With growing understanding of the pathogenesis of diabetic disease, alternative approaches aiming at repair and restoration of failing β-cell function are increasingly considered as complements to current diabetes therapy regimens. This study highlights advances in β-cell regeneration strategies with a focus on pluripotent stem cell platforms in the context of translational applications.
Keywords: Diabetes mellitus, Regenerative medicine, Stem cells, Translation, Transplantation
Abstract
Diabetes engenders the loss of pancreatic β-cell mass and/or function, resulting in insulin deficiency relative to the metabolic needs of the body. Diabetic care has traditionally relied on pharmacotherapy, exemplified by insulin replacement to target peripheral actions of the hormone. With growing understanding of the pathogenesis of diabetic disease, alternative approaches aiming at repair and restoration of failing β-cell function are increasingly considered as complements to current diabetes therapy regimens. To this end, emphasis is placed on transplantation of exogenous pancreas/islets or artificial islets, enhanced proliferation and maturation of endogenous β cells, prevention of β-cell loss, or fortified renewal of β-like-cell populations from stem cell pools and non-β-cell sources. In light of emerging clinical experiences with human embryonic stem cells and approval of the first in-human trial with induced pluripotent stem cells, in this study we highlight advances in β-cell regeneration strategies with a focus on pluripotent stem cell platforms in the context of translational applications.
Introduction
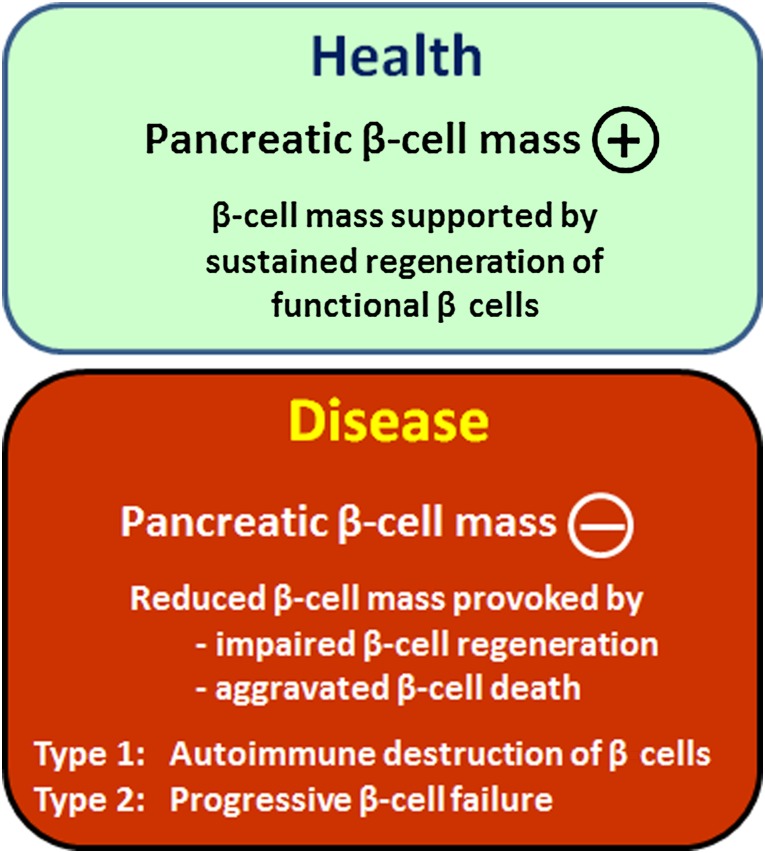
Diabetes is a staggering health problem affecting more than 300 million people worldwide. By 2030, an estimated 440 million adults will be afflicted with diabetes [1, 2]. Premature morbidity and mortality create a substantial and escalating burden on the global health system and society. Type 1 diabetes (T1D) is defined by insulin deficiency brought about by autoimmune destruction of islet β cells. Type 2 diabetes (T2D) is defined by the progressive inability of the insulin secretory capacity to match peripheral insulin needs. Defective innate β-cell regeneration, because of either β-cell destruction or insufficient β-cell replenishment, is increasingly recognized as central to the pathobiology of both T1D and T2D (Fig. 1) [3–6].
Figure 1.
Inadequate β-cell regeneration because of β-cell destruction and/or insufficient β-cell replenishment causes diabetes. The healthy state is characterized by sustained and adequate regeneration of the pancreatic β-cell mass. Disease is precipitated by loss of β-cell mass, through impaired innate regeneration or excessive apoptosis, underlying the pathological substrate of type 1 and type 2 diabetes.
