Human induced pluripotent stem cells (hiPSCs) can be easily and efficiently generated from finger-prick blood. Using this collection method will accelerate the development of large-scale hiPSC banks.
Keywords: Finger prick, Reprogramming, Induced pluripotent stem cells, Human peripheral blood, Human induced pluripotent stem cell banking
Abstract
Induced pluripotent stem cells (iPSCs) derived from somatic cells of patients can be a good model for studying human diseases and for future therapeutic regenerative medicine. Current initiatives to establish human iPSC (hiPSC) banking face challenges in recruiting large numbers of donors with diverse diseased, genetic, and phenotypic representations. In this study, we describe the efficient derivation of transgene-free hiPSCs from human finger-prick blood. Finger-prick sample collection can be performed on a “do-it-yourself” basis by donors and sent to the hiPSC facility for reprogramming. We show that single-drop volumes of finger-prick samples are sufficient for performing cellular reprogramming, DNA sequencing, and blood serotyping in parallel. Our novel strategy has the potential to facilitate the development of large-scale hiPSC banking worldwide.
Introduction
Human induced pluripotent stem cells (hiPSCs) were first generated by the overexpression of several transcription factors in dermal fibroblasts [1–3]. Like human embryonic stem cells (hESCs), hiPSCs are capable of differentiation into functional cell lineages and therefore hold great potential for disease modeling, drug screening, and future therapeutic medicine [4, 5].
Blood is considered an ideal source of cells for reprogramming because of its abundance and accessibility. We and others have previously shown that progenitor blood cells from mobilized, bone marrow, and cord blood are highly amenable to reprogramming [6–9]. However, cytokine-induced mobilization may result in side effects [10], bone marrow harvesting is invasive, and cord blood is limited to individuals who have deposited their samples at birth. In comparison, peripheral blood T cells can be easily obtained from donors and reprogrammed with high efficiency [11–13]. However, T-cell-derived hiPSCs carry pre-existing V(D)J rearrangement at the T-cell receptor (TCR) loci, and it is unclear whether these hiPSCs can differentiate normally or if they demonstrate tendencies for development into T-cell lymphomas [14]. Recent studies report the reprogramming of non-T-cell populations in peripheral blood. The reprogramming efficiencies in these studies are generally low, necessitating the addition of more factors (5–7 factors in some cases, including SV40LT, TP53 short hairpin RNA) in the reprogramming protocols [15–17]. Moreover, almost all studies require a considerable amount of starting material (approximately 10 ml), which was obtained via venipuncture performed by experienced phlebotomists. Such requirements could limit the recruitment of large numbers of potential donors. Two studies described the generation of hiPSCs from a relatively small volume of peripheral blood. Nonetheless, 2–6 ml of peripheral blood was still needed to purify enough CD34+ cells for reprogramming [18, 19].
In this study, we report the successful reprogramming from less than a drop of human finger-pricked blood. The hiPSC lines are transgene-free and do not contain genomic rearrangement. Finger-prick-derived hiPSCs were generated from different donors at very high efficiency (100–600 colonies per milliliter of blood). To the best of our knowledge, this is the most efficient approach for generating hiPSCs from human peripheral blood. Our findings will help to accelerate research in hiPSCs and the development of international hiPSC banking from large cohorts of donors.
Materials and Methods
Finger-Pricked and Venous Blood Samples
A total of 10 µl of finger-tip capillary blood was collected in a sterile laboratory setting. The samples were lysed in 2 ml of 1× red blood cell (RBC) lysis buffer (00-4300-54; eBioscience, San Diego, CA, http://www.ebioscience.com) for 10 minutes before spinning at 250g for 5 minutes. The lysis buffer was aspirated immediately after the centrifugation. Purified cells were resuspended with 500 µl of cell expansion medium and seeded into one well of a 24-well tissue culture plate (3536; Corning Enterprises, Corning, NY, http://www.corning.com). For the do-it-yourself (DIY) experiment, the donors were asked to perform a finger prick themselves and to collect the blood into a Microtainer tube containing anticoagulant ([422]365974; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). The tube can be presterilized over flame or under UV illumination. The DIY blood samples were stored on ice, and RBC lysis was performed 12, 24, or 48 hours later. The finger-prick (FP) blood-cell expansion medium [15, 20] contained StemSpan Serum-Free Expansion Medium (09650; StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) supplemented with 1× penicillin/streptomycin (pen/strep) (Gibco, Grand Island, NY, http://www.invitrogen.com), 1× l-glutamine (Gibco), 1× nonessential amino acids (Gibco), 50 µg/ml l-ascorbic acid (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 50 ng/ml stem cell factor (Peprotech, Rocky Hill, NJ, http://www.peprotech.com), 10 ng/ml interleukin-3 (Peprotech), 40 ng/ml insulin-like growth factor-1 (Peprotech), 2 U/ml erythropoietin (R&D Systems, Minneapolis, MN, http://www.rndsystems.com), and 1 µM dexamethasone (Sigma-Aldrich), with or without 10 ng/ml interleukin-6 (Peprotech). Medium was changed every day by carefully pipetting out half of the medium and replacing with fresh medium. Twelve to 16 days later, when the cell population reached 20,000–30,000 cells, they were transduced with Sendai virus. For venipuncture-derived iPSCs (VPiPSCs) derivation, 250 µl or 500 µl of peripheral blood was collected through venipuncture. Peripheral blood mononuclear cells (PBMCs) were purified using Ficoll-Paque PLUS (p = 1.077 ± .001 g/ml) (17-1440-03; GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com), according to the manufacturer's protocol. The cells were then cultured as described for finger-prick samples. The use of finger-prick blood samples was approved by the ethics committee of the National University of Singapore. Written informed consent was obtained from all donors.
Cellular Reprogramming
A total of 20,000–30,000 cells were transduced by OCT4, SOX2, KLF4, and c-MYC Sendai virus (CytoTune-iPS Reprogramming Kit; Life Technologies, Rockville, MD, http://www.lifetech.com) with each factor at a multiplicity of infection of 10 (approximately 5 µl of each factor) [21]. The transduction was terminated after 24 hours by replacing with fresh cell expansion medium. At day 3, cells were transferred to four or five wells of irradiated CF1-mouse embryonic fibroblasts (MEFs) (seeded at density of 200,000 per well) in six-well tissue culture plates (3516; Corning) and cultured with a 1:1 ratio of expansion and hESC medium (Dulbecco’s modified Eagle’s medium [DMEM]/F12 [Gibco] supplemented with 20% Knockout Serum Replacement [Gibco], 100 µM Minimum Essential Medium with nonessential amino acid solution [Gibco], 100 µM β-mercaptoethanol [Gibco], 1× pen/strep [Gibco], 1× l-glutamine [Gibco], and 10 ng/ml basic fibroblast factor [Gibco]). Two days later, the medium was changed to hESC medium with daily medium changes. From day 14, reprogramming continued with MEF-conditioned hESC medium and mTeSR1 (StemCell Technologies) in a 1:1 ratio. The volume of medium used for six-well culture was 2 ml per well. Once hiPSC colonies resembling hESCs in morphology emerged, the colonies were mechanically picked and replated onto MEFs for expansion.
Gene Expression Analysis
Direct application of PureZOL (Bio-Rad, Hercules, CA, http://www.bio-rad.com) onto adherent cells or cell pellets was performed for RNA isolation. RNA cleanup was performed using a Qiagen (Hilden, Germany, http://www.qiagen.com) RNeasy kit. First-strand cDNA synthesis was performed using iScript cDNA synthesis kit (Bio-Rad). Quantitative polymerase chain reaction (PCR) analyses were performed using the CXF384 real-time system (Bio-Rad) and KAPA SYBR FAST qPCR Master Mix (2×) (KK4602).
