Induced pluripotent stem cells (iPSCs) show considerable promise for cell replacement therapies for Huntington’s disease (HD). Results suggest that transplantation of adenovirus-generated iPSCs may provide a potential avenue for therapeutic treatment of HD.
Keywords: Huntington’s disease, iPSC, Transplantation, Adenovirus, Stem cell, 3-Nitropropionic acid
Abstract
Induced pluripotent stem cells (iPSCs) show considerable promise for cell replacement therapies for Huntington’s disease (HD). Our laboratory has demonstrated that tail-tip fibroblasts, reprogrammed into iPSCs via two adenoviruses, can survive and differentiate into neuronal lineages following transplantation into healthy adult rats. However, the ability of these cells to survive, differentiate, and restore function in a damaged brain is unknown. To this end, adult rats received a regimen of 3-nitropropionic acid (3-NP) to induce behavioral and neuropathological deficits that resemble HD. At 7, 21, and 42 days after the initiation of 3-NP or vehicle, the rats received intrastriatal bilateral transplantation of iPSCs. All rats that received 3-NP and vehicle treatment displayed significant motor impairment, whereas those that received iPSC transplantation after 3-NP treatment had preserved motor function. Histological analysis of the brains of these rats revealed significant decreases in optical densitometric measures in the striatum, lateral ventricle enlargement, as well as an increase in striosome size in all rats receiving 3-NP when compared with sham rats. The 3-NP-treated rats given transplants of iPSCs in the 7- or 21-day groups did not exhibit these deficits. Transplantation of iPSCs at the late-stage (42-day) time point did not protect against the 3-NP-induced neuropathology, despite preserving motor function. Transplanted iPSCs were found to survive and differentiate into region-specific neurons in the striatum of 3-NP rats, at all transplantation time points. Taken together, these results suggest that transplantation of adenovirus-generated iPSCs may provide a potential avenue for therapeutic treatment of HD.
Introduction
Huntington’s disease (HD) is an autosomal dominant disorder caused by an expanded and unstable CAG trinucleotide repeat that causes a progressive degeneration of neurons, primarily in the putamen, caudate nucleus, and cerebral cortex [1]. HD occurs when the gene that codes for the huntingtin (HTT) protein, located on the short arm of chromosome 4, contains more CAG repeats [2]. A CAG repeat count that exceeds 38 corresponds to the onset of HD symptoms, which initially include cognitive impairment and psychiatric disturbances, such as irritability, aggressiveness, and depression. These symptoms precede involuntary motor disturbances, rapid weight loss, and eventual death approximately 15–20 years after the onset of motor symptoms [2, 3].
Currently, there is no cure for HD, and only restorative treatments aimed at reducing its motor symptoms are available. Pharmacotherapy is difficult in HD, because of the complexity and amount of damage to the brain, particularly before the onset of self-reported symptoms. As a result of the time and nature in diagnosing HD (following symptomatic motor deficits and neuronal loss), restorative therapies are focusing on creating a neuroprotective environment to slow the loss of neurons and/or replacing lost neurons, either through stimulating endogenous neurogenesis or through transplantation of cells capable of differentiating, integrating, and replacing the types of cells needed to facilitate recovery.
Several long-term clinical studies have been conducted to assess the viability of cells isolated from fetal tissues as a therapeutic treatment for HD. These studies have produced varying degrees of efficacy as a strategy for long-term cell therapy for HD. Bachoud-Lévi [4] found that three of five patients transplanted with fetal ganglionic eminence showed metabolically active graft cells 10 years after transplantation. Others have shown that when the ganglionic eminence is transplanted into HD patients, the cells display a pattern of neurodegeneration that is similar to what is observed in HD [5], or they begin to show aggregation of the mutant HTT protein [6]. Whereas ganglionic tissue transplanted into HD patients has shown some positive effects, there are many problems with the continued use of fetal cells for transplantation therapies, which underscores the need for new sources of pluripotent stem cells.
In a seminal publication, Takahashi and Yamanaka [7] demonstrated that differentiated somatic cells could be reverted back to an embryonic stem cell (ESC)-like state through forced expression of four factors: Oct4, Sox2, Klf4, and c-Myc. Use of these cells, classified as induced pluripotent stem cells (iPSC), holds considerable promise for tissue repair or replacement, while avoiding ethical, immunological, and availability issues that are inherent with the use of ESCs [8]. Initially, the potential utility of iPSCs was met with skepticism, but several independent groups have shown that these cells have morphology, growth, and gene expression similar to ESCs and were also capable of forming adult chimeras [9] and functional germ cells [10].
Because of critical advantages over fetal or embryonic cell transplants, the use of iPSCs as a potential for treating neurodegenerative diseases has been the focus of a new line of research [11]. The use of iPSCs for treating neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and HD, holds considerable promise. Our group has demonstrated that adenovirus-generated rat iPSCs are capable of surviving in the striatum of immune-competent rats for at least 90 days and can differentiate into neurons expressing the medium spiny neuronal marker DARPP32 [12].
The present study focused on the transplantation of these adenovirus-generated iPSCs into the 3-nitropropionic acid (3-NP) rat model of HD. The neurotoxin, 3-NP, crosses the blood-brain barrier and can be administered systematically to induce cell death in the brain, through energy-depleting mechanisms that closely mimic those observed in HD [13]. The evidence supporting the role of energy impairment in the pathology that resembles those in HD has led to the increased use of mitochondrial toxins, such as 3-NP, in animal models to create the neuropathology and behavioral abnormalities of this disease [14]. Intoxication of 3-NP over several days leads to a selective and progressive striatal lesion that mimics the neuropathological phenotype that is observed in HD patients [15]. Although the 3-NP rat model lacks the gene-induced pathogenesis of HD, it provides a more accurate model of cell loss and replicates the progression of many of the motoric features of the disease [16].
The goals of the present experiment were to test the efficacy of transplanted adenovirus-generated iPSCs in the 3-NP rat model of HD. The efficacy of this approach was assessed using behavioral analysis, measures of neuropathology, amounts of neuronal differentiation, and levels of specific gene expression after transplantation of iPSCs in 3-NP-treated rats.
Materials and Methods
Animals
Fifty-six Sprague-Dawley rats (28 male and 28 female) at 7.5–8.5 weeks of age were used in this study. As no gender differences were observed in the toxicity of the 3-NP and behavioral recovery following transplantation of iPSCs, all treatment groups consisted of both male and female rats. Rats were randomly assigned to one of five groups: sham (5 males and 4 female; given injections of phosphate-buffered saline [PBS]); 3-NP iPSC 7 days (5 male and 6 female rats injected with 3-NP and iPSC transplantation 7 days following the start of 3-NP administration); 3-NP iPSC 21 days (6 male and 6 female rats injected with 3-NP and given iPSC transplantation 21 days after the start of 3-NP administration); 3-NP iPSC 42 days (6 male and 6 female rats injected with 3-NP and given iPSC transplantation 42 days after the start of 3-NP administration); and 3-NP (6 male and 6 female) rats injected with 3-NP, but not given transplants of iPSC, served as controls. Experimenters remained blinded to the group identity of the rats until the final analyses were performed.
3-NP Administration
3-NP administration was adapted from a previously established protocol [15]. Briefly, rats were intraperitoneally injected with an increasing dose of 3-NP dissolved in PBS (pH 7.4) over 82 days. Rats received twice daily (at 0700 and 1900 hours) injections for 9 consecutive days, twice daily injections every third day for 5 weeks, and a single injection (at 0700 hours) once per week for the duration of the study. Concentrations of 3-NP (for each injection) were 2.5 mg/kg (days 1–3), 3.75 mg/kg (days 4–6), 5 mg/kg (days 7–9), 6.25 mg/kg (days 12, 15, and 18), 7.5 mg/kg (days 21, 24, and 27), 8.75 mg/kg (days 30, 33, 36, 39, and 42), 10 mg/kg (days 47 and 54), 15 mg/kg (days 61 and 68), and 20 mg/kg (days 75 and 82).