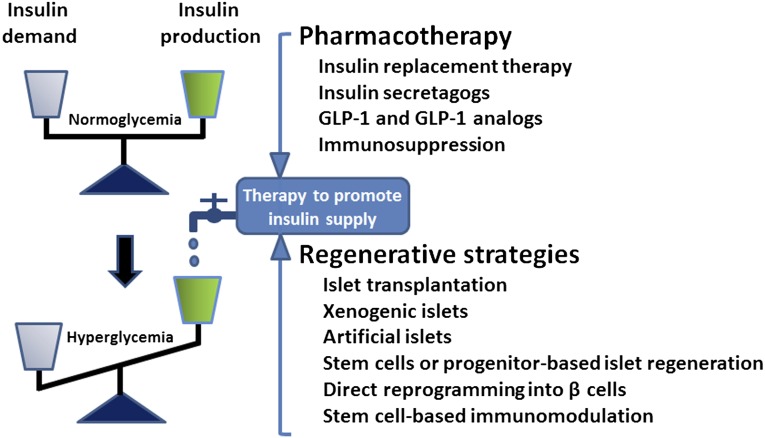
Pharmacological means that promote insulin production or replace insulin function have comprised a primary line of therapy for insulin insufficiency in T1D and a large proportion of patients with T2D, in particular those with advanced disease. Case in point, injection of exogenous insulin is required for people with T1D and advanced T2D. Glucagon-like peptide-1 (GLP-1) analogs have been designed more recently to augment insulin secretion and preserve β-cell function. Alternatively, DPP-4 inhibitors, which prevent inactivation of GLP-1, have been used to promote endogenous insulin production in T2D [7]. Whereas collectively the spectrum of preventive and palliative approaches has led to improved diabetic care, a fail-safe physiological regulation of systemic blood glucose levels remains challenging. Frequent fluctuations in blood glucose levels have been implicated as a culprit of heterogeneous (co)morbidities, such as retinopathy, nephropathy, neuropathy, or cardiovascular complications. In this context, various nonpharmacological approaches have been recently explored to restore functionality of the failing β-cell mass. Such regenerative approaches have evolved rapidly, from prototypic cell replacement therapies through pancreas/islet transplantation to the potential use of artificial insulin-producing cells derived from stem cells or pancreatic progenitor cells (Fig. 2).
Figure 2.
Balancing insulin demand and production is a central objective of diabetes therapy. Complementing pharmacological approaches, a series of regenerative strategies have been developed ranging from islet transplantation to stem cell-based platforms aimed at restoring insulin homeostasis and normoglycemia. Abbreviation: GLP-1, glucagon-like peptide-1.
Adult Cell-Based Therapies for Diabetes
Islet transplantation has provided a foundation for next-generation cell-based therapeutics for diabetes [8]. The Edmonton protocol, a flagship islet transplantation protocol, has achieved long-term islet survival. In early studies, more than 50% of subjects gained insulin independence 1 year post-transplantation and experienced improved glycated hemoglobin levels and protection from hypoglycemia [9]. Twenty percent of islet recipients are insulin therapy-free 5 years after transplantation [10]. Recent studies have shown improvements in primary efficacy, safety outcomes, and insulin independence 3 years post-transplant [11, 12]. Despite notable progress in the field, several issues still need to be addressed. These include the islet isolation methods, sites for transplantation, immunosuppression, or immunoisolation strategies [13–16]. It is also notable that total pancreatectomy with intrahepatic autoislet transplantation for non-T1D patients results in only 30% insulin independence at 3 years [17]. Although this is in part because of low islet yield in some patients [17], failure to achieve high rates of insulin independence in autologous, autoimmunity-free settings highlights the current limitations of islet transplantation. With further improvement, islet transplantation could provide a long-term therapy for patients with insulin deficiency.
An additional key issue is the persistent shortage of postmortem pancreatic tissue for islet isolation. This will be a major hurdle when islet transplantation becomes the standard therapy for T1D. One solution would be the successful combination of an opt-out system and the priority rule to increase organ donation. Additionally, in an effort to obtain a sustainable source of insulin-producing cells, various alternative sources have been explored, including islet transplantation from living donors [18] and xenogeneic islets [19]. Insulin-producing surrogate cells from diverse stem cell sources have also emerged as alternative biotherapeutic candidates for diabetes care [8, 20]. Among adult stem cells, extensive experience has been obtained with multipotent mesenchymal stem cells (MSCs) [21]. In addition to generation of β-like cells from MSCs [21, 22], their potent anti-inflammatory or immune-suppressive effects have been increasingly evaluated. This includes their potential for immunomodulation and tissue damage protection to suppress autoimmunity in T1D or to enhance islet engraftment and survival [23–25], underscoring versatility in their mechanism of action and benefit potential. Because the current efforts using adult mesenchymal stem cells, and also hematopoietic or pancreatic stem cells, have been extensively reviewed [8, 20, 21, 26, 27], this synopsis highlights natural and bioengineered pluripotent stem cells, underscoring their translational potential for diabetes therapies.
Embryonic Stem Cell-Based Therapies for Diabetes
The capacity for unlimited self-renewal and pluripotent lineage specification renders embryonic stem cells (ESCs) a unique platform for regenerative medicine. Various protocols have shown successful and efficient differentiation into ectoderm, endoderm, and mesoderm. Currently, there are two main approaches for generating insulin-producing cells from ESCs. The first is to leverage the spontaneous differentiation propensity of pluripotent cells through embryoid body (EB) formation, followed by selection for β-cell/β-cell-progenitor marker-expressing cells. Use of insulin-producing cells from ESC-derived progeny, selected for β-cell-specific gene expression, has been shown to normalize hyperglycemia in diabetic mice [28, 29]. The second strategy involves stepwise, lineage-specific differentiation protocols, largely adapted from in utero β-cell developmental blueprints. In this way, guided differentiation of ESCs has achieved generation of insulin-producing cells [30], although subsequent studies have questioned the authenticity of derived progeny [31]. Through a marriage of both strategies, spontaneous differentiation of EBs and coaxed differentiation of early pancreatic progenitors, successful generation of insulin-producing cells has also been documented [32].
