ABSTRACT
Purpose: To identify case reports of statin-induced rhabdomyolysis and summarize common predisposing factors, symptoms, diagnostic findings, functional outcomes, characteristics, treatment, and rehabilitation. Method: MEDLINE, CINAHL, SCOPUS, and PEDro databases were searched (1990–2013) for relevant case reports using the search terms “Statins,” “Rhabdomyolysis,” “Myalgia,” “Muscle damage,” “Muscle injury,” and “Myopathy.” Relevance (based on title and abstract) was assessed by one investigator; two investigators independently reviewed the relevant articles to determine inclusion in the review. Results: A total of 112 cases met the inclusion criteria. The majority were in men (70%) and people over 45 years of age (mean 64 [SD 14] years). Simvastatin was the most commonly reported statin (n=55); the majority of cases reported the use of concomitant medications such as fibrates (n=25). Weakness (n=65) and muscle pain (n=64) were the most common symptoms. In 19 cases, the patient was referred to rehabilitation, but the case reports do not include descriptions of the treatment. Conclusion: Statin-induced rhabdomyolysis was more commonly reported when statins were used in conjunction with other drugs, which potentiated its effect. Research is needed to identify the role of exercise and rehabilitation following statin-induced rhabdomyoloysis since muscle damage may be severe and may have long-term effects on muscle function.
Key Words: HMG-CoA, muscular diseases, rhabdomyolysis, statins
RÉSUMÉ
Objectif : Trouver des rapports de cas portant sur la rhabdomyolyse provoquée par les statines et résumer les facteurs prédisposants communs, les symptômes, les résultats diagnostiques, les résultats fonctionnels, les caractéristiques, le traitement et la réadaptation. Méthodes : On a cherché dans les bases de données MEDLINE, CINAHL, SCOPUS et PEDro (1990–2013) des rapports de cas pertinents en utilisant les termes de recherche Statins, Rhabdomyolysis, Myalgia, Muscle damage, Muscle injury et Myopathy. Un chercheur en a évalué la pertinence (en fonction du titre et du résumé) et deux autres ont revu indépendamment les articles pertinents pour déterminer s'il fallait les inclure dans la recherche. Résultats : Au total, 112 cas répondaient aux critères d'inclusion. La majorité des cas portaient sur des hommes (70 %) et les plus de 45 ans (âge moyen de 64 [ET 14] ans). La simvastatine a été la statine incriminée le plus souvent dans les rapports (n=55), la majorité des cas signalant l'utilisation simultanée de médicaments comme des fibrates (n=25). La faiblesse (n=65) et les douleurs musculaires (n=64) étaient les symptômes les plus courants. Dans 19 cas, le patient a été aiguillé vers la réadaptation, mais les rapports ne décrivent pas le traitement. Conclusion : On a signalé une rhabdomyolyse causée par les statines plus souvent lorsqu'elles étaient conjuguées à d'autres médicaments, ce qui en a accentué l'effet. Des recherches s'imposent pour déterminer le rôle de l'exercice et de la réadaptation à la suite d'une rhabdomyoloyse causée par les statines puisque les dommages musculaires peuvent être graves et qu'elles peuvent avoir des effets à long terme sur la fonction musculaire.
Mots clés : Rhabdomyolyse, myopathies, statines, HMG-CoA
Rhabdomyolysis is a syndrome characterized by muscle necrosis and the release of intracellular muscle contents into systemic circulation1; it is most often associated with severe or intense exercise. The manifestations of this syndrome range from asymptomatic elevation of serum muscle enzymes to life-threatening cases associated with extremely high enzyme levels, electrolyte imbalances, and acute renal failure.1 The clinical presentation of rhabdomyolysis includes myalgia, muscle weakness, and brown or “tea-colored” urine. In addition to a recent history of excessive exercise and clinical signs and symptoms, laboratory findings such as elevated serum creatine kinase (CK) and myoglobinuria confirm the diagnosis of rhabdomyolysis.2 The standards of care for rhabdomyolysis include urine alkalization, aggressive intravenous fluids, and in some cases, short-term dialysis.3
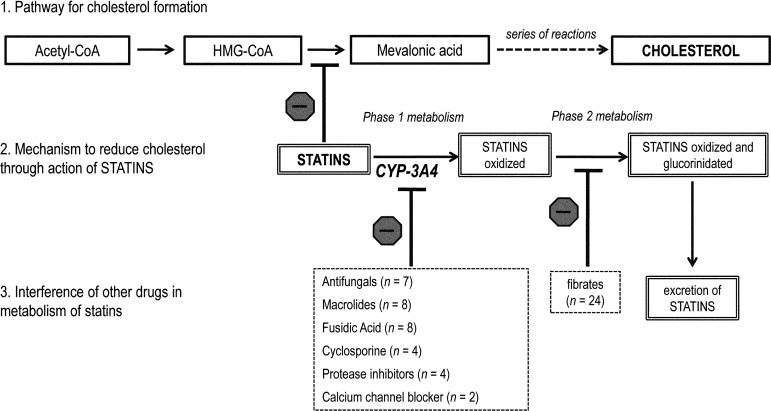
In addition to the known link between excessive exercise and rhabdomyolysis, rehabilitation specialists have recently been interested in the risk of developing rhabdomyolysis from statin medications (hydroxymethylglutaryl–coenzyme A reductase inhibitors [HMG-CoA]).4 Statins are the most effective and widely prescribed drugs currently available for the reduction of low-density lipoprotein (LDL) cholesterol, which is critical for primary and secondary prevention of cardiovascular disease5 (see Figure 1 for schematic representation of the mechanism by which statins reduce cholesterol). Rhabdomyolysis is the most severe form of myotoxicity, which can occur with all statins, either in monotherapy or in combination therapy,6 although the exact mechanism of this link is still unknown.7
Figure 1.
