In contrast to adult patients with acquired heart disease, abnormalities of the right ventricle (RV) are ubiquitous in children and adults with congenital heart disease (CHD). The RV is exposed to volume overload in shunt lesions (e.g., atrial septal defect, anomalous pulmonary venous connections) as well as congenital or acquired tricuspid and/or pulmonary valve regurgitation. RV pressure overload characterizes numerous congenital anomalies, including pulmonary valve stenosis or atresia, large ventricular septal defect, single ventricle, tetralogy of Fallot (TOF), truncus arteriosus, and transposition of the great arteries (TGA), to name a few. Importantly, many surgical and transcatheter treatments of CHD result in persistent or acquired volume and/or pressure overload of the RV. In some CHD patients, the RV functions as the systemic ventricle (e.g., palliated hypoplastic left heart syndrome, physiologically corrected TGA, and D-loop TGA following atrial switch procedure). Furthermore, exposure to cyanosis and to surgical procedures in the RV often lead to myocardial abnormalities, including scar tissue and diffuse fibrosis.
Given the frequent involvement of the RV in CHD, it is not surprising that assessment of RV size and function is key for guiding clinical decisions in these patients.1 Among the diagnostic imaging tools available to clinicians for RV imaging, cardiac magnetic resonance (CMR) has emerged as the reference standard. In the following sections I will review the evidence supporting this contention, highlight how CMR data is used to guide clinical decisions, and discuss the strengths and weaknesses of CMR in comparison with other modalities, including echocardiography, computed tomography (CT), conventional x-ray angiography, and nuclear scintigraphy.
Versatility of CMR
CMR is ideally suited for assessment of the RV because it allows comprehensive assessment of cardiovascular morphology and physiology without most of the limitations that hinder alternative imaging modalities. Specifically, without restrictions related to acoustic windows, body size, scar tissue and other postoperative changes, exposure to harmful ionizing radiation, or the morbidity associated