Abstract
Aim:
To investigate the effects of punicalagin, a polyphenol isolated from Punica granatum, on human U87MG glioma cells in vitro.
Methods:
The viability of human U87MG glioma cells was evaluated using MTT assay. Cell cycle was detected with flow cytometry analysis. The levels of Bcl-2, cleaved caspase-9, cleaved poly(ADP-ribose) polymerase (PARP), phosphor-AMPK and phosphor-p27 at Thr198 were measured using immunoblot analyses. Caspase-3 activity was determined with spectrophotometer. To determine autophagy, LC3 cleavage and punctate patterns were examined.
Results:
Punicalagin (1-30 μg/mL) dose-dependently inhibited the cell viability in association with increased cyclin E level and decreased cyclin B and cyclin A levels. The treatment also induced apoptosis as shown by the cleavage of PARP, activation of caspase-9, and increase of caspase-3 activity in the cells. However, pretreatment of the cells with the pan-caspase inhibitor z-DEVD-fmk (50 μmol/L) did not completely prevent the cell death. On the other hand, punicalagin treatment increased LC3-II cleavage and caused GFP-LC3-II-stained punctate pattern in the cells. Suppressing autophagy of cells with chloroquine (1-10 μmol/L) dose-dependently alleviated the cell death caused by punicalagin. Punicalagin (1-30 μg/mL) also increased the levels phosphor-AMPK and phosphor-p27 at Thr198 in the cells, which were correlated with the induction of autophagic cell death.
Conclusion:
Punicalagin induces human U87MG glioma cell death through both apoptotic and autophagic pathways.
Keywords: punicalagin, human glioma, autophagy, apoptosis, microtubule-associated protein light chain 3, AMPK, p27, z-DEVD-fmk, chloroquine
Introduction
Pomegranate fruit extracts possess antioxidant and anti-atherosclerotic activities and exert cancer chemotherapeutic effects against a number of cancers in humans1,2,3. Pomegranate fruit extract induces the expression of the cyclin kinase inhibitors (CKI) p21/WAF1 and p27/KIP1, but decreases the expression of cyclins E, D1, and D2, and cyclin-dependent kinases (Cdks) 2, 4, and 6, suggesting that the alterations in the CKI-cyclin-Cdk network are involved in the extract's anti-proliferative and pro-apoptotic effects4. Punicalagin is the major antioxidant polyphenol ingredient found in the fruit of the Punica granatum L. tree. Punicalagin has been shown to induce apoptosis in human promyelocytic leukemia HL-60 cells5, HT-29 and HCT116 colon cancer lines2, and colon adenocarcinoma Caco-2 cells6. However, the ability of punicalagin to induce autophagic cell death has not been reported.
Autophagy is a catabolic pathway that increases the turnover of intracellular components by fusing autophagosomes to the lysosome. Autophagosomes are intracellular double-membraned vesicles that encompass damaged organelles and macronutrients and are formed under conditions of ischemia or limited nutrients7. Chloroquine, a lysosomal trophic agent, has been shown to inhibit the fusion of autophagosomes with the lysosome and can thereby suppress autophagy8.
The molecular machinery of autophagy has been elucidated in yeast9. There are 11 autophagy-related genes (Atg) in yeast and 8 orthologs in mammals. Among these genes, Atg8/LC3 and Atg12 are ubiquitin-like proteins. Atg8/LC3, microtubule-associated protein light chain 3, is cleaved at the C-terminal by the Atg4 protease to generate cytosolic LC3-I. LC3-I is then conjugated to phosphatidylethanolamine (PE) to form LC3-phosphatidylethanolamine conjugate (LC3-II), which is further recruited to the membranes of the autophagosomes. The amount of LC3-II present correlates with the number of autophagosomes10. Atg7 is the homologue of an ubiquitin activating enzyme, and the other Atg proteins function during vesicle formation. Autophagy can be regulated by many pathways11. The classical pathway acts through the class III phosphatidylinositol 3-kinase (PI3K-III), which modulates autophagy via the mammalian target of rapamycin (mTOR)11,12. Inhibition of the mTOR-dependent signaling pathway by rapamycin or by the energy sensing AMP-activated protein kinase (AMPK) causes autophagy in both yeast and mammalian cells13,14. Active AMPK has been shown to phosphorylate the cyclin-dependent kinase inhibitor p27 (Kip1) at Thr 198, leading to an increase in p27 stability. Therefore, disrupted nutrient and energy metabolism is linked to cell-cycle progression through the AMPK/p27 signaling pathway15.
