Abstract
The present paper focuses on electrical impedance scanning. The basic science behind the new modality, measurements of breast tissue impedance in vivo and in vitro, and the studies performed with a newly available commercial machine are discussed. Electrical impedance scanning has been generating interest for several reasons, including comfort to the patient, the relatively low cost, and studies suggest that it may be effective in detecting disease in mammographically dense breasts.
Keywords: dielectric properties, electrical impedance scanning
Introduction
Electrical impedance scanning (EIS) maps are a measure of the electrical properties of an object made through surface measurements. The information is obtained quickly and comfortably, and the method shows promise for detecting cancers that may have previously gone undetected. The technology of electrical impedance measurements in the detection of disease has become a topic of great interest for engineering and physiological scientists. As evidence of this, recent issues of IEEE Transactions on Medical Imaging (June 2002) and of Physiological Measurement (February 2001) have been dedicated solely to these topics. Can the technology be brought out of the laboratory? The following sections present information about why EIS may be useful in breast cancer detection and the clinical results to date.
Electrical properties of biological tissues
Electrical properties of living tissue
Interest and experimentation in the electrical properties of tissues began in the late 1800s. This interest has evolved into a branch of physiology and the research has lead to insight into various mechanisms. This section provides an overview of some electrical terminology that is used in tissue impedance studies.
First are basic explanations of resistance (R), conductance (σ), permittivity (ε) and capacitance (C). Resistance is a property that opposes current flow, and conductance is the inverse of resistance. Capacitance is a property that opposes a change in voltage or electric potential across an object and acts to store energy. A capacitor consists of two conductors, each oppositely charged and separated by a dielectric material. Permittivity is a property of the dielectric material and reflects the ability of charges in the material to move in response to an electric field. Capacitance is a function of the permittivity and the physical geometry of the object. For example, the capacitance formula for a two-plate capacitor is C = εA / d, where A is the area of each plate and d is the distance between them. The complex impedance (Z) of an RC series circuit can be described as Z = R + jXc, where Xc = 1 / ωC (ω = 2πf, where f is frequency). The representation of the impedance can also be in the form of polar coordinates with Z = Z / θ, where Z is the magnitude and θ is the phase angle of the impedance. Z is the square root of R2 + Xc 2 and θ = arctan(Xc / R). Permittivity and thus capacitance are usually considered to be constant values, independent of frequency.
Since tissues consist of cells and extracellular medium, their electrical properties determine those of tissues. The extracellular medium consists primarily of ionic solutions. The cell consists of the cellular membrane and the intracellular medium. The cellular membrane consists of a lipid bilayer and proteins. Due to this structure the cellular membrane is primarily capacitive, with the exception of the selective permeability that defines membrane conductivity. Selective permeability is a function of the physiological processes of the living cell. The intracellular material is an ionic solution with microscopic structures and proteins that can become charged and may move in response to applied fields; they contribute to the cell's electrical characteristics.
Tissues exhibit frequency-dependent behavior beyond simple RC circuits. In general terms this is because at different frequencies the different components (extracellular medium, cell membrane and intracellular medium) contribute to the impedance in varying amounts. Unlike in manmade electrical systems, the conductance and permittivity of tissues are not constant values, but are frequency dependent and complex valued. Conductivity has been found to be σ* = σ + jπεoε and permittivity to be ε* = ε + jσ /πεo, where σ* is complex conductivity, σ is the real portion of the conductivity, εo is the permittivity of free space, ε is the relative permittivity of the material in question, and ε* is the complex permittivity.
At low frequencies (< 1000 Hz) most of what is being measured is the ionic extracellular fluid properties, which are primarily conductance values. At higher frequencies permittivity, and thus capacitance, forms a larger portion of the electrical impedance measured. From about 30 kHz to 30 MHz, the capacitive charging of the cell membrane and the dipolar relaxation of proteins in the tissue determine the permittivity. There exists a limited frequency range in which the charging of the organelles within the cells contributes significantly to the permittivity of the tissue. At frequencies in the gigahertz range, the permittivity is primarily due to bipolar relaxation of the water. When permittivity is plotted as a function of frequency, it forms a piecewise linear function. These ranges are known as alpha, beta, delta and gamma, with relaxation frequencies defining the boundaries of the ranges. Since the contribution to the impedance is different due to different components at different frequencies, it may be more useful to obtain measurements over several frequencies.
