport, and in each case it was found that the mutations did not affect the transport of yersiniabactin (5, 14). Yersiniabactin transport is mediated by proteins encoded by ybtP and ybtQ in Y. pestis and the corresponding genes irp6 and irp7 in Y. enterocolitica. Both sets of proteins show homology to ABC transporters with an export function. The ABC transporter genes are within the same operon as ybtX and irp8 in the respective species. It is possible that YbtX and Irp8 represent redundant or inefficient permeases that have been functionally replaced by more efficient ABC transporters for yersiniabactin transport. In S. meliloti, however, there is no obvious ABC transporter located proximal to the rhizobactin 1021 regulon. The effect of the mutations in rhtX on siderophore utilization, the homology of RhtX to AmpG, and the capacity of the gene to effect utilization of the siderophore in the nonutilizing strain of S. meliloti and in the E. coli fhuC mutant combine to suggest that it encodes a dedicated permease for rhizobactin 1021.
A number of the proteins showing highest homology to RhtX are encoded within clusters of genes that are predicted to be involved in iron transport. A homologue of interest in regard to S. meliloti is Smc02889. This gene is located beside a putative outer membrane receptor that shows homology to the yersiniabactin receptor and may confer on S. meliloti the ability to utilize yersiniabactin encountered in the soil environment. Furthermore, located 3′ to Smc02889 and transcribed divergently from it is an Ara-C-like regulator. A number of the homologues of RhtX are found in gene clusters that appear to be regulated by Ara-C-like regulators. Another homologue of particular interest is the product of the gene named PA4218 in the P. aeruginosa genome, which has been assigned the name fptX in this work. It is located adjacent to the region encoding biosynthesis and transport of pyochelin. The bioassay result that we observed for P. aeruginosa PA4218-km1, carrying a mutation in the fptX gene, indicates that the gene functions in pyochelin utilization. Microarray analysis of P. aeruginosa has shown that fptX (PA4218) is upregulated under low-iron growth conditions (32).
There is no confirmed cytoplasmic membrane transport system encoded within the pyochelin region. While the amino acid sequences of proteins encoded by pchH and pchI show similarity to those of ABC transporter proteins (including YbtP and YbtQ of Y. pestis and Irp6 and Irp7 of Y. enterocolitica), no function could be assigned to these proteins in either export or utilization of pyochelin (42). The situation with regard to pyochelin, whereby PchI and PchH, the predicted transporters, have no apparent role in utilization while FptX has a function, is in contrast to that observed for yersiniabactin, where the predicted transporters YbtPQ in Y. pestis (Irp6 and Irp7 in Y. enterocolitica) function in yersiniabactin utilization but YbtX/Irp8 appear to be redundant or of minor importance.
The discovery that rhtX is the first gene in an operon that extends through rhbABCDEF implies that it is under the control of RhrA and iron, both of which were previously demonstrated to regulate this operon at the transcriptional level (30). This led us to examine the sequence between the operon and the upstream open reading frame Sma2335, which is transcribed in the opposite direction. The putative RhrA binding site GTTCGC(N)15GTTCGC that is observed exactly matches a sequence found upstream of rhtA (Fig. 2). RhrA has also been shown to regulate rhtA (30). It is likely that the regulation of iron response is also effected in this region. An iron response regulator of multiple operons, RirA, has been identified in R. leguminosarum (48), and it would be of interest to examine the role of the rirA homologue that exists in S. meliloti in regard to iron regulation of the rhizobactin 1021 regulon. Interestingly, further upstream of the region shown in Fig. 2 and before the start codon of Sma2335 there are 20 CT dinucleotide repeats. Phase variation is known to be mediated by nucleotide repeats that may be lost or gained by strand slippage and repair, leading to frameshifting when the repeat is within an open reading frame or to an effect on RNA polymerase binding when the repeat occurs in a promoter. Variation in the number of repeats was actually confirmed in this case, as we sequenced this region independently and found 19 repeats, in contrast to the 20 repeats in the published genome sequence. It is not clear if the variation affects expression of the operon containing rhtX or expression of neighboring genes in the upstream region and what effect, if any, this may have.
Schizokinen from B. megaterium is structurally identical to rhizobactin 1021 without the lipid moiety and shows a utilization pattern similar to that of rhizobactin 1021 (Fig. 1; Tables 2 and 3). On that basis we can conclude that the unusual lipid of rhizobactin 1021 is not required for its utilization via RhtX. In contrast, aerobactin, which has minor structural differences in comparison to the other two siderphores, is not utilized by strains relying on RhtX to facilitate utilization, even in E. coli, where the outer membrane receptor IutA is present.
