Abstract
Increased levels of plasma troponins and natriuretic peptides are markers of cardiac dysfunction associated with increased risk of cardiovascular disease. Little information exists on cardiac dysfunction and occurrence of venous thromboembolism. In two prospective epidemiological cohorts, we tested the hypothesis that high-sensitivity troponin T (TnT) and N-terminal pro B-type natriuretic peptide (NT-proBNP) are associated positively with venous thromboembolism occurrence. The Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS) measured plasma TnT and NT-proBNP in 13,719 men or women with no history of venous thrombosis, coronary heart disease, or heart failure and followed them for approximately 10 years for venous thromboembolism (VTE) occurrence (n=348 VTEs). In both ARIC and CHS, TnT was associated positively with incidence of total VTE and provoked VTE, but not with unprovoked VTE: age, race, and sex-adjusted hazard ratios for total VTE in the pooled analysis were 1.00, 0.85, 1.36, 1.51, and 1.98 (p trend <0.0001) across five categories of TnT. In contrast, the association of NT-proBNP with VTE was positive in ARIC (hazard ratios approximately 2.5 fold for the highest versus lowest NT-proBNP quintiles), but non-existent in CHS.
Keywords: Venous thrombosis, pulmonary embolism, troponins, natriuretic peptides
Introduction
Much epidemiological information exists on the major risk factors for venous thromboembolism (VTE),1 providing important insights about the causes of VTE and some possibilities to prevent VTE. Yet, research on the relation of cardiac function with VTE incidence is limited.
Troponins and natriuretic peptides, such as N-terminal pro B-type natriuretic peptide (NT-proBNP), are useful plasma diagnostic tests for myocardial infarction and heart failure, respectively. Elevated troponin is considered a marker of cardiac myonecrosis, and elevated NT-proBNP is a marker of myocardial stretch and volume overload. In the general population, these cardiac diagnostic markers also are potent biomarkers for the subsequent development of coronary heart disease (CHD), heart failure, and stroke events as well as total and cardiovascular mortality.2–9
Acute hospitalized cardiac conditions are known to provoke VTE. However, studies have disagreed as to whether chronic CHD or heart failure increase risk of VTE.10,11 It therefore seems useful to determine whether elevations of specific biomarkers of chronic, but often subclinical, cardiac disease are associated with increased risk of VTE. We therefore tested the hypothesis that high-sensitivity troponin T (TnT) and NT-proBNP are associated positively with occurrence of VTE in the population-based Longitudinal Investigation of Thromboembolism Etiology (LITE).
Methods
Study population
The Longitudinal Investigation of Thromboembolism Etiology (LITE) study is a prospective study of VTE occurrence in 2 pooled, multi-center, longitudinal population-based cohort studies: the Atherosclerosis Risk in Communities (ARIC) Study12 and the Cardiovascular Health Study (CHS).13 The LITE study design, methods, and VTE incidence rates have been described in detail elsewhere.14,15 In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC study in 1987–1989, and had subsequent examinations in 1990–92, 1993–95, and 1996–98 (ARIC visit 4) and annual telephone contact thereafter. In CHS, 5,201 men and women aged ≥65 years enrolled in 1989–1990. An additional 687 African Americans were recruited to CHS using similar methods in 1992–1993. CHS contacted participants every six months for follow-up, alternating between a telephone interview and clinic visit for the first 10 years and by telephone interview only after that. The institutional review committees at each study center approved the methods and staff obtained informed participant consent.
Troponin T and NT-proBNP measurements
For both ARIC and CHS, TnT and NT-proBNP were measured in stored plasma samples before the onset of VTE, and the cohorts were followed longitudinally for VTE occurrence. The biological and analytic variability of these biomarkers and stability during frozen storage have been published.16–18
In ARIC, TnT and NT-proBNP were measured at visit 4, because samples from previous visits were depleted. Participants were asked to fast for 12 hours before the visit, and plasma samples were stored at −70°C until assayed several years later. TnT levels were measured at the ARIC laboratory (Baylor College of Medicine) on a Cobas e411 analyzer using the Elecys Troponin T, a high sensitivity assay (Roche Diagnostics, Indianapolis, IN).7 The lower limit was 3 ng/L. The reliability coefficient for blinded quality control replicate measurements (n=418 pairs) of troponin T from single blood draws was 0.98. Plasma NT-proBNP was measured on a Cobas e411 analyzer using the Elecys proBNP II immunoassay (Roche Diagnostics, Indianapolis, IN).19 The reliability coefficient for blind replicate measurements (n=418 pairs) was 0.99.
In CHS, fasting serum samples were stored at −70°C at baseline (1989 to 1990 for the main cohort and 1992 to 1993 for the supplemental African American cohort) and subsequent visits, as described previously.5,20 Frozen samples were pulled for analysis from the earliest sample still available for each participant: approximately 73% of the samples for these biomarkers came from original cohort baseline, 25% from 3 years, and 2% from 5 years after original cohort baseline. TnT and NT-proBNP were measured at the University of Maryland with the NT-proBNP and highly sensitive TnT reagents on an Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, Indiana). The coefficient of variation for TnT was 10% and for NT-proBNP was <5%. According to the vendor, the ARIC and CHS assays were comparable.