Electrical properties of nonliving tissue
Some of the electrical impedance experiments to be discussed have been performed in vitro. It has been proven that there is a significant difference between the impedance of normal living tissue and that of dead tissue. The impedance changes as a function of time after death because the permeability of the cell membrane changes within hours of cell death. To reveal maximum information about living tissue, impedance measurements must occur in vivo or within minutes of death [1]. This fact can be a concern when interpreting the results of in vitro experiments.
Measuring electrical impedance properties
For the purpose of defining the functional or pathological properties of tissue, some researchers have been concerned only with the resistance (inverse of conductance) [2]. However, increasing interest has been generated in identifying the complex impedance characteristics. The primary interest in the past couple of decades has been how the complex impedance might change as the functional properties of the cell change. At present, cellular impedance is frequently modeled by a combination of an intracellular resistance, an extracellular resistance and a transmembrane capacitance. This is a simplification since the cell membrane also has a resistance, but it is considered negligible by most researchers.
Beyond investigating the impedance of tissues at select frequencies, many researchers have looked at complex impedance over ranges of frequencies. Some have chosen to form Cole–Cole plots [3] to observe relationships between pathology and the locus of Z versus frequency. Cole performed experiments on a wide range of cell types. Through his experiments he observed that the variation of impedance with frequency approximates a semicircle on the resistance–reactance plane. The center of the semicircle is displaced positively on the resistance axis, at a frequency known as the characteristic frequency (fc). The semicircle has a radius of r = (R0 - R∞) / 2 where Ro represents the DC frequency and R∞ represents the value that the resistance approaches as the frequency approaches infinity. While the semicircle locus works well in theory, most collected data more closely represent a semicircle with a depressed center. This fact caused Cole to later modify the derived equations; however, not all researchers included this modification in their application. Cole found that when the impedance representing the membrane (z3) was modified such that the phase angle was constant over all frequencies, the locus represented a depressed circle and more closely approximated the experimental data (see Fig. 1).
Figure 1.
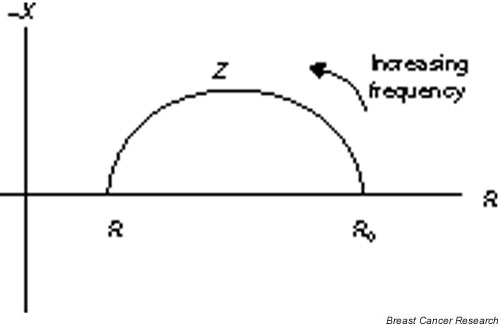
The locus of tissue impedance has a semicircle shape with an offset center. R, resistance; Ro, DC frequency; R∞, value that the resistance approaches as the frequency approaches infinity; X, reactance; Z, magnitude.
Electrical characteristics of breast cancer
Breast cancer is presently detected by palpation (clinical and self), by mammography, and by adjunct imaging methods such as ultrasound and magnetic resonance imaging. When suspicious lesions are detected, biopsies are performed to determine whether the lesion is malignant. At present, some mammographically suspicious lesions are determined to be benign by a subsequent biopsy (false-positive findings). The sensitivity of mammography is decreased in women with mammographically dense breasts. The search continues to locate the means to diagnose breast cancer more accurately and less invasively, and to provide earlier detection.
From as early as 1926, researchers have been studying the electrical properties of breast tumors [4]. While there have been varying results, the consensus is that electrical properties of breast tumors do differ from normal healthy tissue. In 1988 Surowiec and colleagues [5] performed in vitro tests to determine the variability of properties between samples of breast carcinoma, samples with a combination of carcinoma and healthy tissue including the perceived boundary of the lesion, and samples of healthy tissue only. This group concluded that the dielectric constants and the conductivity of cancerous tissues differed between the sample groups (as measured for frequencies from 20 kHz to 100 MHz), although considerable variability existed in the measured data.