Schematic representation of (1) mechanism of cholesterol formation in the liver, (2) inhibition of cholesterol formation by statin medications, and (3) interference of other drugs on statin metabolism. ( indicates “inhibition” of the pathway).
indicates “inhibition” of the pathway).
Statins inhibit the enzyme 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase which is responsible for the conversion of HMG-CoA to mevalonic acid and the subsequent production of cholesterol. CYP-3A4 (a member of the cytochrome P450 family oxidase system) is the liver enzyme responsible for oxidizing statins and allowing them to be excreted out of the body. However, many known drugs inhibit CYP3A4, thereby reducing the oxidation of statins in Phase 1 metabolism, resulting in greater bioavailability of statins and therefore greater inhibition of cholesterol synthesis. Fibrates are a class of drug that inhibit Phase 2 metabolism, which is normally responsible for further metabolism of the statin for excretion out of the body. With inhibition of phase 2 metabolism, statins that are oxidized are not excreted and stay in the body, exerting their inhibitory effect.
The incidence of rhabdomyolysis from all causes is 1.6 per 100,000 person-years8; the US Food and Drug Administration Adverse Event Reporting System database reports rates of statin-induced rhabdomyolysis of 0.3–13.5 cases per 1,000,000 statin prescriptions.9 The literature suggests several risk factors that may predispose a person to develop statin-induced rhabdomyolysis, including frailty or low body mass index, older age, female sex, hypothyroidism, hypertension, polypharmacy, and alcohol or drug abuse.10 However, much of the information on statin-induced rhabdomyolysis is available only from individual case studies, which makes it difficult to determine common factors. Moreover, there is little information about the early symptoms associated with statin-induced rhabdomyolysis or the rehabilitation programs used to restore muscle function following this acute event.
The percentage of adults in the United States taking lipid-lowering medications, including statins, grew from 3.4% to 15.5% between 1988 and 2010.11 In addition to lowering LDL levels, statins are also effective in reducing the risk of cardiovascular events in people without cardiovascular disease (i.e., primary prevention)12 and improving endothelial function.13 Some studies also suggest using statins to treat dementia, but this usage is not well established.14 Because statins are so widely prescribed, rehabilitation specialists need to be aware of the risk and symptoms of rhabdomyolysis, since they are in a position to recognize the onset of myopathic symptoms at an early stage.4 There is also a need to understand the role of rehabilitation in the long term management of muscle myopathy, which may occur as a result of rhabdomyolysis. The primary purpose of our comprehensive review was to identify case studies of statin-induced rhabdomyolysis in the literature to identify the common factors (e.g., statin type, dose, pre-existing conditions) and symptoms associated with this syndrome. The secondary objective was to describe the use of functional measures in patients who experienced statin-induced rhabdomyolysis and their involvement in rehabilitation following their acute illness.
Methods
Search strategy
We identified case reports of statin-induced rhabdomyolysis indexed in MEDLINE, CINAHL, SCOPUS, and PEDro. All searches were based on the strategy developed for MEDLINE and revised appropriately for each database. Several key words were used to retrieve relevant articles with a combination of MeSH headings and “free-text terms”: “statins,” “rhabdomyolysis,” “myalgia,” “muscle damage,” “muscle injury,” and “myopathy.” We limited our search results to reports of studies involving human adults (>19 years), published in English between January 1999 and April 2013. We also manually searched the reference lists of the articles retrieved. A total of 646 articles met our initial criteria.
Study selection
One investigator assessed the retrieved citations for relevance, considering the abstract or title only. Relevant articles were then reviewed independently by two investigators to determine whether they met the inclusion criteria. Any disagreement between the reviewers was discussed, and final decisions were reached by consensus. A case was considered eligible for inclusion if it reported a definitive diagnosis of statin-induced rhabdomyolysis established through laboratory evidence of muscle breakdown and toxicity. Plasma CK values were categorized based on the guidelines for mild (<10× upper limit of normal [ULN]), moderate (10×<ULN<50×), and marked (≥50× ULN) CK elevations; the ULNs for CK levels are 532 international units/litre (IU/L) and 248 IU/L for men and women, respectively.15 We excluded reports that presented summary information about a group of patients with rhabdomyolysis but did not include individual patient data, cohort studies of drug trials, reports of other conditions associated with statin use, or letters to the editor.