Immunohistochemistry Staining
Cells were fixed in 4% paraformaldehyde for 15 minutes. For nonsurface marker staining, the cells were permeabilized with 0.1% Triton X-100 for 30 minutes and blocked in phosphate-buffered saline (PBS) containing 8% fetal bovine serum for 30 minutes. Cells were incubated with primary antibodies overnight at 4°C, washed, and incubated with Alexa Fluor (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) secondary antibodies for 2 hours or incubated with fluorophore-conjugated antibodies for 1 hour. The cells were stained by Hoechst 33342 for 30 minutes before imaging. SSEA-4 Alexa Fluor 647 (560219) and TRA-1-60 Alexa Fluor 647 (560173) antibodies were obtained from BD Biosciences. OCT3/4 antibodies were obtained from Abcam (Cambridge, U.K., http://www.abcam.com) (ab19857). Antibodies against glial fibrillary acidic protein (GFAP) were obtained from Sigma-Aldrich (G9269); anti-β3-tubulin from Covance (Princeton, NJ, http://www.covance.com) (MMS-435P); anti-GATA4 from Santa Cruz Biotechnology (Santa Cruz, CA, http://www.scbt.com) (Sc-25310); anti-smooth muscle α actin (SMA) from Pierce (Rockford, IL, http://www.piercenet.com) (MA1-06110); and anti-Desmin from Cell Signaling Technology (Beverly, MA, http://www.cellsignal.com) (5332S). For live staining, StainAlive TRA-1-60 Antibody (DyLight 488) (09-0068; Stemgent, Cambridge, MA, https://www.stemgent.com) was used, according to the manufacturer’s protocol.
Bisulfite Genomic Sequencing
Bisulfite treatment of genomic DNA (gDNA) was carried out using a CpGenome DNA Modification Kit (Chemicon, Temecula, CA, http://www.chemicon.com), according to the manufacturer’s protocol. Briefly, converted gDNA was amplified by PCR OCT4 primer set 3, as reported previously [22]. PCR products were gel-purified and cloned into pCR2.1-TOPO using the TOPO TA cloning kit (Invitrogen). Bisulfite conversion efficiency of non-CpG cytosines ranged from 80% to 99% for all individual clones for each sample. Every clone of each sample was verified by sequencing with the M13 primers.
Flow Cytometry
The blood cells were stained with a panel of antibodies at the manufacturer’s suggested dilution in PBS at 4°C for 20 minutes. This panel included allophycocyanin (APC)-CD3, phycoerythrin (PE)-CD34, and APC-CD19 from BD Biosciences and APC-CD71 from Miltenyi Biotec (Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). Propidium iodide (Sigma-Aldrich) was used for live/dead discrimination, except for samples stained with PE-CD34. Flow cytometry was performed on a FACSCanto II machine (BD Biosciences), and data were analyzed using FACSDiva software.
DNA Fingerprinting and Karyotyping
Analysis of the DNA fingerprinting was performed on parental blood cells and their derivative hiPSC lines. DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen), according to the manufacturer’s protocol, and sent to Health Sciences Authority (Singapore) for short tandem repeat analysis. Alternatively, PCRs using primers specific to genomic intervals containing highly variable numbers of tandem repeats were used for fingerprinting analysis, as described previously [23]. In brief, the PCR was performed using KAPA SYBR FAST qPCR Master Mix (2×) (KK4602) with 1 ng of genomic DNA and 0.4 µM primers in a 10-µl reaction. The PCR conditions were set at 95°C for 3 minutes, followed by 40 cycles of 95°C for 3 seconds and 58°C for 30 seconds, with a final extension at 72°C for 10 minutes. The PCR products were analyzed on a 2.5% agarose gel in 1× Tris-acetate-EDTA buffer with applied voltage 3 V/cm for 60 minutes. The gel with ethidium bromide stain was visualized under a UV transilluminator. For karyotyping, live hiPSC lines were sent to the Genome Institute of Singapore for analysis.
Embryoid Body Formation
To form embryoid bodies, confluent undifferentiated hiPSCs were mechanically scraped into strips and transferred to six-well low-attachment plates in a differentiation medium consisting of DMEM supplemented with 10% fetal bovine serum (PAN Biotech, Aidenbach, Germany, http://www.pan-biotech.com), 1× nonessential amino acids (Gibco), 1× l-glutamine (Gibco), and 1× pen/strep (Gibco).
Assay for Teratoma Formation
For teratoma formation, hiPSCs grown on MEFs were collected by collagenase treatment, resuspended in Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), and injected into the hind limb muscle of 6-week-old severe combined immunodeficiency (SCID) mice. One well of hiPSCs in a six-well plate of 70% confluence was used for each mouse. Three mice were injected for each hiPSC clone. After 6–10 weeks, teratomas were dissected and fixed in formalin, embedded in paraffin, and subjected to hematoxylin and eosin staining for all histological determinations.
T/B-Cell Rearrangement Assay
DNA was isolated from hiPSCs and control cell lines using DNeasy Blood and Tissue Kit (Qiagen), per the manufacturer’s instructions. For the PCR assay on TCR β, γ, and IgH rearrangement, the primers used were described previously [24]. PCR (35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds) was performed using 1–2 U of Taq DNA polymerase (Qiagen) in a 25-µl reaction with 50 ng of genomic DNA, 10 pmol of each primer, and 200 µM dNTP in 1× PCR buffer. All PCR started with initial denaturation (3 minutes at 95°C) and ended with final extension (10 minutes at 72°C) before holding at 4°C. Alternatively, the PCR was performed using KAPA SYBR FAST qPCR Master Mix (2×) (KK4602) with 1 ng of genomic DNA and 0.2 µM primers in a 10-µl reaction. The PCR and gel-running conditions were same as described in DNA fingerprinting and karyotyping, except that annealing temperature for PCR was set to 65°C.
ABO/Rh Group Typing
A quantity amounting to 1 µl of finger-prick blood was mixed with 1 µl of antibody solution (anti-A, anti-B, anti-A,B, or anti-D, from DIAGAST, Loos, France, http://www.diagast.com) on a glass slide. The reactions were incubated for 30 seconds, and the solutions were then spread and allowed to dry. The glass slides were scanned, and the results were documented. The ABO and Rhesus factor genotyping was carried out as described by Muro et al. [25] and Simsek et al. [26] respectively. The PCR and gel-running conditions were the same as described in DNA fingerprinting and karyotyping, except that annealing temperature for PCR was set to 63.5°C and the cycle number was reduced to 35 cycles.
ADH and ALDH Genotyping
The primers and condition used in PCR of ADH2, ADH3, and ALDH2 are similar to those described by Chen et al. [27], except that 5–10 ng of gDNA was used for each reaction and KAPA HiFi HotStart (KK2601) or KAPA SYBR FAST qPCR Master Mix (2×) (KK4602) was used for the amplifications. The PCR products were subsequently cloned into pcDNA3.3 by using a TOPO TA cloning kit (Invitrogen). At least 10 colonies were picked and sequenced.
Cardiac Differentiation
Human iPSCs were dissociated into single cells by incubating with Accutase (Sigma-Aldrich) at 37°C for 5 minutes and then were seeded onto a Matrigel-coated cell culture dish at 100,000 cells per cm2 in mTeSR1 medium supplemented with 5 μM ROCK inhibitor (Y-27632; Stemgent) and 10 μM CHIR99021 (Stemgent) for 24 hours. Cells were then cultured in RPMI 1640 medium supplemented with B27 without insulin for 2 days. The medium was then changed to RPMI/B27-insulin supplemented with 5 µM IWP-2 (Stemgent) for 2 more days and then changed to RPMI/B27-insulin for another 2 days, after which the cells were maintained in RPMI 1640 medium with B27, with media changed every 3 days [28]. Staining for cardiomyocyte markers were performed with mouse monoclonal antibodies against α-actinin (A7811; Sigma-Aldrich), β-myosin heavy chain (Alexis Biochemical, Lausen, Switzerland, http://www.axxora.com; F36), and titin (Millipore, Billerica, MA, http://www.millipore.com; MAB1553).