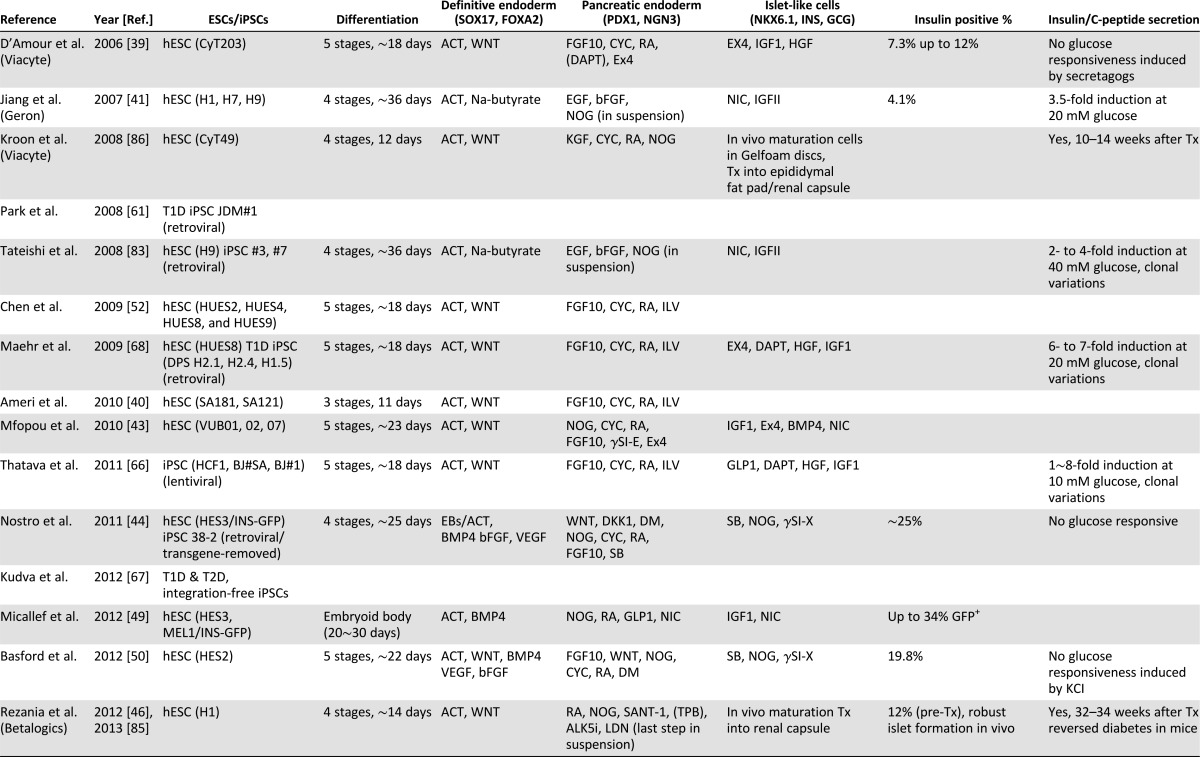
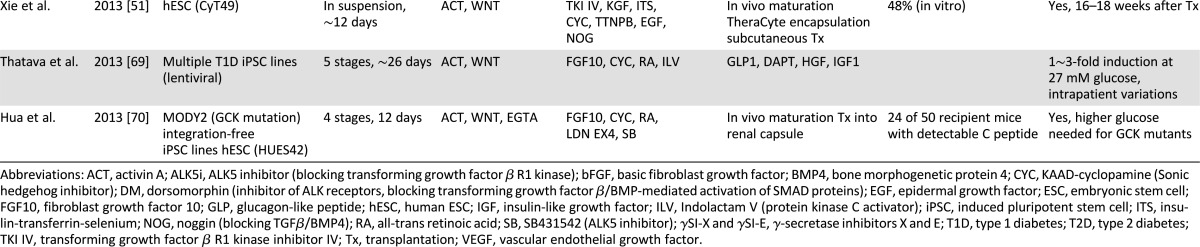
As spontaneous differentiation is limited by inefficiency, guided differentiation has been the primary strategy used for differentiation of human ESC into insulin-producing cells. With renewed focus on decoding embryonic development, the progressive evolution of endoderm into primitive gut tube and ultimately discrete pancreatic β cells has become increasingly defined [33]. The critical first step of guided differentiation protocols is the induction of endoderm from human ESCs [34, 35], which is typically achieved through stimulation with activin A (a Nodal surrogate), Nodal, and/or Wnts, under low serum conditions [36–38]. Derived definitive endoderm expresses markers such as FOXA2, SOX17, and CXCR4 [33]. Further guidance achieves generation of pancreatic multihormonal endocrine cells through foregut, pancreatic endoderm, and endocrine progenitor stages [39]. However, resulting cells demonstrate immature β-cell-like phenotypes [39]; that is, these human ESC-derived β-like cells produce high levels of intracellular C peptide comparable to human islets and respond to insulin secretagogs but fail to respond to high glucose stimulation. Modified or improved protocols have been established using combinations of cytokines and small molecules, such as fibroblast growth factors, noggin, KAAD-cyclopamine Sonic hedgehog pathway inhibitors (KAAD-cyclopamine or SANT-1), retinoic acid, nicotinamide, and GLP-1 (Table 1) [40–51]. Notable improvements in pancreatic differentiation have been reported with use of a small-molecule Indolactam V, which accelerates induction of pancreatic progenitor cells from definitive endoderm through protein kinase C (PKC) activation [52], or suppression of the transforming growth factor (TGF)β/activin/bone morphogenetic protein signaling pathways at specific stages by noggin or SB431542/ALK5 inhibitors [43, 44]. Accordingly, use of noggin, PKC activator (2S,5S)-(E,E)-8-(5-(4-(trifluoromethyl)phenyl)-2,4-pentadienoylamino)benzolactam (TPB) and TGFβ inhibitor in combination has proven effective for induction of pancreatic endoderm and endocrine precursors [46].