Morimoto and colleagues [6,7] measured the electrical impedance of breast tumors in vivo. This was accomplished via a three-electrode method by inserting a fine-needle electrode into the tumor. They calculated the extracellular resistance, the intracellular resistance, and the membrane capacitance based on the measured complex impedance and a model circuit. The model circuit consisted of the extracellular resistance in parallel with a series combination of the intracellular resistance and the capacitance. The measurements were obtained over a frequency range of 0–200 kHz. This group concluded that there are statistically significant differences between pathological and normal tissue. However, the range of values calculated for each tissue type overlap. The authors did not report in this work whether there is always a measurable difference between the two sample types from a single patient.
Jossinet [8-10] studied the impedance of six groups of breast tissue over a frequency range of 488 Hz to 1 MHz. The impedance spectra were obtained for 120 samples obtained from 64 patients, with the sample groups divided into three types of normal breast tissue, two types of benign tissue, and carcinoma. All three articles present studies using the same data. The first study by Jossinet investigates the variability of the impedance data within each group by assessing the standard deviation and the reduced standard error [8].
In the second study by Jossinet, the complex impedance was plotted versus frequency in a desire to compute the Cole–Cole parameters. The study involved calculating parameters to try to distinguish the carcinoma samples from the rest of the samples [9]. Although many of the impedance spectra do not approximate circular arcs, there are obvious differences observed in the shape and location of the various impedance loci on the reactance–resistance plane. The second study suggests that there is a greater difference in characteristics of cancerous tissues at frequencies over 125 kHz.
Jossinet and Schmitt attempted, in the third study, to define a new set of eight parameters by which cancerous tissue can be differentiated from other tissues [10]. They conclude that no one parameter at a single frequency is sufficient to define the tissue but that several parameters spanning a range of frequencies are appropriate.
Chauveau and colleagues [11] have also investigated the need to calculate bio-impedance parameters over a variety of frequency values. For ex vivo samples of normal and pathological tissues, measurements were obtained for frequencies that ranged from 10 KHz to 10 MHz. From these measurements and a model that includes a constant phase element, the extracellular resistance, the intracellular resistance, and the membrane capacitance were calculated from the measurements and a model that includes a constant phase element. From these calculated values, three indices are defined for classifying tissue pathology. Based on the data presented it is suggested that these parameters, and the indexes based on them, would allow cancerous tissues to be differentiated from normal tissues and those with fibrocystic changes.
All of the investigations reviewed have indicated a range of absolute impedance values for a given tissue type. The variability among samples could be due to many causes. Some suggestions have been population variance, experimental errors, or compound tissue samples (cancer and healthy tissue together). Another possibility is that cycling hormones may affect the measured impedance of both healthy and pathological tissues. Perlet and colleagues [12] investigated the stability of the measured impedance of healthy breast tissue. Measurements were obtained once a week over two consecutive menstrual cycles to determine whether EIS images depend on hormones. It was determined that the images obtained varied throughout the cycles with some consistency, and the authors concluded that impedance is dependent on hormone levels.
Electrical impedance scanning
EIS produces an impedance map of an object based upon the spatial electrical characteristics throughout the volume of the object. When a current is injected into an object, by Ohm's law the voltage drop will be proportional to the impedance of the object as long as the object has passive electrical characteristics. In EIS, a known current is injected into the surface and the voltage is measured at a number of points (electrodes) on the surface of the object. The resolution of the resultant image is dependent on the number of electrodes. Areas of low impedance typically appear on an EIS map as areas that have greater intensity (whiter). A measure of the electrical properties of the volume within the surface is obtained from these maps.
TransScan TS2000 and breast cancer
The following reviewed work is limited to research using the Siemens TransScan TS2000 (Siemens Medical, Germany, and TransScan, Ramsey, NJ, USA) as the EIS methodology because it is the only commercially available system for obtaining electrical impedance measurements of the breast.
The information about the following TransScan TS2000 settings was obtained from the user manual document # RD74010348 Rev. C. The TransScan TS2000 maps the capacitance and conductance of the tissue, in two separate images, in a range of frequencies that are selectable for any of the available 26 frequencies. The images are obtained via a square sensor array that is pressed against the breast. The reference electrode is placed in the patient's hand. The images generated can either be high resolution (16 × 16) or low resolution (8 × 8). Each breast is divided into nine sectors, with two images generated for each sector indicating the conductance and capacitance properties. The scans are preprocessed to produce a smoothed image on the screen. Gray-scale mapping is adjustable by the operator to allow enhancement of particular features. As with mammograms, the EIS images are then assessed by a clinician who is trained to interpret the results. The TransScan TS2000 is approved for use in the United States and other countries as an adjunct modality.