with invasive diagnostic catheterization, CMR provides high-resolution time-resolved 3-dimensional (3D) visualization of the right heart (Fig. 1). It allows depiction and quantification of blood flow, measurements of valve regurgitation (Figs. 2 and 3), and assessment of tissue characteristics (e.g., scar tissue) (Fig. 4).2 No other imaging modality currently provides such comprehensive information in the clinical arena. The limitations of CMR — higher cost in comparison with echocardiography (but not in comparison with other modalities),3 lack of portability, limited availability, artifacts from implants containing stainless steel (though no longer used in most modern implants),4 and relative contraindication in patients with pacemaker or defibrillator5 — are well documented. It should be noted that the risk of nephrogenic systemic fibrosis that has been linked to gadolinium-based contrast has largely been eliminated or greatly reduced by avoiding its use in patients with reduced glomerular filtration rate.6 Importantly, when it comes to evaluation of the RV by CMR, use of a contrast agent is not required. Hence, on balance, the clinical benefits of the data obtained by CMR greatly outweigh it limitations as detailed in the following sections.
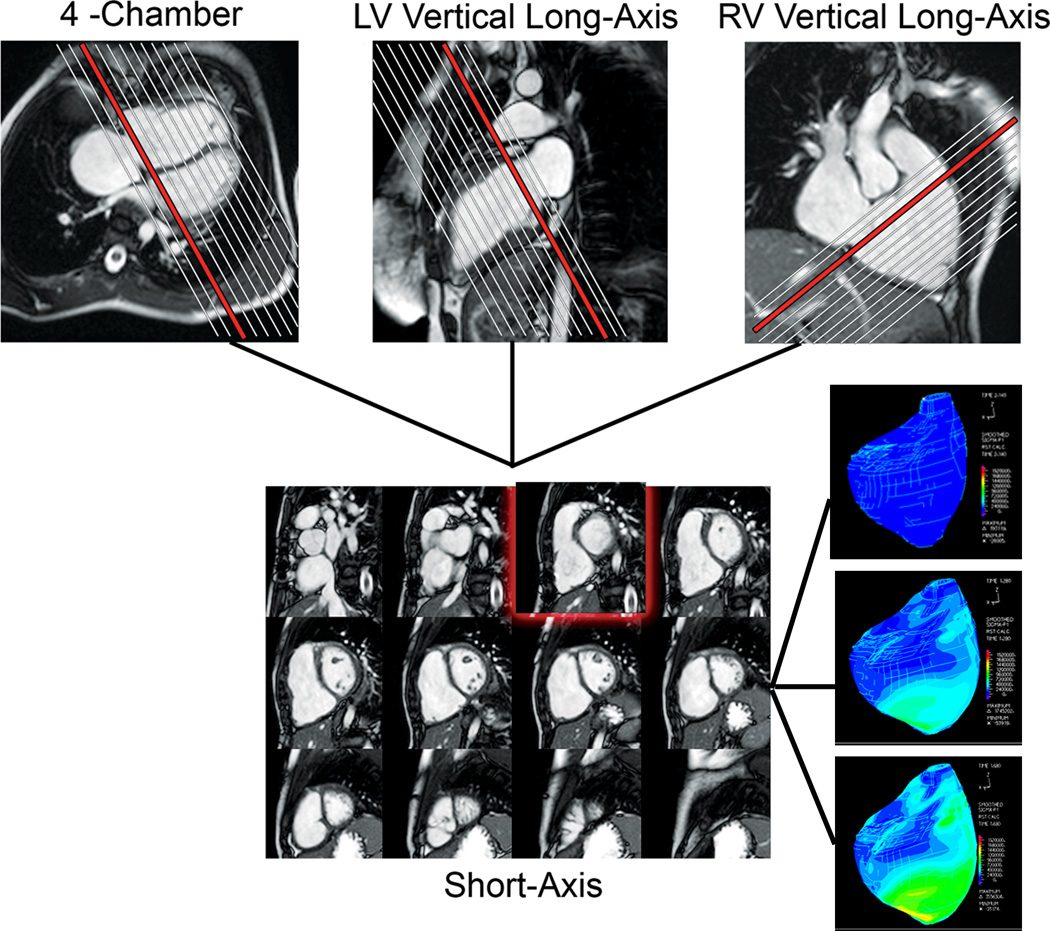
Figure 1.
CMR assessment of biventricular volumes and mass in a patient with repaired TOF. Cross-referencing between ventricular long- and short-axis imaging planes aids determining inclusion of basal slices in the ventricular volume analysis. Right lower panel: 3D strain maps of the RV at end-diastole (top), mid-systole (middle), and late systole (bottom).
Figure 2.
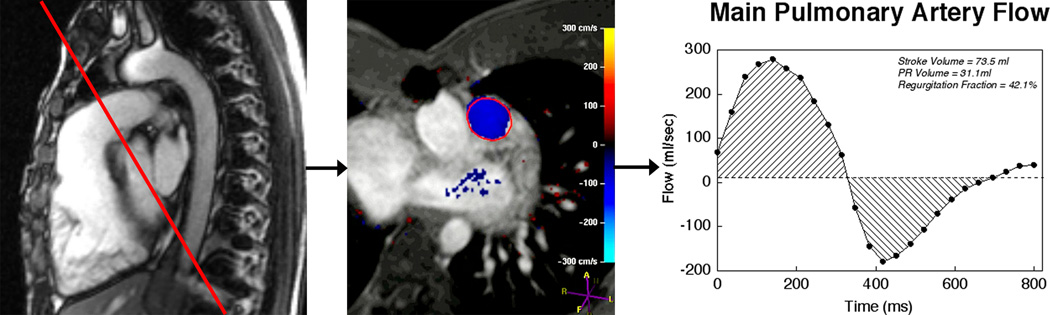
Evaluation of pulmonary regurgitation by ECG-gated cine phase contrast MR. Left panel: The imaging plane is placed perpendicular to the long-axis of the main pulmonary artery (MPA); Middle panel: Color-coded flow map with the region of interest contour shown at peak systole; Right panel: MPA flow rate versus time. Flow above the baseline represents antegrade flow and flow below the baseline represents retrograde (regurgitation) flow.
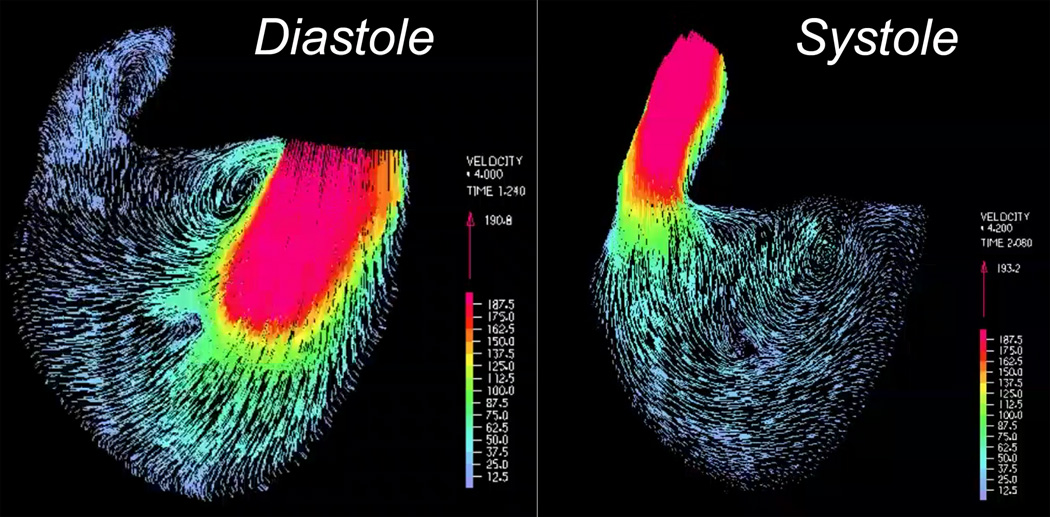
Figure 3.
