The authors developed a novel peptide sequence with only 12 amino acids based on the Ile-Lys-Val-Ala-Val sequence. This novel short peptide shows great promise in artificial niche development for supporting human neural stem/progenitor cell culture in vitro and in vivo and for promoting human neural stem/progenitor cell transplantation in future clinical therapy.
Keywords: Neural stem/progenitor cells, Peptide, Attachment, Migration, Hydrogel
Abstract
Human neural stem/progenitor cells (hNSCs) are very difficult to culture and require human or animal source extracellular matrix molecules, such as laminin or collagen type IV, to support attachment and to regulate their survival and proliferation. These extracellular matrix molecules are difficult to purify from human or animal tissues, have high batch-to-batch variability, and may cause an immune response if used in clinical applications. Although several laminin- and collagen IV-derived peptides are commercially available, they do not support long-term hNSC attachment and growth. To solve this problem, we developed a novel peptide sequence with only 12 amino acids based on the Ile-Lys-Val-Ala-Val, or IKVAV, sequence: Ac-Cys-Cys-Arg-Arg-Ile-Lys-Val-Ala-Val-Trp-Leu-Cys. This short peptide sequence, similar to tissue-derived full laminin molecules, supported hNSCs to attach and proliferate to confluence for continuous passage and subculture. This short peptide also directed hNSCs to differentiate into neurons. When conjugated to poly(ethylene glycol) hydrogels, this short peptide benefited hNSC attachment and proliferation on the surface of hydrogels and promoted cell migration inside the hydrogels with maximum enhancement at a peptide density of 10 μM. This novel short peptide shows great promise in artificial niche development for supporting hNSC culture in vitro and in vivo and for promoting hNSC transplantation in future clinical therapy.
Introduction
Numerous cell types, including stem cells, primary epithelial cells, and primary endothelial cells, require interactions with extracellular matrix (ECM) molecules to support and regulate their survival and proliferation. ECM not only serves as an important structural element in formations such as basement membrane but also interacts with cells to influence adhesion, survival, proliferation, migration, and differentiation by engaging with cellular adhesion receptors, thereby activating a number of downstream signaling pathways [1]. Although animal- and human-derived ECM molecules are often necessary for current cell culture applications, there are many problems related to their use. In particular, the ECM component (e.g., laminin) is an extremely large, complex, post-translationally modified protein that is difficult to produce via recombinant expression systems and thus is commonly purified from mammalian cell lines or tissues. Such protein preparations run the risk of being contaminated with pathogens and immunogens. In addition, there is considerable lot-to-lot variability in animal- and human-derived ECM because of many isoforms present and difficulty in purifying such proteins to homogeneity. The development of synthetic stem cell culture platforms that mimic the physical and biochemical properties of the natural ECM can benefit both scientific studies and clinical therapies.
Several short peptides have been identified from full ECM molecules to support cell adhesion and growth, including Arg-Gly-Asp (RGD); Ile-Lys-Val-Ala-Val (IKVAV); Tyr-Ile-Gly-Ser-Arg (YIGSR); Arg-Tyr-Val-Val-Leu-Pro-Arg, or RYVVLPR; Arg-Asn-Ile-Ala-Glu-Ile-Ile-Lys-Asp-Ile, or RNIAEIIKDI; and so on [2]. RGD and IKVAV are present on the α-laminin chain, whereas YIGSR is found on the β-laminin chain [3, 4]. Short peptide sequences, when compared with whole proteins, are more stable, are more easily synthesized, and are less likely to exhibit steric hindrance [5]. Although many short peptide sequences have been identified, none of these peptides from commercial sources can support attachment, growth, and long-term culture of human neural stem/progenitor cells (hNSCs).
In this study, we developed a novel peptide sequence with only 12 amino acids based on laminin sequence IKVAV: Ac-Cys-Cys-Arg-Arg-Ile-Lys-Val-Ala-Val-Trp-Leu-Cys (CCRRIKVAVWLC). The novel peptide was examined for its ability to support the attachment, proliferation, and neuronal differentiation of hNSCs in two different contexts: coating on the two-dimensional (2D) substrates and conjugated to hydrogels based on polyethylene glycol (PEG) for three-dimensional (3D) culture. On the 2D culture, we compared its effects, relative to commercial laminin peptide Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg (CSRARKQAASIKVAVSADR; lam-IKVAV) and the whole protein laminin, on hNSC attachment, proliferation, and differentiation. When conjugated to PEG-based hydrogels, we examined the effects of this short peptide on hNSC attachment, spreading, and proliferation on the surface of hydrogels as well as cell migration from human neurospheres cultured inside hydrogels.
Materials and Methods
Materials
The commercial laminin IKVAV peptide CSRARKQAASIKVAVSADR was purchased from American Peptide Company (Sunnyvale, CA, http://www.americanpeptide.com). Reagents required for peptide synthesis, including Fmoc-Cys (Trt)-Wang resin, were obtained from EMD Biosciences (Gibbstown, NJ, http://www.emdmillipore.com/life-science-research/?RedirectedFrom=http://www.emdbiosciences.com). Polyethylene glycol tetra-acrylate (PEGTA; 10-kDa molecular weight) was obtained from Creative PEGWorks (Winston Salem, NC, http://www.creativepegworks.com). Recombinant fibroblast growth factor 2 (FGF-2) and epidermal growth factor (EGF) were purchased from Peprotech (Rocky Hill, NJ, http://www.peprotech.com). Accutase was purchased from Innovative Cell Technologies (San Diego, CA, http://www.accutase.com). The CyQuant proliferation kit and mouse laminin were obtained from Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com). Alexa Fluor 543 phalloidin, 4′,6-diamidino-2-phenylindole (DAPI), and the LIVE/DEAD viability kit were obtained from Invitrogen (Carlsbad, CA). Rabbit anti-glial fibrillary acidic protein was obtained from Dako (Glostrup, Denmark, http://www.dako.com), and mouse anti-β-III-tubulin was from Sigma-Aldrich (St. Louis, MO, http://www.invitrogen.com). Fluorophore-conjugated secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA, http://www.jacksonimmuno.com). All other chemical reagents were purchased from Sigma-Aldrich.