Patch-Clamp Analysis
Whole-cell patch-clamp recordings were performed using Axon 200B patch-clamp amplifier (Axon Instruments/Molecular Devices, Union City, CA, http://www.moleculardevices.com) and low-pass filtered at 2–5 kHz. Data acquisition was achieved using the Digidata 1440A (Molecular Devices Corporation, Sunnyvale, CA, http://www.moleculardevices.com). Borosilicate glass electrodes (1.5 mm optical density) were pulled by a horizontal puller (model P-97; Sutter Instrument, Novato, CA, http://www.sutter.com) and fired-polished to a final resistance of 2–3 MΩ when filled with internal solution. The spontaneous action potentials were recorded from hiPSC-derived cardiomyocytes in normal Tyrode’s solution containing the following (in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10, HEPES 10, with the pH value adjusted to 7.40 with NaOH. The solution contained the following (in mM): KCl 130, NaCl 5, MgCl2 1, MgATP 3, EGTA 10, and HEPES 10, with the pH value adjusted to 7.20 with KOH. Cells were maintained by a TC-324B single-channel heater controller (Warner Instruments, Hamden, CT, http://www.warneronline.com) at 35°C–37°C during the recording.
Ethanol Patch Test
Approximately 1 drop of 70% ethanol was added onto a 10 × 10-mm lint pad fixed on an adhesive tape. The patch was attached to the inner surface of the upper arm for 7 minutes before removal. A patch area that showed erythema 10–15 minutes after removal was judged as positive. Controls included lint pad (without any fluid), lint pad with H2O, and lint pad with caffeine [29].
DNA Microarray
Total RNA from PBMCs, hESCs, or hiPSCs from PBMCs was labeled with Cy3. Samples were hybridized to a HumanHT-12 v4 BeadChip (Illumina, San Diego, CA, http://www.illumina.com), according to the manufacturer’s protocol. Arrays were scanned with a BeadChip Reader (Illumina). Data normalization was calculated with the Quantile normalization algorithm implemented in the BioConductor lumi package (http://bioconductor.org/packages/2.12/bioc/html/lumi.html). The cluster analysis was performed and scatter plots were generated in R packages (http://www.r-project.org). Raw data have been deposited at Gene Expression Omnibus under accession number GSE53485.
Results
Generation of hiPSCs From Submilliliter of Human Peripheral Blood
Using 2 ml of human peripheral blood mononuclear cells (PBMCs), we successfully reprogrammed T cells and non-T cells from two individuals (data not shown). This is in good agreement with previous studies that started with similar volumes of blood samples for reprogramming [18, 19]. Next, we asked whether the same Ficoll purification protocol would yield enough cells from blood samples less than 1 ml in volume. We gradually reduced the starting volume of blood and confirmed that reprogramming could be achieved with as little as 250–500 µl of venous blood (supplemental online Fig. 1A). Using immunofluorescence, VPiPSCs stained positive for OCT4, TRA-1-60, and SSEA-4 (supplemental online Fig. 1B). Quantitative reverse transcription-PCR (qRT-PCR) results showed the reactivation of pluripotency markers (supplemental online Fig. 1C). Notably, the Sendai virus transgenes were not detected in the VPiPSCs after four passages in culture (supplemental online Fig. 1D). Consistent with their hESC-like morphology, VPiPSCs show demethylation of CpG dinucleotides at the OCT4 and NANOG promoters (supplemental online Fig. 2A) and are able to differentiate into different lineages in vitro (supplemental online Fig. 2B, 2C). DNA fingerprinting analysis verified that these cells were indeed derived from the parental blood cells (supplemental online Table 1). However, when we used this approach of venipuncture followed by Ficoll-Paque centrifugation, we failed to obtain sufficient PBMCs for reprogramming when the starting blood volume was below 250 µl. This could be because of technical difficulties associated with isolating the thin layer of buffy coat, which results in loss of mononuclear cells.
Derivation of hiPSCs From Finger-Prick Blood Samples
To investigate whether we could generate hiPSCs from minute amounts of blood samples, we modified the purification process to exclude the use of conventional Ficoll purification. We collected finger-prick samples from different individuals (supplemental online Fig. 3A) and subjected the cells to hypotonic lysis solution for depletion of the red blood cells (supplemental online Fig. 3B). Strikingly, we found that the RBC-lysed finger-prick cells from 10 µl of blood could be expanded in medium culture to yield enough dividing cells for reprogramming (supplemental online Fig. 3C). Consistent with previous reports [15, 21], the cells after 15 days of culture expansion were largely CD71+ myeloid progenitors (supplemental online Fig. 3D). Using this approach, we observed the appearance of hESC-like colonies 20 days after transduction with Sendai virus (Fig. 1A). All finger-prick-derived iPSC (FPiPSC) clones have been propagated for at least 20 passages as of this submission and remain stable in culture.
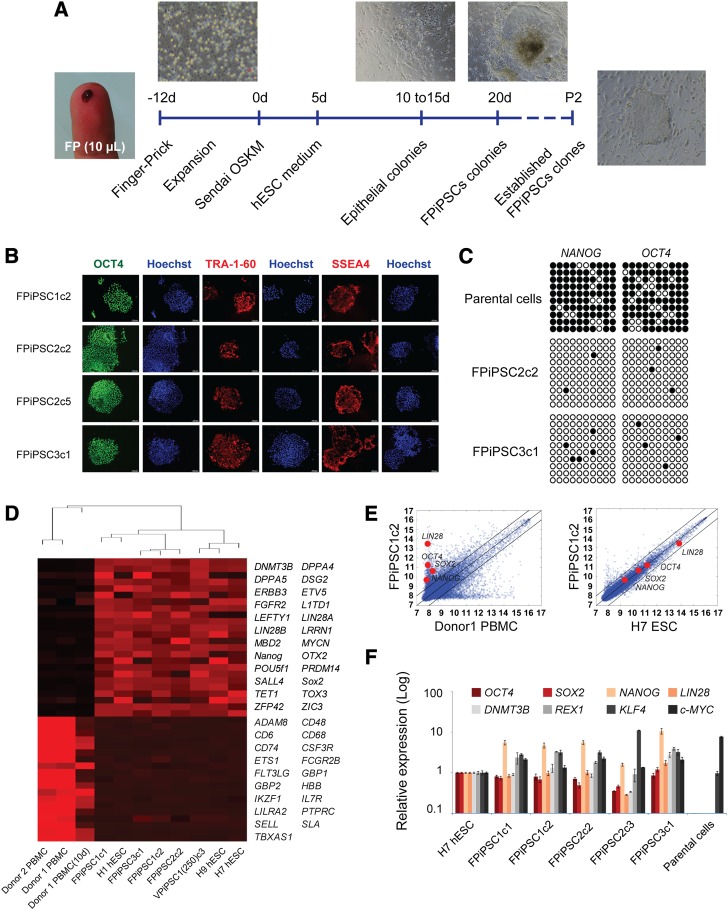
Figure 1.