It is striking that neither RhtX nor FptX, nor any of the other homologues in what appears to be a novel family of permeases, has associated proteins that function in energy coupling or as chaperones of the siderophores across the periplasm. FptX is encoded beside a gene of unknown function (PA4219) with which it would appear to be translationally coupled. However, PA4219 does not have any obvious ATPase or periplasmic binding protein motifs. There is no homologue of PA4219 associated with RhtX or encoded within the S. meliloti genome.
Alignment of the sequences of RhtX homologues revealed that homology is greatest in the N-terminal regions of the proteins (Fig. 4), and there is strong conservation of a motif of four amino acids, QD(V/I)A (at position 180 in the sequence as annotated in Fig. 4), which is predicted to be located in a cytoplasmic loop in RhtX. In the absence of an energizing protein, it is interesting to speculate that this represents a conserved domain for such an interaction. Members of the RhtX family of proteins may function by transporting unaltered siderophores, or they may chemically modify the siderophores while releasing iron. They may function in recycling the siderophores. Also, it may be significant that members of the RhtX family of homologues are found, in most cases, located in clusters of genes regulated by Ara-C-like transcriptional activators. The mechanism by which this family of proteins functions may provide signal molecules for their respective Ara-C-like activators of transcription.
FIG. 4.
Alignment of RhtX, the five proteins showing highest homology, and FptX. Similar residues are shaded. Deletions (dashes) are indicated. The accession numbers of the compared proteins are as follows: RhtX (AAK65915), FptX (NP_252908), Rrub1561 (ZP_00014546), All4027 (NP_488065), Y3421 (AAM86970), YPO0772 (CAC89621), Smc02889 (NP_384320), YbtX (CAC90731), AmpG (P36670).
Acknowledgments
This work was supported in part by the Enterprise Ireland Research Innovation Fund. P.O.C. was supported by an Orla Benson Research Award, and P.C. was supported by the NCSR.
We thank colleagues who supplied strains and Wolfgang Koester for helpful discussion.
REFERENCES
- 1.Altschul, S. F., T. I. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankenbauer, R. L., F. Hanna, and C. D. Cox. 1986. Mapping of mutations in Pseudomonas aeruginosa defective in pyoverdine production. J. Bacteriol. 167:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim, H., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brem, D., C. Pelludat, A. Rakin, C. A. Jacobi, and J. Heesemann. 2001. Functional analysis of yersiniabactin transport genes in Yersinia enterocolitica. Microbiology 147:1115-1127. [DOI] [PubMed] [Google Scholar]
- 6.Carter, R. A., P. S. Worsley, G. Sawers, G. L. Challis, M. J. Dilworth, K. C. Carson, J. A. Lawrence, M. Wexler, A. W. B. Johnston, and K. H. Yeoman. 2002. The vbs genes that direct synthesis of the siderophore vicibactin in Rhizobium leguminosarum: their expression in other genera requires ECF σ factor RpoI. Mol. Microbiol. 44:1153-1166. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., and T. Kuo. 1993. A simple and rapid method for preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosa, J. H., and C. T. Walsh. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 66:223-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz, H., D. Pfeifle, and B. Wiedemann. 1997. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enard, C., A. Diolez, and D. Expert. 1988. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J. Bacteriol. 170:2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expert, D., C. Enard, and C. Masclaux. 1996. The role of iron in plant host interactions. Trends Microbiol. 4:232-237. [DOI] [PubMed] [Google Scholar]
- 13.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 14.Fetherston, J. D., V. J. Bertolino, and R. D. Perry. 1999. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol. Microbiol. 32:289-299. [DOI] [PubMed] [Google Scholar]
- 15.Finan, T. M., B. Kunkel, G. F. De Vos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groeger, W., and W. Koester. 1998. Transmembrane topology of the two FhuB domains representing the hydrophobic components of bacterial ABC transporters involved in the uptake of siderophores, haem and vitamin B12. Microbiology 144:2759-2769. [DOI] [PubMed] [Google Scholar]
- 17.Gross, R., F. Engelbrecht, and V. Braun. 1984. Genetic and biochemical characterisation of the aerobactin synthesis operon on pColV. Mol. Gen. Genet. 196:74-80. [DOI] [PubMed] [Google Scholar]
- 18.Gross, R., F. Engelbrecht, and V. Braun. 1985. Identification of the genes and their polypeptide products responsible for aerobactin synthesis by pColV plasmids. Mol. Gen. Genet. 201:204-212. [DOI] [PubMed] [Google Scholar]