Neural Stem/Progenitor Cell Culture
Human neural stem/progenitor cells were obtained from Millipore (Billerica, MA, http://www.millipore.com). The cells were derived from the ventral mesencephalon region of human fetal brain and immortalized by retroviral transduction with the v-myc oncogene. In conventional culture, laminin (20 μg/ml) was used to coat tissue culture plasticwares at least 2 hours before hNSC seeding. Human neural stem/progenitor cells (NSCs) were maintained in ReNcell neural stem cell medium (Millipore) with FGF-2 (20 ng/ml) and EGF (20 ng/ml). Human NSCs were incubated at 37°C under 5% CO2 and used before passage 5 in this study. For cell adhesion assays, cells were removed from the plate using Accutase, aspirated in growth media, and seeded on peptide- or laminin-coated substrates at a density of 50,000 cells per square centimeter. For differentiation study, hNSCs were cultured with the medium without FGF-2 and EGF.
Neurosphere Fabrication
Neurospheres with uniform sizes were fabricated using a robotic fabrication system developed in our laboratory. Briefly, stamps with a micronipple array were designed in SolidWorks (Dassault Systèmes SolidWorks Corporation, Waltham, MA, http://www.solidworks.com) and fabricated using an ultraprecision lathe. The automatic neurospheres fabricator with electric triggered gripper can pick up the stamp and pipette tips to handle liquids. Agarose solution (2% wt/wt) was prepared by built-in heating elements in the robot platform. The robot was programmed to first add agarose solution to culture dishes. Then the robot picked up the stamp and pressed on the agarose solution at room temperature for 2 minutes. The highly uniformly sized microwells were formed in the agarose gel. Human NSC suspension was taken by the robot and added to the microwells. After 24 hours of culture at 37°C and 5% CO2, uniformly sized neurospheres were formed. For 3D culture, human neurospheres of uniform size were mixed with hydrogel precursor solutions before hydrogel formation.
Peptide Synthesis and Characterization
Short peptide CCRRIKVAVWLC was synthesized from Fmoc-Cys (Trt)-Wang resin using a standard Fmoc solid-phase peptide synthesis protocol. Prior to cleavage from the resin, the free terminal amine was acetylated using 10% acetic anhydride and 10% pyridine in dimethylformamide. Peptide was cleaved from the resin using 94% trifluoroacetic acid, 2.5% ethanedithiol, 2.5% H2O, and 1% triisopropylsilane. Peptide was purified by high-pressure liquid chromatography and characterized using mass spectrometry. High-pressure liquid chromatography was performed using a Waters RCM 25 × 10 C-18 columns (Waters Corporation, Milford, MA, http://www.waters.com). Ion trap mass spectra were obtained using a Finnigan LCQ Advantage Max mass spectrometer (Thermo Electron Corporation, San Jose, CA, http://www.thermoscientific.com). The peptide was stored at −20°C before stock solutions were made.
Hydrogel Preparation
We have developed a series of functionalized hydrogels based on in situ gelable, nonimmunogenic materials, including multiarm thiolated PEG. Polyethylene glycol tetra-acrylate was used as the crosslinker for hydrogel formation. Four-arm PEG was thiolated by an esterification reaction with thioglycolic acid using p-toluenesulfonic acid as a catalyzer, as described previously [6, 7].
Rheological Characterization of Hydrogel
A rheometer (AR1000; TA Instruments, New Castle, DE, http://www.tainstruments.com) was used to measure hydrogel mechanical properties, such as storage modulus (G′) and loss modulus (G′′) and gelation time [8]. Briefly, the time sweep was performed to monitor the in situ gelation at 37°C, recording the temporal evolution of G′ and G′′. A frequency sweep test was used to obtain information about the stability of hydrogel structures. The stress sweep was set up by holding the frequency 1 Hz constant while increasing the stress level from 1 Pa to 10 Pa. The applied range of 1–10 Pa was found to be safe for use in a prior experiment in which we determined the linear viscoelastic region profiles of the hydrogels by shearing them until structural breakdown. The oscillatory stress sweep allows the determination of G′ of the hydrogels.
Peptide Conjugation
Peptide Conjugated to Gold-Coated Cover Slips
The lam-IKVAV and our short peptide were conjugated to gold-coated cover slips. For gold coating on glass surfaces, cover slips were first cleaned with ethanol by sonication for 30 minutes. Then gold was coated on the cover slips by a sputter coater. For peptide and laminin coating, the peptide (150 μg/ml or 100 μM) or laminin (20 μg/ml) solutions were applied on the cover slips for at least 2 hours. Then the solutions were removed from the slips and washed with phosphate-buffered saline three times. An atomic force microscope was used to examine the 3D configuration of the peptides on the surface.
Conjugation of Peptides Into Hydrogels
The lam-IKVAV peptide and our synthesized peptide of different concentrations were conjugated to PEG hydrogels through an additional reaction of the thiol group on peptides at the ends of a PEGTA crosslinker, as described previously [9]. Briefly, peptides containing stock solutions (100 μM) were prepared in the PEGTA stock solution (2% wt/wt). In the peptide-linker stock solutions, the molar ratio of peptides to PEGTA was 1:20. Lower peptide concentrations of 50 μM and 10 μM were prepared by diluting the stock solution with peptide-free PEGTA solution. Then PEGTA solutions containing different concentrations of peptides were added to four-arm thiolated PEG (2% wt/wt) solution and mixed thoroughly to form hydrogels.