Derivation of human induced pluripotent stem cells (hiPSCs) from finger-prick blood samples. (A): Schematic drawing of the strategy used in this study for reprogramming human finger-pricked blood. Images show the collection of finger-pricked blood and expansion of cells in culture and adherent colonies. The first hESC-like colony normally appeared between days 18 and 20 and was picked after day 21. The FPiPSCs derived can be passaged stably for long-term culture. (B): Immunofluorescent staining of FPiPSC colonies expressing pluripotency markers for TRA-1-60, SSEA4, and OCT4. Hoechst staining indicates the total cell content per field. All images were acquired with original magnification ×4 objectives. (C): Bisulfite DNA methylation analysis on the promoter of OCT4 and NANOG in FPiPSCs and in their parental cells. The white and black circles represent unmethylated and methylated CpG islands in the promoter region, respectively. (D): Dendrogram showing unsupervised hierarchical clustering of the global expression profiles of hESCs, hiPSCs, and parental blood cells (top). Heat-map analysis of the hESCs, hiPSCs, and parental blood cells for expression of pluripotency and hematopoietic-specific marker genes (bottom). Red represents upregulation; black represents downregulation. (E): Scatter plots comparing FPiPSC global gene expression profiles with parental cells (left) and H7 human ESCs (right). The black lines indicate the linear equivalent and twofold changes in gene expression levels between the paired cell types. Positions of pluripotency genes OCT4, SOX2, NANOG, and LIN28 in scatter plots are indicated. (F): Quantitative reverse transcription-polymerase chain reaction analyses for the expression of ESC marker genes OCT4, SOX2, NANOG, LIN28, DNMT3B, REX1, KLF4, and c-MYC in FPiPSCs, H7 ESCs, and parental cells. Individual polymerase chain reactions were normalized to H7 ESCs and β-ACTIN as internal control. All the FPiPSC lines examined showed similar expression of ESC marker genes to that of H7 ESCs. Abbreviations: ESC, embryonic stem cell; FP, finger prick; FPiPSC, finger-prick-derived induced pluripotent stem cell; hESC, human embryonic stem cell; OSKM, OCT4, SOX2, KLF4, and c-MYC; P2, passage 2; PBMC, peripheral blood mononuclear cell; VPiPSC, venipuncture-derived induced pluripotent stem cell.
We examined the expression of pluripotency markers in the FPiPSC lines using immunostaining. In line with their hESC-like morphology, we observed expression of OCT4, TRA-1-60, and SSEA-4 (Fig. 1B). Consistent with the activation of pluripotency-associated genes, reprogramming of the finger-prick blood cells was accompanied by the demethylation of CpG dinucleotides at the OCT4 and NANOG promoters (Fig. 1C). We next performed global gene expression analysis of the FPiPSCs comparing them with hESCs and somatic parental cells. Clustering dendrogram revealed a high degree of similarity among the reprogrammed hiPSCs that clustered together with the H1, H7, and H9 ESCs and were distant from the parental somatic cells (Fig. 1D, top). Heat-map analysis indicates the reactivation of pluripotency genes and the suppression of hematopoietic genes in the hiPSC lines (Fig. 1D). Analysis of scatter plots similarly shows a tighter correlation between reprogrammed FPiPSCs and human ESCs (H7 ESCs) rather than with the parental blood cells (Fig. 1E). Quantitative PCR analysis confirmed the expression of endogenous pluripotency markers, including OCT4, SOX2, KLF4, c-MYC, REX1, NANOG, LIN28, and DNMT3B (Fig. 1F). Notably, these genes were restored in the FPiPSCs to levels similar to those observed in H7 hESCs (Fig. 1F).
FPiPSCs Differentiate Into Derivatives of Three Germ Layers
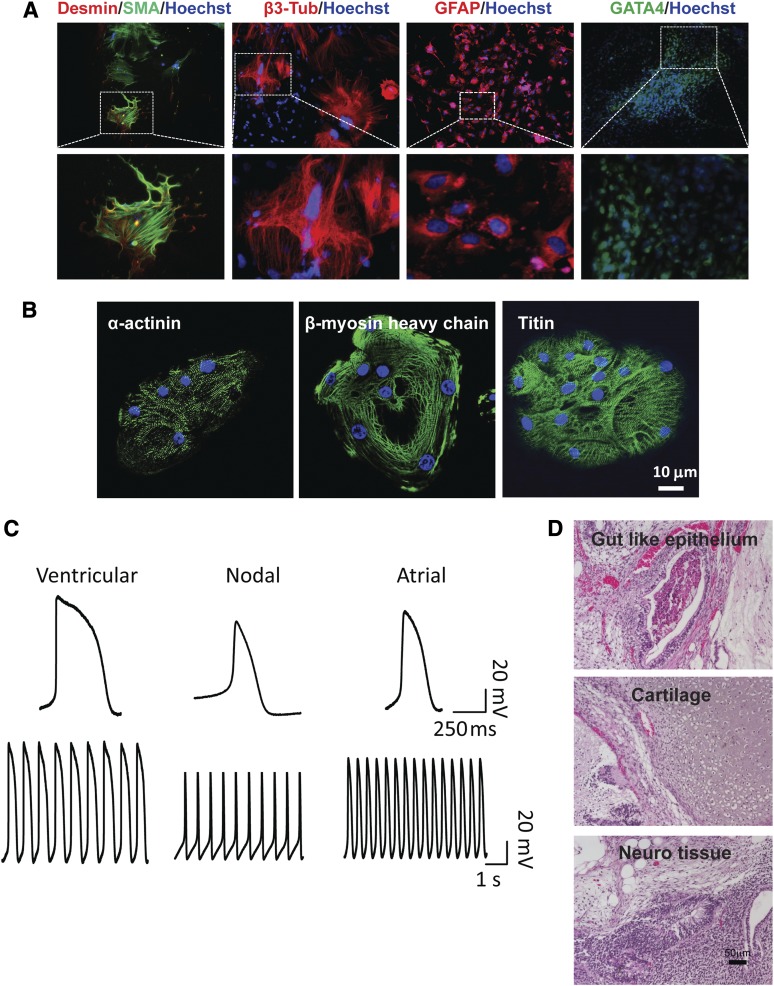
Next, we evaluated the developmental potential of the FPiPSC lines by in vitro embryoid body (EB) differentiation. The hiPSCs readily formed EBs upon induction and were capable of differentiating into mesodermal (Desmin and SMA), neural (β3-tubulin, GFAP), and endodermal (GATA4) lineages (Fig. 2A). Following specific in vitro differentiation, FiPSCs gave rise to rhythmically beating cardiomyocytes (supplemental online Movie 1). Positive staining for α-actinin, β-MHC, and titin confirmed their cardiomyocyte identities (Fig. 2B). Using patch-clamp analysis, we characterized individual cardiomyocytes and showed that they developed distinct ventricular, atrial, and nodal action potentials (Fig. 2C). The most rigorous test for the pluripotency of human iPSCs is the formation of teratomas in immune-deficient mouse hosts [30]. Upon injection into the hind limb muscle of SCID mice, the FPiPSC lines generated well-differentiated cystic teratomas comprising structures and tissues derived from the three embryonic germ layers. These included gut-like epithelium, cartilage, and neural tissues (Fig. 2D).
Figure 2.
Finger-prick-derived induced pluripotent stem cells (FPiPSCs) differentiate into derivatives of three germ layers. (A): Immunofluorescent staining of differentiation markers after in vitro differentiation of FPiPSCs. Ectoderm was stained by GFAP and β3-tubulin antibodies, whereas endoderm was stained by antibody against GATA4. All images in the upper panel were acquired with original magnification ×10 objectives, except for GATA4 staining image, which was acquired with ×4 objectives. Lower panel images are shown in higher magnification. Hoechst staining indicates the nucleus. (B): Immunofluorescent staining of α-actinin, β-myosin heavy chain, and titin in human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes. Hoechst staining indicates the nucleus. (C): Patch-clamp analyses of hiPSC-derived cardiomyocytes. (D): Hematoxylin and eosin staining of teratomas derived from immune-deficient mice injected with FPiPSCs shows tissues representing all three embryonic germ layers, including gut-like epithelium (endoderm), cartilage (mesoderm), and neural tissue (ectoderm). Scale bar = 50 μm. Abbreviations: β3-Tub, β3-tubulin; GFAP, glial fibrillary acidic protein; SMA, smooth muscle α actin.