Measurement of risk factors and cardiovascular diseases
Risk factors were measured at the ARIC or CHS visits in which TnT and NT-proBNP were measured. Body mass index was calculated as weight (kg)/height (m)2. Diabetes was defined as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, a physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks. Glomerular filtration rate (eGFR) was estimated from cystatin C (ARIC) or creatinine (CHS). C-reactive protein (CRP) was measured as previously described.21,22
ARIC defined prevalent CHD at visit 4 as (1) a history at ARIC visit 1 of myocardial infarction or coronary revascularization (self-reported) or a previous myocardial infarction diagnosed by ECG or (2) an incident (validated) definite or probable myocardial infarction or a coronary revascularization between ARIC visit 1 and visit 4. Incident CHD after visit 4 was similarly defined. CHS defined prevalent CHD based on participant self-report and physician review of prior medical records at baseline. Incident CHD included validated definite or probable myocardial infarction, angina, or coronary revascularization.23
ARIC defined prevalent heart failure as (1) visit 1 signs and symptoms of heart failure by the Gothenberg questionnaire or (2) an incident heart failure hospitalization (ICD9 428) between ARIC visit 1 and visit 4. Incident heart failure after visit 4 was defined only by hospital ICD code 428. CHS defined prevalent heart failure based on participant self-report and review of medical records at baseline to verify diagnosis and treatment for heart failure. Incident heart failure was similarly defined, using physician review of medical records.23
VTE occurrence
ARIC and CHS participants were contacted annually or semi-annually by phone and asked about all hospitalizations in the previous year. Hospital records with possible VTE events among the discharge diagnoses were obtained through 2005 in ARIC and through 2001 in CHS. Two physicians validated possible VTE events using standardized criteria.14 Diagnosis of deep venous thrombosis (DVT) or pulmonary embolism (PE) required positive imaging tests. Cases were classified during review as unprovoked (no obvious cause) or provoked (associated with cancer, major trauma, surgery, or marked immobility).14
Statistical analysis
Of the 16,708 LITE participants with TnT or NT-proBNP measurements, we excluded those with a VTE prior to biomarker assessment (n=656), those with CHD or heart failure at or prior to biomarker assessment (n=2,211), and those taking anticoagulants (n=122). This left a maximum of 13,719 participants for the present analyses of new VTE. In ARIC, 9551 had TnT and 9543 had NT-proBNP; in CHS, 4122 had TnT and 4168 had NT-proBNP. Time at risk was computed from the date of biomarker measurement to the earliest of the following: date of hospital discharge with incident VTE, date of death, date of last follow-up contact, or end of follow-up.
Our a priori hypothesis was that the plasma biomarkers, TnT and NT-proBNP, would be associated positively with VTE incidence. We also hypothesized a priori that the association would be stronger for provoked VTE than for unprovoked VTE, because elevated cardiac biomarkers might indicate individuals likely to develop medical conditions, hospitalization, and provoked VTE. For most analyses, we categorized NT-proBNP using quintiles and TnT using previous cutpoints.7 We also ran analyses in which the biomarkers were transformed by their natural logarithms and then modeled as continuous variables. We ran analyses separately for ARIC and CHS and pooled them only when biomarker levels and associations appeared similar for both studies. In particular, the distribution of NT-proBNP varied markedly, with higher levels in CHS than ARIC, necessitating study-specific analyses. Crude incidence rates per 1000 person-years were calculated by biomarker category. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals of incident VTE. We verified the proportional hazards assumption of the Cox models by inspection of ln(-ln) survival curves for TNT and NT-proBNP categories. We tested trends in VTE HRs across risk factor categories by including an ordinal variable for each category in the Cox models. We selected possible confounding variables for regression models based on previous prospective findings from LITE.15 Model 1 adjusted for age (continuous), sex, race; Model 2 additionally for diabetes status (yes or no), body mass index (BMI), eGFR, and CRP (all continuous); Model 3 additionally censored follow-up at the occurrence of incident CHD or heart failure. Adjustment for hormone replacement therapy was explored but dropped due to little confounding. We also conducted a sensitivityanalysis, which returned to the analysis participants excluded because of baseline histories of CHD or heart failure, with statistical adjustment for these conditions.
Results
The medians and distributions of TnT values were similar between ARIC and CHS participants free of VTE, and within each study TnT was associated positively with age, male sex, African American (vs. white) race, diabetes, CRP, and BMI (ARIC) and associated negatively with eGFR (Table 1). In contrast, NT-proBNP values were much higher in CHS than ARIC (medians: 106 vs. 63 pg/mL). NT-proBNP was associated positively with age, female sex (ARIC more clearly than CHS), white race, and CRP, but negatively associated with BMI, diabetes, and eGFR (Table 2). The correlation between natural logarithmic transformed TnT and NT-proBNP was r=0.14 in ARIC and 0.38 in CHS.
Table 1.
