Abstract
OBJECTIVE:
This study compares the performance of urine dipstick alone with urine microscopy and with both tests combined as a screen for urinary tract infection (UTI) in febrile infants aged 1 to 90 days.
METHODS:
We queried the Intermountain Healthcare data warehouse to identify febrile infants with urine dipstick, microscopy, and culture performed between 2004 and 2011. UTI was defined as >50 000 colony-forming units per milliliter of a urinary pathogen. We compared the performance of urine dipstick with unstained microscopy or both tests combined (“combined urinalysis”) to identify UTI in infants aged 1 to 90 days.
RESULTS:
Of 13 030 febrile infants identified, 6394 (49%) had all tests performed and were included in the analysis. Of these, 770 (12%) had UTI. Urine culture results were positive within 24 hours in 83% of UTIs. The negative predictive value (NPV) was >98% for all tests. The combined urinalysis NPV was 99.2% (95% confidence interval: 99.1%–99.3%) and was significantly greater than the dipstick NPV of 98.7% (98.6%–98.8%). The dipstick positive predictive value was significantly greater than combined urinalysis (66.8% [66.2%–67.4%] vs 51.2% [50.6%–51.8%]). These data suggest 8 febrile infants would be predicted to have a false-positive combined urinalysis for every 1 infant with UTI initially missed by dipstick screening.
CONCLUSIONS:
Urine dipstick testing compares favorably with both microscopy and combined urinalysis in febrile infants aged 1 to 90 days. The urine dipstick test may be an adequate stand-alone screen for UTI in febrile infants while awaiting urine culture results.
Keywords: urinary tract infection, urinalysis, infant, newborn, predictive value of tests, reagent strips, nitrites, leukocyte esterase, utilization
What’s Known on This Subject:
Urinary tract infection (UTI) is the most common bacterial infection in febrile infants aged 1 to 90 days. It is unclear if urine microscopy offers significant benefit beyond urine dipstick as a screening test for UTI in this population.
What This Study Adds:
Dipstick may be an adequate screening test for UTI in infants aged 1 to 90 days with a negative predictive value (NPV) of 98.7%. Adding microscopy increases the NPV to 99.2% but results in 8 false-positives for every UTI missed by dipstick.
Serious bacterial infection (SBI) occurs in ∼10% to 15% of febrile infants aged 1 to 90 days.1–3 Urinary tract infection (UTI) is the most common SBI diagnosed in febrile infants.4–6 Institutional practice related to the evaluation of febrile infants varies. At our institution, as in many others, infants aged 1 to 28 days with fever routinely undergo laboratory evaluation for bacterial infection, are admitted to the hospital, and treated with antibiotics regardless of screening test results. In contrast, if screening tests do not suggest SBI, infants aged 29 to 90 days may be managed as outpatients with or without antibiotics until culture results are available.7,8
UTI screening methods may include dipstick urinalysis and/or microscopy of centrifuged urine, as well as other methods.3,4,9–11 Urine dipstick is an inexpensive and rapid screening test that can be performed in office settings and other laboratories and is waived by the Clinical Laboratory Improvement Amendment (CLIA).12 Dipstick has been shown to perform well in children ≥2 years old as a screening test for UTI.13 Microscopic examination of urine requires technicians with special training in laboratories using CLIA-certified methods.12 Previous studies have questioned the additional benefit of microscopy over dipstick urinalysis in children; however, these studies included few infants 1 to 90 days of age.14–20 Although dipstick is rapid, inexpensive, and does not require special training, there currently are insufficient data to recommend dipstick urinalysis alone as a screen for UTI in febrile infants.
Providers within Intermountain Healthcare use an evidence-based care process model (EB-CPM) for management of the febrile infant aged 1 to 90 days.3 The EB-CPM currently recommends urethral catheterization for dipstick urinalysis in combination with microscopy of centrifuged urine (here termed “combined urinalysis”) to screen for UTI while urine culture is pending. Systemwide implementation of this EB-CPM was associated with improved infant outcomes and lower costs.3 Additional opportunities may exist to reduce costs associated with the evaluation and management of febrile infants. We recognized the opportunity to evaluate urine-screening tests for UTI in a large population of febrile infants. Our objective was to compare performance characteristics of dipstick, microscopy, and combined urinalysis for UTI screening in febrile infants aged 1 to 90 days.