Data extraction
One investigator independently extracted patient data from each included study. If two or more studies presented the same data from a single patient, we included those data only once in the analysis. Information on demographics (age, sex), pre-existing clinical conditions, type of statin and dose, co-administered medications, mean time of symptom onset, and laboratory markers used to confirm the diagnosis of rhabdomyolysis (e.g., plasma CK, urinalysis) were recorded from the articles. We also recorded information regarding clinical signs and symptoms, treatment, mortality, other functional measures, and rehabilitation.
Statistical analysis
Demographics and laboratory test results are expressed as mean (SD), and other variables are described as frequencies and percentages. Using STATA software (version 11, StataCorp LP, College Station, TX), we performed univariate descriptive analyses to summarize the characteristics of the course of rhabdomyolysis. Specifically, we computed the distribution of the collected variables and correlation coefficients (chi-square or Fisher exact tests and Pearson product-moment correlations) to investigate relationships between mortality and patient characteristics, pre-existing conditions, time to symptom onset, type of statin and dosage, and drug co-administration.
Results
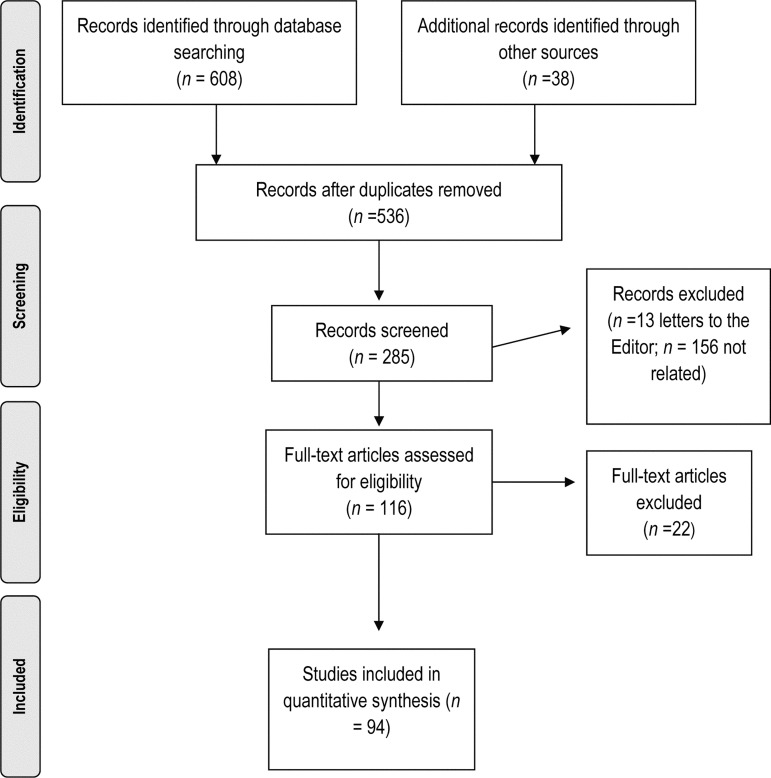
Our search retrieved 646 case reports. After screening for relevance and eligibility, 94 papers, reporting on a total of 112 individual cases of statin-induced rhabdomyolysis, met our inclusion criteria (see Figure 2). A full list of the case reports included in our review is provided in Appendix 1 (available online). The case reports varied widely with respect to the details provided.
Figure 2.
Flowchart of search results and reasons for exclusion.
Patient characteristics
We found 14 cases of statin-induced rhabdomyolysis in adults 45 years old or younger, 42 between the ages of 46 and 65 years, 30 in people aged 66 to 75, and 26 in people 76 years or older (see Table 1); 70% of cases (79/112) were reported in men.
Table 1.
Pre-existing Conditions Classified by Age and Sex in Cases of Statin-Induced Rhabdomyolysis
| No. of patients |
|||||
|---|---|---|---|---|---|
| Age and Sex | Total | Cardio- vascular disease |
Diabetes | Dyslipidemia | Renal impairment |
| Male (n=79) | |||||
| ≤45 y | 10 | 3 | 1 | 1 | 4 |
| 46–65 y | 27 | 19 | 13 | 17 | 8 |
| 66–75 y | 22 | 15 | 13 | 16 | 6 |
| >75 y | 20 | 18 | 4 | 13 | 5 |
| Female (n=33) | |||||
| ≤45 y | 4 | 2 | 1 | 3 | 1 |
| 46–65 y | 15 | 11 | 7 | 11 | 6 |
| 66–75 y | 8 | 6 | 4 | 5 | 5 |
| >75 y | 6 | 5 | 2 | 4 | 4 |
Pre-existing clinical conditions documented in the included studies fell into four major categories: (1) cardiovascular disease (e.g., angina, coronary artery disease, hypertension, myocardial infarction, and congestive heart failure), (2) dyslipidemia, (3) diabetes mellitus, and (4) renal impairment (e.g., chronic renal disease, nephropathy). Cardiovascular disease was the most commonly reported pre-existing diagnosis (n=79), followed by dyslipidemia (n=70), renal disease (n=39), and diabetes mellitus (n=45). Nine people did not fall into any of the major diagnostic groups but had other diagnoses such as rheumatologic conditions, liver cirrhosis or HIV. The majority had more than one pre-existing diagnosis: 24 had one condition, while 41 had two, 29 had three, and 9 had four or more. The most common combination of pre-existing conditions was cardiovascular disease and dyslipidemia (n=50).