Participant characteristics (mean ± SD or %) by category of Troponin T (TnT), LITE
| ARIC | ||||||
| TnT Category | 1 | 2 | 3 | 4 | 5* | |
| Range (ng/L) | < 3 | 3 to ≤ 5 | 5 to ≤ 8 | 8 to ≤ 13 | ≥ 13 | |
| N | 3252 | 2463 | 1928 | 1223 | 685 | |
| Age, years | 60.6 ± 5.0 | 62.4 ± 5.5 | 63.6 ± 5.6 | 64.9 ± 5.6 | 65.1 ± 5.7 | |
| Female, % | 78.6 | 61.2 | 45.5 | 34.2 | 24.7 | |
| African American, % | 22.0 | 19.9 | 22.2 | 24.4 | 31.2 | |
| BMI, kg/m2 | 28.0 ± 5.4 | 28.4 ± 5.4 | 28.9 ± 5.5 | 29.3 ± 5.2 | 29.7 ± 5.5 | |
| Diabetes, % | 9.9 | 11.8 | 16.0 | 20.1 | 36.1 | |
| eGFR, ml/min/1.73m2 | 84.6 ± 19.1 | 82.7 ± 19.1 | 79.7 ± 19.2 | 77.1 ± 19.9 | 72.1 ± 21.4 | |
| CRP, mg/L | 4.58 ± 6.10 | 4.15 ± 6.31 | 3.94 ± 5.80 | 3.97 ± 6.18 | 5.50 ± 9.20 | |
| CHS | ||||||
| N | 1463 | 594 | 742 | 687 | 636 | |
| Age, years | 70.7 ± 4.0 | 72.2 ± 4.6 | 73.1 ± 5.0 | 74.6 ± 5.8 | 76.7 ± 6.6 | |
| Female, % | 78.5 | 68.0 | 55.5 | 47.3 | 38.7 | |
| African American, % | 15.5 | 13.5 | 14.8 | 15.0 | 22.0 | |
| BMI, kg/m2 | 26.6 ± 4.5 | 26.1 ± 4.7 | 26.6 ± 4.8 | 26.9 ± 4.5 | 26.4 ± 4.6 | |
| Diabetes, % | 9.6 | 12.1 | 15.5 | 18.1 | 24.5 | |
| eGFR, ml/min/1.73m2 | 70.9 ± 14.8 | 68.6 ± 14.3 | 66.5 ± 16.2 | 63.2 ± 16.1 | 58.1 ± 17.6 | |
| CRP, mg/L | 3.18 ± 5.02 | 3.28 ± 5.18 | 2.80 ± 3.92 | 3.37 ± 5.72 | 4.22 ± 6.72 | |
All linear trends in risk factors across the TnT categories are statistically significant at p<0.005, except CRP in ARIC (p = 0.98) and BMI in CHS (p = 0.59).
Table 2.
Participant characteristics (mean ± SD or %) by quintile of NT-proBNP, LITE
| ARIC | ||||||
| NT-proBNP Quintile | 1 | 2 | 3 | 4 | 5* | |
| Range (pg/ml) | ≤ 25.6 | 25.7–48.3 | 48.4–80.2 | 80.3–136.9 | ≥137.0 | |
| N | 1908 | 1910 | 1909 | 1909 | 1907 | |
| Age, years | 60.2 ± 5.0 | 61.8 ± 5.4 | 62.6 ± 5.5 | 63.3 ± 5.5 | 64.8 ± 5.7 | |
| Female, % | 38.0 | 50.0 | 62.0 | 68.8 | 70.6 | |
| African American, % | 37.4 | 22.9 | 19.9 | 16.0 | 16.1 | |
| BMI, kg/m2 | 29.5 ± 5.0 | 28.8 ± 5.2 | 28.6 ± 5.4 | 28.1 ± 5.6 | 27.9 ± 5.8 | |
| Diabetes, % | 19.3 | 15.2 | 14.1 | 12.5 | 13.5 | |
| eGFR, ml/min/1.73m2 | 88.9 ± 20.4 | 83.6 ± 18.7 | 81.1 ± 19.0 | 79.0 ± 18.2 | 73.9 ± 18.9 | |
| CRP, mg/L | 3.57 ± 4.43 | 3.96 ± 5.43 | 4.17 ± 6.05 | 4.40 ± 6.17 | 5.52 ± 8.83 | |
| CHS | ||||||
| NT-proBNP Quintile | 1 | 2 | 3 | 4 | 5* | |
| Range (pg/ml) | ≤ 46.9 | 47.0–82.5 | 82.6–133.5 | 133.6–237.1 | ≥237.2 | |
| N | 833 | 835 | 833 | 834 | 833 | |
| Age, years | 70.8 ± 4.2 | 71.4 ± 4.4 | 72.4 ± 5.0 | 73.8 ± 5.5 | 76.4 ± 6.2 | |
| Female, % | 51.9 | 61.3 | 63.6 | 68.1 | 62.8 | |
| African American, % | 23.5 | 15.8 | 14.3 | 14.3 | 13.7 | |
| BMI, kg/m2 | 27.4 ± 4.4 | 27.1 ± 4.7 | 26.6 ± 4.5 | 26.1 ± 4.7 | 25.6 ± 4.6 | |
| Diabetes, % | 17.3 | 15.3 | 14.3 | 12.8 | 13.8 | |
| eGFR, ml/min/1.73m2 | 70.9 ± 15.7 | 69.5 ± 15.3 | 67.1 ± 15.3 | 65.8 ± 16.0 | 59.5 ± 16.8 | |
| CRP, mg/L | 3.04 ± 4.11 | 3.26 ± 5.78 | 2.80 ± 4.03 | 3.34 ± 5.31 | 4.15 ± 6.68 | |
All linear trends in risk factors across the NT-proBNP quintiles are statistically significant at p<0.0001.