We demonstrated that punicalagin induced apoptosis through the activation of the caspase-9/caspase-3 cascade and through the cleavage of poly(ADP-ribose) polymerase (PARP) in U87MG cells. We also showed that punicalagin increased the formation of autophagosomes and the accumulation of LC3-II. Because punicalagin increases the phosphorylation of AMPK and p27T198, it is possible that punicalagin induces autophagic cell death through the AMPK/p27 signaling pathway. The results of the present study indicate that punicalagin-induced cell death is mediated through both the apoptotic and autophagic pathways.
Materials and methods
Materials
Punicalagin was kindly provided by Dr Ta-chen LIN at the Central Taiwan University of Science and Technology in Taiwan. Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS), glutamine, gentamycin, penicillin, and streptomycin were purchased from Life Technologies (Gaithersburg, USA). Benzyloxycarbonyl-Asp-Glu-Val-Ala-Asp (OMe) fluoromethyl ketone (z-DEVD-fmk) was obtained from Sigma-Aldrich Chemical Co (St Louis, USA). Antibodies specific to cyclins A, B, and E, Cdk2, p21CIP1, p27KIP1, PARP, Bcl-2, caspase-9, phosphorylated AMPK, total AMPK, LC3, and α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, Santa Cruz, USA). Horseradish peroxidase-conjugated anti-rabbit IgG antibody was purchased from Bio-Rad (Hercules, USA). Protease inhibitor cocktail tablets were purchased from Boehringer Mannheim (Mannheim, Germany). The human U87MG cell line (ATCC No HTB-14) was purchased from the Institute of Food Sciences (Hsin-Chu, Taiwan, China).
Culture of human glioma cells and preparation of cell lysates
U87MG cells were cultured in DMEM supplemented with 13.1 mmol/L NaHCO3, 13 mmol/L glucose, 2 mmol/L glutamine, 10% heat-inactivated FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were attached to Petri dishes after 24 h incubation and were maintained in a humidified incubator with 5% CO2 at 37 °C. After reaching confluence, the cells were treated with various concentrations of punicalagin and incubated in a humidified incubator at 37 °C for the indicated time intervals. At the end of the incubation, the cell lysates were lysed in lysis buffer containing 10 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EGTA, 1 mmol/L MgCl2, 1 mmol/L sodium orthovanadate, 1 mmol/L DTT, 0.1% mercaptoethanol, 0.5% Triton X-100, and the protease inhibitor cocktail, with final concentrations of 0.2 mmol/L PMSF, 0.1% aprotinin, and 50 μg/mL leupeptin. The lysates were stored at -70 °C for further measurements.
Polyacrylamide gel electrophoresis, immunoblotting, and caspase-3 assay
Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were electrotransferred onto a polyvinyldifluoride (PVDF) membrane. After transfer, the PVDF membrane was washed once with phosphate-buffered saline (PBS) and twice with PBS plus 0.1% Tween 20 (PBST). The PVDF membrane was then blocked with blocking solution containing 5% nonfat dry milk in PBST for 1 h at room temperature. The PVDF membrane was then probed with primary antibodies in the blocking buffer, washed again in PBST, and subsequently incubated with peroxidase-conjugated anti-mouse IgG antibodies for 1 h. The results were visualized using a chemiluminescence kit (Amersham, USA). To determine the caspase-3 activity of the cells, 60 μL cell lysate (∼5×104 cells) was diluted with an equal volume of reaction buffer (40 mmol/L HEPES, pH 7.5, 20% glycerol, 4 mmol/L DTT, and 400 μmol/L Ac-DEVD-pNA substrate; Calbiochem, USA). The mixture was incubated at 37 °C for 3 h, and the caspase activity was determined by measuring the optical density at 405 nm in a spectrophotometer.
MTT assay and determination of the autophagic LC3 punctate pattern
Cell viability, an indication of cytotoxicity, was evaluated using an MTT assay. Glioma cells grown on 150 mm plates were washed twice with PBS and resuspended in DMEM. The suspended cells were plated on 24-well plates at a density of 2×105 cells/well and were treated with the indicated reagent(s) for 24 h. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to the medium at a final concentration of 1 mg/mL and incubated at 37 °C for 4 h. Dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals, and the absorbency of each well was measured at 570 nm. To determine the punctate pattern of microtubule-associated protein light chain 3 (LC3), the cells were transfected with GFP-LC3 or EGFP construct using the Lipofectamine reagent (Invitrogen, USA), followed by treatment with 10 μg/mL punicalagin or the same volume of DMSO for 24 h. The cells were fixed with 4% paraformaldehyde, and fluorescence images were taken.