Methods
Protection of Human Subjects
The institutional review boards of the University of Utah and Intermountain Healthcare (Salt Lake City, UT) approved this study and granted waiver of informed consent.
Setting
This retrospective observational study was performed at Intermountain Healthcare, a not-for-profit integrated health care system that provides care for ∼90% of Utah infants younger than 1 year. Subjects received care at 1 of 23 Intermountain Healthcare hospitals, including a tertiary pediatric referral center (Primary Children’s Hospital [PCH]); regional medical centers located in Salt Lake City, Ogden, Provo, and St George, Utah; and smaller Utah community hospitals. PCH and the regional medical centers provide care for most febrile infants.3 Providers at these facilities include attending physicians specializing in pediatric emergency medicine, pediatrics, emergency medicine, and family medicine; midlevel providers; and supervised residents. Facilities used the same diagnostic technology and electronic record system throughout the study.
Identification of Febrile Infants
Febrile infants were identified from the Intermountain Healthcare Enterprise Data Warehouse (EDW). The EDW contains clinical, laboratory, and administrative data for all facilities. We used a prospectively validated definition for febrile infants based on age, reason for visit, admitting diagnosis, and International Classification of Diseases, Ninth Revision, and All Patient Refined Diagnosis Related Groups coding.21
Identification of Subjects for This Study and Data Collection
Subjects were febrile infants aged 1 to 90 days with an encounter at Intermountain Healthcare facilities between July 1, 2004, and December 31, 2011. All infants included in this analysis had catheterized urine dipstick, microscopic urinalysis, and urine bacterial cultures performed simultaneously. Any subjects with urine obtained by a method specified as bag specimen or suprapubic aspirate were not included. If multiple urinalysis tests were performed during an encounter, only the first urine specimen was included in analysis. All laboratory and culture results were obtained from the EDW. Outcomes of infants aged 29 to 90 days with UTI not identified by urine dipstick were obtained by review of the medical records (E.W.G.).
Definitions
Urine culture results were classified as positive for UTI, negative for UTI, or equivocal. Positive for UTI was defined as growth of ≥1 urine pathogens, each with a quantity of ≥50 000 colony forming units (CFUs) per mL.11,22 Negative for UTI was defined as no bacterial growth or growth only of a contaminant. Contaminants were defined as low numbers (<10 000 CFUs per mL) of commonly identified skin or genitourinary flora such as coagulase-negative Staphylococcus, viridans streptococci, Corynebacterium species, and Micrococcus species and/or growth of multiple bacteria each with colony counts of <10 000 CFUs per mL. Equivocal urine cultures were defined as growth of urine pathogens with quantities between 10 000 and 49 999 CFUs per mL. Subjects with equivocal results were excluded from analysis. The significance determination for each culture was made by a clinical microbiologist (E.K.K.) and a pediatric infectious diseases clinician (C.L.B.) and adjudicated by record review if required. Dipstick was considered positive if either leukocyte esterase or nitrite was positive. Microscopy was considered positive if under high-power microscopic field (HPF) the technician observed either >10 white blood cells (WBCs) or any bacteria. A positive combined urinalysis was defined as any positive finding for either dipstick or microscopy or both.