Statin drugs and dosages
Simvastatin was the most common statin medication reported in association with rhabdomyolysis (55 cases), most commonly taken at a dose of 40 mg/day. Atorvastatin was the second most common drug (20 cases), used in doses of 10 mg/day. Fewer cases reported the use of cerivastatin (12), lovastatin (11), rosuvastatin (6), pravastatin (4), and fluvastatin (3). Only one case did not specify which statin the patient was taking when he developed rhabdomyolysis.
Drug interactions
Concomitant use of medications was reported in the majority of cases: 98% of cases describing the use of simvastatin (51 cases) reported concomitant use of other medications, such as fibrates (12 cases). Of the 19 cases reporting the use of atorvastatin, five (26% percent) described concomitant use with fusidic acid and two others reported the use of fibrates. Also, 50% percent of cases of rhabdomyolysis from cerivastatin, rosuvastatin, provastatin, or fluvastatin also described concomitant use of other drugs; fibrates were the most common across the different statin medications. Figure 1 shows the number of cases reporting concomitant use of major classes of medications and the mechanisms for their interference with metabolism of statins.
Laboratory evidence of rhabdomyolysis
Almost all cases reported elevated plasma CK to confirm rhabdomyolysis; values reported ranged from 785 to 450,000 IU/L. CK elevation was described as “mild,” “moderate,” or “marked” based on international guidelines.15 A total of 15 cases (11 men, 4 women) reported mild CK elevation, 28 (21 men, 7 women) reported moderate CK elevation, and 65 (43 men, 21 women) reported marked CK elevation. Only one case did not report CK levels, stating that the diagnosis of rhabdomyolysis was made based on other lab tests. Elevated myoglobin levels, ranging from 11,400 to 261,070 mg/L (mean 19,655 mg/L), were also reported in 25 cases. Urinalysis was reported in 34 cases: 28 demonstrated the presence of myoglobin in the urine, 9 revealed proteinuria, and 2 were negative for myoglobinuria. Finally, 39 cases reported creatinine levels (mean 3.49 [SD 2.55] mg/dL).
Clinical symptoms and functional measures
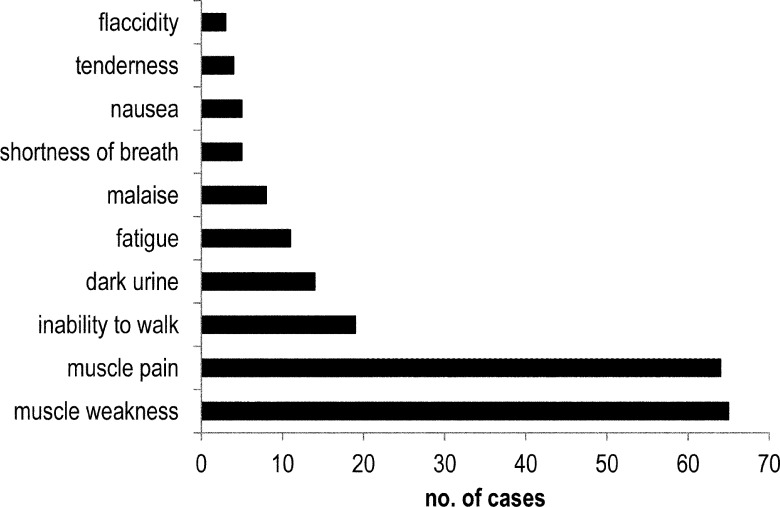
As Figure 3 shows, weakness and muscle pain were the most common presenting symptoms, described in more than 60% of the included case reports (65 and 64 cases, respectively). Other symptoms reported were inability to walk (19 cases), dark urine (14), fatigue (11), malaise (8), shortness of breath (5), nausea (5), muscle tenderness (4), and flaccid muscles (3). Of 39 cases that reported using manual muscle testing to evaluate muscle weakness, only 16 actually provided the results of muscle testing. In these 16 cases, patients presented with a range of muscle test scores for the upper and lower limbs: three presented at grade 5, eight at grade 4, eight at grade 3, four at grade 2, and one at grade 0–1. Deep tendon reflexes were tested to confirm muscle weakness in 14 cases, five of which reported results: 1+ (3 cases), 2+ (1 case), and “positive” (1 case).
Figure 3.
Frequency of symptoms reported in cases of statin-induced rhabdomyolysis.