Table 1.
Progress on β-cell regeneration from human embryonic and induced pluripotent stem cells
Induced Pluripotent Stem Cell-Based Therapies for Diabetes
Despite notable progress, the clinical development of cell replacement therapies using human ESCs has been surrounded by ethical concerns. The use of allogeneic ESC-derived cells is also associated with immunological mismatch. Although a recent study has demonstrated successful generation of personalized human ESCs through somatic cell nuclear transfer (NT-ESCs) [53], derivation of human NT-ESCs remains challenging. In this regard, nuclear reprogramming technology, which allows generation of pluripotent stem cells from adult somatic cells, has opened a new path for generating patient-specific pluripotent stem cells [54, 55]. The induced pluripotent stem cell (iPSC) technology relies on genetic introduction of selected pluripotency-associated factors in adult somatic cell sources, which reprogram cell fate enabling dedifferentiation into a pluripotent stem cell state [56, 57]. Derived human iPSC lines show characteristics similar to human ESCs, including morphology, global gene expression profiles, elongated telomeres, and the propensity to differentiate into all three germ layers [58, 59], offering a self-renewable source of new tissues derived from the patient’s own cell pool [60].
Patient-Derived iPSCs and Their Differentiation Into Insulin-Producing Cells
Unlike their natural counterparts, iPSCs carry patient-specific genetic traits, providing a unique autologous pluripotent platform. Accordingly, differentiation of patient-derived iPSCs into disease-relevant cell types would allow for patient-specific modeling of disease progression and patient-specific drug screening [59, 61]. Several studies have demonstrated the efficacy of recapitulating disease phenotypes using patient-specific iPSCs [62–64], verifying the utility of iPSC technology for in vitro disease modeling. Patient-specific iPSCs would also create the opportunity for immunosuppression-free, autologous stem cell-based regenerative approaches for degenerative disorders.
Derivation of diabetes-specific iPSCs and their differentiation into functional β cells provide the foundation for new diagnostic and therapeutic applications. Diabetes-specific iPSCs have been derived from both T1D and T2D patients [61, 65–68], which demonstrate similar genome-wide gene expression profiles to those of human ESCs [69]. Importantly, iPSC clones derived from patients of different age groups and sex are capable of generating insulin-producing cells [65, 68, 69], a prerequisite in establishing a broader translational platform for diabetes-specific iPSCs. Patient iPSC-derived β-like cells would enable detailed analysis of patient-specific immunity against β cells at the cellular level, whereas autologous properties would facilitate use as a cell-based therapy for diabetes. A recent study demonstrates that iPSC-derived β cells from subjects with maturity-onset diabetes of the young type 2 (MODY2), characterized by impaired glucokinase activity, recapitulate the β-cell-autonomous phenotypes of MODY2 [70].
Challenges for Clinical Applications of Diabetes-Specific iPSCs
Immunogenicity and Epigenetic Abnormalities
Recent iPSC studies have raised several possible concerns for broader application. In particular, the reprogramming process and subsequent expansion of iPSCs have been associated with potential genetic and epigenetic abnormalities [71–74]. Moreover, iPSC-derived cells have been reported to show abnormal gene expression patterns, capable of inducing T-cell-dependent immunity in syngeneic recipients [75]. Yet, these initial studies need further confirmation as, for example, only limited immunogenicity of transplanted iPSC-derived cells has been reported [76].
Teratoma Formation
Another biosafety concern surrounding the therapeutic use of iPSCs or derivatives is the risk of teratoma formation upon transplantation. The primary source of teratoma is the residual undifferentiated pluripotent cell pool. Elimination of undifferentiated cells or purification of fully differentiated cell populations can minimize the risk of teratoma formation upon transplantation [77, 78]. In addition to the risk of teratoma formation inherent in pluripotent stem cells, iPSCs have an additional biosafety risk associated with genetic modification during reprogramming. Originally, iPSCs have been generated by infecting adult somatic cells with integrating reprogramming vectors, such as retroviral or lentiviral vectors [56, 57]. Those vectors permanently integrate into the host genome. Although expression of pluripotency-associated genes from vectors is silenced upon successful reprogramming, the use of integrating vectors has the added risk of provoking insertional mutagenesis, that is, activation of oncogene programs or disruption of an essential gene set [79]. Additionally, reactivation of one of the reprogramming factors, c-MYC, a recognized oncogene, from an integrating vector can further increase the tumorigenicity of iPSCs [80].