Four-dimensional depiction of right ventricular blood flow based on cine phase contrast MR. Left panel: Early-diastolic frame showing blood flow through the tricuspid valve; Right panel: Mid-systolic frame showing blood flow through the right ventricular outflow tract.
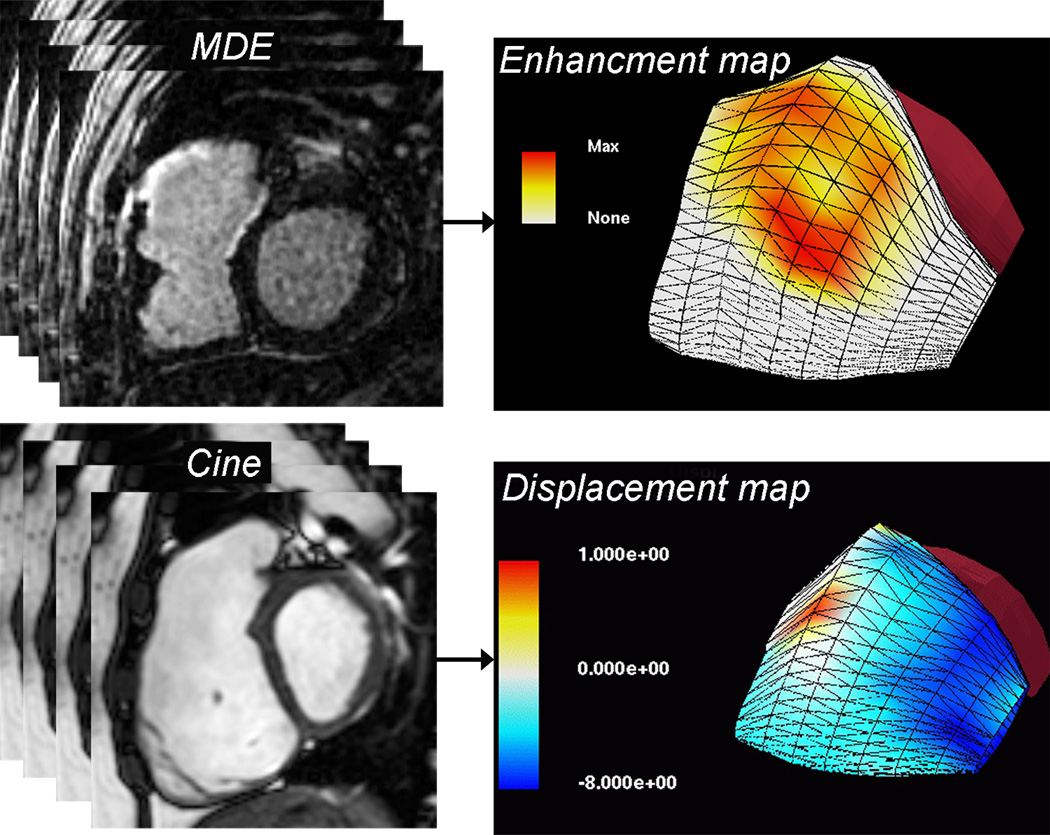
Figure 4.
Three-dimensional surface maps of RV scar tissue and motion.2 The models were reconstructed from multi-slice 2-dimensional short- and long-axis images. Top panel: Scar tissue map based on late gadolinium enhancement (LGE) imaging showing extensive late hyperenhancement of the RVOT (yellow and orange). Bottom panel: Displacement map based on multi-slice cine SSFP showing dyskinesis of the RVOT (red).
CMR is the Gold Standard for Noninvasive Measurements of RV Size and Function
Accuracy and Reproducibility
For any diagnostic test to be clinically useful, it must be accurate and reproducible. Accuracy can be determined by comparing measurements obtained by the technique or modality in question with those obtained by a reference standard. Accuracy determines how close to the “truth” a measurement is. Reproducibility addresses measurement variability, which can relate to the individual(s) performing the measurement (intra- and interobserver variability) as well variability related to repeated measurements (test-retest or interstudy variability). Reproducibility is especially crucial for tests that are being used for clinical surveillance over time, as is the case in serial follow-up of the RV in patients with CHD.