Mortality
We observed an overall mortality rate of 15% (17 cases) in the included studies (see Table 2). Univariate analyses suggest that mortality was independent of age (p=0.84), sex (p=0.12), drug type and dose (p=0.33 and p=0.70), pre-existing conditions (p=0.69), and onset of symptoms (p=0.10). More fatalities were reported in people approaching old age (56–75 years); 15 of the 17 fatal cases involved men at a mean age of 65 (SD 6) years. Simvastatin and atorvastatin, used in six cases each, were the most common drugs associated with deaths and were given at doses>20 mg/day in nine fatal cases. Pre-existing cardiovascular disease and diabetes were reported in most of the fatal cases (13 and 10 cases, respectively).
Table 2.
Key Characteristics of Fatal Cases of Statin-Induced Rhabdomyolysis (n=17)
| Characteristic | No. of cases |
|---|---|
| Age, y | |
| ≤45 | 2 |
| 46–65 | 5 |
| 66–75 | 7 |
| >75 | 3 |
| Sex | |
| Male | 15 |
| Female | 2 |
| Pre-existing conditions | |
| Cardiovascular disease | 13 |
| Diabetes mellitus | 10 |
| Renal impairment | 7 |
| Dyslipidemia | 5 |
| Time to onset, d | |
| ≤7 | 5 |
| 8–14 | 4 |
| ≥15 | 2 |
| Not reported | 6 |
| Type of statin | |
| Simvastatin | 6 |
| Atorvastatin | 6 |
| Lovastatin | 3 |
| Cerivastatin | 1 |
| Fluvastatin | 1 |
| Statin dose, mg | |
| ≤20 | 5 |
| 21–39 | 3 |
| ≥40 | 4 |
| Not reported | 5 |
| Concomitant drugs | |
| Antibiotics | 6 |
| Fibrates | 2 |
| Antifungals | 1 |
| Other | 4 |
| None | 4 |
Treatment and outcomes
Time between initiation of statin medication and onset of rhabdomyolysis symptoms was reported in 79 cases; mean time to onset was 9 (SD 6) days (range 1–60 days). For the 95 people who survived, the mean hospital length of stay was 11.5 (SD 7) days (based on 47 cases; another 48 cases did not specify length of hospitalization); 34 people required treatment with hemodialysis, 20 of them because of acute renal impairment, and 2 required admission to the intensive care unit.
Of 19 cases reporting rehabilitative exercises as part of treatment of rhabdomyolysis, 13 reported that the patients received physical therapy while in the hospital, while in 6 cases the patient was referred to a rehabilitation centre following acute hospitalization. However, none of the case reports provided specific information about the rehabilitation programmes, such as the type of exercises or therapy provided. Time to “free-of-symptoms” recovery and return to normal activities ranged from 7 days to 6 months, based on reports from 65 cases.
Discussion
Because of the increasing global incidence of dyslipidemia and cardiovascular disease, cholesterol-lowering medications are becoming very common, and this will ultimately lead to increased incidence of serious side effects such as rhabdomyolysis. Statins are the most widely prescribed class of drugs worldwide.16 In Canada, retail spending on statins has grown from $0.5 billion in 1998 to $1.9 billion in 2007, making this the fastest-growing class of therapeutic drugs over that period.17 We identified a total of 112 reported cases of statin-induced rhabdomyolysis published between 1999 and 2013, from which the overall mortality rate was 15%. Rhabdomyolysis was most frequently reported in men and in people over 45. The majority of the statin-induced rhabdomyolysis cases reported in the literature occurred when simvastatin and atorvastatin were used concomitantly with other medications, such as fibrates or fusidic acid. We found limited information on muscle strength or other functional outcomes following rhabdomyolysis, although in 20% of cases the patient was discharged to a rehabilitation centre, which indicates a need for rehabilitation following the acute episode. We also identified a gap in information regarding the type of rehabilitation provided and long-term functional recovery, which limits the information we can provide to rehabilitation professionals on the appropriate type and amount of exercise for these patients.
Six statins are currently prescribed: rosuvastatin, atorvastatin, simvastatin, pravastatin, fluvastatin, and lovastatin. A seventh statin drug, cerivastatin, was taken off the market by its manufacturer in 2001 after it was found to have been associated with approximately 100 rhabdomyolysis-related deaths.18 Based on our comprehensive review, cases of rhabdomyolysis were reported most commonly from simvastatin and atorvastatin, at dosages of 40 mg/day and 10 mg/day respectively. In a trial by Marz and colleagues19 with more than 3,000 participants, atorvastatin and simvastatin were reported to be safe for the secondary prevention of cardiovascular disease; the rates of serious adverse events were 2% and 3%, and no drug-induced rhabdomyolysis with either atorvastatin or simvastatin was reported in this trial. Our results may show a greater number of cases from these particular statins because they are more commonly prescribed, rather than because these specific statins carry an increased risk of rhabdomyolysis.