There have been several studies on the use of the TransScan TS2000 to detect breast cancer lesions. A very early study by Piperno and colleagues [13] was performed with the prototype to the TransScan TS2000. 'Mammoscan' consisted of an 8 × 8 matrix of square electrodes, and four images were obtained for each breast rather than nine images. EIS was used to screen 6000 patients, of whom 745 underwent biopsy. All patients underwent an examination that included palpation, thermography, ultrasonic examination, mammography, diaphanoscopy, and EIS. In nine cases, EIS identified cancerous lesions where other tests provided negative results. Furthermore, in five cases, EIS clarified ambiguous findings and identified malignancies. The study by Piperno and colleagues does not specifically address the issue of false-positive findings. They do identify that there are large differences in the dielectric properties of the breast that vary with the individual. They recognized that, with their work, they are identifying inhomogeneities in the breast and are assessing the relative electrical properties within the images.
Melloul and colleagues [14] performed a study to assess the performance of 99mTc-sestamibi scintimammography (SMM) and of the TransScan TS2000 as adjunct modalities. Included in the study were 121 patients, all of whom had breast lesions detected and biopsied. Eighteen of the 121 patients had breast carcinoma. This group concluded that that the TransScan TS2000 did not improve the rate of detection when added to SMM use. There were, however, five cases falsely determined to be malignant by SMM that were correctly identified as benign by the TransScan TS2000, and the TransScan TS2000 found one tumor not detected by SMM. The greatest advantage that the TransScan TS2000 offered in this study was its ability to detect small lesions. The smallest lesion detected by the TransScan TS2000 was 3 mm in diameter. The sensitivity of the TransScan TS2000 was found to be 72.2% and the specificity was 67%.
Malich and colleagues [15] also performed a study to assess the capability of the TransScan TS2000 to detect breast cancer, and concluded it to be a valuable adjunct in the assessment of breast lesions. The methods used in this study include palpation, mammography, and ultrasound. Fifty-eight suspicious lesions were biopsied and EIS provided 10 false-positive results, with some of these false positives considered to be precancerous findings (atypical hyperplasia). Most other studies did not quantify the type of false-positive result, so it is difficult to assess whether the TransScan TS2000 could be used reliably as a method for detection of possibly precancerous lesions.
Malich and colleagues [16] published a more extensive study in 2001, comparing the capabilities of ultrasound, magnetic resonance imaging, and EIS to differentiate mammographically suspicious lesions. The EIS measurements were obtained during a targeted examination using 100 and 200 Hz reading frequencies. Radiologists who had knowledge of the mammography findings evaluated all of the imaging modalities. The EIS findings for sensitivity were not significantly improved over ultrasound at 81% as compared with 77%; however, the authors suggest that EIS supplements ultrasound findings. In the same year, a more comprehensive study [17] including 240 histologically proven breast lesions indicated that the addition of EIS to mammography and ultrasound increased the sensitivity from 86.4% to 95.1%, but the accuracy decreased from 82.3% to 75.7%. The study also suggested that EIS has a poor detection rate for ductal carcinoma in situ at 57.1%.
Assenheimer and colleagues [18] presented a theoretical discussion on the relationship between currents detected at the breast's surface and the electrical field distribution within the breast. They also discuss a theoretical critical measurement frequency at which the obscuring effect of skin impedance is minimized. While the authors do not supply data to support their discussion, it could be a direction for further studies of the inverse problem in EIS imaging. It may also be a direction towards improving the data obtained with EIS.
In 2002, Martin and colleagues [19] studied correlations between the histopathology of the breast and the features of the EIS examination. This group found no correlation between the intensity of the EIS signal and the depth of the lesion. They did find a correlation between disagreement between imaging results and histology to hormonal status and the presence of benign proliferating lesions.