Body Weight
Body weight was measured for 4 consecutive days, before the administration of 3-NP, which served as a baseline (average weight for 4 days) for each animal. Body weight was monitored throughout the duration of the study, as metabolic alterations are common in animals receiving 3-NP [17, 18]. The recorded weights for each animal were averaged at the conclusion of each week and converted to a score representative of percent weight compared with baseline ([average weight/baseline weight] × 100).
Accelerod Testing
Accelerod testing followed previously published protocols [19]. All rats were tested for motor coordination on the accelerod at 2 days before the administration of 3-NP (baseline) and for an additional 10 weeks. The accelerod task (Acceler, Rota-Rod 7750; UGO Basile, Comerio, Italy, http://www.ugobasile.com) was used to assess motor coordination. The rats were required to remain on a 6-cm-diameter rod that increased from 4 RPM to 40 RPM over the course of 120 seconds. If the rat was not able to remain on the rotating rod for the full 120 seconds, it fell onto a foam pad located 75 cm below the apparatus.
iPSC Generation
The generation and characterization of the iPSCs followed a previously established/published protocol [12]. Briefly, iPSCs were generated from tail-tip fibroblasts (TTF) of the adult Sprague-Dawley rats and reprogrammed using our novel adenovirus pair (one adenovirus containing c-Myc, and the other adenovirus containing Oct4, Sox2, and Klf4). Induced pluripotent stem cells were maintained on rat deactivated feeder cells and were transplanted between passages 25 and 30. An in-depth characterization of the expression of genes and proteins, as well as morphology of these iPSCs, has been published previously [12]. Briefly, iPSCs highly expressed regulatory genes and pluripotent proteins, measured by real-time polymerase chain reaction (RT-PCR), immunocytochemistry, and flow cytometry. These cells also displayed typical iPSC morphology and the ability to differentiate into neuronal lineages in vitro.
iPSC Transplantation
Thirty-five rats that had received 3-NP administration were given bilateral transplantations of iPSCs at different time points to mimic the different stages of the progression of HD (i.e., 7-day transplantation, early intervention before the onset of motor disturbances; 21-day transplantation, intermediate intervention during the onset of motor disturbances but before robust cell loss in the striatum; 42-day transplantation, late-stage intervention after robust motor deficits were observed and significant cell loss has occurred).
Rats were anesthetized for the duration of the surgery with a mixture of isoflurane and oxygen. The heads of the rats were shaved and disinfected using chlorhexidine (Molnlycke Healthcare, Norcross, GA, http://www.molnlycke.com). Rats were placed into a stereotaxic, a midline incision was made on the scalp, and the skin was retracted, exposing bregma. Two burr holes were placed above the striatum (coordinates relative to bregma: anterior 0.5 mm; lateral +2.5 mm and −2.5 mm; tooth bar set at 0). iPSCs were prelabeled with Hoechst 33358 (5 µg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and resuspended in Hanks’ balanced salt solution (Gibco, Grand Island, NY, http://www.invitrogen.com) at a density of 200,000 cells per microliter. The cell suspension was loaded into a 10 μl Hamilton microsyringe and injected at a constant rate over 3 minutes (0.33 μl/minute) into the striatum (6 mm ventral from the dura) at the above-mentioned coordinates. Following the first injection, the microsyringe was left in place for 3 minutes to allow for the cells to diffuse, then moved dorsally 0.1 mm, and a second injection at the same rate was performed. Following the second injection, the microsyringe remained in place for an additional 3 minutes before being slowly withdrawn over a 3-minute period and then moved to the opposite hemisphere, where this process was repeated to complete the transplantation. The scalp was then closed using sterile 9-mm wound clips, and the rats were placed in a recovery cage until fully mobile, at which point they were returned to their home cage. Rats were observed for 5 days post-transplantation for any signs of pain, discomfort, distress, or complications from surgery, but none of these signs was observed in any of the rats.
Perfusion
At the conclusion of behavioral testing, one-half of the rats that were designated for immunohistochemical analysis were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with ice-cold 0.1 M PBS, followed by 4% paraformaldehyde (diluted in 0.1 M PBS, pH 7.4). The brains were rapidly removed, postfixed in 4% paraformaldehyde for 24 hours at 4°C, and then transferred to 30% sucrose in 0.1 M PBS for 48 hours at 4°C. The brains were then flash frozen, using methylbutane, and stored at −80°C. Serial, coronal sections (30 µm) were cut at −20°C on a cryostat (Vibratome UltraPro 5000; The Vibratome Co., St. Louis, MO, http://www.vibratome.com) and placed into nine wells containing 0.1 M PBS.
Rapid Decapitation
Rats that were not used for immunohistochemical analysis were rapidly decapitated on the same day, and their brains were extracted, dissecting cortex, striatum, and remaining brain, which were flash frozen using liquid nitrogen, for subsequent mRNA analysis.
Histology
Brain tissue was labeled with cytochrome oxidase (CYO) and immunohistochemical tags using either 3,3′-diaminobenzidine (DAB) or fluorescence labels [20]. Briefly, tissue from a single well (containing striatal sections 270 µm apart) was used for each label.
CYO (metabolically active tissue) was used to visualize the lesion, as previous studies have shown that injections of 3-NP cause significant striatal atrophy [15]. Briefly, tissue designated for CYO analysis was submersed in solution of 800 µg of sucrose, 4 mg of cytochrome C, and 1 mg of DAB dissolved in 20 ml of phosphate-buffer for 4 hours at room temperature. The tissue was then transferred to deionized H2O, mounted onto positively charged glass slides, and coverslipped using Depex (Electron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.com/microscopy).
Tissue designated for CD11b (activated microglia; 1:500; Abcam, Cambridge, U.K., http://www.abcam.com) and IBA1 (macrophages; 1:500; Abcam) DAB immunohistochemistry was used to determine the level of endogenous immune response to the 3-NP and the transplanted iPSCs. Tissue was placed into a solution of 0.3% hydrogen peroxide for 10 minutes. It was placed into the primary antibodies overnight at 4°C and then rinsed twice with PBS plus 0.1% Triton X-100 (Sigma-Aldrich) before incubation in the appropriate conjugated secondary antibodies (biotin; 1/300) for 1 hour at room temperature. The tissue was rinsed twice in PBS plus 0.1% Triton X-100 and placed in an avidin-biotin peroxidase (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Sections remained in the solution for 5 minutes, after which they were moved to the DAB solution (Vector Laboratories). After remaining in the DAB solution for 5 minutes, the tissue was transferred into deionized H20. The tissue was mounted onto charged glass slides, given a series of ascending alcohol rinses (70%, 95%, 100%), placed into xylene for 5 minutes, and coverslipped using Depex (Electron Microscopy Sciences).
Tissue designated for fluorescent labeling had nonspecific binding sites blocked by incubating the tissue in 10% normal goat serum, 0.1% Triton X-100, and 0.1 M PBS for 1 hour at room temperature. The tissue was then transferred into a well containing the primary antibodies for 24 hours at 4°C. Primary antibody pairs used for double labeling included neuronal nuclei (NeuN, for mature neurons; 1:500 dilution; Chemicon, Temecula, CA, http://www.chemicon.com) and dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP32, for D1 receptors located on the medium spiny neurons located in the striatum; 1:500; Abcam). Glial fibrillary acidic protein (GFAP, astrocytes; 1:500; Abcam) was used alone on separate sections. Following incubation in the primary antibodies, the tissue was rinsed and transferred into a well containing the appropriate conjugated secondary antibodies for 1 hour at room temperature. Secondary antibodies included Alexa Fluor 488 and Alexa Fluor 594 (1/300; Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Following incubation, the tissue was rinsed and mounted onto positively charged glass slides, using Fluoromount (Sigma-Aldrich).
RNA Isolation
RNA isolation was performed from the nonperfused brain samples, using a Qiagen (Hilden, Germany, http://www.qiagen.com) All-Prep system. All procedures followed the manufacturer’s guidelines. Purified RNA, in the collection tube, was analyzed using a NanoDrop2000 spectrophotometer (ThermoScientific, Waltham, MA, http://www.thermoscientific.com) and was stored at −20°C until used for cDNA synthesis. A QuantiTect Reverse Transcription Kit (Qiagen) was used for cDNA synthesis following the manufacturer’s guidelines. Briefly, RNA was incubated at 42°C for 2 minutes in a genomic DNA elimination buffer. The solution was transferred to a reverse-transcription master mix and incubated at 42°C for 30 minutes and then 3 minutes at 95°C to inactivate the reverse transcriptase. The cDNA was stored at −20°C until used in quantitative polymerase chain reaction experiments.