Cell Attachment and Proliferation
To determine whether peptide-functionalized surfaces can support cell attachment and long-term culture and proliferation, hNSCs were cultured in complete growth medium, which contains FGF-2 and EGF to maintain hNSCs in the undifferentiated state. Cells were incubated on laminin- or laminin peptide-coated substrates. At days 1, 5, and 10, the cell number from each type of coating was assayed with a CyQuant cell proliferation assay.
Cell Morphology
Morphology of cells was examined by immunostaining of actin. Cells were fixed with 4% (wt/vol) paraformaldehyde, permeabilized with 0.5% Triton X-100 (t-octylphenoxyplyethoxy-ethanol) and stained with Alexa Fluor 543 phalloidin for 30 minutes. The nuclei were counterstained with 1 μg/ml of DAPI for 30 minutes. The cells were imaged using a Leica TCS SP5 laser scanning confocal microscope (Bannockburn, IL, http://www.leica.com).
Cell Viability
Viability of cells inside hydrogels was examined using a LIVE/DEAD viability kit. Live cells were stained with green fluorescent SYTO 10, and dead cells with compromised cell membranes were stained with red fluorescent ethidium homodimer 2. The Leica TCS SP5 laser scanning confocal microscope was used to capture the images of the LIVE/DEAD cell-staining patterns. At least six random fields per sample were analyzed.
Cell Differentiation
The differentiation of hNSCs on the tested surfaces was evaluated by immunocytochemistry. Human NSCs were cultured for 2 weeks with differentiation media without growth factors FGF-2 and EGF. The cells were fixed and stained with primary antibodies, such as β-III-tubulin and glial fibrillary acidic protein. The samples were further stained with fluorophore-conjugated secondary antibodies. The nuclei were counterstained with DAPI. The samples were imaged using the same microscope as described above. At least six random fields per sample were analyzed.
Statistical Analysis
Data are shown as mean ± SD. Statistical analyses were performed using one-way analysis of variance followed by Tukey’s post tests and the paired t test, as appropriate. A p value of <.05 was considered statistically significant.
Results
Peptides Conjugated to Gold Coated Surface
In this study, one new short peptide sequence, CCRRIKVAVWLC, including cell adhesion motif IKVAV, has been successfully developed by solid-phase synthesis protocol. The sequence of polypeptide was confirmed by mass spectrometry with the mass-to-charge ratio of 1,491.7 (calculated at 1,491.77) (supplemental online Fig. 1).
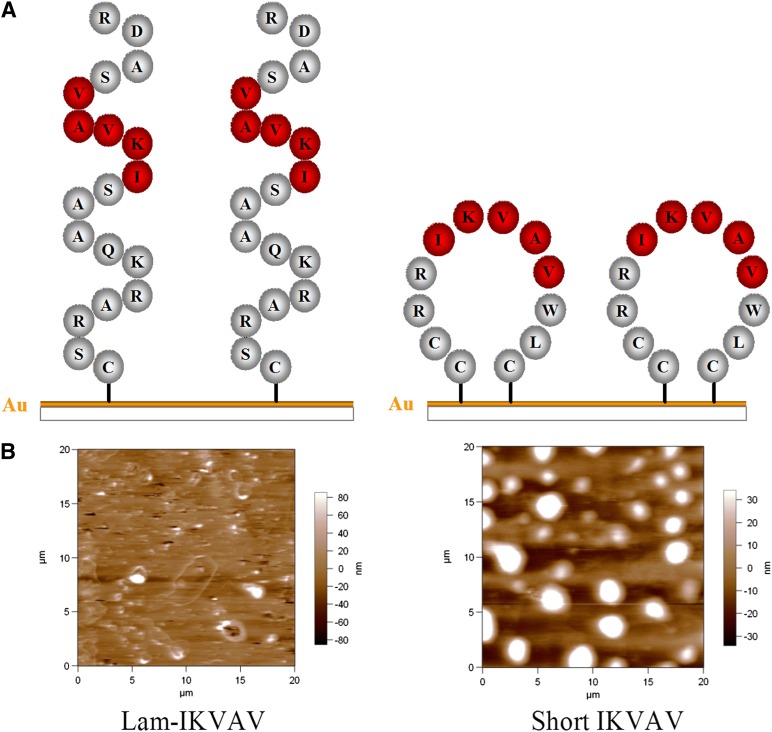
In the sequence designs, two cysteines are located at the N-terminus, and another is located at the C-terminus. Because of the specific interaction between the sulfur of cysteine and the substrate (e.g., gold-coated glass surface), peptides can be immobilized onto the substrates [10–13]. Because another two cysteines are available in the sequences, our short peptides, when conjugated to the substrate, possess the capability to assume a looped conformation, so that it can better present the IKVAV sequence to the cells. In contrast, lam-IKVAV (CSRARKQAASIKVAVSADR), which has only one cysteine in the sequences, cannot form cyclic structures on substrates (Fig. 1A). The morphologies of the lam-IKVAV peptide and our IKVAV conjugated to gold-coated cover slips have been visualized by atomic force microscope (Fig. 1B). The peptide formed 3D tall dots (bright spots) on the surface coated with our short peptide. In contrast, there are very few tall dots (bright spots) on the surface coated with lam-IKVAV peptides. This clearly indicates that our short peptides form 3D loop structures and present the IKVAV sequence better than the lam-IKVAV peptides, which form linear 2D structures rather than 3D loop structures.
Figure 1.
Morphology of the lam-IKVAV peptide and our short IKVAV conjugated to gold-coated cover slips. (A): Scheme of peptides. (B): Morphology of peptides inspected by atomic force microscope. Abbreviations: IKVAV, Ile-Lys-Val-Ala-Val sequence; Lam-IKVAV, Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg sequence.