FPiPSCs Are Free From Transgenes and V(D)J Rearrangement
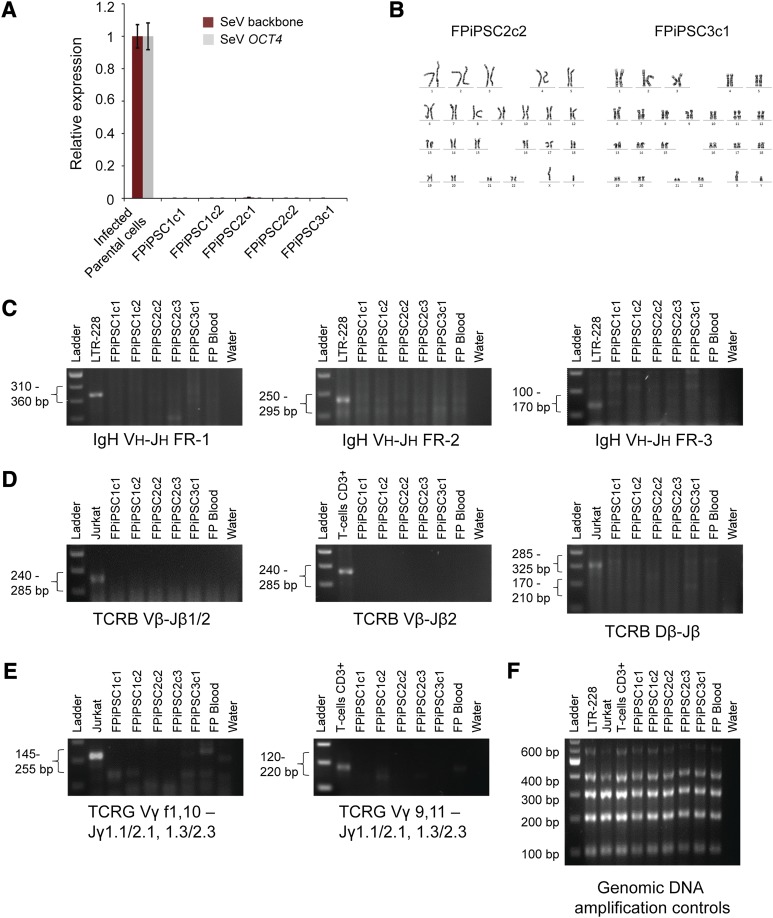
Efficient transgene silencing is essential for the derivation of pluripotent hiPSC lines [3]. qRT-PCR using primers specific for the Sendai vector backbone and the exogenous Sendai OCT4 reprogramming factor confirmed that the FPiPSCs do not carry any Sendai transgenes (Fig. 3A). We observed normal karyotypes for the FPiPSC lines that we examined (Fig. 3B). The DNA fingerprinting analysis verified that these cells were indeed derived from the parental blood cells and were not a result of contamination from existing hESC or hiPSC lines (supplemental online Table 1). Because B and T cells are abundant in circulating blood and the latter can easily be reprogrammed [11–13], we tested the FPiPSC clones for TCR and IgH rearrangement using PCR-based detection. Whereas the clonal controls (Jurkat and LTR-228) and CD3+ T cells showed bands indicative of rearrangement, all the FPiPSC and VPiPSC clones examined did not have any rearrangement at the TCR β, γ, and IgH loci (Fig. 3C–3F; supplemental online Fig. 4). Thus, our results confirm that the FPiPSCs did not originate from lymphoid T and B cells.
Figure 3.
Characterizations of the FPiPSCs. (A): Transgene expression analysis of the FPiPSC lines derived. The SeV backbone and SeV OCT4 expression values were measured by quantitative reverse transcription-polymerase chain reaction and normalized to SeV-infected parental cell with β-ACTIN as internal control. No SeV transgene was detected in the total RNA harvested from these human induced pluripotent stem cell lines. (B): Cytogenetic analysis on FPiPSCs. The FPiPSCs showed normal human karyotype with 46 chromosomes. (C): IgH rearrangement analysis on the FPiPSC clones. LTR-228 (B-cell line) was used as a positive control. No IgH recombination was observed in our FPiPSCs. (D): TCRB rearrangement analysis on the FPiPSC clones. Jurkat (T-cell line) or CD3+ T cells were used as positive controls for this analysis. (E): TCRG rearrangement analysis on the FPiPSC clones. Jurkat cells (T-cell line) or CD3+ T cells were used as positive controls for this analysis. Negative results from all the V(D)J rearrangement polymerase chain reaction (PCR) imply that the FPiPSCs derived did not originate from B or T lymphocytes. In (C), (D), and (E), the expected PCR product range was stated on the left and the target regions were indicated below the gel images. The finger-prick blood used in this assay had been cultured in cell expansion medium for at least 15 days before being harvested. A water sample was always included in the PCR analysis as a negative control. (F): The genomic DNA amplification control experiment was performed on all the samples used in these V(D)J rearrangement analyses. Abbreviations: FPiPSC, finger-prick-derived induced pluripotent stem cell; SeV, Sendai virus; TCR, T-cell receptor.
Reprogramming From DIY Finger-Prick Samples
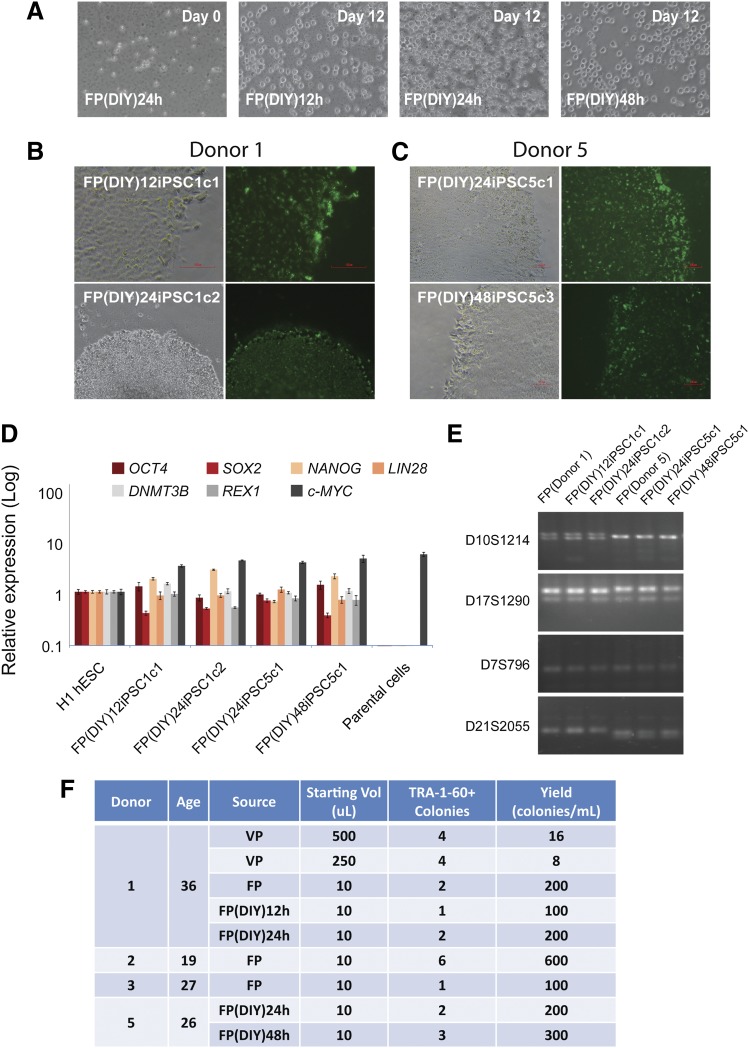
Unlike venipuncture, which can only be performed by trained phlebotomists, finger-prick blood can be collected by the donors themselves with proper instruction. To test this idea, we asked the donors to prick their own fingers in a normal room environment and collect a single drop of blood into an EDTA tube. The tube was placed on ice and delivered to the laboratory for reprogramming. Twelve, 24, or 48 hours later, the cells were treated with RBC lysis buffer and observed under the microscope for viability and signs of contamination (Fig. 4A). Interestingly, after 12 days of expansion in medium, the cells appeared healthy and were actively dividing (Fig. 4A). To commence reprogramming, cells isolated from the finger-prick samples were transduced with Sendai virus. We observed hESC-like colonies approximately 20 days postinfection. In total, we obtained eight TRA-1-60+ colonies from two donors in four independent experiments (Fig. 4B). V(D)J rearrangement PCR confirmed that the FP(DIY)iPSCs were not originated from lymphoid T and B cells (supplemental online Fig. 5). Quantitative PCR analysis indicated the expression of endogenous pluripotency marker genes to the level comparable with H1 ESCs (Fig. 4D). Notably, the FP(DIY)iPSCs were derived from respective donor FP cells and not the result of contamination from existing hESCs or hiPSCs (Fig. 4E). The efficiency of 100–300 colonies per milliliter is similar to the reprogramming results from fresh samples taken under a controlled and clean laboratory setting. Interestingly, we did not observe noticeable reduction in reprogramming efficiency between freshly collected and DIY finger-prick samples (Fig. 4F). In summary, we derived transgene-free and V(D)J rearrangement-free hiPSCs from submilliliter volumes of venipuncture and single-drop volumes of finger-prick samples (Fig. 4F; supplemental online Table 2). We report a high reprogramming yield of 100–600 colonies per milliliter of blood (Fig. 4F).