In another 2002 study, Piperno and Lenington [20] investigated the ability of EIS to reflect the level of estrogen activity in postmenopausal women. The study concluded that nipple conductivity values were consistently higher for women who use hormone replacement therapy. Since increased estrogen activity may be associated with increased risk of breast cancer, they believe EIS may be useful for identifying women at increased risk.
The viability of electrical impedance measurements to assist the clinician in the diagnosis of breast cancer is clearly still under intense investigation. Some recent clinical studies, such as the work by Wersebe and colleagues [21] and Kneeshaw and colleagues [22], again provide conflicting results. Wersebe and colleagues concluded that EIS does not improve diagnostic accuracy, while Kneeshaw and colleagues found favorable results in the ability of EIS to detect occult microcalcification in the breast.
Although the aforementioned comprises much of the work published by journals on the use of the TransScan TS2000 in detecting breast cancer, more clinical data has been obtained. Piperno has incorporated EIS into the breast screening system at Pistoria, Italy since 1995. Also, according to US Food and Drug Administration documents from January 1999, systems were operating at 41 sites in Israel, the United States, France, Italy, Greece, Russia, Germany, China and South Korea. The applicability of EIS is clearly under widespread investigation as an adjunct imaging modality.
Other electrical impedance measurement methods
There are several research groups investigating other forms of EIS, some of which will be discussed. One group is located at Dartmouth College, Hanover, NH, USA. Paulson and colleagues are using their own prototype machine to obtain spectroscopic images of the breast. With this system, the patient lays prone on a table containing a concave-shaped portion complete with electrodes. The images obtained are of the absolute electrical conductivity and permittivity over the range of frequencies from DC to 1 MHz. When reporting the work of this group, Osterman and colleagues [23] have indicated that the impedance values are particularly sensitive to changes in tissue properties when they are measured at or near the center frequency of the tissue's dispersion. They believe that since they are measuring changes in tissue composition, one frequency is not likely to produce optimal images in all cases. Kerner and colleagues [24] recently outlined results of a study on the repeatability of the EIS examination using this system.
There is also an electrical impedance tomography research group located in the Institute of Radio Engineering and Electronics of the Russian Academy of Sciences. They have a system that is very similar in appearance to the TransScan TS2000. Using the surface measurements, Cherepenin and colleagues create three-dimensional tomography images through a reconstruction algorithm [25].
Newell, Issacson and Mueller at the Renesselaer Polytechnical Institute, Troy, NY, USA, have been actively working on an electrical impedance tomography system and mathematical reconstruction algorithms. With publications dating back to 1986, this group has a significant level of experience in the field. Rather than list all of their publications, the interested reader is left to investigate online themselves (http://www.rpi.edu/~newelj/eit.html).
Discussion and conclusion
Based on the information reviewed EIS shows promise as an adjunct modality, but still more work is required. Studies show that the specificity and the sensitivity in detection are increased when more than one modality is used. Several reasons why EIS continues to be investigated include the following:
• EIS measures a property of tissue not otherwise measured. Studies published as far back as 1926 indicate that the electrical properties of cancerous tissues differ from those of healthy tissue.
• EIS equipment is easy to use, is noninvasive, and obtains the data in a way that is comfortable to patients.
• EIS equipment is inexpensive, a fraction of the cost of ultrasound machines.
• EIS has been shown to detect cancerous lesions as small as 3 mm in diameter.
• Electrical impedance maps may be obtained easily; acquisition takes about 15 min.
While the use of EIS imaging has some positive aspects to it, there are several areas that need further work:
• The differences in electrical properties of various breast pathologies are not well documented. More data are required to better categorize the information contained in EIS mappings.
• The frequency response of both normal and pathological breast tissue needs to be better understood. Key features to identify breast cancer may be located at frequencies not typically used.
• While some groups like Glickman and colleagues [26] are looking at post-processing the output maps to automatically discriminate between benign and normal tissue, post-processing of EIS breast information is still limited. Some valuable clinical information may be presently overlooked.
• While three-dimensional reconstruction algorithms are a topic of great interest, with Cherepenin and colleagues [25] and Mueller and colleagues [27] publishing some promising results, there have been limited data to indicate their robustness, which is required for clinical application of these algorithms.