Analyses of transcripts were performed by quantitative RT-PCR, using 2× QuantiTect SYBR Green RT-PCR Master Mix (Qiagen). The glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) was used as an endogenous control gene. Primers consisted of brain-derived neurotrophic factor (BDNF) and tumor necrosis factor-α (TNF-α; supplemental online Table 1). RT-PCR was performed on MyiQ (Bio-Rad, Hercules, CA, http://www.bio-rad.com), using the following cycling conditions: 10 minutes at 95°C; then 40 cycles of 10 seconds at 95°C; 10 seconds at 50°C; and 30 seconds at 72°C. The fold change of each iPSC lineage was calculated using the δ-δ cycle threshold, normalizing the cycle threshold of each primer pair to GAPDH and then comparing this value to the normalized cycle threshold of a control sample.
Imaging and Image Analysis
Cell counts, area, and optical densitometry were measured using ImageJ, at 100, 300, 500, 700, and 900 µm anterior from bregma, as this area contained the transplanted iPSC [21]. Densitometric measures of CYO, GFAP, IBA1, and CD11b within the entire striatum were taken from sequential sections from the levels described above, and the average intensities were normalized to the corpus callosum of each respective section. CYO-labeled slides were used to measure the optical densitometry of metabolically active tissue in the striatum, as well as for measurements of the lateral ventricle and striosome area, as CYO labeling clearly delineates these structures. The striatum, from the levels described above, was measured by tracing along the border of the lateral ventricle, corpus callosum, and the anterior commissure for both hemispheres. Striosome area was calculated as the sum of the areas of each striosome in each of the selected sections. Methods for rare-event stereology for cell counts were modified from previously published protocols [22]. Briefly, a small number of sections at uniform intervals from the entire set of sections containing the area of interest was selected (at 100, 300, 500, 700, and 900 µm anterior to bregma). Counts of cells were made with optical dissectors, at predetermined x and y coordinates, within each section. Cells were counted as positive cells if they showed the following: (a) antibody immunoreactivity within the cell body; (b) the nucleus of that cell was within the counting frame without touching the exclusion lines; and (c) the nucleus of that cell was in focus. For cell counts, five sections (as described above) were analyzed using ImageJ and the average number of cells per section was calculated.
Statistics
All statistical analyses were performed using SPSS v16 with an α level equal to 0.05. All behavioral data were analyzed using a repeated-measures analysis of variance (ANOVA), to measure changes between groups and treatments across weeks. Histological data were analyzed using a multivariate ANOVA. When appropriate, a Tukey’s honestly significant difference (HSD) post hoc test was performed.
Results
No significant differences were present between male and female rats in the percent change from baseline weight or latency to fall from the accelerod. As such, male and female rats were pooled together for all subsequent analyses.
Weight Analysis
A repeated-measures ANOVA revealed a significant interaction between groups over time for the percentage change in weight (F[13,494] = 6.686, p < .001) (supplemental online Fig. 1). A significant between-group difference was also observed (F[4,38] = 15.203, p < .001). Tukey’s HSD analysis revealed significant differences between sham rats and all other groups across all weeks of testing. Significant differences in body weight across all weeks of testing were also observed between 3-NP rats that received iPSC transplants at either 7 or 21 days and those that did not receive transplants.
Behavioral Results
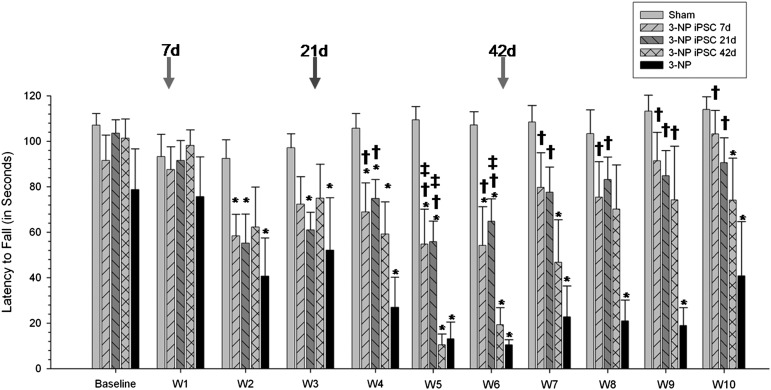
A repeated-measures ANOVA revealed significant between-group differences for the latency to fall in the accelerod task (F[4,31] = 6.021, p = .001) (Fig. 1). Tukey’s HSD analysis revealed significant differences between Sham and 3-NP rats, starting at week 2 and continuing for the duration of the study. The latency to fall for 3-NP-treated rats transplanted with iPSCs after 7 days was significantly different from sham rats at weeks 2, 4, 5, and 6. 3-NP rats transplanted with iPSCs after 21 days were significantly different from sham animals at weeks 4, 5, and 6.
Figure 1.
Accelerod data from iPSC-transplanted 3-NP rats. Analysis of motor coordination revealed significant between-group differences for the latency to fall in the accelerod task. Starting at week 4, and continuing throughout the study, 3-NP rats that received iPSC transplantation at either 7 or 21 days had significantly higher latencies to fall than 3-NP rats that did not receive transplants. Also, 3-NP rats that received transplantation of iPSCs at 42 days showed significantly higher latencies to fall at week 9 than 3-NP rats that did not receive transplants. (Bar graph represents mean value; error bars represent SEM; arrows indicate the time of iPSC transplantation for each group; ∗, significantly different from sham rats, p < .05; †, significantly different from 3-NP rats, p < .05; #, significantly different from 3-NP iPSC 7-day rats, p < .05; ‡, significantly different from 3-NP iPSC 42-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; iPSC, induced pluripotent stem cell.
The latency to fall for 3-NP rats transplanted with iPSCs after 42 days was significantly different from sham rats at weeks 4, 5, 6, 7, and 10. However, starting at week 4, and continuing throughout the study, 3-NP animals that received iPSC transplantation at either 7 or 21 days had significantly higher latencies to fall than untreated 3-NP rats. Also, 3-NP rats that received transplantation of iPSCs at 42 days showed significantly higher latencies to fall at week 9 than untreated 3-NP rats. Interestingly, rats in the 42-day group displayed significant motor impairments, similar to rats receiving 3-NP without transplants before receiving transplantation, but then displayed significant motor recovery 3 weeks after transplantation.
At 9 weeks after the administration of 3-NP, all rats that received transplantation of iPSCs performed at levels that were not significantly different from sham controls, but had significantly longer latencies to fall off the accelerod task, relative to rats that received 3-NP without transplants.
Histological Results
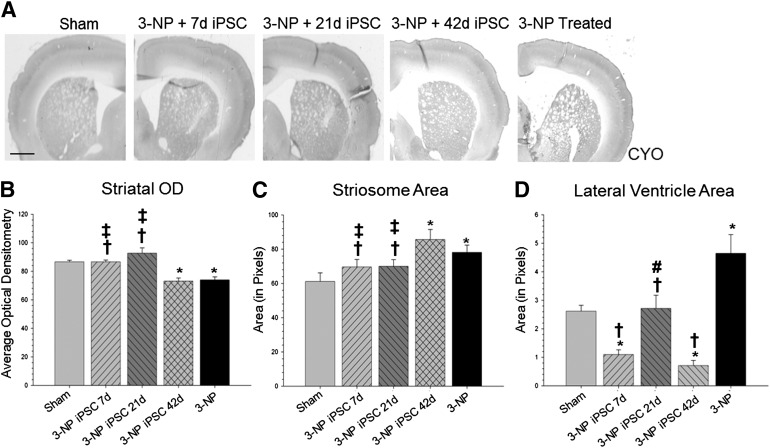
A one-way ANOVA of the brain scans labeled with CYO (used to delineate structures of the brain) revealed significant between-group differences in optical densitometry of striatal tissue (F[4,64] = 14.770, p < .001) (Fig. 2A), striosome area (F[4,119] = 3.783, p < .01) (Fig. 2B), and in the area of the lateral ventricles (F[4,32] = 13.377, p < .001) (Fig. 2C). A Tukey’s HSD revealed that the optical densitometry measure in the striata of 3-NP control rats and 3-NP rats receiving iPSC transplantation at 42 days was significantly lower than sham rats. A Tukey’s HSD also revealed that rats receiving iPSC transplantation at 7 or 21 days had significantly higher measures of optical densitometry in the striatum than untreated 3-NP rats or 3-NP rats that received iPSC transplantation at 42 days.