Figure 4.
DIY concept for finger-prick-derived induced pluripotent stem cell (FPiPSC) generation. (A): FP blood samples collected 12 hours, 24 hours, and 48 hours before cell culture. The cells were red blood cell-lysed at day 0 (left) and expanded for 12 days in medium. All images were acquired with original magnification ×20 objectives. (B): Human induced pluripotent stem cell (hiPSC) colonies derived from donor 1 DIY FP blood (12 hours and 24 hours) stained positive for a pluripotency marker, TRA-1-60. The upper image was acquired with original magnification ×20 objectives, whereas the lower image was acquired with original magnification ×4 objectives. (C): hiPSC colonies derived from donor 5 DIY FP blood (24 hours and 48 hours) stained positive for a pluripotency marker, TRA-1-60. All images were acquired with original magnification ×10 objectives. (D): Quantitative reverse transcription-polymerase chain reaction analyses for the expression of embryonic stem cell (ESC) marker genes OCT4, SOX2, NANOG, LIN28, DNMT3B, REX1, and c-MYC in FP(DIY)iPSCs, H1 ESCs, and parental cells. Individual polymerase chain reactions (PCRs) were normalized to H1 ESC and β-ACTIN as internal control. All the FP(DIY)iPSC lines examined showed similar expression of ESC marker genes to that of H1 ESCs. (E): DNA fingerprinting analysis confirms that FPiPSC lines are derived from their parental lines. PCR primer sets D10S1214, D17S1290, D7S796, and D21S2055 were used to detect for variable tandem repeats. (F): Summary of hiPSCs derived from donors. The VP and FP indicated were the venipuncture blood and finger-pricked blood, respectively. The reprogramming yield was calculated based on the TRA-1-60 colonies observed and normalized to 1 ml of blood. Abbreviations: DIY, do-it-yourself; FP, finger prick; hESC, human embryonic stem cell; iPSC, induced pluripotent stem cell; VP, venipuncture.
Toward an Integrative Strategy for hiPSC Banking
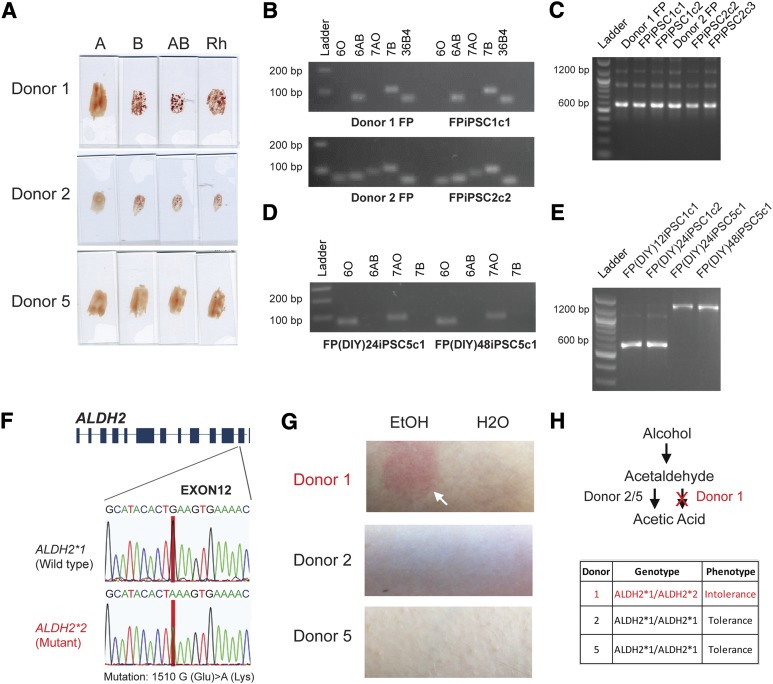
Because a single drop of blood is approximately 20 µl and only 10 µl is required for reprogramming, we asked whether the remaining amount of the donors’ blood could be used for other analyses. Pluripotent stem cells can potentially be an unlimited resource for cell therapy, but immune rejection may pose problems during transplantation. Apart from the human leukocyte antigen [31], a recent study discovered that in vitro hESCs differentiated hepatocytes and cardiomyocytes express ABO antigens [32]. Using 4 µl of finger-prick blood, we performed a serological analysis of A, B, and Rh antigen groups for three donors (Fig. 5A). We then asked whether we could harvest gDNA from finger-prick samples. We found that 2 µl of blood would yield approximately 150 ng of PCR-grade gDNA for molecular studies. ABO/Rh typing could also be determined using PCR-based technology. Moreover, PCR analysis could decipher the exact genotype of the ABO groups. We showed that although both donor 1 and 2 had a phenotype of B+, donor 1 had a genotype of BB+, whereas donor 2 was BO+ (Fig. 5B, 5C). In contrast, donor 5 was recessive for both the ABO and Rh antigens, as shown by the O− phenotype (Fig. 5A). Genotypic analysis of donor 5 finger-prick blood corroborated with the serological results (supplemental online Fig. 6A, 6B). Interestingly, DIY finger-prick blood samples showed no sign of deterioration (24 hours and 48 hours after sample collection) as they faithfully recapitulated the donors’ serological ABO/Rh blood type (supplemental online Fig. 6C). As expected, both the FPiPSCs and FP(DIY)iPSCs derived from the respective donors exhibited the same ABO and Rh genotype as the parental blood cells (Fig. 5B–5E; supplemental online Fig. 6D).
Figure 5.