Human NSCs Cultured on Our Peptide-Coated Surface
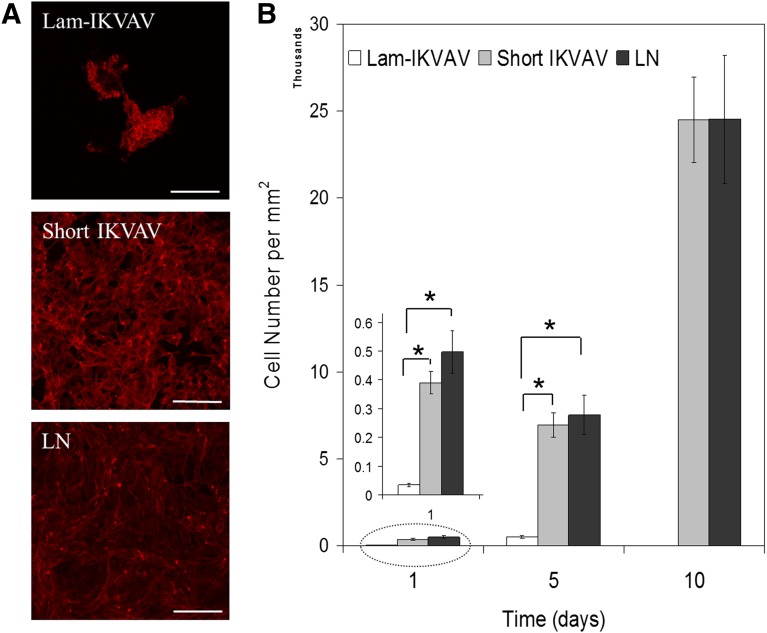
Human NSCs were cultured on the substrates with different coatings in maintenance media with growth factors of FGF-2 and EGF for 1 week. As shown in Figure 2A, on the lam-IKVAV-coated surface, hNSCs preferred to aggregate together. In contrast, on the surface coated with our shorter peptide, they spread more evenly, similar to those on whole-laminin-coated surfaces. As for cell attachment, very few hNSCs attached to the lam-IKVAV-coated surface. These loosely adhered cells formed cell aggregates from day 2 and floated off the surface on day 7–10. In contrast, on our new short peptide-coated surface, significantly more hNSCs were attached compared with the lam-IKVAV-coated substrates (Fig. 2B, insert, p < .05). These attached cells spread evenly and proliferated quickly on our short peptide-coated surface. Total confluence can be reached in about a week. There was no significant difference between our short peptide-coated surface and whole-laminin-coated surface. When the lam-IKVAV peptide was coated on the surface, it did not elicit a stable attachment for hNSCs. Our short IKVAV peptide, just like the whole-laminin molecule, supports hNSC attachment, spreading and proliferating until total confluence on the whole surface is achieved. When laminin-α1 antibody was applied to the surface coated with our short IKVAV peptide, as shown in supplemental online Figure 2, hNSCs aggregated together and loosely attached on the surface. The laminin-α1 antibody blocked the short peptide and then inhibited the adhesion of hNSCs onto the short IKVAV peptide-coated surface. This result confirmed the same integrin attachment sites for our short IKVAV peptide and laminin with human neural stem cells.
Figure 2.
Morphology, attachment and proliferation of human neural stem/progenitor cells cultured on substrates coated with lam-IKVAV peptides, short IKVAV peptides, and whole LN. (A): Human neural stem/progenitor cells were stained with phalloidin (red). Scale bars = 100 μm. (B): Quantification of cell attachment and proliferation. Cells were assayed with CyQuant proliferation kit. A greater number of cells attached on cover slips conjugated with short IKVAV peptide than lam-IKVAV peptide at day 1 (inserted, ∗, p < .05). Abbreviations: IKVAV, Ile-Lys-Val-Ala-Val sequence; Lam-IKVAV, Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg sequence; LN, laminin.
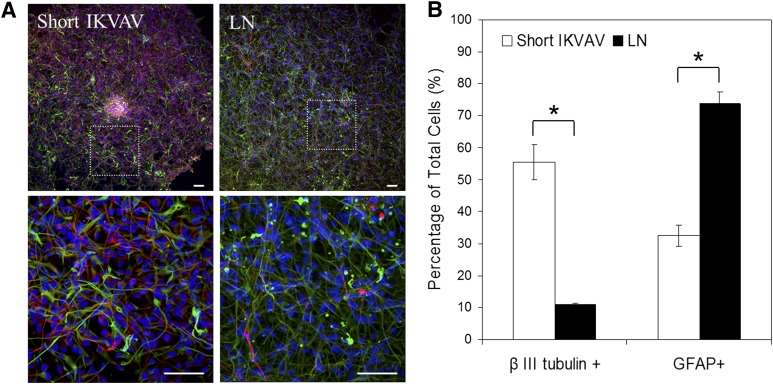
Lam-IKVAV could not support long-term culture of hNSCs; therefore, to investigate the effects of our short peptide on cell differentiation, a whole-laminin-coated surface was used as the control for the comparison. The cells were cultured in differentiation media without FGF-2 and EGF for 2 weeks. Immunocytochemistry was used to establish in vitro differentiation of hNSCs. In addition to cellular morphology, neurons and glial cells can be identified by β-III-tubulin and glial fibrillary acidic protein staining, respectively (Fig. 3A). More than half of hNSCs differentiated into neurons, and about 30% of hNSCs differentiated into glial cells on our short IKVAV peptide-coated surfaces after 2 weeks in culture. In contrast, only about 10% of hNSCs differentiated into neurons, and more than 70% of hNSCs turned into glial cells on the whole-laminin-coated substrates (Fig. 3B).
Figure 3.