PI staining and flow cytometry
Cells were treated with different concentrations of punicalagin for 24 h, fixed with alcohol, and stained with 50 μg/mL propidium iodide (PI) to determine the DNA content. Apoptosis was detected using flow cytometry analysis.
Statistical analysis
The data are expressed as the mean±SEM of triplicate experiments. Comparisons between groups were made using Student's t-test, and a P value <0.05 was considered statistically significant.
Results
Punicalagin inhibits growth of U87MG glioma cells
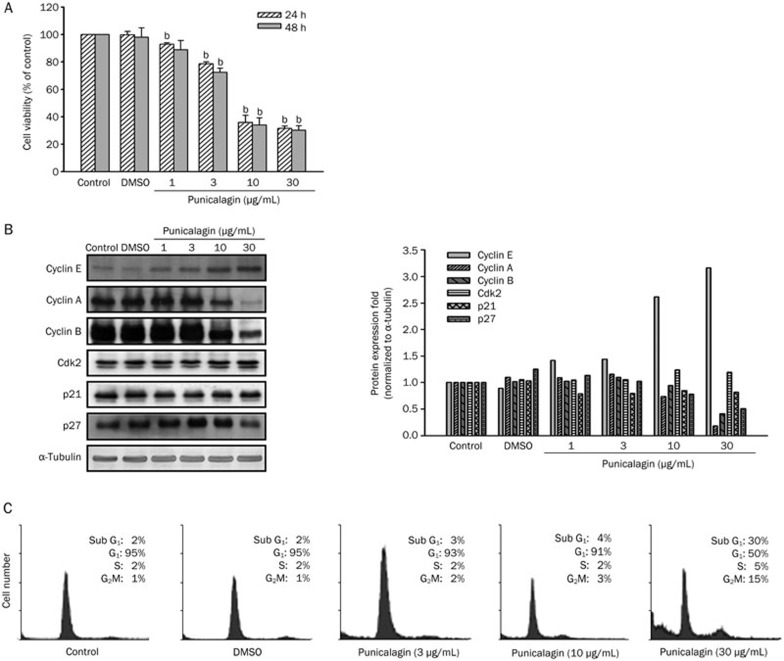
To determine whether punicalagin exerts anti-proliferative effects, U87MG cells were incubated with different concentrations of punicalagin, and the viable cell number was determined using an MTT assay. Treatment of cells with punicalagin resulted in a decrease in the number of viable cells after 24 or 48 h (Figure 1A, P<0.05). Because cell-cycle progression is regulated by the activity of cyclin/cyclin-dependent kinase (Cdk) complexes, uncontrolled expression of cyclins and/or Cdks may lead to either tumorigenesis or cell-cycle arrest. We next examined whether punicalagin affected cyclin/cdk expression. Treatment of cells with punicalagin down-regulated the expression of cyclins A and B, but up-regulated cyclin E expression in a dose-dependent manner (Figure 1B). Punicalagin 10 μg/mL also induced p27Kip1, but not p21Cip1, expression in U87MG cells (Figure 1B). Altered expression of the cyclins and Cdk inhibitors were associated with cell-cycle arrest and an accumulation of sub-G1 cells as determined by flow cytometry (Figure 1C).
Figure 1.
Punicalagin-induced cell death and growth inhibition of U87MG glioma cells. (A) Cell viability after 24 or 48 h of incubation with different concentrations of punicalagin was determined using an MTT assay, and the data are presented as the mean±SEM of triplicate experiments. The viability of untreated cells was included as 100% control. A significant difference (bP<0.05) between the experimental group and the control. (B) Cells were treated with different concentrations of punicalagin for 24 h, and the lysates were collected and immunoblotted with antibodies specific for cyclins A, B, and E, Cdk2, p21, p27, and α-tubulin. Protein quantitation data are located in the right panel and were normalized to α-tubulin. (C) Cell cycle distribution from cultures treated with different concentrations of punicalagin for 24 h. The cell cycle assignment was based on the DNA content detected following PI staining using flow cytometry analysis and was representative of more than two experiments.