The work performed to date suggests that electrical properties of breast tissue change with the evolution from normal healthy tissue to malignant tissue. The existence of this property may provide an additional means to detect breast cancer. The ability of the clinician to use this property has been investigated over the past two decades, with interest increasing with the development of a commercial machine. While this technology will probably never become the gold standard that mammography is today, further clinical study may lead to EIS being reclassified from an experimental modality to an acceptable adjunct modality.
Competing interests
None declared.
Abbreviations
C = capacitance; ε = permittivity; EIS = electrical impedance scanning; R = resistance; σ = conductance; SMM = 99mTc-sestamibi scintimammo-graphy; Z = magnitude.
Acknowledgments
Acknowledgement
Funding for this work has been provided by the Canadian Breast Cancer Foundation, Atlantic Chapter.
References
- Polk C, Postow E (Eds) CRC Handbook of Biological Effects of Electromagnetic Fields. Boca Raton, FL: CRC Press; 1986. pp. 27–96. [Google Scholar]
- Faes T, van der Meij HA, de Munck JC, Heethaar RM. The electric resistivity of human tissues (100 Hz–10 MHz): a meta-analysis of review studies. Physiol Meas. 1999;20:R1–R10. doi: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- Cole KS. Membranes, Ions and Impulses: A Chapter of Classical Biophysics. Berkley, CA: University of California Press; 1968. pp. 6–102. [Google Scholar]
- Fricke H, Morse S. The electrical properties of tumors of the breast. J Cancer Res. 1926;16:310–376. [Google Scholar]
- Surowiec A, Stanislaw SS, Barr JR, Swarup A. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans Biomed Eng. 1988;35:257–263. doi: 10.1109/10.1374. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kinouchi Y, Iritani T, Kimura S, Konishi Y, Mitsuyama N, Komaki K, Monden Y. Measurement of the electrical bio-impedance of breast tumors. Eur Surg Res. 1990;22:86–92. doi: 10.1159/000129087. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kimura S, Konishi Y, Komaki K, Uyama T, Monden Y, Kinouchi Y, Iritani T. A study of the electrical bio-impedance of tumors. J Invest Surg. 1993;6:25–32. doi: 10.3109/08941939309141189. [DOI] [PubMed] [Google Scholar]
- Jossinet J. Variability of impedivity in normal and pathological breast tissue. Med Biol Eng Comput. 1996;34(No 5):346–350. doi: 10.1007/BF02520002. [DOI] [PubMed] [Google Scholar]
- Jossinet J. The impedivity of freshly excised human breast tissue. Physiol Meas. 1998;19(No 1):61–75. doi: 10.1088/0967-3334/19/1/006. [DOI] [PubMed] [Google Scholar]
- Jossinet J, Schmitt M. A review of parameters for the bioelectrical characterization of breast tissue. Ann NY Acad Sci. 1999;873:30–41. doi: 10.1111/j.1749-6632.1999.tb09446.x. [DOI] [PubMed] [Google Scholar]
- Chauveau N, Hamzaoui L, Rochaix P, Rigaud B, Voigt JJ, Morucci JP. Ex vivo discrimination between normal and pathological tissues in human breast surgical biopsies using bioimpedance spectroscopy. Ann NY Acad Sci. 1999;873:42–50. doi: 10.1111/j.1749-6632.1999.tb09447.x. [DOI] [PubMed] [Google Scholar]
- Perlet C, Kessler M, Lenington S, Sittek H, Reiser M. Electrical impedance measurement of the breast: effect of hormonal changes associated with the menstrual cycle. Eur Radiol. 2000;10:1550–1554. doi: 10.1007/s003300000554. [DOI] [PubMed] [Google Scholar]
- Piperno G, Frei EH, Moshitzky M. Breast cancer screening by impedance measurements. Frontiers Med Biol Eng. 1990;2:111–117. [PubMed] [Google Scholar]