Laboratory Methods
Leukocyte esterase and nitrite were considered negative or positive including any result ≥trace, as determined by colorimetric interpretation of the dipstick by a semiautomated urine chemistry analyzer. Two methods of urine dipstick were used at the participating centers. A Siemens Multistix 8 SG dipstick interpreted on CLINITEK Advantus urine chemistry analyzer (Siemens Medical Solutions USA, Inc., Malvern, PA) was used at the PCH. All other facilities used an Aution 9EB strip interpreted on Aution AX-4280 urine chemistry analyzer (Iris Diagnostics, Chatsworth, CA). Microscopic tests (WBCs and bacteria) were performed using uniform standardized CLIA-certified methods on centrifuged urine specimens. A total of 10 mL of urine was centrifuged for 5 to 7 minutes at a relative centrifugal force of 400 g, and the technician averaged the findings of least 10 unstained HPFs under the 40× objective. WBCs were considered positive if the laboratory reported >10 WBCs per HPF. Bacteria were considered negative or positive including any ≥1 (rare) bacteria per HPF.
Statistical Analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each of the urinalysis methods against the gold standard of urine culture. Statistical calculations were made by using the ROCR package in R.13.1.23 To compare the performance characteristics between methodologies, the z-statistic test for proportions was used. Dipstick urinalysis was compared with microscopic urinalysis and with the combination of dipstick and microscopic urinalysis. Comparisons were made for the entire cohort aged 1 to 90 days and 2 subgroups: infants aged 1 to 28 and those aged 29 to 90 days.
Results
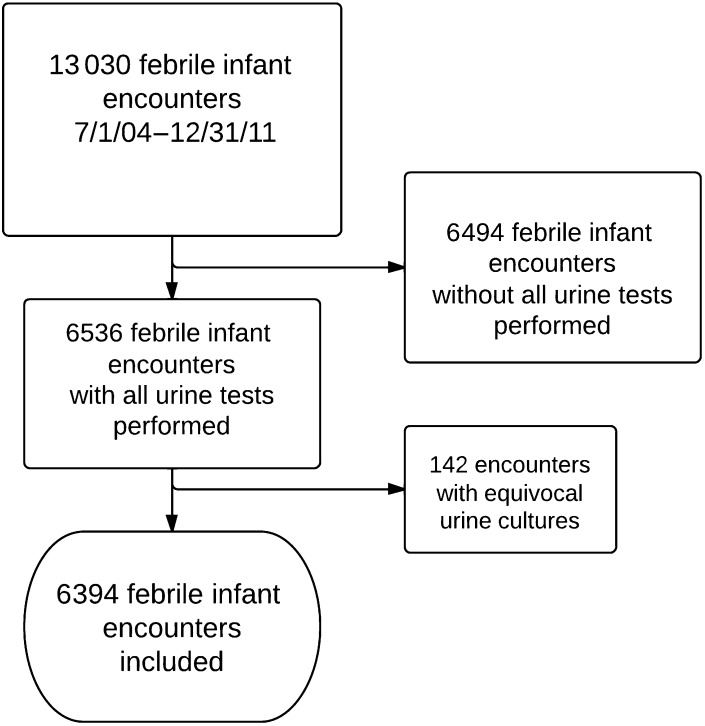
Between July 1, 2004, and December 31, 2011, we identified a total of 13 030 febrile infant encounters at Intermountain Healthcare facilities (Fig 1). Of these, 6536 (50%) had all urine studies including dipstick urinalysis, centrifuged microscopic urinalysis, and urine culture performed. One hundred forty-two of 6536 infants (2%) had a urine culture meeting our definition of equivocal and were excluded from the primary analysis, leaving 6394 febrile infants aged 1 to 90 days for analysis. Of the infants included in analysis, 770 of 6394 (12%) had a urine culture meeting our definition of positive for UTI and 5624 had negative urine cultures. Of the 6394 infants, 1745 were aged 1 to 28 days (27%) and 4649 were 29 to 90 days old (73%). Most subjects (79%) were evaluated at PCH.
FIGURE 1.
Flow diagram of included infants. Urine tests included dipstick urinalysis, centrifuged microscopic urinalysis, and urine culture.