Punicalagin causes apoptotic cell death in U87MG cells
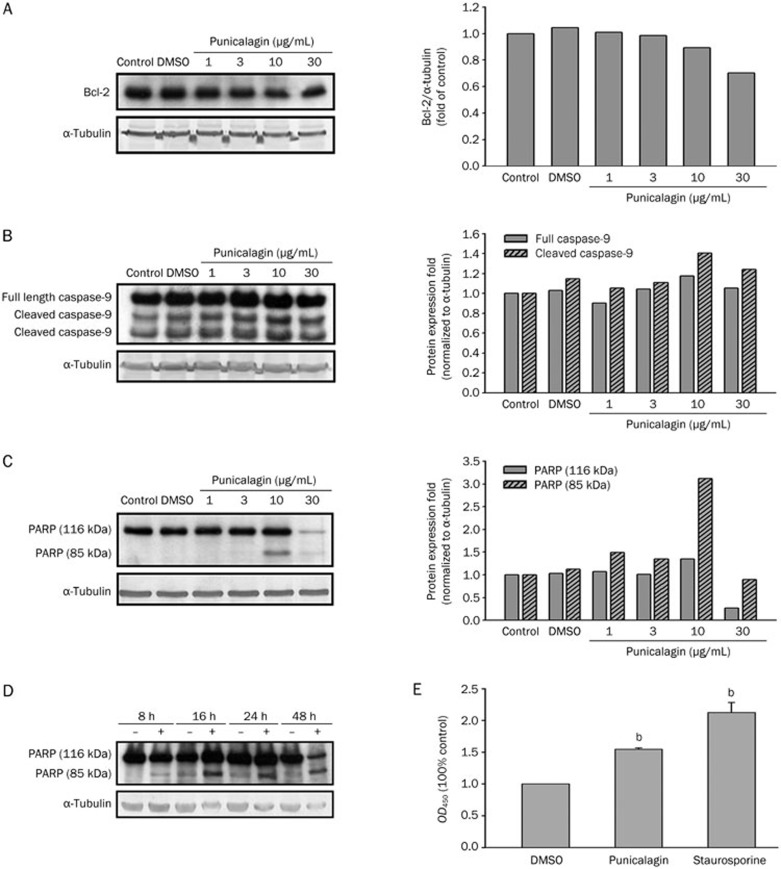
Apoptosis, the process of programmed cell death, is morphologically characterized by membrane blebbing, cell shrinkage, chromatin condensation, and DNA fragmentation, as well as changes in mitochondrial and caspase activities. To investigate whether punicalagin induced apoptosis in U87MG cells, apoptotic features, such as the expression of Bcl-2, the cleavage of caspase-9 and poly(ADP-ribose) polymerase (PARP), and the activation of caspase-3, were examined. When cells were treated with different concentrations of punicalagin (1, 3, 10, or 30 μg/mL), the abundance of Bcl-2 protein decreased, and the cleaved forms of caspase-9 and PARP increased (Figure 2A–2C); the cleavage of caspase-9 was also determined to occur in a time-dependent manner (Figure 2D). The progression of apoptosis was also reflected by an increase in caspase-3 activity (Figure 2E, P<0.05), as determined by incubation with the Ac-DEVD-pNA substrate.
Figure 2.
Molecular features of apoptosis induced by punicalagin. U87MG cells were treated with different concentrations of punicalagin for 24 h, and the cell lysates were prepared and immunoblotted with antibodies to detect the protein amounts of (A) Bcl-2, (B) cleaved caspase-9, and (C, D) cleaved PARP; cells treated with DMSO were included as a control. (E) Cells were treated with 20 μg/mL punicalagin or DMSO for 24 h, and lysates were incubated with 200 μmol/L caspase-3 substrate Ac-DEVD-pNA at 37 °C for 3 h. The caspase activity of the punicalagin-treated cells was determined by reading the optical density at 405 nm, and the samples were normalized to the DMSO-treated cells. The activity of the cells treated with 100 nmol/L staurosporine was included as a control. Bars represent the mean±SEM of triplicate measurements. bP<0.05 versus DMSO control. Protein quantitation data are located in the right panels and were normalized to α-tubulin (A–C).