There were 207 VTE events over a median of 8.3 years of follow-up in ARIC [interquartile range; 7.5 to 9.1 years], and 141 VTE events over a median of 9.9 years in CHS [interquartile range: 7.9 to 12.0 years]. TnT was associated positively with incidence of total VTE and provoked VTE, but not with unprovoked VTE, in both ARIC and CHS (Table 3). In the pooled analysis, the Model 1 hazard ratios for total VTE across the five categories of TnT were 1.00, 0.85, 1.36, 1.51, and 1.98 (p trend π.0001). Further adjustment for other risk factors in Model 2 attenuated the hazard ratios, but the positive associations of TnT with total and provoked VTE mostly remained. Figure 1 illustrates the total VTE association for Model 2 in ARIC. The elevated provoked VTE hazard ratios for TnT were largely related to non-cancer related VTEs (Table 3). When the association between TnT and VTE was further censored for incident CHD or heart failure (Model 3, Table 3), the hazard ratios were similar to Model 2, but the p-trend values were weakened due to fewer person years and VTE events (38 fewer in ARIC and 33 fewer in CHS). When TnT was used as a continuous variable, the hazard ratio of total VTE in Model 2, per study-specific standard deviation increment of natural logarithm-transformed TnT, was 1.21 (95% CI: 1.05, 1.40) in ARIC and 1.19 (95% CI: 0.99, 1.44) in CHS. In other words, VTE incidence increased approximately 20% for every standard deviation higher level of transformed TnT.
Table 3.
Hazard ratios (95% CIs) for venous thromboembolism according to categories of Troponin T (TnT), LITE
| ARIC | |||||||
| TnT Category | 1 | 2 | 3 | 4 | 5 | P-trend | |
| Range (ng/L) | < 3 | 3 to ≤ 5 | 5 to ≤ 8 | 8 to ≤ 13 | ≥ 13 | ||
| N | 3252 | 2463 | 1928 | 1223 | 685 | ||
| Total VTE | |||||||
| N events | 58 | 42 | 44 | 39 | 24 | ||
| Incidence rate per 1000 person-yrs | 2.15 | 2.08 | 2.87 | 4.11 | 4.86 | ||
| Model 1 | 1.00 | 0.94 (0.63, 1.41) | 1.26 (0.84, 1.91) | 1.76 (1.13, 2.74) | 2.09 (1.24, 3.51) | 0.0007 | |
| Model 2 | 1.00 | 0.92 (0.61, 1.38) | 1.15 (0.75, 1.74) | 1.40 (0.88, 2.22) | 1.65 (0.95, 2.87) | 0.04 | |
| Model 3 | 1.00 | 1.02 (0.66, 1.57) | 1.23 (0.78, 1.95) | 1.39 (0.82, 2.35) | 1.66 (0.87, 3.16) | 0.08 | |
| Unprovoked VTE | |||||||
| N events | 21 | 11 | 22 | 17 | 3 | ||
| Model 2 | 1.00 | 0.70 (0.33, 1.49) | 1.69 (0.88, 3.27) | 1.72 (0.81, 3.68) | 0.64 (0.17, 2.32) | 0.32 | |
| Provoked VTE | |||||||
| N events | 37 | 31 | 22 | 22 | 21 | ||
| Model 2 | 1.00 | 1.02 (0.63, 1.66) | 0.86 (0.50, 1.50) | 1.23 (0.68, 2.21) | 2.16 (1.15, 4.06) | 0.06 | |