Differentiation of human neural stem/progenitor cells cultured on substrates coated with short IKVAV peptides and whole LN at the day 14. (A): Cells were stained with DAPI (blue), GFAP (green), and β-III-tubulin (red). Scale bars = 100 μm. (B): Qualification of cells that differentiated into neurons (β-III-tubulin-positive) and glial cells (GFAP-positive). Our short IKVAV peptide induced significantly higher rate of differentiation into neurons and lower rate to glial cells compared with LN (∗,∗p < .05). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; IKVAV, Ile-Lys-Val-Ala-Val sequence; LN, laminin.
The Attachment and Proliferation of hNSCs on PEG Hydrogels Conjugated With Our Peptide
Four-arm thiolated PEG was crosslinked by PEGTA through a Michael-type addition reaction. As shown in supplemental online Figure 3, the solutions of four-arm thiolated PEG (2% wt/wt) and PEGTA (2% wt/wt) started the gelation process at 30 minutes (when G′ > G′′, no peptide) (supplemental online Fig. 3A) and formed the stable hydrogel at 2 hours with G′ about 600 Pa (supplemental online Fig. 3B, 3C). Peptides were incorporated into the hydrogel by conjugation addition of thiol groups in the peptide onto the ends of PEGTA. The conjugation reaction was complete within 5 minutes according to the 5,5′-dithiobis-(2-nitrobenzoic acid) test (data not shown). The reaction between thiol and acrylate rather than the formation of disulfide bond is strongly preferred because, with regard to kinetics, the rate of reaction of the thiol to the acrylate is much more rapid than the rate of reaction between thiol groups (11 hours) [14]. Because this conjugation reaction consumed a very small amount of PEGTA (less than 2.5% of the total amount), even with the highest concentration of our short peptide (100 μM), the peptide addition did not affect the subsequent use of PEGTA for hydrogel crosslinking. Hydrogels conjugated with different amounts of our short peptide (10 μM, 50 μM, and 100 μM) expressed similar stiffness at about 600 Pa (supplemental online Fig. 3B, 3C), although the gelation process of all of our peptide-conjugated hydrogels started a little bit later (40 minutes) than both blank hydrogel and lam-IKVAV-conjugated hydrogel (30 minutes).
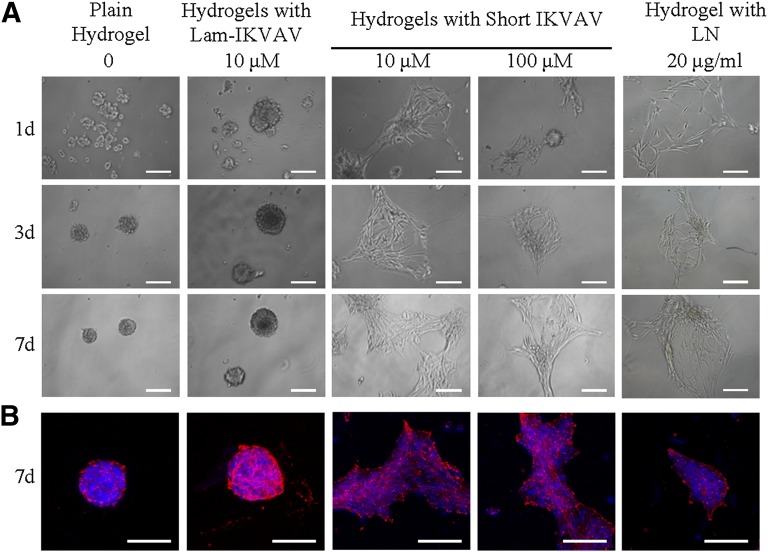
Human NSCs were cultured on the surface of hydrogels with maintenance media for 7 days. Without any bioactive peptide, hNSCs could not attach on the surface of plain PEG hydrogels (Fig. 4A). The cells formed spheres by themselves around day 3. Similar to those on plain hydrogels, cells on hydrogels conjugated with lam-IKVAV (10 μM and 100 μM) also aggregated together. In contrast, on the hydrogels conjugated with our short peptide of both 10 μM and 100 μM, cells attached quickly. These attached cells proliferated on the surface as the function of time. Shown in Figure 4B, at day 7, only on the hydrogel conjugated with our short peptide (10 μM and 100 μM) did hNSCs display spreading cytoskeletons. There was no difference in the morphology of hNSCs between these two concentrations. Moreover, as for the cell attachment and morphology on the surface of the hydrogels, there was no significant difference between hydrogels loaded with laminin (20 μg/ml) and our short peptide.
Figure 4.
Human neural stem/progenitor cells (hNSCs) cultured on the surface of the plain hydrogels, the hydrogels conjugated with lam-IKVAV (10 μM), the hydrogels with short IKVAV peptide of 10 and 100 μM at days 1, 3, and 7, and the hydrogel mixed with LN (20 μg/ml). (A): Phase contrast of hNSCs on the surface of hydrogels. (B): Immunostaining of hNSCs on hydrogels at day 7. Cells were stained with DAPI for nuclei (blue) and phalloidin for actin (red). Scale bars = 100 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; IKVAV, Ile-Lys-Val-Ala-Val sequence; Lam-IKVAV, Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg sequence; LN, laminin.
Human NSCs Migrated Inside the Hydrogels Conjugated With Our Short Peptide
A uniformly sized neurosphere is a good in vitro model in which to study cell migration in different biomaterial structures and formulations. Human neurospheres with uniform size of about 400 μm are shown in supplemental online Fig. 4. Human NSCs inside of our fabricated spheroids maintained their stemness, as demonstrated by nestin-positive staining. The size of neurospheres could be manipulated by adjusting the concentrations of cell suspensions.