CMR has been shown to be both accurate and reproducible with regard to quantitative RV assessment. The combination of a time-resolved 3D dataset, clear distinction between the blood pool and the myocardium, and high spatial and temporal resolutions allow for accurate measurements of the RV regardless of its morphology or orientation within the thorax, and without geometrical assumptions. The accuracy of ventricular volume measurements by CMR was determined in the late 1980s and early 1990s using in-vitro phantoms, animal models, and in human subjects.7–9 Experiments aimed specifically at the RV showed similarly excellent results.10 For example, Koch et al. compared the accuracy of in-vivo RV volume assessment by CMR with ex-vivo measurements in 8 pig hearts.10 Compared with volume measurements in the explanted hearts, observers 1 and 2 underestimated RV volume by a mean of 0.70 mL and 0.2 mL (1.6% and 0.45%), respectively. In another study, Beygui et al. compared the accuracy CMR measurements of RV mass with ex-vivo measurements in minipigs.11 The correlation coefficient between in-vivo and ex-vivo measurements was 0.98 and the mean bias was 2.5 g.
The reproducibility of RV measurements is a notable strength of CMR over other modalities. Over the last decade, several groups have reported on inter- and intraobserver as well as interstudy reproducibility of CMR measurements of RV volumes, ejection fraction (EF), and mass (Table 1).12–15 Mooij et al. demonstrated low intra- and interobserver coefficients of variation in 60 children, most with abnormalities affecting the right heart.12 The interobserver coefficient of variation for RV volumes and mass ranged from 6.4% to 11.3%; for LV volumes and mass variations ranged from 3.6% to 10.5%. Studies by Hudsmith et al.16 and Grothues et al.15 reported similar interobserver coefficients of variations for RV measurements. Clarke et al.17 compared the observer variability of RV volume measurements between images obtained in the short-axis plane versus the axial plane in 50 patients with CHD. The intra- and interobserver reliability of RV end-diastolic volume, end-systolic volume, and stroke volume measurements was excellent for both contouring methods. In most measurements observer reliability was not influenced by the imaging plane except for RV end-systolic volume, which slightly favored the axial plane (p = 0.047). Blalock et al. demonstrated good interstudy reproducibility of RV measurements in 30 patients with repaired TOF, demonstrating the utility of CMR for serial evaluations of the RV in patients with CHD.18
Table 1.
| Mooij et al.12 | Grothues et al.15 |
Hudsmith et al.16 |
Karamitsos et al. (post-training)13 |
Karamitsos et al. (expert)13 |
Moon et al.9 | |
|---|---|---|---|---|---|---|
| No. of patients | 60 | 60 | 12 | 10 | 10 | 20 |
| Diagnosis | Normal/ASD/TOF | Normal/CHF/LVH | Normal | Normal | Normal | Normal/CHF |
| CMR technique | SSFP | FLASH | SSFP | SSFP | SSFP | SSFP |
| Right ventricle | ||||||
| EDV | 6.4% | 6.2% | 9.6% | |||
| ESV | 13.0% | 14.1% | ||||
| EF | 8.0% | 8.3% | 10.7% | |||
| Mass | 11.3% | 8.7% | ||||
| Left ventricle | ||||||
| EDV | 3.6% | 2.7% | 4.6% | 2.6% | 2.6% | |
| ESV | 10.5% | 7.4% | 6.9% | 10.5% | ||
| EF | 5.8% | 3.3% | 3.7% | 2.9% | 6% | |
| Mass | 5.3% | 5.2% | 6.7% | 5.8% | 6% |
Reproducibility in this table is expressed as coefficient of variability (expressed as percentage)
Abbreviations: ASD, atrial septal defect; CHF, congestive heart failure; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; FLASH, fast low-angle shot; LVH, left ventricular hypertrophy; SSFP, steady-state free precession; TOF, tetralogy of Fallot
Use of CMR as a Reference Standard for Other Modalities
CMR has been considered by many investigators as the gold standard for RV assessment since the late 1990s.19 Over the past 15 years numerous publications have documented the use of CMR as reference standard for comparison of echocardiographic (2D, 3D, tissue Doppler, strain),20–26 computed tomography,27, 28 and radionuclear scintigraphy29 measurements. In general, the level of agreement between echocardiographic variables and CMR depends on the subjects included (with influence from factors such as diagnosis or age) and the parameters evaluated. The overall picture that emerges from the literature highlights several consistent observations: 1) compared with CMR, the reliability of 2D echocardiographic measurements of RV size and function is modest with large limits of agreement; 2) RV volumes by 3D echocardiography correlate better with CMR measurements than 2D measurements, though systematic underestimation is common;30, 31 3) unlike promising results in adult patients with acquired cardiopulmonary diseases,32 echocardiographic indices of longitudinal shortening (e.g., tricuspid annular plane excursion, TAPSE) in CHD are not as robust;33, 34 and 4) RV myocardial velocities (by tissue Doppler) and deformation (by speckle tracking) are topics of intense interest but results are too preliminary to draw firm conclusions.35 These and numerous other reports confirm that CMR is the reference standard for noninvasive assessment of the RV in patients with CHD.