| Model 2, not cancer related | 1.00 | 1.45 (0.72, 2.93) | 1.14 (0.51, 2.57) | 1.75 (0.75, 4.09) | 3.99 (1.70, 9.41) | 0.007 | |
| Model 2, cancer related | 1.00 | 0.73 (0.37, 1.44) | 0.66 (0.31, 1.42) | 0.87 (0.38, 2.00) | 1.03 (0.37, 2.82) | 0.86 | |
| CHS | |||||||
| N | 1463 | 594 | 742 | 687 | 636 | P-trend | |
| Total VTEa | |||||||
| N events | 41 | 11 | 33 | 25 | 28 | ||
| Incidence rate per 1000 person-yrs | 2.74 | 1.86 | 4.67 | 4.03 | 6.11 | ||
| Model 1 | 1.00 | 0.65 (0.33, 1.26) | 1.58 (0.98, 2.53) | 1.25 (0.73, 2.13) | 1.87 (1.09, 3.21) | 0.01 | |
| Model 2 | 1.00 | 0.60 (0.30, 1.20) | 1.47 (0.90, 2.38) | 1.14 (0.66, 1.96) | 1.70 (0.97, 2.99) | 0.05 | |
| Model 3 | 1.00 | 0.56 (0.26, 1.20) | 1.27 (0.73, 2.21) | 0.98 (0.53, 1.82) | 1.48 (0.78, 2.83) | 0.74 | |
| Unprovoked VTE | |||||||
| N events | 15 | 4 | 12 | 11 | 7 | ||
| Model 2 | 1.00 | 0.46 (0.13, 1.59) | 1.29 (0.58, 2.91) | 1.15 (0.48, 2.74) | 0.93 (0.33, 2.61) | 0.74 | |
| Provoked VTE | |||||||
| N events | 26 | 7 | 21 | 14 | 21 | ||
| Model 2 | 1.00 | 0.69 (0.30, 1.59) | 1.58 (0.86, 2.88) | 1.11 (0.55, 2.24) | 2.29 (1.17, 4.51) | 0.03 | |
| Model 2, not cancer related | 1.00 | 0.78 (0.28, 2.13) | 1.49 (0.69, 3.10) | 0.95 (0.37, 2.42) | 2.79 (1.22, 6.38) | 0.04 | |
| Model 2, cancer related | 1.00 | 0.53 (0.12, 2.43) | 1.73 (0.66, 4.56) | 1.38 (0.47, 4.05) | 1.51 (0.45, 5.11) | 0.33 | |
| POOLED - ARIC + CHS | |||||||
| N | 4715 | 3057 | 2670 | 1910 | 1321 | P-trend | |
| Total VTE | |||||||
| N events | 99 | 53 | 77 | 64 | 52 | ||
| Incidence rate per 1000 person-yrs | 2.36 | 2.03 | 3.44 | 4.08 | 5.46 | ||
| Model 1 | 1.00 | 0.85 (0.61, 1.19) | 1.36 (1.00, 1.86) | 1.51 (1.07, 2.12) | 1.98 (1.36, 2.87) | <.0001 | |
| Model 2 | 1.00 | 0.83 (0.59, 1.17) | 1.25 (0.91, 1.72) | 1.28 (0.90, 1.81) | 1.70 (1.15, 2.51) | 0.004 | |
| Model 3 | 1.00 | 0.87 (0.61, 1.26) | 1.22 (0.86, 1.73) | 1.18 (0.80, 1.76) | 1.63 (1.04, 2.56) | 0.03 | |
| Unprovoked VTE | |||||||
| N events | 36 | 15 | 34 | 28 | 10 | ||
| Model 2 | 1.00 | 0.61 (0.33, 1.15) | 1.48 (0.89, 2.46) | 1.41 (0.80, 2.48) | 0.84 (0.38, 1.82) | 0.37 | |
| Provoked VTE | |||||||
| N events | 63 | 38 | 43 | 36 | 42 | ||
| Model 2 | 1.00 | 0.95 (0.63, 1.44) | 1.11 (0.74, 1.67) | 1.19 (0.76, 1.87) | 2.27 (1.43, 3.59) | 0.003 | |
Model 1: Adjusted for age, sex, and race.
Model 2: Adjusted for Model 1 + diabetes status, BMI, eGFR and CRP.
Model 3: Adjusted for Model 2 + time-dependent censoring of CHD or heart failure during follow-up.
Three CHS events had missing data for TnT.
Figure 1.
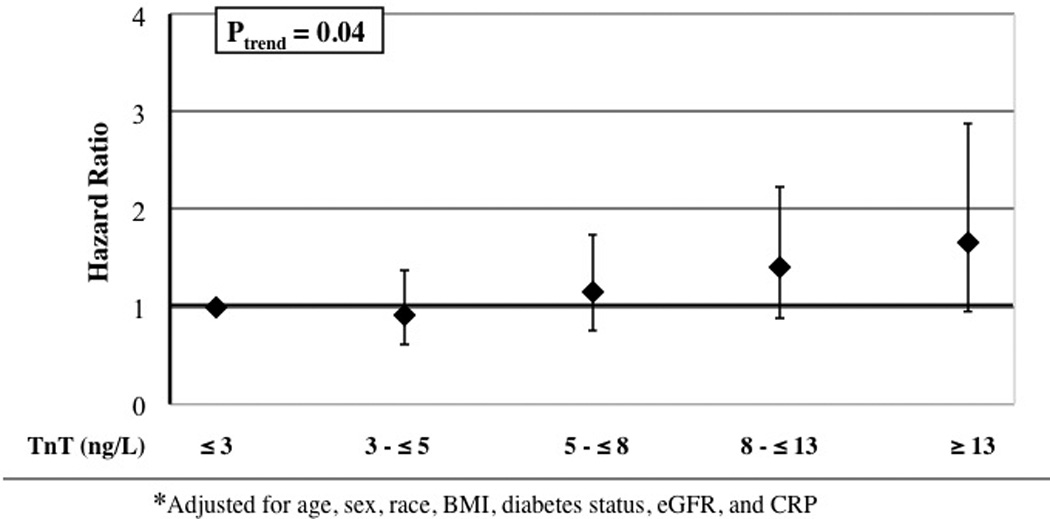
Adjusted* hazard ratios and 95 percent confidence intervals of venous thromboembolism in relation to troponin T level, ARIC.