- Melloul M, Paz A, Ohana G, Laver O, Michalevich D, Koren R, Wolloch Y, Gal R. Double phase Tc-sectamibi scintimammography and Trans-Scan in diagnosing breast cancer. J Nucl Med. 1999;40:376–380. [PubMed] [Google Scholar]
- Malich A, Fritsch T, Anderson R, Boehm T, Freesmeyer MG, Fleck M, Kaiser WA. Electrical impedance scanning for classifying suspicious lesions: first results. Eur Radiol. 2000;10:1555–1561. doi: 10.1007/s003300000553. [DOI] [PubMed] [Google Scholar]
- Malich A, Boehm T, Facius M, FreesMeyer MG, Fleck M, Anderson R, Kaiser WA. Differentiation of mammographically suspicious lesions: evaluation of breast ultrasound, MRI mammography and electrical impedance scanning as adjunctive technologies in breast cancer detection. Clin Radiol. 2001;56:278–283. doi: 10.1053/crad.2000.0621. [DOI] [PubMed] [Google Scholar]
- Malich A, Bohm T, Facius M, Freessmeyer M, Fleck M, Anderson R, Kaiser WA. Additional value of electrical impedance scanning: experience of 240 histologically-proven breast lesions. Eur J Cancer. 2001;37:2324–2330. doi: 10.1016/S0959-8049(01)00283-0. [DOI] [PubMed] [Google Scholar]
- Assenheimer M, Laver-Moskovitz O, Malonek D, Manor D, Nahaliel U, Nitzan R, Saad A. The T-SCAN technology: electrical impedance as a diagnostic tool for breast cancer detection. Physiol Meas. 2001;22:1–8. doi: 10.1088/0967-3334/22/1/301. [DOI] [PubMed] [Google Scholar]
- Martin G, Martin R, Brieva MJ, Santamaria L. Electrical impedance scanning in breast cancer imaging: correlation with mammographic and histologic diagnosis. Eur Radiol. 2002;12:1471–1478. doi: 10.1007/s00330-001-1275-0. [DOI] [PubMed] [Google Scholar]
- Piperno G, Lenington S. Breast electrical impedance and estrogen use in postmenopausal women. Maturitas. 2002;41:17–22. doi: 10.1016/S0378-5122(01)00249-3. [DOI] [PubMed] [Google Scholar]
- Wersebe A, Siegmann K, Krainick U, Fersis N, Vogel U, Claussen Claus, Muller-Schimpfle M. Diagnostic potential of targeted electrical impedance scanning in classifying suspicious breast lesions. Invest Radiol. 2002;37:65–72. doi: 10.1097/00004424-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Kneeshaw PJ, Drew PJ, Hubbard A. Electrical impedance scanning: a new imaging technique for evaluating microcalcification in the breast? Breast Cancer Res. 2002;4(Suppl 1):20. doi: 10.1186/bcr476. [DOI] [Google Scholar]
- Osterman K, Kerner T, Williams D, Hartov A, Poplack S, Paulsen K. Multifrequency electrical impedance imaging: preliminary in vivo experience in breast. Physiol Meas. 2000;21:99–109. doi: 10.1088/0967-3334/21/1/313. [DOI] [PubMed] [Google Scholar]
- Kerner TE, Hartov A, Soho SK, Poplack SP, Paulsen K. Imaging the breast with EIS, an initial study of exam consistency. Physiol Meas. 2002;23:221–236. doi: 10.1088/0967-3334/23/1/323. [DOI] [PubMed] [Google Scholar]
- Cherepenin V, Karpov A, Korjenevsky A, Kornienko V, Mazaletskaya A, Mazourov D, Meister D. A 3D electrical impedance tomography (EIT) system for breast cancer detection. Physiol Meas. 2001;22:9–18. doi: 10.1088/0967-3334/22/1/302. [DOI] [PubMed] [Google Scholar]
- Glickman Y, Filo O, Nachaliel U, Lenington S, Amin-Spector S, Ginor R. Novel EIS postprocessing algorithm for breast cancer diagnosis. IEEE Trans Med Imaging. 2002;21:710–712. doi: 10.1109/TMI.2002.800605. [DOI] [PubMed] [Google Scholar]
- Mueller J, Issacson D, Newell J. A reconstruction algorithm for electrical impedance tomography data collected on rectangular electrode arrays. IEEE Trans Biomed Eng. 1999;46:1379–1386. doi: 10.1109/10.797998. [DOI] [PubMed] [Google Scholar]