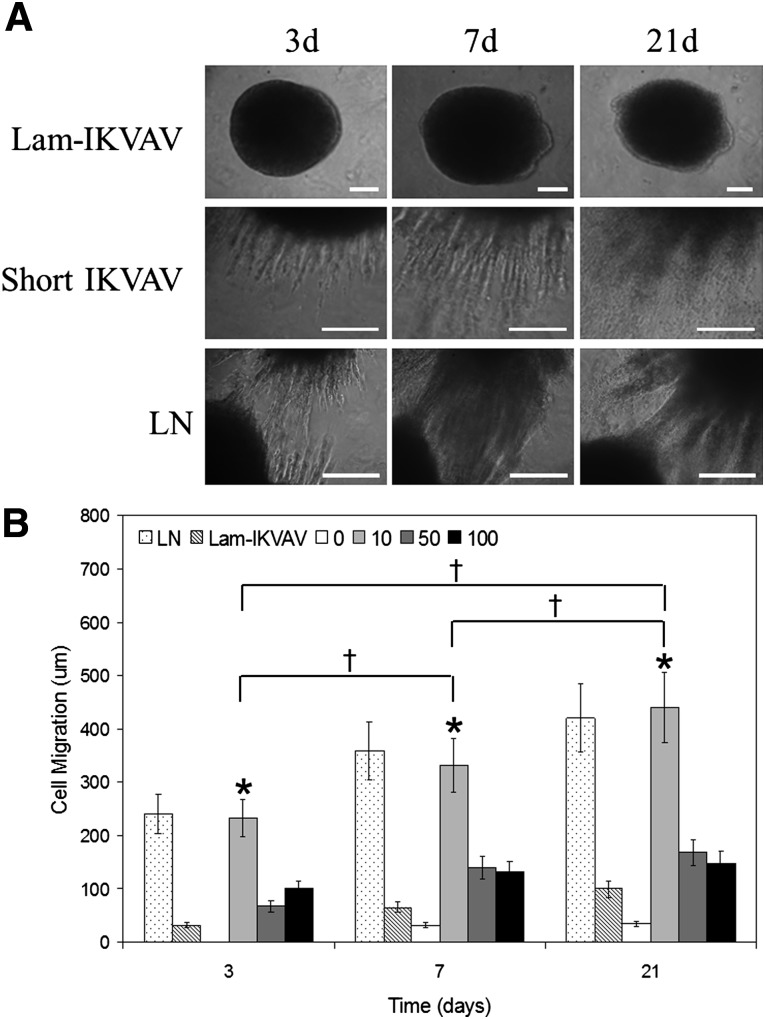
Human neurospheres with uniform size were mixed with hydrogel precursor solutions including 2% (wt/wt) thiolated four-arm PEG and 2% (wt/wt) PEGTA conjugated with lam-IKVAV of 10 μM, 50 μM, and 100 μM; blank hydrogel; hydrogels with our short peptide of different concentrations of 10 μM, 50 μM, and 100 µM; and hydrogel mixed with laminin (20 μg/ml). After hydrogel formation, cells were cultured in differentiation media without FGF-2 and EGF for 3 weeks. As presented in Figure 5, the cells survived the in situ crosslinking process and remained viable during the time of observation (21 days). The viability of hNSCs inside hydrogels was very high (>95%), regardless of the type and concentration of peptide.
Figure 5.
Human neurospheres survived inside the plain hydrogel, the hydrogel conjugated with lam-IKVAV (10 μM), and the hydrogels with short IKVAV peptide of different concentrations of 10 μM, 50 μM, and 100 μM, and the hydrogel mixed with LN (20 μg/ml) at day 21. The bottom panel shows the larger review of neurospheres inside hydrogels. Cells were stained with LIVE/DEAD (Green/Red) kit. Scale bars = 100 μm. Abbreviations: IKVAV, Ile-Lys-Val-Ala-Val sequence; Lam-IKVAV, Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg sequence; LN, laminin.
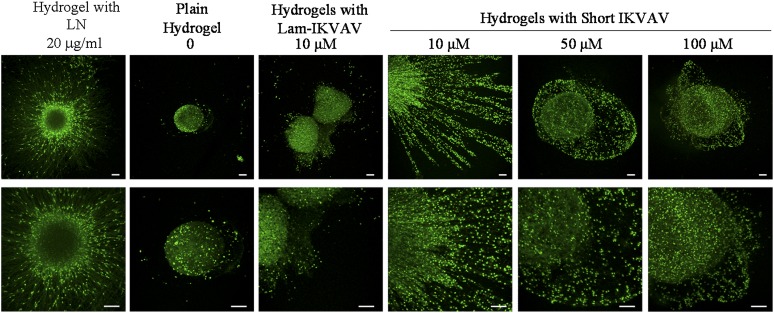
The conjugated peptides resulted in a different response on cell migration. Individual cells could be seen to migrate away from the edge of the neurospheres inside the hydrogels conjugated with our short peptide rather than lam-IKVAV (Fig. 6A). We quantified the cell migration by taking measurements of the distance between the edges of spheres and the cell bodies at their outer perimeters. Figure 6 presents the quantification of cell migration from neurospheres cultured inside the hydrogel without peptide conjugation, the hydrogel mixed with laminin (20 μg/ml), the hydrogel conjugated with lam-IKVAV of 10 μM, and the hydrogels with our novel IKVAV peptide of different concentrations of 10μM, 50μM, and 100 μM at days 3, 7, and 21. Human NSCs encapsulated in the hydrogel without peptide failed to migrate at day 3. Very few hNSCs migrated out from the neurospheres after day 7. When compared with the blank hydrogel group, hNSCs in all of the peptide-conjugated hydrogels showed a greater ability to migrate. By contrast, hNSCs encapsulated in hydrogels with our short peptides of 10 μM expressed the greatest ability to migrate, at more than twofold longer distance at all time points (Fig. 6B, p < .05). The distance of migration was statistically significant as the function of time (Fig. 6B, p < .05). There was no significant difference in cell migration between groups of the hydrogels mixed with laminin and the hydrogels conjugated with short IKVAV peptide at 10 μM.