Clonal Variation of iPSCs
For diagnostic and therapeutic applications of iPSCs, it is critical to achieve reliable and efficient differentiation into insulin-producing islet-like cells. However, human pluripotent stem cell lines show substantial differences in spontaneous differentiation propensities [81–83]. Moreover, intrapatient divergence has been reported in the propensity for pancreatic differentiation among T1D-specific iPSC lines [69]. Such intrapatient variations will in principle impose a translational challenge for individualized applications for diagnostic or therapeutic purposes.
Inefficient Differentiation Into Insulin-Producing Cells
The major barrier preventing clinical applications of pluripotent cells in diabetes therapy is the low efficiency in generating insulin-producing cells. Although several studies have demonstrated in vitro differentiation achieving more than 20% insulin-producing progeny from selected human ESC (hESC) lines [39] (Table 1), a yield of more than 10% insulin-positive cells from human pluripotent stem cells has been challenging, possibly because of intrinsic differences among permissive hESC lines, such as Cyt49 [39], and other less permissive hESC and iPSC lines. It is also possible that current protocols miss critical signals driving pancreatic differentiation of those less permissive lines, highlighting the necessity of a well-defined, improved protocol for guided differentiation into insulin-producing cells.
Lack of Glucose Responsiveness in Derived Insulin-Producing Cells
Another limitation is the difficulty in generating glucose-responsive and insulin-producing cells from human pluripotent stem cells. Despite promising results and proof-of-principle studies, most protocols yield populations of β-like cells lacking glucose responsiveness [39, 84]. Such glucose-unresponsive stem cell-derived β cells mirror neonatal immature β cells. For instance, in vitro guided differentiation of human pluripotent stem cells has achieved islet-like cells responsive to insulin secretagogs, but not high glucose stimulation [39]. Necessitating improvement, the field has shifted toward in vivo differentiation/maturation of pancreatic progenitor cells to generate glucose-responsive insulin-producing cells [70, 85]. To this end, derived pancreatic progenitors are transplanted into immune-compromised hosts and allowed to mature into glucose-responsive insulin-secreting cells capable of treating drug-induced or pre-existing diabetes [46, 86]. One caveat of this approach is the extended in vivo maturation with a required 5- to 8-month period before achieving definitive glucose responsiveness [46, 85, 86].
Potential T1D Recurrence After Transplantation of iPSC-Derived Islets
In the absence of immunosuppression, pancreas transplantation from human leukocyte antigen (HLA)-identical twins or HLA-identical siblings frequently results in T1D recurrence. This secondary T1D is characterized by rapid return of hyperglycemia without pancreatic rejection [87, 88]. Damaged islets demonstrate infiltration of mononuclear cells and selective β-cell destruction, emphasizing cellular mediated autoimmunity in the pathogenicity of T1D. In fact, pre-existing cellular islet autoimmunity prevents islet survival upon transplantation [89]. Together, these observations support the notion that autologous iPSC-derived islets are subject to autoimmune destruction and therefore require an immunosuppressive coregimen for survival of transplanted progeny.
Complex iPSC Technology/Guided Differentiation for Clinical Grade Manufacturing
Use of iPSC-derived insulin-producing cell products in the clinic necessitates multiple elaborate steps, including (a) somatic cell preparation, (b) reprogramming through ex vivo gene delivery using good manufacturing practice (GMP)-grade reprogramming vectors, (c) expansion of iPSC lines under GMP protocols, (4) extensive characterization of derived iPSC lines, and (5) preparation of pancreatic progenitor cells for transplantation through stepwise differentiation. Each step in the process requires regulatory agency-approved reagents, such as specific cell culture media, cytokines, small molecules, and animal component-free coating matrices. Thus, the complexity of iPSC technology and multiple in vitro differentiation steps have impeded rapid translation of iPSCs into scalable diabetes therapy (Table 2).
Table 2.