Punicalagin induces autophagy in U87MG cells
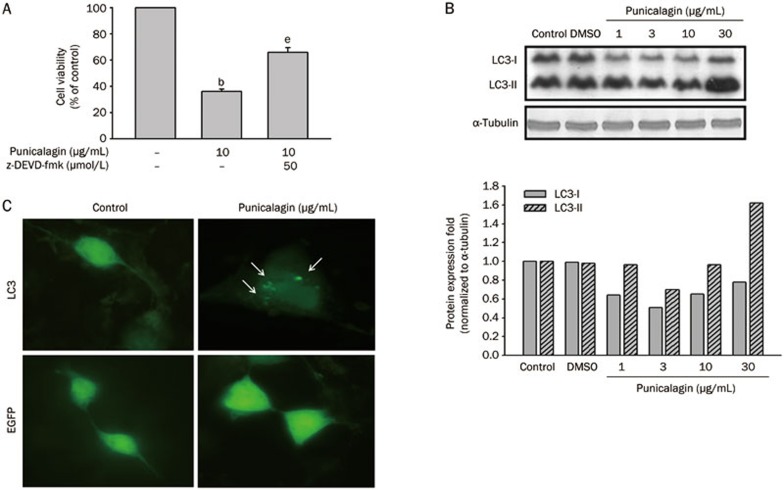
Autophagy, also known as type II cell death, is a process used to maintain homeostasis between synthesis and degradation and involves the degradation of intracellular long-lived proteins or damaged organelles via the lysosomal machinery. To further investigate whether punicalagin alternatively induced cell death through a non-apoptotic pathway, U87MG cells were pretreated with the pan-caspase inhibitor z-DEVD-fmk prior to incubation with punicalagin. As shown in Figure 3A, punicalagin-induced cell death was not completely inhibited by pretreatment with z-DEVD-fmk, indicating that the cell death response was not fully derived from the activation of the caspases. To determine the possibility of punicalagin-induced autophagy, cells were treated with different concentrations of punicalagin, and the accumulation of microtubule-associated protein light chain 3 II (LC3-II, with a molecular weight of 16 kDa) was analyzed by immunoblot analysis (Figure 3B). When treated with 30 μg/mL punicalagin, the transient accumulation of the short half-lived LC3-II, converted from LC3-I (18 kDa), was observed in association with a decrease in the amount of LC3-I protein. On the other hand, U87MG cells expressing green fluorescence-LC3 fusion proteins (GFP-LC3) were used to obtain independent evidence that supports the conclusion that punicalagin triggered autophagy. As shown in Figure 3C, the punctate fluorescence pattern of GFP-LC3 observed in the punicalagin-treated cells is presumably due to the incorporation of the GFP-LC3 into autophagosomes; this pattern was not observed in the control cells, indicating the progression of autophagy.
Figure 3.
Punicalagin induces autophagic cell death in U87MG cells. (A) Cells were pretreated with or without z-DEVD-fmk for 15 min prior to treatment with 10 μg/mL punicalagin for 24 h. Cell viability was determined using an MTT assay, and the viability of the untreated cells was measured as the 100% control. The data are presented as the mean±SEM of triplicate experiments. bP<0.05 vs the 100% control. eP<0.05 vs the 100% control and the inhibitor control. (B) Cells were treated with 10 μg/mL punicalagin for 24 h, and the lysates were collected and immunoblotted with antibodies specific for LC3 and α-tubulin. Protein quantitation data are located in the right panel and were normalized to α-tubulin. (C) Cells were transfected with GFP-LC3 or enhanced green fluorescence protein (EGFP) and were then treated with 10 μg/mL punicalagin for 24 h. All images were taken with an Olympic fluorescence microscope (Model IX71) immediately after fixation by paraformaldehyde. Arrows indicate the punctate dots.
Chloroquine protects U87MG cells from punicalagin-induced autophagic cell death
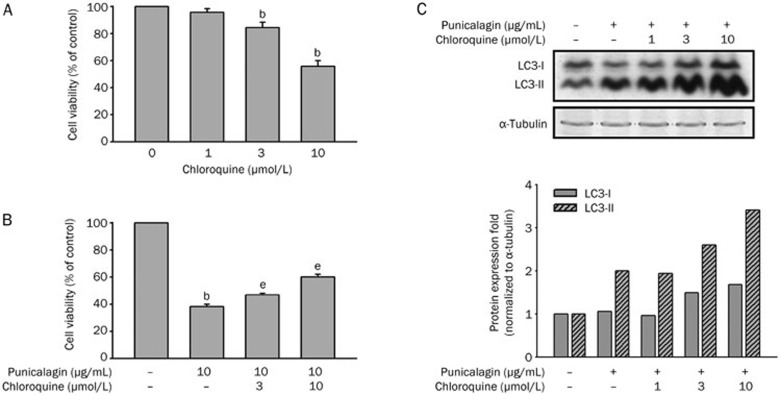
Chloroquine, a lysosomotropic agent, has been shown to increase the intra-lysosomal pH and destroy the lysosomal enzymes, which inhibits the fusion of the autophagosome with the lysosome, thereby suppressing autophagy16,17. Chloroquine treatment exhibited a mild cytotoxic effect in U87MG cells (Figure 4A, P<0.05). To examine whether the inhibition of autophagy by chloroquine protects the cells from punicalagin-induced autophagic cell death, U87MG cells were pretreated with different concentrations of chloroquine for 30 min prior to incubation with 10 μg/mL punicalagin for 48 h. The results showed that chloroquine prevented the punicalagin-induced cell death (Figure 4B, P<0.05) and caused a more significant increase in the accumulation of LC3-II (Figure 4C).