Blood typing and DNA genotyping the finger-prick samples. (A): Blood grouping of the donors from finger-prick samples. The upper panel shows the antibodies used. If the erythrocytes of the donor carry the corresponding antigen, agglutination will take place and the donors’ blood groups can be determined. The phenotypic blood group for donors 1 and 2 were type B+, whereas donor 5 was O−. (B): Genotyping of the donors’ blood group from finger-prick blood and derived human induced pluripotent stem cells (hiPSCs). Specific primers were used for ABO allele detection. The upper panel shows the specific allele that the primers amplified. The primers 6O, 6AB, 7AO, and 7B were designed to target the O allele exon 6, A or B allele exon 6, A or O allele exon 7, and B allele exon 7, respectively. A clear DNA band (∼100 bp) indicates a positive PCR result and shows that the donor carries these alleles. Donor 1 FP blood and FPiPSC show the BB genotype (upper panel), whereas donor 2 FP blood and FPiPSC show the BO genotype (lower panel). The primers targeting the reference gene 36B4 were included as a positive control for genomic DNA amplification. (C): Rhesus D antigen genotyping of the finger-prick samples and derived hiPSCs. The primers were designed to target both Rh CcEe and Rh D regions. The 1,200-bp band is amplified from the Rh CcEe alleles, and 600 bp is from Rh D allele. For Rh D-positive samples, a clear 600-bp band and a 1,200-bp band can be observed; otherwise, only 1,200-bp band can be detected. Donors 1 and 2 and their hiPSCs showed a Rh D-positive genotype. (D): Genotyping of the donors’ blood group from DIY finger-prick blood-derived hiPSCs. Donor 5 FP(DIY)iPSCs showed a OO genotype. (E): Rhesus D antigen genotyping of the DIY finger-prick samples derived hiPSCs. Donor 1 FP(DIY)iPSCs showed Rh D-positive genotype, whereas donor 5 FP(DIY)iPSCs is Rh D-negative. (F): Single-nucleotide polymorphism on the ALDH2 loci. ALDH2 is responsible for conversion of acetaldehyde to acetic acid. ALDH2*2 allele consists of the glutamate to lysine mutation (1510 G→A) and encodes a defective ALDH2. (G): Ethanol patch test on the donors. Donor 1 showed a flush reaction to ethanol, whereas donors 2 and 5 showed no effect to ethanol when it was applied onto the donor’s skin. (H): The upper panel shows the metabolic pathway for degradation of ethanol in the body. The ethanol is first oxidized into toxic acetaldehyde by alcohol dehydrogenase and subsequently oxidized to acetic acid by ALDH. In our study, donor 1 possesses an ALDH2*2 allele and thus manifested an intolerance to ethanol. Abbreviations: ALDH, aldehyde dehydrogenase; DIY, do-it-yourself; FP, finger prick; FPiPSC, finger-prick-derived induced pluripotent stem cell; iPSC, induced pluripotent stem cell.
To further evaluate whether the gDNA isolated from the small amount of finger-prick blood samples can be used to detect single-nucleotide mutations, we performed sequencing analysis on the aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) genes involved in the alcohol metabolism. Interestingly, our sequencing results on ALDH2 indicated that donor 1 carried a mutant ALDH2*2 allele (1510 G > A mutation) (Fig. 4F). The mutation was observed in both the blood sample and the derivative hiPSCs (supplemental online Fig. 7A, 7B). Various reports have suggested that the single-nucleotide polymorphism (SNP) on ALDH2 could lead to protein malfunction, resulting in the accumulation of toxic acetaldehyde in the body, and manifests as a red flush on the skin [33, 34]. We further explored the DIY concept and asked whether the donors could perform a simple test when the finger-prick samples were collected. Consistent with the ALDH2 genotype, an ethanol patch test performed by donor 1 resulted in redness of the skin, whereas donors 2 and 5, who carried the homozygous normal ALDH2*1 alleles, did not display any flush syndrome (Fig. 5G; supplemental online Fig. 7C). In summary, using a combination of DNA sequencing and a DIY ethanol test, we profiled the level of alcohol tolerance for donors 1, 2, and 5 (Fig. 5H; supplemental online Fig. 7D). Importantly, the hiPSCs generated faithfully recapitulated the mutant genotypes and will be a good resource for future study of alcohol metabolism (supplemental online Fig. 7D).
Discussion
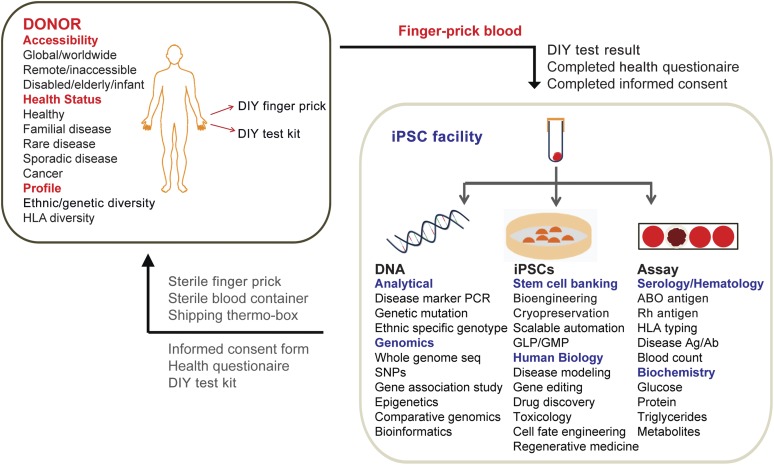
Numerous hiPSC banking initiatives are currently being established around the world [35, 36]. The potential of such stem cell repositories can only be realized if they have access to a wide range of donors/patients of diverse ethnicity, genotype, and disease. The ability to reprogram from a small volume of finger-prick samples provides a strategy for hiPSC banks to access a large cohort of donors. In this model (Fig. 6), the hiPSC facility provides a package consisting of the DIY finger-prick collection kit, shipping thermo box, informed consent form, health questionnaire, and DIY test kit to the donors. In particular, donors with difficulties in traveling because of logistics, financial conditions, or physical conditions (for example, the bedridden, the elderly, the disabled, and infants) will benefit from this strategy. It will also be possible to recruit donors with rare disease phenotypes from remote locations. As shown in our study, the donor can perform a finger prick to collect blood samples in a tube containing anticoagulant and store it at 4°C (Fig. 5A). The health questionnaires will help to detail the clinical conditions and medical history of the donors. Finally, the donors can perform specific DIY tests, as demonstrated by our study (Fig. 5G). These tests can be tailored according to specific questions that the study or facility aims to address. The completed documents, consent form, and finger-prick samples can simply be returned to the hiPSC bank via global courier services.
Figure 6.
Integrative strategy for human induced pluripotent stem cells (hiPSC) banking. Illustration of the integrative strategy for hiPSC banking. Using finger-prick (FP) blood reprogramming, hiPSC facilities can recruit a diverse cohort of donors worldwide. hiPSC facility provides a kit containing sterile finger-prick and blood container to the donor. The donors will complete the informed consent form and the health questionnaire and return them back to the facility together with their FP blood. The facility is able to do a series of DNA sequencing, serological assays, as well as hiPSC derivation from a single drop of FP blood sample. Abbreviations: Ag/Ab, antigen/antibody; DIY, do-it-yourself; HLA, human leukocyte antigen; iPSC, induced pluripotent stem cell; GLP, good laboratory practice; GMP, good manufacturing practice; PCR, polymerase chain reaction; seq, sequencing; SNP, single-nucleotide polymorphism.
At the facility, the samples can be tested for disease genotype or undergo deep genome sequencing to uncover an extensive catalog of genetic variations, SNPs, and haplotypes (Fig. 5F). Analysis of blood provides a general assessment of the donor’s health and phenotype, as the facility can perform serotyping and measure blood count and levels of various metabolites. Finally, the reprogrammed hiPSCs can be used for stem cell banking, disease modeling, drug screening, toxicology tests, and cell fate engineering. Taken together, our model presents an unprecedented opportunity for banking hiPSCs with full annotations of associated genotype (DNA sequencing), phenotype (serological and DIY test), and clinical information (blood biochemistry and health questionnaires) (Fig. 6).
Several previous studies described the reprogramming of nonlymphoid human peripheral blood cells. One of the first studies, by Chou et al. [15], targeted CD71+ mononuclear cells and established iPSCs at the frequency of 0.00018%–0.0008%. Other groups, including Mack et al. [16], purified CD34+ progenitor cells from peripheral blood and observed an efficiency of 0.077%. Although our reprogramming efficiency of 0.008%–0.024% is comparable to these studies, the use of episomal vectors carrying additional factors (5–8 factors) can potentially improve the reprogramming process [15, 16]. Also, a newer version of temperature-sensitive Sendai virus, which allows for rapid removal of residual virus genomic RNA, could further increase the robustness of the current finger-prick reprogramming protocol [37]. As compared with the work by Merling et al. [18], which described the isolation of 20 hiPSC colonies starting with 1 ml of human peripheral blood, the reprogramming yield of 100–600 colonies per milliliter from our finger-prick samples is a significant improvement.