Performance Characteristics of Urine Screening Tests
Performance characteristics of the urine screening tests are presented in Table 1. The sensitivity of combined urinalysis for UTI was greater than for dipstick (94.7% [95% confidence interval (CI): 94.4%–95.0%] vs 90.8% [90.4%–91.2%]; P < .001). The specificity of dipstick for UTI was greater than combined urinalysis (93.8% [93.5%–94.1%] vs 87.6% [87.2%–88.0%]; P < .001) or microscopic urinalysis (93.8% [93.5%–94.1%] vs 91.3% [90.9%–91.7%]; P < .001). The PPV of dipstick for UTI was also greater than combined urinalysis (66.8% [66.2%–67.4%] vs 51.2% [50.6%–51.8%]; P < .001) or microscopic urinalysis (66.8% [66.2%–67.4%] vs 58.6% [58.0%–59.2%]; P < .001). The NPV was ≥98.6% for dipstick, microscopic, and combined urinalysis.
TABLE 1.
Performance Characteristics and Comparisons of Dipstick to Microscopic and Combined Urinalysis
| Performance Characteristic and Infant Age Group | Performance, % (95% CI) | z-Statistic (P) | |||
|---|---|---|---|---|---|
| Dipstick Urinalysis | Microscopic Urinalysis | Combined Urinalysis | Dipstick Versus Microscopic Urinalysis | Dipstick Versus Combined Urinalysis | |
| Sensitivity | |||||
| 1–90 days | 90.8 (90.4–91.2) | 90.3 (89.9–90.7) | 94.7 (94.4–95.0) | 1.01 (.157) | 9.19 (<.001) |
| 1–28 days | 91.7 (91.0–92.4) | 90.7 (90.0–91.4) | 94.4 (93.8–95.0) | 0.15 (.441) | 3.69 (<.001) |
| 29–90 days | 90.4 (90.0–90.8) | 90.1 (89.7–90.5) | 94.8 (94.5–95.1) | 0.41 (.342) | 9.01 (<.001) |
| Specificity | |||||
| 1–90 days | 93.8 (93.5–94.1) | 91.3 (90.9–91.7) | 87.6 (87.2–88.0) | 3.56 (<.001) | 3.31 (<.001) |
| 1–28 days | 90.4 (89.7–91.1) | 89.0 (88.3–89.7) | 83.7 (82.8–84.6) | 5.43 (<.001) | 1.38 (.084) |
| 29–90 days | 95.1 (94.8–95.4) | 92.1 (91.7–92.5) | 89.1 (88.6–89.6) | 6.63 (<.001) | 2.82 (.002) |
| PPV | |||||
| 1–90 days | 66.8 (66.2–67.4) | 58.6 (58.0–59.2) | 51.2 (50.6–51.8) | 5.63 (<.001) | 10.72 (<.001) |
| 1–28 days | 57.4 (56.2–58.6) | 53.8 (52.6–55.0) | 45.0 (43.8–46.2) | 1.35 (.088) | 4.66 (<.001) |
| 29–90 days | 71.4 (70.7–72.1) | 60.8 (60.1–61.5) | 54.1 (53.4–54.8) | 6.22 (<.001) | 10.14 (<.001) |
| NPV | |||||
| 1–90 days | 98.7 (98.6–98.8) | 98.6 (98.5–98.7) | 99.2 (99.1–99.3) | 0.01 (.497) | 3.23 (.001) |
| 1–28 days | 98.7 (98.4–99.0) | 98.6 (98.3–98.9) | 99.1 (98.9–99.3) | 0.01 (.498) | 1.32 (.093) |
| 29–90 days | 98.7 (98.5–98.9) | 98.6 (98.4–98.8) | 99.2 (99.1–99.3) | 0.01 (.498) | 2.77 (.003) |
N = 6394 for infants aged 1–90 days, n = 1745 for those aged 1–28 days, and n = 4649 for those aged 29–90 days.