The included studies most often reported that the statin medication was being taken concomitantly with other medications; for instance, simvastatin in combination with fibrates was reported in 95% of the case reports we examined. The greatest risk of developing a statin-related myopathy appears to be associated with exposure to another medication that interferes with statin metabolism, rendering the statin dose more toxic and thus increasing the risk of rhabdomyolysis.20,21 Although individual differences in statin sensitivity make it difficult to predict the probability of developing rhabdomyolysis from a drug interaction,22 rehabilitation specialists need to be aware of the major classes of drugs that may potentiate the effect of statins, such as fibrates, antifungals, macrolides, and fusidic acid. Fibrates are often co-prescribed with statins to reduce triglycerides and cholesterol23; co-prescription of statins with fusidic acid appears to be on the rise, especially in the elderly population, because of increased prevalence of infections such as methicillin-resistant Staphylococcus aureus.24
It has been suggested that several demographic or health-related factors may predispose a person to develop statin-induced rhabdomyolysis, including older age, frailty, multisystem diseases, and multiple medications. Hypothyroidism, impaired liver function, and impaired kidney function are also thought to increase the incidence of statin-induced myopathy.10 In our review, we found that most reported cases were in men, people over 45 years of age, and those with more than one pre-existing condition. Although our findings are based on a comprehensive review of published case reports and not on a large-scale prospective study, these results suggest that rehabilitation specialists should pay particular attention to patients with this “risk profile” if signs of myopathy such as muscle pain or weakness are observed.
In the case reports we reviewed, the time between the initiation of statin medication, an increase in dosage, or the addition of an interacting drug and the onset of rhabdomyolysis symptoms ranged from 1 to 60 days (mean 9 [SD 6] days; based on 79 cases), indicating that the temporal relationship between initiation of statins and onset of symptoms is widely variable. It is important for clinicians to be aware that statin-induced rhabdomyolysis can occur in people who have been stable for some time, especially if the dose is changed, if a new medication is added that can increase the potency of the statin or if there is a change in exercise status. The time between cessation of statin treatment and resolution of symptoms was also highly variable, ranging from 7 days to 6 months. This variability may be accounted for by many factors, such as pre-existing level of function or age, but these factors could not be determined from the current review.
Rhabdomyolysis is diagnosed by CK values exceeding 10–25× ULN, irrespective of renal function2,25 as well as by the presence of symptoms. In our review, muscle pain and weakness were the most common symptoms of rhabdomyolysis, as indicated in previous literature.26,27 Although muscle weakness is one of the most common symptoms, manual muscle testing to determine the extent of weakness was conducted in only 36 cases, and the results were reported in only 13. No quantitative results from muscle pain or soreness were reported in the case studies, despite the use of pain rating scales commonly used to evaluate these symptoms.28–30 Nor did the case reports we reviewed include testing of functional performance using common outcome measures (e.g., timed up-and-go test, gait speed) although 17 cases reported an “inability to walk.” Quantitative testing is essential to both evaluating the extent of functional impairment and documenting the recovery process through rehabilitation. Systematic evaluation of function is needed in the clinical setting, especially by rehabilitation professionals, and also needs to be reported in the literature.
We identified a gap in the literature on rehabilitation following acute rhabdomyolysis. Although 19 cases reported rehabilitative exercises as part of treatment of rhabdomyolysis, either during hospitalization or after discharge, none of these reports provided information on the exercise programs used. The physiologic impact of rhabdomyolysis causing widespread muscle damage can be a challenge for rehabilitation. The fact that time to “free of symptoms” recovery can be as long as 6 months in some cases only reinforces the need for rehabilitation strategies to augment functional recovery. Although we did not specifically identify cases in which exercise in addition to statin use may have exacerbated symptoms, reports of muscle symptoms has been shown to be higher in people who are engaging in exercise.31 This has important implications for our clients engaging in physical therapy and exercise who are taking statins. More information on a cautious but effective approach to exercise training is needed both for people exercising while taking statins and for those recovering from statin-induced muscle damage.
We found an overall mortality rate of 15% in the reported cases. A review by Thompson and colleagues32 of the Qscan FDA database from 1990 to 2002 found that 7.8% of patients who developed statin-induced rhabdomyolysis died; however, these data relied on voluntary physician reporting, and a uniform definition of rhabdomyolysis was not used. Omar and colleagues'21 retrospective analysis from an FDA database (1997 to 2000) found a very low incidence of fatality from statin-induced rhabdomyolysis (0.15 per million prescriptions). Our review likely overestimated the mortality rate, since it was based solely on published case reports; such reports often document unique or more severe cases, and it is likely as a result of this publication bias that our mortality estimate was higher than those previously reported. When we examined predictors of mortality, we found that all reported fatalities were in men and that pre-existing cardiovascular disease and diabetes were reported in most of these fatal cases; however, we did not find that mortality was significantly related to age, sex, drug type and dose, onset of symptoms, or pre-existing conditions. The lack of such associations in our data may be due to the limited sample size (particularly for the factors of sex and onset of symptoms, where p-values approached significance), heterogeneity in publication of case reports, and the retrospective nature of this review.
As a comprehensive review of published case reports, our study has certain limitations. Findings from such a review cannot replicate those from the large-scale prospective trials of statins that provide information on adverse effects. This is partly because of the selective publication of case reports, which often report on unique, individual cases, and partly because case reports are heterogeneous in nature and comparisons of drug types, dosages, medical treatment, and other factors related to patients' health care and pre-existing condition cannot be controlled. On the other hand, case reports provide information on individual patients who are not represented in larger studies, since most drug trials have strict inclusion and exclusion criteria to exclude patients who may be at higher risk or toxicity and side effects.