Figure 2.
Cytochrome oxidase labeling. (A): Analysis of the brain scans labeled with CYO revealed significant between-group differences in optical densitometry measures in the striatum (B). It was also revealed that rats receiving iPSC transplantation at 7 or 21 days had significantly higher optical densitometry measures in the striatum than 3-NP rats that did not receive transplants or 3-NP rats that received iPSC transplantation at 42 days. In addition, analysis of the striosome area revealed significant between-group differences (C). Rats receiving iPSC transplantation at 7 or 21 days had significantly smaller striosome area when compared with 3-NP rats that did not receive transplants or 3-NP rats that received iPSC transplantation at 42 days. Scans from the same sections also revealed significant between-group differences in the area of the lateral ventricles (D). All groups receiving iPSC transplantation had significantly smaller lateral ventricles when compared with 3-NP rats that did not receive transplants. (Bar graph represents mean value; error bars represent SEM; ∗, significantly different from sham rats, p < .05; †, significantly different from 3-NP rats without transplants, p < .05; #, significantly different from 3-NP iPSC 7-day rats, p < .05; ‡, significantly different from 3-NP iPSC 42-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; iPSC, induced pluripotent stem cell; OD, optical density.
A Tukey’s HSD analysis revealed that the area of the striosomes was significantly larger for 3-NP rats and 3-NP rats receiving iPSC transplantation at 42-day groups, as compared with sham rats. A Tukey’s HSD revealed that rats receiving iPSC transplantation at 7 or 21 days had significantly smaller striosome areas when compared with 3-NP controls or 3-NP rats receiving iPSC transplantation at 42 days. A significant increase in striosome size was observed in 3-NP rats without transplants, when compared with sham rats. This striosome enlargement was prevented by the transplants in the 7-day and 21-day transplant groups.
Lastly, Tukey’s HSD analysis revealed that 3-NP controls had significantly larger lateral ventricles when compared with sham rats. All groups receiving iPSC transplantation had significantly smaller lateral ventricles when compared with untreated 3-NP rats. Interestingly, rats receiving iPSC transplantations at either 7 or 42 days had significantly smaller lateral ventricles than sham rats.
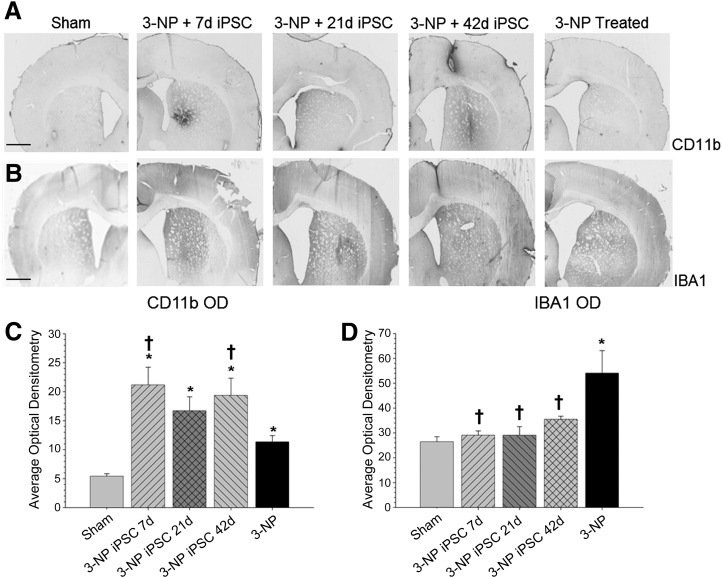
A one-way ANOVA revealed significant between-group differences in the average optical densitometry of CD11b (F[4,103] = 9.758, p < .001) (Fig. 3A), IBA1 (F[4,213] = 7.252, p < .001) (Fig. 3B), and GFAP (F[4,100] = 5.807, p < .001) (Fig. 4A). A Tukey’s HSD analysis revealed that groups receiving 3-NP, regardless of whether they received an iPSC transplant, had higher optical densitometric levels of CD11b than sham rats. It was also shown that 3-NP rats receiving iPSC transplantation at either 7 or 42 days had significantly higher optical densitometry for CD11b labeling in the striatum than did 3-NP control rats.
Figure 3.
Measures of inflammation. Analysis of the immune response as measured by optical densitometry of CD11b (A) and IBA1 (B) to 3-NP and transplanted iPSCs. Analysis of expression levels revealed significant between-group differences in the average optical densitometry of CD11b, with all groups receiving 3-NP having higher optical densitometry measures for CD11b than sham rats (C). Significant between-group differences in the average optical densitometry of IBA1 (D) were also observed, revealing that 3-NP rats without transplants had significantly more macrophage infiltration than sham rats and all rats receiving iPSC transplantation. (Bar graph represents mean value; error bars represent SEM; ∗, significantly different from sham rats, p < .05; †, significantly different from 3-NP rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; iPSC, induced pluripotent stem cell; OD, optical density.
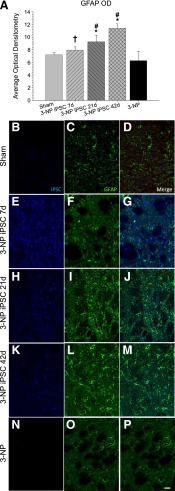
Figure 4.
Optical densitometry of GFAP from the transplant site. Astrocytes (GFAP; green) were observed around the transplanted iPSCs (blue), but no colocalization was observed between the two labels. A significant between-group difference in the average optical densitometry of GFAP (A) was observed. It was revealed that 3-NP rats receiving iPSC transplant at either 21 or 42 days displayed significantly higher GFAP labeling than sham rats and 3-NP rats that received transplantation of iPSCs at 7 days. The 3-NP rats receiving iPSC transplantation at 7 days displayed significantly more astrocyte activation than 3-NP rats that did not receive transplants. Sham rats are shown in (B–D); 7-day iPSC rats are shown in (E–G); 21-day iPSC rats are shown in (H–J); 42-day rats are shown in (K–M); 3-NP rats that did not receive transplants are shown in (N–P). (Scale bar = 50 μm; bar graph represents mean value; error bars represent SEM; ∗, significantly different from sham rats, p < .05; †, significantly different from 3-NP rats, p < .05; #, significantly different from 3-NP iPSC 7-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; GFAP, glial fibrillary acidic protein; iPSC, induced pluripotent stem cell; OD, optical density.
A Tukey’s HSD analysis revealed that 3-NP rats receiving iPSC transplant at either 21 or 42 days displayed significantly higher GFAP labeling than sham rats and 3-NP rats that received transplantation of iPSCs at 7 days. The 3-NP rats receiving iPSC transplantation at 7 days displayed significantly more astrocyte activation than 3-NP control rats. A Tukey’s HSD analysis revealed that 3-NP control rats had a significantly higher macrophage response, as determined by IBA1 labeling, relative to sham rats and all rats receiving iPSC transplantation.