Given differences in practice, infants aged 1 to 90 days were subdivided into those aged 1 to 28 days and those aged 29 to 90 days. In infants 1 to 28 days old only, there was no difference in the NPV of combined urinalysis and dipstick (99.1% [95% CI: 98.9%–99.3%] vs 98.7% [98.4%–99.0%]; P = .093). In infants 29 to 90 days old, the NPV of combined urinalysis was greater than dipstick (99.2% [99.1%–99.3%] vs 98.7% [98.5%–98.9%]; P < .003). When analyzed by age, urinalysis tests in infants aged 29 to 90 days had higher specificity and PPV compared with infants aged 1 to 28 days (Table 1). Dipstick sensitivity was greater in infants aged 1 to 28 days (91.7%; 95% CI: 91.0%–92.4%) compared with infants aged 29 to 90 days (90.4%; 95% CI: 90.0%–90.8%). No differences by age in urinalysis test performance were seen for NPV (Table 1).
Evaluation of Cases of Culture-Confirmed UTI Not Identified by Dipstick
Demographic and laboratory characteristics of cases of culture-confirmed UTI not identified by urine dipstick testing in infants 29 to 90 days old are shown in Table 2. Infants 1 to 28 days old were excluded because most were admitted for at least 24 hours and UTI not identified by dipstick could be identified by culture during hospitalization.
TABLE 2.
Demographic and Laboratory Characteristics of 53 Infants Aged 29 to 90 Days With UTI Missed by Dipstick Urinalysis
| Descriptor | Infants With UTI Missed by Dipstick | Infants With UTI and Bacteremia Missed by Dipstick |
|---|---|---|
| Total N | 53 | 2 |
| Male gender | 34 (64) | 1 (50) |
| Microscopic urinalysis performed | 53 (100) | 2 (100) |
| Urine WBC count (>10 cells/HPF) | 3 (5) | 0 (0) |
| Bacteriuria on unstained microscopic urinalysis | 24 (45) | 0 (0) |
| Admitted to hospital at time of urine testing | 44 (83) | 2 (100) |
| CBC performed | 50 (94) | 2 (100) |
| CBC WBC count ≥15 000 or ≤5000 | 15 (28) | 1 (50) |
| Absolute band count ≥1500 | 8 (15) | 0 (0) |
| Positive cerebrospinal fluid culture | 0 (0) | 0 (0) |
Data are presented as n (%) unless otherwise indicated. CBC, complete blood count.
Fifty-three infants aged 29 to 90 days had UTI with normal dipstick testing. Twenty-seven of these screened positive by urine microscopy, leaving 26 not identified by either screening method. Eighty-three percent (44 of 53) of these infants were admitted on initial encounter and most received antibiotic treatment effective against UTI. Nine of the 53 infants (17%) were not admitted to the hospital at the time of initial evaluation. Positive urine cultures were identified in all 9 of these infants by 24 hours (mean: 19.7 hours). Six of these 9 infants had follow-up and appropriate treatment documented in the in the EDW of the Intermountain Healthcare system.
Two of the 53 infants (3.8%) with normal dipstick testing had bacteremia with the same organism causing UTI (Table 2). Both were admitted to the hospital at the time of initial evaluation, one for poor feeding and irritability and the other for a peripheral WBC count >15 000, which is one of the Intermountain EB-CPM high-risk criteria. None of the 53 infants had positive cerebrospinal fluid cultures. There were no adverse outcomes identified in these 53 infants with subsequent identification of UTI after initial negative dipstick screening.
Prediction of Outcomes on the Basis of Test Performance
Given the UTI prevalence in a population and screening test performance characteristics, we calculated the expected outcomes in a hypothetical cohort on the basis of institutional treatment paradigms. UTI prevalence in infants aged 29 to 90 days was 11.9%. If 1000 febrile infants undergo testing for UTI, 119 would have culture-positive UTI and 881 would not. If dipstick alone were used as a screen for UTI, 108 of 119 (90.4%) would screen positive and 55 of 881 (6.2%; 1-specificity) would screen false positive for UTI. These infants would all be expected to receive antibiotic treatment, and most would be admitted. If combined urinalysis were used, 113 of 119 (94.8%) would screen positive and 96 of 881 (10.9%; 1-specificity) would screen false positive for UTI. The addition of urine microscopy to dipstick testing in 1000 febrile infants would be predicted to correctly identify 5 additional UTIs and falsely predict UTI in 41 additional infants when compared with dipstick alone. Performing urine microscopy in this cohort would be expected to result in up to 8 febrile infants aged 29 to 90 days with a false-positive screen for UTI, and their additional health care utilization associated with a UTI diagnosis, for every 1 infant with UTI not identified by dipstick at the time of initial evaluation.