Milestones in translating stem cell-based regenerative technologies into clinical grade practice-conducive products
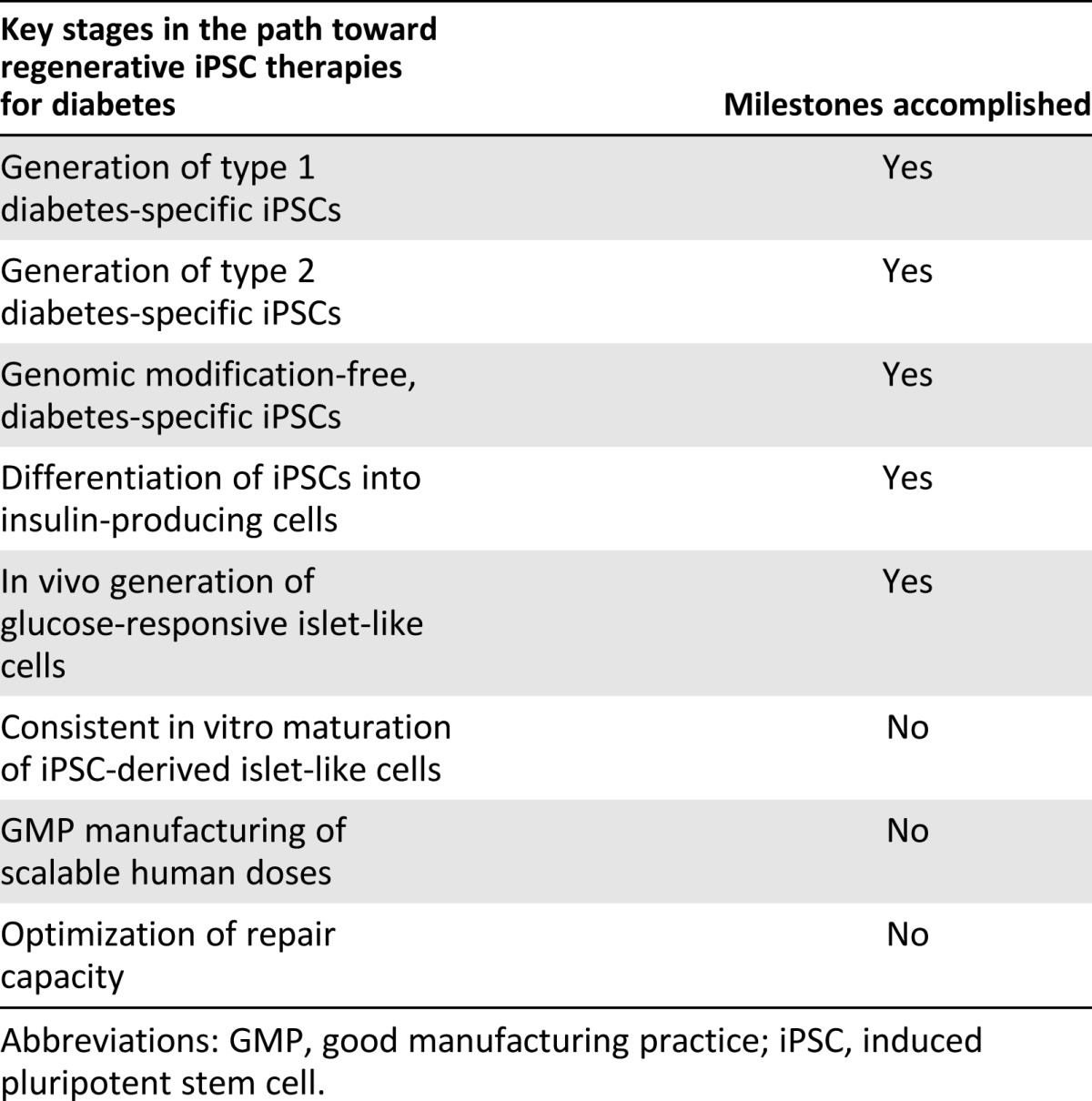
Toward Clinical Applications of iPSCs
Genomic Modification-Free iPSCs
Generation of iPSCs independently from integrating vectors can in principle avoid some of the biosafety concerns. Accordingly, much effort has been made to produce iPSCs without integrating vectors. In this regard, nonintegrating viral vectors (e.g., adenoviral and Sendai viral vectors), nonviral vectors (removable transposon, episomal, and plasmid vectors), or direct transfection of reprogramming proteins or encoding mRNA have been successfully used to derive iPSCs [57, 90–92]. Minimizing the use of pluripotency factors has led to the identification of microRNAs and small molecules with the potential of facilitating reprogramming [93]. Genomic modification-free iPSC generation is also likely to reduce clonal variation among derived iPSCs. Of note, reproducible generation of genomic modification-free, T1D- and T2D-specific iPSCs has been demonstrated with nonintegrating Sendai reprogramming vectors [67].
Improved Biosafety and Differentiation Propensity Through Selection of Somatic Cell Source for Nuclear Reprogramming
iPSCs are typically derived from skin-derived fibroblasts. Other cell sources such as keratinocytes, adult stem cells, blood cells, stomach, and liver cultures have been used as somatic cell sources for iPSC derivation [58, 94, 95]. Notably, reprogramming of mature B lymphocytes requires depletion of the key B-cell transcription factor [96], emphasizing the importance of the intracellular environment in cell reprogramming. Reprogramming efficiency, levels of chromosomal damage, or even epigenetic memory of derived iPSCs can all be affected by the properties of the somatic cell source [65]. Conversely, residual epigenetic memory may inform protocols for improved pancreatic differentiation [73]. For instance, the use of endoderm cell sources, such as hepatocytes, rather than ectoderm-derived dermal cells, may at least in principle improve the iPSC differentiation propensity into insulin-producing cells. It is also notable that somatic cell sources can affect the persistence of undifferentiated cells upon differentiation, substantially affecting the teratoma-formation propensity after transplantation of iPSC-derived progeny [97]. It should, however, be underscored that most recent studies have not demonstrated the correlation between somatic sources and derivation of iPSC progeny [98].