Role of CMR in Guiding Clinical Decisions
The ultimate goal of any diagnostic test is to guide clinical management. In the context of managing patients with CHD that involves the RV, assessment of chamber size, global and regional function, pressure, scar tissue, thrombus formation, AV valve and semilunar valve regurgitation, and shunt quantification are all essential pieces of the diagnostic puzzle used to inform clinical decisions. Although some of these data can be determined by different diagnostic modalities, CMR has an advantage because it is capable of accurately and reproducibly providing most diagnostic information noninvasively and without exposure to harmful ionizing radiation.36
CMR has been shown to be useful in informing clinical decisions in several types of CHD that affect the RV. Repaired TOF is a good example in which CMR data is paramount to clinical management, as stated in the ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease: “MRI is now seen as the reference standard for assessment of RV volume and systolic function.”37 Indeed, RV size and function, pulmonary regurgitation fraction, tricuspid regurgitation, differential pulmonary artery blood flow and anatomy, right ventricular outflow tract aneurysm, and residual shunts and sites of obstruction impact management decisions.38–40 For example, criteria for pulmonary valve replacement rely on CMR-measured parameters such as RV volumes and ejection fraction (Table 2).2 Several investigators have proposed threshold criteria for CMR-measured RV end-diastolic volume index as an important criterion for pulmonary valve replacement. Others have emphasized the importance of RV end-systolic volume index as an important criterion because it integrates both RV size and function.41 Similarly, the importance of RV dysfunction measured by ejection fraction as a criterion for pulmonary valve replacement has been shown by several groups and accepted by the ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease.37 More recently, data from an international multicenter cohort of patients with repaired TOF showed that lower left and right ventricular ejection fractions and higher RV mass-to-volume ratio measured by CMR are strong independent predictors of major adverse clinical outcomes, namely death and sustained ventricular tachycardia.42 These observations highlight the utility of CMR in assessing prognosis and guiding clinical decisions in patients with repaired TOF, which comprises a substantial proportion of adolescents and adult patients with moderate or severe CHD.37, 38, 43
Table 2.
Role of CMR in informing the decision for pulmonary valve replacement in patients with repaired tetralogy of Fallot. Criteria based on CMR are marked with (*)
| Indications for pulmonary valve replacement in patients with repaired TOF or similar physiology with moderate or severe pulmonary regurgitation (regurgitation fraction ≥25%) |
|---|
|
Adapted from Geva T.2
CMR is also valuable in other CHD that affects the RV. Examples in patients with unrepaired CHD include superior and inferior sinus venosus defects,44 partially or totally anomalous pulmonary venous connection,45 atypical atrial communications such as coronary sinus defect, Ebstein anomaly and other forms of dysplastic tricuspid valve,46 anomalies of the RV myocardium such as arrhythmogenic RV cardiomyopathy,47 RV outflow tract obstruction in patients with poor echocardiographic windows, absent pulmonary valve syndrome, and pulmonary hypertension.48 In patients who underwent transcatheter and/or surgical management of lesions affecting the right heart, CMR is frequently being used to inform clinical management. Examples include assessment of pulmonary regurgitation and RV size and function following balloon dilation of pulmonary valve stenosis,49 tricuspid valvuloplasty, residual shunts after management of septal defects,36 and residual or recurrent RV outflow tract obstruction or pulmonary regurgitation.41
Role of Multimodality Imaging
Although CMR is the preferred modality for RV assessment, multiple diagnostic tools are used in clinical practice. The choice of which and when to obtain an echocardiogram, CT, nuclear scintigraphy, diagnostic catheterization, or a combination of these diagnostic procedures is dictated by the clinical question and by a host of patient-, modality-, provider-, and institution-related considerations.3 The patient’s clinical circumstance and the specific information sought constitute the first step in the decision-making process. Once those are determined, patient-, modality- provider-, and institution-related considerations are weighted. Examples of patient-related factors include age, body size, ability to cooperate with the test, and presence of implantable metallic devices or pacemaker/defibrillator. Examples of modality-related considerations include accuracy, reproducibility, patient acceptance, and procedural risk versus benefit. Examples of provider-related factors include level of comfort and trust with specific modalities and their interpretation. Examples of institution-related considerations include access to different modalities, quality of hardware and software, level of expertise, and charges.