In contrast, the associations of NT-proBNP with VTE differed between ARIC and CHS (Table 4). In ARIC, there was a positive association of study-specific quintiles of NT-proBNP with total, unprovoked and provoked VTE incidence. Hazard ratios were elevated approximately 2.5 fold for the highest versus lowest NT-proBNP quintiles in all ARIC models (see Figure 2 for Model 2). In CHS, total VTE hazard ratios were approximately 1.00 for all quintiles of NT-proBNP, indicating no association. These different findings for ARIC and CHS did not change if we swapped study cutpoints for NT-proBNP categories or if in Model 2 we used a clinical cutpoint of ≥400 pg/mL vs <400 pg/mL [hazard ratio = 2.19 in ARIC (95% CI: 1.22, 3.91) and 0.94 in CHS (95% CI: 0.51, 1.73)]. The different findings for ARIC and CHS also persisted when we treated NT-proBNP as a continuous variable: the Model 2 total VTE hazard ratio was 1.37 (95% CI: 1.17, 1.61) in ARIC and 0.99 (95% CI: 0.82, 1.20) in CHS, per study-specific standard deviation increment of natural logarithm NT-proBNP.
Table 4.
Hazard ratios (95% CIs) for venous thromboembolism according to study-specific quintiles of NT-proBNP, LITE
| ARIC | |||||||
| NT-pro BNP Quintile | 1 | 2 | 3 | 4 | 5 | P-trend | |
| Range (pg/ml) | ≤ 25.6 | 25.7–48.3 | 48.4–80.2 | 80.3–136.9 | ≥137.0 | ||
| N | 1908 | 1910 | 1909 | 1909 | 1907 | ||
| Total VTE | |||||||
| N events | 29 | 35 | 41 | 34 | 68 | ||
| Incidence rate per 1000 person-yrs | 1.85 | 2.25 | 2.64 | 2.20 | 4.61 | ||
| Model 1 | 1.00 | 1.26 (0.77, 2.07) | 1.50 (0.92, 2.44) | 1.26 (0.75, 2.11) | 2.54 (1.59, 4.05) | 0.0001 | |
| Model 2 | 1.00 | 1.27 (0.77, 2.09) | 1.40 (0.85, 2.29) | 1.28 (0.76, 2.14) | 2.45 (1.52, 3.95) | 0.0003 | |
| Model 3 | 1.00 | 1.39 (0.82, 2.38) | 1.29 (0.74, 2.25) | 1.18 (0.66, 2.11) | 2.64 (1.56, 4.48) | 0.001 | |
| Unprovoked VTE | |||||||
| N events | 9 | 10 | 15 | 15 | 25 | ||
| Model 2 | 1.00 | 1.05 (0.43, 2.62) | 1.33 (0.56, 3.18) | 1.49 (0.63, 3.54) | 2.15 (0.93, 4.97) | 0.04 | |
| Provoked VTE | |||||||
| N events | 20 | 25 | 26 | 19 | 43 | ||
| Model 2 | 1.00 | 1.39 (0.77, 2.52) | 1.44 (0.79, 2.64) | 1.15 (0.60, 2.22) | 2.65 (1.49, 4.74) | 0.003 | |
| Model 2, not cancer related | 1.00 | 1.37 (0.59, 3.21) | 1.55 (0.66, 3.62) | 1.39 (0.57, 3.39) | 2.83 (1.26, 6.36) | 0.02 | |
| Model 2, cancer related | 1.00 | 1.40 (0.61, 3.23) | 1.34 (0.57, 3.19) | 0.94 (0.36, 2.46) | 2.51 (1.10, 5.75) | 0.08 | |
| CHS | |||||||
| NT-pro BNP Quintile | 1 | 2 | 3 | 4 | 5 | P-trend | |
| Range (pg/ml) | ≤ 46.9 | 47.0–82.5 | 82.6–133.5 | 133.6–237.1 | ≥237.2 | ||
| N | 833 | 835 | 833 | 834 | 833 | ||
| Total VTE | |||||||
| N events | 30 | 31 | 29 | 25 | 26 | ||
| Incidence rate per 1000 person-yrs | 3.56 | 3.72 | 3.59 | 3.25 | 3.97 | ||
| Model 1 | 1.00 | 1.03 (0.62, 1.71) | 0.99 (0.59, 1.66) | 0.85 (0.49, 1.48) | 0.97 (0.55, 1.70) | 0.69 | |
| Model 2 | 1.00 | 0.94 (0.56, 1.58) | 0.98 (0.58, 1.64) | 0.80 (0.46, 1.39) | 0.86 (0.49, 1.53) | 0.49 | |
| Model 3 | 1.00 | 0.87 (0.48, 1.56) | 1.08 (0.62, 1.89) | 0.65 (0.34, 1.24) | 0.79 (0.41, 1.53) | 0.33 | |
| Unprovoked VTE | |||||||
| N events | 16 | 10 | 7 | 12 | 7 | ||
| Model 2 | 1.00 | 0.56 (0.25, 1.29) | 0.42 (0.17, 1.03) | 0.62 (0.28, 1.39) | 0.32 (0.12, 0.89) | 0.05 | |
| Provoked VTE | |||||||
| N events | 14 | 21 | 22 | 13 | 19 | ||
| Model 2 | 1.00 | 1.36 (0.68, 2.73) | 1.63 (0.83, 3.21) | 0.96 (0.44, 2.09) | 1.52 (0.73, 3.19) | 0.54 | |
| Model 2, not cancer related | 1.00 | 0.69 (0.26, 1.83) | 1.37 (0.60, 3.10) | 0.95 (0.39, 2.35) | 1.50 (0.63, 3.57) | 0.27 | |
| Model 2, cancer related | 1.00 | 3.12 (1.00, 9.78) | 2.35 (0.70, 7.92) | 0.92 (0.20, 4.24) | 1.40 (0.33, 5.98) | 0.65 | |
Model 1: Adjusted for age, sex, and race.