Figure 4.
Punicalagin-induced autophagic cell death is blocked by chloroquine. (A) U87MG cells were treated with different concentrations of chloroquine, and the cell viability was determined using an MTT assay. Data represent the mean±SEM of three independent experiments. bP<0.05 vs the control. (B) U87MG cells were pretreated without or with different concentrations of chloroquine (0–10 μmol/L) for 15 min prior to incubation in the presence or absence of 10 μg/mL punicalagin for 24 h. Cell viability was determined using an MTT assay, and the data are presented as the mean±SEM of three independent experiments. bP<0.05 vs the 100% control. eP<0.05 vs the 100% control and the chloroquine control. (C) U87MG cells were pretreated with or without different concentrations of chloroquine (0−10 μmol/L) for 15 min prior to incubation in the presence or absence of 10 μg/mL punicalagin for 24 h. Cell lysates were collected and immunoblotted with antibodies specific for LC3 and α-tubulin. Protein quantitation data are located in the right panel and were normalized to α-tubulin.
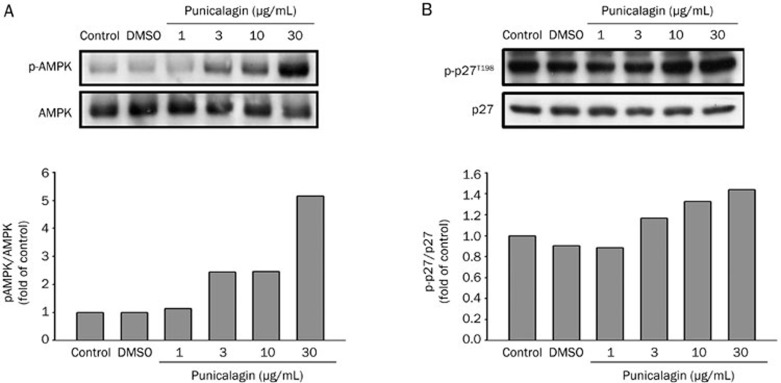
Punicalagin increases AMPK and p27T198 phosphorylation
AMP-activated protein kinase (AMPK) has emerged as a critical regulator of autophagy, possibly through the regulation of the mTOR-dependent signaling pathway18,19. Compound C, an inhibitor of AMPK, or a dominant-negative form of AMPK, have been reported to largely inhibit autophagy in mammalian hepatocyte, HT29, and HeLa cells20. We therefore investigated whether punicalagin-induced autophagy was associated with increased AMPK activity in U87MG cells. Activation of AMPK, as noted by increased phosphorylation, was observed when cells were treated with different concentrations of punicalagin for 15 min; this result was observed in a dose-dependent manner (Figure 5A). Furthermore, increased phosphorylation of p27 at threonine 198 has been implicated as an autophagic signal15. Our results showed that punicalagin elicited a dose-dependent increase in p27 phosphorylation at Thr 198 (Figure 5B), supporting the hypothesis that the induction of autophagy by punicalagin is through the LKB1-AMPK-p27 signaling pathway.
Figure 5.
Punicalagin increases the phosphorylation of AMPK and p27Kip1(Thr198) . U87MG cells were treated with different concentrations of punicalagin for 15 min and lysed. Cell lysates were electrophoresed and probed using specific antibodies to (A) phospho-AMPK or (B) p27Kip1 (Thr198). These experiments were repeated with similar results. Protein quantitation data are located in the right panel and were normalized to the amount of total protein.