- 19.Heinrichs, D. E., and K. Poole. 1996. PchR, a regulator of ferric pyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J. Bacteriol. 178:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, C., L. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effector for β-lactamase induction. EMBO J. 13:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadner, R. J., K. Heller, J. W. Coulton, and V. Braun. 1980. Genetic control of hydroxamate mediated iron uptake in Escherichia coli. J. Bacteriol. 143:256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koester, W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152:291-301. [DOI] [PubMed] [Google Scholar]
- 24.Koester, W., and V. Braun. 1990. Iron(III) hydroxamate transport into Escherichia coli: substrate binding to the periplasmic FhuD protein. J. Biol. Chem. 265:21407-21410. [PubMed] [Google Scholar]
- 25.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 26.Leong, S., and J. B. Neilands. 1981. Relationship of siderophore-mediated iron assimilation to virulence in crown gall disease. J. Bacteriol. 147:482-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letain, T. E., and K. Postle. 1997. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol. Microbiol. 24:271-283. [DOI] [PubMed] [Google Scholar]
- 28.Lindquist, S., K. Weston-Hafer, H. Schmidt, C. Pul, G. Korfmann, J. Erikson, C. Sanders, H. H. Martin, and S. Normark. 1993. AmpG, a signal transducer in chromosomal β-lactamase induction. Mol. Microbiol. 9:703-715. [DOI] [PubMed] [Google Scholar]
- 29.Little, P. F. R. 1987. Choice and use of cosmid vectors, p. 33-34. In D. M. Glover (ed.), DNA cloning, vol. III. IRL Press, Washington, D.C. [Google Scholar]
- 30.Lynch, D., J. O'Brien, T. Welch, P. Clarke, P. Ó Cuív, J. H. Crosa, and M. O'Connell. 2001. Genetic organisation of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 183:2576-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 33.O'Connell, M., M. F. Hynes, and A. Puehler. 1987. Incompatibility between a Rhizobium Sym plasmid and a Ri plasmid of Agrobacterium. Plasmid 18:156-163. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa, J., H. L. Brierley, and S. R. Long. 1991. Analysis of Rhizobium meliloti nodulation mutant WL131: novel insertion sequence ISRm3 in nodG and altered nodH protein product. J. Bacteriol. 173:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persmark, M., P. Pittman, J. S. Buyer, B. Schwyn, P. R. Gill, and J. B. Neilands. 1993. Isolation and structure of rhizobactin 1021, a siderophore from the alfalfa symbiont Rhizobium meliloti 1021. J. Am. Chem. Soc. 115:3950-3956. [Google Scholar]
- 36.Platero, R. A., M. Jaureguy, F. J. Battistoni, and E. R. Fabiano. 2003. Mutations in sitB and sitD genes affect manganese-growth requirements in Sinorhizobium meliloti. FEMS Microbiol. Lett. 218:65-70. [DOI] [PubMed] [Google Scholar]
- 37.Poole, K., and G. A. McKay. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:661-686. [DOI] [PubMed] [Google Scholar]
- 38.Priefer, U. B., R. Simon, and A. Puehler. 1985. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J. Bacteriol. 163:324-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 40.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 41.Reigh, G., and M. O'Connell. 1993. Siderophore-mediated iron transport correlates with the presence of specific iron-regulated proteins in the outer membrane of Rhizobium meliloti. J. Bacteriol. 175:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimmann, C., H. M. Patel, L. Serino, M. Barone, C. T. Walsh, and D. Haas. 2001. Essential PchG-dependent reduction in pyochelin biosynthesis of Pseudomonas aeruginosa. J. Bacteriol. 183:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 45.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilisation of replicons, generation of operon fusions and induction of genes in Gram negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 46.Stevens, J. B., R. A. Carter, H. Hussain, K. C. Carson, M. J. Dilworth, and A. W. B. Johnston. 1999. The fhu genes of Rhizobium leguminosarum, specifying siderophore uptake proteins: fhuDCB are adjacent to a pseudogene version of fhuA. Microbiology 145:593-601. [DOI] [PubMed] [Google Scholar]
- 47.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todd, J. D., M. Wexler, G. Sawers, K. H. Yeoman, P. S. Poole, and A. W. B. Johnston. 2002. RirA, an iron responsive regulator in the symbiotic bacterium Rhizobium leguminosarum. Microbiology 148:4059-4071. [DOI] [PubMed] [Google Scholar]
- 49.Wacek, T., and W. J. Brill. 1976. Simple, rapid assay for screening nitrogen fixing ability in soybean. Crop. Sci. 16:519-522. [Google Scholar]