In clinical pediatric/congenital practice echocardiography is the first line of investigation. With regard to RV assessment, echocardiography is capable of providing the necessary diagnostic information to inform clinical decisions in many scenarios. Examples include the presence or absence of RV volume overload in a young child with a secundum atrial septal defect, abnormalities of the tricuspid valve with mild regurgitation, pulmonary valve regurgitation in a patient followed after balloon dilation of pulmonary valve stenosis, and infants and young children after repair of tetralogy of Fallot with uncomplicated clinical course and reassuring echocardiographic findings. Common to these circumstances is that precise determination of RV size and function and flow measurements (e.g., pulmonary regurgitation, differential pulmonary artery flow) are not essential for clinical decision making. In contrast, when accurate assessment of the RV is essential for clinical management (e.g., adolescent or adult patient with repaired TOF), CMR is the best tool currently available in the clinical arena. Due the to increased risk of cancer associated with ionizing radiation exposure,50 CT, nuclear scintigraphy, and diagnostic catheterization are used for RV assessment in this patient population only when the diagnostic information cannot be obtained by echocardiography or CMR.
Summary
A large body of evidence published during the past 15 years clearly indicates that CMR is presently the best diagnostic modality for assessment of RV size and function in patients with CHD. Moreover, a growing literature informs clinicians on how to use CMR data to guide patient management. Echocardiography, which is more widely available, provides useful diagnostic information in many clinical circumstances that affect the right heart. However, when precise quantitative data is required to make important clinical decisions (e.g., when to recommend pulmonary valve replacement), CMR remains the diagnostic modality of choice. As new echocardiographic, CMR, and other imaging techniques continue to evolve, it would be interesting to revisit this controversy in the future.
Acknowledgments
Sources of Funding
Dr. Geva is supported in part by NIH/NHLBI 1 R01 HL089269-01A2 and by the Higgins Family Noninvasive Research Fund at Boston Children's Hospital.
Footnotes
Disclosures
Dr. Geva is a member of the screening committee of Medtronic’s Native Outflow Tract Transcatheter Pulmonary Valve Clinical Study.
References
- 1.Williams RG, Pearson GD, Barst RJ, Child JS, del Nido P, Gersony WM, Kuehl KS, Landzberg MJ, Myerson M, Neish SR, Sahn DJ, Verstappen A, Warnes CA, Webb CL. Report of the national heart, lung, and blood institute working group on research in adult congenital heart disease. J Am Coll Cardiol. 2006;47:701–707. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 2.Geva T. Repaired tetralogy of fallot: The roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prakash A, Powell AJ, Geva T. Multimodality noninvasive imaging for assessment of congenital heart disease. Circ Cardiovasc Imaging. 2010;3:112–125. doi: 10.1161/CIRCIMAGING.109.875021. [DOI] [PubMed] [Google Scholar]
- 4.Garg R, Powell AJ, Sena L, Marshall AC, Geva T. Effects of metallic implants on magnetic resonance imaging evaluation of fontan palliation. Am J Cardiol. 2005;95:688–691. doi: 10.1016/j.amjcard.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino RA. A novel mri-safe dual-chamber pacemaker system: Its time has come. Heart Rhythm. 2011;8:74–75. doi: 10.1016/j.hrthm.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Reiter T, Ritter O, Prince MR, Nordbeck P, Wanner C, Nagel E, Bauer WR. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:31. doi: 10.1186/1532-429X-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caputo GR, Tscholakoff D, Sechtem U, Higgins CB. Measurement of canine left ventricular mass by using mr imaging. AJR Am J Roentgenol. 1987;148:33–38. doi: 10.2214/ajr.148.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Koch JA, Poll LW, Godehardt E, Korbmacher B, Jung G, Modder U. In vitro determination of cardiac ventricular volumes using mri at 1.0 t in a porcine heart model. Int J Cardiovasc Imaging. 2001;17:237–242. doi: 10.1023/a:1010621126727. [DOI] [PubMed] [Google Scholar]
- 9.Moon JC, Lorenz CH, Francis JM, Smith GC, Pennell DJ. Breath-hold flash and fisp cardiovascular mr imaging: Left ventricular volume differences and reproducibility. Radiology. 2002;223:789–797. doi: 10.1148/radiol.2233011181. [DOI] [PubMed] [Google Scholar]
- 10.Koch JA, Poll LW, Godehardt E, Korbmacher B, Modder U. Right and left ventricular volume measurements in an animal heart model in vitro: First experiences with cardiac mri at 1.0 t. Eur Radiol. 2000;10:455–458. doi: 10.1007/s003300050075. [DOI] [PubMed] [Google Scholar]