Figure 6.
Cell migration inside the hydrogels. (A): The morphology of human neurospheres inside hydrogel conjugated with lam-IKVAV (10 μM), hydrogel with short IKVAV peptide (10 μM), and hydrogel mixed with LN (20 μg/ml). (B): Quantification of cell migration from human neurospheres inside hydrogel conjugated with lam-IKVAV (10 μM), short IKVAV peptide of different concentrations of 10 μM, 50 μM, and 100 μM, and hydrogel mixed with LN (20 μg/ml). A greater rate of migration was found in the hydrogel conjugated with short IKVAV peptide at 10 μM than other concentrations at all time points (∗p < .05). Migration of cells was statistically significant as a function of time (†p < .05). There was no significant difference in cell migration between groups of the hydrogels mixed with LN and the hydrogels conjugated with short IKVAV peptide at 10 μM. Abbreviations: IKVAV, Ile-Lys-Val-Ala-Val sequence; Lam-IKVAV, Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg sequence; LN, laminin.
Discussion
Human NSCs Cultured on Our Peptide-Coated Surface
The greater efficacy and consistency of our short peptide for hNSC adhesion may have resulted from the better presentation of the IKVAV sequence to cell receptors than the linear lam-IKVAV peptide, as described above. Similar to our result, Kämmerer and coworkers demonstrated that cyclic-RGD modified surfaces showed a significantly increased influence on endothelial cell adhesion and proliferation compared with the linear-RGD modified surfaces [15].
Our short IKVAV peptide has significant higher potential in inducing hNSCs to differentiate into neurons than laminin. The reason lies in the fact that our short peptide has presented higher density of bioactive IKVAV to hNSCs than whole-molecule laminin. IKVAV has been shown to promote neuronal differentiation [16]. Previous studies have shown that the concentration of surface peptide conjugated to gold surface is between 2 × 10−11 and 1 × 10−10 mol/cm [2, 17–19]. Thus, our peptide presents to cells at least 1.2 × 1013 IKVAV epitopes per square centimeter. By contrast, closely packed laminin protein molecules in a 2D lattice on a solid substrate have an estimated 7.5 × 1011 IKVAV epitopes per centimeter [2, 16]. Our short peptide could amplify the epitope density relative to a whole laminin coating at least more than 10 times. Moreover, in comparison with whole laminin, our short peptide is less likely to exhibit steric hindrance and is much more stable [5]. In addition, our peptide is very short and very easy to synthesize and is also very low cost. With this peptide sequence, the need for human and animal sources of laminin in cell culture will be minimal or even eliminated.
The Attachment and Proliferation of hNSCs on PEG Hydrogels Conjugated With Our Peptide
PEG acts as an inert structural platform because of its hydrophilicity and resistance to protein adsorption [20]. Lam-IKVAV, when conjugated to PEG hydrogels, did not support any hNSC attachment. This result is consistent with the previous studies in which the same peptide failed to induce adhesion or proliferation of rat hippocampus NSCs when grafted to an interpenetrating poly(acrylamide) hydrogel surface [21]. In contrast, our novel peptide improved hNSC attachment, spreading, and proliferation on the surface of our short peptide-conjugated PEG hydrogels. The greater efficacy of our short peptide on cell adhesion may have resulted from the better presentation of the IKVAV sequence to cell receptors than the linear lam-IKVAV peptide in a hydrogel configuration. Yu and coworkers also demonstrated that Cys-Asp-Pro-Gly-Tyr-Ile-Gly-Ser-Arg, which more closely mimics the native region conformation of the active sites, exhibited greater cellular responses compared with Gly-Tyr-Ile-Gly-Ser-Arg when conjugated to poly(2-hydroxyethyl methacrylate) hydrogel [22].
Human NSCs Migrated Inside the Hydrogels Conjugated With Our Short Peptide
In this study, when our short IKVAV peptide was conjugated to hydrogels in different concentrations, the highest migration rate of hNSCs inside the hydrogels was found at the short IKVAV peptide density of 10 μM. Higher concentrations of our short IKVAV peptides exhibited inhibitory effects on cell migration. It has been shown that migration of cells within hydrogels involves the dynamic interaction between cell surface receptors and adhesion ligands in the hydrogels and the microscopic porosity of hydrogels [23, 24]. The concentration of available ligands, the specific binding affinity of the receptor-ligand pairs, and the strength of the binding affect the binding dynamics and, in turn, the migration behaviors. Our result is consistent with previous studies that demonstrated that the biphasic response of cell migration depending on peptide density was more prevalent at higher receptor-ligand binding affinity [2, 23, 25].
The application of specific peptides has received much attention for use in hydrogel functionalization for neural tissue regeneration [26]. One group, for example, used factor XIIIa to covalently attach certain exogenous bioactive peptides within fibrin hydrogels during crosslinking. It was found that these peptides sequences, including RGD; IKVAV; YIGSR; His-Ala-Val, or HAV; and RNIAEIIKDI all enhanced neurite extension of chicken embryo dorsal root ganglions individually. Although different behavior types, including inhibitory, additive, noninteractive, and synergistic, were seen when the peptides were incorporated in combination [2, 27]. Some groups developed a more complex structure by self-assembly of peptide amphiphile molecules. By incorporating the IKVAV sequence, these nanofiber hydrogels have been shown to inhibit glial scar formation, enhance neuronal differentiation of neural progenitor cells, and promote axon elongation, which ultimately resulted in behavioral improvements after spinal cord injury in mice once implanted in vivo [16, 28]. Our PEG hydrogels conjugated with our short peptide show great potential for neural tissue regeneration. First, an in situ crosslinkable hydrogel is preferred because the injectable material could be formed into any desired shape at the site of injury and the crosslinkable polymer mixture would adhere to the tissue during gelation. In addition, the mechanical interlocking arising from irregular interfaces would strengthen the tissue-hydrogel integration. Second, attracting endogenous stem cells to injured or diseased areas has been a much researched endeavor in past years for neural tissue regeneration [29, 30]. Our short IKVAV peptide functionalized hydrogels allowing for 3D cell migration inside the hydrogels are highly desirable during the regeneration process.