Discussion
Punicalagin is a polyphenol found in many fruits, vegetables, and derived products such as wine and tea. The antiproliferative and pro-apoptotic effects of pomegranates have been attributed to the presence of polyphenols in the fruit's extract. The anticancer effects of punicalagin have been shown in many cancer cell lines2,5,6. Our results are in line with many previous reports that have shown the association of the punicalagin's antiproliferative effects with the up-regulation of cyclin E and the down-regulation of cyclins A and B, resulting in cell cycle arrest at the G2-S checkpoint in many cells4,6. Based on the results in Figure 3C, punicalagin-treated cells were arrested in G2/M phase. The percentage of the arrested cells increased from 2% to 15% when the cells were incubated with punicalagin at concentrations ranging from 3 to 30 μg/mL. In addition, the percentage of cells in the sub-G1 phase increased from 3% to 30%; the sub-G1 population represents the cells that are in the late stages of apoptosis. However, cyclin E is a positive regulator of cell cycle progression and is expressed during late G1 phase of the cell cycle; cyclin E mediates the initiation of DNA synthesis by activating cyclin-dependent kinase 2 (Cdk2). Abnormally high expression levels of cyclin E have frequently been found in cancer cells21. Up-regulation of cyclin E and p27Kip1 expression was considered to be potentially useful in predicting the prognosis of patients with astrocytomas22. Transfection of short hairpin RNAs (shRNA) against cyclin E induces apoptosis in cancer cells and abolishes the ability of U-373-MG glioma cells to induce tumor growth in nude mice23. These contradictory findings pave the way for additional experiments to elucidate whether cyclin E is specifically expressed in cells undergoing autophagy or apoptosis and to determine a potential role of cyclin E and increased p27 phosphorylation at Thr 198 in autophagy.
Malignant gliomas are resistant to various pro-apoptotic therapies, such as radiotherapy and conventional chemotherapy. In this study, we showed that punicalagin was cytotoxic to human glioma cells by inducing both apoptotic and autophagic cell death. When cells were pretreated with the caspase inhibitor z-DEVD-fmk, only a portion of the observed cell death was prevented, indicating that punicalagin may also activate non-apoptotic cell death. These results point to autophagy or type II cell death as another possible mode of cell death induced by punicalagin. Consistently, incubation of cells with punicalagin resulted in an increase in LC3-I cleavage, and the inhibition of autophagy by chloroquine partly reversed the punicalagin-induced cell death.
The autophagosome fuses with the lysosome to become the autolysosome, in which lysosomal hydrolases degrade the sequestered cellular constituents12. Therefore, cells degrade and recycle macronutrients or damaged subcellular organelles through autophagy, which enhances cell survival under a limited supply of nutrients and oxygen. Excessive autophagy, however, may result in autophagic cell death. Whether autophagy represents a mechanism that allows tumor cells to survive from therapy or a mechanism for initiating a non-apoptotic form of programmed cell death is strongly debated24. Autophagy may serve as a survival pathway in tumor cells treated with apoptosis activators, and inhibition of autophagy by chloroquine has been used to enhance the ability of either p53 activation or alkylating drug therapies to induce tumor cell death25. Chloroquine, a weak base, is concentrated in the acidic lysosomal vesicles and raises the intra-lysosomal pH, thereby disrupting the function of the lysosomal enzymes required for autophagy. Indeed, chloroquine may improve mid-term survival when used as an adjuvant in combination with conventional therapy for glioblastoma multiforme26,27. However, this study showed that chloroquine alleviated, instead of enhanced, the punicalagin-induced cell death, suggesting punicalagin may initiate autophagic cell death. Consistent with our findings, autophagic cell death can be induced by arsenic trioxide, rapamycin, or cucurmin in malignant glioma cells28,29,30,31.
The mTOR kinase has been demonstrated to be a critical regulator of autophagy; activation of mTOR via Akt and MAPK signaling suppresses autophagy, and the suppression of mTOR via AMPK and p53 signaling promotes autophagy. The role of AMPK in determining autophagy appears to be controversial. Activation of AMPK by metformin or 5′-amino-4-imidazolecarboxamide (AICAR) has been shown to exert cytoprotective effects in cadiomyocytes and neuronal cells32,33, and AMPK activation can arrest cell cycle progression and induce cell apoptosis in prostate cancer34 and a number of chemo-resistant colon cancer cells35. Our data suggest that AMPK may mediate multiple mechanisms underlying the in vitro anticancer effects of punicalagin. Treatment of cells with punicalagin resulted in many molecular features of apoptosis, and we demonstrated that although punicalagin can trigger apoptosis, an alternative pathway of cell death, autophagy, is also activated. AMPK has been considered as an universal regulator of autophagy19. AMPK can be activated by liver kinase B1 (LKB1) or by various Ca2+ mobilizing agents; active AMPK inhibits the mammalian target of rapamycin complex 1 (mTORC1), resulting in cell autophagy. In this study, we showed that punicalagin activated AMPK and, at the same time, increased the phosphorylation of p27 at Thr 198. Although the detailed mechanism underlying autophagy induction remains unclear, our data agree with the results of Liang et al, who showed that activation of the LKB1-AMPK pathway increases the phosphorylation of p27Kip1 at Thr 198, resulting in the stabilization of p27Kip1 and subsequently the induction of cell autophagy15.