Improved Pancreatic Differentiation and Glucose Responsiveness of iPSC Progeny
Currently, most promising protocols require lengthy in vivo maturation to obtain glucose-responsive islet-like cells [46]. In this context, it is notable that expression of urocortin 3 (UCN3), which regulates glucose-stimulated insulin secretion in β cells [99], gradually increases during β-cell maturation in vivo [100]. Importantly, UCN3 is also expressed in derived β-like cells after in vivo maturation, but not after in vitro differentiation, suggesting the potential role of UCN3 in achieving glucose responsiveness. Conversely, a lack of induction of key pancreatic factors, PDX1 and NKX6.1, is responsible for poor iPSC differentiation into insulin-producing cells [69] or in vivo maturation of iPSC-derived islets [85]. Thus, pharmacological or genetic induction of UCN3, PDX1, and/or NKX6.1 may gauge the efficiency of generating iPSC-derived insulin-producing cells with authentic glucose responsiveness. Moreover, neonatal β cells do not show typical glucose-responsive insulin secretion and are considered immature [101–103], a property regulable through thyroid hormone signaling [104], offering a physiological means to enhance functional maturation of derived β cells.
Direct Reprogramming to Insulin-Producing Cells
An alternative reprogramming approach leverages β-cell-specific factors to directly derive insulin-producing cells without generating iPSCs. Studies have demonstrated that overexpression of a set of three pancreatic factors, PDX1, NEUROG3, and MAFA, can reprogram the fate of hepatocytes, pancreatic exocrine tissues, or liver ductal cells into insulin-producing cells in vivo [105–107]. Although derived insulin-producing cells do not necessarily exhibit complete β-cell phenotypes, those cells are able to control blood glucose levels in diabetic mice, expanding the available regenerative platforms for diabetes care.
Conclusion
The epidemic of diabetes requires new means to address a rampant global need, ensuring effective solutions beyond the current standard of care. In this context, regenerative technologies offer a radical innovation with potential significant impact in advancing diabetes care. New knowledge in developmental biology and disease pathophysiology has fueled the evolution of management approaches increasingly targeted to address the root cause of the problem. Pertinent to the future of diabetes therapy, regenerative modalities aim to restitute pancreatic β-cell structure and function. Such reparative approaches may prove particularly useful with the recognition that diabetes reflects a defective innate β-cell regeneration capacity because of augmented destruction or insufficient replenishment of the existing β-cell pool. Stem cells, including pluripotent platforms highlighted in this work, have the remarkable aptitude to form specialized tissues and promote repair signaling, restoring organ structure and function. Translation of regenerative principles into practice, however, presents significant challenges requiring careful optimization to maximize safe and effective clinical application.
Acknowledgments
We thank Dr. Cristina Aguayo-Mazzucato (Joslin Diabetes Center) for helpful suggestions. This work was supported by the Eisenberg Stem Cell Trust, the Marriott Foundation, a Bernard and Edith Waterman Pilot Grant, and the Mayo Clinic Center for Regenerative Medicine. A.T. is recognized through the Michael S. and Mary Sue Shannon Family Directorship, the Mayo Clinic Center for Regenerative Medicine, and the Marriott Family Professorship of Cardiovascular Diseases Research.
Author Contributions
S.J.H.: manuscript writing; A.T. and Y.I.: conception and design, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Terzic A, Waldman S. Chronic diseases: The emerging pandemic. Clin Transl Sci. 2011;4:225–226. doi: 10.1111/j.1752-8062.2011.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler AE, Galasso R, Meier JJ, et al. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X, Chen Z, Shiota C, et al. No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123:990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 8.Ricordi C, Inverardi L, Domínguez-Bendala J. From cellular therapies to tissue reprogramming and regenerative strategies in the treatment of diabetes. Regen Med. 2012;7:41–48. doi: 10.2217/rme.12.70. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 10.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 11.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PR, Jones KE. Pancreatic islet transplantation. Semin Pediatr Surg. 2012;21:272–280. doi: 10.1053/j.sempedsurg.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig B, Reichel A, Steffen A, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110:19054–19058. doi: 10.1073/pnas.1317561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smink AM, Faas MM, de Vos P. Toward engineering a novel transplantation site for human pancreatic islets. Diabetes. 2013;62:1357–1364. doi: 10.2337/db12-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–424; discussion 424–426. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto S, Okitsu T, Iwanaga Y, et al. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet. 2005;365:1642–1644. doi: 10.1016/s0140-6736(05)66383-0. [DOI] [PubMed] [Google Scholar]
- 19.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 20.Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Translational Medicine. 2013;2:328–336. doi: 10.5966/sctm.2012-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domínguez-Bendala J, Lanzoni G, Inverardi L, et al. Concise review: Mesenchymal stem cells for diabetes. Stem Cells Translational Medicine. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 23.Abdi R, Fiorina P, Adra CN, et al. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staeva TP, Chatenoud L, Insel R, et al. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62:9–17. doi: 10.2337/db12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hematti P, Kim J, Stein AP, et al. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando) 2013;27:21–29. doi: 10.1016/j.trre.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Lanzoni G, Oikawa T, Wang Y, et al. Concise review: Clinical programs of stem cell therapies for liver and pancreas. Stem Cells. 2013;31:2047–2060. doi: 10.1002/stem.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lysy PA, Weir GC, Bonner-Weir S. Concise review: Pancreas regeneration: Recent advances and perspectives. Stem Cells Translational Medicine. 2012;1:150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria B, Roche E, Berná G, et al. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 29.León-Quinto T, Jones J, Skoudy A, et al. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2004;47:1442–1451. doi: 10.1007/s00125-004-1458-8. [DOI] [PubMed] [Google Scholar]