- 11.Beygui F, Furber A, Delepine S, Helft G, Metzger JP, Geslin P, Le Jeune JJ. Routine breath-hold gradient echo mri-derived right ventricular mass, volumes and function: Accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–516. doi: 10.1007/s10554-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 12.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of mri measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28:67–73. doi: 10.1002/jmri.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karamitsos TD, Hudsmith LE, Selvanayagam JB, Neubauer S, Francis JM. Operator induced variability in left ventricular measurements with cardiovascular magnetic resonance is improved after training. J Cardiovasc Magn Reson. 2007;9:777–783. doi: 10.1080/10976640701545073. [DOI] [PubMed] [Google Scholar]
- 14.Hudsmith L, Petersen S, Francis J, Robson M, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 15.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 17.Clarke CJ, Gurka MJ, Norton PT, Kramer CM, Hoyer AW. Assessment of the accuracy and reproducibility of rv volume measurements by cmr in congenital heart disease. JACC Cardiovasc Imaging. 2012;5:28–37. doi: 10.1016/j.jcmg.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of fallot: A prospective observational study. J Magn Reson Imaging. 2013;38:829–835. doi: 10.1002/jmri.24050. [DOI] [PubMed] [Google Scholar]
- 19.Aebischer N, Meuli R, Jeanrenaud X, Koerfer J, Kappenberger L. An echocardiographic and magnetic resonance imaging comparative study of right ventricular volume determination. Int J Card Imaging. 1998;14:271–278. doi: 10.1023/a:1006055512362. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Prakasa K, Bomma C, Tandri H, Dalal D, James C, Tichnell C, Corretti M, Bluemke D, Calkins H, Abraham TP. Comparison of novel echocardiographic parameters of right ventricular function with ejection fraction by cardiac magnetic resonance. J Am Soc Echocardiogr. 2007;20:1058–1064. doi: 10.1016/j.echo.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Puchalski MD, Williams RV, Askovich B, Minich LL, Mart C, Tani LY. Assessment of right ventricular size and function: Echo versus magnetic resonance imaging. Congenit Heart Dis. 2007;2:27–31. doi: 10.1111/j.1747-0803.2007.00068.x. [DOI] [PubMed] [Google Scholar]
- 22.Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24:691–698. doi: 10.1007/s10554-008-9314-4. [DOI] [PubMed] [Google Scholar]
- 23.Niemann PS, Pinho L, Balbach T, Galuschky C, Blankenhagen M, Silberbach M, Broberg C, Jerosch-Herold M, Sahn DJ. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-tesla magnetic resonance imaging. J Am Coll Cardiol. 2007;50:1668–1676. doi: 10.1016/j.jacc.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Tsujino I, Ohira H, Oyama-Manabe N, Yamada A, Ito YM, Goto C, Watanabe T, Sakaue S, Nishimura M. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J Am Soc Echocardiogr. 2012;25:280–286. doi: 10.1016/j.echo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Alghamdi MH, Grosse-Wortmann L, Ahmad N, Mertens L, Friedberg MK. Can simple echocardiographic measures reduce the number of cardiac magnetic resonance imaging studies to diagnose right ventricular enlargement in congenital heart disease? J Am Soc Echocardiogr. 2012;25:518–523. doi: 10.1016/j.echo.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Lytrivi ID, Lai WW, Ko HH, Nielsen JC, Parness IA, Srivastava S. Color doppler tissue imaging for evaluation of right ventricular systolic function in patients with congenital heart disease. J Am Soc Echocardiogr. 2005;18:1099–1104. doi: 10.1016/j.echo.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Bartolles R, Baumann R, Schummers G, Lang RM, Nesser HJ. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–18. doi: 10.1016/j.jcmg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Raman SV, Cook SC, McCarthy B, Ferketich AK. Usefulness of multidetector row computed tomography to quantify right ventricular size and function in adults with either tetralogy of fallot or transposition of the great arteries. Am J Cardiol. 2005;95:683–686. doi: 10.1016/j.amjcard.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Kjaer A, Lebech AM, Hesse B, Petersen CL. Right-sided cardiac function in healthy volunteers measured by first-pass radionuclide ventriculography and gated blood-pool spect: Comparison with cine mri. Clinical physiology and functional imaging. 2005;25:344–349. doi: 10.1111/j.1475-097X.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Nadvoretskiy V, Bu L, Stolpen A, Ayres N, Pignatelli RH, Kovalchin JP, Grenier M, Klas B, Ge S. Accuracy and reproducibility of real-time three-dimensional echocardiography for assessment of right ventricular volumes and ejection fraction in children. J Am Soc Echocardiogr. 2008;21:84–89. doi: 10.1016/j.echo.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Soriano BD, Hoch M, Ithuralde A, Geva T, Powell AJ, Kussman BD, Graham DA, Tworetzky W, Marx GR. Matrix-array 3-dimensional echocardiographic assessment of volumes, mass, and ejection fraction in young pediatric patients with a functional single ventricle: A comparison study with cardiac magnetic resonance. Circulation. 2008;117:1842–1848. doi: 10.1161/CIRCULATIONAHA.107.715854. [DOI] [PubMed] [Google Scholar]