Model 2: Adjusted for Model 1 + diabetes status, BMI, eGFR and CRP.
Model 3: Adjusted for Model 2 + time-dependent censoring of CHD or heart failure during follow-up.
Figure 2.
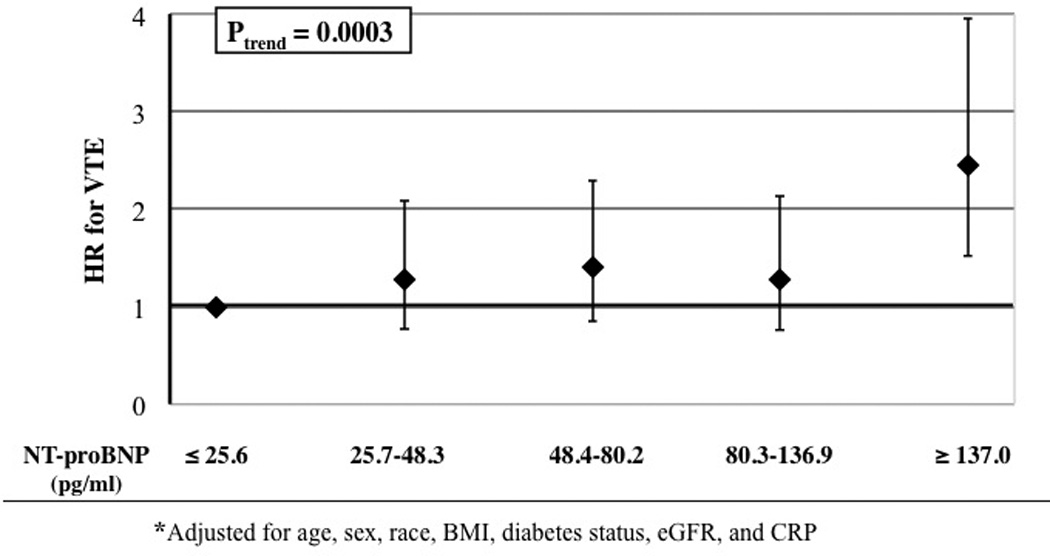
Adjusted* hazard ratios and 95 percent confidence intervals of venous thromboembolism in relation to NT---proBNP, ARIC.
In the sensitivity analysis (Supplemental Table 1), when we replaced excluded participants with histories of CHD or heart failure at baseline and adjusted for these histories, Model 2 hazard ratios were generally similar to those in Tables 3 and 4.
Discussion
This large prospective epidemiological investigation found that, independent of other measured risk factors, a high blood level of TnT in the general population was a moderately strong risk marker for total and provoked VTE, but not unprovoked VTE. The observed association with provoked VTE suggests that greater TnT levels may reflect existing medical conditions which themselves lead to VTE. The association was primarily driven by non-cancer related VTEs. To try to determine the independence of the TnT and VTE association, we not only excluded participants who at baseline had a history of CHD or heart failure and adjusted for several VTE risk factors, but we also conducted analyses that (1) censored participants at the time of myocardial infarction and heart failure events during follow-up and (2) replaced participants with baseline CHD and heart failure and adjusted for these. Regardless of the analysis strategy, a positive association between TnT and provoked VTE persisted. Because TnT is cardiac-specific, a TnT association with VTE may implicate cardiac disease or chronic atherosclerotic disease in the etiology of VTE. This latter association has been a matter of controversy9 and was not observed previously in the LITE study.24,25
NT-proBNP, in contrast, was a risk marker for incidence of both provoked and unprovoked VTE in the younger ARIC cohort, but there was no association in CHS, no matter how we analyzed the CHS data. Although it is well established that some CHD risk factors often carry lower hazard ratios for CHD in older than younger adults,26 this age-related dimorphism was unexpected for NT-proBNP and VTE. We do not believe it relates to methodological differences between ARIC and CHS, because the NT-proBNP assays were the same, VTEs were validated using identical methods, and NT-proBNP was positively associated with CHD and heart failure incidence similarly in ARIC and CHS.5,7 BNP preferentially acts on the venous system, in contrast with atrial natriuretic peptide which acts on the arterial system.27 The positive association between NT-proBNP and VTE in ARIC but not CHS may be simply a chance difference between two subgroups. More speculatively, the difference could suggest (1) a more prominent role of vascular volume overload and venous stasis to VTE etiology in younger adults than older ones or (2) a different etiology for increased BNP in younger than older adults. However, the difference in age between ARIC and CHS was only about 20 years, so different age-related mechanisms seem unlikely. If an association between NT-proBNP and VTE were verified, further exploration of the mechanisms and potential clinical implications would be warranted.