- 30.Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopal J, Anderson WJ, Kume S, et al. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder IS, Rolletschek A, Blyszczuk P, et al. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 33.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin Cell Dev Biol. 2012;23:701–710. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borowiak M, Maehr R, Chen S, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean AB, D’Amour KA, Jones KL, et al. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 36.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 37.D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 38.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 39.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 40.Ameri J, Ståhlberg A, Pedersen J, et al. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 42.Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 43.Mfopou JK, Chen B, Mateizel I, et al. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138:2233–2245, 2245.e1–14. doi: 10.1053/j.gastro.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 44.Nostro MC, Sarangi F, Ogawa S, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezania A, Riedel MJ, Wideman RD, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Browning VL, Odorico JS. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. 2011;128:412–427. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Micallef SJ, Li X, Schiesser JV, et al. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia. 2012;55:694–706. doi: 10.1007/s00125-011-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basford CL, Prentice KJ, Hardy AB, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–371. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 51.Xie R, Everett LJ, Lim HW, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 53.Tachibana M, Amato P, Sparman M, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 55.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohmine S, Dietz AB, Deeds MC, et al. Induced pluripotent stem cells from GMP-grade hematopoietic progenitor cells and mononuclear myeloid cells. Stem Cell Res Ther. 2011;2:46. doi: 10.1186/scrt87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thatava T, Armstrong AS, De Lamo JG, et al. Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther. 2011;2:48. doi: 10.1186/scrt89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terzic A, Nelson TJ. Regenerative medicine primer. Mayo Clin Proc. 2013;88:766–775. doi: 10.1016/j.mayocp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee TH, Song SH, Kim KL, et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106:120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 64.Carvajal-Vergara X, Sevilla A, D’Souza SL, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohmine S, Squillace KA, Hartjes KA, et al. Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency. Aging (Albany NY) 2012;4:60–73. doi: 10.18632/aging.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thatava T, Nelson TJ, Edukulla R, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:289–293. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kudva YC, Ohmine S, Greder LV, et al. Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Translational Medicine. 2012;1:451–461. doi: 10.5966/sctm.2011-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maehr R, Chen S, Snitow M, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thatava T, Kudva YC, Edukulla R, et al. Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol Ther. 2013;21:228–239. doi: 10.1038/mt.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hua H, Shang L, Martinez H, et al. iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123:3146–3153. doi: 10.1172/JCI67638. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 72.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 75.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 76.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 77.Hattori F, Chen H, Yamashita H, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 78.Darabi R, Gehlbach K, Bachoo RM, et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 79.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: Basic to translational. Biochem J. 2012;443:603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 80.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 81.Osafune K, Caron L, Borowiak M, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 82.Bock C, Kiskinis E, Verstappen G, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tateishi K, He J, Taranova O, et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 84.Thatava T, Nelson TJ, Edukulla R, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:283–293. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rezania A, Bruin JE, Xu J, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 86.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 87.Sibley RK, Sutherland DE, Goetz F, et al. Recurrent diabetes mellitus in the pancreas iso- and allograft: A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–144. [PubMed] [Google Scholar]
- 88.Sibley RK, Sutherland DE. Pancreas transplantation: An immunohistologic and histopathologic examination of 100 grafts. Am J Pathol. 1987;128:151–170. [PMC free article] [PubMed] [Google Scholar]
- 89.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seki T, Yuasa S, Fukuda K. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat Protoc. 2012;7:718–728. doi: 10.1038/nprot.2012.015. [DOI] [PubMed] [Google Scholar]
- 91.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 95.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanna J, Markoulaki S, Schorderet P, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 98.Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14:357–368. doi: 10.1038/nrm3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Chen P, Vaughan J, et al. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blum B, Hrvatin SS, Schuetz C, et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30:261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hawdon JM, Aynsley-Green A, Alberti KG, et al. The role of pancreatic insulin secretion in neonatal glucoregulation. I. Healthy term and preterm infants. Arch Dis Child. 1993;68(3 Spec No):274–279. doi: 10.1136/adc.68.3_spec_no.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tuch BE, Jones A, Turtle JR. Maturation of the response of human fetal pancreatic explants to glucose. Diabetologia. 1985;28:28–31. doi: 10.1007/BF00276996. [DOI] [PubMed] [Google Scholar]
- 103.Espinosa de los M, Driscoll SG, Steinke J. Insulin release from isolated human fetal pancreatic islets. Science. 1970;168:1111–1112. doi: 10.1126/science.168.3935.1111. [DOI] [PubMed] [Google Scholar]
- 104.Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, et al. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569–1580. doi: 10.2337/db12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banga A, Akinci E, Greder LV, et al. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci USA. 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang AY, Ehrhardt A, Xu H, et al. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]