Conclusion
In this study, we developed a short peptide sequence with only 12 amino acids, CCRRIKVAVWLC. This novel peptide, when compared with the lam-IKVAV peptide, enhanced NSC attachment and proliferation and directed NSC differentiation into neurons. When conjugated to PEG hydrogels, this specific peptide supported NSC attachment and proliferation on the surface of hydrogels and enhanced cell migration, with the maximum enhancement at peptide density of 10 μM. This novel short peptide shows great promise in stem cell culture and neural tissue regeneration applications.
Supplementary Material
Acknowledgments
This work was made possible by the U.S. National Science Foundation Faculty Early Career Development award (1055922), the Ministry of Science and Technology of China (2012CB966300), the U.S. Department of Defense (0810187), the National Natural Science Foundation of China (81271369), the U.S. National Institutes of Health National Institute of Neurological Disorders and Stroke (R01 NS050243), and the American Heart Association (10PRE4280017). We thank Dr. Delphine Dean for her help with AFM data collection.
Author Contributions
X.W., N.Z., X. Li, X. Liu: conception and design, experiments, data analysis and interpretation, manuscript writing; B.J., C.J.C., Y.T.: data analysis and interpretation.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Tzu J, Marinkovich MP. Bridging structure with function: Structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40:199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schense JC, Bloch J, Aebischer P, et al. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18:415–419. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 3.Graf J, Ogle RC, Robey FA, et al. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman HK, Weeks BS, Cannon FB, et al. Identification of a 110-kDa nonintegrin cell surface laminin-binding protein which recognizes an A chain neurite-promoting peptide. Arch Biochem Biophys. 1991;290:320–325. doi: 10.1016/0003-9861(91)90547-v. [DOI] [PubMed] [Google Scholar]
- 5.Hersel U, Dahmen C, Kessler H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Feng Z, Zhang A, et al. Synthesis and characterization of three-dimensional crosslinked networks based on self-assemly of α-cyclodextrins with thiolated 4-arm PEG using a three-step oxidation. Soft Matter. 2006;2:343–349. doi: 10.1039/b517206c. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Liu X, Zhao W, et al. Manipulating neural-stem-cell mobilization and migration in vitro. Acta Biomater. 2012;8:2087–2095. doi: 10.1016/j.actbio.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh K, Shu XZ, Mou R, et al. Rheological characterization of in situ cross-linkable hyaluronan hydrogels. Biomacromolecules. 2005;6:2857–2865. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 9.Shu XZ, Ghosh K, Liu Y, et al. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68:365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 10.Bastús NG, Sánchez-Tilló E, Pujals S, et al. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano. 2009;3:1335–1344. doi: 10.1021/nn8008273. [DOI] [PubMed] [Google Scholar]
- 11.Bain CD, Troughton EB, Tao YT, et al. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989;111:321–335. [Google Scholar]
- 12.Nuzzo RG, Allara DL. Adsorption of bifunctional organic disulfides on gold surfaces. J Am Chem Soc. 1983;105:4481–4483. [Google Scholar]
- 13.Nuzzo RG, Fusco FA, Allara DL. Spontaneously organized molecular assemblies. 3. Preparation and properties of solution adsorbed monolayers of organic disulfides on gold surfaces. J Am Chem Soc. 1987;109:2358–2368. [Google Scholar]
- 14.Elbert DL, Pratt AB, Lutolf MP, et al. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Kämmerer PW, Heller M, Brieger J, et al. Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur Cell Mater. 2011;21:364–372. doi: 10.22203/ecm.v021a27. [DOI] [PubMed] [Google Scholar]
- 16.Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 17.McMillan R, Meeks B, Bensebaa F, et al. Cell adhesion peptide modification of gold-coated polyurethanes for vascular endothelial cell adhesion. J Biomed Mater Res. 2001;54:272–283. doi: 10.1002/1097-4636(200102)54:2<272::aid-jbm15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Sheardown H, Tengvall P, et al. Peptide modified gold-coated polyurethanes as thrombin scavenging surfaces. J Biomed Mater Res. 2000;49:66–78. doi: 10.1002/(sici)1097-4636(200001)49:1<66::aid-jbm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Saneinejad S, Shoichet MS. Patterned glass surfaces direct cell adhesion and process outgrowth of primary neurons of the central nervous system. J Biomed Mater Res. 1998;42:13–19. doi: 10.1002/(sici)1097-4636(199810)42:1<13::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Whitesides G. Poly(ethylene glycol) chemistry biotechnical and biomedical applications J. Milton Harris, Ed. Appl Biochem Biotechnol. 1993;41:233–234. [Google Scholar]
- 21.Saha K, Irwin EF, Kozhukh J, et al. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J Biomed Mater Res A. 2007;81:240–249. doi: 10.1002/jbm.a.30986. [DOI] [PubMed] [Google Scholar]
- 22.Yu TT, Shoichet MS. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials. 2005;26:1507–1514. doi: 10.1016/j.biomaterials.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–6818. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 24.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palecek SP, Loftus JC, Ginsberg MH, et al. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 26.Sur S, Pashuck ET, Guler MO, et al. A hybrid nanofiber matrix to control the survival and maturation of brain neurons. Biomaterials. 2012;33:545–555. doi: 10.1016/j.biomaterials.2011.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaff M, Tangemann K, Müller B, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 28.Tysseling-Mattiace VM, Sahni V, Niece KL, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin K, Mao X, Xie L, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura M, Refaat MM, Shinoyama M, et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.