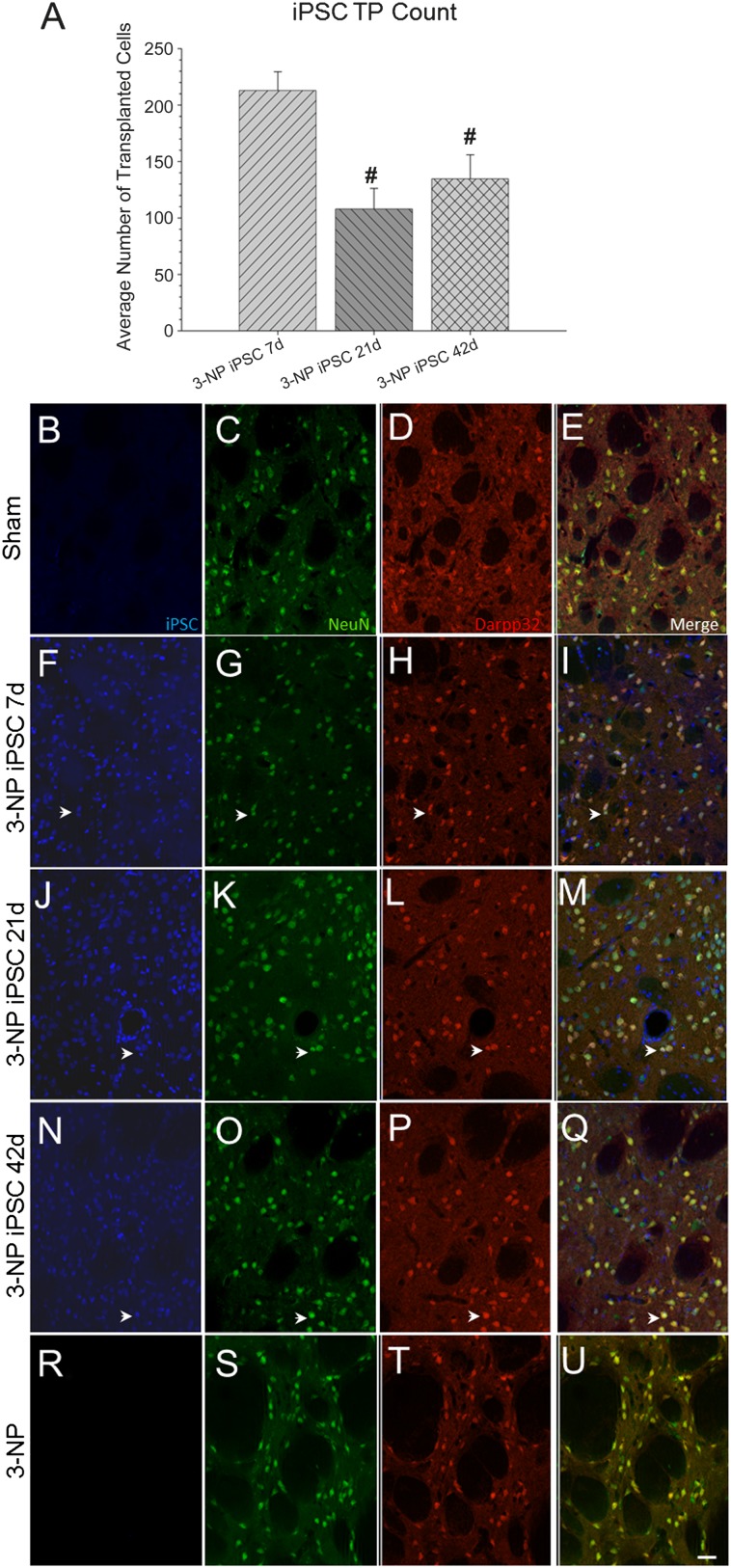
All rats receiving transplants displayed surviving iPSCs around the injection site at the conclusion of the study. However, using rare-event stereology, a significant between-group effect was observed for the average number of surviving iPSCs (F[2,39] = 8.709, p = .001) (Fig. 5A). A Tukey’s HSD revealed that the 7-day transplantation time point had significantly more surviving iPSCs than the 21- or 42-day transplantation group.
Figure 5.
Immunohistochemical analysis of transplanted iPSCs (blue), mature neurons (NeuN; green), and medium spiny neurons (Darpp32; red). All transplanted rats displayed surviving iPSCs (blue) around the injection site at the conclusion of the study. However, a significant between-group effect was observed for the average number of surviving iPSCs (A). It was revealed that the 7-day transplantation time point had significantly more surviving iPSCs than the 21- or 42-day transplantation group. Sham rats are shown in (B–E); 7-day iPSC rats are shown in (F–I); 21-day iPSC rats are shown in (J–M); 42-day rats are shown in (N–Q); 3-NP rats that did not receive transplants are shown in (R–U). (Scale bar = 50 μm; colocalization is shown by white arrowheads; bar graph represents mean value; error bars represent SEM; #, significantly different from 3-NP iPSC 7-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; iPSC, induced pluripotent stem cell.
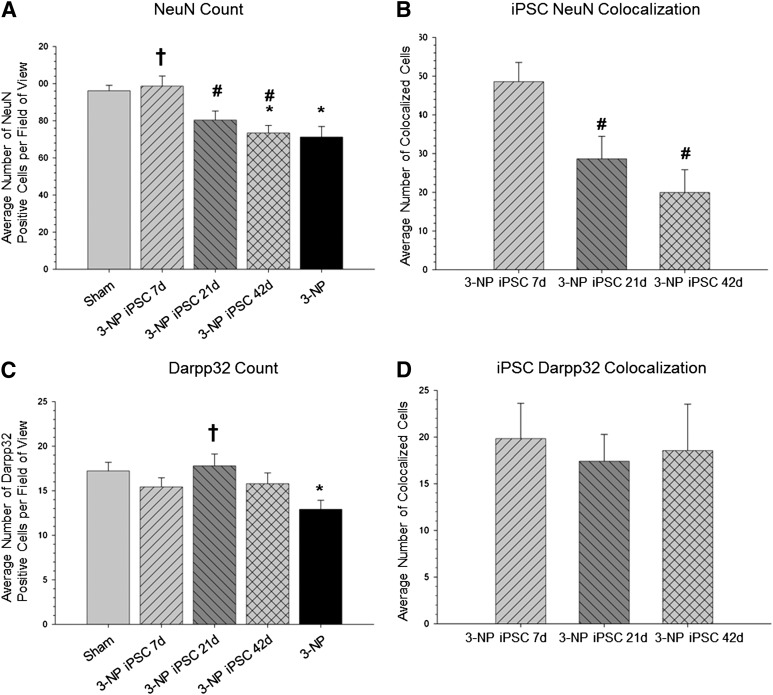
One-way ANOVA revealed a significant between-group difference in the number of NeuN-positive cells in the area around the transplant site (F[4,95] = 4.374, p < .05) (Fig. 6A). A Tukey’s HSD revealed that 3-NP control rats and 3-NP rats receiving iPSC transplants at 42 days had significantly fewer NeuN-positive cells than sham rats. A Tukey’s HSD revealed that 3-NP rats that received iPSC transplantation at 7 days had a significantly greater amount of NeuN-positive cells than the 3-NP control rats or the 3-NP rats receiving transplantation at either 21 or 42 days. A significant between-group difference was found in the number of transplanted iPSC cells that were colocalized with NeuN (F[2,39] = 7.896, p = .001) (Fig. 6B). Tukey’s HSD revealed that the 7-day transplantation group had more iPSCs colocalized with NeuN than either the 21- or 42-day group.
Figure 6.
Cell counts of mature neurons, medium spiny neurons, and colocalization with transplanted iPSCs. A significant between-group difference in the number of NeuN-positive cells in the area around the transplant site (A) was observed. It was revealed that 3-NP rats that received iPSC transplantation at 7 days had a significantly greater amount of NeuN-positive cells than the 3-NP rats that did receive transplants or the 3-NP rats receiving transplantation at either 21 or 42 days. A significant between-group difference was found in the number of transplanted iPSC cells colocalizing with NeuN (B). It was found that the 7-day transplantation group had more iPSCs colocalized with NeuN than either the 21- or 42-day group. A significant between-group difference was observed in the number of DARPP32-positive cells in the area around the transplant site (C). It was found that 3-NP rats receiving iPSC transplantation at 21 days had significantly more DARPP32-positive cells than 3-NP control rats. However, no between-group differences were observed in the number of iPSCs colocalizing with DARPP32 (D). (∗, Significantly different from sham rats, p < .05; †, significantly different from 3-NP rats, p < .05; #, significantly different from 3-NP iPSC 7-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; iPSC, induced pluripotent stem cell; NeuN, neuronal nuclei.