Drawbacks of this study need consideration. Firstly, we used only single measures of the plasma biomarkers. In so far as there is random biological variability in the biomarkers, observed associations with VTE would tend to be weakened. Also, with a single measure, we could not examine the association of change in biomarkers with VTE. Secondly, TnT and NT-proBNP are correlated with other VTE risk factors that could have confounded our results. However, we adjusted for many VTE risk factors to control confounding. One missing potential confounder was statin use during follow-up. Use of statins is associated with reduced VTE occurrence,28 has increased over time, and might have affected TnT and NT-proBNP or underlying subclinical cardiac disease. Thirdly, the numbers of VTEs for ARIC and CHS individually were modest, which limited study-specific statistical power and possibly obscured a weak association between NT-proBNP and VTE in CHS. Finally, although many methodologic aspects of ARIC and CHS were identical, their different NT-proBNP associations with VTE still may be the result of unidentified or statistically uncontrolled differences in study populations; definitions of variables (eg, eGFR, CHD, and heart failure); study design with regard to blood sampling; or the use of two separate laboratories.
Conclusion
TnT and NT-proBNP cannot yet be added to the list of proven risk markers for VTE. However, the observed associations suggest that further exploration of connections between cardiac disease or atherosclerosis and VTE is warranted.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC Study and CHS for their important contributions.
Funding
The Atherosclerosis Risk in Communities Study is supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C through HHSN268201100012C. CHS was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, and N01HC85079 through N01HC85086, and grant HL080295, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS) and AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at https:chs-nhlbi.org/PI. Roche Diagnostics, Inc. provided funding and laboratory reagents for the NT-proBNP and TnT assays.
Dr. Ballantyne has received grant support from Roche Diagnostics. Dr. Ballantyne and Dr. Nambi, along with Roche Diagnostics and Baylor College of Medicine, have a provisional patent filed on the use of biomarkers in the prediction of heart failure. Dr. deFilippi receives honorarium, consulting, and grant support from Roche Diagnostics and Siemens Healthcare Diagnostics. (but receive National Institutes of Health grant funding)
Footnotes
Declaration of conflicting interest
The other authors declare no commercial conflicts of interest.
References
- 1.Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis – current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824–833. doi: 10.1111/j.1365-2141.2010.08206.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 3.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 4.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankenberg S, Zeller T, Saarela O, et al. for the MORGAM Project. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The MONICA, Risk, Genetics, Archiving, and Monograph (MORGAM) Biomarker Project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 7.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutten JH, Mattace-Raso FU, Steyerberg EW, et al. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55:785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Nambi V, Bell EJ, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: The Atherosclerosis Risk in Communities Study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lijfering WM, Flinterman LE, Vandenbroucke JP, et al. Relationship between venous and arterial thrombosis: a review of the literature from a causal perspective. Semin Thromb Hemost. 2011;37:885–896. doi: 10.1055/s-0031-1297367. [DOI] [PubMed] [Google Scholar]
- 11.Dean SM, Abraham W. Venous thromboembolic disease in congestive heart failure. Congest Heart Fail. 2010;16:164–169. doi: 10.1111/j.1751-7133.2010.00148.x. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal SK, Avery CL, Ballantyne CM, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: The Atherosclerosis Risk in Communities Study. Clin Chem. 2011;57:891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowatzke WL, Cole TG. Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin Chem. 2003;49:1560–1562. doi: 10.1373/49.9.1560. [DOI] [PubMed] [Google Scholar]
- 18.Vasile VD, Saenger AK, Kroning JM, et al. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:1086–1090. doi: 10.1373/clinchem.2009.140616. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.deFilippi CR, Christenson RH, Gottdiener JS, et al. Dynamic cardiovascular risk assessment in elderly people: the role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology (LITE) Am J Med. 2002;113:636–642. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 22.Folsom AR, Lutsey PL, Astor BC, et al. C-reactive protein and venous thromboembolism: a prospective investigation in the ARIC Cohort. Thromb Haemost. 2009;102:615–619. doi: 10.1160/TH09-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 24.Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost. 2006;4:1909–1913. doi: 10.1111/j.1538-7836.2006.02121.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost. 2006;4:1903–1908. doi: 10.1111/j.1538-7836.2006.02096.x. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Seeman TE, Merrill SS, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272:1335–1340. [PubMed] [Google Scholar]
- 27.Houben AJ, van der Zander K, de Leeuw PW. Vascular and renal actions of brain natriuretic peptide in man: physiology and pharmacology. Fundam Clin Pharmacol. 2005;19:411–419. doi: 10.1111/j.1472-8206.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 28.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