- 32.Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Rande JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (tapse) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Kowalik E, Kowalski M, Rozanski J, Kusmierczyk M, Hoffman P. The impact of pulmonary regurgitation on right ventricular regional myocardial function: An echocardiographic study in adults after total repair of tetralogy of fallot. J Am Soc Echocardiogr. 2011;24:1199–1204. doi: 10.1016/j.echo.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Koestenberger M, Nagel B, Ravekes W, Everett AD, Stueger HP, Heinzl B, Sorantin E, Cvirn G, Fritsch P, Gamillscheg A. Systolic right ventricular function in pediatric and adolescent patients with tetralogy of fallot: Echocardiography versus magnetic resonance imaging. J Am Soc Echocardiogr. 2011;24:45–52. doi: 10.1016/j.echo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 35.van der Hulst AE, Delgado V, Holman ER, Kroft LJ, de Roos A, Hazekamp MG, Blom NA, Bax JJ, Roest AA. Relation of left ventricular twist and global strain with right ventricular dysfunction in patients after operative "correction" of tetralogy of fallot. Am J Cardiol. 2010;106:723–729. doi: 10.1016/j.amjcard.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the european society of cardiology. Eur Heart J. 2010;31:794–805. doi: 10.1093/eurheartj/ehp586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr., Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW. Acc/aha 2008 guidelines for the management of adults with congenital heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the american society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2008;52:e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Warnes CA. Adult congenital heart disease importance of the right ventricle. J Am Coll Cardiol. 2009;54:1903–1910. doi: 10.1016/j.jacc.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 39.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 40.Egidy Assenza G, Cassater D, Landzberg M, Geva T, Schreier J, Graham D, Volpe M, Barker N, Economy K, Valente AM. The effects of pregnancy on right ventricular remodeling in women with repaired tetralogy of fallot. Int J Cardiol. 2013;168:1847–1852. doi: 10.1016/j.ijcard.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 41.Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, del Nido P. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. 2010;122:S201–S208. doi: 10.1161/CIRCULATIONAHA.110.951178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valente AM, Gauvreau K, Babu-Narayan SV, Assenza GE, Evans SP, Gatzoulis MA, Groenink M, Inuzuka R, Kilner P, Koyak Z, Landzberg MJ, Mulder BJ, Powell AJ, Wald R, Geva T. Ventricular size and function measured by cardiac mri improve prediction of major adverse clinical outcomes independent of prolonged qrs duration in patients with repaired tetralogy of fallot. Circulation. 2011;124:A11414. [Google Scholar]
- 43.Dolk H, Loane M, Garne E European Surveillance of Congenital Anomalies Working G. Congenital heart defects in europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–849. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 44.Valente AM, Sena L, Powell AJ, Del Nido PJ, Geva T. Cardiac magnetic resonance imaging evaluation of sinus venosus defects: Comparison to surgical findings. Pediatr Cardiol. 2007;28:51–56. doi: 10.1007/s00246-006-1477-y. [DOI] [PubMed] [Google Scholar]
- 45.Grosse-Wortmann L, Al-Otay A, Goo HW, Macgowan CK, Coles JG, Benson LN, Redington AN, Yoo SJ. Anatomical and functional evaluation of pulmonary veins in children by magnetic resonance imaging. J Am Coll Cardiol. 2007;49:993–1002. doi: 10.1016/j.jacc.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 46.Tobler D, Yalonetsky S, Crean AM, Granton JT, Burchill L, Silversides CK, Wald RM. Right heart characteristics and exercise parameters in adults with ebstein anomaly: New perspectives from cardiac magnetic resonance imaging studies. Int J Cardiol. 2013;165:146–150. doi: 10.1016/j.ijcard.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Murphy DT, Shine SC, Cradock A, Galvin JM, Keelan ET, Murray JG. Cardiac mri in arrhythmogenic right ventricular cardiomyopathy. AJR Am J Roentgenol. 2010;194:W299–W306. doi: 10.2214/AJR.09.3450. [DOI] [PubMed] [Google Scholar]
- 48.Hoeper MM, Tongers J, Leppert A, Baus S, Maier R, Lotz J. Evaluation of right ventricular performance with a right ventricular ejection fraction thermodilution catheter and mri in patients with pulmonary hypertension. Chest. 2001;120:502–507. doi: 10.1378/chest.120.2.502. [DOI] [PubMed] [Google Scholar]
- 49.Harrild DM, Powell AJ, Tran TX, Geva T, Lock JE, Rhodes J, McElhinney DB. Long-term pulmonary regurgitation following balloon valvuloplasty for pulmonary stenosis risk factors and relationship to exercise capacity and ventricular volume and function. J Am Coll Cardiol. 2010;55:1041–1047. doi: 10.1016/j.jacc.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of internal medicine. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]