Similarly, a significant between-group difference was observed in the number of DARPP32-positive cells in the area around the transplant site (F[4,114] = 2.814, p < .05) (Fig. 6C). A Tukey’s HSD revealed that 3-NP control rats had significantly fewer DARPP32-positive cells in the area around the transplant site, compared with sham rats. It was also found that 3-NP rats receiving iPSC transplantation at 21 days had significantly more DARPP32-positive cells than 3-NP control rats. However, no between-group differences were observed in the number of iPSCs colocalizing with DARPP32 (F[2,39] = 0.083, p > .05) (Fig. 6D).
mRNA Expression
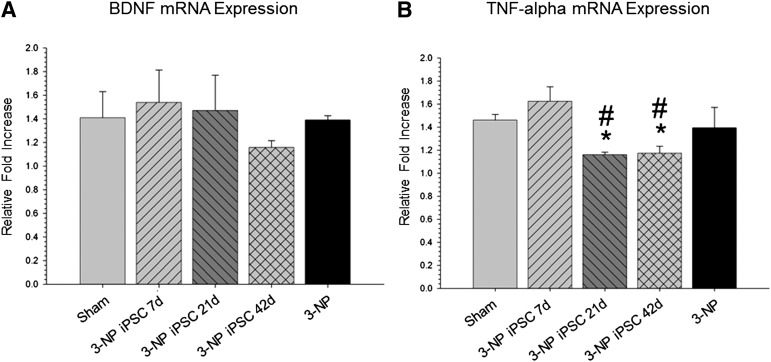
Quantitative RT-PCR of striatal tissue revealed no significant differences in the mRNA expression of BDNF between all groups (F[4,18] = 0.443, p > .05) (Fig. 7A). Quantitative RT-PCR of striatal tissue did, however, reveal a significant difference in the mRNA expression of TNF-α (F[4,18] = 4.776, p < .05) (Fig. 7B), with the 3-NP 21-day and 42-day group having significantly lower levels than sham group.
Figure 7.
Quantitative real-time polymerase chain reaction (RT-PCR) of striatal tissue for mRNA expression of BDNF and TNF-α. Quantitative RT-PCR of striatal tissue revealed no significant differences in the mRNA expression of BDNF between all groups (A), but did reveal a significant between-group difference in the mRNA expression of TNF-α (B). No reduction in BDNF expression was observed in the 3-NP rats without transplantation, indicating that behavioral deficits are not the result of depletion of this trophic factor. The downregulation of TNF-α in the rats that received transplantation of iPSCs at 21 and 42 days is indicative of a general deactivation of the immune response to the transplanted cells. (Bar graph represents mean value; error bars represent SEM; ∗, significantly different from sham rat, p < .05; #, significantly different from 3-NP iPSC 7-day rats, p < .05.) Abbreviations: 3-NP, 3-nitropropionic acid; BDNF, brain-derived neurotrophic factor; iPSC, induced pluripotent stem cell; TNF, tumor necrosis factor.
Quantitative RT-PCR revealed significant between-group differences in the mRNA expression for TNF-α, with the 21-day and 42-day transplant groups showing reduced levels of TNF-α, relative to those in the sham and 7-day transplant groups.
Discussion
Chronic administration of 3-NP caused a significant decrease in weight and impaired motor coordination, as measured on the accelerod, in rats. Whereas motor impairment was prevented or rescued after transplantation of iPSCs, no effect on weight loss was observed. All rats injected with 3-NP displayed a significant decrease in weight, regardless of therapeutic intervention. However, as shown in supplemental online Figure 1, rats given transplants had a nonsignificant trend toward having lower body weights. Further work is needed to ensure that such transplants do not exacerbate toxic- or disease-induced weight loss.
Interestingly, after transplantation of iPSCs, 3-NP-treated rats displayed either a preservation of motor function or behavioral recovery. Although 3-NP-treated rats in the 7-day and 21-day transplant groups displayed motor dysfunction when compared with sham rats at 1 week after their therapeutic intervention, there was evidence of some preservation of motor abilities, as indicated by a significantly longer latency to fall when compared with rats receiving only 3-NP. The 3-NP-treated rats in the 42-day transplant group mimicked the progressive decline in motor performance, when compared with the 3-NP-treated rats that did not receive transplants. However, after transplantation of iPSCs, rats in the 42-day group performed significantly better than 3-NP-treated rats that did not receive transplants and were not significantly different from sham animals at weeks 8 and 9. Data from the early- and middle-stage intervention indicated that iPSCs may be able to prevent the progressive loss of motor coordination, as measured on the accelerod, and that late-stage transplantation of iPSCs can promote behavioral recovery. Transplantation of pluripotent cells, isolated from fetal tissue, has demonstrated similar preservation of motor function in animal models of HD [23–28], but our findings provide the first demonstration of iPSC-induced functional recovery in a HD rodent model do date.
Optical densitometric measures, taken from the striatum, revealed that 3-NP-treated rats that did not receive transplants and 3-NP-treated rats receiving iPSC transplantation at 42 days had significantly lower values than did sham rats. This decrease indicates a loss in metabolic activity in striatal tissue at the end point of the study. These results were not unexpected, as administration of 3-NP causes a significant lesion in the striatum of rats [15]. However, rats receiving iPSC transplantation at 7 or 21 days had significantly higher levels of optical densitometry of CYO labeling in the striatum than untreated 3-NP rats or 3-NP rats that received iPSC transplantation at 42 days, suggesting a preservation of the metabolic activity in the striatum. This finding corresponds to other studies that have shown transplantation of human embryonic neuronal stem cells reduces striatal atrophy in animals with surviving grafts [29–31].
Measures of striosome size were also responsive to transplants of iPSCs. The striatum is made of two complementary chemical components, the extracellular matrix, thought to contain many of the input-output connections of the medium spiny neurons, and the striosomes, which are thought to project mainly to the dopamine-containing neurons in the substantia nigra pars compacta [32]. Striosomes are widely distributed throughout the striatum and are thought to exert a global influence on striatal processing and that imbalances within the striosome/matrix relationship are related to symptomatology of HD [32]. A significant increase in striosome size was observed in 3-NP-treated rats that did not receive transplants, when compared with sham rats, indicating an imbalance in the striatal tissue. This striosome enlargement was not observed in the 7-day and 21-day transplant groups, suggesting that transplantation of iPSCs at these time points prevents this reorganization of striatal tissue. Interestingly, 3-NP-treated rats that received iPSC transplantation at 42 days, while displaying behavioral recovery, showed similar levels of striosome enlargement, suggesting a disjunction between behavioral recovery and striosome size.
Histological analysis revealed that rats receiving 3-NP without transplants had significant larger lateral ventricles, when compared with sham rats. Rats in the 7-day or 21-day transplant groups did not have the significant lateral ventricle enlargements that were observed in the 3-NP rats that did not receive the transplants. Interestingly, rats in the 42-day group had no significant enlargement of the lateral ventricles.
Taken together, it is apparent that administration of 3-NP over the time course of this study induced striatal degeneration, as observed by a decrease in the optical densitometric measures of CYO in the striatum, an enlargement of the striosome area within the striatum, and lateral ventricle enlargement. Rats in the 7-day or 21-day transplant groups did not display these same 3-NP-induced deficits, and the anatomy of these structures more closely resembled those of the sham-injected rats. These data suggest that the early and middle intervention time points were successful in preventing this specific striatal degeneration. Interestingly, rats in the 42-day transplant group did not show the same level of striatal sparing as rats in the 7- or 21-day transplant group, despite displaying significant behavioral recovery. Rats in the 42-day transplant group displayed a similar decrease in striatal optical densitometry and striosome enlargement as the 3-NP-treated rats that did not receive transplants but did not have lateral ventricle enlargement. As a result of the late-stage intervention in the 42-day transplant group, it is possible that much of the striatal loss and reorganization may have already occurred, making it difficult for the transplanted cells to effectively rebuild the striatum. It is possible that the iPSCs in the 42-day transplant group may require more time in vivo, following transplantation, to fully integrate into the striatum and reverse some of the HD-related pathology.