In total, we recruited five donors (19–36 years old), and samples from four of them resulted in successful reprogramming. We did not observe noticeable reduction in reprogramming efficiency as a function of donors’ age. Nevertheless, there remains an information gap on whether finger-prick samples from elderly donors can be effectively reprogrammed. The one donor (aged 29 years old) from whom we collected the finger-prick samples in a laboratory setting did not give rise to enough cells in culture. Because of that, we did not commence viral infection for reprogramming. This suggests that finger-prick samples collected from different individuals may have varied ability for adapting to growth in culture conditions. Furthermore, when we tried to expand the DIY finger-prick samples collected 72 hours before cell culture, we observed high degree of cell death. Future studies using more advanced medium conditions that promote better cell growth and reprogramming [38] will help to further optimize the process for higher reprogramming efficiency.
A recent study used human urine samples for derivation of epithelial-like cells that can be reprogrammed to pluripotency [39]. This method provides an unlimited source of cells that can be collected conveniently and noninvasively. Nevertheless, the current protocol requires the collection of large volume of urine samples resulting in logistic difficulties for downstream storage and transportation [40]. The technique that we describe in this work provides the possibility of collecting small volume of DIY finger-prick samples from a remote location. However, this does not eliminate the benefits of collecting the samples in the hospital. Furthermore, the key advantage of hospital-originated samples is the availability of complete patient medical and diagnostic information.
Conclusion
We have successfully developed a highly efficient reprogramming technique requiring only 10 µl of finger-prick blood samples. Finger-prick samples can be easily and conveniently collected without the need for trained phlebotomists. We show for the first time that donors can collect their own blood samples (DIY) for sending to the hiPSC facility. Expanding on its utility, we demonstrated that a single drop of finger-prick blood sample can be used for parallel experiments in cellular reprogramming, DNA sequencing, and blood typing/biochemistry. Our ability to reprogram finger-prick samples will aid the recruitment of donors from geographically, genetically, and ethnically diverse populations. Our strategy when applied together with recent advances in gene editing [41], as well as automation and miniaturization of hiPSC derivation technologies [42], will increase the functionality, throughput, and feasibility of operating a large-scale international hiPSC bank.
Supplementary Material
Acknowledgments
We are grateful to Yang Lin, Samantha Seah, and Chong Zheng Shan for helpful discussion and comments on the manuscript. We thank Everett Koh and Chadi El Farran for technical assistance. J.L. is supported by the National Research Foundation (NRF2008-CRP001-68) of Singapore and a Duke-NUS GOH Cardiovascular Research Award (Duke-NUS-GCR/2012/0005R). H.L. is supported by Mayo Clinic Center for Individualized Medicine. Y.-H.L. is supported by the A*STAR Investigatorship research award. We are grateful to the Biomedical Research Council, Agency for Science, Technology, and Research, Singapore, for research funding.
Author Contributions
H.-K.T.: experimental design and performance of research, data analysis, manuscript writing; C.-X.D.T., D.M., B.Y., T.M.L., J.L., C.-W.W., T.-K.T., and H.L.: design and performance of research; C.S., E.-L.T., B.L., Y.-P.L., and S.A.C.: data analysis; Y.-H.L.: experimental design, data analysis, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Park I-H, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Asai M, Tsukita K, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Z, Zhan H, Mali P, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgetti A, Montserrat N, Aasen T, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase A, Olmer R, Schwanke K, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Sohn SK, Kim JG, Seo KW, et al. GM-CSF-based mobilization effect in normal healthy donors for allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2002;30:81–86. doi: 10.1038/sj.bmt.1703598. [DOI] [PubMed] [Google Scholar]
- 11.Loh YH, Hartung O, Li H, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Staerk J, Dawlaty MM, Gao Q, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serwold T, Hochedlinger K, Inlay MA, et al. Early TCR expression and aberrant T cell development in mice with endogenous prerearranged T cell receptor genes. J Immunol. 2007;179:928–938. doi: 10.4049/jimmunol.179.2.928. [DOI] [PubMed] [Google Scholar]
- 15.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack AA, Kroboth S, Rajesh D, et al. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One. 2011;6:e27956. doi: 10.1371/journal.pone.0027956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okita K, Yamakawa T, Matsumura Y, et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 18.Merling RK, Sweeney CL, Choi U, et al. Transgene-free iPSCs generated from small volume peripheral blood nonmobilized CD34+ cells. Blood. 2013;121:e98–e107. doi: 10.1182/blood-2012-03-420273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye L, Muench MO, Fusaki N, et al. Blood cell-derived induced pluripotent stem cells free of reprogramming factors generated by Sendai viral vectors. Stem Cells Translational Medicine. 2013;2:558–566. doi: 10.5966/sctm.2013-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Akker E, Satchwell TJ, Pellegrin S, et al. The majority of the in vitro erythroid expansion potential resides in CD34(-) cells, outweighing the contribution of CD34(+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010;95:1594–1598. doi: 10.3324/haematol.2009.019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Mills JA, Sullivan S, et al. iPSC reprogramming from human peripheral blood using Sendai virus mediated gene transfer. StemBook. 2012. [PubMed]
- 22.Freberg CT, Dahl JA, Timoskainen S, et al. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell. 2007;18:1543–1553. doi: 10.1091/mbc.E07-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prigione A, Fauler B, Lurz R, et al. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 24.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 25.Muro T, Fujihara J, Imamura S, et al. Determination of ABO genotypes by real-time PCR using allele-specific primers. Leg Med. 2012;14:47–50. doi: 10.1016/j.legalmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Simsek S, Faas BH, Bleeker PM, et al. Rapid Rh D genotyping by polymerase chain reaction-based amplification of DNA. Blood. 1995;85:2975–2980. [PubMed] [Google Scholar]
- 27.Chen WJ, Loh EW, Hsu YP, et al. Alcohol dehydrogenase and aldehyde dehydrogenase genotypes and alcoholism among Taiwanese aborigines. Biol Psychiatry. 1997;41:703–709. doi: 10.1016/S0006-3223(96)00072-8. [DOI] [PubMed] [Google Scholar]
- 28.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muramatsu T, Higuchi S, Shigemori K, et al. Ethanol patch test—a simple and sensitive method for identifying ALDH phenotype. Alcohol Clin Exp Res. 1989;13:229–231. doi: 10.1111/j.1530-0277.1989.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 30.Lensch MW, Schlaeger TM, Zon LI, et al. Teratoma formation assays with human embryonic stem cells: A rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–258. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Mölne J, Björquist P, Andersson K, et al. Blood group ABO antigen expression in human embryonic stem cells and in differentiated hepatocyte- and cardiomyocyte-like cells. Transplantation. 2008;86:1407–1413. doi: 10.1097/TP.0b013e31818a6805. [DOI] [PubMed] [Google Scholar]
- 33.Rao VR, Bhaskar LV, Annapurna C, et al. Single nucleotide polymorphisms in alcohol dehydrogenase genes among some Indian populations. Am J Hum Biol. 2007;19:338–344. doi: 10.1002/ajhb.20589. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Q, Weiner H, Johnston T, et al. The aldehyde dehydrogenase ALDH2*2 allele exhibits dominance over ALDH2*1 in transduced HeLa cells. J Clin Invest. 1995;96:2180–2186. doi: 10.1172/JCI118272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao M. Public private partnerships: A marriage of necessity. Cell Stem Cell. 2013;12:149–151. doi: 10.1016/j.stem.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Turner M, Leslie S, Martin NG, et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad, Ser B, Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou T, Benda C, Duzinger S, et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22:1221–1228. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Y, Cai X, Wang L, et al. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS One. 2013;8:e70573. doi: 10.1371/journal.pone.0070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geti I, Ormiston ML, Rouhani F, et al. A practical and efficient cellular substrate for the generation of induced pluripotent stem cells from adults: Blood-derived endothelial progenitor cells. Stem Cells Translational Medicine. 2012;1:855–865. doi: 10.5966/sctm.2012-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.