Conclusion
Rhabdomyolysis is a rare but clinically important adverse event that can occur as a result of statin use, either alone or, in particular, in combination with drugs that increase the potency of the statin. Because an increasing number of people require statins to control high cholesterol and reduce their risk of cardiovascular disease, identifying patients with an increased risk of statin-induced rhabdomyolysis could facilitate the prevention of potential complications and further improve the already overwhelmingly positive benefit-risk ratio of statins. We found that statin-induced rhabdomyolysis was most commonly reported in men, in people over 45 years of age, and in those with multiple pre-existing conditions such as cardiovascular disease and dyslipidemia, which are often treated using statins. There was a lack of information in the case reports on functional outcomes, long-term recovery, and rehabilitation guidelines in people who suffered from rhabdomyolysis. Rehabilitation professionals need to be involved in the long-term evaluation and management of people with rhabdomyolysis, and more information is needed on the type and intensity of exercise that is safe and effective for myopathic muscles.
Key Messages
What is already known on this topic
There is a growing need for statins in the population to control high cholesterol and reduce the risk of cardiovascular disease, which may result in greater occurrence of statin-induced rhabdomyolysis. This condition is characterized by severe muscle damage (high plasma creatine kinase and myoglobin in the urine), as well as symptoms of muscle weakness, fatigue and pain; it may result in death in certain severe cases.
What this study adds
The present study provides a comprehensive review of the published case studies of statin-induced rhabdomyolysis and identifies key factors that physical therapists should be aware of in their patients on statins that may increase their risk of developing myopathy or rhabdomyolysis. These include concomitant use of medications that potentiate the effect of statins, male sex, people over 45 years old, and people with more than one pre-existing condition (e.g., cardiovascular disease, diabetes). Physical therapists should also be alert to the early symptoms of rhabdomyolysis in their clients who are taking statins, such as muscle weakness and pain and/or fatigue, as these may become exacerbated with exercise. Finally, this review identifies a gap in the published literature on the role for rehabilitation following acute rhabdomyolysis and calls attention to the need to report on the type of exercise programs used to optimize their recovery, as well as the long-term effects of acute rhabdomyolysis on muscle structure and function.
Physiotherapy Canada 2014; 66(2);124–132; doi:10.3138/ptc.2012-65
References
- 1.Line RL, Rust GS. Acute exertional rhabdomyolysis. Am Fam Physician. 1995;52(2):502–6. Medline:7625324. [PubMed] [Google Scholar]
- 2.Arrington ED, Miller MD. Skeletal muscle injuries. Orthop Clin North Am. 1995;26(3):411–22. Medline:7609956. [PubMed] [Google Scholar]
- 3.Landesman KA, Stozek M, Freeman NJ. Rhabdomyolysis associated with the combined use of hydroxymethylglutaryl-coenzyme A reductase inhibitors with gemfibrozil and macrolide antibiotics. Conn Med. 1999;63(8):455–7. Medline:10500341. [PubMed] [Google Scholar]
- 4.Di Stasi SL, MacLeod TD, Winters JD, et al. Effects of statins on skeletal muscle: a perspective for physical therapists. Phys Ther. 2010;90(10):1530–42. doi: 10.2522/ptj.20090251. http://dx.doi.org/10.2522/ptj.20090251. Medline:20688875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway JR, Genest J, Habib R, et al. Canadian Lipid Guidelines Update. Montréal: E.O.C.I. Pharmacomm Ltd.; 2009. [Google Scholar]
- 6.Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J. 2004;147(6):956–65. doi: 10.1016/j.ahj.2003.12.037. http://dx.doi.org/10.1016/j.ahj.2003.12.037. Medline:15199341. [DOI] [PubMed] [Google Scholar]
- 7.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38(1):41–57. doi: 10.2165/00003088-200038010-00003. http://dx.doi.org/10.2165/00003088-200038010-00003. Medline:10668858. [DOI] [PubMed] [Google Scholar]
- 8.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C. doi: 10.1016/j.amjcard.2005.12.010. Epub 2006 Feb 3. http://dx.doi.org/10.1016/j.amjcard.2005.12.010. Medline:16581329. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MH, Clark JA, Glass LM, et al. Statin safety: an appraisal from the adverse event reporting system. Am J Cardiol. 2006;97(8A):32C–43C. doi: 10.1016/j.amjcard.2005.12.008. Epub 2006 Feb 3. http://dx.doi.org/10.1016/j.amjcard.2005.12.008. Medline:16581327. [DOI] [PubMed] [Google Scholar]
- 10.Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119(5):400–9. doi: 10.1016/j.amjmed.2006.02.007. http://dx.doi.org/10.1016/j.amjmed.2006.02.007. Medline:16651050. [DOI] [PubMed] [Google Scholar]
- 11.Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308(15):1545–54. doi: 10.1001/jama.2012.13260. http://dx.doi.org/10.1001/jama.2012.13260. Medline:23073951. [DOI] [PubMed] [Google Scholar]
- 12.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338(jun30 1):b2376. doi: 10.1136/bmj.b2376. http://dx.doi.org/10.1136/bmj.b2376. Medline:19567909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15(5):467–78. doi: 10.2174/138161209787315684. http://dx.doi.org/10.2174/138161209787315684. Medline:19199975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuinness B, O'Hare J, Craig D, et al. Cochrane review on ‘Statins for the treatment of dementia’. Int J Geriatr Psychiatry. 2013;28(2):119–26. doi: 10.1002/gps.3797. http://dx.doi.org/10.1002/gps.3797. Medline:22473869. [DOI] [PubMed] [Google Scholar]
- 15.Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta. 1999;279(1–2):107–15. doi: 10.1016/s0009-8981(98)00180-6. http://dx.doi.org/10.1016/S0009-8981(98)00180-6. Medline:10064122. [DOI] [PubMed] [Google Scholar]
- 16.Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60(10):875–81. doi: 10.1016/j.jacc.2012.07.007. http://dx.doi.org/10.1016/j.jacc.2012.07.007. Medline:22902202. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Institute for Health Information. Drivers of prescription drug spending in Canada. Ottawa: The Institute; 2011. [Google Scholar]
- 18.Fuhrmans V. Bayer discloses higher death toll from Baycol. Wall Street Journal. 2002. Jan 21, p. Sect. A10.