When examining the immune response to these transplanted cells, an analysis of activated microglia (CD11b) revealed that all rats that received 3-NP had significantly more activated microglia than sham rats and that rats in the 7-day and 42-day groups had more activated microglia than rats receiving 3-NP without transplantation, indicating possible local immune responses to the transplanted cells. An analysis of macrophage response (IBA1) revealed that the 3-NP rats that did not receive transplants had significantly more positive IBA1 labeling than all other groups, indicating that the transplants did not activate a significant host macrophage response. The immune response, in terms of the iPSC-transplant-induced activated microglia and macrophage response observed in this study, has also been reported previously in a study [12, 33]. Although an immune response was observed in terms of CD11b and IBA1 labeling present in the brain of these rats, transplanted cells were still visualized. In addition, none of the 3-NP iPSC-transplanted groups showed an upregulation of mRNA expression of TNF-α, suggesting that there was not a continuous immune response to these cells. TNF-α is implicated to play a central role in initiating the cascade of cytokines that are responsible for an immune response in the brain [34] and can be a general indicator of the immune response after cell transplantation. These data, along with immunohistochemistry of IBA1 and CD11b, suggest that the transplanted cells were tolerated relatively well by the host immune system.
Immunohistochemical analysis of astrocyte activation around the transplant site revealed that astrocyte activation was dependent on the time of iPSC transplantation. Rats in the 21-day or 42-day transplant groups displayed more astrocyte activation than the sham rats or rats in the 7-day transplant group. Less than 1% of iPSCs displayed colocalization with the astrocyte marker GFAP, suggesting that very few transplanted iPSC differentiated into astrocytes. It has been previously reported that, following transplantation of embryonic neural stem cells, the majority of the transplanted cells differentiated into glial lineage [33]. This discrepancy in the amount of GFAP labeling between our study and the previous work may be due in part to the specific location in which the neural stem cells were harvested and/or the type of culture media used before transplantation.
Similar to transplantation of these iPSCs into a healthy rat striatum [12], transplantation into the striata of a 3-NP brain revealed survival and differentiation into mature, region-specific neurons in the brain of the rats at all time points. The number of surviving iPSC at the conclusion of the study was dependent on the transplantation time point. It was observed that the 7-day transplant group had the most surviving cells at the conclusion of the study, with the 21-day transplant group having fewer than the 7-day group, and the 42-day transplant group having the fewest. Perhaps early intervention, before widespread cell loss in the striatum, would allow for the cells in the 7-day transplant group to integrate more efficiently. It is possible that the lower number of surviving cells in the 42-day transplant group is a result of the microenvironment into which the cells were transplanted. Chronic exposure to 3-NP causes mitochondrial dysfunction and eventual cell death in a time-dependent manner. Early- and middle-intervention time points used in this study may have preceded changes in the microenvironment that made it unsuitable for cell survival following transplantation, and this could account for the time-dependent decrease in transplant survival.
As previously described [14, 15, 35], chronic administration of 3-NP leads to significant cell death, specifically of the medium spiny neurons. In our study, we observed significant neuronal loss in the 3-NP rats without transplants, when compared with sham rats. In the 7-day transplant group, there was no significant decrease in the number of neuronal cells within the striatum, suggesting that iPSC transplantation at this time point prevented or restored neuronal loss. An intermediate effect was observed in the 21-day transplant group, as they did not significantly differ from either the sham- or 3-NP-treated animals. Rats in the 42-day transplant group resembled 3-NP-treated rats that did not receive transplants, which corresponds to earlier data suggesting that this intervention time point may have been too late to prevent 3-NP-induced neuronal loss or rescue affected cells. The significant sparing of NeuN-positive cells in the striatum after iPSC transplantation can be accounted by examining the percentage of iPSCs that differentiated into neuronal lineages (as measured by colocalization with NeuN; white arrowheads in Fig. 5E–5T). This examination revealed that the 7-day transplant group had significantly more iPSCs colocalized with NeuN than iPSCs that were transplanted at 21 or 42 days, suggesting an increase in neuronal differentiation at this early time point.
It was observed that the neurotoxin 3-NP significantly reduced the number of Darpp32-positive cells, indicating a loss of striatal neurons in the 3-NP-treated rats that did not receive transplants. However, this loss of medium spiny neurons was not observed in all 3-NP-treated rats that received transplantation of iPSCs. There was also no difference between transplantation time points with regard to the number of iPSCs that displayed colocalization with Darpp32, suggesting that the time point in which these cells are transplanted did not affect their ability to differentiate into medium spiny neurons. The lack of differences between transplantation time points and DARPP32 colocalization may explain why the 7-day iPSC transplant group demonstrated such robust survival, even with longer exposure to the 3-NP toxin. Although it was clearly demonstrated that a portion of the transplanted iPSCs can express neuronal phenotypes after transplantation, further characterization of transplanted cells, at multiple time points, is needed to determine the fate of the transplanted cells. Given that only mature neuronal cells or astrocytes were visualized through colocalization analysis, it is possible that the remaining transplanted iPSCs expressed markers of immature and developing neurons. This question will be the focus of future research with the transplantation of iPSCs into neurodegenerative diseases, as well as protocols to enhance the cell population to differentiate into neuronal lineages after transplantation.
When examining the role of trophic support from transplanted iPSCs, it was found that no significant differences existed between all groups in mRNA expression of BDNF, suggesting that the motor and histological deficits associated with 3-NP administration were not because of a depletion of BDNF and that the motor recovery and anatomical preservation observed in the iPSC transplant group were not the result of trophic support alone, as was observed in previous work with mesenchymal stem cell transplantation in our laboratory [15, 36–38].
Conclusion
Taken together, these data represent an initial finding of behavioral recovery and neuronal/morphological sparing after transplantation of iPSCs in an animal model of HD. It has been previously demonstrated that transplantation of human ESC-derived neurons can promote restoration of motor function in a quinolinic-acid lesion mouse model of HD [39]. In their study, the transplanted cells were able to survive for at least 4 months and express both markers of GABAergic neurons and βIII-tubulin. However, their transplant was performed in immune-deficient SCID mice [39]. Our study is the first to date to demonstrate that cells, reprogrammed to a pluripotent state, can reduce HD-like deficits in a nonimmune-suppressed, progressive model of HD. These data also suggest that transplantation of iPSCs, derived from tail-tip fibroblasts and reprogrammed with our adenovirus pair, is safe (i.e., no evidence of tumor formation or observable side effects), can survive in a neurodegenerating brain, and can differentiate into region-specific neuronal phenotypes. Our results also highlight the need for nonintegrative strategies for producing iPSCs for therapeutic use. Recently, it was reported that iPSCs generated by retroviral reprogramming have incomplete transgene silencing and that these cells did not differentiate as efficiently as iPSC generated from a Sendai virus [40]. As a result of the concern regarding tumor formation following iPSC transplantation, most likely because of the reactivation of c-Myc, protocols have been developed to generate iPSCs in the absence of c-Myc [41–43]. It has been found that iPSC generated, in the absence of c-Myc, with Oct4, Sox2, and Klf4, generated high-quality pluripotent cells that did not develop teratomas in mice, although the reprogramming time was longer for iPSCs without c-Myc [41]. In an attempt to characterize whether iPSCs derived without c-Myc were safe, in vivo, after transplantation, another group generated iPSCs without c-Myc and showed that these cells mobilized to the damaged liver in mice and rescued them from lethal acute hepatic failure [42]. Interestingly, in that study, no tumor formation was found at 6 months following transplantation, indicating that these cells provide a safer iPSC for transplantation into the liver. This group further tested the efficacy of iPSCs, derived without c-Myc, in a rat model of retinal ischemia and found that the transplanted cells survived in the absence of tumor formation for 6 months [43]. In that study, it was found that the non-c-Myc iPSC restored many of the deficits observed after retinal ischemia [43]. The presence of the oncogene c-Myc in iPSC may lead to tumorigenicity by overstimulating cell growth and/or cell metabolism. In our method of generating iPSCs from rat TTF, only transient expression of c-Myc is induced via adenovirus reprogramming, and the reprogramming factors are no longer present once iPSCs are formed [12]. Whereas our method for generating iPSC differs from the above-mentioned studies, the same theory of eliminating the potentially oncogenentic factors from our iPSCs is used to create a safer cell type for transplantation studies.