Discussion
We report data from the largest study of urinary diagnostic testing in febrile infants aged 1 to 90 days. In our study, dipstick performed well as a stand-alone screening test to rule out UTI, with an overall NPV of 98.7%. In cases in which dipstick screening was negative in infants later identified to have culture-confirmed UTI, the mean time to positive culture was 19.7 hours and no adverse events related to delayed treatment were documented. Although the addition of microscopy to dipstick increased the NPV to 99.2%, the combined testing would be predicted to cause additional false-positive results that might lead to unnecessary interventions, including hospital admission. Urine dipstick test alone may be an adequate screen for UTI in febrile infants aged 1 to 90 days while awaiting urine culture results.
Urine dipstick compared favorably with urine microscopy and combined urinalysis. On all measurements, dipstick was equivalent or superior to microscopy.
Dipstick NPV is statistically inferior to combined urinalysis (98.7% [95% CI: 98.6%–98.8%] vs 99.2% [99.1%–99.3%]; P < .001), but this difference may not be clinically significant. Dipstick has a superior PPV compared with combined urinalysis (66.8% [66.2%–67.4%] vs 51.2% [50.6%–51.8%]; P < .001). Dipstick testing does not require CLIA certification and can be performed rapidly in a variety of care settings by personnel with minimal training. Our data suggest that the use of dipstick without microscopy may be an effective screen and the use of dipstick alone could reduce the time required and costs associated with the laboratory evaluation of febrile infants.
Regardless of the UTI screening method used, a small number of UTIs in febrile infants aged 1 to 90 days will initially be missed. Febrile infants in our study with UTI and negative dipstick screening did not experience adverse outcomes. Eighty-three percent of the infants aged 29 to 90 days with UTI and false-negative dipstick were admitted on initial encounter. The infants who were discharged after the initial evaluation were given instructions for observation in the outpatient settings, decreasing the risk of unrecognized progression of their infection. The urine culture results were positive in <24 hours, and infants returned for additional evaluation and treatment in most cases. The expected rapid turnaround time for urine cultures offers the opportunity for close follow-up and initiation of antimicrobial therapy if indicated.
The risk of failing to identify UTI at initial encounter should be balanced with clinical risks to the patient and the potential increase in health care expenditures associated with incorrectly predicting UTI when none exists. Our data suggest that false-positive screens for UTI will be higher in febrile infants when urine microscopy is routinely performed. Up to 8 febrile infants aged 29 to 90 days would be predicted to have the additional health care utilization associated with a false-positive UTI diagnosis for every 1 infant with true UTI not identified by dipstick at the time of initial evaluation.
No urine-screening test was completely accurate in predicting UTI in our population. In addition, urine screening is not the only factor used to guide the management of febrile infants. Clinical appearance, other laboratory test results, health system, and social and geographic factors are also used in determining the disposition of febrile infants aged 29 to 90 days. Our data revealed that 83% of infants found to have UTI after normal dipstick screen, were admitted to the hospital at the time of initial evaluation. Microscopy also adds additional cost to the evaluation of the febrile infant and may also increase length of stay in the emergency department while awaiting results.
Although our study population had a prevalence of UTI (12%) similar to populations from previous publications,5,24,25 there are some differences in urinalysis test performance. The 90.8% sensitivity of laboratory-performed dipstick in this study is higher than previous estimates of 75% to 85%.9,16,26,27 However, these other studies defined UTI at a lower threshold of ≥10 000 CFUs per mL and/or emphasized infants and children older than 90 days.