In summary, we have identified a novel activity for punicalagin in human glioma cells, namely, the ability to induce apoptosis and autophagy. Therefore, punicalagin may be useful in the development of adjuvant therapies to treat glioma.
Author contribution
Shyang-guang WANG, Horng-mo LEE, and Yuan-nian HSU designed the experiments. Ming-hung HUANG, Jui-hsiang LI, and Fu-i LAI performed the experiments. Shyang-guang WANG, Horng-mo LEE, and Yuan-nian HSU analyzed the data. Shyang-guang WANG and Horng-mo LEE wrote the manuscript.
Acknowledgments
This work was supported by a joint research grant from Central Taiwan University of Science and Technology and Taoyuan General Hospital (Grant No DOH100-HO-2060) in Taiwan. We would like to thank Dr Ta-chen LIN for providing the punicalagin.
References
- Mehta R, Lansky EP. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur J Cancer Prev. 2004;13:345–8. doi: 10.1097/01.cej.0000136571.70998.5a. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;5:371–3. doi: 10.4161/cc.5.4.2486. [DOI] [PubMed] [Google Scholar]
- Chen LG, Huang WT, Lee LT, Wang CC. Ellagitannins from Terminalia calamansanai induced apoptosis in HL-60 cells. Toxicol In Vitro. 2009;23:603–9. doi: 10.1016/j.tiv.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Larrosa M, Tomás–Barberán FA, Espín JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611–25. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;11:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Klocke BJ, Roth KA. Autophagy, bafilomycin and cell death: the “a-B-cs” of plecomacrolide-induced neuroprotection. Autophagy. 2006;2:228–30. doi: 10.4161/auto.2703. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Noda T, Ohsumi Y. Interrelationships among Atg proteins during autophagy in Saccharomyces cerevisiae. Yeast. 2004;21:1057–65. doi: 10.1002/yea.1152. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–12. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy. 2007;3:238–40. doi: 10.4161/auto.3710. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Autophagy: regulation by energy sensing. Curr Biol. 2011;21:R227–9. doi: 10.1016/j.cub.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, et al. The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- Fedorko M. Effect of chloroquine on morphology of cytoplasmic granules in maturing human leukocytes — an ultrastructural study. J Clin Invest. 1967;46:1932–42. doi: 10.1172/JCI105683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;25:220–33. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy. Autophagy. 2007;3:381–3. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–9. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Herliczek TW. The role of cyclin E in cell proliferation, development and cancer. Prog Cell Cycle Res. 1997;3:171–91. doi: 10.1007/978-1-4615-5371-7_14. [DOI] [PubMed] [Google Scholar]
- Tamiya T, Mizumatsu S, Ono Y, Abe T, Matsumoto K, Furuta T, et al. High cyclin E/low p27Kip1 expression is associated with poor prognosis in astrocytomas. Acta Neuropathol (Berl) 2001;101:334–40. doi: 10.1007/s004010000261. [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Izquierdo M. Cyclin E1 knockdown induces apoptosis in cancer cells. Neurol Res. 2006;28:493–9. doi: 10.1179/016164106X115143. [DOI] [PubMed] [Google Scholar]
- Marx J. Autophagy: is it cancer's friend or foe. Science. 2006;26:1160–1. doi: 10.1126/science.312.5777.1160. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceno E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus. 2003;14:e3. doi: 10.3171/foc.2003.14.2.4. [DOI] [PubMed] [Google Scholar]
- Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–43. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–8. [PubMed] [Google Scholar]
- Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- Iwamaru A, Kondo Y, Iwado E, Aoki H, Fujiwara K, Yokoyama T, et al. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;22:1840–51. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally. J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Kewalramani G, Chan JK, Qi D, Ghosh S, Pulinilkunnil T, et al. Metformin influences cardiomyocyte cell death by pathways that are dependent and independent of caspase-3. Diabetologia. 2006;49:2174–84. doi: 10.1007/s00125-006-0338-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil M, Bertini F, Pesi R, Voccoli V, Tozzi MG, Camici M. 5′-Amino-4-imidazolecarboxamide riboside induces apoptosis in human neuroblastoma cells via the mitochondrial pathway. Nucleosides Nucleotides Nucleic Acids. 2006;25:1265–70. doi: 10.1080/15257770600890905. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–21. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]