Furthermore, our study suggests that when transplantation of iPSCs is done early in the progression of the disease, it not only prevents a decline in motor function but also prevents anatomical alterations in the brain. Overall, rats that received iPSC transplantation at 7 days after the administration of 3-NP did not display motor dysfunction, did not exhibit a decrease in metabolic activity or enlargement of the lateral ventricles, did not display significant neuronal loss, and had the highest number of surviving iPSCs at the end point of this study. As such, these findings support the hypothesis that our newly described method of producing iPSCs may prove to be therapeutically efficacious and clinically relevant for the treatment of HD. Although this study is one of the first to describe functional and neuropathological improvements after transplantation of undifferentiated, adenovirus-generated iPSCs, future studies are needed to more fully characterize the safety of these cells following transplantation, as well as improving methods to increase the neuronal, and region-specific, differentiation of iPSCs after transplantation, and to examine this approach in a transgenic model of HD.
Supplementary Material
Acknowledgments
This work was supported by a Partner University Fund grant (Chateaubriand fellowship from the Embassy of France in the U.S. to K.D.F.), INSERM U643 (to K.D.F.), and the John G. Kulhavi Professorship and Field Neurosciences Institute (to G.L.D.).
Author Contributions
K.D.F.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; A.T.C.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; X.L.: collection and/or assembly of data, data analysis and interpretation; D.J.D., L.D.H., A.C.M., D.T.S., R.E.D., A.A., and P.A.S.: collection and/or assembly of data; M.L.: conception and design, data analysis and interpretation; L.L. and J.R.: conception and design, administrative support, data analysis and interpretation; G.L.D.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Estrada Sánchez AM, Mejía-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39:265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Southwell AL, Ko J, Patterson PH. Intrabody gene therapy ameliorates motor, cognitive, and neuropathological symptoms in multiple mouse models of Huntington’s disease. J Neurosci. 2009;29:13589–13602. doi: 10.1523/JNEUROSCI.4286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachoud-Lévi A-C. Neural grafts in Huntington’s disease: Viability after 10 years. Lancet Neurol. 2009;8:979–981. doi: 10.1016/S1474-4422(09)70278-9. [DOI] [PubMed] [Google Scholar]
- 5.Cicchetti F, Saporta S, Hauser RA, et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci USA. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuter I, Tai YF, Pavese N, et al. Long-term clinical and positron emission tomography outcome of fetal striatal transplantation in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2008;79:948–951. doi: 10.1136/jnnp.2007.142380. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 10.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 11.Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: Neurodegenerative diseases. Stem Cell Res Ther. 2011;2:32. doi: 10.1186/scrt73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink KD, Rossignol J, Lu M, et al. Survival and differentiation of adenovirus-generated induced pluripotent stem cells transplanted into the rat striatum. Cell Transplant. 2013 doi: 10.3727/096368913X670958. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Túnez I, Tasset I, Pérez-De La Cruz V, et al. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: Past, present and future. Molecules. 2010;15:878–916. doi: 10.3390/molecules15020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shear DA, Haik KL, Dunbar GL. Creatine reduces 3-nitropropionic-acid-induced cognitive and motor abnormalities in rats. Neuroreport. 2000;11:1833–1837. doi: 10.1097/00001756-200006260-00007. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol J, Boyer C, Lévèque X, et al. Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington’s disease: Morphological and behavioral outcomes. Behav Brain Res. 2011;217:369–378. doi: 10.1016/j.bbr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 16.El Massioui N, Ouary S, Chéruel F, et al. Perseverative behavior underlying attentional set-shifting deficits in rats chronically treated with the neurotoxin 3-nitropropionic acid. Exp Neurol. 2001;172:172–181. doi: 10.1006/exnr.2001.7766. [DOI] [PubMed] [Google Scholar]
- 17.Beal MF. Huntington’s disease, energy, and excitotoxicity. Neurobiol Aging. 1994;15:275–276. doi: 10.1016/0197-4580(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 18.Brouillet E, Guyot MC, Mittoux V, et al. Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J Neurochem. 1998;70:794–805. doi: 10.1046/j.1471-4159.1998.70020794.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossignol J, Fink K, Davis K, et al. Transplants of adult mesenchymal and neural stem cells provide neuroprotection and behavioral sparing in a transgenic rat model of Huntington’s disease. Stem Cells. 2014;32:500–509. doi: 10.1002/stem.1508. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol J, Boyer C, Thinard R, et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med. 2009;13:2547–2558. doi: 10.1111/j.1582-4934.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. London, U.K.: Elsevier, 1998. [Google Scholar]
- 22.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong RJ, Watts C, Svendsen CN, et al. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington’s disease. Cell Transplant. 2000;9:55–64. doi: 10.1177/096368970000900108. [DOI] [PubMed] [Google Scholar]
- 24.Bernreuther C, Dihné M, Johann V, et al. Neural cell adhesion molecule L1-transfected embryonic stem cells promote functional recovery after excitotoxic lesion of the mouse striatum. J Neurosci. 2006;26:11532–11539. doi: 10.1523/JNEUROSCI.2688-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunnett SB, Carter RJ, Watts C, et al. Striatal transplantation in a transgenic mouse model of Huntington’s disease. Exp Neurol. 1998;154:31–40. doi: 10.1006/exnr.1998.6926. [DOI] [PubMed] [Google Scholar]
- 26.McBride JL, Behrstock SP, Chen E-Y, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JK, Kim J, Cho SJ, et al. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol Dis. 2004;16:68–77. doi: 10.1016/j.nbd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Lee S-T, Kang W, et al. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett. 2007;423:58–61. doi: 10.1016/j.neulet.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 29.Lee S-T, Chu K, Park J-E, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res. 2005;52:243–249. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Pineda JR, Rubio N, Akerud P, et al. Neuroprotection by GDNF-secreting stem cells in a Huntington’s disease model: Optical neuroimage tracking of brain-grafted cells. Gene Ther. 2007;14:118–128. doi: 10.1038/sj.gt.3302847. [DOI] [PubMed] [Google Scholar]
- 31.Roberts TJ, Price J, Williams SCR, et al. Preservation of striatal tissue and behavioral function after neural stem cell transplantation in a rat model of Huntington’s disease. Neuroscience. 2006;139:1187–1199. doi: 10.1016/j.neuroscience.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johann V, Schiefer J, Sass C, et al. Time of transplantation and cell preparation determine neural stem cell survival in a mouse model of Huntington’s disease. Exp Brain Res. 2007;177:458–470. doi: 10.1007/s00221-006-0689-y. [DOI] [PubMed] [Google Scholar]
- 34.Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Shear DA, Dong J, Haik-Creguer KL, et al. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp Neurol. 1998;150:305–311. doi: 10.1006/exnr.1998.6767. [DOI] [PubMed] [Google Scholar]
- 36.Dey ND, Bombard MC, Roland BP, et al. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav Brain Res. 2010;214:193–200. doi: 10.1016/j.bbr.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Dunbar GL, Sandstrom MI, Rossignol J, et al. Neurotrophic enhancers as therapy for behavioral deficits in rodent models of Huntington’s disease: Use of gangliosides, substituted pyrimidines, and mesenchymal stem cells. Behav Cogn Neurosci Rev. 2006;5:63–79. doi: 10.1177/1534582306289367. [DOI] [PubMed] [Google Scholar]
- 38.Lescaudron L, Unni D, Dunbar GL. Autologous adult bone marrow stem cell transplantation in an animal model of Huntington’s disease: Behavioral and morphological outcomes. Int J Neurosci. 2003;113:945–956. doi: 10.1080/00207450390207759. [DOI] [PubMed] [Google Scholar]
- 39.Ma L, Hu B, Liu Y, et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toivonen S, Ojala M, Hyysalo A, et al. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl Med. 2013;2:83–93. doi: 10.5966/sctm.2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 42.Li H-Y, Chien Y, Chen Y-J, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011;32:5994–6005. doi: 10.1016/j.biomaterials.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Fang I-M, Yang C-M, Yang C-H, et al. Transplantation of induced pluripotent stem cells without C-Myc attenuates retinal ischemia and reperfusion injury in rats. Exp Eye Res. 2013;113:49–59. doi: 10.1016/j.exer.2013.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.