Our study has several limitations. Data analysis was retrospectively performed from a data set collected for other purposes.3 Patients without urine culture or for whom both urine dipstick and microscopy were not performed were not included. Subjects with urine cultures considered indeterminate were excluded from analysis. Our use of centrifuged urine specimens for microscopy has been reported elsewhere9 but differs from other methods16 and does not include urine Gram-stain.28 Dipstick was performed in the clinical laboratory and automated colorimetric interpretation was used. The results may not be the same in point-of-care settings using visual dipstick interpretation. Although we excluded subjects with bag urine–labeled specimens, the limitations of the EDW did not allow us to confirm all samples obtained by urethral catheterization. We assume very few, if any, specimens were obtained by a method other than urethral catheterization because of the EB-CPM. Because UTI was defined by culture results alone and some of the subjects with UTI did not have pyuria, one may raise the question of asymptomatic bacteriuria. However, we believe very few, if any, of the infants in this study were asymptomatic because subjects were identified in the EDW by using a definition for fever and other diagnostic codes.21 A potential criticism of this analysis is that it does not include a subanalysis of cutoff points for microscopy. However, we elected to analyze urine microscopy by using the cutoffs used in the EB-CPM.
Our study is also limited by lack of chart review of all subjects, preventing an analysis of urinalysis test performance by clinical appearance. Although our EB-CPM is specifically written for well-appearing infants, the inclusion of ill-appearing infants would be expected to both increase the prevalence of UTI and result in an underestimate of NPV, which would strengthen rather than weaken our conclusions.
Conclusions
The urine dipstick test alone may be an adequate screen for UTI in febrile infants aged 29 to 90 days while urine culture is pending. Adding microscopy increased the NPV from 98.7% to 99.2% but potentially results in 8 false-positives for every UTI missed by dipstick. Additional health care resource utilization, antibiotic exposure, and cost may be incurred when urine microscopy is performed routinely.
Acknowledgments
We thank Ms Carolyn Reynolds and the Intermountain Healthcare Pediatric Clinical Program for their support of this work.
Glossary
- CFU
colony-forming unit
- CI
confidence interval
- CLIA
Clinical Laboratory Improvement Amendment
- EB-CPM
evidence-based care process model
- EDW
Enterprise Data Warehouse
- HPF
high-power microscopic field
- NPV
negative predictive value
- PCH
Primary Children’s Hospital
- PPV
positive predictive value
- SBI
serious bacterial infection
- UTI
urinary tract infection
- WBC
white blood cell
Footnotes
Drs Glissmeyer and Byington conceptualized and designed the study, acquired data by chart review, drafted the initial manuscript, and critically reviewed and revised the manuscript; Drs Schunk and Blaschke assisted with study design and interpretation of data and revised the manuscript critically; Mr Wilkes and Mr Korgenski assisted with the study design, acquired data by database query, analyzed data, and critically revised the manuscript; Dr Sheng provided expert statistical input for data analysis and critically revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Drs Byington and Sheng received support from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1RR025764, and the HA and Edna Benning Society. Drs Byington and Sheng and Mr Korgenski received support from the NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (K24-HD047249). Dr Blaschke received support through the National Institute of Allergy and Infectious Disease at the NIH, grant number 1K23AI079401. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Unrelated to urinary tract infection, Drs Byington and Blaschke have intellectual property in and receive royalties from BioFire Diagnostics, Inc. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662–1666 [DOI] [PubMed] [Google Scholar]
- 2.Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11(4):257–264 [DOI] [PubMed] [Google Scholar]