- 19.März W, Wollschläger H, Klein G, et al. Safety of low-density lipoprotein cholestrol reduction with atorvastatin versus simvastatin in a coronary heart disease population (the TARGET TANGIBLE trial) Am J Cardiol. 1999;84(1):7–13. doi: 10.1016/s0002-9149(99)00183-6. http://dx.doi.org/10.1016/S0002-9149(99)00183-6. Medline:10404843. [DOI] [PubMed] [Google Scholar]
- 20.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109(23) Suppl 1:III50–7. doi: 10.1161/01.CIR.0000131519.15067.1f. Medline:15198967. [DOI] [PubMed] [Google Scholar]
- 21.Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36(2):288–95. doi: 10.1345/aph.1A289. http://dx.doi.org/10.1345/aph.1A289. Medline:11847951. [DOI] [PubMed] [Google Scholar]
- 22.Bottorff M, Hansten P. Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors: the role of metabolism-monograph for physicians. Arch Intern Med. 2000;160(15):2273–80. doi: 10.1001/archinte.160.15.2273. http://dx.doi.org/10.1001/archinte.160.15.2273. Medline:10927723. [DOI] [PubMed] [Google Scholar]
- 23.Davidson MH, Armani A, McKenney JM, et al. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99(6A):S3–S18. doi: 10.1016/j.amjcard.2006.11.016. Epub 2006 Dec 8. http://dx.doi.org/10.1016/j.amjcard.2006.11.016. Medline:17368275. [DOI] [PubMed] [Google Scholar]
- 24.Whitby M. Fusidic acid in the treatment of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1999;12(Suppl 2):S67–71. doi: 10.1016/s0924-8579(98)00075-2. http://dx.doi.org/10.1016/S0924-8579(98)00075-2. Medline:10528788. [DOI] [PubMed] [Google Scholar]
- 25.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–72. doi: 10.1016/s0735-1097(02)02030-2. http://dx.doi.org/10.1016/S0735-1097(02)02030-2. Medline:12142128. [DOI] [PubMed] [Google Scholar]
- 26.Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150(12):858–68. doi: 10.7326/0003-4819-150-12-200906160-00009. http://dx.doi.org/10.7326/0003-4819-150-12-200906160-00009. Medline:19528564. [DOI] [PubMed] [Google Scholar]
- 27.Kordas K. Clinical characteristics of 1053 patients with statin associated muscle complaints. Arterioscler Thromb Vasc Biol. 2004;24:e51. [Google Scholar]
- 28.Bishop MD, Horn ME, Lott DJ, et al. Magnitude of spinal muscle damage is not statistically associated with exercise-induced low back pain intensity. Spine J. 2011;11(12):1135–42. doi: 10.1016/j.spinee.2011.11.005. http://dx.doi.org/10.1016/j.spinee.2011.11.005. Medline:22208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur S, Sheel AW, Road JD, et al. Delayed onset muscle soreness after inspiratory threshold loading in healthy adults. Cardiopulm Phys Ther J. 2010;21(1):5–12. Medline:20467514. [PMC free article] [PubMed] [Google Scholar]
- 30.Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain. 2011;152(7):1540–7. doi: 10.1016/j.pain.2011.02.038. http://dx.doi.org/10.1016/j.pain.2011.02.038. Medline:21419575. [DOI] [PubMed] [Google Scholar]
- 31.Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40(4):188–94. doi: 10.1097/JES.0b013e31826c169e. Medline:23000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13):1681–90. doi: 10.1001/jama.289.13.1681. http://dx.doi.org/10.1001/jama.289.13.1681. Medline:12672737. [DOI] [PubMed] [Google Scholar]