- 3.Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1). Available at: www.pediatrics.org/cgi/content/full/130/1/e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr. 1985;107(6):855–860 [DOI] [PubMed] [Google Scholar]
- 5.Zorc JJ, Levine DA, Platt SL, et al. Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005;116(3):644–648 [DOI] [PubMed] [Google Scholar]
- 6.Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS ONE. 2010;5(8):e12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baraff LJ, Bass JW, Fleisher GR, et al. Agency for Health Care Policy and Research . Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med. 1993;22(7):1198–1210 [DOI] [PubMed] [Google Scholar]
- 8.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36(6):602–614 [DOI] [PubMed] [Google Scholar]
- 9.Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60–65 [DOI] [PubMed] [Google Scholar]
- 10.Hoberman A, Wald ER, Penchansky L, Reynolds EA, Young S. Enhanced urinalysis as a screening test for urinary tract infection. Pediatrics. 1993;91(6):1196–1199 [PubMed] [Google Scholar]
- 11.Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994;124(4):513–519 [DOI] [PubMed] [Google Scholar]
- 12.Tests granted waived status under CLIA. Available at: www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/waivetbl.pdf. Centers for Medicare and Medicaid Services. Accessed January 12, 2014
- 13.Mori R, Yonemoto N, Fitzgerald A, Tullus K, Verrier-Jones K, Lakhanpaul M. Diagnostic performance of urine dipstick testing in children with suspected UTI: a systematic review of relationship with age and comparison with microscopy. Acta Paediatr. 2010;99(4):581–584 [DOI] [PubMed] [Google Scholar]
- 14.Gorelick MH, Shaw KN. Screening tests for urinary tract infection in children: a meta-analysis. Pediatrics. 1999;104(5). Available at: www.pediatrics.org/cgi/content/full/104/5/e54 [DOI] [PubMed] [Google Scholar]
- 15.Shaw KN, Hexter D, McGowan KL, Schwartz JS. Clinical evaluation of a rapid screening test for urinary tract infections in children. J Pediatr. 1991;118(5):733–736 [DOI] [PubMed] [Google Scholar]
- 16.Shaw KN, McGowan KL, Gorelick MH, Schwartz JS. Screening for urinary tract infection in infants in the emergency department: which test is best? Pediatrics. 1998;101(6). Available at: www.pediatrics.org/cgi/content/full/101/6/E1 [DOI] [PubMed] [Google Scholar]
- 17.Sharief N, Hameed M, Petts D. Use of rapid dipstick tests to exclude urinary tract infection in children. Br J Biomed Sci. 1998;55(4):242–246 [PubMed] [Google Scholar]
- 18.Wiggelinkhuizen J, Maytham D, Hanslo DH. Dipstick screening for urinary tract infection. S Afr Med J. 1988;74(5):224–228 [PubMed]
- 19.Marsik FJ, Owens D, Lewandowski J. Use of the leukocyte esterase and nitrite tests to determine the need for culturing urine specimens from a pediatric and adolescent population. Diagn Microbiol Infect Dis. 1986;4(2):181–183 [DOI] [PubMed] [Google Scholar]
- 20.Woodward MN, Griffiths DM. Use of dipsticks for routine analysis of urine from children with acute abdominal pain. BMJ. 1993;306(6891):1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gesteland PVK, Korgenski EK, Raines B, Byington C. A Method for Identifying Febrile Infants Presenting to the Emergency Department Using Administrative Data. Toronto, Canada: Pediatric Academic Societies; 1997 [Google Scholar]
- 22.Long SS, Pickering LK, Prober CG. Principles and Practice of Pediatric Infectious Disease. Edinburgh, United Kingdom: Churchill Livingstone; 2012 [Google Scholar]
- 23.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. Oct 15 2005;21(20):3940–3941 [DOI] [PubMed] [Google Scholar]
- 24.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302–308 [DOI] [PubMed] [Google Scholar]
- 25.Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44–54 [DOI] [PubMed] [Google Scholar]
- 26.Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants < or = 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12–14 [DOI] [PubMed] [Google Scholar]
- 27.Kazi BA, Buffone GJ, Revell PA, Chandramohan L, Dowlin MD, Cruz AT. Performance characteristics of urinalyses for the diagnosis of pediatric urinary tract infection. Am J Emerg Med. 2013;31(9):1405–1407 [DOI] [PubMed] [Google Scholar]
- 28.Lockhart GR, Lewander WJ, Cimini DM, Josephson SL, Linakis JG. Use of urinary gram stain for detection of urinary tract infection in infants. Ann Emerg Med. 1995;25(1):31–35 [DOI] [PubMed] [Google Scholar]